Bifosfonatos para a osteoporose induzida por corticoesteróides
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT; study duration 12 months | |
| Participants | N: 67 participants; men (45%) and premenopausal women (55%) Conditions: inflammatory bowel disease Mean age (range) Intervention: 30 (19‐50) Comparator: 30 (21‐51) Baseline vertebral fractures: yes Serious adverse events: not reported Withdrawals due to adverse events: not reported | |
| Interventions | Active group: clodronate 900 mg IV every 3 months, daily elemental calcium/vitamin D Comparator: placebo IV every 3 months, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry1 | |
| Mean steroid dose | 5‐7.5 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States “randomised in blocks of four” |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States double blinded, intravenous placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | DEXA results interpreted by central blinded outcome assessor. No mention of how radiographs were assessed |
| Incomplete outcome data (attrition bias) | Low risk | 7/67 dropouts all accounted for, none due to adverse events. No outcome data to carry forward so not an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 141 participants; men (38%), premenopausal women (12%) and postmenopausal women (50%) Comparison: rheumatoid arthritis and polymyalgia rheumatica Mean age (range) Intervention: 62 (31‐83) Comparator: 60 (19‐87) Baseline vertebral fractures: yes Serious adverse events: one death in bisphosphonate group (pneumonia) Withdrawals due to adverse events: details incomplete, one withdrawal from intervention group due to increased serum creatinine | |
| Interventions | Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment Criteria: semiquantitative2 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified then randomised, no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | No mention of blinding investigators, used placebo |
| Blinding of outcome assessment (detection bias) | Low risk | All radiographs interpreted by central blinded outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis, 24/141 participants did not complete the study, reasons given, numbers given for those who withdrew for adverse events |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by grant from drug manufacturer, no industry authorship |
| Methods | RCT; study duration 12 months (extension trial from 12‐24 months) | |
| Participants | N: 116 participants; men (29%), premenopausal women (27%) and postmenopausal women (44%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, pemphigus, asthma, inflammatory myopathy, inflammatory bowel disease, giant cell arteritis, sarcoidosis, myasthenia gravis, COPD, and nephrotic syndrome Mean age (range) Intervention: 53 (21‐78) Comparator: 54 (23‐76) Baseline vertebral fractures: yes Serious adverse events: see Saag 1998 Withdrawals due to adverse events: see Saag 1998 | |
| Interventions | Active group: alendronate 10 mg/day orally, daily elemental calcium/vitamin D Comparator: daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | *Treatment study | |
| Incident vertebral fractures | Assessment Criteria: semiquantitative2 | |
| Mean steroid dose | 10‐5 mg/day | |
| Notes | Extension trial of Saag 1998 Fracture/harm data not included ‐ partial cohort of Saag 1998 *Majority of participants had previous steroid use > 3 months Other treatment groups of 5 mg and 2.5/10 mg not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | as per Saag 1998 |
| Allocation concealment (selection bias) | Unclear risk | as per Saag 1998 |
| Blinding of participants and personnel (performance bias) | Unclear risk | as per Saag 1998 |
| Blinding of outcome assessment (detection bias) | Unclear risk | as per Saag 1998 |
| Incomplete outcome data (attrition bias) | Low risk | as per Saag 1998 |
| Selective reporting (reporting bias) | Low risk | as per Saag 1998 |
| Other bias | Unclear risk | extension trial ‐ risk of unblinding |
| Methods | RCT; study duration 12 months | |
| Participants | N: 27 participants; men (19%), premenopausal women (11%) and postmenopausal women (70%) Conditions: polymyalgia rheumatica, temporal arteritis, rheumatoid arthritis, haemolytic anaemia, inflammatory bowel disease, asthma, uveitis, sarcoidosis, reactive arthritis Mean age (SD) Intervention: 60 (16) Comparator: 61 (12) Baseline vertebral fractures: not explicitly stated Serious adverse events: one death due to severe pulmonary infection in control group Withdrawals due to adverse events: not reported | |
| Interventions | Active group: pamidronate 90 mg loading dose IV then 30 mg every 3 months IV, daily elemental calcium Comparator: daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment Criteria: spinal deformity index3 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software, including minimisation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | No placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | 1 death, 4 dropouts all in control group, 1 protocol violation |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 27 participants; men (44%), premenopausal women (12%) and postmenopausal women (44%) Conditions: polymyalgia rheumatica, temporal arteritis, rheumatoid arthritis, inflammatory bowel disease, reactive arthritis, asthma Mean age (SD) Intervention: 55 (17) Comparator: 57 (18) Low dose: 59 (21) Baseline vertebral fractures: none, exclusion criteria Serious adverse events: none occurred Withdrawals due to adverse events: not reported | |
| Interventions | Active group: pamidronate 90 mg IV loading dose then 30 mg IV every 3 months, daily elemental calcium Comparator: daily elemental calcium Low dose: pamidronate IV 90 mg single infusion, daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: spinal deformity index3 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly matched, 3 x 3, taking into account starting dose of steroid, sex and menopausal status |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | Only participants who were matched in the other 2 groups were analysed. 30 matched ‐ 1 dropped out, only 27 analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 228 participants; men (34%), premenopausal women (20%) and postmenopausal women (46%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, giant cell arteritis, vasculitis, asthma, chronic interstitial lung disease, polymyositis, dermatomyositis Mean age (SD) Intervention: 61.9 (14.3) Comparator: 57.2 (14.7) Low dose: 59.5 (14.0) Baseline vertebral fractures: yes Serious adverse events: details on type of serious adverse events not provided Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided | |
| Interventions | Active group: risedronate 5 mg/day orally, daily elemental calcium Comparator: placebo, daily elemental calcium Low dose: risedronate 2.5 mg/day orally, daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry4 | |
| Mean steroid dose | > 20 mg/day | |
| Notes | Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified then randomised, no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Stated double‐blinded, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Low risk | X‐ray data reviewed by single observer blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | 77/224 participants dropped out. Note 2.5 mg risedronate group stopped halfway through study |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Unclear risk | One author and study sponsorship from Proctor & Gamble |
| Methods | RCT; study duration 12 months | |
| Participants | N: 83 participants; men (34%), premenopausal women (11%) and postmenopausal women (55%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, giant cell arteritis Mean age (SD) Intervention: 61.4 (12.5) Comparator: 63.3 (11.5) Baseline vertebral fractures: not explicitly stated Serious adverse events: reported 2 deaths but did not specify in which treatment group they occurred Withdrawals due to adverse events: 1 withdrawal due to myocardial infarction, 1 due to heart failure, 1 due to lung cancer. Did not specify in which treatment group they occurred | |
| Interventions | Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium Daily vitamin D permitted in all participants (set maximum dose) | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | ˜ 7.5 mg/day | |
