| Bisphosphonates (alone or with calcium and/or vitamin D) compared with control (calcium and/or vitamin D and/or placebo) for adults with GIOP |
| Patient or population: adults with GIOP Settings: ambulatory Intervention: bisphosphonates (alone or with calcium and/or vitamin D) Comparison: control (calcium and/or vitamin D and/or placebo) |
| Incident vertebral fractures Radiographic follow‐up: 12‐24 months | 77 per 1000 | 44 per 1000
(27 to 70) | RR 0.57 (0.35 to 0.91) RD ‐0.02 (‐0.05 to 0.01) | 1343
(12 RCTs) | ⊕⊕⊕⊕
high1 | Absolute increased benefit 2% fewer people with fractures using bisphosphonates (95% CI 5.00% fewer to 1.00% more) Relative per cent change 43% improvement with bisphosphonates (95% CI 9.00% to 65.00% better) NNTB = 31 (95% CI 20 to 145) |
| Incident nonvertebral fractures Radiographic follow‐up: 12‐24 months | 55 per 1000 | 42 per 1000
(25 to 69) | RR 0.79 (0.47 to 1.33) RD ‐0.01 (‐0.04 to 0.01) | 1245
(9 RCTs) | ⊕⊕⊝⊝
low2,3 due to risk of bias and imprecision | Absolute increased benefit 1% fewer people with fractures using bisphosphonates (95% CI 4.00% fewer to 1.00% more) Relative per cent change 21% improvement with bisphosphonates (95% CI 33.00% worse to 53.00% better) NNTB = n/a4 |
