Ultrasonido terapéutico para la úlcera venosa de pierna
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial in Scotland, UK | |
| Participants | 108 people with chronic leg ulcers attending participating physiotherapy clinics Exclusion criteria: allergy to standard treatments, peripheral vascular disease | |
| Interventions | US group: once weekly pulsed, direct US 0.5 W/cm2 at a frequency of 1 MHz, applied directly to the tissue surrounding the ulcer for 12 weeks or until healing (whichever occurred first) plus standard treatment (see below) | |
| Outcomes | Tracings of ulcer at 0, 4, 8, 12 weeks. Analysed using computerised planimetry. Number of ulcers completely healed at 12 weeks (losses considered as treatment failures) | |
| Notes | Withdrawn participants were censored at the point of withdrawal except for those who withdrew due to deterioration, who were regarded as unhealed at 12 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into a control group ... and a treatment group" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was made through a central office and was based on the use of randomised permuted blocks, with stratification to ensure that appropriate balance between the treatment groups was maintained at each centre" |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors: tracings of the ulcer circumference were completed by people who were not blind to treatment group, however, analysis of the tracings (calculation of percentage area ulcer remaining) was blinded to treatment group. Quote: "The tracings were identified only by a code number to exclude observer bias" |
| Incomplete outcome data (attrition bias) | Low risk | Similar numbers withdrew from treatment groups for similar reasons; 21% (11/52) withdrawals in US group and 27% (15/56) withdrawals in control group due to allergy, pain, withdrawal of consent, deterioration, arterial disease and death. These data were considered in intention‐to‐treatment analysis by study authors. |
| Selective reporting (reporting bias) | Unclear risk | Expected outcomes reported, though we did not request a study protocol. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised controlled trial in Poland. | |
| Participants | 70 participants post venous surgery, whose venous disease was diagnosed by Duplex scan (to rule out arterial disease and locate the venous insufficiency) Exclusion criteria: diabetes, and rheumatoid arthritis US plus standard care group: n = 33; Standard care group: n = 37 | |
| Interventions | US group: US via a water bath at 0.5 W/cm2; 1 MHz frequency, US probe 10 cm2 placed 2 cm above ulcer. An ulcer of 5 cm2 or less had 5 minutes treatment with 1 minute extra of treatment for every 1 cm2 by which the ulcer exceeded an area of 5 cm2. Treatment provided daily for 6 days/week for 7 weeks. Between treatments ulcers were covered with saline‐soaked gauze, received compression and 1 g flavonoid fraction daily. US commenced 5 days after surgery. | |
| Outcomes | Proportion of ulcers completely healed | |
| Notes | Ulcers were observed for complete healing and measured for area, volume and a range of dimensions using planimetry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "70 patients ... were included and allocated into two comparative groups", "A prospective, randomised, controlled clinical trial was conducted" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned, see above. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: not blinded, since they did not receive sham US. Personnel: unclear, but presumably not blinded since study was not sham controlled. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors: unclear Quote: "Treatment progress was evaluated by observing the number of completely healed ulcers, and measuring the area ... by planimetry" |
| Incomplete outcome data (attrition bias) | Unclear risk | Final numbers not stated; complete follow‐up implied. |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised trial comparing US plus standard care with sham US plus standard care in Sweden. | |
| Participants | People with venous leg ulcers referred from departments of internal medicine and surgery, and primary care providers Exclusion criteria: allergy to the standard treatment, or evidence of peripheral arterial disease, rheumatoid arthritis, diabetic ulcers, or traumatic venous ulcers US group: n = 19; Sham US group: n = 19 | |
| Interventions | US group: US 1 W/cm2 at 1 MHz, for 10 minutes twice a week for 8 weeks, plus standard treatment | |
| Outcomes | Number of ulcers known to be completely healed at 8 weeks (of those randomised) | |
| Notes | Duration of follow‐up: 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to either a control group ... or a treatment group" |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants: this was a placebo (sham) US controlled trial, therefore, it was implied that the participants did not know their allocation. Personnel: unclear (they may have been responsible for setting the ultrasound machine to zero). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors: unclear whether those responsible for taking ulcer tracings were blinded. Those responsible for analysing the tracings were blinded, quote: "At the end of the 8 week study all tracings were analysed using a computer graphics program to calculate the areas of each ulcer...The tracings were identified by code numbers to exclude observer bias." |
| Incomplete outcome data (attrition bias) | Unclear risk | 38 people randomised; 13 withdrew. Not clear how these were handled. Quote: "The cumulative percentage of healed ulcers in the two groups was compared by the use of life table methods" (censoring not mentioned), and. In the Results section: "If analysed by intention to treat there were similar non‐significant findings between the groups". |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised trial comparing two US densities (0.5 W/cm2 and 1 W/cm2) with no US and pharmacotherapy | |
| Participants | 65 people with signs of venous disease and an ABPI > 1.0, were admitted to dermatology departments. People were excluded if they had diabetes mellitus or advanced sclerosis. Mean (median) baseline area (cm2): The authors did not publish the SD or SE around the mean. | |
| Interventions | US group 1: pulsed 1 MHz, 1 W/cm2 in a water bath with a temperature of 34 °C plus standard treatment of topical wet dressings of isotonic salt solution and compression therapy. Participants were admitted to the Dermatology Clinic of the Silesian Medical University in Katowice. These 3 treatment groups differed systematically not only in the US treatment but the pharmacotherapy received by the pharmacotherapy group and its place of treatment (different from that of the US groups). | |
| Outcomes | Number of ulcers completely healed at 3 weeks | |
| Notes | No withdrawals reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 65 patients with venous ulcers were randomly divided into three groups ...". |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no (no sham US). |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors: no: "To check how the ulcers healed we measured the longest dimensions ... and the widest dimensions perpendicular to the former ... measurements were taken before the treatment, every week during treatment and upon completion ...". |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete follow‐up implied but not stated. No mention of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | High risk | Major performance bias. Control group patients (pharmacotherapy group) received topical ulcer treatments that were not received by the US patients, and they were admitted to a different hospital. |