| Notes | Missing data: we assumed authors incorrectly reported SE as SD. This is justified by the P value as per our biostatistician. We have corrected for this error in our data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States double blind, placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | DEXA and biochemical results interpreted by central blinded outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | 7/87 participants did not complete the study, reasons given. No adverse events |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 18 months | |
| Participants | N: 200 participants; men (38%), premenopausal women (9%) and postmenopausal women (53%) Conditions: polymyalgia rheumatica, rheumatoid arthritis or other rheumatic disease Mean age (SD) Intervention: 60 (14) Comparator: 62 (15) Baseline vertebral fractures: yes Serious adverse events: only deaths reported Intervention: 1 death due to diverticulitis with perforation, 1 death due to non‐Hodgkin's lymphoma Comparator: 1 death due to cerebrovascular accident Withdrawals due to adverse events: Intervention: 5 due to gastrointestinal side effects, 2 due to cancer, 3 due to "other conditions" Comparator: 5 due to gastrointestinal side effects, 1 due to cancer, 6 due to "other conditions" | |
| Interventions | Active group: alendronate 10 mg/day orally, daily placebo (vitamin D look‐alike) Comparator: placebo, daily vitamin D Any participant with dietary intake below set threshold received daily calcium Any participant with serum levels below set threshold received daily vitamin D | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: semiquantitative5 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Missing data: SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisations |
| Allocation concealment (selection bias) | Low risk | Pharmacist did allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Stated double blinded, placebo tablets |
| Blinding of outcome assessment (detection bias) | Low risk | Radiographs assessed by blinded individuals |
| Incomplete outcome data (attrition bias) | Low risk | 163/201 completed study. See Figure 1 and text page 678, right column 3rd paragraph for details |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | No industry sponsorship or authorship although industry supplied drug |
| Methods | RCT; study duration 48 months | |
| Participants | N: 163 participants; premenopausal (24%) and postmenopausal women (68%) Conditions: rheumatoid arthritis and psoriatic arthritis Mean age (SD) Intervention: 61.1 (12.2) Comparator: 62.4 (13.4) Baseline vertebral fractures: yes Serious adverse events: not reported Withdrawals due to adverse events: Intervention: due to gastralgia and/or local pain at injection site Comparator: due to gastralgia | |
| Interventions | Active group: clodronate 100 mg/week IM, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: measured at 4 years, not included in analysis | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Missing data: SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | No mention of double blind or blinding of personnel, placebo given |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | 31/163 participants dropped out for "gastralgia" or "GI intolerance". Twice as many in the clodronate group |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 24 months | |
| Participants | N: 37 participants; all postmenopausal women Conditions: rheumatoid arthritis, polymyalgia rheumatica, osteoarthritis, chronic bronchitis, inflammatory bowel disease, idiopathic eosinophilia, sarcoidosis Mean age (SD) Intervention: 61.1 (12.2) Comparator: 62.4 (13.4) Baseline vertebral fractures: yes Serious adverse events: 1 death due to ruptured aortic aneurysm in bisphosphonate group Withdrawals due to adverse events: Intervention: none Comparator: 1 due to anaphylaxis, 1 due to shoulder fracture | |
| Interventions | Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | 5‐7.5mg/day | |
| Notes | Missing data: 12 months BMD mean and SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in blocks of two |
| Allocation concealment (selection bias) | Low risk | Code located in sponsor's office and only broken after full statistical analysis |
| Blinding of participants and personnel (performance bias) | Low risk | Explicit "blinding was successful among participants, doctors, data managers," identical placebo given |
| Blinding of outcome assessment (detection bias) | Low risk | Explicitly stated as above |
| Incomplete outcome data (attrition bias) | Low risk | 11/37 dropouts. Stated they used an intention‐to‐treat population for their ANCOVA analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 140 participants; all postmenopausal women Conditions: rheumatoid arthritis, polymyalgia rheumatica and other rheumatic diseases Mean age (SD) Intervention: 64 (8) Comparator: 63 (7) Baseline vertebral fractures: yes, but those with symptomatic or 2 or more radiographic vertebral fractures were excluded from the study Serious adverse events: Intervention: gastrointestinal bleeding, transient ischaemic attack, acute pancreatitis, death (agranulocytosis and sepsis), acute pyelonephritis, concussion, poisoning, follicle center lymphoma, malignant tongue neoplasm, deep vein thrombosis, pulmonary embolism Comparator: erysipelas, pneumonia, radius fracture, hip fracture, headache Withdrawals due to adverse events: reasons for withdrawal included anaemia, palpitations, reflux oesophagitis, stomach discomfort, pyrexia, arthralgia, back pain associated with a prestudy operation, myalgia, dizziness, headache, tremor, and cough. Did not specify in which treatment groups these occurred | |
| Interventions | Active group: ibandronate 150 mg/month orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | 5‐7.5 mg/day | |
| Notes | *Majority of participants had previous steroid use > 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States investigators and co‐ordinators were blinded to BMD results, placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | Data processing of BMD was done centrally |
| Incomplete outcome data (attrition bias) | Low risk | 124/140 completed, intention‐to‐treat analysis included any participant with one follow‐up data point |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Unclear risk | One author worked for Roche |
| Methods | RCT; study duration 12 months | |
| Participants | N: 74 participants; men (45%) and postmenopausal women (55%) Conditions: COPD and asthma Mean age (range) Intervention: 56.1 (43‐71) Comparator: 57.3 (39‐73) Baseline vertebral fractures: not explicitly stated Serious adverse events: Intervention: 1 death due to end‐stage COPD (clodronate 2400 mg/day group) Comparator: 1 death due to asthma attack Withdrawals due to adverse events: 7 withdrawals due to gastrointestinal reasons but did not specify in which treatment groups these occurred | |
| Interventions | Active group: clodronate 800 mg/day orally Comparator: placebo | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Other treatment arms (1600 mg/day and 2400 mg/day) not included Missing data: SD calculated from 95% CI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by using table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Refer to study as double blinded, identical number of tablets |
| Blinding of outcome assessment (detection bias) | Low risk | BMD data analysed at end of study by one technician blinded to treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | 61/74 completed study. 68 participants had BMD data from 2 visits and were analysed, 7 dropouts due to GI adverse events |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 28 participants; men (39%) and women (61%) Conditions: rheumatoid arthritis and polymyalgia rheumatica Mean age (SD) Intervention: 68.7 (10.9) Comparator: 65.9 (9.7) Baseline vertebral fractures: not explicitly stated Serious adverse events: 1 death in control group, other types of serious events not reported Withdrawals due to adverse events: none occurred | |
