Bifosfonatos para a osteoporose induzida por corticoesteróides
Appendices
Appendix 1. Original Review search strategies
MEDLINE (1966‐1997)
1. exp "osteoporosis"/
2. exp "adrenal cortex hormones"/
3. exp "anabolic steroids"/
4. exp "bone density"/
5. exp "anti‐inflammatory agents, steroidal"/
6. 1 or 4
7. 2 or 3 or 5
8. 6 and 7
9. exp "diphosphonates"/
10. 9 and 6
11. exp "osteoporosis"/ci
12. 8 or 10 or 11
13. limit 12 to human
14. limit 13 to English language
15. exp osteoporosis/dt
16. exp bone diseases/
17. 16 and 7
18. limit 17 to human
19. limit 18 to English language
20. 14 or 15 or 19
Embase (1988‐1997)
1. exp bone demineralization/
2. exp bone density/
3. exp bone disease/
4. bone demineralization/
5. osteopenia/
6. osteoporosis/
7. postmenopause osteoporosis/
8. posttraumatic osteoporosis/
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp corticosteroid/
11. exp antirheumatic agent/
12. antiinflammatory agent/
13. exp antiinflammatory agent/
14. exp nonsteroid antiinflammatory agent/
15. 13 not 14
16. 10 or 11 or 12 or 15
17. exp bisphosphonic acid derivative/
18. 9 and 17
19. 9 and 16
20. exp bone demineralization/si
21. exp osteopenia/si
22. exp bone demineralization/dt
23. 18 or 19 or 20 or 21 or 22
Appendix 2. CENTRAL search strategy 2015
Database: Cochrane Central Register of Controlled Trials (CENTRAL)
Date Searched: 07 May 2015
1. exp Osteoporosis/
2. (osteoporo* or osteopeni*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
3. Bone Density/
4. exp Fractures, Bone/
5. (bone* adj fragil*).mp.
6. exp Bone Resorption/
7. bone loss.mp.
8. (bmd or bone mineral densit* bone deminerali*).mp.
9. or/1‐8
10. exp Steroids/
11. exp Adrenal Cortex Hormones/
12. (steroid* or corticosteroid* or glucocorticoid*).mp.
13. or/10‐12
14. 9 and 13
15. Bone Diseases, Metabolic/ci [Chemically Induced]
16. 14 or 15
17. exp Diphosphonates/
18. (diphosphonate* or bisphosphonate*).mp.
19. etidronate.mp.
20. alendronate.mp.
21. pamidronate.mp.
22. clodronate.mp.
23. tiludronate.mp.
24. olpadronate.mp.
25. incadronate.mp.
26. zolendronate.mp.
27. risedronate.mp.
28. zolendronic acid.mp.
29. ibandronate.mp.
30. medronate.mp.
31. minodronate.mp.
32. neridronate.mp.
33. oxidronate.mp.
34. or/17‐33
35. 16 and 34
36. randomized controlled trial.pt.
37. controlled clinical trial.pt.
38. clinical trial.pt.
39. randomi?ed.ti,ab.
40. placebo.ti,ab.
41. dt.fs.
42. randomly.ti,ab.
43. trial.ti,ab.
44. groups.ti,ab.