| Methods | Randomised trial in different hospitals in Poland | |
| Participants | 92 people with venous leg ulcers presenting symptoms of chronic venous insufficiency, some had varicose veins and symptoms of postthrombotic syndrome. ABPI > 0.8 Exclusion criteria: presence of diabetes or atherosclerosis Number of male: female participants: Electrostimulation group: 8:18; Laser therapy group: 4:17; US group: 4:11; Compression + pharmacological agents: 3:21 Mean (range) participant age in years: Electrostimulation group: 69.8 (48‐90); Laser therapy group: 65.2 (44‐80); US group: 63.6 (37‐82); Compression + pharmacological agents: 67 (43‐86) Mean (range) initial ulcer area in cm2: Electrostimulation group: 17.6 (2.6‐65.8); Laser therapy group: 15.8 (0.5‐59.6); US group: 15.6 (0.4‐84.7); Compression + pharmacological agents: 17.3 (1.9‐84) Mean (range) ulcer duration in months/years: Electrostimulation group: 4.5 years (2 months‐12 years); Laser therapy group: 3.5 years (2 months‐24 years); US group: 1.7 years (3 months‐8 years); Compression + pharmacological agents: 2.7 years (3 months‐11 years) | |
| Interventions | All groups received compression therapy, bandages were removed for purposes of physical therapy and then put back on. Electrostimulation group: 50‐minute session once daily, for 6 consecutive days, for a total of 4 weeks total (2 weeks katodic and 2 weeks anodic stimulation), NaCl 0.9% locally (no further details provided) Laser therapy group: 65 mW laser therapy session once daily, for 5 consecutive days, the duration of each session depended on the size of ulceration area – device was set up to develop 4 J/cm2 on average power 65 mW, various pharmacological agents applied locally, for a total of 4 weeks US group: 0.5 W/cm2 once daily, duration of each session depended on the size of ulceration area: 5 minutes of therapy given for 5 cm2 ulcer, 1 additional minute of therapy given for each additional 1 cm2 of ulceration area, for a total of 4 weeks, NaCl 0.9% locally Compression (no further details provided) plus pharmacological agents: compression and local application of collistin (no further details provided), chloramphenicol, gentamycin, fibrolan, potassium permanganate, copper sulphate, according to medical indications, no phlebotropic drugs), for a total of 4 weeks | |
| Outcomes | Changes in the area, length, width and volume of the tissue defect after above physical therapies | |
| Notes | No withdrawals reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From translator: "... random assignment ... " Comment: no randomisation method specified. Authors did not state whether participants were randomized before or after surgery. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | No report of withdrawals, and not clear from report whether all participants were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised controlled trial of high‐frequency US compared with sham US | |
| Participants | 44 people with venous leg ulcers referred from departments of internal medicine, surgery, and primary care Exclusion criteria: peripheral vascular disease, rheumatoid arthritis, diabetes mellitus, or traumatic venous ulcer | |
| Interventions | US group: US 0.5 W/cm2, at 1 MHz for 10 minutes. US was directly applied to the ulcer and surrounding tissue. Treatment frequency: 3 times a week for 4 weeks, twice a week for 4 weeks, and once weekly for 4 weeks, unless healing had occurred. Participants also received standard treatment (see below). | |
| Outcomes | Number of ulcers completely healed at 12 weeks | |
| Notes | Duration of follow‐up: 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomly assigned ...The distribution of the patients was based on the use of randomised permuted blocks” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants: blinded (sham compared with active). Personnel: unclear whether they were blinded, as they might have been responsible for setting the ultrasound machine to zero. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors: unclear whether person taking the ulcer tracing was aware of allocation. Person analysing the tracing was blinded, quote: "At the end of the 12 week study all tracings were analysed using a computer graphics program to calculate the areas of each ulcer ... tracings were identified by code numbers to exclude observer bias" |
| Incomplete outcome data (attrition bias) | High risk | 44 participants were randomised; 12 withdrew (evenly distributed between groups and for similar reasons). Quote: "Patients refused to continue or withdrew from the study for any of the following reasons: allergy to treatment; excessive pain; intervening illness ...". The analysis was by "life table methods" but it is not clear if withdrawn patients were censored. A quote from the Results: "The lack of difference was also maintained when taking withdrawals into consideration. If analysed by intention to treat there were similar non‐significant findings ..." would suggest they were not. |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised controlled trial in Germany. | |
| Participants | 24 people attending an outpatient clinic, with a venous leg ulcer of minimum area 2 cm2, and minimum duration of 3 months. Clinical diagnosis of venous disease confirmed by history, Doppler US, light reflection rheography, ABPI ≥ 0.8 Exclusion criteria: arterial disease, liver disease, cardiac or renal insufficiency, haemorrhagic gastroduodenitis, colitis, leukaemia, diabetes, rheumatoid arthritis, treatment allergy Mean ulcer area (cm2) (SD): Mean ulcer duration (SD) (months): | |
| Interventions | US group: US 30 kHz, at 0.1 W/cm2 for 10 minutes 3 times a week plus standard therapy (comprised of hydrocolloid dressings and "strong" compression therapy). The US (indirect method) was delivered by placing legs in a footbath of 32 °C‐34 °C water at filled to 10 cm above the ulcer. The US probe was immersed in the bath 5 cm from the ulcer. Continuous US was given for 10 minutes. | |
| Outcomes | The ulcer was measured using planimetry at 2, 4, 6, 8, 10, 12 weeks. The initial ulcer radius was calculated from the initial area and thereafter the daily ulcer radius reduction calculated at each time. Photographs were taken at the same time points. | |
| Notes | No variance data supplied for continuous outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised in parallel groups ..." |
| Allocation concealment (selection bias) | Unclear risk | See above; no further information provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants: blinded via sham control |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors: unclear, Quote: "the ulcer area was measured using planimetry ... prior to treatment and after 2, 4, 6, 8, 10 and 12 weeks of therapy". |
| Incomplete outcome data (attrition bias) | High risk | Two patients (both control group) were withdrawn due to "non‐compliance". |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised trial in an outpatients clinic in Poland | |
