Peripheral nerve blocks for hip fractures in adults
Appendices
Appendix 1. Search strategies
MEDLINE ALL (Ovid) 1946 to 15 November 2019
1 exp Femoral Fractures/
2 exp Hip Fractures/
3 ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) adj5 fracture*).mp.
4 1 or 2 or 3
5 exp Anesthesia/
6 exp nerve block/
7 ((an?est* or analg*) adj5 (regional* or local* or block* or nerv*)).mp.
8 (((nerv* or plexus or femoral or femur* or psoas or compartment or regional) adj3 block*) or lumbar plexus or fascia iliac*).mp.
9 5 or 6 or 7 or 8
10 ((randomized controlled trial or controlled clinical trial).pt. or random*.ab. or placebo.ab. or drug therapy.fs. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
11 Meta‐analysis.pt. or exp Meta‐analysis/ or exp Meta‐analysis as topic/ or (meta analy* or metaanaly*).tw. or ((review* or search*) adj10 (literature* or medical database* or medline or pubmed or embase or cochrane or cinahl or biosis or current content* or systemat*)).tw.
12 10 or 11
13 4 and 9 and 12
Embase (Ovid) 1974 to 2019 November 13
1 exp femur fracture/
2 exp hip fracture/
3 ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) adj5 fracture*).mp.
4 1 or 2 or 3
5 exp regional anesthesia/
6 exp nerve block/
7 ((an?est* or analg*) adj5 (regional* or local* or block* or nerv*)).mp.
8 (((nerv* or plexus or femoral or femur* or psoas or compartment or regional) adj3 block*) or lumbar plexus or fascia iliac*).mp.
9 5 or 6 or 7 or 8
10 (randomized controlled trial/ or crossover procedure/ or double blind procedure/ or single blind procedure/ or controlled clinical trial/ or ((single or double or triple or treble or doubly or singly) adj2 (blind* or mask*)).ti,ab. or (controlled adj5 (study or design or trial)).ti,ab. or (parallel group* or open label).ti,ab. or (allocat* or assign* or crossover* or cross over* or multicenter* or multi center* or placebo* or random* or factorial or volunteer* or (trial or groups)).tw.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti,ab.))
11 4 and 9 and 10
CENTRAL (Cochrane Library)
#1 MeSH descriptor: [Hip Fractures] explode all trees
#2 MeSH descriptor: [Femoral Fractures] explode all trees
#3 (hip* or femor* or femur* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) NEAR fracture*
#4 #1 OR #2 OR #3
#5 MeSH descriptor: [Anesthesia] explode all trees
#6 MeSH descriptor: [Nerve Block] explode all trees
#7 ((anesth* or anaesth* or analg*) NEAR (regional* or local* or block* or nerv*))
#8 ((nerv* or plexus or femoral or femur* or psoas or compartment or regional) NEAR block*) or lumbar plexus or fascia iliac*
#9 #5 or #6 or #7 or #8
#10 #4 and #9
#11 #10 in Trials
CINAHL (Ebsco)
| S1 | (MH "Femoral Fractures+") | |
| S2 | (MH "Hip Fractures+") | |
| S3 | TX ((hip* or femur* or femoral* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) N5 fracture*) | |
| S4 | S1 OR S2 OR S3 | |
| S5 | (MH "Anesthesia+") | |
| S6 | (MH "Nerve Block+") | |
| S7 | TX ((anesth* or anaesth* or analg*) N5 (regional* or local* or block* or nerv*)) | |
| S8 | TX (((nerv* or plexus or femoral or femur* or psoas or compartment or regional) N3 block*) or lumbar plexus or fascia iliac*) | |
| S9 | S5 OR S6 OR S7 OR S8 | |
| S10 | S4 AND S9 | |
| S11 | ((MH "Randomized Controlled Trials") OR (MH "Clinical Trials+") OR (MH "Random Assignment") OR (MH "Prospective Studies+") OR (MH "Clinical Trial Registry") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Multicenter Studies") OR (MH "Placebos") OR (PT Clinical trial) OR (MH "Quantitative Studies")) OR TX (random* or placebo* or trial* OR cross over OR crossover) OR TX ((singl* OR doubl* OR trebl* OR tripl*) N3 (blind* OR mask*)) OR TX (clinic* N1 trial*) | |
| S12 | S10 AND S11 |
Appendix 2. Risk of bias assessment
Supplement toMethods.
For bias due to the randomization process, we evaluated allocation sequence generation, allocation sequence concealment, and baseline imbalances suggesting a problem in the randomization process.
For bias due to deviations from intended interventions, we evaluated the effect of assignment to intervention. To assess the effect of assignment to intervention, we evaluated if participants were aware of their assigned intervention during the trial, if carers and people delivering the interventions were aware of participants' assigned intervention during the trial, if there were deviations from the intended intervention that arose because of the trial context, if these deviations were likely to have affected the outcome, if these deviations from the intended intervention were balanced between groups, if an appropriate analysis was used to estimate the effect of assignment to the intervention, and if there was potential for a substantial impact (on the result) of the failure to analyse participants in the groups to which they were randomized.
For bias due to missing outcome data, we evaluated if data for this outcome were available for all, or nearly all, participants randomized, if there was evidence that the result was not biased by missing outcome data, if missingness in the outcome could depend on its true value, and if it was likely that missingness in the outcome depended on its true value.
For bias due to measurement of the outcome, we evaluated if the method of measuring the outcome was inappropriate, if measurement or ascertainment of the outcome could have differed between intervention groups, if outcome assessors were aware of the intervention received by study participants, if assessment of the outcome could have been influenced by knowledge of intervention received, and if it was likely that assessment of the outcome was influenced by knowledge of intervention received.
For bias due to selection of the reported result, we evaluated if the data that produced this result were analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis, and if the numerical result being assessed was likely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data.