| Interventions | Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: screened < 50% participants, not included in analysis | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Update of Jenkins 1997 from original review Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of allocation method |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Paper states that study is a double blind placebo controlled trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | 25/28 completed study and dropouts accounted for. Vertebral radiographs available on only 13/28 participants despite protocol stating screening of all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 163 participants*; men (44%), premenopausal women (9%) and postmenopausal women (47%) Conditions: rheumatoid arthritis Mean age (SD) Intervention + low dose: 61.7 (11.0) Comparator: 61.6 (11.3) Baseline vertebral fractures: yes Serious adverse events: details on type of serious events not provided Withdrawals due to adverse events: did not specify in which treatment groups these occurred nor provide details on adverse events leading to withdrawal | |
| Interventions | Active group: alendronate 10 mg/day orally Comparator: placebo Low dose: alendronate 5 mg/day orally Any participant with self‐reported low dietary intake received daily calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry1 | |
| Mean steroid dose | ˜7.5mg/day | |
| Notes | *Active group = postmenopausal women; comparator group = pre/postmenopausal women and men; low‐dose group = premenopausal women and men Did not provide data on withdrawals from each group: not included in meta‐analysis Missing data: SD calculated from P value | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind" but not sure if this refers to all personnel or just outcome assessors, identical placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | Radiographs assessed by blinded individuals |
| Incomplete outcome data (attrition bias) | Low risk | Accounted for dropouts. Intention‐to‐treat analysis on any participant with a follow‐up BMD, 144/163 |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 40 participants; all Chinese premenopausal (40%) and postmenopausal women (60%) Conditions: systemic lupus erythematosus Median age (IQR) Intervention: 47 (33.5, 50) Comparator: 45.5 (0.5, 49) Baseline vertebral fractures: no Serious adverse events: not reported Withdrawals due to adverse events: none occurred | |
| Interventions | Active group: ibandronate 150 mg/month orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | Unclear: began with ˜ 25‐30 mg/day | |
| Notes | Missing data: median used, SD calculated from IQR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomizations conducted with a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Project co‐ordinator and investigators blinded to group assignment, the method of concealed random allocation was used |
| Blinding of participants and personnel (performance bias) | Low risk | Project co‐ordinators and study investigators blinded, placebo tablets used |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed study |
| Selective reporting (reporting bias) | Low risk | The authors published the planned outcomes in a trial protocol and provided results for each planned outcome |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 104 weeks | |
| Participants | N: 49 participants; men (39%), premenopausal and postmenopausal women (61%) Conditions: asthma, polymyalgia rheumatica, systemic lupus erythematosus, emphysema, fasciitis, giant cell arteritis, polyarteritis nodosa, bronchiectasis, fibrosing alveolitis, and scleroderma Mean age (SD) Intervention: 58.9 (13.7) Comparator: 59.2 (10.8) Baseline vertebral fractures: not explicitly stated Serious adverse events: details on types of serious events other than death not provided Intervention: 1 death due to respiratory failure, 1 death due to adenocarcinoma of lung Comparator: 1 death due to perforated bowel Withdrawals due to adverse events: Intervention: 1 withdrawal due to myocardial infarction Comparator: none occurred | |
| Interventions | Active group: cyclic etidronate 400 mg orally and elemental calcium/vitamin D Comparator: cyclic placebo and elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Assessment criteria: semiquantitative3 | |
| Mean steroid dose | ˜ 7.5 mg/day | |
| Notes | Update of Pitt 1997 from original review Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind, placebo given |
| Blinding of outcome assessment (detection bias) | Low risk | X‐rays were assessed centrally by a blinded outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | 41/49 participants completed, dropouts were explained, intention‐to‐treat analysis included any participant who took 1 dose of drug |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 290 participants; men (37%), premenopausal women (9%) and postmenopausal women (54%) Conditions: rheumatoid arthritis, asthma, systemic lupus erythematosus, temporal arteritis, vasculitis, COPD, polymyositis, chronic interstitial lung disease and other Mean age (SD) Intervention: 58 (12) Comparator: 59 (12) Low dose: 59 (14) Baseline vertebral fractures: yes Serious adverse events: details on type of serious events not provided Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided | |
| Interventions | Active group: risedronate 5 mg/day orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D Low dose: risedronate 2.5 mg/day orally, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry1 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind", placebo given |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | 22% dropouts ‐ adverse events only in text ‐ "no difference between groups" |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Unclear risk | Clear articulation regarding the role of the funding pharmaceutical company in design, implementation and analysis of study. It indicates a potential for bias |
| Methods | RCT; study duration 12 months | |
| Participants | N: 117 participants; men (36%), premenopausal women (15%) and postmenopausal women (49%) Conditions: vasculitis, rheumatoid arthritis, polymyalgia rheumatica, temporal arteritis, systemic lupus erythematosus, asthma, chronic interstitial lung disease, polymyositis, dermatomyositis, and skin disease Mean age (SD) Intervention: 58.5 (13.9) Comparator: 59.0 (13.6) Baseline vertebral fractures: not explicitly stated Serious adverse events: not reported Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided | |
| Interventions | Active group: cyclic etidronate 400 mg orally and daily elemental calcium Comparator: cyclic placebo and daily elemental calcium Daily vitamin D permitted in all participants (set maximum dose) | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Update of Roux 1997 from original review Missing data: total number of participants provided with BMD results so we estimated as equal per group; SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States double blinded, placebo used |
| Blinding of outcome assessment (detection bias) | Unclear risk | X‐rays were assessed using qualitative scale, no mention of blinding. BMD interpreted centrally, no mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 107/117 participants completed, dropouts were explained, intention‐to‐treat analysis included any participant who was randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 18 months | |
| Participants | N: 72 participants; men (10%) and premenopausal women (90%) Conditions: systemic lupus erythematosus, polymyositis, dermatomyositis etc. Mean age: 36.6 Baseline vertebral fractures: not explicitly stated Serious adverse events: not reported Withdrawals due to adverse events: not reported | |
| Interventions | Active group: alendronate 10 mg/day orally, daily elemental calcium, twice weekly vitamin D Comparator: daily elemental calcium, twice weekly vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Assessment criteria: not specified, not included in analysis | |
| Mean steroid dose | > 20 mg/day | |
| Notes | * Type of study unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised into two groups," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding, no placebo used |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | 100 participants consented: 28 did not follow treatment and were excluded, 72 did follow treatment (calcium + vitamin D or calcium + vitamin D + alendronate) |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 48 weeks | |
