Peripheral nerve blocks for hip fractures in adults
Abstract
Background
This review was published originally in 1999 and was updated in 2001, 2002, 2009, 2017, and 2020. Updating was deemed necessary due to the high incidence of hip fractures, the large number of official societies providing recommendations on this condition, the possibility that perioperative peripheral nerve blocks (PNBs) may improve patient outcomes, and the major role that PNBs may play in reducing preoperative and postoperative opioid use for analgesia.
Objectives
To compare PNBs used as preoperative analgesia, as postoperative analgesia, or as a supplement to general anaesthesia versus no nerve block (or sham block) for adults with hip fracture. Outcomes were pain on movement at 30 minutes after block placement, acute confusional state, myocardial infarction, chest infection, death, time to first mobilization, and costs of an analgesic regimen for single‐injection blocks.
We undertook the update to look for new studies and to update the methods to reflect Cochrane standards.
Search methods
For the updated review, we searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 11), in the Cochrane Library; MEDLINE (Ovid SP, 1966 to November 2019); Embase (Ovid SP, 1974 to November 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1982 to November 2019), as well as trial registers and reference lists of relevant articles.
Selection criteria
We included randomized controlled trials (RCTs) assessing use of PNBs compared with no nerve block (or sham block) as part of the care provided for adults 16 years of age and older with hip fracture.
Data collection and analysis
Two review authors independently screened new trials for inclusion, assessed trial quality using the Cochrane Risk of Bias‐2 tool, and extracted data. When appropriate, we pooled results of outcome measures. We rated the certainty of evidence using the GRADE approach.
Main results
We included 49 trials (3061 participants; 1553 randomized to PNBs and 1508 to no nerve block (or sham block)). For this update, we added 18 new trials. Trials were published from 1981 to 2020. Trialists followed participants for periods ranging from 5 minutes to 12 months. The average age of participants ranged from 59 to 89 years. People with dementia were often excluded from the included trials. Additional analgesia was available for all participants.
Results of 11 trials with 503 participants show that PNBs reduced pain on movement within 30 minutes of block placement (standardized mean difference (SMD) ‐1.05, 95% confidence interval (CI) ‐1.25 to ‐0.86; equivalent to ‐2.5 on a scale from 0 to 10; high‐certainty evidence). Effect size was proportionate to the concentration of local anaesthetic used (P = 0.0003). Based on 13 trials with 1072 participants, PNBs reduce the risk of acute confusional state (risk ratio (RR) 0.67, 95% CI 0.50 to 0.90; number needed to treat for an additional beneficial outcome (NNTB) 12, 95% CI 7 to 47; high‐certainty evidence). For myocardial infarction, there were no events in one trial with 31 participants (RR not estimable; low‐certainty evidence). From three trials with 131 participants, PNBs probably reduce the risk for chest infection (RR 0.41, 95% CI 0.19 to 0.89; NNTB 7, 95% CI 5 to 72; moderate‐certainty evidence). Based on 11 trials with 617 participants, the effects of PNBs on mortality within six months are uncertain due to very serious imprecision (RR 0.87, 95% CI 0.47 to 1.60; low‐certainty evidence). From three trials with 208 participants, PNBs likely reduce time to first mobilization (mean difference (MD) ‐10.80 hours, 95% CI ‐12.83 to ‐8.77 hours; moderate‐certainty evidence). One trial with 75 participants indicated there may be a small reduction in the cost of analgesic drugs with a single‐injection PNB (MD ‐4.40 euros, 95% CI ‐4.84 to ‐3.96 euros; low‐certainty evidence).
We identified 29 ongoing trials, of which 15 were first posted or at least were last updated after 1 January 2018.
Authors' conclusions
PNBs reduce pain on movement within 30 minutes after block placement, risk of acute confusional state, and probably also reduce the risk of chest infection and time to first mobilization. There may be a small reduction in the cost of analgesic drugs for single‐injection PNB. We did not find a difference for myocardial infarction and mortality, but the numbers of participants included for these two outcomes were insufficient. Although randomized clinical trials may not be the best way to establish risks associated with an intervention, our review confirms low risks of permanent injury associated with PNBs, as found by others.
Some trials are ongoing, but it is unclear whether any further RCTs should be registered, given the benefits found. Good‐quality non‐randomized trials with appropriate sample size may help to clarify the potential effects of PNBs on myocardial infarction and mortality.
PICO
Plain language summary
Do local anaesthetic nerve blocks provide effective pain relief for adults with a hip fracture?
What is a peripheral nerve block?
A peripheral nerve block (PNB) is an injection of local anaesthetic close to nerves to block pain signals to the brain. PNBs can be used alone or together with other pain relief medicines. They may be given as a single injection or continuously, using a catheter (drip).
Why is this question important?
Hip fractures commonly occur in older people. Surgery is usually needed to repair the bone. Hip fractures are very painful. Opioids such as morphine, which are strong painkillers, are often used to manage hip fracture pain. Older people do not tolerate high doses of opioids well. Also, people with hip fracture may have complications such as confusion, myocardial infarction and chest infection.
By reducing the use of opioids and better treating pain, PNBs may improve the mobility of people with hip fracture and reduce risks of complications.
What did we want to find out?
We wanted to know whether using PNBs compared to no nerve block (no block at all or a placebo nerve block), in people with hip fracture could reduce:
• pain on movement;
• confusion, myocardial infarction, and chest infection;
• death from any cause within six months;
• length of time until people were mobile after surgery; and
• costs of drugs used to manage pain.
What did we do?
We searched medical databases for studies that investigated the use of PNBs versus no effective nerve block (i.e. no block at all or a placebo block) for pain in people with hip fracture. Study participants had to be over 16 years of age and had to have a hip fracture. We looked for randomized controlled trials (RCTs), where the treatment people receive is decided randomly.
What we found
We included 49 studies with 3061 participants (average age 59 to 89 years); 1553 participants received PNBs and 1508 received no nerve block. Additional pain relief, including opioids, was available for all participants when required. Studies were conducted in various countries and published between 1980 and 2020. Twenty‐six studies received non‐commercial funding, and the source of funding was not stated for the other studies.
Main results
PNBs reduced pain on movement by 2.5 on a scale of 1 to 10, compared with no nerve block (11 studies, 503 participants). PNBs reduced the risk of confusion; for every 12 people with a hip fracture, one person less will become confused with PNBs (13 studies, 1072 participants). We did not find a difference in risk of myocardial infarction (1 study, 31 participants).
PNBs probably reduce the risk of chest infection (3 studies, 131 participants) and time to first mobilization after surgery by 11 hours (3 studies, 208 participants). We did not find a difference in deaths from any cause within six months (11 studies, 617 participants). Costs of drugs used for pain management were slightly lower when a single‐injection PNB was compared to no PNB (1 study, 75 participants).
How reliable are the results?
Our confidence (certainty) in the evidence for reduced pain on movement and for reduced confusion was high; we are moderately confident in the evidence for reduced chest infection. However, we are less confident about the evidence for myocardial infarction, death, time to first mobilization, and costs of drugs used for pain management, mainly because this evidence came from small studies with few participants.
What does this mean?
We found enough good‐quality evidence to support the use of PNBs in patients with hip fracture. Larger studies are required to clarify the effects of PNBs on myocardial infarction and death.
How up‐to‐date is this review?
This is an updated review. Evidence is up‐to‐date to 16 November 2019.
Authors' conclusions
Summary of findings
| Peripheral nerve blocks for hip fracture | ||||||
| Patient or population: patients with hip fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain on movement at 30 minutes after block placement | Mean pain on movement at 30 minutes after block placement in the intervention groups was | 503 | ⊕⊕⊕⊕ | |||
| Acute confusional state Follow‐up: 0 to 30 days | Study population | RR 0.67 | 1072 | ⊕⊕⊕⊕ | ||
| 181 per 1000 | 121 per 1000 | |||||
| Low | ||||||
| 150 per 1000 | 101 per 1000 | |||||
| High | ||||||
| 350 per 1000 | 235 per 1000 | |||||
| Myocardial infarction Follow‐up: 0 to 30 days | N/A | N/A | 31 | ⊕⊕⊝⊝ | ||
| Chest infections Follow‐up: 0 to 30 days
| Study population | RR 0.41 (0.19 to 0.89) | 131 (3 studies) | ⊕⊕⊕⊝ moderatee,f |
| |
| 269 per 1000 | 110 per 1000 (51 to 239) | |||||
| Low | ||||||
| 50 per 1000 | 20 per 1000 (9 to 44) | |||||
| High | ||||||
| 200 per 1000 | 82 per 1000 (38 to 178) | |||||
| Death | Study population | RR 0.87 | 617 | ⊕⊕⊝⊝ | ||
| 68 per 1000 | 59 per 1000 | |||||
| Low | ||||||
| 25 per 1000 | 22 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 131 per 1000 | |||||
| Time to first mobilization Follow‐up: in‐hospital | Mean time to first mobilization in intervention groups was | 208 | ⊕⊕⊕⊝ | |||
| Cost of analgesic regimens for single‐injection blocks Follow‐up: in‐hospital | Mean cost of analgesic regimens for single‐injection blocks in intervention groups was | 75 | ⊕⊕⊕⊝ | |||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades for certainty of evidence. | ||||||
| aThe effect was still present even when trials at high risk of bias were withdrawn from the analysis, or when a correction for the possibility of publication bias was applied. bThe difference was equivalent to 2.5 on a scale from 0 to 10. cThe number needed to treat for additional beneficial outcome was 12 (95% confidence interval 7 to 47). dDowngraded by two levels for imprecision. eDowngraded by one level for imprecision. fThe number needed to treat for additional beneficial outcome was 7 (95% confidence interval 5 to 72). gMean costs in 2009 euros. | ||||||
Background
Description of the condition
Among women 55 years of age and older in the USA, the Nationwide Inpatient Sample (NIS) for 2000 to 2010 reported 4.9 million hospitalizations for osteoporotic fractures (2.6 million for hip fractures) − a higher number of hospitalizations than for myocardial infarction (2.9 million), stroke (3.0 million), and breast cancer (0.7 million) (Singer 2015). Osteoporotic fractures accounted for more than 40% of hospitalizations for these four outcomes, with an age‐adjusted rate of 1124 admissions per 100,000 person‐years. The annual total population facility‐related hospital cost was highest for hospitalizations due to osteoporotic fractures (USD 5.1 billion), followed by myocardial infarction (USD 4.3 billion), stroke (USD 3.0 billion), and breast cancer (USD 0.5 billion) (Singer 2015).
The term 'hip fracture' refers to a fracture of the proximal femur down to about 5 cm below the lower border of the lesser trochanter. Costs of care for hip fractures are high and, when both acute care and the care needed for subsequent dependency were included, exceeded GBP 2 billion in 2012 for the UK as a whole. That same year, the overall rate of return home by 30 days was 44.6% in the UK (National Hip Fracture Database 2019; www.nhfd.co.uk). In the USA, from 2003 to 2005, 5.3% of patients with hip fracture returned home in 30 days, and 52.8% were discharged to a skilled nursing facility (Brauer 2009). Hip fractures are associated with reduced life expectancy when they occur in individuals over 50 years of age. Pooled data from cohort studies revealed that the relative hazard (RH) for all‐cause mortality during the first three months after hip fracture was 5.75 (95% confidence interval (CI) 4.94 to 6.67) in women and 7.95 (95% CI 6.13 to 10.30) in men (Haentjens 2010). However, improved care has resulted in encouraging figures. Indeed, data collected in UK in 2018 show a 6.1% death rate, representing a decrease of one in eight when compared with the mortality figure of 6.9% reported for 2017, implying that 564 fewer people died within a month of breaking their hip in 2018 (National Hip Fracture Database 2019).
Description of the intervention
Regional blockade refers to injection of local anaesthetics around neural structures to transiently prevent pain transmission to the brain, and may also produce motor blockade of the muscle in a specific area, depending on the type and concentration of local anaesthetic used. Local anaesthetics can be used at the spine level (neuraxial blocks = epidural or spinal) or around the nerves outside the spine (plexus blocks or peripheral nerve blocks (PNBs)). Local anaesthetic may also be infiltrated directly into wound tissues. All of these blocks can be given as a single injection or by continuous infusion through a catheter to prolong their beneficial effects. PNBs may be used as a replacement for general anaesthesia during surgery, as adjunctive treatment for preoperative and postoperative pain, or as a means of decreasing the use of intraoperative systemic drugs during general anaesthesia. Use of regional blockade as a replacement for general anaesthesia in individuals with hip fracture is covered in another review (Guay 2016). For the present review, the intervention is limited to PNBs used for analgesia (i.e. before surgery), in addition to other anaesthetic methods for surgery or for postoperative analgesia. Although neuraxial blocks may have been used in some trials included here (usually as replacement for general anaesthesia for the surgery), they will not be evaluated in the present review but, once again, are covered separately in another review (Guay 2016).
How the intervention might work
Most hip fractures occur in an elderly population; more than 30% of individuals with hip fracture are 85 years of age or older (Brauer 2009). Opioid‐related respiratory depression may result in severe brain damage or death (Lee 2015). By reducing the quantity of opioids used before, during, and after surgery (Guay 2006; Guay 2017), regional blockade may improve the mobility of persons with hip fracture (Saunders 2010), potentially facilitating their participation in rehabilitation and hence reducing complications related to prolonged immobilization such as pneumonia (Guay 2017). Hip fractures in the elderly have also been associated with a high rate of postoperative delirium. In a recent review on 8439 geriatric hip fracture patients, Arshi and colleagues reported a 30.4% rate of postoperative delirium (Arshi 2018). Patients with postoperative delirium had significantly higher risk‐adjusted 30‐day mortality (12.0% vs 4.8%; odds ratio (OR) 2.22, 95% CI 1.74 to 2.84) (Arshi 2018). Some study authors have suggested that the rate of perioperative delirium might be lower when PNBs are added to a multi‐modal regimen of perioperative analgesia (Mouzopoulos 2009).
Why it is important to do this review
Despite their advantages, PNBs still are not widely used for people with hip fracture (Haslam 2013). Many official clinical societies recommend preoperative regional anaesthesia (e.g. American Academy of Orthopaedic Surgeons 2014: "strong recommendation"; NICE 2017: "consider adding nerve blocks if paracetamol and opioids do not provide sufficient preoperative pain relief, or to limit opioid dosage") and postoperative multi‐modal analgesia including regional anaesthesia (e.g. American Academy of Orthopaedic Surgeons 2014: "strong recommendation") for patients with hip fracture. It is not the mandate of Cochrane reviewers to make any recommendations but rather to summarize the evidence, hence providing official societies, policy makers, clinicians, and patients with high‐quality systematic reviews to help them make decisions as to what intervention should or should not be used for a specific clinical condition in their specific environment.
In addition, exclusive use of opioids for perioperative pain has become a controversial clinical practice. Between 1999 and 2016, more than 630,000 people in the United States died from a drug overdose, and a record number of drug overdose deaths occurred in 2016: 63,632 − a rate of 19.8 per 100,000 persons (Centers for Disease Control and Prevention 2018). Within the first six months of 2018 alone, 2066 opioid‐related deaths were reported in Canada (11.2 deaths per 100 000 people) (Ball 2019). Up to 75% of all heroin users were first introduced to narcotics through an initial physician‐ or surgeon‐related opioid prescription (Ball 2019). Reduction in perioperative opioid consumption with the use of regional anaesthesia has already been reported (Guay 2016; Guay 2017).
Some adverse events may happen with the use of PNBs. Severe and permanent nerve injuries have occurred, at an estimated incidence of approximately 1:2500 to 1:5000 blocks (Neal 2015). Although systemic local anaesthetic toxicity has probably decreased in both incidence and severity with the use of ultrasound, seizures are still reported, with an incidence of 1.3 (95% CI 0.3 to 3.8) per 10,000 PNBs (Sites 2014). Finally, although infections are rarely seen with single PNBs, they may occur with catheter insertion (Bomberg 2017).
The topic of the present review is very important to update, considering: (1) the high prevalence of hip fractures, (2) the large number of official societies providing recommendations on this condition, (3) the possibility that perioperative PNBs may improve patient outcomes, and (4) the major role that PNBs may play in reducing preoperative and postoperative opioid use for analgesia.
Therefore, we have decided to re‐evaluate the beneficial effects of PNBs for hip fracture.
This is an update of a previously published review (Guay 2017; Parker 2002).
Objectives
To compare PNBs used as preoperative analgesia, as postoperative analgesia, or as a supplement to general anaesthesia versus no nerve block (or sham block) for adults with hip fracture. Outcomes were pain on movement at 30 minutes after block placement, acute confusional state, myocardial infarction, chest infection, death, time to first mobilization, and costs of an analgesic regimen for single‐injection blocks.
We undertook the update to look for new studies and to update the methods to reflect Cochrane standards.
Methods
Criteria for considering studies for this review
Types of studies
We included all parallel randomized controlled trials (RCTs) and cluster trials comparing PNBs inserted preoperatively, intraoperatively, or postoperatively (intervention) versus no nerve block (or sham block) (comparator).
For the purpose of this review, a sham nerve block and no nerve block were considered as equivalent. We excluded quasi‐RCTs (e.g. alternation) and cross‐over trials. These two categories of trials were also excluded from previously published versions of our review. Cross‐over trials were considered unsuitable for our review. Indeed, it would not be possible to evaluate the effects of adding PNBs on the risk of perioperative acute confusional state, pneumonia, myocardial infarction, or mortality if all participants had received a PNB at some point during their perioperative period (unless we had considered only the first part of the cross‐over trial, when results would be available as such).
Types of participants
We included adults aged 16 years of age and older with a proximal femoral fracture (hip fracture).
Types of interventions
PNBs of any type versus no nerve block (or sham block).
Types of outcome measures
Primary outcomes
-
Pain on movement 30 minutes after block placement (study author's scale; Thong 2018)
-
Acute confusional state (study author's definition), 0 to 30 days
-
Myocardial infarction (study author's definition), 0 to 30 days
Secondary outcomes
-
Chest infection (study author's definition), 0 to 30 days
-
Mortality (all death from any cause), 0 to 6 months
-
Time to first mobilization after surgery
-
Costs of analgesic regimens (at any time points chosen by study authors)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 11), in the Cochrane Library; MEDLINE ALL (Ovid SP, 1966 to 16 November 2019); Embase (Ovid SP, 1974 to 16 November 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1982 to 16 November 2019). We searched for studies as described in the Cochrane Handbook of Systematic Reviews of Interventions, Chapter 4 (Lefebvre 2019). We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE (Lefebvre 2019). For MEDLINE (Ovid SP), we designed a subject‐specific search strategy, and we used this as the basis for search strategies used in Embase, CENTRAL, and CINAHL. When appropriate, we supplemented the search strategy with search terms used to identify RCTs. All search strategies can be found in Appendix 1. We searched the bibliographic references and citations of relevant studies and systematic reviews for further potentially relevant studies. We applied no language or publication status restrictions.
Searching other resources
We also searched http://www.clinicaltrials.gov (18 January 2020) and http://apps.who.int/trialsearch/ (January 2020) to identify trials in progress. We screened the reference lists of all studies retained (during data extraction) and from other recently published systematic reviews related to the topic (December 2019). We screened conference proceedings of anaesthesiology societies for 2017, 2018, and 2019, as published in two major anaesthesiology journals: European Journal of Anaesthesiology (January 2020) and Regional Anesthesia and Pain Medicine (January 2020). In addition, we looked for abstracts on the website of the American Society of Anesthesiologists for the same years (2017 to 2019; American Society of Anesthesiologists 2020) (18 January 2020).
Data collection and analysis
Selection of studies
We independently assessed potentially eligible trials for inclusion. We resolved disagreements by discussion.
Data extraction and management
We independently extracted data for the outcomes listed above for all new trials and resolved differences through discussion. When we were unable to extract relevant data or information, we contacted the study authors for whom we could find an email address (N = 38; Albrecht 2014; Altermatt 2013; Antonopoulou 2006; Bang 2016; Brownbridge 2018; Cuvillon 2007; De La Tabla 2010; Diakomi 2014; Domac 2015; Fletcher 2003; Foss 2005a; Gille 2006; Godoy Monzon 2010; Graham 2008; Gürtan Bölükbasi 2013; Jadon 2014; Jang 2018; Kullenberg 2004; Landsting 2008; Liebmann 2012; Luger 2012; Ma 2018a; Madabushi 2016; Morrison 2008; Mosaffa 2005; Mouzopoulos 2009; Murgue 2006; Nie 2015; Ranjit 2016; Segado Jimenez 2009; Szucs 2010; Thompson 2019; Tuncer 2003; Unneby 2017; Uysal 2018; Wang 2015; Yamamoto 2016; Yun 2009).
Assessment of risk of bias in included studies
We evaluated the quality of all included studies using the new Cochrane Risk of Bias‐2 tool for each outcome (summary of findings Table 1) (last accessed July 2020; Sterne 2019).
-
Pain on movement at 30 minutes after block placement.
-
Acute confusional state (0 to 30 days).
-
Myocardial infarction (0 to 30 days).
-
Pneumonia (0 to 30 days).
-
Death (0 to 6 months).
-
Time to first mobilization (in‐hospital).
-
Cost of analgesic regimens for single‐injection PNBs (in‐hospital).
Risks of bias for all outcomes were independently assessed by two review authors with respect to the effect of assignment to the intervention at baseline. We first read the detailed guidance document (available at drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view). We completed a Word document template (available at drive.google.com/file/d/18Zks7k4kxhbUUlbZ51Ya5xYa3p3ECQV0/view) for each included trial and for each outcome to allow agreement between the two review authors. We settled any disagreement by discussion. Then, one review author (JG) entered data into the Excel tool (available at drive.google.com/file/d/1KSFASeBJP8FjBMpEbNlDiYxp4CKuOZgM/view). The Word document was converted into a PDF document and stored online in an open repository (Figshare) (Guay 2020).
Briefly, we considered bias arising from the following domains: bias in the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of outcomes, and bias in selection of the reported result. For each signalling question, we answered yes, probably yes, probably no, no, or no information, based on information retrieved from the reports or from the study authors. We inserted brief direct quotations into the text box to support those answers.
Subsequently, each outcome result was given an overall judgement for risk of bias.
-
Low risk of bias overall, if all domains for this result were assessed as ‘low’ risk.
-
Some concerns overall, if at least one domain for this result was assessed as ‘some concerns’ but none were assessed as ‘high’ risk.
-
High risk of bias overall, if at least one domain was assessed as ‘high’ risk, or if we had ‘some concerns’ about several domains that, when considered together, could indicate ‘high’ risk of bias.
Additional details can be found in Appendix 2.
We planned to evaluate risks of bias of cluster trials using the cluster trial extension for Risk of Bias‐2 (Eldridge 2016).
When possible, we mentioned the direction of the bias.
Measures of treatment effect
We presented results as risk ratios (RRs) or risk differences (RDs), along with the 95% confidence intervals (95% CIs) for dichotomous data, and as mean differences (MDs) and 95% CIs for continuous data. Although hazard ratio would have been optimal for time to event data (time to first mobilization; Deeks 2019), data were unfortunately not available in this format. If some of the continuous data were reported using different scales, or when results were not provided as mean and standard deviation (SD) (therefore extracted as P values), we produced the results as standardized mean differences (SMDs) and 95% CIs. For SMDs, we considered 0.2 to be a small effect, 0.5 to be a moderate effect, and 0.8 to be a large effect (Pace 2011). A clinical equivalence was calculated for results produced as SMD. When results for dichotomous data showed an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH), using the odds ratio. We provided results for dichotomous data as RRs as often as was feasible, as the odds ratio (OR) is not easily understood by clinicians (Deeks 2002; McColl 1998). We used the OR for calculation of NNTB and NNTH (http://www.nntonline.net/visualrx/), as this value is less likely to be affected by the side (benefit or harm) on which data are entered (Cates 2002; Deeks 2002). When we noted no effect, we calculated the optimal information size to make sure that enough participants were included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998; http://www.stat.ubc.ca/~rollin/stats/ssize/b2.html). We arbitrarily defined a difference of 25% (increase or decrease) as the minimal clinically relevant difference (Schünemann 2019).
Unit of analysis issues
If a trial included more than two groups, we fused two groups (by using the appropriate formula for adding standard deviations, when required) when we thought that they were equivalent according to the criteria chosen a priori for exploration of heterogeneity; we separated them and split the control group in half if we thought that they were different (Higgins 2019). For cluster trials, we planned to simply extract odds ratios and their confidence intervals when an appropriate analysis was used by study authors. If not, we planned to correct the sample sizes or inflate the standard errors, as recommended by Cochrane (Higgins 2019).
Dealing with missing data
We contacted study authors to ask for apparently missing data. We did not consider medians as equivalent to means. Instead, we used the P value and the number of participants included in each group to calculate the effect size. We did not use imputed results. We entered data as intention‐to‐treat (ITT) as much as was feasible in accordance with our choice for risk of bias assessment (i.e. "assignment to the intervention at baseline"). If this was not possible, we entered the data on a per‐protocol basis and took this into account in our risk of bias assessment.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results, and we examined statistical heterogeneity. We visually examined all forest plots. We quantified statistical heterogeneity by using the I² statistic with data entered in the way (benefit or harm) that yielded the lowest amount. We qualified the amount as follows: might not be important (0% to 40%), may represent moderate heterogeneity (30% to 60%), may represent substantial heterogeneity (50% to 90%), or considerable heterogeneity (75% to 100%), depending on the value obtained for the I2 statistic (Deeks 2019).
Assessment of reporting biases
We examined publication bias by using a funnel plot, then performed Duval and Tweedie’s trim and fill technique for each outcome. When publication bias is present, this technique yields an adjusted point of estimate that takes into account the number of theoretically missing studies.
Data synthesis
We analysed the data using RevMan 5.3 and Comprehensive Meta‐Analysis Version 2.2.044 (www.Meta-Analysis.com; visual inspection of forest plots with data placed in a specific order, Egger's regression intercept, Duval and Tweedie's trim and fill analysis, and meta‐regression) with fixed‐effect models. We avoided random‐effects models due to a large number of small studies. Random‐effects models give greater weight to small studies. We presented study characteristics in relevant tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies). We presented risk of bias assessments in graphs and results for each comparison as forests plots or narratively (in the case of comparisons with fewer than two available trials or for results with a high level of heterogeneity unexplained by heterogeneity exploration).
Subgroup analysis and investigation of heterogeneity
For exploration of heterogeneity, we focused specifically on comparisons with more than a small amount of heterogeneity (I2 > 40%) (Deeks 2019). We used Egger’s regression intercept to assess the possibility of a small‐study effect (Rucker 2011; Sterne 2001). We visually inspected forest plots with trials placed in order according to a specific moderator. If forest plots suggested a specific moderator to be relevant, we used subgroup analysis or meta‐regression with Comprehensive Meta‐Analysis Version 2.2.044 (www.Meta-Analysis.com).
We explored heterogeneity by conducting subgroup analysis based on the following categories.
-
Type of nerve block (e.g. psoas compartment, fascia iliaca, femoral nerve (we considered three‐in‐one and triple nerve blocks as femoral nerve blocks), lateral femoral cutaneous, obturator).
-
Single‐injection PNB versus continuous infusion.
-
Technique of localization (landmark, nerve stimulator, or ultrasound).
-
American Society of Anesthesiologists (ASA) physical status of participants.
We used meta‐regression for ages of participants included, year the study was published, and local anaesthetic concentration in lidocaine equivalent (used for single‐injection PNBs only and calculated as follows: lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3, and tetracaine = 4) (Berde 2009)).
Sensitivity analysis
We performed a sensitivity analysis based on risk of bias of the study, or if a study was a clear outlier, as long as a reason differentiating this study from the other studies (difference in study design, population, intervention, comparator, or outcome measurement) could be identified. For risk of bias, for each outcome, we reported the results obtained while excluding trials at high risk of bias based on overall risk of bias judgements.
Summary of findings and assessment of the certainty of the evidence
We used the principles of the GRADE approach to assess the certainty of evidence associated with all outcomes (pain on movement 30 minutes after block placement, acute confusional state, myocardial infarction, pneumonia, death, time to first mobilization, and cost of analgesic regimen for single PNBs) (Schünemann 2019), and we constructed summary of findings Table 1 using GRADEPro.
For uncertainty resulting from risk of bias, we judged the certainty of evidence as presenting low risk of bias when exclusion of trials at high risk of bias did not change the conclusion. We downgraded quality by one or two levels when excluding trials at high risk of bias changed the conclusion, or when evidence was based mainly on trials with multiple domains with some concerns.
For uncertainty resulting from inconsistency, we downgraded the certainty of evidence by one level when the I2 statistic was 50% or higher without satisfactory explanation, and by two levels when the I2 statistic was 75% or higher without an explanation. We also considered clinical heterogeneity as a potential contributor to inconsistency.
For uncertainty resulting from indirectness and applicability, we planned to downgrade the certainty of evidence if outcomes were not measured on the population of interest, involved differences in intervention (different setting or related interventions), involved differences in outcomes measures (surrogate markers) or were based on indirect comparisons (Schünemann 2013).
For uncertainty resulting from imprecision (Zhang 2019), we downgraded the certainty of evidence by one or two levels when the CI around the effect size was large or overlapped with absence of effect and failed to exclude an important benefit or harm, or when the number of participants was smaller than the optimal information size. The outcome itself was also taken into account.
For uncertainty resulting from publication bias, we downgraded the certainty of evidence by one level when correcting for the possibility of publication bias as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion.
Results
Description of studies
Characteristics of included studies, excluded studies, and ongoing trials can be found in Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables, respectively.
Results of the search
Details of the search for this update can be found in Figure 1. We found 477 titles from the Cochrane Central Register of Controlled Trials (CENTRAL), 211 from the Cumulative Index to Nursing and Allied Health Literature (CINHAL), 410 from Embase, and 418 from MEDLINE. Upon adding articles from the latest previously published version, titles from references lists of articles retained and from relevant reviews, conference proceedings, and ongoing trials, we retrieved 158 articles. We excluded 46 trials due to ineligible study design, 20 because they studied a different population, 40 because they studied a different intervention, and five because they were withdrawn or were terminated by study authors. Twenty‐nine trials were ongoing.