Appendix 3. Diagnostic criteria for acute confusional state
| Study ID | Diagnostic criteria |
| CAM‐ICU scoring system will be used daily to measure delirium (time frame: during hospital stay up to 1 month) | |
| Clinical evaluation "somnolence‐confusion" and Mini Mental Test | |
| "episodes of delirium" | |
| "acute confusional state" | |
| Pfeiffer test, graded according to a 4‐degree scale (0 to 3: no, light, moderate, and pronounced confusion) | |
| "agitation or confusion" | |
| Confusion Assessment Method daily supplemented by chart review | |
| Perioperative delirium: syndrome defined using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV), and Confusion Assessment Method (CAM) criteria "Daily patient assessments using the MMSE, DRS‐R‐ 98, and Digit Span test [assessment of attention, range 0 (no attention) to 42 (good attention)] were used to enable the DSM‐IV and CAM diagnoses and assess delirium severity"; "CAM and DRS‐R‐98 assessments were continued once delirium was diagnosed" | |
| "The Confusion Assessment Method was used to diagnose delirium pre‐ and postsurgery" | |
| "Delirium Rating Scale‐R‐98 (DRS‐R‐98)" | |
| "confusion" | |
| "Delirium occurring within 24 hour after surgery was diagnosed by the Confusion Assessment Method" | |
| "delirium" |
Appendix 4. Diagnostic criteria for myocardial infarction
| Study ID | Diagnostic criteria |
| Serial electrocardiograms and troponin concentration measurements were performed daily until postoperative day 3, or more frequently if an ischaemic episode was suspected |
Appendix 5. Diagnostic criteria for chest infection
| Study ID | Diagnostic criteria |
| "lower respiratory tract infections" | |
| “chest infections which required antibiotics” | |
| "pneumonia" |
Appendix 6. Results from other recent reviews on the topic published in English
| Review | Pain | Acute confusional state | Myocardial infarction | Chest infections | Death | Time to first mobilization | Cost of analgesic regimen | Remarks |
|---|---|---|---|---|---|---|---|---|
| FICB is safe and effective in controlling perioperative pain | N/A | N/A | N/A | N/A | N/A | N/A | NR | |
| Utilize various strategies to reduce pain including RA | N/A | N/A | N/A | N/A | N/A | N/A | NR | |
| FICB reduces acute pain on movement Variable results for pain at rest | N/A | N/A | N/A | N/A | N/A | N/A | MA | |
| FICB is part of recommended | Use multi‐modal analgesia to | N/A | N/A | N/A | N/A | N/A | NR | |
| FICB is suitable for pre‐hospital use and has few adverse effects Comparisons with systemic opioids are required | N/A | N/A | N/A | N/A | N/A | N/A | SR
| |
| FNB seemed to be more effective than IV fentanyl | N/A | N/A | N/A | N/A | N/A | N/A | SR | |
| FICB reduced pain at 1 to 8, 12, 24, and 48 hours No difference at 72 hours | N/A | N/A | N/A | N/A | N/A | N/A | MA | |
| Limited evidence for reduced pain on movement at 30 minutes and at 6 hours after surgery with FICB No significant complications
| N/A | N/A | N/A | N/A | N/A | N/A | MA | |
| FNB achieved lower pain scores on movement at 30 minutes than IV analgesia | N/A | N/A | N/A | N/A | N/A | N/A | MA
| |
| N/A | N/A | N/A | N/A | Nerve blocks may reduce mortality or morbidity Continuing research | N/A | N/A | NR | |
| ONB plus LFCNB had the highest probability of being effective against acute postoperative pain More trials comparing multiple nerve blocks in hip fractures are required | FICB had the highest probability of being the most effective | N/A | N/A | N/A | N/A | N/A | SR | |
| Consistent evidence that PNBs reduce pain and are more effective than standard systemic analgesia alone | Moderate evidence for a reduction | N/A | N/A | Limited evidence for a reduction | N/A | N/A | NR | |
| Limited quantity of evidence for decreased pain scores leading to very low certainty of evidence supporting preoperative single‐injection | N/A | N/A | N/A | N/A | N/A | N/A | SR with MA | |
| PNBs and non‐opioid multi‐modal analgesic agents are suggested preoperatively | N/A | N/A | N/A | N/A | N/A | N/A | ER | |
|
| FICB superior to opioids during movement Very few adverse effects | Insufficient evidence | N/A | N/A | Insufficient evidence | N/A | N/A | SR
|
| CR: Case report; ER: evidence review; FICB: fascia iliaca compartment block; FNB: femoral nerve block; | ||||||||

Flow diagram for the 2020 update.
CENTRAL: The Cochrane Central Register of Controlled Trials; CINHAL: Cumulative Index to Nursing and Allied Health Literature.
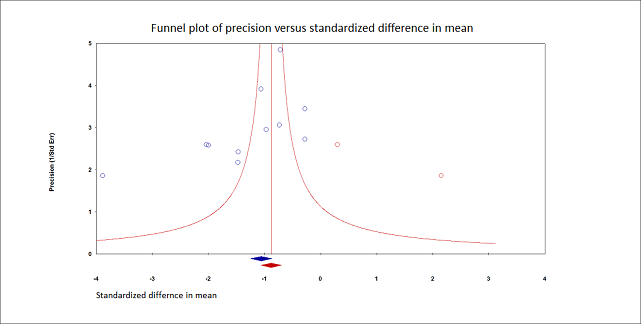
Pain on movement at 30 minutes after block placement.
Duval and Tweedie's trim and fill analysis: blue circles indicate studies found, and red circles are imputed studies. Correcting for the possibility of publication bias would give an estimated standardized mean difference of ‐0.88 (95% confidence interval ‐1.07 to ‐070).
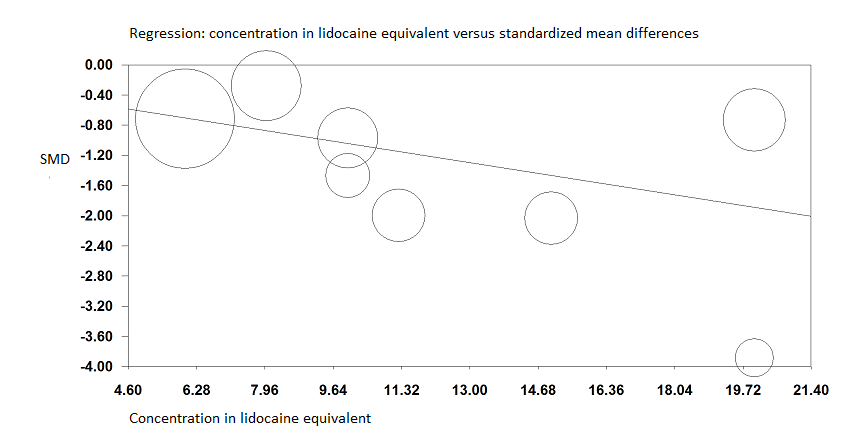
Pain on movement at 30 minutes after block placement.
A meta‐regression indicates that the effect size was proportional to the concentration of local anaesthetic injected in lidocaine equivalents; P = 0.0003.

Forest plot of comparison: 1 Nerve block versus other modes of analgesia, outcome: 1.11 Acute confusional state.
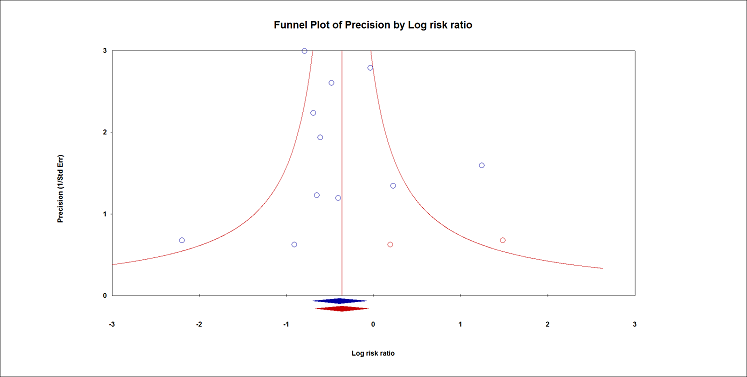
Acute confusional state.
Duval and Tweedie's trim and fill analysis: blue circles indicate studies found, and red circles are imputed studies. Correcting for the possibility of publication bias would give an estimated risk ratio 0.70 (95% CI 0.51 to 0.94).