| Participants | N: 477 participants; men (29%), premenopausal women (22%) and postmenopausal women (49%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, pemphigus, asthma, inflammatory myopathy, inflammatory bowel disease, giant cell arteritis, sarcoidosis, myasthenia gravis, COPD, and nephrotic syndrome Mean age (SD) Intervention: 55 (15) Comparator: 54 (15) Low dose: 56 (15) Baseline vertebral fractures: yes Serious adverse events: details on type of serious adverse events incomplete Intervention: serious gastro‐intestinal events in 2 participants (alendronate 10 mg/day group) Comparator: serious gastro‐intestinal events in 2 participants Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided | |
| Interventions | Active group: alendronate 10 mg/day orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D Low dose: alendronate 5 mg/day orally, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Assessment criteria: semiquantitative2 | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Update of Saag 1997 from original review Aledronate 5 mg and 10 mg are combined in analyses of fractures *Majority of participants with > 3 months steroid use Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding, USA arm had matching placebo. No mention of placebo in Europe arm |
| Blinding of outcome assessment (detection bias) | Unclear risk | X‐ray analysis read centrally but not mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Used an intention‐to‐treat analysis with last observation carried forward (12 week result). All dropouts accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by a grant from Merck |
| Methods | RCT; study duration 24 months | |
| Participants | N: 195 participants; men (31%), premenopausal women (14%) and postmenopausal women (55%) Conditions: polymyalgia rheumatica/giant cell arteritis, rheumatoid arthritis, systemic lupus erythematosus, polymyositis, inflammatory bowel disease, respiratory disease, neurologic disease, and other Mean age (SD) Intervention: 62.4 (13.5) Comparator: 57.9 (13.0) Baseline vertebral fractures: yes Serious adverse events: reported 2 deaths but did not specify in which treatment group they occurred Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided and did not specify in which treatment groups these occurred | |
| Interventions | Active group: alendronate 10 mg/day orally, daily elemental calcium Comparator: ergocalciferol 0.25 mg orally 3 times weekly, daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Assessment criteria: semiquantitative2 | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | 12 months BMD data insufficient for analysis: not included in meta‐analysis Calcitriol treatment arm not included Did not report which groups withdrawals came from: not included in meta‐analysis *Majority of participants had prior steroid therapy > 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisations by CRO using adaptive assignment |
| Allocation concealment (selection bias) | Low risk | Allocation performed centrally |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Low risk | X‐ray analysis read centrally by blinded individual and densitometry technician was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | 177/195 completed 1 year, authors stated no apparent differences between groups, dropouts fully accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by a grant from Merck |
| Methods | RCT; study duration 24 months | |
| Participants | N: 38 participants; men (24%), premenopausal women (8%) and postmenopausal women (68%) Conditions: polymyalgia rheumatica, temporal arteritis and COPD Mean age Intervention: 65 Comparator: 64 Baseline vertebral fractures: yes Serious adverse events: not reported Withdrawals due to adverse events: not reported | |
| Interventions | Active group: cyclic etidronate 400 mg orally, daily elemental calcium Comparator: daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Incomplete data: reported total number of fractures and not participants with fractures, not included in analysis | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Update of Skingle 1994 from original review Missing data: median used; SD calculated from P value | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Open study, no placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Spinal X‐rays interpreted by single blinded outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | 17/55 did not complete first year of study. 7 because prednisone dose too low, 10 for non compliance, lost to follow‐up. Completer analysis. Only 23 participants out of 38 completers had X‐rays |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 173 participants; men (42%), premenopausal women (31%) and postmenopausal women (27%) Conditions: rheumatoid arthritis, Still's disease, connective tissue disorder, arthritis, osteoarthritis, polymyalgia, polymyalgia rheumatica, polymyositis, psoriatic arthritis, scleroderma, and systemic lupus erythematosus Mean age (SD) Intervention: 51.9 (14.4) Comparator: 54.6 (14.8) Baseline vertebral fractures: not explicitly stated Serious adverse events: details on types of serious events other than death not provided Intervention: one death due to cardiac arrest Comparator: no deaths Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided | |
| Interventions | Active group: alendronate 70 mg/week orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | *Majority of participants had prior steroid therapy > 3 months Missing data: SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned “randomised in a 2:1 ratio," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States all study personnel blinded, placebo used and same administration instructions given to both |
| Blinding of outcome assessment (detection bias) | Low risk | States central lab and DEXA personnel blinded |
| Incomplete outcome data (attrition bias) | Low risk | Modified intention‐to‐treat analysis (as long as 1 dose of medication and 1 follow‐up outcome measured), used last observation carried forward, provided detailed patient flow diagram |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 44 participants; men (57%), premenopausal women (11%) and postmenopausal women (32%) Conditions: immunobullous skin diseases Mean age (SD) Intervention: 56.8 (16.2) Comparator: 61.5 (15.2) Baseline vertebral fractures: none, exclusion criteria Serious adverse events: Intervention: 1 death, cause not reported Comparator: 1 participant suffered myocardial infarction Withdrawals due to adverse events: Intervention: 1 due to nausea and vomiting, 1 due to drug‐related rash, 1 due to leukopenia Comparator: 1 due to abdominal pain, 1 due to leukopenia | |
| Interventions | Active group: alendronate 10 mg/day orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: semiquantitative2 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | BMD data reported as change in T‐score, unable to include in meta‐analysis as per our biostatistician | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified and randomly assigned in blocks of 6 |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind, placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | States X‐ray assessment blinded and performed independently by two assessors |
| Incomplete outcome data (attrition bias) | High risk | 30% dropout rate, stated main reason due to unavailability for follow‐up, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 20 participants; men (30%) and premenopausal and postmenopausal women (70%) Conditions: rheumatoid arthritis Mean age (range) Intervention: 56 (35‐77) Comparator: 62 (41‐77) Baseline vertebral fractures: not explicitly stated Serious adverse events: not reported Withdrawals due to adverse events: not reported | |
| Interventions | Active group: pamidronate 60 mg IV every 3 months, daily elemental calcium Comparator: placebo IV every 3 months, daily elemental calcium Vitamin D provided at baseline to any participant with level below set minimum threshold | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Did not provide numerical data for BMD at femoral neck Missing data: median used, SD calculated from range | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no details of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | States double blind, placebo used |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinded outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of dropouts or adverse events |
| Selective reporting (reporting bias) | Unclear risk | No mention of adverse events |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 12 months | |