Flow diagram for the 2020 update.
CENTRAL: The Cochrane Central Register of Controlled Trials; CINHAL: Cumulative Index to Nursing and Allied Health Literature.
Included studies
We included 49 trials with 3061 participants; 1553 participants were randomized to PNBs and 1508 to no nerve block (or sham block). Forty‐three trials with 2750 participants could be included in the analysis: 1368 participants randomized to PNBs and 1382 randomized to no nerve block (or sham block).
Trials were published between 1980 and 2020 and were funded by a charitable organization (N = 5; Cuvillon 2007; Foss 2005a; Liebmann 2012; Ma 2018a; Unneby 2017), by a governmental organization (N = 5; Altermatt 2013; Jang 2018; Landsting 2008; Morrison 2008; Nie 2015), or by departmental/institutional resources (N = 16; Albrecht 2014; Bang 2016; Brownbridge 2018; Domac 2015; Gille 2006; Henderson 2008; Godoy Monzon 2010; Jadon 2014; Luger 2012; Madabushi 2016; Szucs 2010; Thompson 2019; Uysal 2018; Wang 2015; Yamamoto 2016; Yun 2009). Remaining trials did not specify the source of funding.
Some trials were registered at an official trial registry outside the institution (N = 13; Albrecht 2014; Altermatt 2013; Bang 2016; Brownbridge 2018; Diakomi 2014; Foss 2005a; Hogg 2009; Jang 2018; Landsting 2008; Liebmann 2012; Morrison 2008; Wang 2015; Yamamoto 2016).
Trials were performed in Argentina (N = 1; Godoy Monzon 2010), Austria (N = 1; Luger 2012), Canada (N = 1; Brownbridge 2018), Chile (N = 1; Altermatt 2013), China (N = 5; Graham 2008; Nie 2015; Ma 2018a; Wang 2015; Yang 2016), Denmark (N = 2; Foss 2005a; Spansberg 1996), France (N = 2; Cuvillon 2007; Murgue 2006), Greece (N = 3; Antonopoulou 2006; Diakomi 2014; Mouzopoulos 2009), Germany (N = 1; Gille 2006), India (N = 2; Jadon 2014; Madabushi 2016), Iran (N = 1; Mosaffa 2005), Ireland (N = 1; Szucs 2010), Israel (N = 1; Chudinov 1999), Japan (N = 1; Yamamoto 2016), Korea (N = 3; Bang 2016; Jang 2018; Yun 2009), Nepal (N = 1; Ranjit 2016), South Africa (N = 1; White 1980), Spain (N = 2; De La Tabla 2010; Segado Jimenez 2009), Sweden (N = 3; Kullenberg 2004; Landsting 2008; Unneby 2017), Switzerland (N =1; Albrecht 2014), Turkey (N = 5; Deniz 2014; Domac 2015; Gürtan Bölükbasi 2013; Tuncer 2003; Uysal 2018), United Kingdom (N = 6; Coad 1991; Fletcher 2003; Haddad 1995; Hogg 2009; Hood 1991; Jones 1985), and United States of America (N = 4; Henderson 2008; Liebmann 2012; Morrison 2008; Thompson 2019).
The average age of participants ranged from 59 to 89 years. Participants included had an American Society of Anesthesiologists (ASA) physical status between I and IV. The proportion of included females varied between 33% and 95%. The proportion of arthroplasty varied between 0 and 100%.
Details of the PNBs, anaesthetic techniques, comparators, and rescue analgesics used are included in Table 1.
| Study | Purpose of blockade | Time of block placement | Surgical anaesthesia | Block technique | Comparison | Supplemental analgesia for both groups |
| Preoperative analgesia | In the emergency department | No information | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.5% with epinephrine 1:200,000 30 mL Operator: trained emergency physicians | Sham block with normal saline | Acetaminophen Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | Preoperatively, probably in the emergency department | Spinal anaesthesia | Psoas compartment block Nerve stimulator (quadriceps contraction at 0.5 mA, 1 Hz, Continuous infusion Bupivacaine 0.1% 20 mL followed by patient‐controlled analgesia: basal rate 8 mL/hour, bolus 5 mL, lock‐out time 30 minutes for 72 hours Operator: no information | No nerve block IV PCA with Morphine | Acetaminophen Ketorolac | |
| Postoperative analgesia | After recovery of anaesthesia | Spinal anaesthesia | Femoral nerve block Nerve stimulator Continuous infusion Levobupivacaine 0.25% 18 mL followed by levobupivacaine 0.125% at 3 to 4 mL/hour for 24 hours after surgery Operator: no information | No nerve block | Acetaminophen Pethidine | |
| Postoperative analgesia | After surgery and after confirmation of patient’s mental status to be alert, able to communicate, and obey commands | Spinal anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Single injection Ropivacaine 0.2% 40 mL Operator: no information | No nerve block | Ketorolac Celecoxib IV PCA with Fentanyl Tramadol | |
| Preoperative, intraoperative, and postoperative analgesia | Preoperatively, after patients had been assigned to a bed on the ward | Spinal (53% for intervention group and 40% for comparator group) or general anaesthesia | Fascia iliaca compartment block Landmarks Continuous infusion Ropivacaine 0.125% 40 mL followed by ropivacaine 0.2% 10 mL/hour until surgery. In the operating room, catheters were Operator: anaesthesiology department | No nerve block | Acetaminophen NSAIDs Opioids | |
| Preoperative, intraoperative, and postoperative analgesia Surgery for some participants | Preoperatively, within 6 hours after admission to the orthopaedic ward | Intervention: psoas block alone (3/20) with sciatic block (5/20), spinal (11/20) or general anaesthesia (1/20) Comparator: neuraxial block (19/20) or general anaesthesia (1/20) | Psoas compartment block Landmarks and loss of resistance to air, lateral decubitus Continuous infusion: started preoperatively (16 to 48 hours) and kept for 72 hours after surgery Test dose with 3 mL of 0.5% bupivacaine with epinephrine 5 mcg/mL followed by bupivacaine 0.25% with epinephrine Operator: anaesthesiologists | No nerve block IM Meperidine Diclofenac | IM Meperidine | |
| Postoperative analgesia | At completion of surgery before awakening from general anaesthesia | General anaesthesia | 1) Lateral femoral cutaneous nerve block Landmarks Single injection 1) Bupivacaine 0.5% with epinephrine 5 mcg/mL 15 mL Operator: anesthesiology department | No nerve block | Pethidine | |
| Postoperative analgesia | After ending of effects of spinal blockade | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps for patella ascension with 0.3 to 0.5 mA at 0.1 ms and catheter 10 to 15 cm passed over the needle tip) Continuous infusion Lidocaine 1.5% plus epinephrine 30 mL of lidocaine 1.5% followed by ropivacaine 0.2% at 10 mL/hour for 48 hours Operator: anesthesiology department | No nerve block IV Paracetamol for half of participants in the comparator group | 1 dose of paracetamol in the emergency department Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | Upon hospital arrival | No information | Femoral nerve block Dual technique: ultrasound‐guided plus nerve stimulator Continuous infusion Ropivacaine 0.2% 15 mL followed by ropivacaine 0.2% at 5 mL/hour basal rate plus boluses of 10 mL every 30 Operator: no information | No nerve block IV Metamizole | IV Tramadol | |
| Intraoperative and postoperative analgesia | In the operating room, before induction of general anaesthesia | General anaesthesia | 1) Fascia iliaca compartment block 1) Ultrasound‐guided 2) Dual technique: ultrasound‐guided plus nerve stimulator Single injection 1) Bupivacaine 0.25% 30 mL Operator: anesthesiology department | No nerve block | Tenoxicam IV PCA with Tramadol | |
| Spinal positioning, intraoperative and postoperative analgesia | Before positioning for spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.5% 40 mL Operator: anesthesiology department | No nerve block IV Fentanyl for positioning for spinal block | IV PCA with Morphine | |
| Spinal positioning, intraoperative and postoperative analgesia | In the regional anaesthetic technique room, before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.5% 15 mL and lidocaine 2% 15 mL Operator: anesthesiology department | No nerve block | IV PCA with Morphine Tramadol | |
| Preoperative analgesia | In the emergency department, after radiographic confirmation | No information | 3‐in‐1 femoral nerve block Paraesthesia Single injection Bupivacaine 0.5% 20 mL Operator: trained emergency physicians | No nerve block | IV Morphine | |
| Preoperative analgesia | Upon arrival in the emergency department | No information | Fascia iliaca compartment block Landmarks Single injection Mepivacaine 1% with epinephrine 5 mcg/mL 40 mL Operator: junior anaesthesiologists with less than 2 years of training | Sham block with 0.9% saline plus IM Morphine | IV Morphine Epidural analgesia after 3‐hour study period | |
| Preoperative, intraoperative. and postoperative analgesia | Upon arrival in the emergency department | Intervention: spinal anaesthesia for 37/50 and general anaesthesia for 13/50 Comparator: spinal anaesthesia for 38/50 and general anaesthesia for 12/50 | Femoral nerve block Nerve stimulator (0.5 mA and 0.1 millisecond) Continuous infusion (non‐stimulating catheters advanced about 10 cm past the needle tip) Prilocaine 1% 40 mL followed 2 hours later by ropivacaine 0.2% 30 mL, repeated every 6 hours (up to 40 mL; N = 5) and at intervals (up to every 4 hours; N = 8) or both (N = 6), adjusted on pain scores Operator: anaesthesiology department | No nerve block IV Metamizole Oral Tilidine and Naloxone | Ibuprofen Tilidine | |
| Preoperative analgesia | In the emergency department, after confirmation of diagnosis | No information | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.25% 0.3 mL/kg Operator: physicians (first study author is an orthopaedic surgeon) | Sham block with saline and IV NSAIDs | NSAIDs Opioids | |
| Preoperative analgesia | In the emergency department | No information | Femoral (3‐in‐1) nerve block Single injection Nerve stimulator Bupivacaine 0.5% 30 mL (not exceeding 3 mg/kg) Operator: specialist emergency physician or higher trainee resident, post intermediate examination level | No nerve block IV Morphine | IV Morphine Dihydrocodeine Diclofenac Paracetamol | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | No information | Fascia iliaca compartment block Single injection Ultrasound‐guided Levobupivacaine 0.375% 30 mL Operator: anesthesiology department | No nerve block IV Remifentanil | Additional analgesia | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Single injection Bupivacaine 0.25%.0.3 mL/kg Paraesthesia technique with a short bevel needle Operator: 1 orthopaedic registrar | No nerve block | Co‐dydramol Voltarol Pethidine | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Nerve stimulator Single injection Bupivacaine 0.5% Operator: trained emergency physicians | No nerve block | Opioids | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block No information on localizing technique Single injection Lidocaine 1% 2 mg/kg Operator: anaesthesiology department | No nerve block IV Ketamine 0.2 mg/kg IV Midazolam 0.025 mg/kg | Ketamine | |
| Intraoperative and postoperative analgesia | Before induction of general anaesthesia | General anaesthesia | 1) Femoral "3‐in‐1" nerve block 1) Nerve stimulator (quadriceps contraction with < 1 mA) 2) Landmarks Single injection 1) Prilocaine 0.75% 35 mL Operator: anaesthesiology department | No nerve block | Papaveratum | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps contraction with 0.3 to 0.5 mA) Single injection Lidocaine 1.5% (2% diluted with distilled water) with epinephrine 5 mcg/mL 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | IV Fentanyl | |
| Preoperative analgesia | In the emergency department, 48 hours before surgery | No information | Femoral nerve block Single injection Ultrasound‐guided (in‐plane) Bupivacaine 0.5% 0.3 mL/kg (maximum 20 mL) Operator: 1 physician experienced in administering ultrasound‐guided femoral nerve blocks | Sham block with saline | IV Tramadol | |
| Postoperative analgesia | At completion of surgery, while still under general anaesthesia | General anaesthesia | Lateral femoral cutaneous nerve block Single injection Landmarks Bupivacaine 0.5% with epinephrine 5 mcg/mL 15 mL Operator: anaesthesiology department | No nerve block | IM Pethidine | |
| Preoperative analgesia | As soon as the diagnosis of hip fracture was radiologically confirmed | No information | Femoral nerve block Nerve stimulator Single injection Ropivacaine 0.75% 30 mL. Operator: 1 orthopaedic surgeon | No nerve block | Paracetamol Tramadol Ketobemidon | |
| Preoperative analgesia | Within 1 hour of hospital admission | No information | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.2% 30 mL Operator: orthopaedic surgeons | Sham block with saline | IV Morphine Paracetamol | |
| Preoperative analgesia | In the emergency department | No information | 3‐in‐1 femoral nerve block Ultrasound‐guided (in‐plane) Single injection Bupivacaine 0.5% 25 mL Operator: emergency physicians experienced with the technique | Sham block with saline | Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | In the emergency department | Spinal anaesthesia | Femoral "3‐in‐1" nerve block Ultrasound‐guided Continuous infusion (catheters inserted ≥ 12 to 15 cm past the needle tip) Bupivacaine 0.25% 30 mL (additional 10 mL if required for adequate sensory blockade) followed by bupivacaine Operator: anesthesiology department | No nerve block | Piritramide Paracetamol | |
| Preoperative analgesia | After hospital admission | No information | Fascia iliaca compartment block Ultrasound‐guided (in‐plane) Continuous infusion (catheters 5 to 10 cm beyond the tip of the needle) Ropivacaine 0.4% 30 mL followed by ropivacaine 0.2% at 5 mL/hour plus 5 mL for breakthrough pain until surgery (mean 3.5 days). Catheters removed on the morning of surgery Operator: 1 anaesthesiologist experienced in ultrasound‐guided nerve block | No nerve block | Tramadol Acetaminophen Pethidine | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.375% 30 mL Operator: anaesthesiologists | No nerve block IV Fentanyl | Paracetamol Tramadol Diclofenac | |
| Preoperative analgesia, intraoperative and postoperative analgesia | In the emergency department for femoral nerve block and within 24 hours of femoral block for continuous fascia iliaca block | Regional anaesthesia for 62.1% | 1) Femoral nerve block Ultrasound‐guided (out‐of‐plane for insertion, but advancement visualized) 1) Single injection Bupivacaine 0.5% 20 mL 2) Continuous infusion Ropivacaine 0.2% 15 mL followed by 5 mL/hour for 72 hours after surgery Operators: 1) Trained emergency physicians 2) Anaesthesiologists (mobile peripheral nerve block service) | No nerve block | Opioids Acetaminophen | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca block with 20 mL of 1.5% lidocaine No information for localizing technique Single injection Lidocaine 1.5% 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | No information | |
| Preoperative and postoperative analgesia | Started upon admission to the orthopaedic ward | Epidural anaesthesia | Fascia iliaca compartment blocks daily (from admission until surgery, restarted at 24 hours after surgery until discharge, stopped earlier (before or after surgery) if delirium occurred) Landmarks Bupivacaine 0.3 mL/kg (0.25%?) Operator: orthopaedic surgeons | Sham blocks with water | IV Paracetamol Pethidine | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Nerve stimulator (quadriceps contraction with patellar ascension) Single injection Mepivacaine 20 mL Operator: unclear, published by emergency physicians | No nerve block IV Morphine or IV Paracetamol and Ketoprofen | Nitrous oxide | |
| Postoperative analgesia | After closure of the surgical wound | General anaesthesia | Fascia iliaca block Landmarks Continuous infusion (catheter inserted ≥ 10 cm cranially) Ropivacaine 0.5% according to body weight (20 mL if Operator: no information, probably anaesthesiology department | No nerve block IV PCA with Fentanyl | Acetaminophen Dihydrocodeine Morphine | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Femoral nerve block Dual technique: nerve stimulator plus in‐plane ultrasound Single injection Lidocaine 2% 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | IV Fentanyl | |
| Postoperative analgesia | In post‐anaesthesia care unit after full recuperation of motor blockade from the spinal block | Spinal anaesthesia | 1) Lateral femoral cutaneous nerve block Landmarks Single injections 1) Bupivacaine 0.5% with vasoconstrictor 5 mL Operator: anaesthesiology department | No nerve block | IV Metamizole Dexketoprofen trometamol Tramadol Morphine | |
| Postoperative analgesia | Catheters inserted before spinal anaesthesia Administration of local anaesthetics started after surgery | Spinal anaesthesia | Femoral nerve block Nerve stimulator Continuous infusion (non‐stimulating catheter advanced 8 to 15 cm past needle tip) Bupivacaine 0.5% 0.4 mL/kg followed by bupivacaine 0.25% at 0.14 mL/kg/hour for 16 hours after surgery Operator: anaesthesiology department | Sham block with saline | Morphine Acetylsalicylic acid | |
| Preoperative, intraoperative, and postoperative analgesia | Catheters inserted in the emergency department Administration of local anaesthetics started during catheter installation | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps contraction resulting in Continuous infusion (non‐stimulating catheter, space dilated with 10 mL of lidocaine 2%, catheter advanced cephalad 3 cm past the needle tip) Bupivacaine 0.5% 10 mL followed by 0.25% bupivacaine at 4 mL/hour for 72 hours Operator: anaesthesiology department | No nerve block | Paracetamol Morphine | |
| Intraoperative and postoperative analgesia | Immediately before induction of anaesthesia | General or spinal anaesthesia (38%) | Fascia iliaca compartment block Ultrasound‐guided Single injection Ropivacaine 0.25% 30 mL Operator: a board‐certified anaesthesiologist | No nerve block | Acetaminophen Tramadol Opioids | |
| Postoperative analgesia | After surgery and reversal of neuromuscular blockade | General anaesthesia | Femoral (3‐in‐1) nerve block Nerve stimulator (quadriceps contraction with patellar ascension with < 1 mA) Continuous infusion (non‐stimulating catheter advanced 4 to 5 cm past the needle tip) Lidocaine 2% with epinephrine 5 mcg/mL 30 mL followed by bupivacaine 0.125% patient‐controlled analgesia: basal rate 4 mL/hour, boluses 3 mL, lockout time 20 minutes Operator: probably anaesthesiology department | No nerve block IV PCA with Morphine | Tenoxicam | |
| Preoperative analgesia | Before surgery, as soon as possible after admission to the orthopaedic ward | No information | Femoral nerve block Nerve stimulator (quadriceps contraction) Single injection Levobupivacaine 0.25% 20 to 40 mL In case of delayed surgery or if otherwise necessary, participants could receive 1 additional block Operator: 36 anaesthesiologists with various training | No nerve block | Opioids | |
| Preoperative analgesia | In the emergency department | Spinal anaesthesia | Femoral nerve block | No nerve block IV Paracetamol | IV Tramadol Epidural analgesia after surgery | |
| Preoperative, intraoperative, and postoperative analgesia | Upon admission, after radiographic confirmation of the diagnosis | Combined spinal‐epidural anaesthesia | Fascia iliaca compartment block Ultrasound‐guided (out‐of‐plane for needle insertion and in‐plane for solution diffusion, injected cephalad) Continuous infusion (catheter inserted 5 to 10 cm past the needle tip) Ropivacaine 0.4% 50 mL followed by ropivacaine 0.2% at 5 mL/hour (plus 5 mL top‐up doses) Operator: anaesthesiologist with experience in ultrasound‑guided nerve block | Sham block with saline Paracetamol Tramadol | IVPCA with Sufentanil after surgery | |
| Intraoperative and postoperative analgesia | After induction of anaesthesia, before surgery | General anaesthesia | Psoas compartment block Landmarks Single injection Mepivacaine 2% 30 mL Operator: anaesthesiology department | No nerve block | Usual surgical care | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Single injection Levobupivacaine 0.25% 40 mL Operator: an orthopaedic surgeon with extensive experience in this block procedure | No nerve block IV Acetaminophen | Diclofenac Rescue analgesics | |
| Intraoperative and postoperative analgesia | Catheter insertion and local anaesthetic administration started before induction of anaesthesia | General anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Continuous infusion Ropivacaine 0.33% 30 mL followed by 0.15% ropivacaine at 2 mL/hour plus a bolus of 30 mL Operator: anaesthesiology department | No nerve block IV PCA with Sufentanil | Rescue analgesics | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.375% 30 mL Operator: 1 experienced anaesthesiologist | No nerve block IV Alfentanil | IV Alfentanil for spinal block Pethidine before spinal block and after surgery |
G: gram.
h: hour.
IM: intramuscular.
IV: inteavenous.
mA: milliAmpere.
mcg/mL: microgram/millilitre.
mg/kg: milligram/kilogram.
MHz: megahertz.
mL: millilitre.
msec: millisecond.
n: number.
NSAIDs: non‐steroidal anti‐inflammatory drugs.
PCA: patient‐controlled analgesia.
SC: subcutaneous.
PNBs performed included a femoral nerve block (femoral or three‐in‐one block or triple nerve block) (N = 22; Antonopoulou 2006; Coad 1991; Cuvillon 2007; De La Tabla 2010; Deniz 2014; Fletcher 2003; Gille 2006; Graham 2008; Haddad 1995; Henderson 2008; Jadon 2014; Jang 2018; Kullenberg 2004; Liebmann 2012; Luger 2012; Murgue 2006; Ranjit 2016; Spansberg 1996; Szucs 2010; Tuncer 2003; Unneby 2017; Uysal 2018), a femoral nerve block plus an infiltration above the iliac crest (N = 1; Hood 1991), a femoral nerve block followed by a fascia iliaca block (N =1; Morrison 2008), a fascia iliaca compartment block (N = 21; Albrecht 2014; Bang 2016; Brownbridge 2018; Deniz 2014; Diakomi 2014; Domac 2015; Foss 2005a; Godoy Monzon 2010; Gürtan Bölükbasi 2013; Hogg 2009; Landsting 2008; Ma 2018a; Madabushi 2016; Mosaffa 2005; Mouzopoulos 2009; Nie 2015; Thompson 2019; Wang 2015; Yamamoto 2016; Yang 2016; Yun 2009), a lateral femoral cutaneous nerve block (N = 2; Coad 1991; Jones 1985), a lateral femoral cutaneous nerve block plus an obturator nerve block (N = 1; Segado Jimenez 2009), an obturator nerve block (N = 1; Segado Jimenez 2009), or a psoas compartment block (N = 3; Altermatt 2013; Chudinov 1999; White 1980).
Techniques of localization used for PNBs included loss of resistance to air (N = 1; Chudinov 1999), use of nerve stimulator (N = 14; Altermatt 2013; Antonopoulou 2006; Cuvillon 2007; Gille 2006; Graham 2008; Henderson 2008; Hood 1991; Jadon 2014; Kullenberg 2004; Murgue 2006; Spansberg 1996; Szucs 2010; Tuncer 2003; Unneby 2017), paraesthesia (N = 2; Fletcher 2003; Haddad 1995), ultrasound with or without a nerve stimulator (N = 15; Bang 2016; De La Tabla 2010; Deniz 2014; Gürtan Bölükbasi 2013 ; Jang 2018; Liebmann 2012; Luger 2012; Ma 2018a; Morrison 2008; Ranjit 2016; Thompson 2019; Uysal 2018; Wang 2015; Yamamoto 2016; Yang 2016), or landmarks (N = 15; Albrecht 2014; Brownbridge 2018; Coad 1991; Diakomi 2014; Domac 2015; Foss 2005a; Godoy Monzon 2010; Jones 1985; Landsting 2008; Madabushi 2016; Mouzopoulos 2009; Nie 2015; Segado Jimenez 2009; White 1980; Yun 2009). Hogg 2009 and Mosaffa 2005 provided no information on the localizing technique.
PNBs were single‐injection PNBs or continuous PNBs (infusion or repeated doses) (N = 17; Altermatt 2013; Antonopoulou 2006; Brownbridge 2018; Chudinov 1999; Cuvillon 2007; De La Tabla 2010; Gille 2006; Luger 2012; Ma 2018a; Morrison 2008; Mouzopoulos 2009; Nie 2015; Spansberg 1996; Szucs 2010; Tuncer 2003; Wang 2015; Yang 2016) given for a duration ranging from 15 to 72 hours.
Investigators performed PNBs for preoperative analgesia (N = 14; Albrecht 2014; Fletcher 2003; Foss 2005a; Godoy Monzon 2010; Graham 2008; Haddad 1995; Henderson 2008; Jang 2018; Kullenberg 2004; Landsting 2008; Liebmann 2012; Ma 2018a; Murgue 2006; Uysal 2018); for preoperative, intraoperative, and postoperative analgesia (N = 10; Altermatt 2013; Brownbridge 2018; Chudinov 1999; De La Tabla 2010; Gille 2006; Luger 2012; Morrison 2008; Szucs 2010; Unneby 2017; Wang 2015); for spinal positioning and intraoperative and postoperative analgesia (N = 10; Diakomi 2014; Domac 2015; Gürtan Bölükbasi 2013; Hogg 2009; Jadon 2014; Madabushi 2016; Mosaffa 2005; Ranjit 2016; Yamamoto 2016; Yun 2009); for preoperative and postoperative analgesia (N = 1; Mouzopoulos 2009); for intraoperative and postoperative analgesia (N = 5; Deniz 2014; Hood 1991; Thompson 2019; White 1980; Yang 2016); or for postoperative analgesia (N = 9; Antonopoulou 2006; Bang 2016; Coad 1991; Cuvillon 2007; Jones 1985; Nie 2015; Segado Jimenez 2009; Spansberg 1996; Tuncer 2003). Exact time of block placement can be found in Table 1.
Excluded studies
We excluded 46 studies based on study design (Akhtar 2015; Arsoy 2017; Arsoy 2017a; Barnes 2019; Beaudoin 2010; Bendtsen 2015b; Callear 2016; Candal‐Couto 2005; Castillon 2017; Chang 2011; Christos 2010; Dulaney‐Cripe 2012; Elkhodair 2011; Evans 2019; Finlayson 1988; Foss 2009; Fujihara 2013; Godoy Monzon 2007; Gosavi 2001; Gozlan 2005; Grigg 2009; Groot 2015; Haines 2012; Hauritz 2009; Helsø 2016; Hogh 2008; Irwin 2012; Isalgue 2014; Ishioka 2018; Kassam 2018; Klukowski 2017; Kumar 2016; Kumie 2015; Leeper 2012; Levente 2017; Lopez 2003; McGlone 1987; Perrier 2010; Randall 2008; Rapchuk 2013; Rojas Rivera 2002; Tao 2016; Thakur 2018; Vats 2016; Wang 2019; Williams 2016); 20 trials because they studied a different population (Anaraki 2012; Bhadani 2017; Bulger 2015; Carlisle 2004; Durrani 2013; Iamaroon 2010; Kacha 2018; Levine 2003; Li 2013; Masoumi 2014; McRae 2015; Memary 2015; Mostafa 2015; Mutty 2007; Pakhare 2016; Reddy 2016; Segado Jimenez 2010; Shi 2018; Sia 2004; Singh 2016); and 40 trials because they studied a different intervention (Amini 2012; Amiri 2012; Aprato 2018; Bech 2011; Bendtsen 2015a; Bhattacharya 2019; Bouhours 2010; Dodd 2019; Foss 2005; Gasanova 2019; George 2016; Ghimire 2015; Gorodetskyi 2007; Hao 2018; Hoffmann 2015; Hussain 2014; Inan 2009; Kang 2013; Kristek 2019; Lee 2015; Lee 2016; Li 2013; Mannion 2005; Manohara 2015; Marhofer 1998; Matot 2003; Nielsen 2015; Parras 2016; Piangatelli 2004; Rashwan 2013; Reavley 2015; Sahota 2011; Scheinin 2000; Sonawane 2019; Swart 2017; Turker 2003; Van Leeuwen 2000; Wei 2018; Zadeh 2015; Zheng 2017). Five trials were either terminated or withdrawn by study authors (Bendtsen 2014; Bendtsen 2015; Hallberg 2012; Siguira 2014; WHO Int 2007). Details on reasons for exclusion can be found in Characteristics of excluded studies tables.
Studies awaiting classification
We have no studies awaiting classification.
Ongoing studies
We found 29 ongoing trials (Capelleri 2017; Carvalho 2015; Chinachoti 2010; Chiu 2016; ClinicalTrials.gov 2019; Compere 2012; Cong 2016; Dhimar 2017; Diakomi 2015; El Sharkawy 2016; Kulkarni 2018; Levins 2006; Li 2018; Luo 2019; Mathijssen 2015; Nguyen 2018; Park 2009; Postma 2017; Qiu 2018; Ridderikhof 2015; Saga 2019; Sahiti 2019; Shah 2016; Tsui 2015; Winso 2009; Xi 2014; Xuesheng 2019; Yuan 2017; Yun 2018). Details on ongoing trials can be found under Characteristics of ongoing studies. Fifteen trials were first posted (N = 10; ClinicalTrials.gov 2019; Kulkarni 2018; Li 2018; Luo 2019; Nguyen 2018; Qiu 2018; Saga 2019; Sahiti 2019; Xuesheng 2019; Yun 2018), or they were at least last updated (N = 5; Capelleri 2017; Dhimar 2017; Diakomi 2015; Postma 2017; Ridderikhof 2015), after 1 January 2018.
Risk of bias in included studies
A summary of the risks of bias of studies included in each analysis can be found in forest plots of each outcome (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7). Risk of bias assessments for each outcome, including all domain judgements and support for judgement, is located in the Risk of bias section (located after the Characteristics of included studies). Additional details on how the Risk of Bias‐2 tool was applied for each trial for each outcome can be found in the supplemental data file available in Figshare (Guay 2020).
Briefly, the number of results at high risk of bias was low. Reasons to judge risk of bias as high were: possible problems with randomization (one trial), missing data and inability to determine whether or not missingness was related to the outcome (one trial for pain on movement at 30 minutes after block placement and one trial for acute confusional state), deviation from pre‐planned analysis (one trial for pain on movement at 30 minutes after block placement), and possible unplanned outcome at the specific time point measured (one trial for mortality). Details on the implications of assessments of risk of bias for each specific result are reported in the Effects of interventions section.
Effects of interventions
See: Summary of findings 1 Peripheral nerve blocks for hip fracture
Primary outcomes
1. Pain
1.1 Pain on movement and at rest within 30 minutes after block placement
Pain on movement at 30 minutes after block placement
We did not retain data from three studies for this analysis due to inappropriate timing of outcome measurement. Jadon 2014 evaluated pain scores during positioning for spinal anaesthesia five minutes after a femoral nerve block performed with a nerve stimulator and 20 mL of a solution containing 15 mL of lidocaine 2% and 5 mL of distilled water. Parkinson 1989 reported that at five minutes after a femoral nerve block with lidocaine‐HCl and a nerve stimulator, only 6 and 11 participants out of 20 would have a complete or partial femoral nerve block, and 15 minutes would be required for a complete or partial femoral nerve block in all participants. Mosaffa 2005 evaluated pain scores during positioning for spinal anaesthesia five minutes after a fascia iliaca block with 20 mL of lidocaine 1.5%. Although some effects on pain scores can be seen at 10 minutes after a fascia iliaca block with lidocaine, maximal effects are more likely to occur at 30 minutes or later (Dochez 2014; Gozlan 2005). For Brownbridge 2018, the exact time point was unclear.
We retained 11 trials that included 503 participants and evaluated pain on movement within 30 minutes after block placement (Albrecht 2014; Diakomi 2014; Domac 2015; Foss 2005a; Gille 2006; Hogg 2009; Landsting 2008; Murgue 2006; Ranjit 2016; Szucs 2010; Yun 2009). The specific intervention was a femoral nerve block ‐ Gille 2006; Murgue 2006; Ranjit 2016; Szucs 2010 ‐ or a fascia iliaca block ‐ Albrecht 2014; Diakomi 2014; Domac 2015; Foss 2005a; Hogg 2009; Landsting 2008; Yun 2009. Pain scores were lower with PNBs (standardized mean difference (SMD) ‐1.05, 95% CI ‐1.25 to ‐0.86; I2 = 83%; Analysis 1.1; Figure 2). There was no statistical difference between a femoral nerve block versus a fascia iliaca block (P value for difference between subgroups 0.16). On the basis of a typical standard deviation in the control group of one study (2.4 (Diakomi 2014)), this was equivalent to ‐2.5 on a scale from 0 to 10.

