禁煙における個別の行動カウンセリング
アブストラクト
背景
禁煙専門家が個別に行うカウンセリングは、喫煙者に禁煙を成功させる助けになるかもしれない。
目的
このレビューは、次の仮説に取り組むものである。
1.個別のカウンセリングは、無治療や簡潔なアドバイスよりも禁煙の推進により効果的である。2.個別のカウンセリングは、自助教材よりも禁煙の推進により効果的である。3.より集中的なカウンセリングの介入は、あまり集中的でない介入より効果的である。
検索戦略
2016年5月にCochrane Tobacco Addiction Group Specialized Registerの全ての領域でcounsel* を含む研究を検索した。
選択基準
普段の臨床ケアに関与していない医療従事者による対面式個別カウンセリングからなる、少なくとも1つの治療群があるランダム化または準ランダム化試験。アウトカムは、カウンセリング開始から少なくとも6ヶ月後のフォローアップ時の禁煙であった。
データ収集と分析
2名の著者がデータを二重に抽出した。介入と対象集団の特徴、ランダム化の方法とフォローアップの完全性を記録した。私たちはそれぞれの試験において、禁煙の最も厳密な定義を使用し、生化学的に妥当な割合を利用した。分析において、私たちは追跡できなかった参加者を継続的な喫煙者と仮定した。私たちは、効果を禁煙のリスク比(RR)として表した。可能な場合は、固定効果(Mantel‐Haenszel)モデルを使用して、メタ分析を実施しました。Cochraneの'Risk of bias' ツールとGRADE基準を使用して、各研究のエビデンスの質を評価した。
主な結果
私たちは約19,000人の参加者を含む49の試験を同定した。33の臨床試験は、個別のカウンセリングと最小の行動介入を比較していた。薬物療法が参加者に提供されなかった場合、個別のカウンセリングは最小のコンタクトの対照群(簡単なアドバイス、通常のケア、または自助教材の提供)よりも効果的であったという高い質のエビデンスがあった。(RR 1.57,95%信頼区間(CI)1.40〜1.77; 27の研究、11,100人の参加者; I 1 2 1 = 50%)。全ての参加者が薬物療法を受けていた場合(ニコチン置換療法)、カウンセリングの利益には中程度の質のエビデンスがあった(不正確さのため、ダウングレード)。(RR 1.24, 95% CI 1.01 to 1.51; 6 studies, 2662 participants; I121 = 0%)短いカウンセリングと比較して、より集中的なカウンセリングには小さな利益があるという、中程度の質のエビデンス(不正確さのためにダウングレード)があった(RR 1.29, 95% CI 1.09 to 1.53; 11 studies, 2920 participants; I121 = 48%)。似たような強さの、異なるカウンセリングモデルを比較する5 つの試験は、いずれも有意差が検出されなかった。
著者の結論
個別に行われる禁煙カウンセリングは喫煙者の禁煙を支援するという高い質のエビデンスがある。カウンセリングが薬物療法に加えて使用される場合、短いカウンセリング介入と比較して、より集中的なカウンセリングに相対的に少ない利益の中程度の質のエビデンスがある。
PICOs
一般語訳
個別のカウンセリングは人々が禁煙するのを助けるか。
背景
個別カウンセリングは、喫煙をやめようとしている人々 を助けるためによく使われます。このレビューでは、医療とは別に1回以上の対面セッションを行った、訓練を受けたセラピストによるカウンセリングの臨床試験に注目した。アウトカムは、少なくとも6ヵ月後に非喫煙者であることであった。
試験の特性
私たちは2016年5月に臨床試験を検索し、1約19,000人の参加者を含む49の試験を同定した。全ての試験は少なくとも10分以上続く対面カウンセリング セッションを1回以上含んでいたが、ほとんどはそれ以上長く行われた。多くは追加的支援として、さらに電話によるコンタクトを含んでいた。33の試験は、通常のケア、禁煙に関する簡単な助言、または書面による最小限の支援しかない対照群と、個別のカウンセリングを比較していた。そのうち、27の試験は人々が喫煙をやめるのを助けるニコチン置換療法(NRT)などの薬を提供しなかった。33の試験のうち6つは、全員にNRTや他の薬を提供した。12の研究はより集中的でないカウンセリングと比較し、5つの研究は異なるタイプの研究と比較した。
結果とエビデンスの質
研究結果を統合することにより、個別のカウンセリングを受けることは最小限の支援と比較して40%から80%の間で喫煙をやめる機会を増加させうることを示していた。つまり、簡潔な支援のみが与えられた対照群では、100名のうち7名の喫煙者がなんとか喫煙をやめられたが、カウンセリングを受けた群は100名のうち10~12名が喫煙をやめることが期待された。私たちはこのエビデンスの質を高いと判断した。誰もがNRTや他の投薬を受けていて、対照群の100名中11名が喫煙をやめられた場合、カウンセリングを追加することで、100名中11~16名が成功すると期待される。利益の大きさがあまり確かではないため、このエビデンスの質は中程度であると評価した。より多くのセッションなど、より集中的なカウンセリングのサポートがあれば、おそらくもっと役立ちますが、利益の大きさが不確かであるため、追加の利益は小さくなる可能性があり、やはり中程度の質でしょう。異なるタイプのカウンセリングを比較した研究はほとんどなく、それらの間に相違は見られなかった。
Authors' conclusions
Summary of findings
| Patient or population: People who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Numbers quit in control condition | Numbers quit after individual counselling | |||||
| Smoking cessation at longest follow‐up ‐ 6 months or more No systematic pharmacotherapy | Study population | RR 1.57 | 11,100 | ⊕⊕⊕⊕ | Limiting to studies at low risk of bias on all assessed domains marginally increases estimate of effect | |
| 7 per 100 | 11 per 100 | |||||
| Smoking cessation at longest follow‐up ‐ 6 months or more Pharmacotherapy offered to all participants | Study population | RR 1.24 | 2662 | ⊕⊕⊕⊝ | Higher control group quit rate reflecting use of pharmacotherapy | |
| 11 per 100 | 13 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded due to wide confidence intervals. | ||||||
| More intensive compared to less intensive counselling for smoking cessation | ||||||
| Patient or population: People who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Numbers quit with less intensive counselling | Numbers quit with more intensive counselling | |||||
| Smoking cessation at longest follow‐up | Without pharmacotherapy | RR 1.29 | 2920 | ⊕⊕⊕⊕ | Effect estimates for subgroups of studies with and without pharmacotherapy for all participants overlapped, so the overall pooled estimate is used with alternative control group estimates from subgroups | |
| 9 per 100 1 | 12 per 100 | |||||
| With pharmacotherapy | ||||||