| Participants | N: 12 participants; men (25%) and women (75%) Conditions: primary biliary cirrhosis Mean age (SD) Intervention: 57 (11) Comparator: 49 (6) Baseline vertebral fractures: no, exclusion criteria Serious adverse events: not reported Withdrawals due to adverse events: none occurred | |
| Interventions | Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Prevention study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified then randomised, no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding, no placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of a blinded outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed |
| Selective reporting (reporting bias) | Unclear risk | X‐rays of the spine were done to look for fractures, but only to validate DEXA measurement, not as an outcome. Not reported |
| Other bias | Low risk | None apparent |
| Methods | RCT; study duration 24 months | |
| Participants | N: 98 participants; all premenopausal women Conditions: systemic lupus erythematosus Mean age (SD) Intervention: 31.13 (8.44) Comparator: 28.09 (6.49) Baseline vertebral fractures: no Serious adverse events: 3 deaths due to infective complications of lupus but did not specify in which treatment group these occurred Withdrawals due to adverse events: 3 with renal impairment, 1 with fractured tibia and fibula, 1 avascular necrosis of hip (control group), and 1 severe thrombocytopenia. Did not specify in which treatment groups these occurred | |
| Interventions | Active group: alendronate 70 mg/week orally, daily elemental calcium Comparator group: daily elemental calcium | |
| Outcomes |
| |
| Types of studies | Treatment study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Calcitriol treatment group not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisations used |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Specifically stated not blinded, no placebo |
| Blinding of outcome assessment (detection bias) | Low risk | BMD assessor was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 77/98 participants completed the study, adverse events were detailed, no intention‐to‐treat analysis done |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by grant from two pharmaceutical companies |
ANCOVA: analysis of covariance
BMD: bone mineral density
DEXA: dual energy X‐ray absorptiometry
GIOP: glucocorticoid‐induced osteoporosis
IM: intramuscular
IV: intravenous
RCT: randomised controlled trial
1Black 1996 and Genant 1996; 2Genant 1993 and Van Kujik 1995; 3Minne 1988; 4Kiel 1995 and Melton 1993; 5Kleerekoper 1984
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Low‐dose bisphosphonates: neridronate 25 mg/day IM | |
| Low‐dose bisphosphonates: risedronate 2.5 mg/day orally | |
| Low‐dose bisphosphonates: cyclic etidronate 200 mg orally | |
| Low‐dose bisphosphonates: risedronate 2.5 mg/day orally | |
| Low‐dose bisphosphonates: alendronate 5 mg/day orally | |
| Low‐dose bisphosphonates: cyclic etidronate 200 mg orally | |
| Low‐dose bisphosphonates: alendronate 5 mg/day orally | |
| Low‐dose bisphosphonates: cyclic etidronate 200 mg orally | |
| Low‐dose bisphosphonates: alendronate 5 mg/day orally | |
| Low‐dose bisphosphonates: risedronate 2.5 mg/day orally | |
| Non‐standard‐dose bisphosphonates: pamidronate 100 mg/week orally |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Not yet known |
| Participants | Not yet known |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese with abstract in English, awaiting translation |
| Methods | Not yet known |
| Participants | N: 34 participants; all women Conditions: not yet known Mean age (SD) not yet known Baseline vertebral fractures: not yet known |
| Interventions | Active group: Cyclic etidronate 400 mg orally Comparator group: Not yet known Low dose group: Cyclic etidronate 200 mg orally |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese with abstract in English, awaiting translation |
| Methods | RCT; completed trial, no publication |
| Participants | Men, ages 25‐85 |
| Interventions | zoledronic acid vs alendronate (unsure if vitamin D or calcium control) |
| Outcomes | Per cent change in BMD at the lumbar spine and femoral neck at 24 months |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not included |
| Mean steroid dose | Unsure if GIOP population included |
| Notes | Title: Efficacy and safety of zoledronic acid for the treatment of osteoporosis in men |
| Methods | RCT; completed trial, no publication |
| Participants | Men and women, ages 18‐75 |
| Interventions | Risedronate vs placebo |
| Outcomes | BMD, incident vertebral fractures, adverse events |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Pulse methylprednisolone or oral prednisolone (> = 0.8mg/kg/day) or equivalent for at least 6 weeks |
| Notes | Title: The efficacy of risedronate in prevention of bone loss in patients receiving high‐dose corticosteroid treatment |
| Methods | RCT; completed trial, no publication |
| Participants | Men and women with Crohn's disease |
| Interventions | Risedronate vs placebo |
| Outcomes | Per cent change in BMD at the lumbar spine and hip at 12 months |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Unsure if GIOP population included |
| Notes | Title: A randomized, data collection program to determine the efficacy and safety of risedronate (Actonel) therapy plus calcium and vitamin D supplementation versus placebo plus calcium and vitamin D supplementation in the treatment of low bone mineral density in Crohn's disease patients |
| Methods | RCT; completed trial, no publication |
| Participants | Women with rheumatoid arthritis |
| Interventions | Ibandronate vs placebo |
| Outcomes | Per cent change in BMD at the lumbar spine and femoral neck at 12 months Incident vertebral fractures |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Minimum 5 mg/day prednisolone for 3 months |
| Notes | Title: Efficacy of monthly ibandronate in women with rheumatoid arthritis and reduced bone mineral density receiving long‐term glucocorticoids |
| Methods | Not yet known |
| Participants | Not yet known |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese, awaiting translation |
| Methods | RCT, study duration 12 months |
| Participants | N: 50 participants; men (14%) and women (86%) Conditions: rheumatoid arthritis Mean age (SD) Intervention: 49.9 (11.6) Comparator: 47.3 (13.6) Baseline vertebral fractures: not explicitly stated |
| Interventions | Active group: alendronate 70 mg/week orally, daily calcium/vitamin D Comparator group: daily calcium/vitamin D |
| Outcomes | Insufficient reporting of BMD data, pending clarification from authors |
| Types of studies | Treatment study |
| Incident vertebral fractures | Not reported as outcome |
| Mean steroid dose | ˜7.5 mg/day |
| Notes | Calcitriol and alendronate + calcitriol groups not included |
| Methods | Not yet known |
| Participants | Not yet known |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese, awaiting translation |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A phase III randomized study of zoledronate bisphosphonate therapy for the prevention of bone loss in men with prostate cancer receiving long‐term androgen deprivation |
| Methods | RCT; study ongoing |
| Participants | Men, ages 18 and older with stage III and IV prostate cancer |
| Interventions | Zolendronate IV vs calcium gluconate and cholecalciferol |
| Outcomes | BMD changes |
| Starting date | March 2003 |
| Contact information | Study Chair: Charles L. Bennett, MD, PhD, Robert H. Lurie Cancer Center |
| Notes | Steroids allowed, unsure if meet minimum 5 mg/day dose |
| Trial name or title | ZEST II for osteoporotic fracture prevention |
| Methods | RCT; recruiting participants |
| Participants | Women in LTC facilities with osteoporosis, ages 65 and older |
| Interventions | zoledronic acid vs placebo |
| Outcomes | Clinical vertebral and nonvertebral fractures |
| Starting date | January 2016 |
| Contact information | Principal Investigator: Susan L Greenspan, MD, University of Pittsburgh |
| Notes | Unsure if GIOP population included |
| Trial name or title | Drug therapy for the prevention of glucocorticoid induced osteoporosis in elderly patients: teriparatide or bisphosphonates? |
| Methods | RCT |
| Participants | Women and men, ages 65 and older with collagen vascular disorders on steroid therapy |
| Interventions | Teriparative vs alendronate or risedronate |