We identified possible significant risk of bias for two trials for this outcome (Figure 2). Landsting 2008 was judged as at high risk of bias for bias due to missing outcome data, as results for this outcome were available for 33 out of 66 participants randomized to the intervention group and for 38 out of 61 participants randomized to the comparator group. No information was provided on possible differences between participants with and without missing values. We had no information to help us determine whether or not missingness in the outcome could depend on its true value. Albrecht 2014 was judged as at high risk of bias in selection of the reported result due to the fact that study authors elected to deviate from the original planned analysis when they realized that the two groups had different mean baseline scores.
When the two trials at high risk of bias for this outcome were excluded (Albrecht 2014; Landsting 2008), SMD was ‐1.12 (95% CI ‐1.34 to ‐0.90). Egger's regression intercept showed the possibility of a small‐study effect as a source of heterogeneity (P = 0.03; 2‐tailed). Duval and Tweedie's trim and fill analysis showed the possibility of publication bias. Correcting for the possibility of publication bias would give an SMD of ‐0.88 (95% CI ‐1.07 to ‐0.70; Figure 3). Excluding trials at high risk of bias and one study that did not provide the exact concentration of local anaesthetic injected ‐ Murgue 2006 ‐ led to an effect size that was correlated with the concentration of local anaesthetic used in lidocaine equivalent (P = 0.0003; Figure 4). We calculated equivalences as mentioned in the methods section (i.e. lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3, and tetracaine = 4) (Berde 2009). Therefore, for Diakomi 2014, the concentration in lidocaine equivalent was calculated as 15 mg/mL (ropivacaine 0.5% or ropivacaine 5 mg/mL multiplied by 3 = 15 mg/mL). For Domac 2015, the concentration in lidocaine equivalent was calculated as 20 mg/mL (mixture of 15 mL bupivacaine 0.5% or bupivacaine 5 mg/mL multiplied by 4 = 20 mg/mL and 2% lidocaine or lidocaine 20 mg/mL). For Foss 2005a, the equivalence was calculated as 8 mg/mL (mepivacaine 1% or mepivacaine 10 mg/mL multiplied 0.8 = 8 mg/mL). For Gille 2006, the lidocaine equivalent was calculated as 9 mg/mL (1% prilocaine or prilocaine 10 mg/mL multiplied by 0.9 = 9 mg/mL). For Hogg 2009, the solution injected was lidocaine 1% (or 10 mg/mL). For Ranjit 2016, the solution injected was lidocaine 2% (or 20 mg/mL). For Szucs 2010, the equivalence was calculated as 20 mg/mL (10 mL of 2% lidocaine or lidocaine 20 mg/mL and 10 mL of 0.5% bupivacaine or bupivacaine 5 mg/mL multiplied by 4 = 20 mg/mL). For Yun 2009, the equivalence was calculated as 11.25 mg/mL (ropivacaine 0.375% or ropivacaine 3.75 mg/mL multiplied by 3 = 11.25 mg/mL). Results from Diakomi 2014 (mean and SD of the control group 7.5 and 2.4) show that 182 participants (91 per group) would be required in a simple trial to eliminate a difference of 1 on a 0 to 10 scale (alpha 0.05; beta 0.2; two‐sided test) (http://stat.ubc.ca/~rollin/stats/ssize/n2a.html).
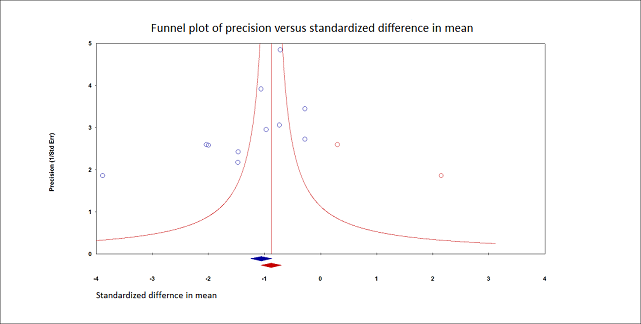
Pain on movement at 30 minutes after block placement.
Duval and Tweedie's trim and fill analysis: blue circles indicate studies found, and red circles are imputed studies. Correcting for the possibility of publication bias would give an estimated standardized mean difference of ‐0.88 (95% confidence interval ‐1.07 to ‐070).
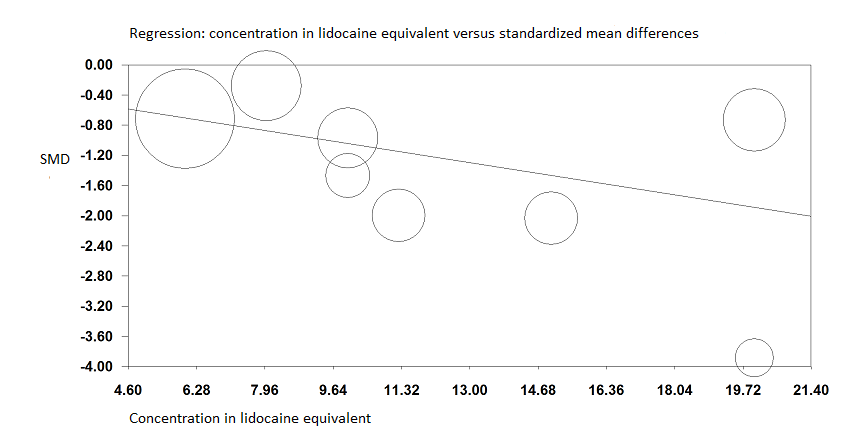
Pain on movement at 30 minutes after block placement.
A meta‐regression indicates that the effect size was proportional to the concentration of local anaesthetic injected in lidocaine equivalents; P = 0.0003.
Level of certainty for pain on movement at 30 minutes after block placement
We did not downgrade for risk of bias because the effect was still present when trials at high risk of bias were excluded from the analysis. We did not downgrade the level of certainty on the basis of inconsistency because we found a reasonable explanation for heterogeneity. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. The optimal information size was achieved. We did not downgrade for publication bias because the effect was still present after correction for this possibility. We rated the level of certainty as high.
2. Acute confusional state
We have provided in Appendix 3 definitions for acute confusional state used by study authors. Based on 13 trials with 1072 participants (Brownbridge 2018; Cuvillon 2007; Godoy Monzon 2010; Graham 2008; Kullenberg 2004; Liebmann 2012; Morrison 2008; Mouzopoulos 2009; Nie 2015; Uysal 2018; White 1980 ; Yamamoto 2016; Yang 2016), the risk of acute confusional state was reduced by the use of PNBs (RR 0.67, 95% CI 0.50 to 0.90; I2 = 16%; Analysis 1.2; Figure 5). There was no statistical difference according to the type of localizing technique used (landmark versus nerve stimulation versus ultrasound guidance; P value for difference between subgroups 0.75).

Forest plot of comparison: 1 Nerve block versus other modes of analgesia, outcome: 1.11 Acute confusional state.
Godoy Monzon 2010 was judged as at high risk of bias for this outcome due to a large quantity of missing data in the comparator group yielding two very unequal groups (i.e. 92 for the intervention group and 62 for the comparator group). We had no information to help us determine whether or not missingness in the outcome could depend on its true value. Excluding Godoy Monzon 2010, the estimate would be RR 0.70 (95% CI 0.52 to 0.95; I2 = 9%).
Egger's regression intercept showed no evidence of small‐study effect. Duval and Tweedie's trim and fill analysis calculated that two trials might be missing to right of mean for an adjusted point of estimate of RR 0.70 (95% CI 0.51 to 0.94; Figure 6). Given a rate of 30% (Arshi 2018), the number of participants required in a large trial to eliminate a 25% decrease would be 850 (425 per group) (alpha 0.05; beta 0.2; one‐sided test). The NNTB was 12 (95% CI 7 to 47).

Acute confusional state.
Duval and Tweedie's trim and fill analysis: blue circles indicate studies found, and red circles are imputed studies. Correcting for the possibility of publication bias would give an estimated risk ratio 0.70 (95% CI 0.51 to 0.94).
Level of certainty for acute confusional state
We did not downgrade the level of certainty for risk of bias because the effect was still present when we excluded the trial at high risk of bias. We did not downgrade for heterogeneity (I2 < 25%). We included only direct comparisons performed on the population of interest, and this is not a surrogate marker. We did not downgrade for imprecision because the optimal information size was achieved. We did not downgrade the level of certainty on the basis of the possibility of publication bias because applying a correction for the possibility of one would not modify the conclusion. We rated the level of certainty of evidence as high.
3. Myocardial infarction
Only one small trial with 31 participants reported data suitable for extraction for myocardial infarction (Altermatt 2013). There were no events (Analysis 1.3). The definition used can be found in Appendix 4.
Altermatt 2013 was judged as at low risk of bias for this outcome.
Level of certainty for myocardial infarction
The trial was not at high risk of bias. We downgraded the level by two for imprecision and rated the level of certainty as low.
Secondary outcomes
1. Chest infection
Results of three trials with 131 participants show that PNBs reduced the risk of chest infection (RR 0.41, 95% CI 0.19 to 0.89; I2 = 3%; Analysis 1.4; Figure 7) (Fletcher 2003; Haddad 1995; White 1980). Definitions used by study authors are provided in Appendix 5.

The three trials were judged as at low risk of bias for this outcome. Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis revealed no evidence of publication bias. Given a basal rate of 27%, the NNTB would be 7 (95% CI 5 to 72) and the number of participants required to eliminate a 25% decrease in a large trial would be 978 (489 per group) (alpha 0.05; beta 0.2; one‐sided test).
Level of certainty for chest infection
We did not downgrade for risk of bias because no trial was judged as at high risk of bias. Statistical heterogeneity was less than 25% (I2 = 3%). We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. The optimal information size was not achieved, so we downgraded by one level for imprecision. We found no evidence of publication bias. We rated the level of certainty as moderate.
2. Mortality
Based on 11 trials including 617 participants (Albrecht 2014; Brownbridge 2018; Cuvillon 2007; De La Tabla 2010; Fletcher 2003; Haddad 1995; Hood 1991; Jones 1985; Morrison 2008; Wang 2015; White 1980), we did not find a difference in short‐term (within six months) mortality (RR 0.87, 95% CI 0.47 to 1.60; I2 = 0%; Analysis 1.5; Figure 8). There was no statistical difference according to the type of block (i.e. single injection versus continuous infusion) (P value for the difference between subgroups 0.67).

Two trials were judged at high risk of bias for this result (Albrecht 2014; De La Tabla 2010). The study Albrecht 2014 was judged as at high risk for selection of the reported result due to the fact that mortality was not an outcome for this trial, and that no other outcome had this specific time point for measurement when the trial was registered. The study De La Tabla 2010 was judged at high risk for randomization process due to the fact that groups were of very unequal sizes (i.e. 11 participants allocated to the intervention group and 38 participants allocated to the comparator group).
With exclusion of the two trials at high risk of bias (Albrecht 2014; De La Tabla 2010), the estimate would be RR 0.81 (95% CI 0.42 to 1.59).
Egger's regression intercept showed no significant evidence of a small‐study effect. Correcting for the possibility of publication bias with Duval and Tweedie's trim and fill analysis would yield an estimate of RR 0.78 (95% CI 0.41 to 1.51). Given an incidence of 9.8%, 3228 participants (1614 per group) would have been required to eliminate a 25% reduction (alpha 0.05; beta 0.2; one‐sided test).
Level of certainty for mortality within six months
We did not downgrade for risk of bias because excluding the two trials judged as at high risk of bias would not change the conclusion. We noted no heterogeneity. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. Correcting for the possibility of publication bias would not change the conclusion. We downgraded the level of evidence by two for imprecision because the confidence interval included both absence of effect and important benefit. We rated the level of certainty as low.
3. Time to first mobilization
Findings of three trials with 208 participants show that PNBs reduced time to first mobilization (MD ‐10.80, 95% CI ‐12.83 to ‐8.77 hours; I2 = 41%; Analysis 1.6) (Kullenberg 2004; Segado Jimenez 2009; Yamamoto 2016).
All three trials were judged as at low risk of bias for this outcome.
Egger's regression intercept showed no evidence of a small‐study effect. Correcting for the possibility of publication bias would yield an estimate of MD ‐11.17 hours (95% CI ‐13.07 to ‐9.26).
Level of certainty for time to first mobilization
We did not downgrade the level of certainty for risk of bias because no trial was judged at high risk of bias. We downgraded certainty by one level for a moderate amount of heterogeneity. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. We did not downgrade evidence for imprecision. The effect was still present with correction for the possibility of publication bias. We rated the level of certainty as moderate.
4. Costs of analgesic regimens
One trial with 75 participants reported that costs related to analgesia were reduced when PNBs were given as a single‐injection PNB (MD ‐4.40 euros (2009 value), 95% CI ‐4.84 to ‐3.96; Analysis 1.7) compared to no nerve block (Segado Jimenez 2009). Segado Jimenez 2009 was judged as at low risk of bias for this outcome.
Level of certainty for costs of analgesic regimens
The trial was not at high risk of bias. The comparison was a direct one. We downgraded the evidence by two levels for the small number of trials included. We could not assess publication bias. We rated the level of certainty as low.
Complications
Complications of analgesic techniques can be found in Table 2.
| Study | Complications related to regional anaesthesia | Complications related to analgesic technique |
| Not reported | Not reported | |
| Not reported | Not reported | |
| No complications such as motor block. local haematoma or infection, inadvertent arterial puncture, direct nerve damage, and cardiovascular or neurological toxicity were observed Five participants had accidental removal of the catheter: 4 during the procedure or while the catheter was secured, and 1 while in the ward | Not reported | |
| No patient developed any residual sensory‐motor deficit during the postoperative period | Patients in the non‐block group had nausea (N=2) | |
| Not reported | Respiratory complications in 5 out of 15 participants for each group Opioid side effects after enrolment: 3/15 in the block group; 7/15 in the non‐block group | |
| No major complications in group regional blockade were described. Three participants developed local erythema at the catheter insertion site at the end of the study period No signs of local anaesthetic toxicity were documented One participant developed bilateral blockade (L1‐L3 on the opposite side) | Not reported | |
| No complications related to nerve blocks and no case of prolonged motor blockade | Not reported | |
| Four catheters were prematurely removed: 1 by a confused participant, 2 by nurses (unexplained fever), and 1 by a surgeon (unconfirmed suspicion of local anaesthetic toxicity) (ropivacaine blood level < 2 ng/mL)) | More constipation (47% vs 19% for regional blockade) | |
| Not reported | Not reported | |
| Hypotension occurred in 1 participant in the fascia iliaca compartment block group (1/20) and in 1 participant in the femoral nerve block group (1/20) There was no complication that might be relevant to fascia iliaca compartment block in our study In 1 case, prolonged (4 months) temporary motor and sensory neurological deficits occurred due to 3‐in‐1 block | Hypotension occurred in 2 patients with IV patient‐controlled analgesia (2/20), requiring stopping of IV patient‐controlled analgesia | |
| Complications such as local anaesthetic toxicity recorded as well (none reported in results section) Nor did complication rates vary between groups | Complications such as hypoventilation (breathing rate < 8 breaths/min) were recorded as well Moreover, the 2 groups did not differ in these parameters at any time point until study completion at 24 hours after surgery. Nor did complication rates vary between groups | |
| Not reported | Not reported | |
| Among study participants, none experienced adverse effects as a result of nerve block administration | No clinically important differences between groups with respect to pulse rate, oxygen saturation, or respiratory rate at any time interval. Oxygen saturation 94.87% | |
| No side effects attributable to femoral nerve block were noted in any participants during their hospital stay | More participants (P = 0.05) in the morphine group were sedated at 180 minutes after block placement No difference in nausea and vomiting was noted between groups, with 3 participants in each group having these side effects Tendency towards lower saturation was noted in the opioid group at 60 and 180 minutes after the block despite oxygen supplementation (P = 0.08) | |
| One inadvertent arterial puncture and blood aspiration positive for 3 participants Two transient paraesthesias No catheter site infection Ten catheters accidentally removed No severe complications related to analgesia | No respiratory depression from systemic analgesia and no allergic reactions All complications were reversible | |
| The only complications were local bruises at the site of injection | Two participants with nausea and 2 with nausea and vomiting | |
| No immediate complications occurred in either group defined as inadvertent vascular puncture, anaphylaxis or collapse, severe pain, or inability to tolerate the procedure | No immediate complications were noted in either group | |
| No local or systemic complications of femoral nerve blocks were noted | Not reported | |
| No complications associated with femoral nerve block were noted | Not reported | |
| One patient was withdrawn from the fascia iliaca compartment block group due to new‐onset arrhythmia | Not reported | |
| No untoward sequelae were associated with nerve blocks All plasma prilocaine concentrations (maximum 3 pg/mL) were below the suggested threshold for toxicity for prilocaine of 6 pg/mL | Not reported | |
| Not reported | In participants of fentanyl group, drowsiness was observed that required the presence of more persons holding the participant during positioning SpO2 was significantly lower in the fentanyl group (P = 0.001). However, no participant in either group had SpO2 < 90% during the procedure Mean arterial blood pressure was significantly lower in the fentanyl group (P = 0.0019) | |
| All femoral nerve block procedures required a single attempt and no complications were observed | Nausea and vomiting 4 vs 6, hypotension 2 vs 4, pruritus 0 vs 1, and desaturation 3 vs 2 for intervention and comparator, respectively | |
| No untoward sequelae associated with the nerve block were seen | Not reported | |
| No complications related to the nerve blockade were noted in this study | Not reported | |
| No serious adverse events due to the fascia iliaca compartment block were reported in this study | Not reported | |
| No other adverse events were noted during the study period, and no other adverse events were reported to study investigators | Four‐hour oxygen saturation (%) 96 (93 to 99) vs (%) 98 (95 to 99) for regional blockade Adverse events: One participant had an episode of rapid atrial fibrillation requiring diltiazem, but the participant had a history of chronic atrial fibrillation | |
| Not reported | Not reported | |
| Two patients’ catheters kinked. This problem was solved after the catheter was adjusted No other complications (local anaesthetic toxicity, puncture site infection, haematoma, catheter dislodgment) occurred | The occurrence of nausea and vomiting in group fascia iliaca compartment block were lower than those in group control. No patients experienced respiratory depression and over‐sedation in 2 groups during the waiting period | |
| No complications were noted in either group | No complications were noted in either group | |
| There were no episodes of bleeding, falls, or catheter‐related infections in the intervention group | Intervention participants were significantly less likely to report opioid side effects | |
| Not reported | Not reported | |
| No complications of femoral nerve block administration occurred, except 3 local haematomas developed at the injection site, which resolved spontaneously | Not reported | |
| Not reported | Not reported | |
| No adverse effects such as pain at the insertion site or paraesthesia were observed No positive cultures were observed with the fascia iliaca block catheter tip, nor were any signs of infection noted in the current study | Not reported | |
| There was no inadvertent vascular puncture nor adverse effect of systemic local anaesthetic toxicity in the study group | SpO₂ was significantly lower in the IV fentanyl group during positioning (95 vs 97; P < 0.001) and 5 minutes after (95 vs 98; P < 0.001). | |
| We did not observe any complications in the realization of regional anaesthetic techniques during or subsequent to these techniques | The incidence of side effects (sleepiness, hypotension, constipation, pruritus) was greater in the group with no block than in groups with blocks (P < 0.01) | |
| No haematomas at the site of femoral catheters | Two participants in each group experienced nausea and vomiting | |
| For 1 participant, the elastomeric pump failed, resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, and another participant, suffering from acute confusional state, disconnected his pump after 12 hours | The incidence of nausea/vomiting, pruritus, or excessive sedation was similar in the 2 groups | |
| Of the 23 patients in group fascia iliaca compartment block, there were no intervention‐related complications or adverse events. None of the patients receiving a block reported residual injection site pain, sensory or motor deficits, intravascular injections, cardiopulmonary events, or other adverse events | Not reported | |
| Not reported | Side effects (vomiting and pruritus) were observed significantly more frequently with intravenous analgesia | |
| No adverse events related to the femoral nerve block were noted | Not reported | |
| Not reported | Not reported | |
| The study group did not develop complications (local anaesthetic toxicity, puncture site infection, hematoma in preoperative waiting period) | All patients in the present study did not demonstrate symptoms of respiratory depression and excessive sedation in the preoperative waiting period Nausea 7 vs 12 and vomiting 5 vs 5 for intervention and comparator, respectively | |
| No participants showed any evidence of local anaesthetic toxicity | Not reported | |
| Patients were also evaluated for potential drug‐ or block‐related complications during the course of the trial No complications | Patients were also evaluated for potential drug‐ or block‐related complications during the course of the trial No complications | |
| Not reported | Fewer side effects for fascia iliaca compartment block group Nausea and vomiting 0 vs 3, respiratory depression 0 vs 1 for intervention and comparator, respectively | |
| No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited No complications such as haematoma or persistent paraesthesia were observed in participants with a femoral nerve block within 24 hours after the operation | Hypoventilation (ventilatory rate 6 to 8/min) or pulse oximetric desaturation (oxygen saturation 88% or 89%) was encountered in 4 participants (20%) in the intravenous analgesia group. This was reverted with assisted manual mask ventilation All participants in the intravenous group experienced mild dizziness, and mild drowsiness was present in 12/20 of them |
Brief summary: For peripheral nerve block, there was no case of systemic local anaesthetic toxicity and no infection. One case of prolonged (4 months) temporary motor and sensory neurological deficit occurred due to a 3‐in‐1 block (Deniz 2014). One new‐onset arrhythmia was reported (Hogg 2009). Four cases of respiratory depression requiring face mask ventilation were reported with intravenous analgesia (Yun 2009). Other opioid side effects such as drowsiness, hypoventilation, desaturation, hypotension, nausea and vomiting, pruritus, and constipation were reported in both groups. No allergic reaction was reported.
%: percentage.
L: litre.
mg: milligram.
min: minute.
ng/mL: nanogram/millilitre.
pg/mL: picogram/millilitre.
Discussion
Summary of main results
We found some advantages of peripheral nerve block (PNB) versus systemic analgesia alone for pain treatment in people with hip fracture. Compared with systemic analgesia, pain on movement within 30 minutes after block placement will be less by approximately 2.5 out of 10 (Analysis 1.1; summary of findings Table 1). This represents a clear and undeniable advantage over systemic analgesia, especially in this era of opioid crisis.
Acute confusional state is common after hip fracture and may delay rehabilitation, may increase hospital length of stay, and may impede nursing home placement and even increase risk for mortality (Pompei 1994). PNBs reduce the risk of acute confusional state (risk ratio (RR) 0.67, 95% confidence interval (CI) 0.50 to 0.90; Analysis 1.2; summary of findings Table 1). The pathophysiology of acute confusional state in these patients may be multifactorial and may include side effects of medications used, hypoxaemia, immobilization, infection, and systemic inflammation (Mouzopoulos 2009). PNBs (or local anaesthetics) may have an influence on any of these factors. Also, PNBs are associated with a reduction in opioid consumption (Guay 2017).
We could not demonstrate a reduction in the incidence of myocardial infarction (summary of findings Table 1). We found it odd that only one trial reported on the risk of myocardial infarction with PNBs (Altermatt 2013). Although the number of participants included in this trial was relatively small, study authors monitored ST segments continuously up to three days after surgery. They reported no difference in ischaemic episodes with a continuous psoas compartment block. This contrasts with results reported by Schenin and colleagues (i.e. a reduction in myocardial ischaemic episodes with an epidural infusion of bupivacaine and fentanyl) (Scheinin 2000). Epidural analgesia has been reported to reduce myocardial infarction in high‐risk patients undergoing high‐risk surgery (Guay 2016a).
Chest infections were reduced with PNBs (Analysis 1.4; summary of findings Table 1). This could be due to reduced time to first mobilization (Analysis 1.6).
We did not find a reduction in short‐term (up to six months) mortality rate (Analysis 1.5; summary of findings Table 1), but participants were too few to allow definitive conclusions on this.
Compared with systemic analgesia alone, adding a single‐injection PNB will make little or no difference in the cost of analgesic drugs (equivalent to ‐4 euros per patient in 2009).
Only one trial (Deniz 2014) reported one major complication: a sensory/motor deficit lasting four months with a femoral nerve block (Table 2). This is consistent with information derived from large prospective studies indicating that the incidence of nerve injury lasting longer than six months associated with femoral nerve block would be relatively low, at 0 to 1.2 per 1000 procedures (Auroy 2002; Brull 2007; Sites 2012).
Overall completeness and applicability of evidence
We are confident that our results reflect the actual available literature. More data may be required to evaluate the effects of PNBs on myocardial infarction and death. Indeed the number of participants included for these two outcomes was still below the optimal information size. The population included in these trials reflects quite well the overall adult population with hip fracture, with the exception of patients with dementia, who were often excluded from randomized controlled trials. Furthermore, the low incidence of major complications related to PNBs in this review has probably been made possible by adherence of study authors to recommendations of major societies on the topic. Some recommendations on the prevention of infectious and bleeding complications for each type of regional anaesthetic technique are available at www.asra.com/advisory-guidelines.
Quality of the evidence
We have summarized the certainty of evidence in summary of findings Table 1. We quantified the level of certainty as high for reduced pain on movement and for acute confusional state, and as moderate for reduced chest infection. Although some studies might not have been perfect, excluding studies at high risk of bias did not change any of our conclusions. The quality of evidence was most often reduced by insufficient numbers of included participants (myocardial infarction, chest infection, death, time to first mobilization, and cost of analgesia).
Potential biases in the review process
Our search was extensive. We chose factors for exploration of heterogeneity a priori. Trials reporting on outcomes included in our summary of findings were evaluated with the Cochrane Risk of Bias‐2 tool. Certainty was evaluated according to the GRADE system.
Cochrane is introducing a new tool for quality evaluation of randomized controlled trials: Risk of Bias‐2. Compared with the previous tool, all trials are now assessed for each domain specifically for each outcome. Indeed evaluation of the quality of a trial may vary according to the outcome for which it is evaluated. Domains are also reorganized differently, and the process of evaluation is much more detailed and extensive (see details under Characteristics of included studies). Using this new tool, very few results had trials at high risk of bias.
Regional blockade is a topic for which adequate blinding of participants and personnel taking care of participants is rarely feasible. A simple evaluation of block effectiveness is incompatible with preserved blinding. Blinding of outcome assessors and at least of the researcher analysing data should, however, often be feasible. Therefore, clarity on how allocation is concealed until the time the participant has been included in the trial and formally attributed to his/her treatment group and to blinding of outcome assessors, as well as of the researcher analysing data, represents domains on which study authors could try to improve the quality of future trials.
Agreements and disagreements with other studies or reviews
Even at rest, the level of pain after hip fracture is relatively high, particularly among those with subtrochanteric fracture (median 5 out of 10) (Foss 2005a). Movement by these individuals immediately after injury is unavoidable: transport from the scene of injury to the hospital, unclothing for medical examination, transport for X‐ray diagnostic confirmation, transfer to the operating room table, positioning for spinal anaesthesia, etc. Movement‐associated median pain ranges from 8 to 10 out of 10, depending on the type of fracture (intracapsular = 8; trochanteric = 9; subtrochanteric = 10) (Foss 2005a).
In our latest previous version of this review (Other published versions of this review), we included 31 trials with 1760 participants. We found that PNBs reduce pain and chest infection. Based on the evidence available at the time, we did not find a difference between PNBs and other modes of analgesia in terms of acute confusional state, but the number of participants included in the 2017 version was insufficient to eliminate a difference in the risk of acute confusional state. In the present version, we included 49 trials with 3061 participants. We confirmed that PNBs reduced pain on movement within 30 minutes after block placement and chest infection. We also found a reduction in acute confusional state.
In Appendix 6, we have summarized the main findings of recent reviews on this topic published in the English language (Amin 2017; Dizdarevic 2019; Fadhlillah 2019; Freeman 2016; Hards 2018; Hartmann 2017; Hong 2019; Hsu 2018; Hsu 2019; Parker 2016; Rashiq 2013; Scurrah 2018; Skjold 2019; Soffin 2019; Steenberg 2018). These reviews included between 2 and 25 trials. Most reviews focused on effects of PNBs on acute pain and confirmed our findings for this outcome. Many reviews evaluated only one specific block compared to systemic analgesia alone (i.e. either a fascia iliaca compartment block or a femoral nerve block). Therefore it is not surprising that none of these reviews included sufficient participants for evaluation of effects of PNBs on major morbidity or mortality. Indeed, chest infection and acute confusional state were not included as outcomes in most of these reviews.
Our review did not include enough participants with adequate follow‐up to evaluate the effects of adding PNBs on mortality in this population with a high level of certainty. A retrospective chart review on 535 patients evaluated the effects of a comprehensive programme, including a switch from systemic opiates to a local anaesthetic femoral nerve catheter block, an earlier assessment by the anaesthesiologist, and a more systematic approach to nutrition, fluid, oxygen therapy, and urinary retention (Pedersen 2008). Investigators reported that overall 12‐month mortality was 29% in the control group and 23% in the intervention group (P = 0.2).