| Participants | 73 people with venous leg ulcers recruited after surgery for ligation and stripping (Babcock procedure) on saphenous or sagittal veins Inclusion criteria: venous leg ulcer confirmed with Doppler ultrasound Exclusion criteria: presence of diabetes, atherosclerosis or rheumatoid arthritis; steroid treatment; metal implants present at ultrasound application site; ulcer aetiology other than venous Number of participants: US group: n = 24; Compression group: n = 25; Standard care group: n = 24 Number of male:female participants: US group: 9:15; Compression group: 9:16; Standard care group: 13:11 Mean ± SD (range) participant age in years: US group: 62.0 ± 9.8 (47‐85); Compression group: 61.6 ± 8.3 (43‐78); Standard care group: 62.3 ± 9.5 (40‐79) Number of participants with superficial vs superficial and deep venous insufficiency: US group: 9 vs 15; Compression group: 9 vs 16; Standard care group: 9 vs 15 Mean ± SD ulcer area in cm2: US group: 26.5 ± 17.0; Compression group: 24.4 ± 12.9; Standard care group: 22.0 ± 15.5 Mean ± SD (range) ulcer duration in weeks: US group: 33 ± 27 (4‐124); Compression group: 36 ± 39 (6‐176); Standard care group: 32 ± 35 (2‐120) | |
| Interventions | US group: US therapy, moist normal saline dressing, and pharmacotherapy (diosmin 450 mg and hesperidin 50 mg combined as proprietary preparation (Detralex) Compression group: moist normal saline dressing, 2‐component compression system comprising an elastic bandage (Sigvaris) applied at 30 mm Hg ankle pressure for superficial venous insufficiency, and 40 mm Hg for superficial and deep venous insufficiency (unclear whether pressure was verified) plus stocking (no further details of this) and pharmacotherapy as above Standard care group: moist normal saline dressing plus pharmacotherapy as above Treatment duration was 7 weeks for all participants. | |
| Outcomes | Mean percentage change in ulcer area (relative to baseline) at 7 weeks Mean percentage change in ulcer area/week (NB: values read from figure) Mean ± SD ulcer area in cm2 at 7 weeks No secondary outcomes reported. No report of withdrawals from the trial. | |
| Notes | Ulcers assessed at baseline and weekly during treatment using a digitiser combined with computerised planimetry. In addition, ulcers were photographed (frequency and other details of this unclear). No information provided about experience or skill of care providers. Participants were the unit of randomisation. Trial report was in Polish; we extracted data with the assistance of a translator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From translator: " ... random assignment ..." Comment: no randomisation method specified. Authors did not state whether participants were randomised before or after surgery. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | No report of withdrawals, and not clear from report whether all participants were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised controlled trial in Poland. | |
| Participants | People assessed as having venous disease by assessment of symptoms and Duplex scanning. Number of participants: Surgery + US group: n = 21; No surgery ‐ US group: n = 20 Baseline characteristics: mean duration of ulcer (months) (SD): No surgery ‐ US group: 30.87 (20.12) Mean baseline area (cm2) (SD): | |
| Interventions | Surgery: as appropriate for each person included crossectomy, partial stripping of the greater or lesser saphenous veins, local phlebectomy and ligation of insufficient perforators. Compression: Sigvaris compression stockings (30‐40 mmHg at ankle) | |
| Outcomes | Treatment progress evaluated by observation of number of healed ulcers, measuring area by planimetry by projecting image onto transparency paper using a digitising pallet. Measurements of area and volume made at baseline, and before treatment each week. | |
| Notes | Duration of follow‐up 7 weeks. People who refused surgery were also randomised to US or standard care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “In this randomised controlled clinical trial ...”. Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eighty one patients with venous leg ulcers were included ... Forty one individuals ‐ who agreed on surgical operation ... were ultimately allocated into two comparative groups 1 and 2. Other individuals ‐ who did not agree on surgical procedure ‐ were ultimately allocated into two comparative groups 3 and 4 ..." |
| Blinding of participants and personnel (performance bias) | High risk | Participants: not blinded, since study was not sham controlled. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors: almost certainly not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. Withdrawals not mentioned (100% follow‐up implied but not stated). |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
| Methods | Randomised controlled trial in a variety of settings in UK and Ireland. | |
| Participants | 337 people with hard to heal venous leg ulcers (defined as more than 6 months' duration or area greater than 5cm2 or both); ABPI ≥0.8. Variety of settings in UK and Ireland (community nursing services, hospital outpatients clinics). US group: n = 168; Standard care group: n = 169 | |
| Interventions | US group: low dose (0.5 W/cm2) ultrasound at 1 MHz with a pulsed pattern of 1:4 applied to the periulcer skin (using a water‐based contact gel) once a week for up to 12 weeks plus standard care. US was applied for a period of 5‐10 minutes per treatment to the reference ulcer; the actual time being determined by a protocol based on ulcer area. Standard care group: simple low adherent dressing and high compression (4‐layer bandaging), reduced compression or no compression according to the clinician's assessment of the level of pressure tolerated by the participant | |
| Outcomes | Time to healing of the reference ulcer Cost effectiveness Proportion of participants with healed ulcers at 3, 6, 12 months Percentage and absolute change in ulcer size HRQoL and adverse events | |
| Notes | Maximal duration of follow‐up was of 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised equally between the two trial arms: ultrasound plus standard care and standard care alone. Randomisation was carried out using varying block sizes of four and six participants ... The computerised randomisation system was checked periodically during the trial following standard operating procedures." |
| Allocation concealment (selection bias) | Low risk | Quote: "To maintain allocation concealment the generation of the randomisation sequence and subsequent treatment allocation were performed by an independent, secure, remote, telephone randomisation service (York Trials Unit)." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Owing to the nature of the intervention, it was not possible to conceal the treatment allocation from either the patient or the nurse" This lack of blinding leaves the study susceptible to performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote for 12‐week outcome: "The reference ulcer was the largest ulcer on either leg (as assessed at the time of trial entry). The date of healing was recorded by the research nurses on the Ulcer Healed Form and the photographs of the reference ulcer were assessed independently by two people blind to treatment group. Any disagreements were resolved by discussion or referral to a third blinded assessor. The primary outcome was calculated using the date of healing as decided by the blind assessors. If the blinded assessors did not agree on a healing date, then the date as recorded on the Ulcer Healed Form was used." This blinded, remote adjudication of healing reduced the risk of detection bias. It is difficult to accurately judge the risk of bias in this scenario because an unmasked research nurse took a photograph. However, the blinded adjudication gives some reassurance that the risk of detection bias is low. 12‐month outcome: unclear. Quote: "The number of leg ulcers that had completely healed by 12 months was based on nurse‐reported data and not on blinded photographs ..." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Time to healing was derived as the number of days between randomisation and the first date that healing was confirmed. Patients who withdrew unhealed from the trial or died prior to healing were treated as censored in the analysis. Their time to censoring was derived using the date of trial exit, the date of their last ulcer assessment or the date of trial closure." Quote: "All randomised participants were included in the analysis and numbers of full withdrawals were low (only 10 patients ceased contributing data on the primary endpoint)." Analysis was by intention to treat. |