| Outcomes | Vertebral and nonvertebral fractures, BMD changes |
| Starting date | 2012/12/01 |
| Contact information | Principle Investigator: Koichi Amano, Saitama Medical University |
| Notes | Unsure if placebo and/or vitamin D and calcium control |
| Trial name or title | Efficacy of once every four week oral minodronate in patients with glucocorticoid‐induced osteoporosis after switching from weekly oral bisphosphonate |
| Methods | RCT |
| Participants | Women and men, ages 20 and older with rheumatic diseases on steroid therapy |
| Interventions | Minodronate vs alendronate or risedronate |
| Outcomes | BMD changes |
| Starting date | 2013/10/25 |
| Contact information | Principle Investigator: Taio Naniwa, Nagoya City University Hospital |
| Notes | Unsure if placebo and/or vitamin D and calcium control |
| Trial name or title | Efficacy of a human anti‐RANKL antibody (Denosumab) on prevention of steroid‐induced osteoporosis in patients with autoimmune hepatitis (AIH) |
| Methods | RCT |
| Participants | Women and men, ages 20‐75 with autoimmune hepatitis on steroid therapy |
| Interventions | Denosumab vs bisphosphonate |
| Outcomes | BMD changes |
| Starting date | 2014/04/08 |
| Contact information | Principle Investigator: Kenichi Ikejima, Juntendo University School of Medicine |
| Notes | unsure if placebo and/or vitamin D and calcium control |
| Trial name or title | Glucocorticoid‐induced osteoporosis treated with bisphosphonate and denosumab |
| Methods | RCT |
| Participants | Women and men, ages 18 and older on steroid therapy |
| Interventions | Bisphosphonate vs denosumab |
| Outcomes | Incident vertebral fractures, BMD changes |
| Starting date | 2014/06/24 |
| Contact information | Principle Investigator: Hisaji Oshima, Tokyo Medical Center, National Hospital Organization |
| Notes | Unsure if placebo and/or vitamin D and calcium control |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident radiographic vertebral fractures 12‐24 months Show forest plot | 12 | 1343 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.35, 0.91] |
| Analysis 1.1  Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 1 Incident radiographic vertebral fractures 12‐24 months. | ||||
| 2 Incident radiographic nonvertebral fractures 12‐24 months Show forest plot | 9 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.33] |
| Analysis 1.2  Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 2 Incident radiographic nonvertebral fractures 12‐24 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LS BMD change 12 months: all trials Show forest plot | 23 | 2042 | Mean Difference (IV, Random, 95% CI) | 3.50 [2.90, 4.10] |
| Analysis 2.1  Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 1 LS BMD change 12 months: all trials. | ||||
| 1.1 Prevention trials | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 1.2 Treatment trials | 11 | 1112 | Mean Difference (IV, Random, 95% CI) | 3.19 [2.64, 3.73] |
| 2 LS BMD change 12 months: oral treatment Show forest plot | 18 | 1767 | Mean Difference (IV, Random, 95% CI) | 3.25 [2.88, 3.63] |
| Analysis 2.2  Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 2 LS BMD change 12 months: oral treatment. | ||||
| 3 LS BMD change 12 months: parenteral treatment Show forest plot | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| Analysis 2.3 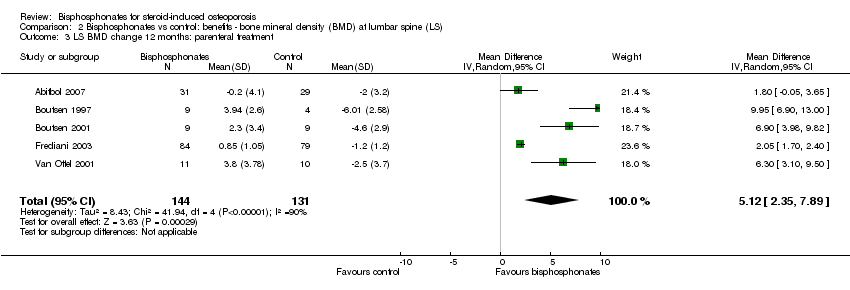 Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 3 LS BMD change 12 months: parenteral treatment. | ||||
| 4 LS BMD change 12 months: low‐ vs standard‐dose Show forest plot | 5 | 642 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.37, 1.53] |
| Analysis 2.4  Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 4 LS BMD change 12 months: low‐ vs standard‐dose. | ||||
| 5 LS BMD change 18‐24 months Show forest plot | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 5.49 [3.47, 7.51] |
| Analysis 2.5  Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 5 LS BMD change 18‐24 months. | ||||
| 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups Show forest plot | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| Analysis 2.6 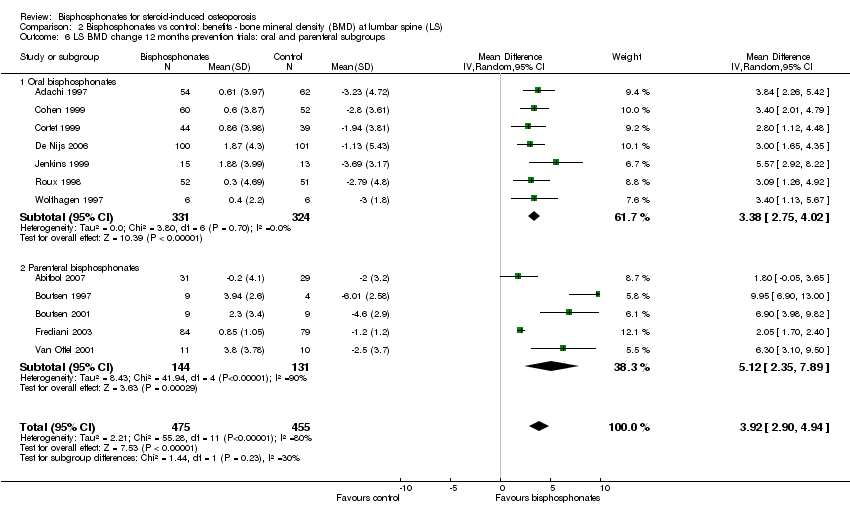 Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups. | ||||
| 6.1 Oral bisphosphonates | 7 | 655 | Mean Difference (IV, Random, 95% CI) | 3.38 [2.75, 4.02] |
| 6.2 Parenteral bisphosphonates | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 7 LS BMD change 12 months: gender/menopausal status subgroups Show forest plot | 5 | 840 | Mean Difference (IV, Random, 95% CI) | 3.86 [2.03, 5.68] |
| Analysis 2.7 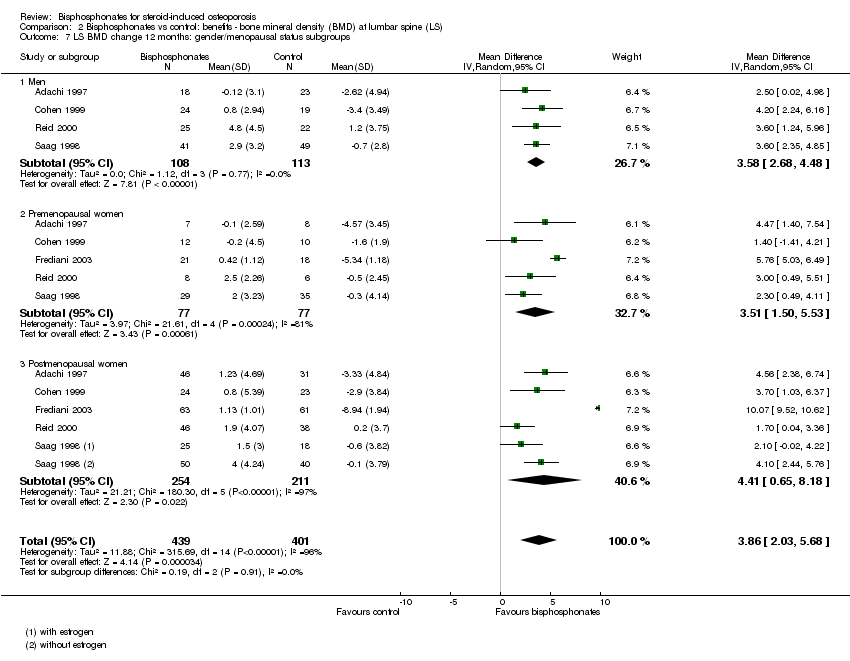 Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 7 LS BMD change 12 months: gender/menopausal status subgroups. | ||||
| 7.1 Men | 4 | 221 | Mean Difference (IV, Random, 95% CI) | 3.58 [2.68, 4.48] |
| 7.2 Premenopausal women | 5 | 154 | Mean Difference (IV, Random, 95% CI) | 3.51 [1.50, 5.53] |
| 7.3 Postmenopausal women | 5 | 465 | Mean Difference (IV, Random, 95% CI) | 4.41 [0.65, 8.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FN BMD change 12 months: all trials Show forest plot | 18 | 1665 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.45, 2.68] |
| Analysis 3.1 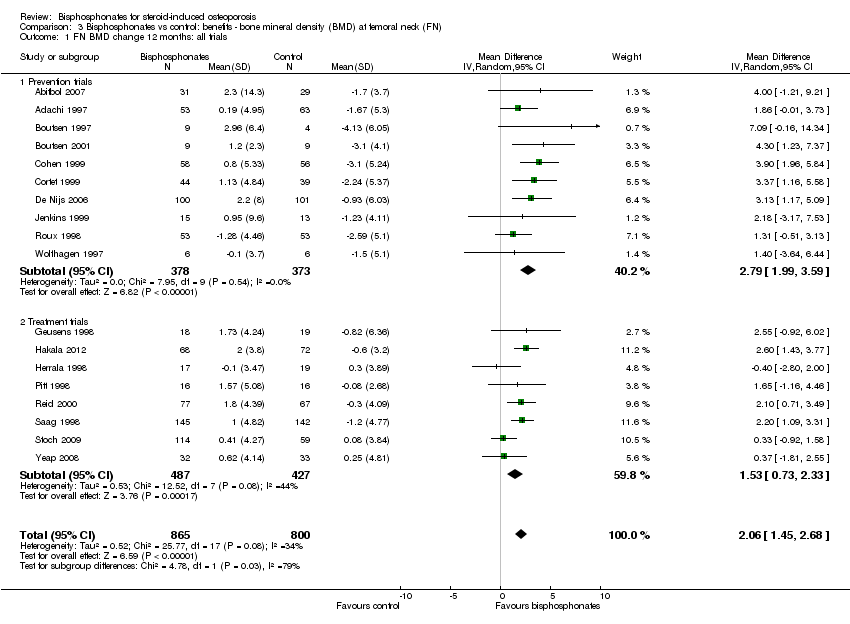 Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 1 FN BMD change 12 months: all trials. | ||||
| 1.1 Prevention trials | 10 | 751 | Mean Difference (IV, Random, 95% CI) | 2.79 [1.99, 3.59] |
| 1.2 Treatment trials | 8 | 914 | Mean Difference (IV, Random, 95% CI) | 1.53 [0.73, 2.33] |
| 2 FN BMD change 12 months: oral treatment Show forest plot | 15 | 1574 | Mean Difference (IV, Random, 95% CI) | 1.92 [1.31, 2.53] |
| Analysis 3.2 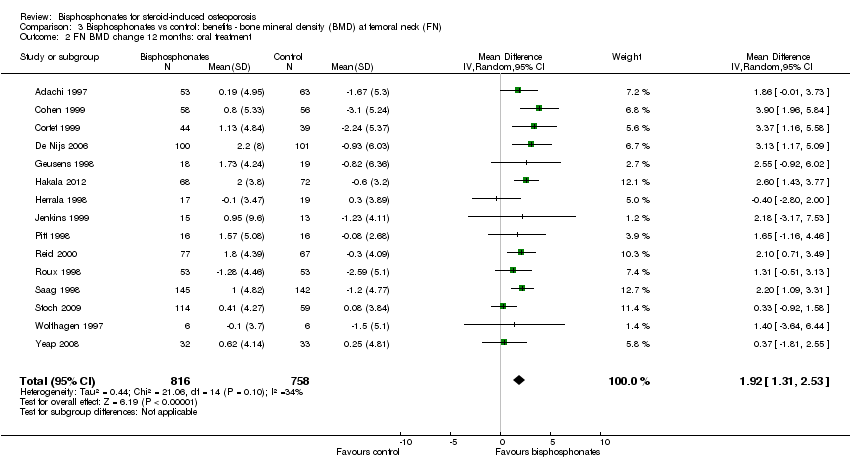 Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 2 FN BMD change 12 months: oral treatment. | ||||
| 3 FN BMD change 12 months: parenteral treatment Show forest plot | 3 | 91 | Mean Difference (IV, Random, 95% CI) | 4.56 [2.07, 7.05] |
| Analysis 3.3 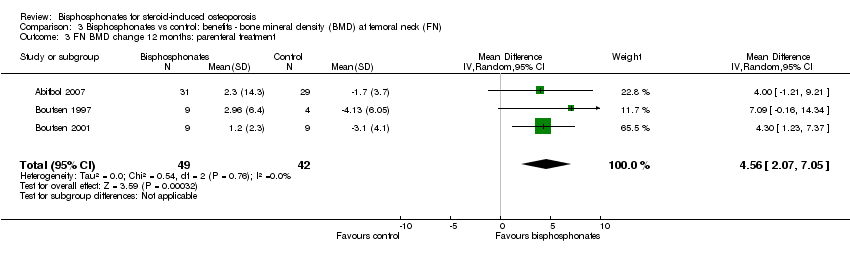 Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 3 FN BMD change 12 months: parenteral treatment. | ||||
| 4 FN BMD change 12 months: low‐ vs standard‐dose Show forest plot | 4 | 542 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.42, 1.90] |
| Analysis 3.4  Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 4 FN BMD change 12 months: low‐ vs standard‐dose. | ||||
| 5 FN BMD change 18‐24 months Show forest plot | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 3.28 [1.70, 4.87] |
| Analysis 3.5 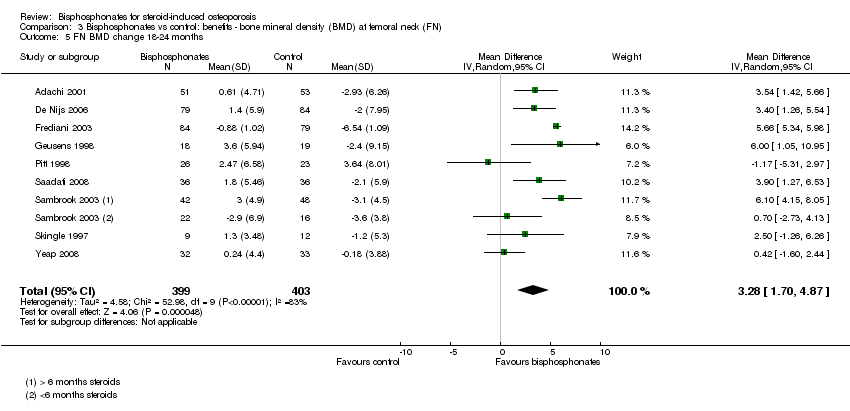 Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 5 FN BMD change 18‐24 months. | ||||
| 6 FN BMD change 12 months: gender/menopausal status subgroups Show forest plot | 4 | 537 | Mean Difference (IV, Random, 95% CI) | 3.29 [1.65, 4.94] |
| Analysis 3.6  Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 6 FN BMD change 12 months: gender/menopausal status subgroups. | ||||
| 6.1 Men | 3 | 134 | Mean Difference (IV, Random, 95% CI) | 2.91 [1.15, 4.68] |
| 6.2 Premenopausal women | 4 | 88 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐0.96, 6.35] |
| 6.3 Postmenopausal women | 4 | 315 | Mean Difference (IV, Random, 95% CI) | 3.62 [‐0.37, 7.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse events 12‐24 months Show forest plot | 15 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| Analysis 4.1  Comparison 4 Bisphosphonates vs control: harms, Outcome 1 Serious adverse events 12‐24 months. | ||||
| 2 Withdrawals due to adverse events 12‐24 months Show forest plot | 15 | 1790 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |
| Analysis 4.2  Comparison 4 Bisphosphonates vs control: harms, Outcome 2 Withdrawals due to adverse events 12‐24 months. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 1 Incident radiographic vertebral fractures 12‐24 months.

Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 2 Incident radiographic nonvertebral fractures 12‐24 months.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 1 LS BMD change 12 months: all trials.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 2 LS BMD change 12 months: oral treatment.
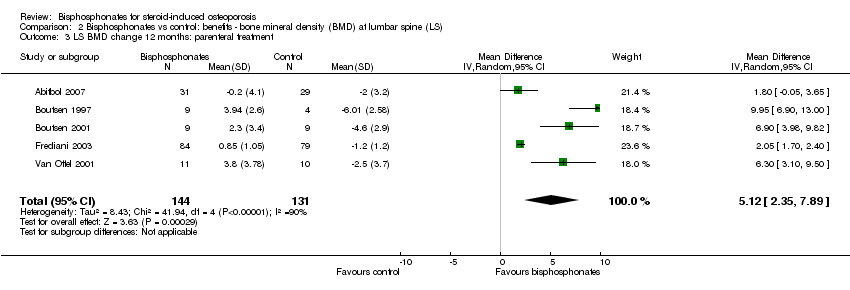
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 3 LS BMD change 12 months: parenteral treatment.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 4 LS BMD change 12 months: low‐ vs standard‐dose.