Flow diagram for the 2020 update.
CENTRAL: The Cochrane Central Register of Controlled Trials; CINHAL: Cumulative Index to Nursing and Allied Health Literature.
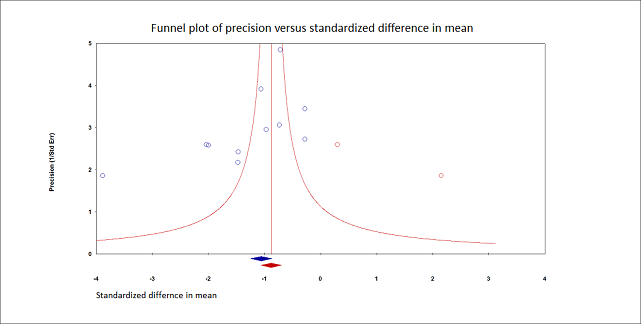
Pain on movement at 30 minutes after block placement.
Duval and Tweedie's trim and fill analysis: blue circles indicate studies found, and red circles are imputed studies. Correcting for the possibility of publication bias would give an estimated standardized mean difference of ‐0.88 (95% confidence interval ‐1.07 to ‐070).
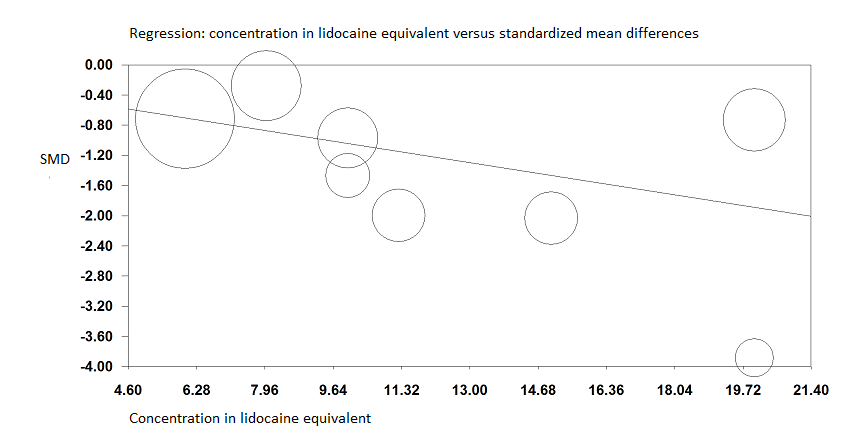
Pain on movement at 30 minutes after block placement.
A meta‐regression indicates that the effect size was proportional to the concentration of local anaesthetic injected in lidocaine equivalents; P = 0.0003.

Forest plot of comparison: 1 Nerve block versus other modes of analgesia, outcome: 1.11 Acute confusional state.
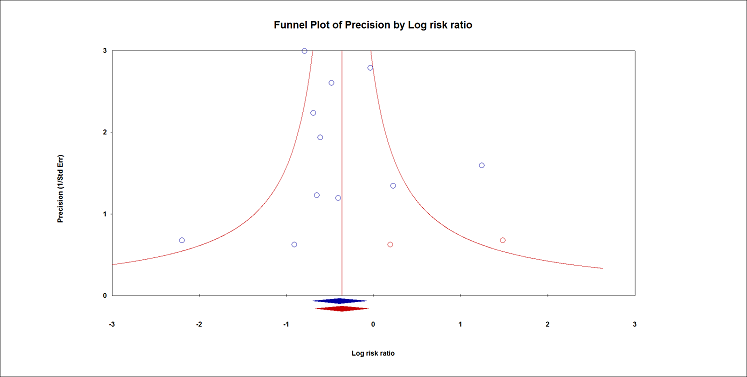
Acute confusional state.
Duval and Tweedie's trim and fill analysis: blue circles indicate studies found, and red circles are imputed studies. Correcting for the possibility of publication bias would give an estimated risk ratio 0.70 (95% CI 0.51 to 0.94).

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 1: Pain on movement within 30 minutes of block placement

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 2: Acute confusional state

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 3: Myocardial infarction

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 4: Chest infections

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 5: Mortality

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 6: Time to first mobilization