| Selective reporting (reporting bias) | Low risk | A full protocol was available and the published trial followed the protocol. Amendments to the original protocol were detailed and justified. |
| Other bias | Low risk | No other serious bias. |
| Methods | Randomised controlled trial in Germany. | |
| Participants | People admitted to an outpatient clinic for chronic leg ulcers. 38 participants with chronic venous leg ulceration of 3 months minimum duration plus evidence of incompetent perforating or superficial veins | |
| Interventions | US group: 30 kHz, intensity 0.1 W/cm2 for 10 minutes, delivered via the indirect (water‐bath) method, plus conventional therapy | |
| Outcomes | Number of ulcers healed at 8 weeks | |
| Notes | Duration of follow‐up: 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Each patient was randomly assigned to receive ...”, and “Randomisation was performed with sequential treatment cards which labelled the patient as either control or treatment. The cards were produced with a computer random number generator, preserving balance for each group” |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no blinding, since study was not sham controlled. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors: highly unlikely that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 ineligible participant was excluded from the analysis, quote: "Within the control group only 18 patients were evaluated for the study endpoints because at the end of the study evidence of arterial vascular disease was present in one patient, who was therefore excluded from the evaluation." Otherwise complete follow‐up and analysis by intention‐to‐treat analysis implied, but not stated. |
| Selective reporting (reporting bias) | Unclear risk | No details provided. |
| Other bias | Unclear risk | No details provided. |
Abbreviations
> = greater than
≥ = greater than or equal to
ABPI = ankle‐brachial pressure index
HRQoL: health‐related quality of life
n = number of participants in group(s)
RCT = randomised controlled trial
SD = standard deviation
US = ultrasound
mS = millisiemens
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a trial | |
| Not a randomised trial | |
| Trial predominantly involved people with ulcers secondary to critical limb ischaemia. | |
| Trial was an open‐label, non‐randomised, baseline‐controlled clinical case series. | |
| Not a randomised trial | |
| Non‐controlled pilot study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | A UK‐based, assessor‐blinded, randomised, controlled trial, conducted in a single dedicated unit specialising in wound healing research. The trial consisted of a 4‐week run‐in phase, followed by an 8‐week treatment phase. |
| Participants | Adults with chronic venous leg ulcers (duration ≥ 6 weeks and ≤ 5 years, and area 5 cm2‐100 cm2 at randomisation) and an ABPI of > 0.8. Those whose wounds reduced by > 40% during the first 4 weeks (the run‐in phase) did not progress to randomisation. 36 people were randomised. |
| Interventions | US group: non‐contact low‐frequency US (NLFU) + standard care (SOC) 3 times a week. NLFU consisted of the application of MIST US therapy (Therapy System; Celleration Inc., Eden Prairie, MN) to a clean wound bed for 3‐12 minutes (depending on the wound area) 3 times a week for up to 8 weeks; a non‐adherent dressing and strong compression therapy was applied after NLFU application. Standard care alone at least once a week. |
| Outcomes | The primary outcome was the change in wound area from baseline (week 5) to week 13 (or the point of healing) controlling for the baseline wound area measurement. |
| Notes | We are seeking independent guidance as to whether this is a distinct intervention for the purpose of debridement. |
Abbreviations
ABPI = ankle‐brachial pressure index
HRQoL = health‐related quality of life
NLFU: non‐contact low‐frequency
US = ultrasound
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed at 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 High frequency US vs no US, Outcome 1 Proportion of ulcers completely healed at 3 weeks. | ||||
| 2 Proportion of ulcers completely healed at 7 or 8 weeks Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 High frequency US vs no US, Outcome 2 Proportion of ulcers completely healed at 7 or 8 weeks. | ||||
| 2.1 Losses as unhealed | 6 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.86, 1.71] |
| 2.2 Complete case analysis | 6 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.88, 1.67] |
| 3 Proportion of ulcers completely healed at 12 weeks Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 High frequency US vs no US, Outcome 3 Proportion of ulcers completely healed at 12 weeks. | ||||
| 3.1 Losses as unhealed | 3 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.92, 1.73] |
| 3.2 Complete case analysis | 3 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.62] |
| 4 Proportion of ulcers completely healed at 12 months (nurse‐reported data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 High frequency US vs no US, Outcome 4 Proportion of ulcers completely healed at 12 months (nurse‐reported data). | ||||
| 5 HRQoL: 12‐week SF‐12 Physical Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 High frequency US vs no US, Outcome 5 HRQoL: 12‐week SF‐12 Physical Component Score. | ||||
| 6 HRQoL: 12‐week SF‐12 Mental Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6 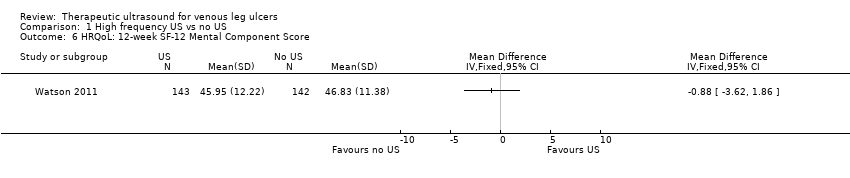 Comparison 1 High frequency US vs no US, Outcome 6 HRQoL: 12‐week SF‐12 Mental Component Score. | ||||
| 7 HRQoL: 12‐month SF‐12 Physical Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 High frequency US vs no US, Outcome 7 HRQoL: 12‐month SF‐12 Physical Component Score. | ||||
| 8 HRQoL: 12‐month SF‐12 Mental Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 High frequency US vs no US, Outcome 8 HRQoL: 12‐month SF‐12 Mental Component Score. | ||||
| 9 Non‐serious and serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 High frequency US vs no US, Outcome 9 Non‐serious and serious adverse events. | ||||
| 9.1 Non‐serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed at 8‐12 weeks Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [0.47, 32.85] |
| Analysis 2.1  Comparison 2 Low frequency US vs no US, Outcome 1 Proportion of ulcers completely healed at 8‐12 weeks. | ||||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
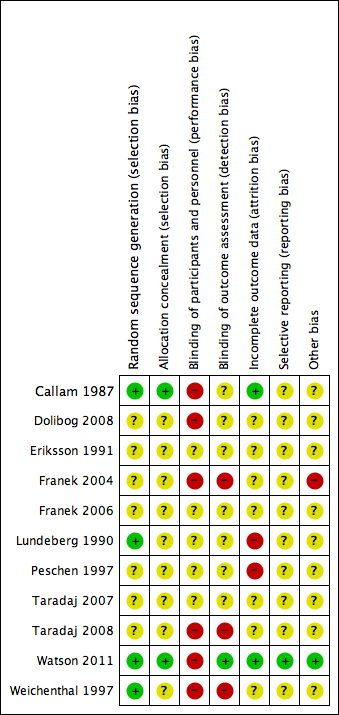
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Study flow diagram

Comparison 1 High frequency US vs no US, Outcome 1 Proportion of ulcers completely healed at 3 weeks.