45. or/36‐44
46. animals/
47. humans/
48. 46 not (46 and 47)
49. 45 not 48
50. 35 and 49
51. limit 50 to yr="2010 ‐Current"
Appendix 3. MEDLINE search strategy 1997‐2010
Database: Medline via Ovid <1946 to Present>
Date Searched: 25 January 2010
| 1. exp Osteoporosis/ 2. osteoporo$.tw. 3. osteopeni$.tw. 4. exp Bone Density/ 5. exp Fractures, Bone/ 6. (bone$ adj fragil$).tw. 7. (bone adj loss).tw. 8. bmd.tw. 9. (bone adj2 densit$).tw. |
| 10. or/1‐9 |
| 11. exp Adrenal Cortex Hormones/ 12. exp Steroids/ 13. exp Glucocorticoids/ 14. (corticosteroid$ or $corticoid$).tw. 15. (adrenal adj cortex adj hormone$).tw. 16. steroid$.tw. |
| 17. or/11‐16 |
| 18. exp Diphosphonates/ 19. risedronate.tw. 20. pamidronate.tw. 21. zolendronate$.tw. 22. (bisphosphonate$ or biphosphonate$ or diphosphonate).tw. 23. tiludronate.tw. 24. etidronate.tw. 25. alendronate.tw. 26. olpadronate.tw. 27. incadronate.tw. 28. exp Clodronic Acid/ 29. clodronate.tw. |
| 30. or/18‐29 |
| 31. and/10,17,30 |
| 32. randomized controlled trial.pt. 33. controlled trial.pt. 34. randomized.ab. 35. placebo.ab. 36. drug therapy.fs. 37. randomly.ab. 38. trial.ab. 39. groups.ab. |
| 40. or/32‐39 |
| 41. (animals not (humans and animals)).sh. 42. 40 not 41 |
| 43. 31 and 42 44. limit 43 to yr="1997 ‐ current" |
Appendix 4. MEDLINE search strategy 2010‐2015
Database: Medline via Ovid <1946 to Present>
Date Searched: 03 April 2013; 07 May 2015
1. exp Osteoporosis/
2. (osteoporo* or osteopeni*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
3. Bone Density/
4. exp Fractures, Bone/
5. (bone* adj fragil*).mp.
6. exp Bone Resorption/
7. bone loss.mp.
8. (bmd or bone mineral densit* bone deminerali*).mp.
9. or/1‐8
10. exp Steroids/
11. exp Adrenal Cortex Hormones/
12. (steroid* or corticosteroid* or glucocorticoid*).mp.
13. or/10‐12
14. 9 and 13
15. Bone Diseases, Metabolic/ci [Chemically Induced]
16. 14 or 15
17. exp Diphosphonates/
18. (diphosphonate* or bisphosphonate*).mp.
19. etidronate.mp.
20. alendronate.mp.
21. pamidronate.mp.
22. clodronate.mp.
23. tiludronate.mp.
24. olpadronate.mp.
25. incadronate.mp.
26. zolendronate.mp.
27. risedronate.mp.
28. zolendronic acid.mp.
29. ibandronate.mp.
30. medronate.mp.
31. minodronate.mp.
32. neridronate.mp.
33. oxidronate.mp.
34. or/17‐33
35. 16 and 34
36. randomized controlled trial.pt.
37. controlled clinical trial.pt.
38. clinical trial.pt.
39. randomi?ed.ti,ab.
40. placebo.ti,ab.
41. dt.fs.
42. randomly.ti,ab.
43. trial.ti,ab.
44. groups.ti,ab.