| 14 per 100 2 | 18 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on average in studies without pharmacotherapy. 2Based on average in studies with pharmacotherapy. | ||||||
Background
Psychological interventions to aid smoking cessation include self‐help materials, brief therapist‐delivered interventions such as advice from a physician or nurse, intensive counselling delivered on an individual basis or in a group, and combinations of these approaches. Previous reviews have shown a small but consistent effect of brief, therapist‐delivered interventions (Stead 2013a). The effect of self‐help interventions is less clear (Hartmann‐Boyce 2014). More intensive intervention in a group setting increases quit rates (Stead 2017).
In this review, we assess the effectiveness of more intensive counselling delivered by a smoking cessation counsellor to a person on a one‐to‐one basis. One problem in assessing the value of individual counselling is that of confounding with other interventions. For example, counselling delivered by a physician in the context of a clinical encounter may have different effects from that provided by a non‐clinical counsellor. One approach to this problem is to employ statistical modelling (logistic regression) to control for possible confounders, an approach used by the US Public Health Service in preparing clinical practice guidelines (AHCPR 1996; Fiore 2000; Fiore 2008). An alternative approach is to review only unconfounded interventions. This is the approach we have adopted in the Cochrane Tobacco Addiction Review Group. We therefore specifically exclude from this review counselling provided by doctors or nurses during the routine clinical care of the patient, and focus on smoking cessation counselling delivered by specialist counsellors. We define counselling broadly, based only on a minimum time spent in contact with the smoker, not according to the use of any specific behavioural approach.
Objectives
The review addresses the following hypotheses:
1. Individual counselling is more effective than no treatment or brief advice in promoting smoking cessation.
2. Individual counselling is more effective than self‐help materials in promoting smoking cessation.
3. A more intensive counselling intervention is more effective than a less intensive intervention.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials (RCTs) with a minimum follow‐up of six months, where at least one treatment arm consisted of an unconfounded intervention from a counsellor. Studies in which the treatment arm combined counselling and pharmacotherapy, and the control condition had neither, are covered in a separate review (Stead 2016).
Types of participants
Any smokers, except pregnant women (smoking cessation interventions in pregnancy are addressed by a separate review, Chamberlain 2013). We also exclude trials recruiting only children and adolescents.
Types of interventions
We defined individual counselling as a face‐to‐face encounter between a smoker and a counsellor trained in assisting smoking cessation. This review specifically excludes studies of counselling delivered by doctors and nurses as part of clinical care, which are covered in separate reviews (Rice 2013; Stead 2013a). It also excludes studies of interventions that combined counselling with provision of pharmacotherapy, compared to brief support (Stead 2016), studies of motivational interviewing (Lindson‐Hawley 2015) and interventions which address multiple risk factors in addition to smoking. We include studies that evaluate the effect of counselling as an addition to pharmacotherapy.
We include studies comparing different counselling approaches if they are not covered by other Cochrane Reviews of specific interventions. Comparisons between individual counselling and behavioural therapy conducted in groups are covered in the Cochrane Review of group behavioural therapy (Stead 2017).
