Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight 1000 g to 2000 g (appropriate birth weight for gestational age and of gestational age < 35 weeks at birth), who were starting formula feeds Setting: Neonatal Unit, Department of Pediatrics, University of Texas Medical School, Houston, Texas, USA | |
| Interventions | Feeds advancement at 20 ml/kg/day (N = 74) versus 30 ml/kg/day (N = 84) | |
| Outcomes | NEC (Bell stage II/III) | |
| Notes | Feeds were ceased if the residual gastric aspirate was more than one‐third of the previous feed volume, or if there was frequent vomiting, abdominal distention, or bloody stools (including occult blood). We have not been able to obtain data on all‐cause mortality from the principal investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence |
| Allocation concealment (selection bias) | Low risk | Blinded draw from envelope by caregivers not involved in study |
| Blinding (performance bias and detection bias) | High risk | Caregivers and clinical investigators were not blinded once allocation to intervention groups had occurred. |
| Blinding (performance bias and detection bias) | Low risk | Radiologists interpreting X‐rays were blinded to the intervention group. |
| Incomplete outcome data (attrition bias) | Low risk | Three infants excluded after enrolment because of protocol violations have been included in this review and meta‐analysis. Two infants (one in each group) were excluded because they were determined not eligible for enrolment because of an in utero gastrointestinal perforation and fetal alcohol syndrome; these infants were not included in the meta‐analysis. |
| Methods | Randomised controlled trial | |
| Participants | Preterm infants < 32 weeks gestation with birth weights of 750 g to 1250 g. 32% of participants weighed < 1000 g. Exclusion criteria included major congenital malformations, severe respiratory distress, presence of umbilical vessel catheters, contraindications to enteral feeding, perinatal asphyxia, or cardiovascular compromise. Setting: Division of Neonatology, Dr Sami Ulus Maternity, Children's Education and Research Hospital, Ankara, Turkey | |
| Interventions | Slow advancement at 20 ml/kg/day (N = 46) versus rapid advancement at 30 ml/kg/day (N = 46) | |
| Outcomes | NEC (stage II/III), all‐cause mortality, time to regain birth weight, time to reach full enteral feeds, feed intolerance, duration of hospital stay, rates of invasive infection Subgroup analysis for ELBW infants | |
| Notes | Feeds were ceased if any of the following occurred: gastric residuals > 5 ml/kg or > 50% of feed volume, vomiting > 3 times in 24 hours, increase in abdominal girth > 2 cm between feeds, abdominal tenderness or erythema, reduced bowel sounds, blood in the stools, or recurrent apnoea. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Caregivers and study investigators were not blinded. |
| Blinding (performance bias and detection bias) | Unclear risk | No reference to whether staff interpreting radiological images were blinded to the study groups |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow‐up |
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (birth weight 1000 g to 1499 g) and gestational age < 34 weeks at birth. Exclusion criteria included respiratory distress, mechanical ventilation, inotrope support, and umbilical arterial or venous catheterisation. Setting: Department of Paediatrics, University College of Medical Sciences, Dehli, India | |
| Interventions | Feeds advancement at 20 ml/kg/day (N = 50) versus 30 ml/kg/day (N = 50) | |
| Outcomes | NEC (stage II/III) Incidence of nosocomial infection, in‐hospital mortality, time to regain birth weight, time to achieve full enteral feeds, and time to hospital discharge | |