45. or/36‐44
46. animals/
47. humans/
48. 46 not (46 and 47)
49. 45 not 48
50. 35 and 49
51. limit 50 to yr="2010 ‐Current"
Appendix 5. Embase search strategy 1997‐2010
Database: Embase via Ovid <1980 to present>
Date Searched: 25 January 2010
| 1. exp osteoporosis/ 2. osteoporo$.tw. 3. osteopeni$.tw. 4. exp bone density/ 5. exp fracture/ 6. (bone$ adj fragil$).tw. 7. (bone adj loss).tw. 8. bmd.tw. 9. (bone adj2 densit$).tw. |
| 10. or/1‐9 |
| 11. exp steroid/ 12. steroid$.tw. 13. exp corticosteroid/ 14. exp glucocorticoid/ 15. (corticosteroid$ or $corticoid$).tw. |
| 16. or/11‐15 |
| 17. 10 and 16 |
| 18. exp corticosteroid induced osteoporosis/ |
| 19. 17 or 18 |
| 20. exp bisphosphonic acid derivative/ 21. (diphosphonate$ or biphosphonate$ or bisphosphonate$).tw. 22. etidronate.tw. 23. alendronate.tw. 24. tiludronate.tw. 25. olpadronate.tw. 26. incadronate.tw. 27. risendronate$.tw. 28. pamidronate$.tw. 29. clodronate$.tw. 30. zolendronate$.tw. 31. exp clodronic acid/ |
| 32. or/20‐31 |
| 33. 19 and 32 |
| 34. (random$ or placebo$).ti,ab. 35. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab. 36. (controlled adj clinical adj trial$).ti,ab. 37. RETRACTED ARTICLE/ |
| 38. or/34‐37 |
| 39. (animal$ not human$).sh,hw. 40. 38 not 39 |
| 41. 33 and 40 42. limit 41 to yr="1997 ‐ current" |
Appendix 6. Embase search strategy 2010‐2015
Database: Embase via Ovid <1974 to present>
Date Searched: 03 April 2013; 07 May 2015
1. exp osteoporosis/
2. (osteoporo* or osteopeni*).mp.
3. bone demineralization/
4. bone density/
5. exp fracture/
6. (bone* adj fragil*).mp.
7. osteolysis/
8. bone loss.mp.
9. (bmd or bone mineral densit* bone deminerali*).mp.
10. or/1‐9
11. exp steroid/
12. (steroid* or corticosteroid* or glucocorticoid*).mp.
13. 11 or 12
14. 10 and 13
15. corticosteroid induced osteoporosis/
16. 14 or 15
17. exp bisphosphonic acid derivative/
18. (diphosphonate* or bisphosphonate*).mp.
19. etidronate.mp.
20. alendronate.mp.
21. pamidronate.mp.
22. clodronate.mp.
23. tiludronate.mp.
24. olpadronate.mp.
25. incadronate.mp.
26. zolendronate.mp.
27. risedronate.mp.
28. zolendronic acid.mp.
29. ibandronate.mp.
30. medronate.mp.
31. minodronate.mp.
32. neridronate.mp.
33. oxidronate.mp.
34. or/17‐33
35. 16 and 34
36. controlled clinical trial/ or clinical trial/ or controlled study/ or randomized controlled trial/ or major clinical study/
37. randomi?ed.ti,ab.
38. placebo.ti,ab.
39. randomly.ti,ab.
40. trial.ti.
41. or/36‐40
42. (exp vertebrate/ or animal/ or exp experimental animal/ or nonhuman/ or animal.hw.) not (exp human/ or human experiment/)
43. (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. not (exp human/ or human experiment/)
44. 42 or 43
45. 41 not 44
46. 35 and 45
47. limit 46 to yr="2010 ‐ current"
Appendix 7. IPA search strategy 1970‐2012
Database: International Pharmaceutical Abstracts via Ovid <1970 to present>
Date Searched: 27 January 2012
| 1. osteoporosis.hw,sh. 2. bone density.hw,sh. 3. fracture$.hw,sh. 4. osteoporo$.tw. 5. osteopeni$.tw. 6. (bone adj2 density).tw. 7. bmd.tw. 8. (bone$ adj fragil$).tw. |
| 9. or/1‐8 |
| 10. Adrenal cortex hormones.hw,sh. 11. steroid$.hw,sh,tw. 12. (adrenal adj cortex adj hormone$).tw. 13. anabolic agents.hw,sh. 14. (anabolic adj agent$).tw. 15. glucocorticoids.hw,sh. 16. corticosteroid.hw,sh. 17. (corticosteroid$ or $corticoid$).tw. |
| 18. or/10‐17 |
| 19. bisphosphonates.hw,sh. 20. (diphosphonate$ or bisphosphonate$ or biphosphonate$).tw. 21. risedronate.hw,sh,tw. 22. pamidronate.hw,sh,tw. 23. clodronic acid.hw,sh,tw. 24. clodronate.hw,sh,tw. 25. etidronate.hw,sh,tw. 26. alendronate.hw,sh,tw. 27. tiludronate.hw,sh,tw. 28. olpadronate.hw,sh,tw. 29. incadronate.hw,sh,tw. |
| 30. or/19‐29 |
| 31. random$.hw,sh,tw. 32. ((double or single) and procedure$).mp. 33. (clin$ adj25 trial$).mp. 34. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp. 35. placebo$.mp. 36. (comparative adj25 stud$).mp. 37. evaluation$.mp. 38. (control$ or prospectiv$ or volunteer$).mp. |
| 39. or/31‐38 |
| 40. (animals not (humans and animals)).sh,hw. 41. 39 not 40 |
| 42. and/9,18,30 43. 41 and 42 |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 1 Incident radiographic vertebral fractures 12‐24 months.

Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 2 Incident radiographic nonvertebral fractures 12‐24 months.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 1 LS BMD change 12 months: all trials.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 2 LS BMD change 12 months: oral treatment.
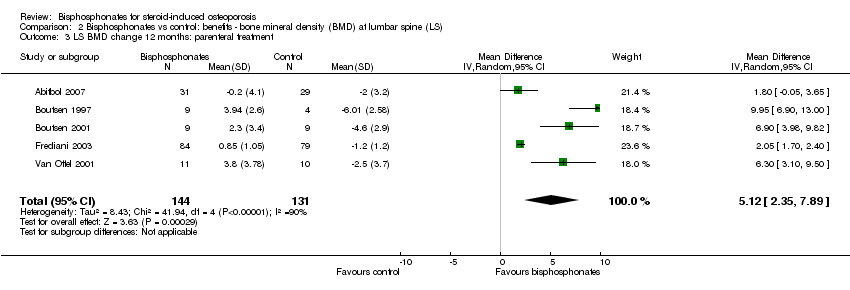
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 3 LS BMD change 12 months: parenteral treatment.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 4 LS BMD change 12 months: low‐ vs standard‐dose.
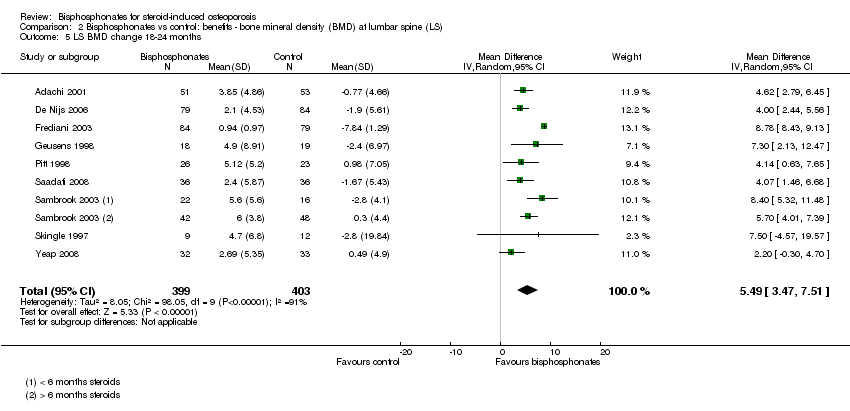
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 5 LS BMD change 18‐24 months.