Types of outcome measures
The outcome was smoking cessation at the longest reported follow‐up. We used sustained abstinence where available, or multiple point prevalence. We included studies using self‐report with or without biochemically‐validated cessation, and performed sensitivity analyses to determine whether the estimates differed significantly in studies without verification.
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Group Specialized Register for studies with counsel* in title, abstract or keyword fields. At the time of the search the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 4, 2016; MEDLINE (via OVID) to update 20160513; EMBASE (via OVID) to week 201621; PsycINFO (via OVID) to update 20160516. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and list of other resources searched. We also checked previous reviews and meta‐analyses for relevant studies, including all studies in the previous US guidelines (AHCPR 1996; Fiore 2000; Fiore 2008). The most recent search was conducted in May 2016.
Data collection and analysis
One author (LS, who is also the Cochrane Information Specialist for the Tobacco Addiction Group) prescreened results of the searches. Both authors checked reports of studies of potentially relevant interventions.
Both authors extracted data independently.
Information extracted included descriptive details on the setting of the study, the population, and details of intervention(s) and control conditions, including number and duration of planned sessions.
Assessment of risk of bias in included studies
We assessed risk of selection, detection and attrition bias, based on the reported methods of randomization and allocation concealment (selection bias), use of biochemical validation of self‐reported abstinence (detection bias) and numbers lost to follow‐up (attrition bias).
Measures of treatment effect & data synthesis
We summarized individual study results as a risk ratio (RR), calculated as: (number of quitters in intervention group/number randomized to intervention group) / (number of quitters in control group/number randomized to control group). We assumed that participants lost to follow‐up continued to smoke and included them as such in denominators. Where appropriate we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect method to estimate a pooled risk ratio with a 95% confidence interval (CI) (Greenland 1985). We estimated the amount of statistical heterogeneity between trials using the I2 statistic (Higgins 2003). Values over 50% can be regarded as moderate heterogeneity, and values over 75% as high.
We made the following comparisons:
-
Individual counselling versus no treatment, brief advice or self‐help materials
-
More intensive versus less intensive individual counselling
-
Comparisons between counselling methods matched for contact time
Results
Description of studies
We include 49 studies in this updated review, with around 19,000 participants. Thirty‐three studies (11 new for this update) contribute to the primary analysis comparing individual counselling to a minimal contact behavioural intervention. Eleven studies (six new) compared different intensities of counselling and five (two new) compared different counselling approaches which were similar in intensity of contact.
In a few cases we resolved difficulties in applying the inclusion criteria by discussion. In two cases (Wiggers 2006; Aveyard 2007) we were uncertain whether the providers were acting as specialist counsellors or were providing interventions as part of usual care in other healthcare roles. We included both after discussion about this aspect of their designs. We included one study that had only five months follow‐up (Kim 2005).
Study populations
Nineteen of the 49 studies recruited medical or surgical hospital inpatients (Pederson 1991; Ockene 1992; Stevens 1993; Rigotti 1997; Simon 1997; Dornelas 2000; Molyneux 2003; Simon 2003; Hennrikus 2005; Pedersen 2005; Brunner 2012), or outpatients (Weissfeld 1991; Kim 2005; Tonnesen 2006; Hennrikus 2010; Chan 2012; Ramon 2013; Thankappan 2013; Chen 2014). One recruited some inpatients (Schmitz 1999). Four other studies recruited drug‐ and alcohol‐dependent veterans attending residential rehabilitation (Bobo 1998; Burling 1991; Burling 2001; Mueller 2012). One study recruited new mothers in maternity wards (Hannover 2009); we considered the subgroup of trial participants who were smoking at this point. Other studies recruited smokers in primary care clinics (Fiore 2004; Aveyard 2007; Marley 2014; Ramos 2010), dental clinics (Nohlert 2009), primary care and local community (Aleixandre 1998), local community and university (Alterman 2001), communities and worksites (Nakamura 2004), at a periodic healthcare examination (Bronson 1989), at a Planned Parenthood clinic (Glasgow 2000), employees volunteering for a company smoking cessation programme (Windsor 1988), participants in a lung cancer screening study (Marshall 2016), and community volunteers (Jorenby 1995; Lifrak 1997; Ahluwalia 2006; Killen 2008; McCarthy 2008, Wu 2009; Garvey 2012; Kim 2015). Lack of interest in quitting was not an explicit exclusion criterion in any study, but the level of motivation to quit smoking was sometimes difficult to assess. One trial enrolled all smokers admitted to hospital (Stevens 1993), whilst one enrolled 90% of smokers approached (Rigotti 1997). In one large study in primary care 68% of smokers agreed to participate and 52% met the inclusion criteria and were recruited (Fiore 2004). In other studies a larger proportion of eligible smokers may have declined randomization because of lack of interest in quitting.