Comparison 1 High frequency US vs no US, Outcome 2 Proportion of ulcers completely healed at 7 or 8 weeks.

Comparison 1 High frequency US vs no US, Outcome 3 Proportion of ulcers completely healed at 12 weeks.

Comparison 1 High frequency US vs no US, Outcome 4 Proportion of ulcers completely healed at 12 months (nurse‐reported data).

Comparison 1 High frequency US vs no US, Outcome 5 HRQoL: 12‐week SF‐12 Physical Component Score.

Comparison 1 High frequency US vs no US, Outcome 6 HRQoL: 12‐week SF‐12 Mental Component Score.

Comparison 1 High frequency US vs no US, Outcome 7 HRQoL: 12‐month SF‐12 Physical Component Score.

Comparison 1 High frequency US vs no US, Outcome 8 HRQoL: 12‐month SF‐12 Mental Component Score.
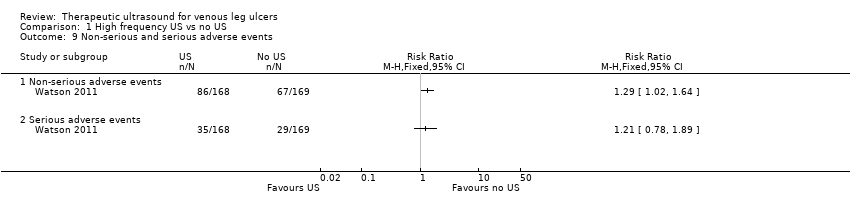
Comparison 1 High frequency US vs no US, Outcome 9 Non‐serious and serious adverse events.

Comparison 2 Low frequency US vs no US, Outcome 1 Proportion of ulcers completely healed at 8‐12 weeks.
| High frequency ultrasound compared with no ultrasound for people with venous leg ulcers | ||||||
| Patient or population: people with venous leg ulcers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no ultrasound | Risk with high frequency ultrasound | |||||
| Proportion of ulcers completely healed at 3 weeks | Study population | RR 2.05 | 65 | ⊕⊝⊝⊝ | Highly uncertain whether high frequency ultrasound affects healing at 3 weeks. | |
| 45 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 45 per 1000 | 92 per 1000 | |||||
| Proportion of ulcers completely healed at 7 or 8 weeks: losses to follow‐up regarded as unhealed | Study population | RR 1.21 | 678 | ⊕⊕⊝⊝ | Highly uncertain whether high frequency ultrasound affects healing at 7 to 8 weeks. | |
| 166 per 1000 | 198 per 1000 | |||||
| Moderate | ||||||
| 218 per 1000 | 259 per 1000 | |||||
| Proportion of ulcers completely healed at 12 weeks: losses to follow‐up regarded as unhealed | Study population | RR 1.26 | 489 | ⊕⊕⊕⊝ | Uncertain whether high frequency ultrasound affects healing at 12 weeks. | |
| 202 per 1000 | 255 per 1000 | |||||
| Moderate | ||||||
| 304 per 1000 | 383 per 1000 | |||||
| High10 | ||||||
| 500 per 1000 | 630 per 1000 | |||||
| Healing at 12 months (nurse‐reported data) | Study population | RR 0.93 | 337 | ⊕⊕⊝⊝ | Uncertain whether high frequency ultrasound affects healing at 1 year. | |
| 462 per 1000 | 429 per 1000 | |||||
| Moderate | ||||||
| 461 per 1000 | 429 per 1000 | |||||
| High11 | ||||||
| 800 per 1000 | 744 per 1000 | |||||
| Change in ulcer size at 4 or 7 weeks | Mean percentage change in ulcer area was reported in both studies. Data were insufficient to conduct a meta‐analysis. One study (4‐week follow‐up) found a difference in change in ulcer size between groups. The other study reported no clear difference. | 165 (2 RCTs) | ⊕⊝⊝⊝ | Highly uncertain whether high frequency ultrasound affects change in ulcer size at 4 or 7 weeks. | ||
| Non‐serious adverse events | Study population | RR 1.29 | 337 | ⊕⊕⊕⊝ | The data refer to the number of people experiencing adverse events, rather than the number of adverse events. | |
| 172 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 222 per 1000 | |||||
| Serious adverse events | Study population | RR 1.21 | 337 | ⊕⊕⊕⊝ | The data refer to the number of people experiencing adverse events, rather than the number of adverse events. | |
| 396 per 1000 | 480 per 1000 | |||||
| Moderate | ||||||
| 396 per 1000 | 479 per 1000 | |||||
| HRQoL: 12‐week SF‐12 mean Physical/Mental Component Scores | Physical Component Score (PCS) mean (SD): 34.96 (11.39) Mental Component Score (MCS) mean (SD): 46.83 (11.38) | PCS in the ultrasound group was 1.09 lower (3.75 lower to 1.57 higher) MCS in the ultrasound group was 0.88 lower (3.62 lower to 1.86 higher) | See comment | 285 | ⊕⊕⊕⊝ | No clear differences in physical or mental HRQoL at 12 weeks |
| HRQoL: 12‐month SF‐12 Physical Component Score | PCS mean (SD): 35.57 (1.88) MCS mean (SD): 45.41 (12.15) | PCS in ultrasound group was 0.96 lower (3.17 lower to 1.25 higher) MCS in ultrasound group was 2.1 higher (0.97 lower to 5.17 higher) | See comment | 229 | ⊕⊕⊕⊝ | No clear differences in physical or mental HRQoL at 12 months |
| Cost Follow‐up: 12 months | Addition of ultrasound treatment to standard care cost GBP 197.88 more per participant per year (95% bias‐corrected CI GBP ‐35.19 to GBP 420.32) | 337 | ⊕⊕⊕⊝ | No clear differences in cost at 12 months | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (two levels) ‐ at high or unclear risk of performance bias; the use of US was not the only difference in treatment between groups 2 Downgraded due to imprecision (one level) ‐ 95% CIs were very wide 3 Downgraded due to imprecision (one level) ‐ very short follow‐up time 4 Only 5 participants reached the endpoint (complete ulcer healing) and 4 of them were in the intervention group 5 Downgraded due to risk of bias (one level) ‐ most studies at high or unclear risk of bias 6 Downgraded due to imprecision (one level) ‐ 95% CIs were wide with only 122 participants reaching the endpoint 7 Downgraded due to imprecision (one level) ‐ only 111 participants across the three trials reached the endpoints and the OIS is hard to reach (Guyatt 2011) 8 Downgraded due to risk of bias (one level) since the outcome of healed wounds was based on nurse‐reported data 9 Downgraded due to imprecision estimate (one level) ‐ low event rate; OIS is hard to reach 10 High risk of healing at 12 weeks of 50% taken from a large, well conducted RCT where patients all received best practice care (Iglesias 2004). Moderate risk taken from median control group healing rate in these trials 11 With best practice (i.e. high compression bandaging), a baseline risk of healing at 12 months would be approximately 80% (Iglesias 2004) | ||||||