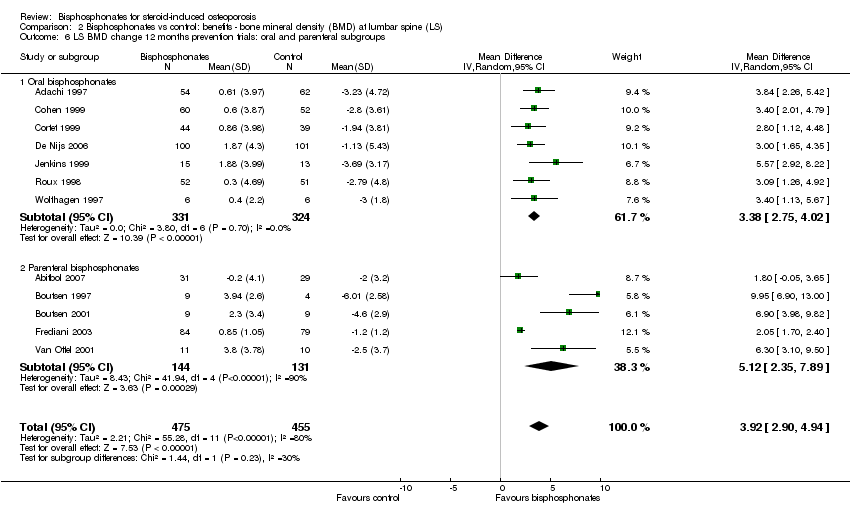
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups.

Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 7 LS BMD change 12 months: gender/menopausal status subgroups.
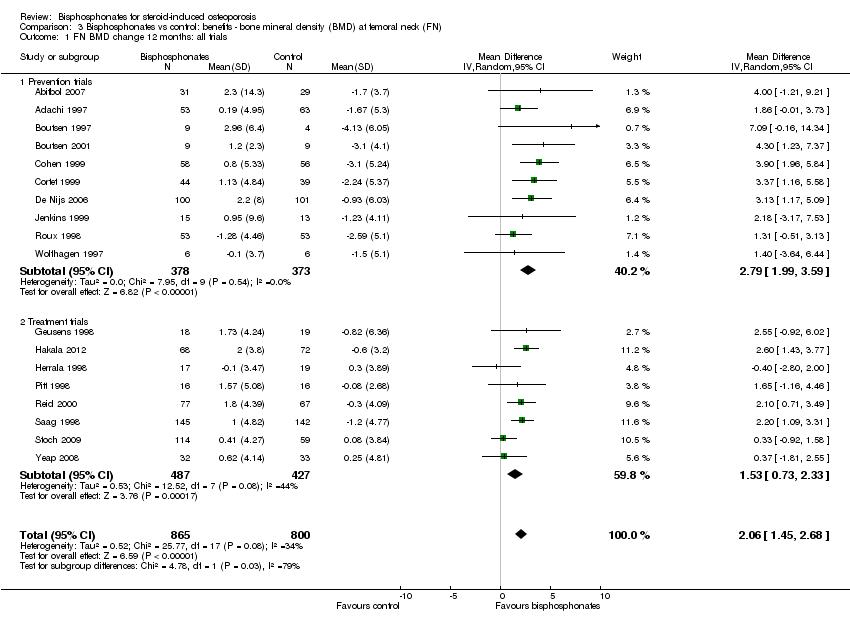
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 1 FN BMD change 12 months: all trials.
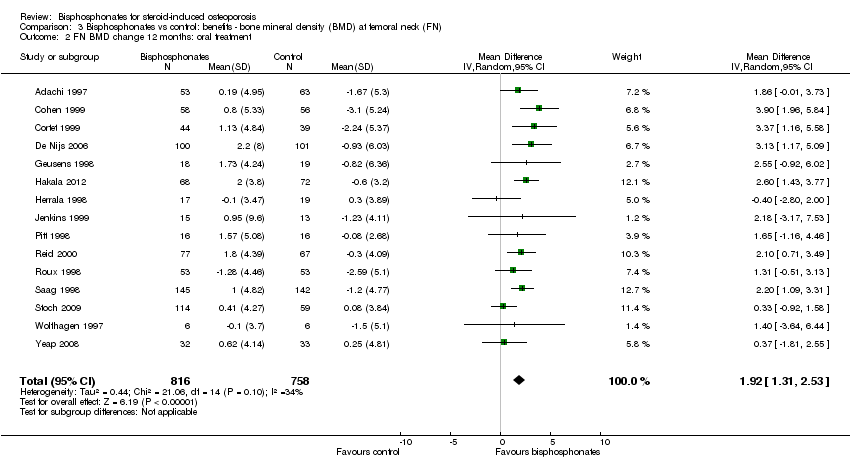
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 2 FN BMD change 12 months: oral treatment.

Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 3 FN BMD change 12 months: parenteral treatment.
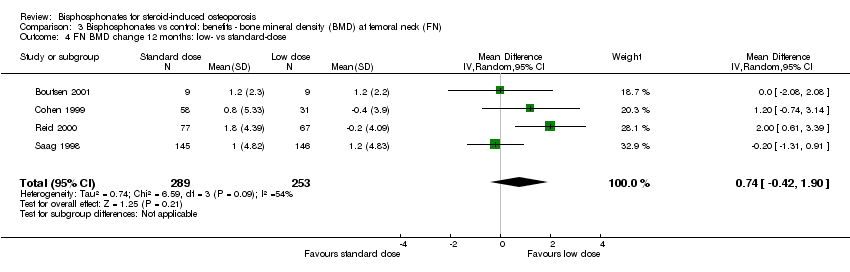
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 4 FN BMD change 12 months: low‐ vs standard‐dose.

Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 5 FN BMD change 18‐24 months.

Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 6 FN BMD change 12 months: gender/menopausal status subgroups.

Comparison 4 Bisphosphonates vs control: harms, Outcome 1 Serious adverse events 12‐24 months.
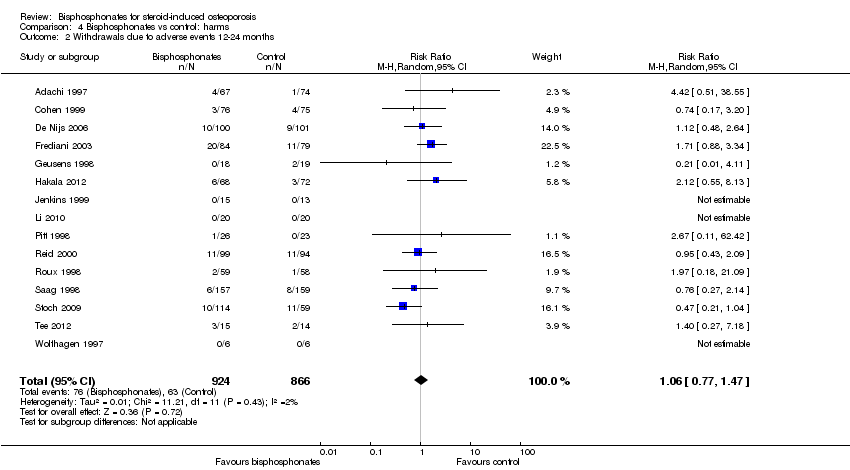
Comparison 4 Bisphosphonates vs control: harms, Outcome 2 Withdrawals due to adverse events 12‐24 months.