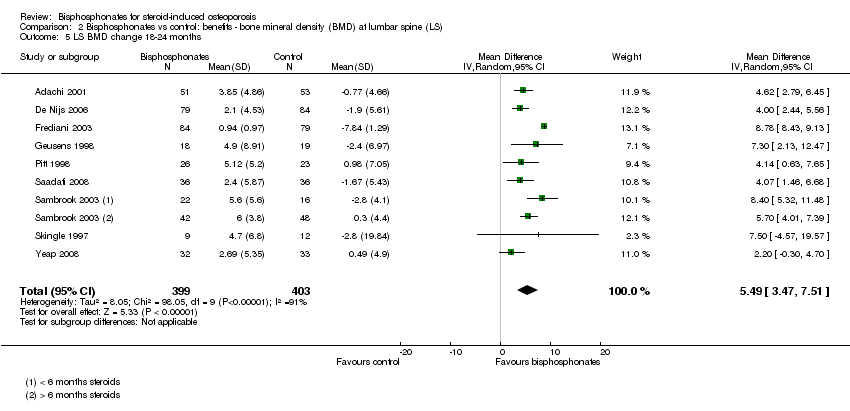
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 5 LS BMD change 18‐24 months.
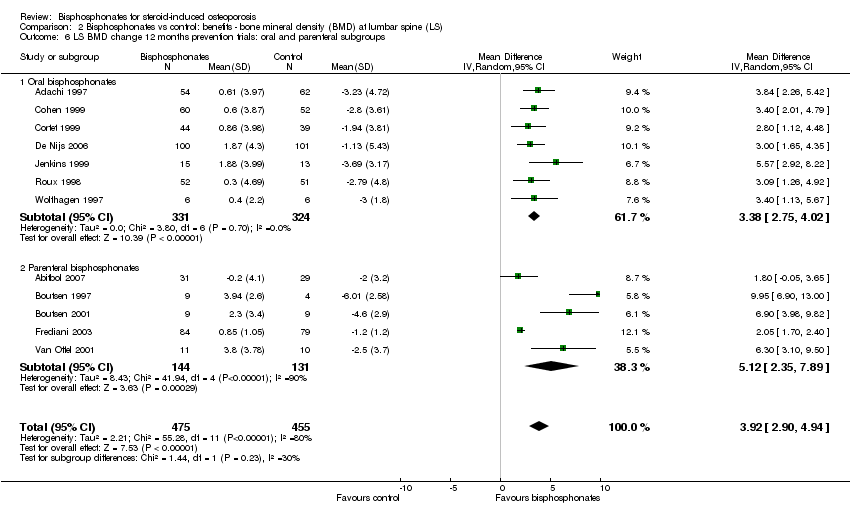
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 7 LS BMD change 12 months: gender/menopausal status subgroups.
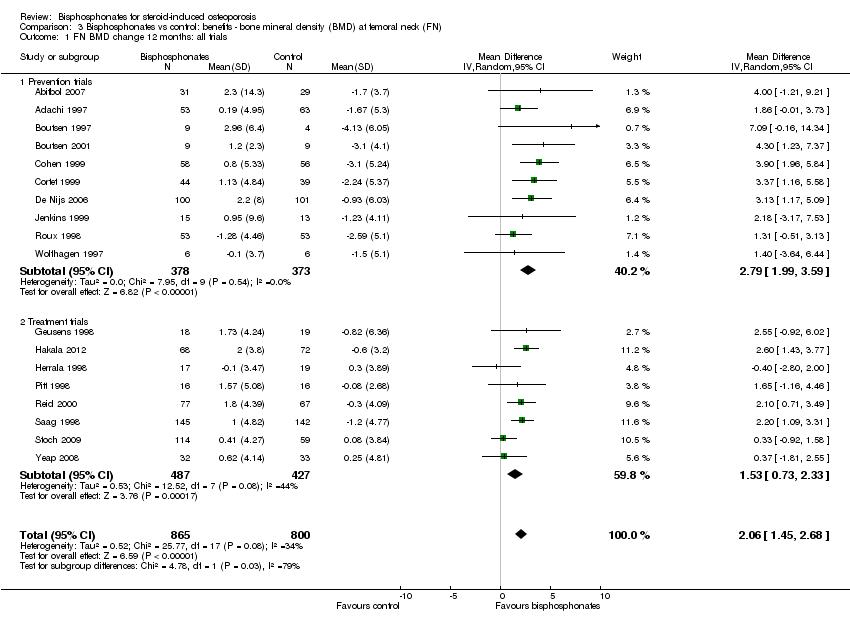
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 1 FN BMD change 12 months: all trials.
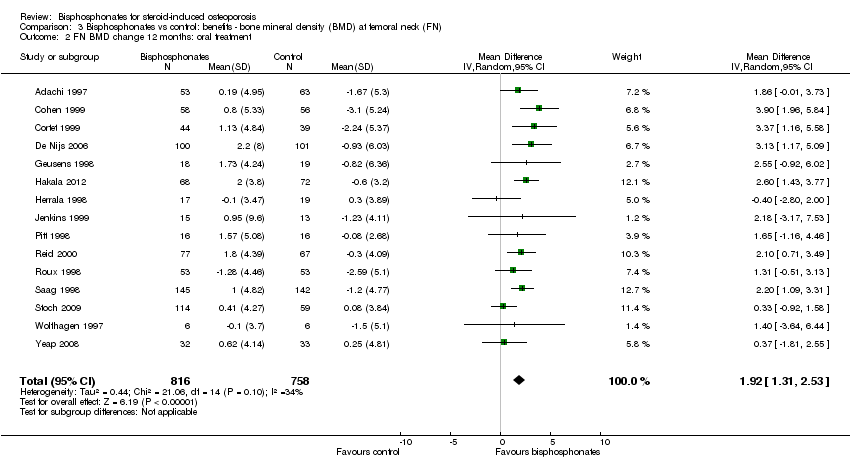
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 2 FN BMD change 12 months: oral treatment.

Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 3 FN BMD change 12 months: parenteral treatment.
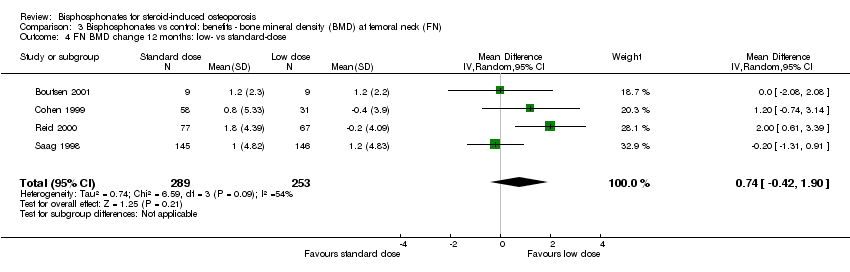
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 4 FN BMD change 12 months: low‐ vs standard‐dose.

Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 5 FN BMD change 18‐24 months.

Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 6 FN BMD change 12 months: gender/menopausal status subgroups.

Comparison 4 Bisphosphonates vs control: harms, Outcome 1 Serious adverse events 12‐24 months.
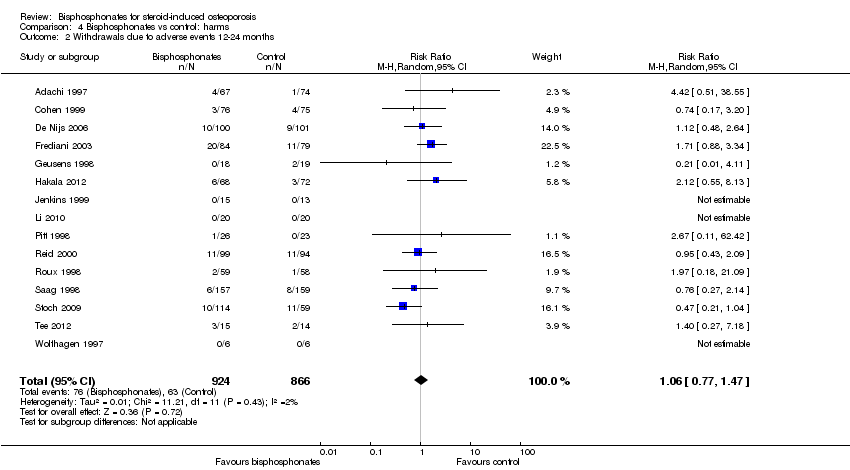
Comparison 4 Bisphosphonates vs control: harms, Outcome 2 Withdrawals due to adverse events 12‐24 months.