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 1: Pain on movement within 30 minutes of block placement

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 2: Acute confusional state

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 3: Myocardial infarction

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 4: Chest infections

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 5: Mortality

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 6: Time to first mobilization

Comparison 1: Peripheral nerve blocks (PNBs) versus no nerve block (or sham block), Outcome 7: Costs of analgesic drugs
| Peripheral nerve blocks for hip fracture | ||||||
| Patient or population: patients with hip fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain on movement at 30 minutes after block placement | Mean pain on movement at 30 minutes after block placement in the intervention groups was | 503 | ⊕⊕⊕⊕ | |||
| Acute confusional state Follow‐up: 0 to 30 days | Study population | RR 0.67 | 1072 | ⊕⊕⊕⊕ | ||
| 181 per 1000 | 121 per 1000 | |||||
| Low | ||||||
| 150 per 1000 | 101 per 1000 | |||||
| High | ||||||
| 350 per 1000 | 235 per 1000 | |||||
| Myocardial infarction Follow‐up: 0 to 30 days | N/A | N/A | 31 | ⊕⊕⊝⊝ | ||
| Chest infections Follow‐up: 0 to 30 days
| Study population | RR 0.41 (0.19 to 0.89) | 131 (3 studies) | ⊕⊕⊕⊝ moderatee,f |
| |
| 269 per 1000 | 110 per 1000 (51 to 239) | |||||
| Low | ||||||
| 50 per 1000 | 20 per 1000 (9 to 44) | |||||
| High | ||||||
| 200 per 1000 | 82 per 1000 (38 to 178) | |||||
| Death | Study population | RR 0.87 | 617 | ⊕⊕⊝⊝ | ||
| 68 per 1000 | 59 per 1000 | |||||
| Low | ||||||
| 25 per 1000 | 22 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 131 per 1000 | |||||
| Time to first mobilization Follow‐up: in‐hospital | Mean time to first mobilization in intervention groups was | 208 | ⊕⊕⊕⊝ | |||
| Cost of analgesic regimens for single‐injection blocks Follow‐up: in‐hospital | Mean cost of analgesic regimens for single‐injection blocks in intervention groups was | 75 | ⊕⊕⊕⊝ | |||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades for certainty of evidence. | ||||||
| aThe effect was still present even when trials at high risk of bias were withdrawn from the analysis, or when a correction for the possibility of publication bias was applied. bThe difference was equivalent to 2.5 on a scale from 0 to 10. cThe number needed to treat for additional beneficial outcome was 12 (95% confidence interval 7 to 47). dDowngraded by two levels for imprecision. eDowngraded by one level for imprecision. fThe number needed to treat for additional beneficial outcome was 7 (95% confidence interval 5 to 72). gMean costs in 2009 euros. | ||||||
| Study | Purpose of blockade | Time of block placement | Surgical anaesthesia | Block technique | Comparison | Supplemental analgesia for both groups |
| Preoperative analgesia | In the emergency department | No information | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.5% with epinephrine 1:200,000 30 mL Operator: trained emergency physicians | Sham block with normal saline | Acetaminophen Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | Preoperatively, probably in the emergency department | Spinal anaesthesia | Psoas compartment block Nerve stimulator (quadriceps contraction at 0.5 mA, 1 Hz, Continuous infusion Bupivacaine 0.1% 20 mL followed by patient‐controlled analgesia: basal rate 8 mL/hour, bolus 5 mL, lock‐out time 30 minutes for 72 hours Operator: no information | No nerve block IV PCA with Morphine | Acetaminophen Ketorolac | |
| Postoperative analgesia | After recovery of anaesthesia | Spinal anaesthesia | Femoral nerve block Nerve stimulator Continuous infusion Levobupivacaine 0.25% 18 mL followed by levobupivacaine 0.125% at 3 to 4 mL/hour for 24 hours after surgery Operator: no information | No nerve block | Acetaminophen Pethidine | |
| Postoperative analgesia | After surgery and after confirmation of patient’s mental status to be alert, able to communicate, and obey commands | Spinal anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Single injection Ropivacaine 0.2% 40 mL Operator: no information | No nerve block | Ketorolac Celecoxib IV PCA with Fentanyl Tramadol | |
| Preoperative, intraoperative, and postoperative analgesia | Preoperatively, after patients had been assigned to a bed on the ward | Spinal (53% for intervention group and 40% for comparator group) or general anaesthesia | Fascia iliaca compartment block Landmarks Continuous infusion Ropivacaine 0.125% 40 mL followed by ropivacaine 0.2% 10 mL/hour until surgery. In the operating room, catheters were Operator: anaesthesiology department | No nerve block | Acetaminophen NSAIDs Opioids | |
| Preoperative, intraoperative, and postoperative analgesia Surgery for some participants | Preoperatively, within 6 hours after admission to the orthopaedic ward | Intervention: psoas block alone (3/20) with sciatic block (5/20), spinal (11/20) or general anaesthesia (1/20) Comparator: neuraxial block (19/20) or general anaesthesia (1/20) | Psoas compartment block Landmarks and loss of resistance to air, lateral decubitus Continuous infusion: started preoperatively (16 to 48 hours) and kept for 72 hours after surgery Test dose with 3 mL of 0.5% bupivacaine with epinephrine 5 mcg/mL followed by bupivacaine 0.25% with epinephrine Operator: anaesthesiologists | No nerve block IM Meperidine Diclofenac | IM Meperidine | |
| Postoperative analgesia | At completion of surgery before awakening from general anaesthesia | General anaesthesia | 1) Lateral femoral cutaneous nerve block Landmarks Single injection 1) Bupivacaine 0.5% with epinephrine 5 mcg/mL 15 mL Operator: anesthesiology department | No nerve block | Pethidine | |
| Postoperative analgesia | After ending of effects of spinal blockade | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps for patella ascension with 0.3 to 0.5 mA at 0.1 ms and catheter 10 to 15 cm passed over the needle tip) Continuous infusion Lidocaine 1.5% plus epinephrine 30 mL of lidocaine 1.5% followed by ropivacaine 0.2% at 10 mL/hour for 48 hours Operator: anesthesiology department | No nerve block IV Paracetamol for half of participants in the comparator group | 1 dose of paracetamol in the emergency department Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | Upon hospital arrival | No information | Femoral nerve block Dual technique: ultrasound‐guided plus nerve stimulator Continuous infusion Ropivacaine 0.2% 15 mL followed by ropivacaine 0.2% at 5 mL/hour basal rate plus boluses of 10 mL every 30 Operator: no information | No nerve block IV Metamizole | IV Tramadol | |
| Intraoperative and postoperative analgesia | In the operating room, before induction of general anaesthesia | General anaesthesia | 1) Fascia iliaca compartment block 1) Ultrasound‐guided 2) Dual technique: ultrasound‐guided plus nerve stimulator Single injection 1) Bupivacaine 0.25% 30 mL Operator: anesthesiology department | No nerve block | Tenoxicam IV PCA with Tramadol | |
| Spinal positioning, intraoperative and postoperative analgesia | Before positioning for spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.5% 40 mL Operator: anesthesiology department | No nerve block IV Fentanyl for positioning for spinal block | IV PCA with Morphine | |
| Spinal positioning, intraoperative and postoperative analgesia | In the regional anaesthetic technique room, before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.5% 15 mL and lidocaine 2% 15 mL Operator: anesthesiology department | No nerve block | IV PCA with Morphine Tramadol | |
| Preoperative analgesia | In the emergency department, after radiographic confirmation | No information | 3‐in‐1 femoral nerve block Paraesthesia Single injection Bupivacaine 0.5% 20 mL Operator: trained emergency physicians | No nerve block | IV Morphine | |
| Preoperative analgesia | Upon arrival in the emergency department | No information | Fascia iliaca compartment block Landmarks Single injection Mepivacaine 1% with epinephrine 5 mcg/mL 40 mL Operator: junior anaesthesiologists with less than 2 years of training | Sham block with 0.9% saline plus IM Morphine | IV Morphine Epidural analgesia after 3‐hour study period | |