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 7: Costs of analgesic drugs
| Peripheral nerve blocks for hip fracture | ||||||
| Patient or population: patients with hip fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain on movement at 30 minutes after block placement | Mean pain on movement at 30 minutes after block placement in the intervention groups was | 503 | ⊕⊕⊕⊕ | |||
| Acute confusional state Follow‐up: 0 to 30 days | Study population | RR 0.67 | 1072 | ⊕⊕⊕⊕ | ||
| 181 per 1000 | 121 per 1000 | |||||
| Low | ||||||
| 150 per 1000 | 101 per 1000 | |||||
| High | ||||||
| 350 per 1000 | 235 per 1000 | |||||
| Myocardial infarction Follow‐up: 0 to 30 days | N/A | N/A | 31 | ⊕⊕⊝⊝ | ||
| Chest infections Follow‐up: 0 to 30 days
| Study population | RR 0.41 (0.19 to 0.89) | 131 (3 studies) | ⊕⊕⊕⊝ moderatee,f |
| |
| 269 per 1000 | 110 per 1000 (51 to 239) | |||||
| Low | ||||||
| 50 per 1000 | 20 per 1000 (9 to 44) | |||||
| High | ||||||
| 200 per 1000 | 82 per 1000 (38 to 178) | |||||
| Death | Study population | RR 0.87 | 617 | ⊕⊕⊝⊝ | ||
| 68 per 1000 | 59 per 1000 | |||||
| Low | ||||||
| 25 per 1000 | 22 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 131 per 1000 | |||||
| Time to first mobilization Follow‐up: in‐hospital | Mean time to first mobilization in intervention groups was | 208 | ⊕⊕⊕⊝ | |||
| Cost of analgesic regimens for single‐injection blocks Follow‐up: in‐hospital | Mean cost of analgesic regimens for single‐injection blocks in intervention groups was | 75 | ⊕⊕⊕⊝ | |||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades for certainty of evidence. | ||||||
| aThe effect was still present even when trials at high risk of bias were withdrawn from the analysis, or when a correction for the possibility of publication bias was applied. bThe difference was equivalent to 2.5 on a scale from 0 to 10. cThe number needed to treat for additional beneficial outcome was 12 (95% confidence interval 7 to 47). dDowngraded by two levels for imprecision. eDowngraded by one level for imprecision. fThe number needed to treat for additional beneficial outcome was 7 (95% confidence interval 5 to 72). gMean costs in 2009 euros. | ||||||
| Study | Purpose of blockade | Time of block placement | Surgical anaesthesia | Block technique | Comparison | Supplemental analgesia for both groups |
| Preoperative analgesia | In the emergency department | No information | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.5% with epinephrine 1:200,000 30 mL Operator: trained emergency physicians | Sham block with normal saline | Acetaminophen Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | Preoperatively, probably in the emergency department | Spinal anaesthesia | Psoas compartment block Nerve stimulator (quadriceps contraction at 0.5 mA, 1 Hz, Continuous infusion Bupivacaine 0.1% 20 mL followed by patient‐controlled analgesia: basal rate 8 mL/hour, bolus 5 mL, lock‐out time 30 minutes for 72 hours Operator: no information | No nerve block IV PCA with Morphine | Acetaminophen Ketorolac | |
| Postoperative analgesia | After recovery of anaesthesia | Spinal anaesthesia | Femoral nerve block Nerve stimulator Continuous infusion Levobupivacaine 0.25% 18 mL followed by levobupivacaine 0.125% at 3 to 4 mL/hour for 24 hours after surgery Operator: no information | No nerve block | Acetaminophen Pethidine | |
| Postoperative analgesia | After surgery and after confirmation of patient’s mental status to be alert, able to communicate, and obey commands | Spinal anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Single injection Ropivacaine 0.2% 40 mL Operator: no information | No nerve block | Ketorolac Celecoxib IV PCA with Fentanyl Tramadol | |
| Preoperative, intraoperative, and postoperative analgesia | Preoperatively, after patients had been assigned to a bed on the ward | Spinal (53% for intervention group and 40% for comparator group) or general anaesthesia | Fascia iliaca compartment block Landmarks Continuous infusion Ropivacaine 0.125% 40 mL followed by ropivacaine 0.2% 10 mL/hour until surgery. In the operating room, catheters were Operator: anaesthesiology department | No nerve block | Acetaminophen NSAIDs Opioids | |
| Preoperative, intraoperative, and postoperative analgesia Surgery for some participants | Preoperatively, within 6 hours after admission to the orthopaedic ward | Intervention: psoas block alone (3/20) with sciatic block (5/20), spinal (11/20) or general anaesthesia (1/20) Comparator: neuraxial block (19/20) or general anaesthesia (1/20) | Psoas compartment block Landmarks and loss of resistance to air, lateral decubitus Continuous infusion: started preoperatively (16 to 48 hours) and kept for 72 hours after surgery Test dose with 3 mL of 0.5% bupivacaine with epinephrine 5 mcg/mL followed by bupivacaine 0.25% with epinephrine Operator: anaesthesiologists | No nerve block IM Meperidine Diclofenac | IM Meperidine | |
| Postoperative analgesia | At completion of surgery before awakening from general anaesthesia | General anaesthesia | 1) Lateral femoral cutaneous nerve block Landmarks Single injection 1) Bupivacaine 0.5% with epinephrine 5 mcg/mL 15 mL Operator: anesthesiology department | No nerve block | Pethidine | |
| Postoperative analgesia | After ending of effects of spinal blockade | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps for patella ascension with 0.3 to 0.5 mA at 0.1 ms and catheter 10 to 15 cm passed over the needle tip) Continuous infusion Lidocaine 1.5% plus epinephrine 30 mL of lidocaine 1.5% followed by ropivacaine 0.2% at 10 mL/hour for 48 hours Operator: anesthesiology department | No nerve block IV Paracetamol for half of participants in the comparator group | 1 dose of paracetamol in the emergency department Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | Upon hospital arrival | No information | Femoral nerve block Dual technique: ultrasound‐guided plus nerve stimulator Continuous infusion Ropivacaine 0.2% 15 mL followed by ropivacaine 0.2% at 5 mL/hour basal rate plus boluses of 10 mL every 30 Operator: no information | No nerve block IV Metamizole | IV Tramadol | |
| Intraoperative and postoperative analgesia | In the operating room, before induction of general anaesthesia | General anaesthesia | 1) Fascia iliaca compartment block 1) Ultrasound‐guided 2) Dual technique: ultrasound‐guided plus nerve stimulator Single injection 1) Bupivacaine 0.25% 30 mL Operator: anesthesiology department | No nerve block | Tenoxicam IV PCA with Tramadol | |
| Spinal positioning, intraoperative and postoperative analgesia | Before positioning for spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.5% 40 mL Operator: anesthesiology department | No nerve block IV Fentanyl for positioning for spinal block | IV PCA with Morphine | |
| Spinal positioning, intraoperative and postoperative analgesia | In the regional anaesthetic technique room, before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.5% 15 mL and lidocaine 2% 15 mL Operator: anesthesiology department | No nerve block | IV PCA with Morphine Tramadol | |
| Preoperative analgesia | In the emergency department, after radiographic confirmation | No information | 3‐in‐1 femoral nerve block Paraesthesia Single injection Bupivacaine 0.5% 20 mL Operator: trained emergency physicians | No nerve block | IV Morphine | |
| Preoperative analgesia | Upon arrival in the emergency department | No information | Fascia iliaca compartment block Landmarks Single injection Mepivacaine 1% with epinephrine 5 mcg/mL 40 mL Operator: junior anaesthesiologists with less than 2 years of training | Sham block with 0.9% saline plus IM Morphine | IV Morphine Epidural analgesia after 3‐hour study period | |
| Preoperative, intraoperative. and postoperative analgesia | Upon arrival in the emergency department | Intervention: spinal anaesthesia for 37/50 and general anaesthesia for 13/50 Comparator: spinal anaesthesia for 38/50 and general anaesthesia for 12/50 | Femoral nerve block Nerve stimulator (0.5 mA and 0.1 millisecond) Continuous infusion (non‐stimulating catheters advanced about 10 cm past the needle tip) Prilocaine 1% 40 mL followed 2 hours later by ropivacaine 0.2% 30 mL, repeated every 6 hours (up to 40 mL; N = 5) and at intervals (up to every 4 hours; N = 8) or both (N = 6), adjusted on pain scores Operator: anaesthesiology department | No nerve block IV Metamizole Oral Tilidine and Naloxone | Ibuprofen Tilidine | |
| Preoperative analgesia | In the emergency department, after confirmation of diagnosis | No information | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.25% 0.3 mL/kg Operator: physicians (first study author is an orthopaedic surgeon) | Sham block with saline and IV NSAIDs | NSAIDs Opioids | |
| Preoperative analgesia | In the emergency department | No information | Femoral (3‐in‐1) nerve block Single injection Nerve stimulator Bupivacaine 0.5% 30 mL (not exceeding 3 mg/kg) Operator: specialist emergency physician or higher trainee resident, post intermediate examination level | No nerve block IV Morphine | IV Morphine Dihydrocodeine Diclofenac Paracetamol | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | No information | Fascia iliaca compartment block Single injection Ultrasound‐guided Levobupivacaine 0.375% 30 mL Operator: anesthesiology department | No nerve block IV Remifentanil | Additional analgesia | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Single injection Bupivacaine 0.25%.0.3 mL/kg Paraesthesia technique with a short bevel needle Operator: 1 orthopaedic registrar | No nerve block | Co‐dydramol Voltarol Pethidine | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Nerve stimulator Single injection Bupivacaine 0.5% Operator: trained emergency physicians | No nerve block | Opioids | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block No information on localizing technique Single injection Lidocaine 1% 2 mg/kg Operator: anaesthesiology department | No nerve block IV Ketamine 0.2 mg/kg IV Midazolam 0.025 mg/kg | Ketamine | |
| Intraoperative and postoperative analgesia | Before induction of general anaesthesia | General anaesthesia | 1) Femoral "3‐in‐1" nerve block 1) Nerve stimulator (quadriceps contraction with < 1 mA) 2) Landmarks Single injection 1) Prilocaine 0.75% 35 mL Operator: anaesthesiology department | No nerve block | Papaveratum | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps contraction with 0.3 to 0.5 mA) Single injection Lidocaine 1.5% (2% diluted with distilled water) with epinephrine 5 mcg/mL 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | IV Fentanyl | |
| Preoperative analgesia | In the emergency department, 48 hours before surgery | No information | Femoral nerve block Single injection Ultrasound‐guided (in‐plane) Bupivacaine 0.5% 0.3 mL/kg (maximum 20 mL) Operator: 1 physician experienced in administering ultrasound‐guided femoral nerve blocks | Sham block with saline | IV Tramadol | |
| Postoperative analgesia | At completion of surgery, while still under general anaesthesia | General anaesthesia | Lateral femoral cutaneous nerve block Single injection Landmarks Bupivacaine 0.5% with epinephrine 5 mcg/mL 15 mL Operator: anaesthesiology department | No nerve block | IM Pethidine | |
| Preoperative analgesia | As soon as the diagnosis of hip fracture was radiologically confirmed | No information | Femoral nerve block Nerve stimulator Single injection Ropivacaine 0.75% 30 mL. Operator: 1 orthopaedic surgeon | No nerve block | Paracetamol Tramadol Ketobemidon | |
| Preoperative analgesia | Within 1 hour of hospital admission | No information | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.2% 30 mL Operator: orthopaedic surgeons | Sham block with saline | IV Morphine Paracetamol | |
| Preoperative analgesia | In the emergency department | No information | 3‐in‐1 femoral nerve block Ultrasound‐guided (in‐plane) Single injection Bupivacaine 0.5% 25 mL Operator: emergency physicians experienced with the technique | Sham block with saline | Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | In the emergency department | Spinal anaesthesia | Femoral "3‐in‐1" nerve block Ultrasound‐guided Continuous infusion (catheters inserted ≥ 12 to 15 cm past the needle tip) Bupivacaine 0.25% 30 mL (additional 10 mL if required for adequate sensory blockade) followed by bupivacaine Operator: anesthesiology department | No nerve block | Piritramide Paracetamol | |
| Preoperative analgesia | After hospital admission | No information | Fascia iliaca compartment block Ultrasound‐guided (in‐plane) Continuous infusion (catheters 5 to 10 cm beyond the tip of the needle) Ropivacaine 0.4% 30 mL followed by ropivacaine 0.2% at 5 mL/hour plus 5 mL for breakthrough pain until surgery (mean 3.5 days). Catheters removed on the morning of surgery Operator: 1 anaesthesiologist experienced in ultrasound‐guided nerve block | No nerve block | Tramadol Acetaminophen Pethidine | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.375% 30 mL Operator: anaesthesiologists | No nerve block IV Fentanyl | Paracetamol Tramadol Diclofenac | |
| Preoperative analgesia, intraoperative and postoperative analgesia | In the emergency department for femoral nerve block and within 24 hours of femoral block for continuous fascia iliaca block | Regional anaesthesia for 62.1% | 1) Femoral nerve block Ultrasound‐guided (out‐of‐plane for insertion, but advancement visualized) 1) Single injection Bupivacaine 0.5% 20 mL 2) Continuous infusion Ropivacaine 0.2% 15 mL followed by 5 mL/hour for 72 hours after surgery Operators: 1) Trained emergency physicians 2) Anaesthesiologists (mobile peripheral nerve block service) | No nerve block | Opioids Acetaminophen | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca block with 20 mL of 1.5% lidocaine No information for localizing technique Single injection Lidocaine 1.5% 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | No information | |
| Preoperative and postoperative analgesia | Started upon admission to the orthopaedic ward | Epidural anaesthesia | Fascia iliaca compartment blocks daily (from admission until surgery, restarted at 24 hours after surgery until discharge, stopped earlier (before or after surgery) if delirium occurred) Landmarks Bupivacaine 0.3 mL/kg (0.25%?) Operator: orthopaedic surgeons | Sham blocks with water | IV Paracetamol Pethidine | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Nerve stimulator (quadriceps contraction with patellar ascension) Single injection Mepivacaine 20 mL Operator: unclear, published by emergency physicians | No nerve block IV Morphine or IV Paracetamol and Ketoprofen | Nitrous oxide | |
| Postoperative analgesia | After closure of the surgical wound | General anaesthesia | Fascia iliaca block Landmarks Continuous infusion (catheter inserted ≥ 10 cm cranially) Ropivacaine 0.5% according to body weight (20 mL if Operator: no information, probably anaesthesiology department | No nerve block IV PCA with Fentanyl | Acetaminophen Dihydrocodeine Morphine | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Femoral nerve block Dual technique: nerve stimulator plus in‐plane ultrasound Single injection Lidocaine 2% 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | IV Fentanyl | |
| Postoperative analgesia | In post‐anaesthesia care unit after full recuperation of motor blockade from the spinal block | Spinal anaesthesia | 1) Lateral femoral cutaneous nerve block Landmarks Single injections 1) Bupivacaine 0.5% with vasoconstrictor 5 mL Operator: anaesthesiology department | No nerve block | IV Metamizole Dexketoprofen trometamol Tramadol Morphine | |
| Postoperative analgesia | Catheters inserted before spinal anaesthesia Administration of local anaesthetics started after surgery | Spinal anaesthesia | Femoral nerve block Nerve stimulator Continuous infusion (non‐stimulating catheter advanced 8 to 15 cm past needle tip) Bupivacaine 0.5% 0.4 mL/kg followed by bupivacaine 0.25% at 0.14 mL/kg/hour for 16 hours after surgery Operator: anaesthesiology department | Sham block with saline | Morphine Acetylsalicylic acid | |
| Preoperative, intraoperative, and postoperative analgesia | Catheters inserted in the emergency department Administration of local anaesthetics started during catheter installation | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps contraction resulting in Continuous infusion (non‐stimulating catheter, space dilated with 10 mL of lidocaine 2%, catheter advanced cephalad 3 cm past the needle tip) Bupivacaine 0.5% 10 mL followed by 0.25% bupivacaine at 4 mL/hour for 72 hours Operator: anaesthesiology department | No nerve block | Paracetamol Morphine | |
| Intraoperative and postoperative analgesia | Immediately before induction of anaesthesia | General or spinal anaesthesia (38%) | Fascia iliaca compartment block Ultrasound‐guided Single injection Ropivacaine 0.25% 30 mL Operator: a board‐certified anaesthesiologist | No nerve block | Acetaminophen Tramadol Opioids | |
| Postoperative analgesia | After surgery and reversal of neuromuscular blockade | General anaesthesia | Femoral (3‐in‐1) nerve block Nerve stimulator (quadriceps contraction with patellar ascension with < 1 mA) Continuous infusion (non‐stimulating catheter advanced 4 to 5 cm past the needle tip) Lidocaine 2% with epinephrine 5 mcg/mL 30 mL followed by bupivacaine 0.125% patient‐controlled analgesia: basal rate 4 mL/hour, boluses 3 mL, lockout time 20 minutes Operator: probably anaesthesiology department | No nerve block IV PCA with Morphine | Tenoxicam | |
| Preoperative analgesia | Before surgery, as soon as possible after admission to the orthopaedic ward | No information | Femoral nerve block Nerve stimulator (quadriceps contraction) Single injection Levobupivacaine 0.25% 20 to 40 mL In case of delayed surgery or if otherwise necessary, participants could receive 1 additional block Operator: 36 anaesthesiologists with various training | No nerve block | Opioids | |
| Preoperative analgesia | In the emergency department | Spinal anaesthesia | Femoral nerve block | No nerve block IV Paracetamol | IV Tramadol Epidural analgesia after surgery | |
| Preoperative, intraoperative, and postoperative analgesia | Upon admission, after radiographic confirmation of the diagnosis | Combined spinal‐epidural anaesthesia | Fascia iliaca compartment block Ultrasound‐guided (out‐of‐plane for needle insertion and in‐plane for solution diffusion, injected cephalad) Continuous infusion (catheter inserted 5 to 10 cm past the needle tip) Ropivacaine 0.4% 50 mL followed by ropivacaine 0.2% at 5 mL/hour (plus 5 mL top‐up doses) Operator: anaesthesiologist with experience in ultrasound‑guided nerve block | Sham block with saline Paracetamol Tramadol | IVPCA with Sufentanil after surgery | |
| Intraoperative and postoperative analgesia | After induction of anaesthesia, before surgery | General anaesthesia | Psoas compartment block Landmarks Single injection Mepivacaine 2% 30 mL Operator: anaesthesiology department | No nerve block | Usual surgical care | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Single injection Levobupivacaine 0.25% 40 mL Operator: an orthopaedic surgeon with extensive experience in this block procedure | No nerve block IV Acetaminophen | Diclofenac Rescue analgesics | |
| Intraoperative and postoperative analgesia | Catheter insertion and local anaesthetic administration started before induction of anaesthesia | General anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Continuous infusion Ropivacaine 0.33% 30 mL followed by 0.15% ropivacaine at 2 mL/hour plus a bolus of 30 mL Operator: anaesthesiology department | No nerve block IV PCA with Sufentanil | Rescue analgesics | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.375% 30 mL Operator: 1 experienced anaesthesiologist | No nerve block IV Alfentanil | IV Alfentanil for spinal block Pethidine before spinal block and after surgery | |
| G: gram. h: hour. IM: intramuscular. IV: inteavenous. mA: milliAmpere. mcg/mL: microgram/millilitre. mg/kg: milligram/kilogram. MHz: megahertz. mL: millilitre. msec: millisecond. n: number. NSAIDs: non‐steroidal anti‐inflammatory drugs. PCA: patient‐controlled analgesia. SC: subcutaneous. | ||||||
| Study | Complications related to regional anaesthesia | Complications related to analgesic technique |
| Not reported | Not reported | |
| Not reported | Not reported | |
| No complications such as motor block. local haematoma or infection, inadvertent arterial puncture, direct nerve damage, and cardiovascular or neurological toxicity were observed Five participants had accidental removal of the catheter: 4 during the procedure or while the catheter was secured, and 1 while in the ward | Not reported | |
| No patient developed any residual sensory‐motor deficit during the postoperative period | Patients in the non‐block group had nausea (N=2) | |
| Not reported | Respiratory complications in 5 out of 15 participants for each group Opioid side effects after enrolment: 3/15 in the block group; 7/15 in the non‐block group | |
| No major complications in group regional blockade were described. Three participants developed local erythema at the catheter insertion site at the end of the study period No signs of local anaesthetic toxicity were documented One participant developed bilateral blockade (L1‐L3 on the opposite side) | Not reported | |
| No complications related to nerve blocks and no case of prolonged motor blockade | Not reported | |
| Four catheters were prematurely removed: 1 by a confused participant, 2 by nurses (unexplained fever), and 1 by a surgeon (unconfirmed suspicion of local anaesthetic toxicity) (ropivacaine blood level < 2 ng/mL)) | More constipation (47% vs 19% for regional blockade) | |
| Not reported | Not reported | |
| Hypotension occurred in 1 participant in the fascia iliaca compartment block group (1/20) and in 1 participant in the femoral nerve block group (1/20) There was no complication that might be relevant to fascia iliaca compartment block in our study In 1 case, prolonged (4 months) temporary motor and sensory neurological deficits occurred due to 3‐in‐1 block | Hypotension occurred in 2 patients with IV patient‐controlled analgesia (2/20), requiring stopping of IV patient‐controlled analgesia | |
| Complications such as local anaesthetic toxicity recorded as well (none reported in results section) Nor did complication rates vary between groups | Complications such as hypoventilation (breathing rate < 8 breaths/min) were recorded as well Moreover, the 2 groups did not differ in these parameters at any time point until study completion at 24 hours after surgery. Nor did complication rates vary between groups | |
| Not reported | Not reported | |
| Among study participants, none experienced adverse effects as a result of nerve block administration | No clinically important differences between groups with respect to pulse rate, oxygen saturation, or respiratory rate at any time interval. Oxygen saturation 94.87% | |
| No side effects attributable to femoral nerve block were noted in any participants during their hospital stay | More participants (P = 0.05) in the morphine group were sedated at 180 minutes after block placement No difference in nausea and vomiting was noted between groups, with 3 participants in each group having these side effects Tendency towards lower saturation was noted in the opioid group at 60 and 180 minutes after the block despite oxygen supplementation (P = 0.08) | |
| One inadvertent arterial puncture and blood aspiration positive for 3 participants Two transient paraesthesias No catheter site infection Ten catheters accidentally removed No severe complications related to analgesia | No respiratory depression from systemic analgesia and no allergic reactions All complications were reversible | |
| The only complications were local bruises at the site of injection | Two participants with nausea and 2 with nausea and vomiting | |
| No immediate complications occurred in either group defined as inadvertent vascular puncture, anaphylaxis or collapse, severe pain, or inability to tolerate the procedure | No immediate complications were noted in either group | |
| No local or systemic complications of femoral nerve blocks were noted | Not reported | |
| No complications associated with femoral nerve block were noted | Not reported | |
| One patient was withdrawn from the fascia iliaca compartment block group due to new‐onset arrhythmia | Not reported | |
| No untoward sequelae were associated with nerve blocks All plasma prilocaine concentrations (maximum 3 pg/mL) were below the suggested threshold for toxicity for prilocaine of 6 pg/mL | Not reported | |