| Low frequency ultrasound compared with no ultrasound for people with venous leg ulcers | ||||||
| Patient or population: venous leg ulcers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no ultrasound | Risk with Low frequency US | |||||
| Proportion of ulcers completely healed at 8 to 12 weeks | Study population | RR 3.91 | 61 | ⊕⊝⊝⊝ | There were no events in the control groups so we added 0.5 to the cell as a fixed value (as per Cochrane Handbook). Highly uncertain whether low frequency ultrasound affects healing at 8 to 12 weeks. | |
| 17 per 1000 | 65 per 1000 | |||||
| High3 | ||||||
| 300 per 1000 | 1000 per 1000 | |||||
| Adverse events | No study reported adverse events | Pain was reported; however, this does not appear to have been measured systematically. | ||||
| HRQoL | No study reported HRQoL | |||||
| Cost | No study reported cost | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level) because both studies were at unclear or high risk 2 Downgraded due to imprecision (two levels): the OIS is hard to reach; very wide 95% CIs ‐ ranging in the ultrasound group from a 53% reduction of risk for ulcer healing to a 3285% increased risk (Guyatt 2011) 3 With best practice (i.e. high compression bandaging), a baseline risk of healing at 10 weeks (midpoint of 8 and 12 weeks) would be approximately 30% (Iglesias 2004) | ||||||
| Study | Intervention and Co‐interventions | Comparison intervention | Participants | Results |
|
5 physiotherapy departments
Scotland
NB 2 related abstracts say there were 110 participants
| US group (n = 52): DIRECT and HIGH FREQUENCY
*Lancet paper reports this frequency as 1 mHz. Verified with M Callam in Dec 09 that this should have read 1 MHz. | Standard treatment group (n = 56): cleansing with 1% saline; application of Arachis oil to skin without massage; application of paste bandage (Calaband); application of support bandage (Lestreflex); advice on exercise.
Weekly treatment. | Included: patients attending clinics for treatment of chronic leg ulcers Excluded: non consent, allergy to standard treatment, PVD (lack of ankle pulses) | Ulcers completely healed by 12 wks: US: 25/52 (48%) C: 17/56 (30%)
Read from graph: Ulcers completely healed at 8 wks: US: 23/52 (45%) C: 14/56 (25%)
Ulcers completely healed by 12 wks (complete case): US: 25/41 (61%) C: 17/41 (41%)
US treated healed sig more quickly by log rank ;=0.03. this effect persisted even when withdrawals due to pain and deterioration counted as failures.
Mean % ulcer unhealed at 12 weeks (no variance): US: 9% C: 27% P<0.05
Withdrawals: US: 11/52 (21%) Allergy 4 Pain 4 DNA/refused 2 Death 2
C: 15/56 (27%) Allergy 6 Pain 3 Refused/DNA 3 Deterioration 2 Arterial disease 1 |
|
Poland
| US group: 33 participants treated with US, compression (Sigvaris), and drug therapy.
INDIRECT and HIGH FREQUENCY | Standard care group (n = 37): compression and drug therapy. Dressings changed daily for 7 weeks. | 70 participants with venous leg ulcers who all had venous surgery, and diagnosed as having venous disease with Duplex scanning.
Excluded: diabetes, rheumatoid arthritis Surgery included crossectomy, partial stripping of GSV or LSV, local phlebectomy, ligation of perforators. | Ulcers healed completely: Group 1: 10/33 Group 2: 12/37
Mean area after therapy (SD) Group 1: 13.15 (11.55) Group 2: 13.12 (14.57)
|
|
Hospital and primary care
Sweden | US group: 1.0 W/cm2 at 1 MHz. Enraf Nonius US machine with aquasonic gel. Ultrasound head was 2.8 cm diameter for superficial ulcers and 1.2 cm diameter for deep ulcers. US applied to ulcer surface area and surrounding tissue for 10 minutes twice a week for 8 weeks.
DIRECT and HIGH FREQUENCY | Sham US group: cleaned with saline; paste bandage, support bandage plus exercise advice (no further details provided). | Included; people with venous leg ulcers Excluded: allergy to standard treatment, arterial disease, rheumatoid arthritis, diabetic ulcers, traumatic venous ulcer | Ulcers completely healed at 8 wks: US: 6/19 (6/12 completers) C: 4/19 (4/13 completers)
Cumulative % healed compared using life table methods.
% ulcers completely healed at different times (wks) US:C 2 wks: 8:0 4 wks: 17:8 6 wks: 25:15 8 wks: 41:30
% ulcer area healed at 8 wks (SD): US: 42 (9) C: 48 (13)
Withdrawals: US: 7/19 C: 6/19
For allergy: US: 3 C: 2
For pain: US: 2 C: 1
Refusal/DNA: US: 2 C: 3 |
|
Poland
Hospital inpatients
| US group 1: US at 1 W/cm2 (n = 22) US group 2: US at 0.5 W/cm2 (n = 21)
Both groups received pulsed cycle of 1:5, frequency 1 MHz.
Cointerventions: saline soaked gauze. Single‐layer elastic compression (Hartmann). INDIRECT and HIGH FREQUENCY | Pharmacotherapy group (n = 22): no US. Local baths of potassium permanganate and wet dressings of 0.1M copper sulphate solution plus compresses of fibrolan, chloramphenicol, colistin, gentamicin. Drugs alternated every few days. Single layer elastic compression (Hartmann). Treatment for 3 weeks.