| Preoperative, intraoperative. and postoperative analgesia | Upon arrival in the emergency department | Intervention: spinal anaesthesia for 37/50 and general anaesthesia for 13/50 Comparator: spinal anaesthesia for 38/50 and general anaesthesia for 12/50 | Femoral nerve block Nerve stimulator (0.5 mA and 0.1 millisecond) Continuous infusion (non‐stimulating catheters advanced about 10 cm past the needle tip) Prilocaine 1% 40 mL followed 2 hours later by ropivacaine 0.2% 30 mL, repeated every 6 hours (up to 40 mL; N = 5) and at intervals (up to every 4 hours; N = 8) or both (N = 6), adjusted on pain scores Operator: anaesthesiology department | No nerve block IV Metamizole Oral Tilidine and Naloxone | Ibuprofen Tilidine | |
| Preoperative analgesia | In the emergency department, after confirmation of diagnosis | No information | Fascia iliaca compartment block Landmarks Single injection Bupivacaine 0.25% 0.3 mL/kg Operator: physicians (first study author is an orthopaedic surgeon) | Sham block with saline and IV NSAIDs | NSAIDs Opioids | |
| Preoperative analgesia | In the emergency department | No information | Femoral (3‐in‐1) nerve block Single injection Nerve stimulator Bupivacaine 0.5% 30 mL (not exceeding 3 mg/kg) Operator: specialist emergency physician or higher trainee resident, post intermediate examination level | No nerve block IV Morphine | IV Morphine Dihydrocodeine Diclofenac Paracetamol | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | No information | Fascia iliaca compartment block Single injection Ultrasound‐guided Levobupivacaine 0.375% 30 mL Operator: anesthesiology department | No nerve block IV Remifentanil | Additional analgesia | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Single injection Bupivacaine 0.25%.0.3 mL/kg Paraesthesia technique with a short bevel needle Operator: 1 orthopaedic registrar | No nerve block | Co‐dydramol Voltarol Pethidine | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Nerve stimulator Single injection Bupivacaine 0.5% Operator: trained emergency physicians | No nerve block | Opioids | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block No information on localizing technique Single injection Lidocaine 1% 2 mg/kg Operator: anaesthesiology department | No nerve block IV Ketamine 0.2 mg/kg IV Midazolam 0.025 mg/kg | Ketamine | |
| Intraoperative and postoperative analgesia | Before induction of general anaesthesia | General anaesthesia | 1) Femoral "3‐in‐1" nerve block 1) Nerve stimulator (quadriceps contraction with < 1 mA) 2) Landmarks Single injection 1) Prilocaine 0.75% 35 mL Operator: anaesthesiology department | No nerve block | Papaveratum | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps contraction with 0.3 to 0.5 mA) Single injection Lidocaine 1.5% (2% diluted with distilled water) with epinephrine 5 mcg/mL 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | IV Fentanyl | |
| Preoperative analgesia | In the emergency department, 48 hours before surgery | No information | Femoral nerve block Single injection Ultrasound‐guided (in‐plane) Bupivacaine 0.5% 0.3 mL/kg (maximum 20 mL) Operator: 1 physician experienced in administering ultrasound‐guided femoral nerve blocks | Sham block with saline | IV Tramadol | |
| Postoperative analgesia | At completion of surgery, while still under general anaesthesia | General anaesthesia | Lateral femoral cutaneous nerve block Single injection Landmarks Bupivacaine 0.5% with epinephrine 5 mcg/mL 15 mL Operator: anaesthesiology department | No nerve block | IM Pethidine | |
| Preoperative analgesia | As soon as the diagnosis of hip fracture was radiologically confirmed | No information | Femoral nerve block Nerve stimulator Single injection Ropivacaine 0.75% 30 mL. Operator: 1 orthopaedic surgeon | No nerve block | Paracetamol Tramadol Ketobemidon | |
| Preoperative analgesia | Within 1 hour of hospital admission | No information | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.2% 30 mL Operator: orthopaedic surgeons | Sham block with saline | IV Morphine Paracetamol | |
| Preoperative analgesia | In the emergency department | No information | 3‐in‐1 femoral nerve block Ultrasound‐guided (in‐plane) Single injection Bupivacaine 0.5% 25 mL Operator: emergency physicians experienced with the technique | Sham block with saline | Morphine | |
| Preoperative, intraoperative, and postoperative analgesia | In the emergency department | Spinal anaesthesia | Femoral "3‐in‐1" nerve block Ultrasound‐guided Continuous infusion (catheters inserted ≥ 12 to 15 cm past the needle tip) Bupivacaine 0.25% 30 mL (additional 10 mL if required for adequate sensory blockade) followed by bupivacaine Operator: anesthesiology department | No nerve block | Piritramide Paracetamol | |
| Preoperative analgesia | After hospital admission | No information | Fascia iliaca compartment block Ultrasound‐guided (in‐plane) Continuous infusion (catheters 5 to 10 cm beyond the tip of the needle) Ropivacaine 0.4% 30 mL followed by ropivacaine 0.2% at 5 mL/hour plus 5 mL for breakthrough pain until surgery (mean 3.5 days). Catheters removed on the morning of surgery Operator: 1 anaesthesiologist experienced in ultrasound‐guided nerve block | No nerve block | Tramadol Acetaminophen Pethidine | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.375% 30 mL Operator: anaesthesiologists | No nerve block IV Fentanyl | Paracetamol Tramadol Diclofenac | |
| Preoperative analgesia, intraoperative and postoperative analgesia | In the emergency department for femoral nerve block and within 24 hours of femoral block for continuous fascia iliaca block | Regional anaesthesia for 62.1% | 1) Femoral nerve block Ultrasound‐guided (out‐of‐plane for insertion, but advancement visualized) 1) Single injection Bupivacaine 0.5% 20 mL 2) Continuous infusion Ropivacaine 0.2% 15 mL followed by 5 mL/hour for 72 hours after surgery Operators: 1) Trained emergency physicians 2) Anaesthesiologists (mobile peripheral nerve block service) | No nerve block | Opioids Acetaminophen | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca block with 20 mL of 1.5% lidocaine No information for localizing technique Single injection Lidocaine 1.5% 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | No information | |
| Preoperative and postoperative analgesia | Started upon admission to the orthopaedic ward | Epidural anaesthesia | Fascia iliaca compartment blocks daily (from admission until surgery, restarted at 24 hours after surgery until discharge, stopped earlier (before or after surgery) if delirium occurred) Landmarks Bupivacaine 0.3 mL/kg (0.25%?) Operator: orthopaedic surgeons | Sham blocks with water | IV Paracetamol Pethidine | |
| Preoperative analgesia | In the emergency department | No information | Femoral nerve block Nerve stimulator (quadriceps contraction with patellar ascension) Single injection Mepivacaine 20 mL Operator: unclear, published by emergency physicians | No nerve block IV Morphine or IV Paracetamol and Ketoprofen | Nitrous oxide | |
| Postoperative analgesia | After closure of the surgical wound | General anaesthesia | Fascia iliaca block Landmarks Continuous infusion (catheter inserted ≥ 10 cm cranially) Ropivacaine 0.5% according to body weight (20 mL if Operator: no information, probably anaesthesiology department | No nerve block IV PCA with Fentanyl | Acetaminophen Dihydrocodeine Morphine | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Femoral nerve block Dual technique: nerve stimulator plus in‐plane ultrasound Single injection Lidocaine 2% 20 mL Operator: anaesthesiology department | No nerve block IV Fentanyl | IV Fentanyl | |
| Postoperative analgesia | In post‐anaesthesia care unit after full recuperation of motor blockade from the spinal block | Spinal anaesthesia | 1) Lateral femoral cutaneous nerve block Landmarks Single injections 1) Bupivacaine 0.5% with vasoconstrictor 5 mL Operator: anaesthesiology department | No nerve block | IV Metamizole Dexketoprofen trometamol Tramadol Morphine | |
| Postoperative analgesia | Catheters inserted before spinal anaesthesia Administration of local anaesthetics started after surgery | Spinal anaesthesia | Femoral nerve block Nerve stimulator Continuous infusion (non‐stimulating catheter advanced 8 to 15 cm past needle tip) Bupivacaine 0.5% 0.4 mL/kg followed by bupivacaine 0.25% at 0.14 mL/kg/hour for 16 hours after surgery Operator: anaesthesiology department | Sham block with saline | Morphine Acetylsalicylic acid | |
| Preoperative, intraoperative, and postoperative analgesia | Catheters inserted in the emergency department Administration of local anaesthetics started during catheter installation | Spinal anaesthesia | Femoral nerve block Nerve stimulator (quadriceps contraction resulting in Continuous infusion (non‐stimulating catheter, space dilated with 10 mL of lidocaine 2%, catheter advanced cephalad 3 cm past the needle tip) Bupivacaine 0.5% 10 mL followed by 0.25% bupivacaine at 4 mL/hour for 72 hours Operator: anaesthesiology department | No nerve block | Paracetamol Morphine | |
| Intraoperative and postoperative analgesia | Immediately before induction of anaesthesia | General or spinal anaesthesia (38%) | Fascia iliaca compartment block Ultrasound‐guided Single injection Ropivacaine 0.25% 30 mL Operator: a board‐certified anaesthesiologist | No nerve block | Acetaminophen Tramadol Opioids | |
| Postoperative analgesia | After surgery and reversal of neuromuscular blockade | General anaesthesia | Femoral (3‐in‐1) nerve block Nerve stimulator (quadriceps contraction with patellar ascension with < 1 mA) Continuous infusion (non‐stimulating catheter advanced 4 to 5 cm past the needle tip) Lidocaine 2% with epinephrine 5 mcg/mL 30 mL followed by bupivacaine 0.125% patient‐controlled analgesia: basal rate 4 mL/hour, boluses 3 mL, lockout time 20 minutes Operator: probably anaesthesiology department | No nerve block IV PCA with Morphine | Tenoxicam | |
| Preoperative analgesia | Before surgery, as soon as possible after admission to the orthopaedic ward | No information | Femoral nerve block Nerve stimulator (quadriceps contraction) Single injection Levobupivacaine 0.25% 20 to 40 mL In case of delayed surgery or if otherwise necessary, participants could receive 1 additional block Operator: 36 anaesthesiologists with various training | No nerve block | Opioids | |
| Preoperative analgesia | In the emergency department | Spinal anaesthesia | Femoral nerve block | No nerve block IV Paracetamol | IV Tramadol Epidural analgesia after surgery | |
| Preoperative, intraoperative, and postoperative analgesia | Upon admission, after radiographic confirmation of the diagnosis | Combined spinal‐epidural anaesthesia | Fascia iliaca compartment block Ultrasound‐guided (out‐of‐plane for needle insertion and in‐plane for solution diffusion, injected cephalad) Continuous infusion (catheter inserted 5 to 10 cm past the needle tip) Ropivacaine 0.4% 50 mL followed by ropivacaine 0.2% at 5 mL/hour (plus 5 mL top‐up doses) Operator: anaesthesiologist with experience in ultrasound‑guided nerve block | Sham block with saline Paracetamol Tramadol | IVPCA with Sufentanil after surgery | |
| Intraoperative and postoperative analgesia | After induction of anaesthesia, before surgery | General anaesthesia | Psoas compartment block Landmarks Single injection Mepivacaine 2% 30 mL Operator: anaesthesiology department | No nerve block | Usual surgical care | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Single injection Levobupivacaine 0.25% 40 mL Operator: an orthopaedic surgeon with extensive experience in this block procedure | No nerve block IV Acetaminophen | Diclofenac Rescue analgesics | |
| Intraoperative and postoperative analgesia | Catheter insertion and local anaesthetic administration started before induction of anaesthesia | General anaesthesia | Fascia iliaca compartment block Ultrasound‐guided Continuous infusion Ropivacaine 0.33% 30 mL followed by 0.15% ropivacaine at 2 mL/hour plus a bolus of 30 mL Operator: anaesthesiology department | No nerve block IV PCA with Sufentanil | Rescue analgesics | |
| Spinal positioning, intraoperative and postoperative analgesia | Before spinal anaesthesia | Spinal anaesthesia | Fascia iliaca compartment block Landmarks Single injection Ropivacaine 0.375% 30 mL Operator: 1 experienced anaesthesiologist | No nerve block IV Alfentanil | IV Alfentanil for spinal block Pethidine before spinal block and after surgery | |
| G: gram. h: hour. IM: intramuscular. IV: inteavenous. mA: milliAmpere. mcg/mL: microgram/millilitre. mg/kg: milligram/kilogram. MHz: megahertz. mL: millilitre. msec: millisecond. n: number. NSAIDs: non‐steroidal anti‐inflammatory drugs. PCA: patient‐controlled analgesia. SC: subcutaneous. | ||||||
| Study | Complications related to regional anaesthesia | Complications related to analgesic technique |
| Not reported | Not reported | |
| Not reported | Not reported | |
| No complications such as motor block. local haematoma or infection, inadvertent arterial puncture, direct nerve damage, and cardiovascular or neurological toxicity were observed Five participants had accidental removal of the catheter: 4 during the procedure or while the catheter was secured, and 1 while in the ward | Not reported | |
| No patient developed any residual sensory‐motor deficit during the postoperative period | Patients in the non‐block group had nausea (N=2) | |
| Not reported | Respiratory complications in 5 out of 15 participants for each group Opioid side effects after enrolment: 3/15 in the block group; 7/15 in the non‐block group | |
| No major complications in group regional blockade were described. Three participants developed local erythema at the catheter insertion site at the end of the study period No signs of local anaesthetic toxicity were documented One participant developed bilateral blockade (L1‐L3 on the opposite side) | Not reported | |
| No complications related to nerve blocks and no case of prolonged motor blockade | Not reported | |
| Four catheters were prematurely removed: 1 by a confused participant, 2 by nurses (unexplained fever), and 1 by a surgeon (unconfirmed suspicion of local anaesthetic toxicity) (ropivacaine blood level < 2 ng/mL)) | More constipation (47% vs 19% for regional blockade) | |
| Not reported | Not reported | |
| Hypotension occurred in 1 participant in the fascia iliaca compartment block group (1/20) and in 1 participant in the femoral nerve block group (1/20) There was no complication that might be relevant to fascia iliaca compartment block in our study In 1 case, prolonged (4 months) temporary motor and sensory neurological deficits occurred due to 3‐in‐1 block | Hypotension occurred in 2 patients with IV patient‐controlled analgesia (2/20), requiring stopping of IV patient‐controlled analgesia | |
| Complications such as local anaesthetic toxicity recorded as well (none reported in results section) Nor did complication rates vary between groups | Complications such as hypoventilation (breathing rate < 8 breaths/min) were recorded as well Moreover, the 2 groups did not differ in these parameters at any time point until study completion at 24 hours after surgery. Nor did complication rates vary between groups | |
| Not reported | Not reported | |
| Among study participants, none experienced adverse effects as a result of nerve block administration | No clinically important differences between groups with respect to pulse rate, oxygen saturation, or respiratory rate at any time interval. Oxygen saturation 94.87% | |
| No side effects attributable to femoral nerve block were noted in any participants during their hospital stay | More participants (P = 0.05) in the morphine group were sedated at 180 minutes after block placement No difference in nausea and vomiting was noted between groups, with 3 participants in each group having these side effects Tendency towards lower saturation was noted in the opioid group at 60 and 180 minutes after the block despite oxygen supplementation (P = 0.08) | |
| One inadvertent arterial puncture and blood aspiration positive for 3 participants Two transient paraesthesias No catheter site infection Ten catheters accidentally removed No severe complications related to analgesia | No respiratory depression from systemic analgesia and no allergic reactions All complications were reversible | |
| The only complications were local bruises at the site of injection | Two participants with nausea and 2 with nausea and vomiting | |
| No immediate complications occurred in either group defined as inadvertent vascular puncture, anaphylaxis or collapse, severe pain, or inability to tolerate the procedure | No immediate complications were noted in either group | |
| No local or systemic complications of femoral nerve blocks were noted | Not reported | |
| No complications associated with femoral nerve block were noted | Not reported | |
| One patient was withdrawn from the fascia iliaca compartment block group due to new‐onset arrhythmia | Not reported | |
| No untoward sequelae were associated with nerve blocks All plasma prilocaine concentrations (maximum 3 pg/mL) were below the suggested threshold for toxicity for prilocaine of 6 pg/mL | Not reported | |
| Not reported | In participants of fentanyl group, drowsiness was observed that required the presence of more persons holding the participant during positioning SpO2 was significantly lower in the fentanyl group (P = 0.001). However, no participant in either group had SpO2 < 90% during the procedure Mean arterial blood pressure was significantly lower in the fentanyl group (P = 0.0019) | |