Special populations included Australian Aboriginal people (Marley 2014), homeless people (Okuyemi 2013), people under community corrections supervision (Cropsey 2015) and people with schizophrenia (Williams 2010). Two studies recruited Asian minority populations in the US; Kim 2015 (Koreans) and Wu 2009 (Chinese), and one recruited African Americans (Ahluwalia 2006).
Two studies recruited only women: Schmitz 1999 recruited 53 women hospitalized with coronary artery disease (CAD) and 107 volunteers with CAD risk factors. Glasgow 2000 recruited women attending Planned Parenthood clinics, who were not selected for motivation to quit. Weissfeld 1991 recruited only men, while Simon 2003 and Nakamura 2004 recruited predominantly men.
Thirty studies were conducted in the USA, three in Spain (Aleixandre 1998; Ramos 2010; Ramon 2013), three in Denmark (Pedersen 2005; Tonnesen 2006; Brunner 2012), two in the UK (Molyneux 2003; Aveyard 2007), two in Australia (Marley 2014; Marshall 2016), and one each in Germany (Hannover 2009), Switzerland (Mueller 2012), Sweden (Nohlert 2009), Netherlands (Wiggers 2006), Hong Kong (Chan 2012), China (Chen 2014), Japan (Nakamura 2004), Korea (Kim 2005), and India (Thankappan 2013).
Intervention components
The counselling interventions typically included the following components: review of a participant's smoking history and motivation to quit, help in the identification of high‐risk situations, and the generation of problem‐solving strategies to deal with such situations. Counsellors may also have provided non‐specific support and encouragement. Some studies provided additional components such as written materials, video or audiotapes. The main components used in each study are shown in the Characteristics of included studies tables.
Intervention providers
The therapists who provided the counselling were generally described as smoking cessation counsellors. Their professional backgrounds included social work, psychology, psychiatry, health education and nursing. In one study, the therapist for some of the sessions was a nurse practitioner (Alterman 2001), and in two others the therapists were research doctors or nurses trained in counselling (Molyneux 2003; Hennrikus 2005). In Aveyard 2007 all the support was from primary care nurses who were not full‐time counsellors. We included this study because the nurses were trained to provide counselling support as part of the National Health Service Stop Smoking Services and were not offering it as part of usual care. In Tonnesen 2006 the counselling was provided by nurses employed in a lung clinic, and in Wiggers 2006 it was provided by nurse practitioners in a cardiology outpatient clinic.
Studies with minimal contact controls
In the 33 studies with a minimal contact control the treatments offered to the control comparison group ranged from usual care to up to 15 minutes of advice, with or without the provision of self‐help materials. To be classified as individual counselling the trials had to involve at least one session with face‐to‐face contact lasting more than 10 minutes, although the duration was typically much longer. The face‐to‐face counselling in Kim 2005 was the shortest, at only 11 minutes on average. Three tested a single face‐to‐face session without further support by telephone (Weissfeld 1991 (low‐intensity arm); Molyneux 2003; Marshall 2016). Nine others offered a single face‐to‐face session with further support by telephone (Windsor 1988; Weissfeld 1991 (high‐intensity arm); Stevens 1993; Rigotti 1997; Simon 1997; Dornelas 2000; Glasgow 2000; Hennrikus 2005; Kim 2005). All the other studies planned multiple sessions of face‐to‐face support, and sometimes also telephone contacts.
In the meta‐analysis we have not distinguished between brief advice, usual care or provision of self‐help materials as the control intervention with which counselling is compared. Provision of written materials was generally accompanied by brief advice; no trials directly addressed the effect of providing counselling as an addition to a structured self‐help programme. One trial offered 15 minutes of counselling on a healthy diet to controls (Chan 2012), and one offered autogenic training, a relaxation‐based programme not shown to aid cessation (Mueller 2012).
Within this group of studies, pharmacotherapy was systematically provided to participants in all trial arms in six trials. Nicotine patch was provided to all participants in Jorenby 1995; Simon 2003; Fiore 2004; Okuyemi 2013. Cropsey 2015 provided bupropion to all participants. Wiggers 2006 provided nicotine patches to participants ready to quit in either trial arm. Since the use of pharmacotherapy might change the relative effect of additional counselling, we include these studies in a subgroup analysis. In one trial (Simon 1997) smokers randomized to receive counselling were given a prescription for nicotine gum if there were no contraindications. Although 65% in the counselling condition used gum compared to 17% of the control group, its use was not significantly associated with quitting.
Studies of counselling intensity
Eleven studies compared intensive counselling to less intensive interventions that also met our definition of counselling by involving more than 10 minutes of face‐to‐face contact. We considered these studies separately from those using a minimal‐contact control. Eight of these studies provided pharmacotherapy to all participants and we included subgroups for studies with and without pharmacotherapy. Tonnesen 2006 contributed to both subgroups.
-
Weissfeld 1991 compared two intensities of counselling with a control; both intensities are combined versus control in the first analysis but compared in this analysis.
-
Lifrak 1997 compared two intensities of counselling as an adjunct to nicotine patch therapy. The lower‐intensity one was a four‐session advice and education intervention from a nurse practitioner who reviewed self‐help materials and monitored patch use. The higher‐intensity intervention added 16 weekly sessions of cognitive behavioural relapse prevention therapy.
-
Alterman 2001 used similar interventions to Lifrak 1997, but added a lower‐intensity control of a single 30‐minute session with a nurse practitioner.
-
Tonnesen 2006 compared seven visits and five phone calls with a contact time of 4½ hours to four visits and six calls taking 2½ hours. This trial had a factorial design, also comparing a nicotine sublingual tablet and placebo; we entered the arms with and without NRT in separate subgroups.
-
Aveyard 2007 compared seven weekly contacts with four contacts for people receiving cessation support with nicotine patches.
-
Killen 2008 provided six counselling session and combined NRT and bupropion, and compared different schedules of extended contact.
-
Nohlert 2009 compared eight 40‐minute sessions over four months with a single 30‐minute session introducing a self‐help programme.
-
Wu 2009 compared four 60‐minute culturally‐tailored counselling sessions to four 60‐minute health education sessions covering general health, nutrition, exercise and tobacco. All sessions were in Chinese, and all participants were offered nicotine patch.
-
Williams 2010 compared 24 weekly 45‐minute counselling sessions to nine 20‐minute sessions that focused on medication management. All participants were given nicotine patches.
-
Brunner 2012 provided a 30‐minute counselling session and offer of nicotine patch during a hospital stay and tested the effect of an additional five outpatient sessions including free samples of NRT; we included this in the non‐pharmacotherapy subgroup, as it was not provided as standard to all participants.
-
Kim 2015 compared eight weekly 40‐minute sessions of culturally‐tailored counselling to eight 10‐minute sessions focusing on medication management. All participants received nicotine patches.
Studies of counselling methods or timing
Five studies compared different counselling approaches that had similar contact times. We considered these separately from the groups above.
-
Schmitz 1999 involved six one‐hour sessions. One intervention used a coping skills relapse prevention model. It was compared with a health belief model that focused on smoking‐related health information, the relationship with coronary disease and the benefits of quitting.
-
Ahluwalia 2006 provided three face‐to‐face visits and three phone contacts extending over six weeks, and 2 mg nicotine gum for eight weeks. One intervention used motivational interviewing and the other a health education focus.
-
McCarthy 2008 provided eight 10‐minute counselling sessions during assessment visits in a trial that also compared bupropion to placebo. The counselling was consistent with US practice guidelines. The control focused on medication use and adherence, and general support and encouragement.
-
Garvey 2012 compared two different schedules of 14 counselling sessions, either front‐loaded with six sessions in the first two weeks after quit date, or just two in that period. All participants received nicotine patches.
-
Ramon 2013 directly compared delivery of counselling either entirely face‐to‐face or with a combination of face‐to‐face and telephone to a control group where all contact after the pre‐quit session was by telephone.
Excluded studies
We excluded one study that provided motivational interviewing as part of an intervention to reduce passive smoke exposure in households with young children (Emmons 2001). Cessation was a secondary outcome and there was no significant difference in quit rates, which were not reported separately by group. A sensitivity analysis including this study assuming equal quit rates did not alter the review results.
We list 48 other studies identified as potentially relevant but which did not meet the full inclusion criteria, with their reasons for exclusion in the table Characteristics of excluded studies. We note where studies were included in other Cochrane Reviews.
Risk of bias in included studies
We assessed the risks of selection bias, detection bias and attrition bias.
Twenty‐seven studies reported the method for generating the randomization sequence in sufficient detail to be classified as having a low risk of bias, but only 14 also described a method of allocation likely to ensure that the assignment was concealed until after allocation, and thus being at low risk of selection bias (Simon 1997; Weissfeld 1991; Windsor 1988; Kim 2005; Ahluwalia 2006; Wiggers 2006; Aveyard 2007; Killen 2008; McCarthy 2008; Williams 2010; Chan 2012; Ramon 2013; Marley 2014; Marshall 2016). In most other trials, neither the method of randomization nor the use of allocation concealment was described. We judged five trials to be at high risk of selection bias, due to the method of randomization or concealment, or both (Stevens 1993; Bobo 1998; Dornelas 2000; Hannover 2009; Brunner 2012).
We judged the risk of detection bias to be low if self‐reported abstinence was confirmed biochemically. Eight studies were at high risk of bias because no validation was attempted and trial arms had different amounts of contact with study staff, making differential misreporting of abstinence more likely (Bronson 1989; Stevens 1993; Aleixandre 1998; Pedersen 2005; Nohlert 2009; Thankappan 2013; Kim 2005; Ahluwalia 2006). We rated three studies as unclear; one study tested for cotinine but did not report validated rates (Bobo 1998), and in two others validation was incomplete and results were based on self‐report (Pederson 1991; Marshall 2016).