| Notes | All feeds were delivered by gavage via nasogastric tubes at 2‐hour intervals. Feeds were ceased if any of the following occurred: residual gastric contents more than 50% of the previous feed volume (delayed if volume was 25% to 50%); more than 3 episodes of apnoea in the preceding hour; abdominal distention or tenderness; or bloody stools (including occult blood). Parenteral nutrition was not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Caregivers and investigators were not blinded to the interventions. |
| Blinding (performance bias and detection bias) | Low risk | Radiologist interpreting X‐rays was blinded to the intervention group. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (birth weight 1000 g to 1249 g) and gestational age > 30 weeks at birth who have antenatal evidence of absent end diastolic flow velocities (presumed in umbilical artery) Setting: Department of Paediatrics, Postgraduate Institute of Medical Education & Research, Chandigarh, India | |
| Interventions | Feeds advancement at 20 ml/kg/day (N = 15) versus 30 ml/kg/day (N = 15) | |
| Outcomes | NEC (all stages) Late‐onset bloodstream (culture‐positive) infection, in‐hospital mortality, time to achieve full enteral feeds | |
| Notes | Pre‐specified subgroup of larger trial that enrolled infants with birth weight > 1250 g and compared feed advancement at 30 ml/kg/day versus 40 ml/kg/day Published as conference abstract; further data courtesy of Dr Mukhopadhyay (September 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) | High risk | Caregivers and investigators were not blinded to the interventions. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up for primary outcomes |
| Methods | Randomised controlled trial | |
| Participants | Very low birth weight infants of gestational age < 34 weeks at birth Setting: Neonatal Unit, Department of Pediatrics, University of Alabama, Birmingham, Alabama, USA | |
| Interventions | Feeds advancement at 15 ml/kg/day (N = 98) versus 35 ml/kg/day (N = 87) | |
| Outcomes | NEC (stage II/III) | |
| Notes | Infants for whom full or partial feeding with expressed breast milk was planned were not eligible to participate. Feeding was commenced using standard 'term' artificial formula, then switched to nutrient‐enriched 'preterm' formula when full enteral feeding had been achieved.Feeds were ceased if any of the following occurred: residual gastric contents more than 30% of the previous feed volume, abdominal distention or tenderness, or bloody stools (including occult blood). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Caregivers and investigators not blinded to the intervention groups. |
| Blinding (performance bias and detection bias) | Low risk | Radiologist interpreting the X‐rays was blinded to the study group. |
| Incomplete outcome data (attrition bias) | Low risk | 7 protocol violations occurred after enrolment, but all infants were included the final data analysis. |
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight < 1250 g. More than 95% of the participants were 'small for gestational age'. Exclusion criteria included recurrent apnoea, respiratory distress requiring supplemental oxygen, and receipt of inotrope support. Setting: Neonatal Unit , Maulana Azad Medical College (tertiary‐level teaching hospital), New Delhi, India | |
| Interventions | Feeds advancement at 15 ml/kg/day (N = 26) versus 30 ml/kg/day (N = 27) | |
| Outcomes | NEC (stage II/III) Neonatal mortality, time to regain birth weight, time to achieve full enteral feeds, and time to hospital discharge | |
| Notes | Feeds were ceased if the residual gastric content was more than 30% of the previous feed volume or if there was abdominal distention. Mortality data courtesy of Dr Namasivayam Ambalavanan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Investigators were blinded at allocation stage, but it is unclear if they remained blinded thereafter. Caregivers were not blinded to intervention group. |