| Bisphosphonates (alone or with calcium and/or vitamin D) compared with control (calcium and/or vitamin D and/or placebo) for adults with GIOP | ||||||
| Patient or population: adults with GIOP Settings: ambulatory Intervention: bisphosphonates (alone or with calcium and/or vitamin D) Comparison: control (calcium and/or vitamin D and/or placebo) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (calcium and/or vitamin D and/or placebo) | Bisphosphonates (alone or with calcium and/or vitamin D) | |||||
| Incident vertebral fractures Radiographic follow‐up: 12‐24 months | 77 per 1000 | 44 per 1000 | RR 0.57 (0.35 to 0.91) RD ‐0.02 (‐0.05 to 0.01) | 1343 | ⊕⊕⊕⊕ | Absolute increased benefit 2% fewer people with fractures using bisphosphonates (95% CI 5.00% fewer to 1.00% more) Relative per cent change 43% improvement with bisphosphonates (95% CI 9.00% to 65.00% better) NNTB = 31 (95% CI 20 to 145) |
| Incident nonvertebral fractures Radiographic follow‐up: 12‐24 months | 55 per 1000 | 42 per 1000 | RR 0.79 (0.47 to 1.33) RD ‐0.01 (‐0.04 to 0.01) | 1245 | ⊕⊕⊝⊝ due to risk of bias and imprecision | Absolute increased benefit 1% fewer people with fractures using bisphosphonates (95% CI 4.00% fewer to 1.00% more) Relative per cent change 21% improvement with bisphosphonates (95% CI 33.00% worse to 53.00% better) NNTB = n/a4 |
| Lumbar spine BMD DEXA follow‐up: 12 months | Mean per cent change in BMD across control groups was ‐3.19% (‐8.08% to 1.70%) from baseline5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 3.50% higher than control groups (2.90% to 4.10% higher) | ‐ | 2042 | ⊕⊕⊕⊝ due to indirectness | Absolute increased benefit 3.50% with bisphosphonates (95% CI 2.90 to 4.10) Relative per cent change 1.10% (95% CI 0.91 to 1.29) with bisphosphonates NNTB = 3 (95% CI 2 to 3) |
| Femoral neck BMD DEXA follow‐up: 12 months | Mean per cent change in BMD across control groups was ‐1.59% (‐10.49% to 7.31%) from baseline 5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 2.06% higher than control groups (1.45% to 2.68% higher) | ‐ | 1665 | ⊕⊕⊕⊝ due to indirectness | Absolute increased benefit 2.06% with bisphosphonates (95% CI 1.45 to 2.68) Relative per cent change 1.29% with bisphosphonates (95% CI 0.91 to 1.69) NNTB = 5 (95% CI 4 to 7) |
| Serious adverse events follow‐up: 12‐24 months | 162 per 1000 | 147 per 1000 | RR 0.91 (0.74 to 1.12) RD 0.00 (‐0.02, 0.02) | 1703 | ⊕⊕⊕⊝ due to risk of bias and imprecision | Absolute increased harm 0% more adverse events with bisphosphonates (95% CI 2.00% fewer to 2.00% more) Relative per cent change 9% improvement with bisphosphonates (95% CI 12.00% worse to 26.00% better) NNTH = n/a4 |
| Withdrawals due to adverse events follow‐up: 12‐24 months | 73 per 1000 | 77 per 1000 | RR 1.06 (0.77 to 1.47) RD 0.01 (‐0.01 to 0.03) | 1790 | ⊕⊕⊕⊝ due to risk of bias and imprecision | Absolute increased harm 1% more withdrawals with bisphosphonates (95% CI 1.00% fewer to 3.00% more) Relative per cent change 6% worsening with bisphosphonates (95% CI 47.00% worse to 23.00% better) NNTH = n/a4 |
| Quality of life | 0 per 1000 | 0 per 1000 | Not estimable | (0 studies) | This outcome was not assessed by any of the trials | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Vertebral fractures meet calculated OIS threshold of 1174 (calculation not shown ‐ Brant 2014) 2Downgraded for risk of bias: nonvertebral fractures were a patient‐reported, subjective outcome 3Downgraded for imprecision: total sample size is below calculated optimal information size (OIS) (calculations not shown ‐ Brant 2014) and the 95% confidence interval around the pooled estimate of effect includes both the possibility of no effect and appreciable benefit or harm 4Number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) is not applicable when result is not statistically significant 5We calculated mean baseline risk for the control group in RevMan using generic inverse variance (calculations not shown) 6Most heterogeneity explained through sensitivity analyses 7Downgraded for indirectness: bone density is a surrogate marker for fracture risk 8Clinically relevant change in BMD: the natural history of participants starting steroid therapy based on control arms in our prevention trials is to see a 1%‐6% decrease in lumbar spine BMD and 1%‐4% decrease in femoral neck BMD in the first year of treatment. We have used an SMD of 0.5 as an estimate of the minimal clinically important difference for BMD change to calculate the NNTB (Schünemann 2011b) 9Downgraded for risk of bias: the protocols for the collection of harm data in a large number of trials were unclear | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident radiographic vertebral fractures 12‐24 months Show forest plot | 12 | 1343 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.35, 0.91] |
| 2 Incident radiographic nonvertebral fractures 12‐24 months Show forest plot | 9 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LS BMD change 12 months: all trials Show forest plot | 23 | 2042 | Mean Difference (IV, Random, 95% CI) | 3.50 [2.90, 4.10] |
| 1.1 Prevention trials | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 1.2 Treatment trials | 11 | 1112 | Mean Difference (IV, Random, 95% CI) | 3.19 [2.64, 3.73] |
| 2 LS BMD change 12 months: oral treatment Show forest plot | 18 | 1767 | Mean Difference (IV, Random, 95% CI) | 3.25 [2.88, 3.63] |
| 3 LS BMD change 12 months: parenteral treatment Show forest plot | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 4 LS BMD change 12 months: low‐ vs standard‐dose Show forest plot | 5 | 642 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.37, 1.53] |
| 5 LS BMD change 18‐24 months Show forest plot | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 5.49 [3.47, 7.51] |
| 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups Show forest plot | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 6.1 Oral bisphosphonates | 7 | 655 | Mean Difference (IV, Random, 95% CI) | 3.38 [2.75, 4.02] |
| 6.2 Parenteral bisphosphonates | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 7 LS BMD change 12 months: gender/menopausal status subgroups Show forest plot | 5 | 840 | Mean Difference (IV, Random, 95% CI) | 3.86 [2.03, 5.68] |
| 7.1 Men | 4 | 221 | Mean Difference (IV, Random, 95% CI) | 3.58 [2.68, 4.48] |
| 7.2 Premenopausal women | 5 | 154 | Mean Difference (IV, Random, 95% CI) | 3.51 [1.50, 5.53] |
| 7.3 Postmenopausal women | 5 | 465 | Mean Difference (IV, Random, 95% CI) | 4.41 [0.65, 8.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FN BMD change 12 months: all trials Show forest plot | 18 | 1665 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.45, 2.68] |
| 1.1 Prevention trials | 10 | 751 | Mean Difference (IV, Random, 95% CI) | 2.79 [1.99, 3.59] |
| 1.2 Treatment trials | 8 | 914 | Mean Difference (IV, Random, 95% CI) | 1.53 [0.73, 2.33] |
| 2 FN BMD change 12 months: oral treatment Show forest plot | 15 | 1574 | Mean Difference (IV, Random, 95% CI) | 1.92 [1.31, 2.53] |
| 3 FN BMD change 12 months: parenteral treatment Show forest plot | 3 | 91 | Mean Difference (IV, Random, 95% CI) | 4.56 [2.07, 7.05] |
| 4 FN BMD change 12 months: low‐ vs standard‐dose Show forest plot | 4 | 542 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.42, 1.90] |
| 5 FN BMD change 18‐24 months Show forest plot | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 3.28 [1.70, 4.87] |
| 6 FN BMD change 12 months: gender/menopausal status subgroups Show forest plot | 4 | 537 | Mean Difference (IV, Random, 95% CI) | 3.29 [1.65, 4.94] |
| 6.1 Men | 3 | 134 | Mean Difference (IV, Random, 95% CI) | 2.91 [1.15, 4.68] |
| 6.2 Premenopausal women | 4 | 88 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐0.96, 6.35] |
| 6.3 Postmenopausal women | 4 | 315 | Mean Difference (IV, Random, 95% CI) | 3.62 [‐0.37, 7.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse events 12‐24 months Show forest plot | 15 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| 2 Withdrawals due to adverse events 12‐24 months Show forest plot | 15 | 1790 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |