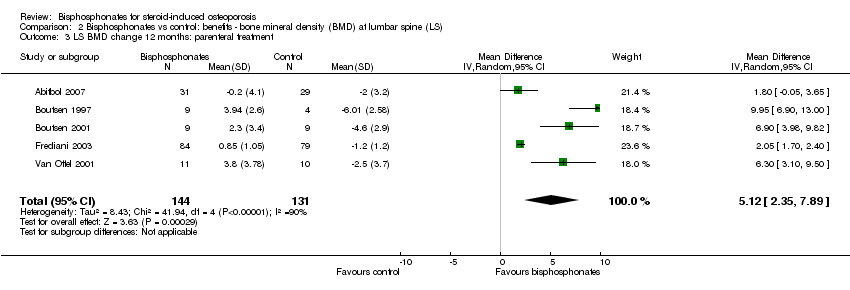
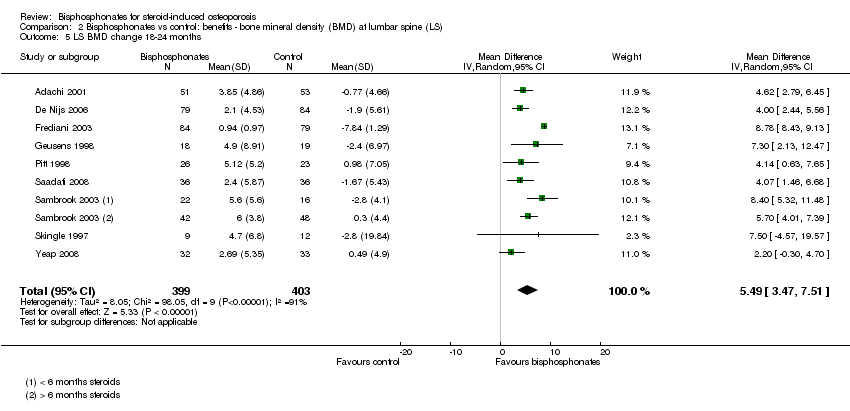
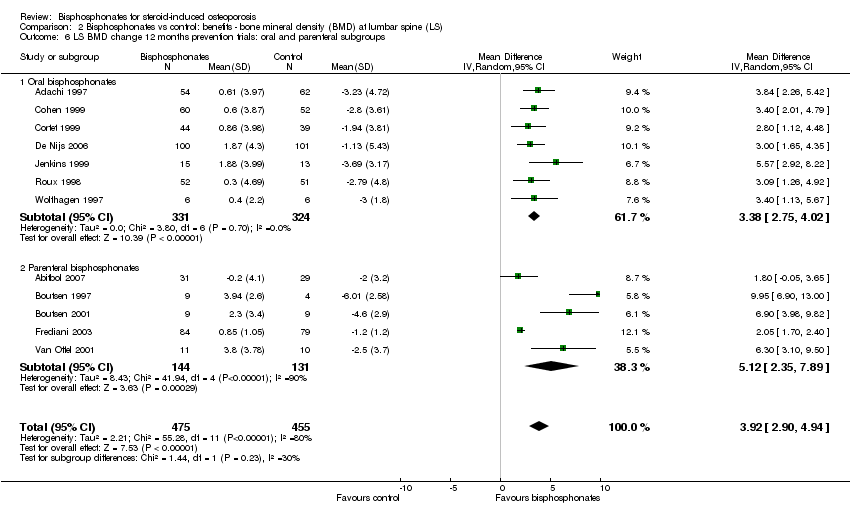
| Lumbar spine BMD DEXA follow‐up: 12 months | Mean per cent change in BMD across control groups was ‐3.19% (‐8.08% to 1.70%) from baseline5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 3.50% higher than control groups (2.90% to 4.10% higher) | ‐ | 2042
(23 RCTs) | ⊕⊕⊕⊝
moderate6,7,8 due to indirectness | Absolute increased benefit 3.50% with bisphosphonates (95% CI 2.90 to 4.10) Relative per cent change 1.10% (95% CI 0.91 to 1.29) with bisphosphonates NNTB = 3 (95% CI 2 to 3) |
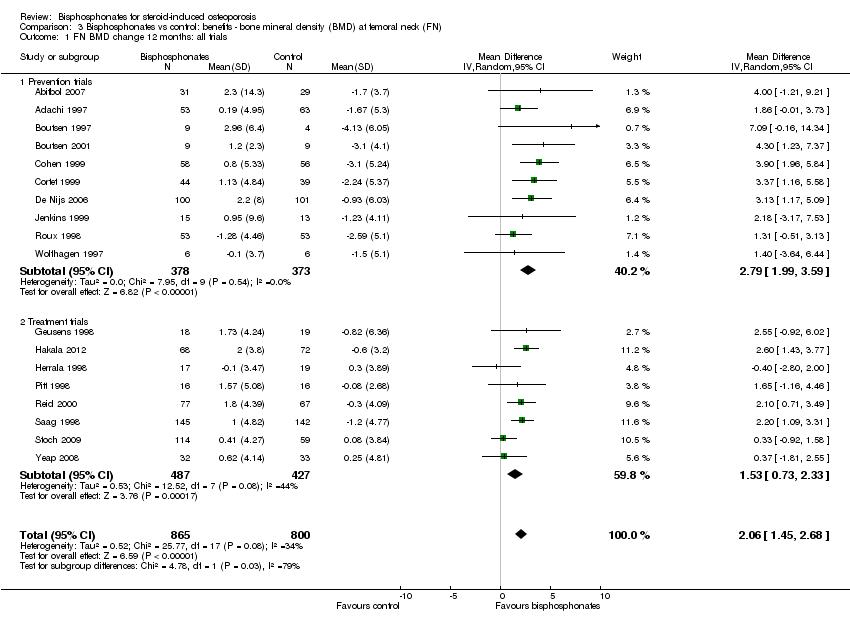
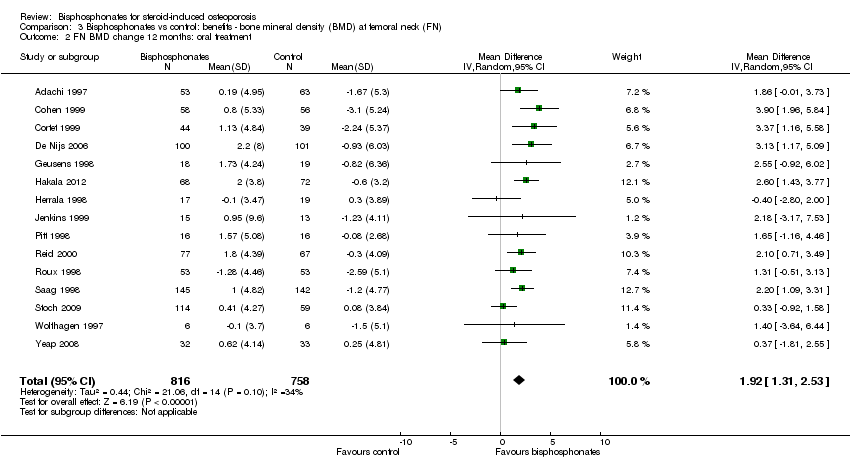
| Femoral neck BMD DEXA follow‐up: 12 months | Mean per cent change in BMD across control groups was ‐1.59% (‐10.49% to 7.31%) from baseline 5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 2.06% higher than control groups (1.45% to 2.68% higher) | ‐ | 1665
(18 RCTs) | ⊕⊕⊕⊝
moderate7,8 due to indirectness | Absolute increased benefit 2.06% with bisphosphonates (95% CI 1.45 to 2.68) Relative per cent change 1.29% with bisphosphonates (95% CI 0.91 to 1.69) NNTB = 5 (95% CI 4 to 7) |