| All femoral nerve block procedures required a single attempt and no complications were observed | Nausea and vomiting 4 vs 6, hypotension 2 vs 4, pruritus 0 vs 1, and desaturation 3 vs 2 for intervention and comparator, respectively | |
| No untoward sequelae associated with the nerve block were seen | Not reported | |
| No complications related to the nerve blockade were noted in this study | Not reported | |
| No serious adverse events due to the fascia iliaca compartment block were reported in this study | Not reported | |
| No other adverse events were noted during the study period, and no other adverse events were reported to study investigators | Four‐hour oxygen saturation (%) 96 (93 to 99) vs (%) 98 (95 to 99) for regional blockade Adverse events: One participant had an episode of rapid atrial fibrillation requiring diltiazem, but the participant had a history of chronic atrial fibrillation | |
| Not reported | Not reported | |
| Two patients’ catheters kinked. This problem was solved after the catheter was adjusted No other complications (local anaesthetic toxicity, puncture site infection, haematoma, catheter dislodgment) occurred | The occurrence of nausea and vomiting in group fascia iliaca compartment block were lower than those in group control. No patients experienced respiratory depression and over‐sedation in 2 groups during the waiting period | |
| No complications were noted in either group | No complications were noted in either group | |
| There were no episodes of bleeding, falls, or catheter‐related infections in the intervention group | Intervention participants were significantly less likely to report opioid side effects | |
| Not reported | Not reported | |
| No complications of femoral nerve block administration occurred, except 3 local haematomas developed at the injection site, which resolved spontaneously | Not reported | |
| Not reported | Not reported | |
| No adverse effects such as pain at the insertion site or paraesthesia were observed No positive cultures were observed with the fascia iliaca block catheter tip, nor were any signs of infection noted in the current study | Not reported | |
| There was no inadvertent vascular puncture nor adverse effect of systemic local anaesthetic toxicity in the study group | SpO₂ was significantly lower in the IV fentanyl group during positioning (95 vs 97; P < 0.001) and 5 minutes after (95 vs 98; P < 0.001). | |
| We did not observe any complications in the realization of regional anaesthetic techniques during or subsequent to these techniques | The incidence of side effects (sleepiness, hypotension, constipation, pruritus) was greater in the group with no block than in groups with blocks (P < 0.01) | |
| No haematomas at the site of femoral catheters | Two participants in each group experienced nausea and vomiting | |
| For 1 participant, the elastomeric pump failed, resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, and another participant, suffering from acute confusional state, disconnected his pump after 12 hours | The incidence of nausea/vomiting, pruritus, or excessive sedation was similar in the 2 groups | |
| Of the 23 patients in group fascia iliaca compartment block, there were no intervention‐related complications or adverse events. None of the patients receiving a block reported residual injection site pain, sensory or motor deficits, intravascular injections, cardiopulmonary events, or other adverse events | Not reported | |
| Not reported | Side effects (vomiting and pruritus) were observed significantly more frequently with intravenous analgesia | |
| No adverse events related to the femoral nerve block were noted | Not reported | |
| Not reported | Not reported | |
| The study group did not develop complications (local anaesthetic toxicity, puncture site infection, hematoma in preoperative waiting period) | All patients in the present study did not demonstrate symptoms of respiratory depression and excessive sedation in the preoperative waiting period Nausea 7 vs 12 and vomiting 5 vs 5 for intervention and comparator, respectively | |
| No participants showed any evidence of local anaesthetic toxicity | Not reported | |
| Patients were also evaluated for potential drug‐ or block‐related complications during the course of the trial No complications | Patients were also evaluated for potential drug‐ or block‐related complications during the course of the trial No complications | |
| Not reported | Fewer side effects for fascia iliaca compartment block group Nausea and vomiting 0 vs 3, respiratory depression 0 vs 1 for intervention and comparator, respectively | |
| No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited No complications such as haematoma or persistent paraesthesia were observed in participants with a femoral nerve block within 24 hours after the operation | Hypoventilation (ventilatory rate 6 to 8/min) or pulse oximetric desaturation (oxygen saturation 88% or 89%) was encountered in 4 participants (20%) in the intravenous analgesia group. This was reverted with assisted manual mask ventilation All participants in the intravenous group experienced mild dizziness, and mild drowsiness was present in 12/20 of them | |
| Brief summary: For peripheral nerve block, there was no case of systemic local anaesthetic toxicity and no infection. One case of prolonged (4 months) temporary motor and sensory neurological deficit occurred due to a 3‐in‐1 block (Deniz 2014). One new‐onset arrhythmia was reported (Hogg 2009). Four cases of respiratory depression requiring face mask ventilation were reported with intravenous analgesia (Yun 2009). Other opioid side effects such as drowsiness, hypoventilation, desaturation, hypotension, nausea and vomiting, pruritus, and constipation were reported in both groups. No allergic reaction was reported. %: percentage. L: litre. mg: milligram. min: minute. ng/mL: nanogram/millilitre. pg/mL: picogram/millilitre. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain on movement within 30 minutes of block placement Show forest plot | 11 | 503 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.25, ‐0.86] |
| 1.1.1 Fascia iliaca compartment block | 7 | 309 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐1.42, ‐0.92] |
| 1.1.2 Femoral nerve block | 4 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.19, ‐0.60] |
| 1.2 Acute confusional state Show forest plot | 13 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 1.2.1 Peripheral nerve block based on landmarks | 4 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.13] |
| 1.2.2 Peripheral nerve block based on nerve stimulator | 3 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.97] |
| 1.2.3 Peripheral nerve blocks inserted on ultrasound guidance | 6 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.44, 1.20] |
| 1.3 Myocardial infarction Show forest plot | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 Chest infections Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.89] |
| 1.5 Mortality Show forest plot | 11 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.47, 1.60] |
| 1.5.1 Single‐injection block | 6 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.44, 2.24] |
| 1.5.2 Continuous infusion | 5 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.89] |
| 1.6 Time to first mobilization Show forest plot | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐10.80 [‐12.83, ‐8.77] |
| 1.7 Costs of analgesic drugs Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐4.84, ‐3.96] |
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.1.1 Fascia iliaca compartment block | ||||||||||||
| Albrecht 2014 |  |  |  |  |  |  | ||||||
| Randomly allocated according to a computer‐generated list of random numbers and allocation concealed in sealed opaque envelopes. Intervention group had lower pain score at baseline. This difference was judged as compatible with what could be expected from chance alone in a study with a sample size. | ||||||||||||
| No deviations from intended interventions identified | ||||||||||||
| 100% of included participants were analyzed | ||||||||||||
| Pain scores collected by a nurse blinded to the intervention group | ||||||||||||
| Study authors elected to deviate from the planned statistical analysis after knowing the results. | ||||||||||||
| This trial was judged as at high risk of bias for this outcome due to the fact that study authors elected to deviate from the planned statistical analysis after knowing the results. | ||||||||||||
| Diakomi 2014 |  |  |  |  |  |  | ||||||
| Patients were randomly assigned, using a sealed envelope method and there was no baseline differences between intervention groups. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 98% of included participants were analysed | ||||||||||||
| Pain scores collected by an anaesthesiologist blinded to the intervention group. | ||||||||||||
| No deviation to the planned statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Domac 2015 |  |  |  |  |  |  | ||||||
| Patients included in the study were divided into two equal groups for this prospective double‐blind study. No difference between intervention groups at baseline identified. | ||||||||||||
| No deviations from intended interventions identified | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Pain scores probably collected by an assessor blinded to the intervention group. | ||||||||||||
| No deviation to the statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Foss 2005a |  |  |  |  |  |  | ||||||
| The randomization was done via a computer‐generated list. Pain at rest before intervention was higher in the intervention group (P = 0.04). The imbalance can be compatible with the one expected due to chance alone in a study with a small sample size. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| One patient did not have a fracture but only a severe contusion and was excluded after x‐ray; an extra patient was therefore included on a new number. 98% of included participants were analyzed | ||||||||||||
| Pain scores collected by an assessor blinded to the intervention group. | ||||||||||||
| No deviation from the plan analysis identified. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Hogg 2009 |  |  |  |  |  |  | ||||||
| Prospective, randomised controlled trial and no baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 98% of included participants were analysed. | ||||||||||||
| Pain scores. Although this is a subjective score, the fact that a correlation between the effect size and the local anaesthetic drug concentration was found in the review (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Landsting 2008 |  |  |  |  |  |  | ||||||
| Randomization was carried out using a computer, and information about the study intervention was sealed in envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| Only 56% of included participants had data available for the time point selected by review authors. We were unable to determine if missingness was related to the outcome or not. We therefore deemed it prudent to judge this trial at high risk of bias for this domain for this outcome. | ||||||||||||
| Pain scores derived from a combination of self‐rating scales collected by a blinded assessor. | ||||||||||||
| No deviation to the statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| Judged as at high risk of bias for this outcome due to high number of missing data at the time point selected by review authors and uncertainty as to whether or not missingness could be related to this outcome. | ||||||||||||
| Yun 2009 |  |  |  |  |  |  | ||||||
| Randomly assigned using an allocation sequence generated by a computer, and allocation sequence concealed in envelopes until group was assigned. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations form intended interventions identified. | ||||||||||||
| 100% of included participants analyzed. | ||||||||||||
| Pain scores collected by an assessor probably blinded to te intervention group. | ||||||||||||
| No deviations from the planned statistical analysis identified and only one result provided for the time point selected by the review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Subgroup 1.1.2 Femoral nerve block | ||||||||||||
| Gille 2006 |  |  |  |  |  |  | ||||||
| Randomization in two groups by the anaesthesiologist. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the planned statistical analysis reported, only one results provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Murgue 2006 |  |  |  |  |  |  | ||||||
| Randomized by “tirage au sort (translated as "hat drawing) ” and no baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 94% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the planned statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Ranjit 2016 |  |  |  |  |  |  | ||||||
| Selected patients were randomized by sealed envelope technique and no baseline differences between intervention groups were identified. | ||||||||||||
| No deviations from the intended interventions were identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviation to the planned statistical analysis reported. Only one result provided for the time point selected by review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Szucs 2010 |  |  |  |  |  |  | ||||||
| Randomized using a random number sequence and sealed envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 89% of included participants were analyzed. | ||||||||||||
| Pain scores. This is a subjective score but the fact that a correlation between the effect size and the local anaesthetic drug concentration was found by the review authors (meta‐regression P value = 0.0003) seems to indicate that scores were valid indicators of pain on movement. | ||||||||||||
| No deviations from the planned statistical analysis identified and only one result provided for the time point selected by the review authors. | ||||||||||||
| No risk of bias identified | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.2.1 Peripheral nerve block based on landmarks | ||||||||||||
| Godoy Monzon 2010 |  |  |  |  |  |  | ||||||
| Randomized using numbers generated by a computer. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 88% of included participants analyzed: 92 for the intervention group and 62 for the comparator group. We were uncertain if missingness was related to the outcome or not. Therefore, we deemed it prudent to judge this trial at high risk of bias for this domain for this outcome. | ||||||||||||
| Delirium. To be qualified as delirious, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as delirious or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| Judged as at high risk of bias for this outcome due to high number of missing data and inability to determine whether or not missingness was related to this outcome | ||||||||||||
| Mouzopoulos 2009 |  |  |  |  |  |  | ||||||
| Computer‐generated randomization code. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. | ||||||||||||
| Diagnosis of the syndrome was defined using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) and Confusion Assessment Method (CAM) criteria [1, 21]. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Nie 2015 |  |  |  |  |  |  | ||||||
| Randomly assigned according to a computer‐generated random number table. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 98% of included participants were analyzed. | ||||||||||||
| The Confusion Assessment Method was used to diagnose delirium pre‐ and postsurgery. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| White 1980 |  |  |  |  |  |  | ||||||
| Patients were randomly allocated. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| Participants with failed block were excluded: 4/20 no other exclusion. So, 90 % of included participants analyzed. | ||||||||||||
| Confusion. To be qualified as confused, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Subgroup 1.2.2 Peripheral nerve block based on nerve stimulator | ||||||||||||
| Cuvillon 2007 |  |  |  |  |  |  | ||||||
| Randomized using sealed numbered envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Results are given for sedation and/or confusion. To be qualified as confused, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. Participants in comparator groups received less morphine than the block group, we therefore have no reason to believe that the highest number of participants with sedation and or confusion in the comparator group were assessed as positive for this outcome because they were excessively sedated from morphine. Also, results of this trial are consistent with results of the other trials included in the analysis. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Graham 2008 |  |  |  |  |  |  | ||||||
| Randomized by numbered, sequential, sealed opaque envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 85% of included participants were analyzed. | ||||||||||||
| Confusion. To be qualified as confused, a patient has to show clear symptoms of confusion. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Kullenberg 2004 |  |  |  |  |  |  | ||||||
| Randomized using the envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| An experienced nurse evaluated patients' mental status with the Short Portable Mental Status Questionnaire, Pfeiffer‐test, graded according to a 4‐degree scale. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Subgroup 1.2.3 Peripheral nerve blocks inserted on ultrasound guidance | ||||||||||||
| Brownbridge 2018 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| CAM‐ICU scoring system will be used daily to measure delirium. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Liebmann 2012 |  |  |  |  |  |  | ||||||
| Randomization occurred using an Internet‐based program. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. | ||||||||||||
| Confusion. To be qualified as confused, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as confused or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Morrison 2008 |  |  |  |  |  |  | ||||||
| Randomized using a computer‐generated, stratified, blocked randomization list, with stratification according to site and allocation concealed in sealed envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. | ||||||||||||
| Confusion Assessment Method supplemented by chart review evaluated by an assessor blinded to the treatment group. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Uysal 2018 |  |  |  |  |  |  | ||||||
| A randomized controlled trial. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 83% of included participants were analyzed. | ||||||||||||
| The delirium status of patients was assessed using “Delirium Rating Scale‐R‐98 (DRS‐R‐98)” in the postoperative period for three days. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Yamamoto 2016 |  |  |  |  |  |  | ||||||
| Randomisation was performed with a random number list generated by a computer software. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Delirium occurring within 24 h after surgery was diagnosed by the confusion assessment method. The method chosen to evaluate the outcome makes it unlikely to be influenced by possible knowledge of assignment. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Yang 2016 |  |  |  |  |  |  | ||||||
| Randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Delirium. To be qualified as delirious, a patient has to show clear symptoms of disorientation. It seems to us that knowledge of the intervention group was not likely to influence the fact that a patient was diagnosed as delirious or not. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Altermatt 2013 |  |  |  |  |  |  | ||||||
| Randomized using a computer generated random number table. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Serial EKGs and troponin concentration measurements were performed daily until postoperative day 3 or more frequently if an ischemic episode was suspected. Analysis of ST segments were evaluated a posteriori by a cardiologist blinded the allocated group. | ||||||||||||
| No deviation to the statistical analysis reported. No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Fletcher 2003 |  |  |  |  |  |  | ||||||
| The randomization sequence was derived from a random number generator, and allocation concealment was achieved by means of the sealed opaque envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Lower respiratory tract infections determined by a blinded assessor. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Haddad 1995 |  |  |  |  |  |  | ||||||
| Randomized by sealed envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 90% of included participants were analyzed. | ||||||||||||
| Chest infections which required antibiotics. We judged it as unlikely to have been influenced by knowledge of the treatment group. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| White 1980 |  |  |  |  |  |  | ||||||
| Patients were randomly allocated. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 80% of included participants were analyzed. | ||||||||||||
| Pneumonia. We judged it as unlikely to have been influenced by knowledge of the treatment group. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.5.1 Single‐injection block | ||||||||||||
| Albrecht 2014 |  |  |  |  |  |  | ||||||
| Randomly allocated according to a computer‐generated list of random numbers and allocation concealed in sealed opaque envelopes. Intervention group had lower pain score at baseline. This difference was judged as compatible with what could be expected from chance alone in a study with a sample size. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Death from all causes | ||||||||||||
| This outcome was not part of the outcomes when the trial was registered. There was no other planned measurement at 3 months. | ||||||||||||
| Judged as at high risk of bias for this outcome due to possibility that this outcome was not pre‐determined for the specific time point at which it was measured | ||||||||||||
| Fletcher 2003 |  |  |  |  |  |  | ||||||
| The randomization sequence was derived from a random number generator, and allocation concealment was achieved by means of the sealed opaque envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Haddad 1995 |  |  |  |  |  |  | ||||||
| Randomized by sealed envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Hood 1991 |  |  |  |  |  |  | ||||||
| Randomly allocated by choosing an unmarked envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Jones 1985 |  |  |  |  |  |  | ||||||
| Prospective controlled randomised trial, an envelope was opened after surgery completion. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| White 1980 |  |  |  |  |  |  | ||||||
| Patients were randomly allocated. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| Participants with failed block were excluded: 4/20 no other exclusion. So, 90 % of included participants analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Subgroup 1.5.2 Continuous infusion | ||||||||||||
| Brownbridge 2018 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Cuvillon 2007 |  |  |  |  |  |  | ||||||
| Randomized using sealed numbered envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| De La Tabla 2010 |  |  |  |  |  |  | ||||||
| Prospective, randomized study. 49 patients were included: 38 in group 1 (77,6%) and 11 in group 2 (22,4%). | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| Judged as at high risk of bias due to a possible problem with randomization leading to two very unequal number of participants per group. | ||||||||||||
| Morrison 2008 |  |  |  |  |  |  | ||||||
| Randomized using a computer‐generated, stratified, blocked randomization list, with stratification according to site and allocation concealed in sealed envelopes. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 95% of included participants were analyzed. Missingness not related to outcome. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Wang 2015 |  |  |  |  |  |  | ||||||
| Randomly assigned using a computer‑generated random number table method with randomized group information sealed in an opaque envelope. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Deaths from all causes. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Kullenberg 2004 |  |  |  |  |  |  | ||||||
| Randomized using the envelope method. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Time to first support of body weight next to the bed in hours after surgery. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Segado Jimenez 2009 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Time to sit down for the first time. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Yamamoto 2016 |  |  |  |  |  |  | ||||||
| Randomisation was performed with a random number list generated by a computer software. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Time to first standing. | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Segado Jimenez 2009 |  |  |  |  |  |  | ||||||
| Patients were randomized. No baseline differences between intervention groups identified. | ||||||||||||
| No deviations from intended interventions identified. | ||||||||||||
| 100% of included participants were analyzed. | ||||||||||||
| Drugs expenses (not including indirect costs or stay). | ||||||||||||
| No deviation to the planned statistical analysis identified. Only one result provided. | ||||||||||||
| No risk of bias identified. | ||||||||||||