We judged the risk of attrition bias to be low if loss to follow‐up was reported by group, was no greater than 50% and not substantially different between groups. Most studies reported the number of participants who dropped out or were lost to follow‐up, and included these people as smokers in analysis denominators. We judged most studies to be at low risk of bias, because the percentage lost was small and similar across conditions. We classified two studies as being at high risk (Ramos 2010; Mueller 2012), and one as unclear (Burling 1991). One study (Fiore 2004) excluded randomized participants who failed to collect their free supply of nicotine patches, and as a consequence also did not receive any additional behavioural components to which they were allocated. The proportions excluded were similar in all the intervention groups, so we have used the denominators as given.
Overall we classified 11 of the 49 included studies (22%) as being at low risk of bias on all the domains we considered. A summary is displayed in Figure 1.
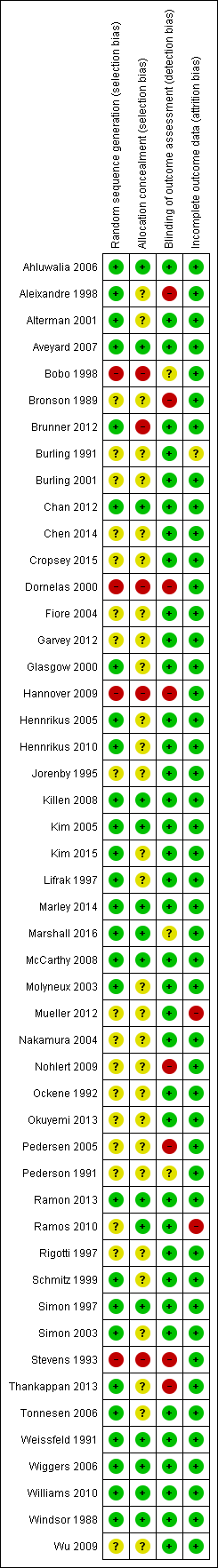
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
We did not formally assess the risk of performance bias. There was little information about blinding of participants or staff during treatment. Whilst the therapists delivering counselling could not have been blinded, in some cases other care providers were noted to be unaware of intervention status. It was unclear what information participants were given, but almost all trials included an active control group that received some information about stopping smoking. Because of this, we do not consider that the risk of bias from this aspect of design for this group of studies is high.
Effects of interventions
See: Summary of findings for the main comparison Individual counselling compared to minimal contact control for smoking cessation; Summary of findings 2 More intensive compared to less intensive counselling for smoking cessation
Counselling versus minimal contact control
We estimated a pooled effect size based on 33 studies of counselling, including one (Burling 1991) where there were no quitters and which therefore did not contribute to the meta‐analysis. The risk ratio (RR) was 1.48 (95% confidence interval (CI) 1.34 to 1.64, n = 13,762; Analysis 1.1), with some evidence of heterogeneity (I2 = 46%). Restricting the analysis to seven studies at low risk of bias on all domains (Windsor 1988; Weissfeld 1991; Simon 1997; Kim 2005; Wiggers 2006; Chan 2012; Marley 2014) did not alter the conclusions; the point estimate increased slightly (RR 1.65, 95% CI 1.32 to 2.06). The estimate was higher in the subgroup of 27 studies where pharmacotherapy was not provided (RR 1.57, 95% CI 1.40 to 1.77; n = 11,100; I2 = 50%) than in the six testing the additional effect of counselling when participants had access to pharmacotherapy (RR 1.24, 95% CI 1.01 to 1.51; n = 2662; I2 = 0%) and a test for subgroup difference detected a difference between subgroups with and without pharmacotherapy. We base the estimates of absolute effect in summary of findings Table for the main comparison on the subgroup estimates.

Forest plot of comparison: 1 Individual counselling compared to minimal contact control, outcome: 1.1 Smoking cessation at longest follow‐up.
More intensive versus less intensive counselling
Eight of the studies compared different levels of counselling as adjuncts to pharmacotherapy, and four did not offer medication (Tonnesen 2006 contributes different arms to each subgroup). The estimates in the two subgroups overlapped. Pooling all 11 studies, there was evidence of a small benefit from more intensive compared to brief counselling (RR 1.29, 95% CI 1.09 to 1.53; n = 2920; I2 = 48%; Analysis 2.1), a change from the previous version of the review in which pooling five studies did not detect evidence of benefit. The moderate heterogeneity was attributable to two new studies with large effects. The control groups in these were distinct, with Wu 2009 offering general health education and Kim 2015 focusing on medication management. A sensitivity analysis excluding these two studies no longer detected evidence of a dose response to counselling intensity. Limiting the analysis to four studies at low risk of bias also failed to suggest evidence of benefit.