| Not reported | In participants of fentanyl group, drowsiness was observed that required the presence of more persons holding the participant during positioning SpO2 was significantly lower in the fentanyl group (P = 0.001). However, no participant in either group had SpO2 < 90% during the procedure Mean arterial blood pressure was significantly lower in the fentanyl group (P = 0.0019) | |
| All femoral nerve block procedures required a single attempt and no complications were observed | Nausea and vomiting 4 vs 6, hypotension 2 vs 4, pruritus 0 vs 1, and desaturation 3 vs 2 for intervention and comparator, respectively | |
| No untoward sequelae associated with the nerve block were seen | Not reported | |
| No complications related to the nerve blockade were noted in this study | Not reported | |
| No serious adverse events due to the fascia iliaca compartment block were reported in this study | Not reported | |
| No other adverse events were noted during the study period, and no other adverse events were reported to study investigators | Four‐hour oxygen saturation (%) 96 (93 to 99) vs (%) 98 (95 to 99) for regional blockade Adverse events: One participant had an episode of rapid atrial fibrillation requiring diltiazem, but the participant had a history of chronic atrial fibrillation | |
| Not reported | Not reported | |
| Two patients’ catheters kinked. This problem was solved after the catheter was adjusted No other complications (local anaesthetic toxicity, puncture site infection, haematoma, catheter dislodgment) occurred | The occurrence of nausea and vomiting in group fascia iliaca compartment block were lower than those in group control. No patients experienced respiratory depression and over‐sedation in 2 groups during the waiting period | |
| No complications were noted in either group | No complications were noted in either group | |
| There were no episodes of bleeding, falls, or catheter‐related infections in the intervention group | Intervention participants were significantly less likely to report opioid side effects | |
| Not reported | Not reported | |
| No complications of femoral nerve block administration occurred, except 3 local haematomas developed at the injection site, which resolved spontaneously | Not reported | |
| Not reported | Not reported | |
| No adverse effects such as pain at the insertion site or paraesthesia were observed No positive cultures were observed with the fascia iliaca block catheter tip, nor were any signs of infection noted in the current study | Not reported | |
| There was no inadvertent vascular puncture nor adverse effect of systemic local anaesthetic toxicity in the study group | SpO₂ was significantly lower in the IV fentanyl group during positioning (95 vs 97; P < 0.001) and 5 minutes after (95 vs 98; P < 0.001). | |
| We did not observe any complications in the realization of regional anaesthetic techniques during or subsequent to these techniques | The incidence of side effects (sleepiness, hypotension, constipation, pruritus) was greater in the group with no block than in groups with blocks (P < 0.01) | |
| No haematomas at the site of femoral catheters | Two participants in each group experienced nausea and vomiting | |
| For 1 participant, the elastomeric pump failed, resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, and another participant, suffering from acute confusional state, disconnected his pump after 12 hours | The incidence of nausea/vomiting, pruritus, or excessive sedation was similar in the 2 groups | |
| Of the 23 patients in group fascia iliaca compartment block, there were no intervention‐related complications or adverse events. None of the patients receiving a block reported residual injection site pain, sensory or motor deficits, intravascular injections, cardiopulmonary events, or other adverse events | Not reported | |
| Not reported | Side effects (vomiting and pruritus) were observed significantly more frequently with intravenous analgesia | |
| No adverse events related to the femoral nerve block were noted | Not reported | |
| Not reported | Not reported | |
| The study group did not develop complications (local anaesthetic toxicity, puncture site infection, hematoma in preoperative waiting period) | All patients in the present study did not demonstrate symptoms of respiratory depression and excessive sedation in the preoperative waiting period Nausea 7 vs 12 and vomiting 5 vs 5 for intervention and comparator, respectively | |
| No participants showed any evidence of local anaesthetic toxicity | Not reported | |
| Patients were also evaluated for potential drug‐ or block‐related complications during the course of the trial No complications | Patients were also evaluated for potential drug‐ or block‐related complications during the course of the trial No complications | |
| Not reported | Fewer side effects for fascia iliaca compartment block group Nausea and vomiting 0 vs 3, respiratory depression 0 vs 1 for intervention and comparator, respectively | |
| No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited No complications such as haematoma or persistent paraesthesia were observed in participants with a femoral nerve block within 24 hours after the operation | Hypoventilation (ventilatory rate 6 to 8/min) or pulse oximetric desaturation (oxygen saturation 88% or 89%) was encountered in 4 participants (20%) in the intravenous analgesia group. This was reverted with assisted manual mask ventilation All participants in the intravenous group experienced mild dizziness, and mild drowsiness was present in 12/20 of them | |
| Brief summary: For peripheral nerve block, there was no case of systemic local anaesthetic toxicity and no infection. One case of prolonged (4 months) temporary motor and sensory neurological deficit occurred due to a 3‐in‐1 block (Deniz 2014). One new‐onset arrhythmia was reported (Hogg 2009). Four cases of respiratory depression requiring face mask ventilation were reported with intravenous analgesia (Yun 2009). Other opioid side effects such as drowsiness, hypoventilation, desaturation, hypotension, nausea and vomiting, pruritus, and constipation were reported in both groups. No allergic reaction was reported. %: percentage. L: litre. mg: milligram. min: minute. ng/mL: nanogram/millilitre. pg/mL: picogram/millilitre. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain on movement within 30 minutes of block placement Show forest plot | 11 | 503 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.25, ‐0.86] |
| 1.1.1 Fascia iliaca compartment block | 7 | 309 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐1.42, ‐0.92] |
| 1.1.2 Femoral nerve block | 4 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.19, ‐0.60] |
| 1.2 Acute confusional state Show forest plot | 13 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 1.2.1 Peripheral nerve block based on landmarks | 4 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.13] |
| 1.2.2 Peripheral nerve block based on nerve stimulator | 3 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.97] |
| 1.2.3 Peripheral nerve blocks inserted on ultrasound guidance | 6 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.44, 1.20] |
| 1.3 Myocardial infarction Show forest plot | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 Chest infections Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.89] |
| 1.5 Mortality Show forest plot | 11 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.47, 1.60] |
| 1.5.1 Single‐injection block | 6 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.44, 2.24] |
| 1.5.2 Continuous infusion | 5 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.89] |
| 1.6 Time to first mobilization Show forest plot | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐10.80 [‐12.83, ‐8.77] |
| 1.7 Costs of analgesic drugs Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐4.84, ‐3.96] |
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.1.1 Fascia iliaca compartment block | ||||||||||||
| Albrecht 2014 |  |  |  |  |  |  | ||||||
| Randomly allocated according to a computer‐generated list of random numbers and allocation concealed in sealed opaque envelopes. Intervention group had lower pain score at baseline. This difference was judged as compatible with what could be expected from chance alone in a study with a sample size. | ||||||||||||
| No deviations from intended interventions identified | ||||||||||||
| 100% of included participants were analyzed | ||||||||||||
| Pain scores collected by a nurse blinded to the intervention group | ||||||||||||
| Study authors elected to deviate from the planned statistical analysis after knowing the results. | ||||||||||||
| This trial was judged as at high risk of bias for this outcome due to the fact that study authors elected to deviate from the planned statistical analysis after knowing the results. | ||||||||||||
| Diakomi 2014 |  |  |  |  |  |  | ||||||
| Patients were randomly assigned, using a sealed envelope method and there was no baseline differences between intervention groups. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 98% of included participants were analysed | ||||||||||||
| Pain scores collected by an anaesthesiologist blinded to the intervention group. | ||||||||||||
| No deviation to the planned statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Domac 2015 |  |  |  |  |  |  | ||||||
| Patients included in the study were divided into two equal groups for this prospective double‐blind study. No difference between intervention groups at baseline identified. | ||||||||||||
| No deviations from intended interventions identified | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Pain scores probably collected by an assessor blinded to the intervention group. | ||||||||||||
| No deviation to the statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Foss 2005a |  |  |  |  |  |  | ||||||
| The randomization was done via a computer‐generated list. Pain at rest before intervention was higher in the intervention group (P = 0.04). The imbalance can be compatible with the one expected due to chance alone in a study with a small sample size. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| One patient did not have a fracture but only a severe contusion and was excluded after x‐ray; an extra patient was therefore included on a new number. 98% of included participants were analyzed | ||||||||||||
| Pain scores collected by an assessor blinded to the intervention group. | ||||||||||||
| No deviation from the plan analysis identified. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Hogg 2009 |  |  |  |  |  |  | ||||||
| Prospective, randomised controlled trial and no baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 98% of included participants were analysed. | ||||||||||||
| Pain scores. Although this is a subjective score, the fact that a correlation between the effect size and the local anaesthetic drug concentration was found in the review (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Landsting 2008 |  |  |  |  |  |  | ||||||
| Randomization was carried out using a computer, and information about the study intervention was sealed in envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| Only 56% of included participants had data available for the time point selected by review authors. We were unable to determine if missingness was related to the outcome or not. We therefore deemed it prudent to judge this trial at high risk of bias for this domain for this outcome. | ||||||||||||
| Pain scores derived from a combination of self‐rating scales collected by a blinded assessor. | ||||||||||||
| No deviation to the statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| Judged as at high risk of bias for this outcome due to high number of missing data at the time point selected by review authors and uncertainty as to whether or not missingness could be related to this outcome. | ||||||||||||
| Yun 2009 |  |  |  |  |  |  | ||||||
| Randomly assigned using an allocation sequence generated by a computer, and allocation sequence concealed in envelopes until group was assigned. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations form intended interventions identified. | ||||||||||||
| 100% of included participants analyzed. | ||||||||||||
| Pain scores collected by an assessor probably blinded to te intervention group. | ||||||||||||
| No deviations from the planned statistical analysis identified and only one result provided for the time point selected by the review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Subgroup 1.1.2 Femoral nerve block | ||||||||||||
| Gille 2006 |  |  |  |  |  |  | ||||||
| Randomization in two groups by the anaesthesiologist. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the planned statistical analysis reported, only one results provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Murgue 2006 |  |  |  |  |  |  | ||||||
| Randomized by “tirage au sort (translated as "hat drawing) ” and no baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 94% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the planned statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Ranjit 2016 |  |  |  |  |  |  | ||||||
| Selected patients were randomized by sealed envelope technique and no baseline differences between intervention groups were identified. | ||||||||||||
| No deviations from the intended interventions were identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the planned statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Szucs 2010 |  |  |  |  |  |  | ||||||
| Randomized using a random number sequence and sealed envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 89% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviations from the planned statistical analysis identified and only one result provided for the time point selected by the review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.2.1 Peripheral nerve block based on landmarks | ||||||||||||
| Godoy Monzon 2010 |  |  |  |  |  |  | ||||||
| Randomized using numbers generated by a computer. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 88% of included participants analyzed: 92 for the intervention group and 62 for the comparator group. We were uncertain if missingness was related to the outcome or not. Therefore, we deemed it prudent to judge this trial at high risk of bias for this domain for this outcome. | ||||||||||||
| Delirium. To be qualified as delirious, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as delirious or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| Judged as at high risk of bias for this outcome due to high number of missing data and inability to determine whether or not missingness was related to this outcome | ||||||||||||
| Mouzopoulos 2009 |  |  |  |  |  |  | ||||||
| Computer‐generated randomization code. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. | ||||||||||||
| Diagnosis of the syndrome was defined using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) and Confusion Assessment Method (CAM) criteria [1, 21]. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Nie 2015 |  |  |  |  |  |  | ||||||
| Randomly assigned according to a computer‐generated random number table. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 98% of included participants were analyzed. | ||||||||||||
| The Confusion Assessment Method was used to diagnose delirium pre‐ and postsurgery. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| White 1980 |  |  |  |  |  |  | ||||||
| Patients were randomly allocated. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| Participants with failed block were excluded: 4/20 no other exclusion. So, 90 % of included participants analyzed. | ||||||||||||
| Confusion. To be qualified as confused, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Subgroup 1.2.2 Peripheral nerve block based on nerve stimulator | ||||||||||||
| Cuvillon 2007 |  |  |  |  |  |  | ||||||
| Randomized using sealed numbered envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Results are given for sedation and/or confusion. To be qualified as confused, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. Participants in comparator groups received less morphine than the block group, we therefore have no reason to believe that the highest number of participants with sedation and or confusion in the comparator group were assessed as positive for this outcome because they were excessively sedated from morphine. Also, results of this trial are consistent with results of the other trials included in the analysis. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Graham 2008 |  |  |  |  |  |  | ||||||
| Randomized by numbered, sequential, sealed opaque envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 85% of included participants were analyzed. | ||||||||||||
| Confusion. To be qualified as confused, a patient has to show clear symptoms of confusion. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Kullenberg 2004 |  |  |  |  |  |  | ||||||
| Randomized using the envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| An experienced nurse evaluated patients' mental status with the Short Portable Mental Status Questionnaire, Pfeiffer‐test, graded according to a 4‐degree scale. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Subgroup 1.2.3 Peripheral nerve blocks inserted on ultrasound guidance | ||||||||||||
| Brownbridge 2018 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| CAM‐ICU scoring system will be used daily to measure delirium. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Liebmann 2012 |  |  |  |  |  |  | ||||||
| Randomization occurred using an Internet‐based program. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. | ||||||||||||
| Confusion. To be qualified as confused, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Morrison 2008 |  |  |  |  |  |  | ||||||
| Randomized using a computer‐generated, stratified, blocked randomization list, with stratification according to site and allocation concealed in sealed envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. | ||||||||||||
| Confusion Assessment Method supplemented by chart review evaluated by an assessor blinded to the treatment group. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Uysal 2018 |  |  |  |  |  |  | ||||||
| A randomized controlled trial. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 83% of included participants were analyzed. | ||||||||||||
| The delirium status of patients was assessed using “Delirium Rating Scale‐R‐98 (DRS‐R‐98)” in the postoperative period for three days. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Yamamoto 2016 |  |  |  |  |  |  | ||||||
| Randomisation was performed with a random number list generated by a computer software. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Delirium occurring within 24 h after surgery was diagnosed by the confusion assessment method. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Yang 2016 |  |  |  |  |  |  | ||||||
| Randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Delirium. To be qualified as delirious, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as delirious or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Altermatt 2013 |  |  |  |  |  |  | ||||||
| Randomized using a computer generated random number table. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Serial EKGs and troponin concentration measurements were performed daily until postoperative day 3 or more frequently if an ischemic episode was suspected. Analysis of ST segments were evaluated a posteriori by a cardiologist blinded the allocated group. | ||||||||||||
| No deviation to the statistical analysis reported. No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Fletcher 2003 |  |  |  |  |  |  | ||||||
| The randomization sequence was derived from a random number generator, and allocation concealment was achieved by means of the sealed opaque envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Lower respiratory tract infections determined by a blinded assessor. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Haddad 1995 |  |  |  |  |  |  | ||||||
| Randomized by sealed envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 90% of included participants were analyzed. | ||||||||||||
| Chest infections which required antibiotics. We judged it as unlikely to have been influenced by knowledge of the treatment group. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| White 1980 |  |  |  |  |  |  | ||||||
| Patients were randomly allocated. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 80% of included participants were analyzed. | ||||||||||||
| Pneumonia. We judged it as unlikely to have been influenced by knowledge of the treatment group. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.5.1 Single‐injection block | ||||||||||||
| Albrecht 2014 |  |  |  |  |  |  | ||||||
| Randomly allocated according to a computer‐generated list of random numbers and allocation concealed in sealed opaque envelopes. Intervention group had lower pain score at baseline. This difference was judged as compatible with what could be expected from chance alone in a study with a sample size. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Death from all causes | ||||||||||||
| This outcome was not part of the outcomes when the trial was registered. There was no other planned measurement at 3 months. | ||||||||||||
| Judged as at high risk of bias for this outcome due to possibility that this outcome was not pre‐determined for the specific time point at which it was measured | ||||||||||||
| Fletcher 2003 |  |  |  |  |  |  | ||||||
| The randomization sequence was derived from a random number generator, and allocation concealment was achieved by means of the sealed opaque envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Haddad 1995 |  |  |  |  |  |  | ||||||
| Randomized by sealed envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Hood 1991 |  |  |  |  |  |  | ||||||
| Randomly allocated by choosing an unmarked envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Jones 1985 |  |  |  |  |  |  | ||||||
| Prospective controlled randomised trial, an envelope was opened after surgery completion. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| White 1980 |  |  |  |  |  |  | ||||||
| Patients were randomly allocated. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| Participants with failed block were excluded: 4/20 no other exclusion. So, 90 % of included participants analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Subgroup 1.5.2 Continuous infusion | ||||||||||||
| Brownbridge 2018 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Cuvillon 2007 |  |  |  |  |  |  | ||||||
| Randomized using sealed numbered envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| De La Tabla 2010 |  |  |  |  |  |  | ||||||
| Prospective, randomized study. 49 patients were included: 38 in group 1 (77,6%) and 11 in group 2 (22,4%). | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| Judged as at high risk of bias due to a possible problem with randomization leading to two very unequal number of participants per group. | ||||||||||||
| Morrison 2008 |  |  |  |  |  |  | ||||||
| Randomized using a computer‐generated, stratified, blocked randomization list, with stratification according to site and allocation concealed in sealed envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. Missingness not related to outcome. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Wang 2015 |  |  |  |  |  |  | ||||||
| Randomly assigned using a computer‑generated random number table method with randomized group information sealed in an opaque envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Kullenberg 2004 |  |  |  |  |  |  | ||||||
| Randomized using the envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Time to first support of body weight next to the bed in hours after surgery. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Segado Jimenez 2009 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Time to sit down for the first time. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Yamamoto 2016 |  |  |  |  |  |  | ||||||
| Randomisation was performed with a random number list generated by a computer software. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Time to first standing. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Segado Jimenez 2009 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Drugs expenses (not including indirect costs or stay). | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||