| Serious adverse events follow‐up: 12‐24 months | 162 per 1000 | 147 per 1000
(120 to 181) | RR 0.91 (0.74 to 1.12) RD 0.00 (‐0.02, 0.02) | 1703
(15 RCTs) | ⊕⊕⊕⊝
low3,9 due to risk of bias and imprecision | Absolute increased harm 0% more adverse events with bisphosphonates (95% CI 2.00% fewer to 2.00% more) Relative per cent change 9% improvement with bisphosphonates (95% CI 12.00% worse to 26.00% better) NNTH = n/a4 |
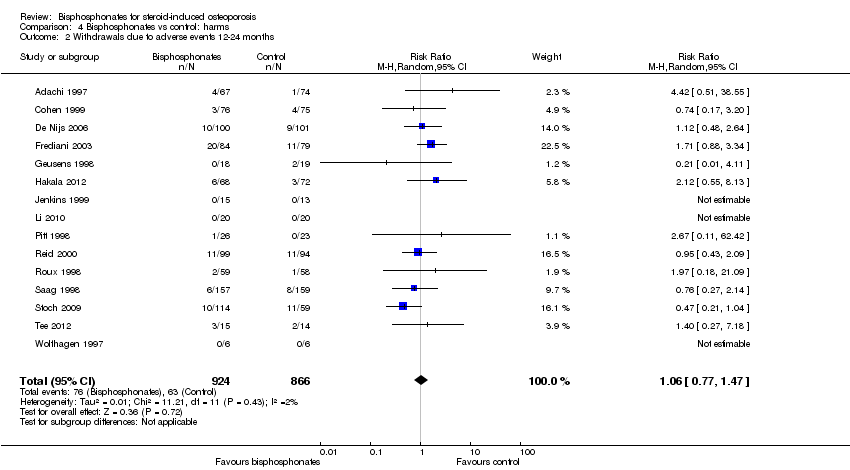
| Withdrawals due to adverse events follow‐up: 12‐24 months | 73 per 1000 | 77 per 1000
(56 to 107) | RR 1.06 (0.77 to 1.47) RD 0.01 (‐0.01 to 0.03) | 1790
(15 RCTs) | ⊕⊕⊕⊝
low3,9 due to risk of bias and imprecision | Absolute increased harm 1% more withdrawals with bisphosphonates (95% CI 1.00% fewer to 3.00% more) Relative per cent change 6% worsening with bisphosphonates (95% CI 47.00% worse to 23.00% better) NNTH = n/a4 |
| Quality of life | 0 per 1000 | 0 per 1000
(0 to 0) | Not estimable | (0 studies) | | This outcome was not assessed by any of the trials |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk Ratio; RD: Risk Difference |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. |