| Blinding (performance bias and detection bias) | Unclear risk | No statement about blinding of radiological assessors to intervention group |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Infants were randomly allocated to either a stable (not progressively increased) trophic feeding volume or to feed volume advancement at 20 ml/kg/day. | |
| Enteral feeding volumes were advanced at 10 ml/kg/day versus 20 ml/kg/day, that is both groups received 'slow' advancement of feed volumes. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of necrotising enterocolitis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 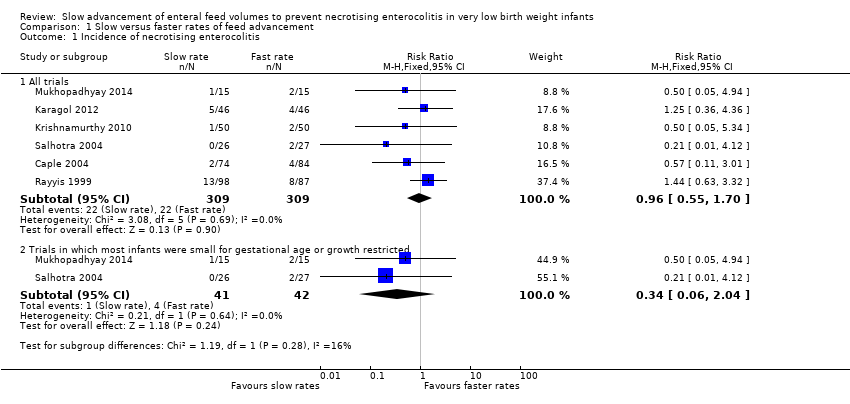 Comparison 1 Slow versus faster rates of feed advancement, Outcome 1 Incidence of necrotising enterocolitis. | ||||
| 1.1 All trials | 6 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.70] |
| 1.2 Trials in which most infants were small for gestational age or growth restricted | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.06, 2.04] |
| 2 Mortality Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 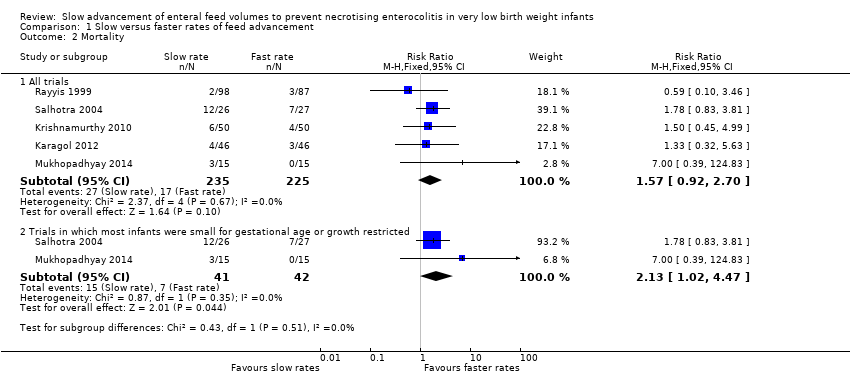 Comparison 1 Slow versus faster rates of feed advancement, Outcome 2 Mortality. | ||||
| 2.1 All trials | 5 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.92, 2.70] |
| 2.2 Trials in which most infants were small for gestational age or growth restricted | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.02, 4.47] |
| 3 Feeds intolerance (causing interruption of enteral feeding) Show forest plot | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.87, 1.73] |
| Analysis 1.3  Comparison 1 Slow versus faster rates of feed advancement, Outcome 3 Feeds intolerance (causing interruption of enteral feeding). | ||||
| 4 Incidence of invasive infection Show forest plot | 3 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.81, 3.09] |
| Analysis 1.4  Comparison 1 Slow versus faster rates of feed advancement, Outcome 4 Incidence of invasive infection. | ||||
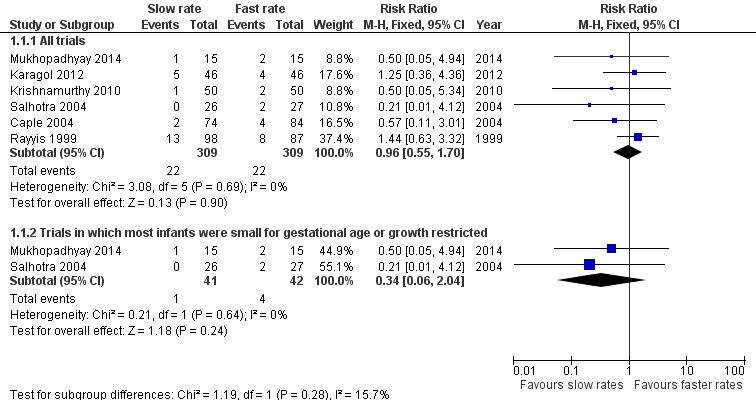
Forest plot of comparison: 1 Slow versus faster rates of feed advancement, outcome: 1.1 Incidence of necrotising enterocolitis.
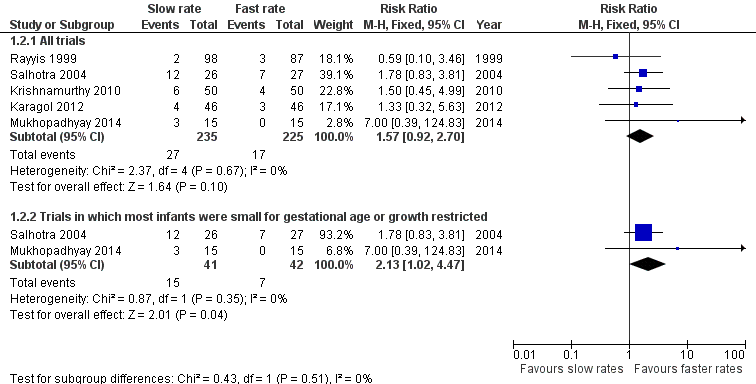
Forest plot of comparison: 1 Slow versus faster rates of feed advancement, outcome: 1.2 Mortality.

Forest plot of comparison: 1 Slow versus faster rates of feed advancement, outcome: 1.3 Feeds intolerance (causing interruption of enteral feeding).
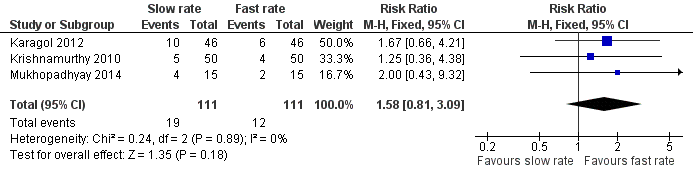
Forest plot of comparison: 1 Slow versus faster rates of feed advancement, outcome: 1.4 Incidence of invasive infection.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Slow versus faster rates of feed advancement, Outcome 1 Incidence of necrotising enterocolitis.

Comparison 1 Slow versus faster rates of feed advancement, Outcome 2 Mortality.

Comparison 1 Slow versus faster rates of feed advancement, Outcome 3 Feeds intolerance (causing interruption of enteral feeding).
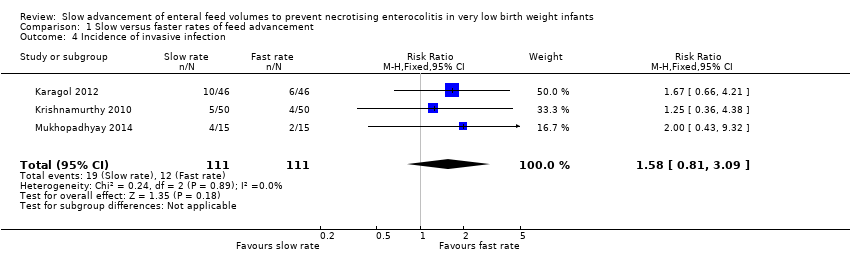
Comparison 1 Slow versus faster rates of feed advancement, Outcome 4 Incidence of invasive infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of necrotising enterocolitis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All trials | 6 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.70] |
| 1.2 Trials in which most infants were small for gestational age or growth restricted | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.06, 2.04] |
| 2 Mortality Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 All trials | 5 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.92, 2.70] |
| 2.2 Trials in which most infants were small for gestational age or growth restricted | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.02, 4.47] |
| 3 Feeds intolerance (causing interruption of enteral feeding) Show forest plot | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.87, 1.73] |
| 4 Incidence of invasive infection Show forest plot | 3 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.81, 3.09] |