This was problematic as the use of US or not was not the only difference in treatment between the groups i.e. performance bias. Also US groups 1 and 2 were hospitalised in the Dermatology Clinic of Katowice and Group C in the Dermatology Dept of Zabrze.
| Included: people with venous ulcers (signs of venous disease) and ABPI > 1.0. Excluded: people with diabetes, advanced sclerosis
| Mean (median) area after treatment (cm2): A: 14 (11.14) p = 0.0001 B: 9.29 (3.78) p = 0.00006 C: 20.58 (9.86) p = 0.002
Complete ulcer healing by 3 weeks: A: 1/22 B: 3/21 C: 1/22
|
| Poland Hospital inpatients | Electrostimulation group: once a day, 50 minutes each session, 6 consecutive days, 4 weeks total (2 weeks cathodic and 2 weeks anodic stimulation), NaCl 0.9% locally (no further details provided) Laser therapy group: 65 mW once a day, 5 consecutive days, duration of each session depending of ulceration area – device was set up to develop 4J/cm2 on average power 65 mW, various pharmacological agents locally, 4 weeks total US group: 0.5 W/cm2 – once a day, duration of each session depending on ulceration area: 5 cm2 received 5 minutes, plus 1 minute more for each 1 cm2 of additional ulcer area, 4 weeks total, 0.9% NaCl locally | Compression therapy provided for all groups. Bandages were removed every time for purposes of physical therapy and then put back on. Compression + pharmacological agents group: 4 weeks total | People with venous leg ulcers | Mean % change in ulcer area (relative to baseline) at 4 weeks: Group 1: ‐55.26%; Group 2: ‐35.97%; Group 3: ‐63.42%; Group 4: ‐30.77% P(Group 3 & Group 4) = 0.007 |
|
Sweden
| US. group (n = 22): US: pulsed 1:9 0.5 W/cm2 at 1 MHz US applied to ulcer surface and surrounding tissue for 10 minutes; probe applied for 1 minute per probe head area (no further details provided). Treated 3 x per week for 4 weeks, then 2 x per week for 4 weeks, then once a week for 4 weeks. Plus standard care, which comprised of cleansing with saline; paste bandage; support bandage; exercise instructions.
DIRECT and HIGH FREQUENCY | Sham US (no further details provided) + standard treatment group (n = 22): standard care consisted of cleansing with saline; paste bandage; support bandage; exercise instructions. | Patients with VLUs referred from depts. of internal medicine, surgery, primary health care.
Exclusion: skin allergy, PVD, RA, DM, traumatic venous ulcer.
| Cumulative % (n) healed at 8 weeks: US: 30% (5) C: 20% (3)
Cumulative % (n) healed at 12 weeks: US: 59% (10) C: 52% (8)
Mean % ulcer area remaining at 8 weeks (SD) in patients completing: US: 47% (8) C: 53% (10)
Mean % ulcer area remaining at 12 weeks (SD) in patients completing: US: 39% (5) C: 43% (6)
12/44 patients withdrew (7 placebo group, 5 US group).
Placebo: 3 allergy, 1 pain, 3 DNA/refused.
US: 2 allergy, 1 pain, 2 DNA/refused |
|
Germany
Outpatient clinic | US group (n = 12): US treatment involved placing legs in footbath of water at 32 °C‐34 °C filled to 10 cm above the ulcer. US sound head transducer immersed in bath and placed in line with ulcer 5 cm away. The continuous US was given for 10 minutes at 30 kHz, 0.1 W/cm2 3 x per week. Standard care was also given. This comprised HCL dressings (Coloplast); compression therapy using “strong‐quality elastic compression bandages (Beiersdorf)”
INDIRECT and LOW FREQUENCY | Sham US group (n = 12): sham US plus standard care
Sham procedure involved placing legs in footbath of water at 32 °C‐34 °C filled to 10 cm above the ulcer. US sound head transducer immersed in bath and placed in line with ulcer 5 cm away. Sham US for 10 minutes 3 x per week. Standard care consisted of HCL dressings (Coloplast); compression therapy using “strong‐quality elastic compression bandages (Beiersdorf)” | 24 people attending outpatients clinic. Included: people with chronic VLUs at least 2 cm2 and 3 months’ duration. Clinical diagnosis of VLU confirmed by history, Doppler US, light reflection rheography, ABPI of 0.8 or above. Excluded: arterial disease, liver, cardiac or renal insufficiency, heamorrhagic gastroduodenitis, colitis, leukaemia, diabetes, RA, treatment allergy.
| Complete ulcer healing at 12 weeks: US: 2/12 C: 0/12 (or 0/10 completers)
Mean % decrease in ulcer area 12 weeks: US: 55.4% C: 16.5% No variance data p<0.007
Micro‐bleeding around the ulcer: US: 5 C: 0
Pain: US: 3/12 C: 4/10 pain free
Irritation: US: 8/12 C: 0
8 US patients felt tingling sensation during US.
After 12 wk treatment phase, standard care continued.
At 3 months post treatment: Mean ulcer area: US: 30.6% C: 70.2%
Mean change ulcer radius (mm) US: 9.9mm (n = 12) C: 5.3mm (n = 10) (P<0.012)
|
| Poland | US group (n = 24): sonotherapy with sonicator 730 device, in water bath, 1 MHZ, 0.5 W/cm2, duration dependent on area of ulceration ‐ e.g. 5 min for ≦ 5 cm2), 6 days/week for 7 weeks plus pharmacotherapy | All participants used moist normal saline dressing, and pharmacotherapy (diosmin 450 mg and hesperidin 50 mg combined as proprietary preparation (Detralex) All patients: treatment duration 7 weeks. Compression group (n = 25): compression plus stocking and pharmacotherapy Standard care group (n = 24): pharmacotherapy | People with venous ulcers who had undergone venous surgery by modified Babcock method. | Mean % change in ulcer area (relative to baseline) at 7 weeks: Group 1: ‐53.6%; Group 2: ‐69.4%; Group 3: ‐62.6% (P > 0.05 for all 3 comparisons between groups). Mean ± SD ulcer area in cm2 at 7 weeks (NB: comparisons are within group vs baseline): Group 1: 14.1 ± 11.7 (P = 0.00002); Group 2: 8.8 ± 10.0 (P = 0.00001); Group 3: 11.4 ± 14.1 (P = 0.00002). |
|
Poland | Surgery + US group (n = 21): surgery plus US, compression stockings (Sigvaris, 30 mmHg‐40 mmHg at ankle), drug therapy
No surgery + US group: US, compression and drug therapy
Drug therapy was flavonoid (450 mg diosmin, 50 mg hesperidin), 2 tabs (one of each) twice daily.