Comparisons between counselling approaches
We did not pool these clinically heterogeneous five studies. Only one of them detected a significant difference between different types of counselling, where number of contacts and general intensity were similar. Schmitz 1999, comparing a relapse prevention approach to a health belief model, showed no significant difference, but with wide confidence intervals (RR 0.94, 95% CI 0.45 to 1.98; n = 160; analysis 3.1.1). Ahluwalia 2006 compared a motivational interviewing to a health education approach and the point estimate favoured the latter (RR 0.51, 95% CI 0.34 to 0.76; n = 755; analysis 3.1.2). Participants were making quit attempts and using nicotine gum or placebo and therefore the motivational aspect may have been less relevant. McCarthy 2008 was also a pharmacotherapy trial with a factorial design and the specific behavioural components did not increase quitting over instructions about medication and general support (RR 0.93, 95% CI 0.62 to 1.39; n = 463; analysis 3.1.3). There was no evidence of an interaction between medication and counselling in either of the factorial trials. Garvey 2012 did not show that front‐loading the schedule of sessions was associated with greater quit success, but CIs did not exclude no effect (RR 1.81, 95% CI 0.79 to 4.15; n = 242; analysis 3.1.4). Ramon 2013 did not detect a difference between face‐to‐face and telephone counselling (RR 1.39, 95% CI 0.89 to 2.19; n = 301; analysis 3.1.5), or combined contact (face‐to‐face plus telephone) versus telephone only (RR 1.44, 95% CI 0.92 to 2.25; n = 299), but confidence intervals were again wide.
Discussion
There is consistent evidence that individual counselling increases the likelihood of cessation compared to less intensive support. Individual counselling, used independently of pharmacotherapy, was estimated to increase cessation by 40% to 80% after at least six months, based on pooling 27 trials with over 11,000 participants. Assuming a control group quit rate of 7% from a brief intervention, the provision of counselling would be expected to result in 10% to 12% quit, an absolute increase of 3% to 5%. We rated the quality of this evidence as high, using the GRADE approach (summary of findings Table for the main comparison). This estimate was based on using counselling without any pharmacotherapy. The six trials that offered pharmacotherapy (typically nicotine replacement therapy) to all participants had a smaller and less certain effect. Assuming a control quit rate of 11% reflecting the benefit of medication, the addition of counselling could result in an absolute increase of 0% to 5%. We rated this as moderate quality using GRADE, because of the imprecision of the estimate. It is possible that the relative additional benefit is smaller when the quit rates in the control group are already increased by the use of an effective pharmacotherapy, but the absolute benefit of counselling could be similar, whether or not pharmacotherapy is used.
Almost half the trials recruited people in hospital settings, but there was no evidence of heterogeneity of results in different settings.
These results are consistent with the US Public Health Service practice guideline (Fiore 2008), which supports the use of intensive counselling. The guideline evidence in this area is based on meta‐analyses conducted for the previous update of the guideline (Fiore 2000), and includes indirect comparisons. These included an analysis of 58 trials where treatment conditions differed in format (self‐help, individual counselling with person‐to‐person contact, proactive telephone counselling or group counselling) and estimated an odds ratio (OR) for successful cessation with individual counselling compared to no intervention of 1.7 (95% confidence interval (CI) 1.4 to 2.0) (Fiore 2008 Table 6.13). Individual counselling in their categorization would have also included counselling from a physician. When they separately analysed the effect of different providers of care the estimates suggest that non‐physician clinicians (a category including psychologists, social workers and counsellors) are similarly effective compared to a no‐provider reference group (OR 1.7, 95% CI 1.3 to 2.1) as physicians (OR 2.2, 95% CI 1.5 to 3.2) (Fiore 2008 Table 6.11).
In our review there was no evidence of significant heterogeneity between relative quit rates in the different trials. Absolute quit rates varied across studies but this is likely to be related to the motivation of the smokers to attempt to quit and the way in which cessation was defined. Cessation rates were generally higher in trials where nicotine replacement therapy (NRT) was also used (Alterman 2001; Jorenby 1995; Lifrak 1997; Simon 2003), although there were exceptions (Ahluwalia 2006; Aveyard 2007). Rates were also higher amongst people with cardiovascular disease (Ockene 1992 ; Dornelas 2000; Pedersen 2005). Quit rates tended to be lower in studies recruiting hospitalized patients unselected for their readiness to quit (Stevens 1993; Rigotti 1997; Molyneux 2003). All these features of a trial are likely to affect absolute quit rates, confounding a possible effect of the exact content of the intervention.
Whilst we took account of the broad nature of the support offered to the control group when pooling studies, variation in the components used as part of, for example, a usual care control, may still give rise to heterogeneity. Treatment effects could be underestimated if those studies using effective interventions tended to provide relatively helpful usual care or brief advice. An ongoing systematic review is conducting a detailed analysis of behavioural intervention and control elements, and is expected to provide more evidence about this (de Bruin 2016).