| Bisphosphonates (alone or with calcium and/or vitamin D) compared with control (calcium and/or vitamin D and/or placebo) for adults with GIOP | ||||||
| Patient or population: adults with GIOP Settings: ambulatory Intervention: bisphosphonates (alone or with calcium and/or vitamin D) Comparison: control (calcium and/or vitamin D and/or placebo) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (calcium and/or vitamin D and/or placebo) | Bisphosphonates (alone or with calcium and/or vitamin D) | |||||
| Incident vertebral fractures Radiographic follow‐up: 12‐24 months | 77 per 1000 | 44 per 1000 | RR 0.57 (0.35 to 0.91) RD ‐0.02 (‐0.05 to 0.01) | 1343 | ⊕⊕⊕⊕ | Absolute increased benefit 2% fewer people with fractures using bisphosphonates (95% CI 5.00% fewer to 1.00% more) Relative per cent change 43% improvement with bisphosphonates (95% CI 9.00% to 65.00% better) NNTB = 31 (95% CI 20 to 145) |
| Incident nonvertebral fractures Radiographic follow‐up: 12‐24 months | 55 per 1000 | 42 per 1000 | RR 0.79 (0.47 to 1.33) RD ‐0.01 (‐0.04 to 0.01) | 1245 | ⊕⊕⊝⊝ due to risk of bias and imprecision | Absolute increased benefit 1% fewer people with fractures using bisphosphonates (95% CI 4.00% fewer to 1.00% more) Relative per cent change 21% improvement with bisphosphonates (95% CI 33.00% worse to 53.00% better) NNTB = n/a4 |
| Lumbar spine BMD DEXA follow‐up: 12 months | Mean per cent change in BMD across control groups was ‐3.19% (‐8.08% to 1.70%) from baseline5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 3.50% higher than control groups (2.90% to 4.10% higher) | ‐ | 2042 | ⊕⊕⊕⊝ due to indirectness | Absolute increased benefit 3.50% with bisphosphonates (95% CI 2.90 to 4.10) Relative per cent change 1.10% (95% CI 0.91 to 1.29) with bisphosphonates NNTB = 3 (95% CI 2 to 3) |
| Femoral neck BMD DEXA follow‐up: 12 months | Mean per cent change in BMD across control groups was ‐1.59% (‐10.49% to 7.31%) from baseline 5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 2.06% higher than control groups (1.45% to 2.68% higher) | ‐ | 1665 | ⊕⊕⊕⊝ due to indirectness | Absolute increased benefit 2.06% with bisphosphonates (95% CI 1.45 to 2.68) Relative per cent change 1.29% with bisphosphonates (95% CI 0.91 to 1.69) NNTB = 5 (95% CI 4 to 7) |
| Serious adverse events follow‐up: 12‐24 months | 162 per 1000 | 147 per 1000 | RR 0.91 (0.74 to 1.12) RD 0.00 (‐0.02, 0.02) | 1703 | ⊕⊕⊕⊝ due to risk of bias and imprecision | Absolute increased harm 0% more adverse events with bisphosphonates (95% CI 2.00% fewer to 2.00% more) Relative per cent change 9% improvement with bisphosphonates (95% CI 12.00% worse to 26.00% better) NNTH = n/a4 |
| Withdrawals due to adverse events follow‐up: 12‐24 months | 73 per 1000 | 77 per 1000 | RR 1.06 (0.77 to 1.47) RD 0.01 (‐0.01 to 0.03) | 1790 | ⊕⊕⊕⊝ due to risk of bias and imprecision | Absolute increased harm 1% more withdrawals with bisphosphonates (95% CI 1.00% fewer to 3.00% more) Relative per cent change 6% worsening with bisphosphonates (95% CI 47.00% worse to 23.00% better) NNTH = n/a4 |
| Quality of life | 0 per 1000 | 0 per 1000 | Not estimable | (0 studies) | This outcome was not assessed by any of the trials | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Vertebral fractures meet calculated OIS threshold of 1174 (calculation not shown ‐ Brant 2014) 2Downgraded for risk of bias: nonvertebral fractures were a patient‐reported, subjective outcome 3Downgraded for imprecision: total sample size is below calculated optimal information size (OIS) (calculations not shown ‐ Brant 2014) and the 95% confidence interval around the pooled estimate of effect includes both the possibility of no effect and appreciable benefit or harm 4Number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) is not applicable when result is not statistically significant 5We calculated mean baseline risk for the control group in RevMan using generic inverse variance (calculations not shown) 6Most heterogeneity explained through sensitivity analyses 7Downgraded for indirectness: bone density is