Ulcers covered by saline soaks. Dressings changed once day only in clinic.
Ultrasound; 0.5 W/cm2 pulsed; impulse 2 mS, interval 8 mS. Frequency 1 MHz. Performed in a bath of water with temp 34 °C. probe head 10 cm2 placed 2 cm above ulcer. An ulcer of 5 cm2 or less had 5 minutes treatment, with 1 minute more for each 1 cm2 by which the ulcer exceeded this size. If larger than 20 cm2 the ulcer was divided in 2. Treatment daily for 6 days/week for 7 weeks.
INDIRECT and HIGH FREQUENCY | Surgery ‐ US group (n = 20): surgery plus compression and drug therapy
No surgery ‐ US group (n = 20): compression and drug therapy
Drug therapy was flavonoid (450 mg diosmin, 50 mg hesperidin), 2 tabs (one of each) twice daily. Ulcers covered by saline soaks. Dressings changed once day only in clinic. | People with venous disease assessed by symptoms and Duplex scanning. All offered venous surgery. Those refusing surgery were randomised to US or no US.
| Group 1 vs. Group 2 Group 3 vs. Group 4
Numbers completely healed at 7 weeks: Group 1: 6/21 Group 2: 6/20
Group 3: 6/20 Group 4: 3/20
|
|
UK Community nurse services, community leg ulcer clinics, and hospital outpatient leg ulcer clinics | US group (n = 168): low‐dose (0.5 W/cm2) US, 1 MHz, with a pulsed pattern of 1:4, applied for 5 to 10 minutes to periulcer skin, weekly for up to 12 weeks, plus standard care, then standard care alone. DIRECT and HIGH FREQUENCY
| Standard care group (n = 169): simple low‐adherent dressing and high compression (4‐layer bandage), reduced compression or no compression depending on participant tolerance.
| 337 patients with hard‐to‐heal venous leg ulcers i.e., ulcer of 6 months’ duration or more and/or area greater than 5 cm2. Considered a venous ulcer if no other obvious causative factor and ulcer appeared clinically venous (moist, shallow, irregular shape, venous eczema, ankle oedema, lipodermatosclerosis, ulcer not confined to the foot). Participants had to have ABPI of 0.8 or greater. Excluded if poorly controlled diabetes, ankle prostheses, thrombophlebitis, active infection including cellulitis, local or metastatic cancer.
| Hazard ratio* for US vs. SC 0.99 (0.70 to 1.40), p = 0.969 (NSD). * the analysis adjusted for centre as a random effect, ulcer area (from baseline tracing), ulcer duration and whether or not the patient was treated with high‐compression bandaging. Median time (for all ulcers) to complete healing: US: 365 days (95% CI 224, inestimable) SC: 328 days (95% CI 235, inestimable) P = 0.9051, log rank.
Ulcers completely healed/not healed (%) at 8 wks (personal communication): US: 9/168 SC: 15/169
Ulcers completely healed/not healed (%) at 12 wks (personal communication): US: 26/168 SC: 25/169 Ulcers completely healed/not healed (%) at 12 month (personal communication): US: 72/168 SC: 78/169 HRQoL by SF‐12: Mean Baseline PCS (SD): US: 36.55 (11.32); n = 160 SC: 35.33 (11.47); n = 167
3 month PCS (SD): US:33.87 (11.49); n = 143 SC: 34.96 (11.39); n = 142
12 month PCS (SD): US:34.61 (12.09); n = 118 SC: 35.57 (11.39); n = 111 Baseline MCS (SD): US: 46.72 (11.52); n = 160 SC: 47.11 (11.29); n = 167
3 month MCS (SD): US: 45.95 (12.22); n = 143 SC: 46.83 (11.38); n = 142
12 month MCS (SD): US: 47.51 (11.54); n = 118 SC: 45.41 (12.15); n = 111 Serious Adverse Events (SAEs): US: 35/168 patients SC: 29/169 patients Non serious AEs: SC: 67/169 patients NS using random effects negative binomial regression (p = 0.3904).
Using random effects negative binomial regression showed that significantly more non serious AEs in US group (p = 0.0411).
For all adverse events in random effects binomial regression, there was a significant effect of treatment (p = 0.0446). Adjusted annual costs ( 95% bias‐corrected CI): US arm 1583.39 (1427.51 to 1728.70) vs. SC arm 1385.51 (1223.84 to 1549.21 |
|
Outpatient clinic
Germany | US group: 'experimental' 30 kHz US applicator mounted to footbath. Transducer positioned within 5 cm of ulcer surface. Surface subjected to 30 kHz US at 0.1 W/cm2 for 10 minutes, plus standard care.
INDIRECT and LOW FREQUENCY | Conventional therapy group: topical fibrinolytic agents, antibiotics or other antiseptics and occlusive dressings. Eczema of surrounding skin could be treated with topical steroids. Compression with elastic bandages. Dressings changed at least 3 x per week.
Participants received foot bathing but participants in US group did not. | Inclusion: presence of ulceration for min. 3 mo. plus evidence of venous incompetence. Excluded: diabetes, arterial disease. | Mean ulcer area at 3 weeks (SD): US: 8.3 (6.4) C: 14.7 (10.4)
Mean ulcer area at 8 weeks (SD): US: 6.2 (5.9) C: 13.4 (12.1)
Ulcers completely healed at 8 weeks: US: 1/19 C: 0/19 (0/18 completers)
US: no/minor complaints about pain with US. Mild to mod erythema often observed with US. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed at 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Proportion of ulcers completely healed at 7 or 8 weeks Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Losses as unhealed | 6 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.86, 1.71] |
| 2.2 Complete case analysis | 6 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.88, 1.67] |
| 3 Proportion of ulcers completely healed at 12 weeks Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Losses as unhealed | 3 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.92, 1.73] |
| 3.2 Complete case analysis | 3 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.62] |
| 4 Proportion of ulcers completely healed at 12 months (nurse‐reported data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 HRQoL: 12‐week SF‐12 Physical Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 HRQoL: 12‐week SF‐12 Mental Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 HRQoL: 12‐month SF‐12 Physical Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 HRQoL: 12‐month SF‐12 Mental Component Score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Non‐serious and serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Non‐serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed at 8‐12 weeks Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [0.47, 32.85] |