The following description of the intervention used in the Coronary Artery Smoking Intervention Study (CASIS) (Ockene 1992) is broadly typical of the interventions used: "The telephone and individual counseling sessions were based on a behavioral multicomponent approach in which counselors used a series of open‐ended questions to assess motivation for cessation, areas of concern regarding smoking cessation, anticipated problems and possible solutions. Cognitive and behavioral self‐management strategies, presented in the self help materials, were discussed and reinforced". Although we cannot exclude the possibility that small differences in components, and in the therapists' training or skills, have an effect on the outcome, it is not possible to detect such differences in the meta‐analysis.
Most of the counselling interventions in this review included repeated contact, but differed according to whether face‐to‐face or telephone contact was used after an initial meeting. There are too few trials to draw conclusions from indirect comparisons about the relative efficacy of the various contact strategies. Again, the homogeneity of the results suggests that the way in which contact is maintained may not be important. A separate Cochrane Review of telephone counselling suggests that telephone support aids quitting (Stead 2013b).
The 11 trials that directly compared different intensities of individual support detected only weak evidence of a dose‐response effect which was sensitive to exclusion of outlying trials, and restriction to trials judged to be at low risk of bias. In some of the trials in this comparison the difference between the counselling protocols may be too small to affect long‐term quitting. The intended difference may also be eroded if the more intensive support cannot be consistently delivered. Eight of the trials provided pharmacotherapy to all participants, so were testing the additional benefit of more intensive individual counselling. As seen in the trials offering pharmacotherapy in the primary analysis, the relative effect of the additional support may be smaller in relation to the higher rates of cessation in the control arm receiving combined behavioural and pharmacological support. A separate Cochrane Review (Stead 2015) has assessed the effect of increasing the amount of any type of behavioural support when used alongside pharmacotherapy. It analysed 47 studies including relevant studies from this review, and concluded that "increasing the amount of behavioural support is likely to increase the chance of success by about 10% to 25%". The estimates in this review are consistent with that range.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Individual counselling compared to minimal contact control, outcome: 1.1 Smoking cessation at longest follow‐up.

Comparison 1 Individual counselling compared to minimal contact control, Outcome 1 Smoking cessation at longest follow‐up.

Comparison 2 More intensive versus less intensive counselling, Outcome 1 Smoking cessation at longest follow‐up.

Comparison 3 Comparisons between counselling approaches of similar intensity, Outcome 1 Smoking cessation at longest follow‐up.
| Patient or population: People who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Numbers quit in control condition | Numbers quit after individual counselling | |||||
| Smoking cessation at longest follow‐up ‐ 6 months or more No systematic pharmacotherapy | Study population | RR 1.57 | 11,100 | ⊕⊕⊕⊕ | Limiting to studies at low risk of bias on all assessed domains marginally increases estimate of effect | |
| 7 per 100 | 11 per 100 | |||||
| Smoking cessation at longest follow‐up ‐ 6 months or more Pharmacotherapy offered to all participants | Study population | RR 1.24 | 2662 | ⊕⊕⊕⊝ | Higher control group quit rate reflecting use of pharmacotherapy | |
| 11 per 100 | 13 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded due to wide confidence intervals. | ||||||
| More intensive compared to less intensive counselling for smoking cessation | ||||||
| Patient or population: People who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Numbers quit with less intensive counselling | Numbers quit with more intensive counselling | |||||
| Smoking cessation at longest follow‐up | Without pharmacotherapy | RR 1.29 | 2920 | ⊕⊕⊕⊕ | Effect estimates for subgroups of studies with and without pharmacotherapy for all participants overlapped, so the overall pooled estimate is used with alternative control group estimates from subgroups | |
| 9 per 100 1 | 12 per 100 | |||||
| With pharmacotherapy | ||||||
| 14 per 100 2 | 18 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on average in studies without pharmacotherapy. 2Based on average in studies with pharmacotherapy. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking cessation at longest follow‐up Show forest plot | 33 | 13762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.34, 1.64] |
| 1.1 Counselling versus control (no systematic pharmacotherapy) | 27 | 11100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.40, 1.77] |
| 1.2 Counselling plus pharmacotherapy versus pharmacotherapy alone | 6 | 2662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.01, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking cessation at longest follow‐up Show forest plot | 11 | 2920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.09, 1.53] |
| 1.1 No pharmacotherapy | 4 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.98, 2.06] |
| 1.2 Adjunct to pharmacotherapy | 8 | 2048 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.04, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking cessation at longest follow‐up Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Relapse prevention versus health belief model | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Motivational interviewing versus health education | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Counselling versus equal sessions of psychoeducation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Front‐loaded versus weekly counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Face‐to‐face versus telephone counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