a surrogate marker for fracture risk 8Clinically relevant change in BMD: the natural history of participants starting steroid therapy based on control arms in our prevention trials is to see a 1%‐6% decrease in lumbar spine BMD and 1%‐4% decrease in femoral neck BMD in the first year of treatment. We have used an SMD of 0.5 as an estimate of the minimal clinically important difference for BMD change to calculate the NNTB (Schünemann 2011b) 9Downgraded for risk of bias: the protocols for the collection of harm data in a large number of trials were unclear | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident radiographic vertebral fractures 12‐24 months Show forest plot | 12 | 1343 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.35, 0.91] |
| 2 Incident radiographic nonvertebral fractures 12‐24 months Show forest plot | 9 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LS BMD change 12 months: all trials Show forest plot | 23 | 2042 | Mean Difference (IV, Random, 95% CI) | 3.50 [2.90, 4.10] |
| 1.1 Prevention trials | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 1.2 Treatment trials | 11 | 1112 | Mean Difference (IV, Random, 95% CI) | 3.19 [2.64, 3.73] |
| 2 LS BMD change 12 months: oral treatment Show forest plot | 18 | 1767 | Mean Difference (IV, Random, 95% CI) | 3.25 [2.88, 3.63] |
| 3 LS BMD change 12 months: parenteral treatment Show forest plot | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 4 LS BMD change 12 months: low‐ vs standard‐dose Show forest plot | 5 | 642 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.37, 1.53] |
| 5 LS BMD change 18‐24 months Show forest plot | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 5.49 [3.47, 7.51] |
| 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups Show forest plot | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 6.1 Oral bisphosphonates | 7 | 655 | Mean Difference (IV, Random, 95% CI) | 3.38 [2.75, 4.02] |
| 6.2 Parenteral bisphosphonates | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 7 LS BMD change 12 months: gender/menopausal status subgroups Show forest plot | 5 | 840 | Mean Difference (IV, Random, 95% CI) | 3.86 [2.03, 5.68] |
| 7.1 Men | 4 | 221 | Mean Difference (IV, Random, 95% CI) | 3.58 [2.68, 4.48] |
| 7.2 Premenopausal women | 5 | 154 | Mean Difference (IV, Random, 95% CI) | 3.51 [1.50, 5.53] |
| 7.3 Postmenopausal women | 5 | 465 | Mean Difference (IV, Random, 95% CI) | 4.41 [0.65, 8.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FN BMD change 12 months: all trials Show forest plot | 18 | 1665 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.45, 2.68] |
| 1.1 Prevention trials | 10 | 751 | Mean Difference (IV, Random, 95% CI) | 2.79 [1.99, 3.59] |
| 1.2 Treatment trials | 8 | 914 | Mean Difference (IV, Random, 95% CI) | 1.53 [0.73, 2.33] |
| 2 FN BMD change 12 months: oral treatment Show forest plot | 15 | 1574 | Mean Difference (IV, Random, 95% CI) | 1.92 [1.31, 2.53] |
| 3 FN BMD change 12 months: parenteral treatment Show forest plot | 3 | 91 | Mean Difference (IV, Random, 95% CI) | 4.56 [2.07, 7.05] |
| 4 FN BMD change 12 months: low‐ vs standard‐dose Show forest plot | 4 | 542 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.42, 1.90] |
| 5 FN BMD change 18‐24 months Show forest plot | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 3.28 [1.70, 4.87] |
| 6 FN BMD change 12 months: gender/menopausal status subgroups Show forest plot | 4 | 537 | Mean Difference (IV, Random, 95% CI) | 3.29 [1.65, 4.94] |
| 6.1 Men | 3 | 134 | Mean Difference (IV, Random, 95% CI) | 2.91 [1.15, 4.68] |
| 6.2 Premenopausal women | 4 | 88 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐0.96, 6.35] |
| 6.3 Postmenopausal women | 4 | 315 | Mean Difference (IV, Random, 95% CI) | 3.62 [‐0.37, 7.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse events 12‐24 months Show forest plot | 15 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| 2 Withdrawals due to adverse events 12‐24 months Show forest plot | 15 | 1790 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |

