Periphere Nervenblockaden bei Hüftfrakturen
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Chile Funding: unspecified NCT01961895 | |
| Participants | 31 older than 60 years, ASA II‐III with risk factors for known coronary artery disease (≥ 2 risk factors for coronary heart disease as defined by Wallace 1987) and hip fracture within 48 hours of fracture Exclusion criteria: receiving orthopaedic treatment, coagulopathy (clinic or laboratory), sepsis or infection of the catheter insertion site of the lumbar plexus, neurological diseases evolving Also, disoriented, dementia, chronic renal failure stage IV National Kidney Foundation, glomerular filtration rate between 15 and 29 mL/min/1.73 m2, unable to assess pain, non‐sinus rhythm or conduction abnormalities (right bundle branch block or left atrioventricular block) on admission EKG, with pacemaker, acute coronary syndrome or decompensated cardiovascular disease at entry, allergy to any drugs of the protocol and inability to understand or sign informed consent unaided | |
| Interventions | Treatment group: continuous lumbar plexus started preoperatively and continued for 72 hours after surgery (n = 17) Control group: IV PCA with morphine (n = 14) | |
| Outcomes | Ischaemic events per participant (extracted as P value): continuous EKG monitoring and serial cardiac enzymes | |
| Notes | Conference abstract. Email sent on 25 May 2015. Study authors responded that the manuscript had not been submitted for publication yet and confirmed the registration number. They noted no major cardiac events during the entire period of observation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Double=blind (participant, caregiver, investigator) |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind (participant, caregiver, investigator) |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | No details on participants enrolled |
| Methods | RCT Setting: Greece Funding; unspecified | |
| Participants | 84 participants (63 female and 21 male) with hip fracture | |
| Interventions | Treatment group: continuous femoral nerve block with 0.125% levobupivacaine at 3‐4 mL/h, started after surgery (n = 49) Control group: IM pethidine (n = 35) Spinal anaesthesia and paracetamol after surgery for all participants | |
| Outcomes | Pain scores during 24 hours (not included in analysis) | |
| Notes | No complications such as motor blockade, local haematoma or infection, inadvertent arterial puncture, direct nerve damage and cardiovascular or neurological toxicity Conference abstract Email sent on 25 May 2015: no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomized", no details. Unequal groups: 48 and 35 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up No failed block Five accidental catheter dislodgements ‐ 4 during procedure of securing the catheter, 1 on the ward |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | No details for each group separately |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: United States of America Funding: charity Registered at ClinicalTrials.gov (Identifier NCT01701414) | |
| Participants | 36 participants Eligible: aged ≥ 55 years, radiographically proven femoral neck or intertrochanteric fracture, normal lower extremity neurovascular examination, able to consent and actively participate in the study, moderate to severe pain (numerical pain rating score 5) at time of enrolment Excluded: known international normalized ratio > 3.0, prior femoral artery vascular surgery on the same side as the fracture, other significant trauma, hypoxia (pulse oximetry < 92%), hypotension (systolic blood pressure < 100 mm Hg), known hypersensitivity to local anaesthetics or morphine | |
| Interventions | Treatment group: ultrasound‐guided femoral nerve block plus subcutaneous morphine (n = 18) Control group: sham‐injection (3 mL of saline under ultrasound probe over 5 minutes) plus subcutaneous morphine (n = 18) | |
| Outcomes | Pain scores at 15 minutes after the block Opioids during 4 hours after the block | |
| Notes | One participant in the SC group had an episode of rapid atrial fibrillation requiring diltiazem, but this participant had a history of chronic atrial fibrillation. No other adverse events (respiratory depression, hypotension, nausea or vomiting) were noted during the study period, and no other adverse events were reported to study investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After consent, participants were randomized by sequentially numbered cards in sealed envelopes Internet‐based programme with a 1:1 allocation ratio performed by the research department |
| Allocation concealment (selection bias) | Low risk | After consent, participants were randomized by sequentially numbered cards in sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | "Blinded" with sham injection |
| Blinding of outcome assessment (detection bias) | Low risk | "Blinded" with sham injection |
| Incomplete outcome data (attrition bias) | Low risk | One participant withdrawn from each group (38 randomized and 36 analysed) Two patients enrolled (1 in each arm) dropped out after randomization but before the study procedure. No reason provided |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee Setting: Israel Funding: unspecified | |
| Participants | 40 participants (30 female and 10 male) with a hip fracture undergoing surgery Excluded: patients with severe cardiac, pulmonary, renal or liver dysfunction; systemic infection; decubitus ulcer; dementia; aspirin or anticoagulant treatment; allergy to local anaesthetics Percentage female: 75% Lost to follow‐up: none No details on surgical technique provided | |
| Interventions | Treatment group: psoas compartment block. Chayen's technique with loss of resistance to air (operated side up), using 2 mg/kg/body weight of 0.25% bupivacaine with adrenaline (0.8 mL/kg) and supplementary doses as required via a catheter inserted 2‐3 cm cephalad past the tip of the needle. Blocks were performed before surgery (16‐48 hours), within 6 hours of admission (n = 20). According to assessment, a sciatic nerve block (n = 5), general anaesthesia (n = 1) or spinal anaesthesia (n = 11) was added for surgery. Catheters were kept for 72 hours after surgery Control group: IM meperidine and diclofenac as required for pain relief (n = 20). Neuraxial block (spinal or epidural n = 19) or general anaesthesia (n =1) for surgery | |
| Outcomes | Pain relief as assessed by VAS at 30 minutes after block placement and at 8, 24, 48 and 72 hours after surgery Complications of the block: 3 participants showed inflammation at the site of insertion; 1 had epidural spread. Local anaesthetic toxicity: none. No major complications associated with the block | |
| Notes | Length of follow‐up: 72 hours after surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized trial: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | No failed block. None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced. No details on surgical technique provided |
| Methods | RCT Approved by the ethics committee and consents obtained Setting: United Kingdom Funding: unspecified | |
| Participants | 50 participants with a hip fracture undergoing surgery with a pin and plate or a sliding hip screw Excluded: receiving analgesic drugs, diagnosis of dementia, regional anaesthesia considered contraindicated | |
| Interventions | Treatment group 1: lateral cutaneous nerve of thigh block with 15 mL 0.5% bupivacaine; Eriksson's technique (n = 17) Treatment group 2: femoral (3‐in‐1) nerve block with 15 mL 0.5% bupivacaine; Winnie's technique (n = 17) Control group : IM meperidine (n = 16) All participants had general anaesthesia consisting of fentanyl, etomidate, vecuronium, nitrous oxide and enflurane | |
| Outcomes | No complications related to the blocks | |
| Notes | Length of follow‐up: 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized trial: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear for participants; nurses administering supplemental analgesia were blinded to the treatment group |
| Blinding of outcome assessment (detection bias) | Low risk | Nurses administering supplemental analgesia were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: France Funding: charity | |
| Participants | 62 participants with a hip fracture undergoing surgery. Arthroplasty: 58% Excluded: more than 72 between fracture and surgery, weight < 40 kg, ASA physical status > IV, neurological disease (alcoholic or diabetic), allergy or contraindication to regional anaesthesia, severe hepatic or renal dysfunction, Mini Mental score < 15/30 | |
| Interventions | Treatment group: continuous femoral nerve block. Nerve stimulator, 0.3 to 0.5 mA, catheter introduced 10 to 15 cm past needle tip and loaded with 30 mL of lidocaine 1.5% plus epinephrine. Infusion of 0.2% ropivacaine at 10 mL/h for 48 hours (n = 21) Control groups: intravenous propacetamol 2 G 6‐hourly (n = 21) or subcutaneous morphine 0.05 mg/kg 4‐hourly (n = 20) Propacetamol and morphine before surgery, spinal anaesthesia for surgery for all participants. Treatment group and propacetamol group participants could also receive morphine after surgery if needed | |
| Outcomes | Pain scores at 8, 24 and 48 hours after surgery Number of participants who required additional opioids during first 48 hours after surgery Confusion/somnolence Transfused Pressure sores Mortality at 6 months Cost of analgesic regimens | |
| Notes | Length of follow‐up: 6 months Study authors contacted 22 May 2015; no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: use of numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Randomized trial: use of numbered envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up Failed blocked excluded, but no immediate failed block. Four catheter dislodgements; these participants were kept in the analysis (intention‐to‐treat) |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced except for delay between admission and surgery (median 40 hours in femoral catheter group and 21 and 23,5 hours in the 2 control groups) |
| Methods | RCT Setting: Spain Funding: unspecified | |
| Participants | 49 participants older than 65 years with a neck fracture scheduled for surgical treatment | |
| Interventions | Treatment group: double guidance (ultrasound and nerve stimulator) femoral nerve block with 15 mL of 0.2% ropivacaine followed by infusion of 0.2% ropivacaine at 5 mL/h plus 10 mL every 30 minutes (n = 11) Control group: intravenous metamizole 2 G every 6 hours (n = 38) Rescue analgesia with 100 mg tramadol and ondansetron 4 mg | |
| Outcomes | Pain scores at rest and on movement at 24 and 48 hours after surgery Death at 6 months | |
| Notes | Conference abstract Additional information on pain scores received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No details and very unequal groups |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Greece Funding: unspecified NCT02037633 | |
| Participants | 41 ASA I‐III participants, aged 38 to 94 years, scheduled for hip fracture repair Excluded: contraindications for central nervous blockade, impaired cognition or dementia, multiple fractures, any previous analgesic administration in last 12 hours before surgery | |
| Interventions | Treatment group: fascia iliaca block, modified Dalen's technique with 40 mL ropivacaine 0.5% injected while caudal pressure maintained, then turned lateral (fracture side up) for spinal 20 minutes later (n = 21) Control group: IV fentanyl 1.5 mcg/kg, then turned lateral (fracture side up) for spinal 5 minutes later (n = 20) Spinal anaesthesia for surgery. IV PCA with morphine after surgery | |
| Outcomes | Pain at rest and on movement at 20 minutes after the block (or 5 minutes after fentanyl administration; movement = positioning for spinal anaesthesia) Opioid requirement during first 24 hours after surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned, using a sealed envelope method" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned, using a sealed envelope method" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Pain scores were assessed by a blind observer who entered the operating room only after the analgesic intervention (IV fentanyl administration or fascia iliaca block performance) had taken place. Landmarks were drawn on all participants, and gauze was applied to the “puncture” site for all participants. Each participant was aware of his/her group allocation because we considered a placebo injection in the inguinal area not acceptable. The observer who recorded participant satisfaction was unaware of group allocation and was not involved in any other step of the study |
| Blinding of outcome assessment (detection bias) | Low risk | Pain scores were assessed by a blind observer who entered the operating room only after the analgesic intervention (IV fentanyl administration or fascia iliaca block performance) had taken place. Landmarks were drawn on all participants, and gauze was applied to the “puncture” site for all participants. Each participant was aware of his/her group allocation because we considered a placebo injection in the inguinal area not acceptable. The observer who recorded participant satisfaction was unaware of group allocation and was not involved in any other step of the study |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up. One participant withdrew consent |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Turkey Funding: departmental | |
| Participants | 40 ASA I‐III participants aged 65 to 80 years undergoing femoral fracture repair under spinal anaesthesia Excluded: patients < 65 years of age or > 80 years of age, with peripheral neurological disease, mental disorders, allergy to amide local anaesthetics, coagulation/haemostasis diseases, moderate or severe liver or kidney failure, contraindication to or refusing fascia iliaca block | |
| Interventions | Treatment group: fascia iliaca block with 15 mL of 0.5% bupivacaine and 15 mL of 2% lidocaine (n = 20) Control group: no block (n = 20) Spinal anaesthesia for surgery and IV patient‐controlled analgesia with morphine for postoperative analgesia for all participants | |
| Outcomes | Pain at rest and on movement (positioning for spinal) and after surgery Opioids requirements for the first 4 and 48 hours Participant satisfaction | |
| Notes | SD of 0.00 entered as 0.001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided into two equal groups for this prospective double‐blind study", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Participants in control group received no block |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study is said to be double‐blinded. No sham block reported. Unclear who was the outcome assessor for pain scores |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and informed consents obtained Seeting: United Kingdom Funding: unspecified | |
| Participants | 50 participants with a hip fracture Excluded: confused (and therefore unable to give informed consent), bleeding diathesis or taking warfarin, local or systemic infection, previous hypersensitivity to local anaesthetics | |
| Interventions | Treatment group: femoral (3‐in‐1) nerve block inserted at the time of admission with 20 mL 0.5% bupivacaine, Winnie's technique and 5 minutes distal compression (n = 24) Control group: intravenous morphine alone (n = 26) Blocks performed by trained emergency physicians | |
| Outcomes | Pneumonia from chart review at 6 months Mortality at 6 months Opioids during first 24 hours after block placement (before surgery) | |
| Notes | Extra information supplied by trialists to confirm secure randomization and that no participants were lost to follow‐up Length of follow‐up: 6 months. Study authors re‐contacted 22 May 2015: no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: use of sealed opaque numbered envelopes with randomization generated by a random number generator |
| Allocation concealment (selection bias) | Low risk | Randomized trial: use of sealed opaque numbered envelopes with randomization generated by a random number generator |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded to group allocation because our research ethics committee |
| Blinding of outcome assessment (detection bias) | Low risk | "blinded assessors"; "the same blinded observer (AKF) abstracted all data" |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Denmark Funding: charity NCT00162630 | |
| Participants | Orthopaedic hospital in Copenhagen, Denmark Excluded: refusal to participate in the study, previous surgery in the affected hip, regular prefracture opioid or glucocorticoid therapy, alcohol or substance abuse, infection at the injection site, morphine intolerance, any previous opioid administration for acute pain and non‐confirmation of hip fracture suspicion on x‐ray | |
| Interventions | Treatment group: fascia iliaca compartment blockade with 40 mL 1% mepivacaine and epinephrine based on landmarks to fractured limb with saline injection into the contralateral gluteal region (n = 24) Control group: saline injection into fractured side at the site of the fascia iliac block and injection of morphine (0.1 mg/kg) into the contralateral gluteal region (n = 24) After 3 hours, all participants received epidural analgesia | |
| Outcomes | Pain scores at rest and on movement (15‐degree leg raise) 30 minutes after block Use of supplementary opiates during first 3 hours after block placement | |
| Notes | Length of follow‐up: till 3 hours after the block No side effects attributable to the fascia iliac block were noted in any participants during their hospital stay Before block placement, pain at rest was significantly less (P = 0.05) in participants with intracapsular fractures (median 2, (interquartile range (IQR) 0–5)) vs those who had trochanteric (median 4, (IQR 2–5)) or subtrochanteric fractures (median 5, IQR 4–7), but no significant difference in movement‐associated pain between fracture types, which was median 8 (IQR 6.5–10), 9 (IQR 8–10) and 10 (IQR 8–10) for intracapsular, trochanteric and subtrochanteric fractures, respectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: method stated as via a computer‐generated list using treatments prepared by a nurse not involved in collection of participant data |
| Allocation concealment (selection bias) | Low risk | "the medicine used for each individual patient was prepared by a nurse not otherwise involved with the collection of patient data" |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | One participant did not have a fracture but only a severe contusion and was excluded after x‐ray; an extra participant was therefore included on a new number Two participants (1 from each group) had protocol violations because they received sufentanil as supplementation instead of morphine; both of these supplementations occurred in the post‐anaesthesia care unit more than 60 minutes after block placement |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | High risk | Groups well balanced except for higher proportion of male participants in the block group. Participants in the block group had higher pain scores on admission (P = 0.04) |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Germany Funding: corresponding study author had no relationship with any mentioned product nor competitors, classified as departmental resources | |
| Participants | Orthopaedic hospital in Leipzig, Germany Excluded: < 18 years old, uncooperative, contraindications to regional anaesthesia or drugs used in the protocol, long‐term use of opioids and/or opioid dependence, history of ulcers, multiple trauma, absence of consent, anaesthetist inexperienced (fewer than 5) with the technique | |
| Interventions | Treatment group: femoral nerve block with catheter inserted at the time of admission (stitched in place) using 40 mL 1% prilocaine, then 30 mL 0.2% ropivacaine 6‐hourly (n = 50) Control group: no injection (n = 50) Operated 14 hours after admission. All participants had ibuprofen every 8 hours after surgery | |
| Outcomes | Pain score on a scale of 1 to 5 (least pain level 1) at rest and passive movement (30 degrees anteflexion) 30 minutes after insertion of the block and at 24, 48 and 72 hours after surgery No severe complications related to analgesia: more specifically, no infection at insertion points of the catheters. 10 catheters were dislodged. No significant respiratory depression due to opioids, no allergic reactions | |
| Notes | Length of follow‐up: until discharge from orthopaedic ward Extra information regarding method of randomization and length of follow‐up supplied by trialists | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by anaesthesiologists called to the emergency room: "Sealed envelopes: information from the authors to previous reviewers" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | 20% rate of catheter dislodgement, resulting in the need for systemic analgesia Unclear whether participants with dislodged catheters were included in pain scores in their treatment group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced, including similar admission pain scores (2.50 and 2.46 at rest and 4.30 and 4.34 on movement) |
| Methods | RCT Approved by the ethics committee and signed informed consents obtained Setting: Argentina Funding: unspecified | |
| Participants | 154 adult participants > 65 years old who presented to the emergency department because of a previously | |
| Interventions | Treatment group: fascia iliaca compartment block with 0.3 mL/kg 0.25% bupivacaine and 5 mL 5% dextrose (n = 92) Control group: normal saline in the fascia iliaca compartment and IV non‐steroidal anti‐inflammatory drugs (diclofenac or ketorolac) (n = 62) | |
| Outcomes | Pain scores at rest at 15 minutes after block placement Confusion | |
| Notes | Protocol included observing participants for 8 hours The only complications were local bruises at the site of injection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized into 2 groups (A and B) with numbers generated by the EPI‐INFO™ (Atlanta, GA: Centers for Disease Control and Prevention) programme |
| Allocation concealment (selection bias) | Low risk | Randomization list was kept by one of the study authors who did not interact with participants. He gave instructions to participants’ ED nurse about which treatment should be administered |
| Blinding of participants and personnel (performance bias) | Unclear risk | Nurse prepared the medication according to physician’s instructions and assigned a letter to the protocol (from a set of 10 letters: 5 for group A and 5 for group B) that designated whether the participant was receiving active medications in the fascia‐iliaca block. The physician administering medications and obtaining VAS scores did not know which medications the participant was |
| Blinding of outcome assessment (detection bias) | Low risk | The physician administering the medications and obtaining the VAS scores did not know which medications the participant was receiving |
| Incomplete outcome data (attrition bias) | Unclear risk | In all, 175 participants were randomized upon presentation to the ED. A total of 21 were excluded from participation (1) because they or their legal decision‐maker declined to participate, (2) owing to systemic or laboratory abnormalities that interfered with their participation or (3) because they were subsequently found to have missing data (pain scores not recorded or incomplete vital signs on scheduled measurements) |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: China Funding: unspecified | |
| Participants | 40 adult participants (> 16 years of age) with adequate Mini Mental tests and hip fracture confirmed by x‐ray Excluded: known allergy or contraindication to morphine or bupivacaine, Mini Mental test score < 9 | |
| Interventions | Treatment group: femoral (3‐in‐1) nerve block with 30 mL 0.5% bupivacaine (not exceeding 3 mg/kg) (n = 18; 15 analysed) Control group : IV morphine 0.1 mg/kg (n = 22; 18 analysed) Intravenous morphine 0.1 mg/kg bolus as required 2‐ to 4‐hourly, oral dihydrocodeine 30‐60 mg 4‐hourly as required (maximum 240 mg per 24 hours), rectal diclofenac 50 mg 8‐hourly as required (maximum 150 mg per 24 hours), oral paracetamol 1 G 4‐ to 6‐hourly (maximum 4 G per 24 hours) | |
| Outcomes | Pain scores at 30 minutes after block placement Confusion 24 hours after block placement or to surgery (whichever came first) Opioids used in 24 hours or to surgery (whichever came first) | |
| Notes | No immediate complications in either group, defined as inadvertent vascular puncture, anaphylaxis or collapse, severe pain or inability to tolerate the procedure for the femoral block group and anaphylaxis or collapse, respiratory depression or requirement for naloxone use within 1 hour of IV morphine administration for the systemic analgesia group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were then randomized using numbered, sequential, sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | "Patients were then randomized using numbered, sequential, sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Seven participants had incomplete data One participant in the morphine group was unable to complete the study owing to development of an acute confusional state. Full data were unavailable for 3 other participants in the IV morphine group owing to incomplete data collection post intervention. Within the '3‐in‐1' nerve block group, 1 participant was found to have an impalpable femoral artery on the side of the hip fracture after randomization and was unable to receive a nerve block owing to lack of anatomical landmarks. Full data were unavailable for 2 other participants owing to incomplete data collection post intervention. Full results were available for 33 participants: 18 in the morphine group and 15 in the femoral nerve block group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced Data were not analysed in intention‐to‐treat |
| Methods | RCT Setting: United Kingdom Funding: unspecified | |
| Participants | Orthopaedic hospital in Stevenage, UK Excluded: dementia, unable to rate their pain | |
| Interventions | Treatment group: femoral nerve block inserted at the time of admission using 0.3 mL/kg 0.25% bupivacaine (n = 25) Control group: no injection (n = 25) | |
| Outcomes | Pneumonia Mortality Wound infection Pressure sores No local or systemic complications of femoral nerve blocks | |
| Notes | Length of follow‐up: 24 hours for analgesia, unclear for other outcomes ("short term"; taken as in hospital) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | No placebo injections used |
| Blinding of outcome assessment (detection bias) | Low risk | Admitting house surgeons and nursing staff who administered analgesia were unaware to which group participants had been allocated |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up One failed block; data included |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: United Kingdom Funding: unspecified | |
| Participants | Orthopaedic hospital in Sheffield, UK Excluded: absolute contraindication to a regional technique, allergy to local anaesthetic agents, systemic disease that indicated an alternative method of anaesthesia | |
| Interventions | Treatment group: femoral (triple nerve block) nerve block with 35 mL 0.75% prilocaine and infiltration above the iliac crest with 8 mL 0.75% prilocaine inserted before induction of anaesthesia (n = 25) | |
| Outcomes | Death | |
| Notes | Length of follow‐up: 24 hours No untoward sequelae were associated with nerve blocks. Venous blood for plasma prilocaine levels in the first 12 participants in group 2 was taken from an indwelling cannula at 0, 5, 10, 15, 20, 25, 30, 45 and 60 minutes after completion of the prilocaine injection. Concentrations were measured using gas chromatography. Cmax occurred no later than 25 minutes. Methemoglobin levels were not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of 'unmarked envelopes' |
| Allocation concealment (selection bias) | Low risk | Use of 'unmarked envelopes' |
| Blinding of participants and personnel (performance bias) | Unclear risk | All participants were prescribed intramuscular papaveretum 0.2 rng/kg; administration was done at the discretion of the nursing staff, who were unaware of participant groups. All participants had their skin prepared and an elastoplast placed over the possible injection site to minimize bias. Unclear for participants: blocks performed before induction of general anaesthesia |
| Blinding of outcome assessment (detection bias) | Low risk | All participants were prescribed intramuscular papaveretum 0.2 rng/kg; administration was done at the discretion of the nursing staff, who were unaware of participant groups. All participants had their skin prepared and an elastoplast placed over the possible injection site to minimize bias The operating theatre recovery sister and ward staff, who were blind to participant groups, were asked to assess the quality of analgesia after the operation |
| Incomplete outcome data (attrition bias) | Low risk | One participant lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Thailand Funding: unspecified | |
| Participants | 64 ASA I–III participants aged 18–80 years undergoing surgery for femur fracture with body weight > 50 kg and scheduled for surgery under spinal block Excluded: multiple fractures, peripheral neuropathy, bleeding disorders, mental disorders, communication failure, allergy to local anaesthetics, use of analgesics for premedication | |
| Interventions | Treatment group: femoral nerve block with a nerve stimulator and 20 mL bupivacaine 0.5% and 10 mL saline 15 minutes before positioning for the spinal (n = 32) Control group: 2 doses of IV fentanyl 0.5 mcg/kg (n = 32) During positioning for the spinal (lateral position with the fracture site up), fentanyl in 0.5 mcg/kg increments was given every 5 minutes until pain scores were < or = 4 | |
| Outcomes | Pain scores at rest and on movement (positioning for the spinal) 15 minutes after block placement No adverse systemic toxicity of bupivacaine, such as seizure, arrhythmia or cardiovascular collapse, was noted in the femoral nerve block group. Neither vascular puncture nor paraesthesia occurred. No complications, such as haematoma, infection or persistent paraesthesia, were observed within 24 hours after the operation. No participant in either group had hypoventilation (ventilatory rate < 10/min) or oxygen saturation < 95% | |
| Notes | Although the vast majority of participants had a proximal fracture, 10 participants had a shaft (6 participants for the femoral nerve block and 1 for the control) or a distal (3 participants in the control group) fracture. The only outcomes retained in the analysis were pain scores. Pain scores at rest and on movement after block placement were finally excluded because we thought that measurement was done before the block could be effective (please see Results) An email was sent on 17 March 2016, to obtain data separately for participants with a proximal fracture. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated by computer‐generated random numbers into 2 groups of 32 participants each |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed in opaque, sealed envelopes until a group was assigned |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded: "All patients were aware of their treatment group allocation" |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded: "Assessors of pain were blinded to the patients’ allocated treatment group" |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up No failed block reported |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced except that time from trauma to surgery was significantly longer in the fentanyl group than in the FNB group (P = 0.03) and most participants in the FNB had femoral neck fractures, whereas most of those in the fentanyl group had intertrochanteric fractures (P = 0.04) |
| Methods | RCT Insitutional approval and informed consents obtained | |
| Participants | Included: patients of both sexes, 18–70 years, weight > 50 kg, American Society of Anesthesiologists physical status I‐III, scheduled for fracture femur operation under central neuraxial block but unable to sit because of pain Excluded: could sit comfortably, any contraindication to spinal anaesthesia, FNB or local anaesthetic | |
| Interventions | Treatment group: femoral nerve block with 20 mL lidocaine 1.5% (15 mL lidocaine 2% and 5 mL distilled water) with epinephrine 5 mcg/mL, nerve stimulator, quadriceps response at 0.3‐0.5 mA, insulated 50 mm 22 G needle (n = 23) Control group: fentanyl 1.0 mcg/kg IV (n = 21) Positioning for spinal 5 minutes after block placement or IV fentanyl. Additional fentanyl 0.5 mcg/kg every 5 minutes in both groups allowed if VAS scores ≥ 4 until VAS scores < 4 or a maximal dose of 3 mcg/kg (whichever came first). No participant required an additional dose of fentanyl | |
| Outcomes | Pain scores on movement (positioning for the spinal) 5 minutes after block placement (scale 0‐10) | |
| Notes | Study also includes participants with shaft fracture. We obtained results for pain scores on movement for participants with proximal fracture only from the study authors. However, we did not keep results in the analysis (see Effects of interventions) owing to the short delay between the block and the evaluation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were distributed in two groups through computer generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up. For 2 participants from each group, surgery was postponed owing to infection at the surgical site and a change in the surgical plan. One participant from each group was excluded owing to refusal for spinal anaesthesia on the table after initial consent. Therefore, 6 participants were subsequently excluded, leaving 60 participants for final analysis All participants for whom a pain score could be obtained during spinal positioning had their score analysed within their assigned group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | "Demographic data and type of surgery were comparable in both the groups" |
| Methods | RCT Informed consents obtained Setting: United Kingdom Funding: unspecified | |
| Participants | Orthopaedic hospital in London, UK Excluded: other painful lesions, signs of moderate or severe dementia, < 65 years of age, systemic disease indicating an alternative method of anaesthesia (e.g. spinal) | |
| Interventions | Tretament group: lateral cutaneous nerve of thigh block with 15 mL 0.5% bupivacaine and adrenaline (n = 10) All participants had general anaesthesia with fentanyl, thiopentone, suxamethonium, nitrous oxide, halothane. Blocks performed at completion of surgery | |
| Outcomes | Death (24 hours) | |
| Notes | One participant died within 24 hours of surgery, and results for this participant were not given Length of follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random envelopes' |
| Allocation concealment (selection bias) | Low risk | 'Random envelopes' opened at completion of surgery |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Low risk | Postoperative analgesia was prescribed before allocation to respective groups, with administration performed at the discretion of the nursing staff, who were unaware of whether a block had been performed. The dose of pethidine was 25 or 50 mg intramuscularly, depending on the participant's estimated weight ‐ not on general condition |
| Incomplete outcome data (attrition bias) | Low risk | One lost to follow‐up Results for 1 participant who died are not included: "One patient in Group 2 who died within the 24‐hour period is not included in analysis of the results: there are thus nine patients in each group" |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Setting: Sweden Funding: no conflict of interest declared, classified as departmental resources | |
| Participants | Orthopaedic hospital in Sweden Excluded: inability to rate their pain | |
| Interventions | Treatment group: femoral nerve block inserted at the time of admission with 30 mL ropivacaine (7.5 mg/mL) (n = 40). Mean bock duration 15.8 ± 5.6 hours. Four participants had their block during transportation to the hospital. The block was repeated for 3 participants owing to a long delay before surgery (23.9 hours, 26.3 hours and 30.9 hours) Control group: no injection (n = 40) | |
| Outcomes | Confusion (Pfeiffer test, graded according to a 4‐degree scale (0‐3: no, light, moderate and pronounced confusion) at 48 hours Time to first mobilization Pressure sores Opioids used per 24 hours | |
| Notes | Length of follow‐up: length of acute hospital stay (mean 11 days) No complication related to the nerve block All participants indicated that they would consider a new future blockade if this would be necessary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up Five failed blocks; participants kept in their treatment groups |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced except for a longer delay in arrival to surgery for the block group (15.5 hours vs 5.8 hours) |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Austria Funding: departmental resources | |
| Participants | Included: 37 very elderly participants (> 80 years) with hip fractures (of whom 3 with dementia had to be excluded) scheduled for surgery under spinal anaesthesia Excluded: score < 18 on the Mini‐Mental State Examination, surgery did not take place within 36 hours, known intolerance or allergies to drugs, planned or required general anaesthesia, refusal of consent, participation in a different study, administration of midazolam as premedication, chronic pain, contraindications and spinal anaesthesia failure, incomplete data records | |
| Interventions | Treatment groups: ultrasound‐guided continuous femoral (3‐in‐1) nerve block with bupivacaine (n = 10) Control group: systemic analgesia with IV/SC piritramide and IV paracetamol (n = 10) | |
| Outcomes | Pain scores at rest and on movement at 24 hours after surgery Opioids at 24 hours after surgery Number of participants with postoperative myocardial ischaemia | |
| Notes | Study also includes a group with epidural analgesia ‐ not retained in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomized according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out for the 2 included subgroups |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups comparable |
| Methods | RCT Setting: Iran Funding: unspecified | |
| Participants | 40 participants with femoral neck fracture | |
| Interventions | Treatment group: fascia iliaca block with 20 mL 1.5% lidocaine (n = 20) Control group: IV fentanyl 1.5 mcg/kg (n = 20) Lateral decubitus position for spinal anaesthesia | |
| Outcomes | Pain on movement 5 minutes after block placement (positioning for spinal) Participant satisfaction | |
| Notes | Conference abstract Email sent on 26 May 2015; no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | No details |
| Methods | RCT Approved by the ethics committee and signed informed consents obtained Setting: Greece Funding: unspecified | |
| Participants | 207 participants aged 70 years or older at intermediate or high risk of delirium scheduled for hip fracture repair Risk classification was based on the presence of 4 predictive risk factors (severity of illness, measured by acute physiology age and chronic health examination; cognitive impairment, measured by the mini‐mental state examination score; index of dehydration, measured by the ratio of blood urea nitrogen to creatinine; and visual impairment, measured by the standardized Snellen test) as described by Inouye. Intermediate risk for postoperative delirium was defined as the presence of 1 or 2 risk factors; high risk was defined as the presence of ≥ 3 risk factors Excluded: delirium at admission, metastatic hip cancer, history of bupivacaine allergy, use of cholinesterase inhibitors, severe coagulopathy, parkinsonism, epilepsy, levodopa treatment, delayed surgery more than 72 hours after admission, inability to participate in interviews (profound dementia, respiratory isolation, intubation, aphasia, coma or terminal illness) | |
| Interventions | Treatment group: fascia iliaca block with bupivacaine 0.3 mL/kg (0.25%?) repeated daily until delirium or surgery and at 24 hours after surgery, and daily until delirium or discharge (n = 108 randomized; n = 102 analysed) Control group: placebo medication (water for injection) identical in appearance to the active drug and administered at the same site and in the same way (n = 111 randomized; n = 105 analysed) Intravenous and intramuscular analgesics were administered as needed in both groups | |
| Outcomes | Confusion (Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) and Confusion Assessment Method criteria) No complications of fascia iliaca block administration report, except 3 local haematomas developed at the injection site, which resolved spontaneously. Exact number of blocks performed was not specified | |
| Notes | Reduction was seen only in participants at intermediate risk of developing delirium ‐ not among those at high risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "sequentially randomly assigned" "according to a computer‐generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | "All participants were blinded to the treatment group." "Placebo medication (water for injection) was identical in appearance to the active drug and was administered at the same site and in the same way as the fascia iliaca block was injected." However, no clear mention of blinding of personal taking care of participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not really clear |
| Incomplete outcome data (attrition bias) | Low risk | A total of 12 participants were further excluded from both groups for different reasons: 6 lost to follow‐up, 3 refused further participation and 3 died (2 pulmonary embolism and 1 stroke) between second and fourth days of admission |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
| Methods | RCT Informed consents obtained Setting: France Funding: unspecified | |
| Participants | Orthopaedic hospital in Feurs, France Excluded: inability to rate their pain (Mini Mental score < 24), contraindication to nitrous oxide, regional anaesthesia, allergy to study drugs, renal dysfunction or prefracture opioid treatment | |
| Interventions | Treatment group: femoral nerve block inserted at the time of admission with 20 mL mepivacaine (n = 16) Control groups: no injection. IV morphine (n = 14) or IV paracetamol and ketoprofen (n = 15). We retained only the IV morphine group as the control group All participants received nitrous oxide for withdrawal of clothing on arrival | |
| Outcomes | Pain score on VAS at movement (x‐ray): 20 minutes after block placement | |
| Notes | Length of follow‐up: duration of time in emergency department | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hat drawing |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up Failed block (defined as pain scores > 4/10 at skin traction installation) 18.7%. Results included in treatment group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and written informed obtained Setting: China Funding: governmental Open reduction and internal fixation surgery with the antirotation proximal femoral nail technique | |
| Participants | 104 participants scheduled for open reduction of hip fracture Excluded: neuropathy involving lower extremities, bladder dysfunction, coagulopathies, known allergy to amide local anaesthetic drugs or opioids, inability to co‐operate, psychological disorders or linguistic difficulties that could interfere with pain assessment | |
| Interventions | Treatment group: fascia iliaca block (n = 51) Control treatment: intravenous patient‐controlled analgesia (n = 53) General anaesthesia with fentanyl, remifentanil, propofol and atracurium for surgery and flurbiprofen 40 mg at completion of surgery, plus acetaminophen and dihydrocodeine or morphine on request as rescue analgesia for all participants | |
| Outcomes | Pain at 2, 4, 6, 12, 24 and 48 hours after surgery (taken as at rest) Acute confusional state (time point unspecified, participants screened daily; length of hospital stay 23 and 21 days for fascia iliaca and IV groups, respectively) Opioid consumption up to 48 hours Participant satisfaction (92.5% of participants receiving a fascia iliaca block were satisfied vs 94.3% of participants receiving IV analgesia) | |
| Notes | Additional information received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned according to a computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | "Blinding could not be conducted due to differences in the analgesia procedures and |
| Blinding of outcome assessment (detection bias) | High risk | "Blinding could not be conducted due to differences in the analgesia procedures and |
| Incomplete outcome data (attrition bias) | Low risk | "Patients who underwent the full protocol were included in the analysis" 2 participants withdrawn from fascia iliaca group owing to catheter failure |
| Selective reporting (reporting bias) | Unclear risk | All results mentioned in methods section given in results section, except preoperative and postoperative mini‐mental state examination. We contacted study authors who informed us that Mini Mental tests were not "collected" |
| Other bias | Unclear risk | Groups well balanced. Prophylaxis against nausea and vomiting given to IV group only Not in intention‐to‐treat |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Spain Funding: unspecified | |
| Participants | 75 participants undergoing hip fracture repair under spinal anaesthesia Excluded: general anaesthesia or intravenous administration of analgesics intraoperatively, pretreatment for chronic pain, or for ischaemic heart rhythm disorders, neurodegenerative and psychiatric diseases, lack of collaboration and/or understanding of the participant, allergy to local anaesthetics and contraindications for regional anaesthesia | |
| Interventions | Treatment group: femoral cutaneous and obturator nerve block (n = 25) or obturator nerve block only (n = 25). Blocks were performed after the spinal had worn off Control group: intravenous analgesia (n = 25) For all groups, investigators administered additional intravenous analgesia according to participants' demands (if VAS scores ≥ 3): metamizole 2 G or dexketoprofen trometamol 50 mg IV up to every 8 hours (depending on allergies). If pain persisted, tramadol 100 mg and metoclopramide 10 mg IIV every 8 hours was added. | |
| Outcomes | Drug expenses Time to first mobilization (sitting) Participant satisfaction (score from 1 to 5; 1 = bad, 2 = regular, 3 = good, 4 = very good and 5 = excellent) Opioids during first 48 hours after surgery Participant satisfaction (score from 1 to 5; 1 = bad, 2 = regular, 3 = good, 4 = very good and 5 = excellent) "We did not observe any complication in the realization of locoregional techniques during or subsequent to the locoregional technical" | |
| Notes | Email sent on 26 May 2015; no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly distributed", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Triple blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Triple blinded |
| Incomplete outcome data (attrition bias) | Low risk | No missing results |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section are given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Denmark Funding: unspecified | |
| Participants | 20 participants with a hip fracture surgically treated | |
| Interventions | All participants had spinal anaesthesia with 3.5 mL 0.5% bupivacaine Control group: saline infusion for 16 hours of same volume of fluid (control) (n = 10) Regular aspirin administration and IM morphine on demand | |
| Outcomes | Opioids used during first 18 hours after surgery No haematomas at the site of femoral catheters. Length of follow‐up unspecified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by a computer after surgery |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled study: participants, recovery staff and observers were blind to the solution used |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo‐controlled study: participants, recovery staff and observers were blind to the solution used |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up Nine of 10 participants receiving bupivacaine were analgesic to pin prick in the distribution of all 3 nerves. The other participant was analgesic only in the distribution of the lateral femoral cutaneous nerve of the thigh |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Ireland Funding: unspecified | |
| Participants | 24 participants presenting with fractured neck of femur, ASA I‐III and aged > 50 years | |
| Interventions | Treatment group : continuous femoral nerve block with bupivacaine 0.25% for 72 hours (n = 12) Control group: IM morphine (n = 12) All participants received paracetamol regularly and parenteral morphine up to 0.1 mg/kg IM 4‐hourly as required | |
| Outcomes | Pain at rest and on passive movement (30 degrees flexion) at 30 minutes after block placement Participant satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number sequence and sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "random number sequence and sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Three participants were excluded for the following reasons: (1) elastomeric pump failure resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, (2) participant confusion with subsequent pump disconnection after 12 hours, (3) late diagnosis of a complicating acetabular fracture |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Turkey Funding: unspecified | |
| Participants | Orthopaedic hospital in Konya, Turkey Excluded: coagulation abnormality, age < 18 or > 80 years, weight < 50 or > 100 kg, known allergy to bupivacaine or opioids, previous analgesic treatment with opioids, inability to understand pain scales or use a patient‐controlled analgesia device | |
| Interventions | All participants had general anaesthesia with fentanyl, propofol atracurium, nitrous oxide, isoflurane. At completion of the operation, participants had: Treatment group: femoral (3‐in‐1) nerve block with 30 mL 2% lidocaine with 2% epinephrine 1:200,000, followed by continuous infusion with 0.125% bupivacaine for 48 hours (n = 20) Control group: patient‐controlled analgesia with morphine (control) (n = 20) | |
| Outcomes | Participant satisfaction (rated as excellent, good, moderate or poor; we attributed scores from 1 to 4 to compare the data) | |
| Notes | Length of follow‐up: 48 hours Email sent to study authors on 24 May 2015, to ask for additional information; no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized at completion of surgery, method unspecified |
| Allocation concealment (selection bias) | Low risk | Randomized after inclusion |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Two missing results for participant satisfaction |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
| Methods | RCT Consents obtained Setting: South Africa Funding: unspecified | |
| Participants | Orthopaedic hospital in Cape Town, South Africa Excluded: fracture sustained more than 8 days before admission; < 60 years old; absolute contraindication to a regional technique, such as localized sepsis, suspicion of bacteraemic process or patients receiving anticoagulant therapy; overt or suspected endocrine disorder other than diabetes mellitus | |
| Interventions | Treatment groups: psoas block (n = 16) or spinal (n = 20) plus 'light" general anaesthesia using althesin, nitrous oxide Control group: general anaesthesia with fentanyl, thiopentone, suxamethonium, nitrous oxide, halothane (n = 20) | |
| Outcomes | Confusion Pneumonia Mortality | |
| Notes | Length of follow‐up: 4 weeks Four of 20 participants allocated to receive psoas nerve block failed to achieve a satisfactory block; outcome for these participants was not given (other than for mortality) No participant showed evidence of toxicity to the local anaesthetic Trial also includes a group with spinal (n = 20) added to light general anaesthesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | Four failed psoas compartment blocks, no other losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced, except for preoperative pneumonia: 1 in the psoas compartment block and 4 in the group general anaesthesia alone Not in intention‐to‐treat |
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Korea Funding: unspecified | |
| Participants | 40 ASA physical status I–III participants aged 62–88 years with isolated femoral neck fracture Excluded: known allergy to amide local anaesthetics, haemorrhagic diathesis, peripheral | |
| Interventions | Treatment group: fascia iliaca with 30 mL 0.375% ropivacaine (n = 20) Control group: IV alfentanil 10 mcg/kg followed by 0.25 mcg/kg/min starting 2 minutes before spinal (n = 20) Participants were moved to the operating suite for the spinal 20 minutes after block placement. Spinals were performed in lateral decubitus position on the side best tolerated by the participant. When a participant reported a VAS 4 during this positioning, the procedure was stopped, and 100 mg of IV alfentanil was administered in both groups | |
| Outcomes | Pain scores on movement at 30 minutes after block placement (positioning for spinal anaesthesia) and at rest at 6 and 24 hours after surgery Participant satisfaction (yes/no: 1 = good (if necessary, I would repeat the procedure) and 2 = bad (I would never repeat the procedure again)) Opioids at 24 hours No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited in the fascia iliaca block group. No complications, such as haematoma or persistent paraesthesia, were observed in participants with a fascia iliaca block within 24 hours after the operation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned, using an allocation sequence (which was generated by Y. H. Kim using a computer)" |
| Allocation concealment (selection bias) | Low risk | "The random allocation sequence was concealed until group was assigned (by J. W. Hwang)" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up No failed block mentioned. 40% (8 of 20) of participants had a complete block (3 nerves) and 60% (12 of 20) had blockade of 2 nerves (lateral femoral cutaneous and femoral) |
| Selective reporting (reporting bias) | Low risk | All results mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
ASA: American Society of Anesthesiologists physical status
DSM: Diagnostic and Statistical Manual of Mental Disorders
ECG or EKG: electrocardiogram
ED: emergency department
FNB: femoral nerve block
G: gram
h: hour
IM: intramuscular
IV: intravenous
IQR: interquartile range
mcg: microgram
mg: milligram
mL: millilitre
n: number
PCA: patient‐controlled analgesia
RCT: randomized controlled trial
SC: subcutaneous
VAS or VRS: = visual or verbal analogue/response scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Different intervention: local anaesthetic infiltration | |
| No outcome of interest. Conference abstract. Not enough details on possible outcomes of interest in the abstract. Study authors contacted on 25 May 2015. Confirmed that they were the authors of the abstracts but did not provide requested information | |
| Different population. 47 proximal fractures, 28 shaft fractures and 9 distal fractures. Mean age 42 years. Email sent 17 March 2016, to request separate data for participants with a proximal fracture. No reply | |
| Different intervention: epidural analgesia | |
| Not randomized: "The included patients were assigned to one of two groups in alternating order" | |
| Different intervention. Comparison between fascia iliaca block and femoral nerve block for positioning for spinal anaesthesia | |
| Different intervention. This was a randomized study of 60 participants with a trochanteric hip fracture fixed with a sliding hip screw or a trochanteric external fixator. After surgery, participants were randomized to an active non‐invasive interactive neurostimulation device or to a sham device. The active device generated biphasic electrical impulses. Participants allocated to the active group had a reduced level of pain, a reduced analgesic requirement and a greater range of flexion of the injured limb. We excluded the study as it was not a study of nerve blocks | |
| Different intervention. The amount of local anaesthetic used (bupivacaine 12.5 mg/kg of body weight) exceeds recommendations | |
| No outcome of interest at our selected time points (a study may be legitimately excluded if outcomes of interest were not measured; Higgins 2011 Section 5.4.1). Here, pain intensity was measured at 2 and 3 hours after block placement only from the results of 2 conference abstracts for this study, and at 1, 4 and 24 hours after block placement in the third abstract. The third abstract also reported absence of a difference in opioid consumption "during the course of the study (no specific time point mentioned for this outcome) on preliminary results:12.0 mg of morphine equivalent (6 participants) versus 12.9 mg for the control group (8 participants); P value 0.88." This result was not retained in the analysis. Adding it would not change the conclusions for opioid consumption standardized mean difference ‐0.71 (95% confidence interval ‐0.94 to ‐0.48); I2 statistic = 44%, with this result vs standardized mean difference ‐0.77 (95% confidence interval ‐0.98 to ‐0.56); I2 statistic = 30% without. No complications were recorded in either group | |
| Retrospective study | |
| Different intervention: local anaesthetic infiltration | |
| Not a RCT. Single‐institution case control study | |
| Different intervention. This was a randomized trial of 36 participants who were having hip fracture surgery. All participants had a psoas block and general anaesthesia. Participants were randomized into 3 groups. A control group received a psoas block and IV saline, another group received psoas block and IV clonidine 1 mg/kg and a third group received a psoas block and peripheral clonidine. The interval from time of completion of block to first supplementary analgesic administration was longer in the IV clonidine group. Results show no significant differences among groups regarding postoperative adverse effects. We excluded the study as investigators included no 'control' group that received no block | |
| Different intervention. Comparison between ultrasound‐guided supra‐inguinal fascia iliaca block and femoral nerve block | |
| Different intervention. This was a randomized trial of 60 participants. 20 received a 3‐in‐1 block with ultrasound guidance with 20 mL 0.5% bupivacaine, 20 received 20 mL of 0.5% bupivacaine and 20 received 30 mL of 0.5% bupivacaine with nerve stimulator guidance. We excluded the study as investigators included no comparison with a group without nerve block | |
| Different intervention: epidural analgesia | |
| Different population: 6 participants with shaft fracture. Letter sent 17 March 2016, to request separate data for participants with a proximal fracture. No reply | |
| Different population. This was a randomized trial comparing femoral nerve block vs no block for 54 participants with a femoral shaft or distal femoral fracture. We excluded the study as it included no proximal femoral fractures | |
| Different intervention. This was a randomized study of 80 participants undergoing lower extremity surgery that compared 4 different methods. A lumbar plexus block with 30 mL 0.5% levobupivacaine or a lumbar plexus block with 30 mL 0.75% ropivacaine or a sciatic nerve block with 10 mL 0.75% ropivacaine or a sciatic nerve block with 10 mL 0.5% levobupivacaine. We excluded the study from this review, as investigators included no 'control' group without nerve block | |
| Different intervention. Comparison between fascia iliaca block and femoral (3‐in‐1) block for preoperative analgesia in the emergency department | |
| Different intervention: epidural analgesia | |
| Different population. This was a randomized trial of 62 participants with femoral trauma who were randomized to receive at the site of the accident a femoral nerve block or intravenous metamizole for pain. Studiy provided a variety of causes for the femoral trauma, including 20 cases of hip fracture. The nerve block was shown to reduce the degree of pain as assessed by the visual analogue scale and to reduce anxiety and heart rate. We excluded the study as it included participants with other conditions. Trialists were unable to provide separate results for hip fracture participants | |
| Study authors informed us that the trial included participants with hip fracture and participants without hip fracture undergoing elective hip arthroplasty. They could not give us data separately for participants with and without hip fracture: "I did not registered which patients were hip fractures, just the type of surgery" | |
| Different population. Femoral shaft fractures | |
| Different intervention. This was a randomized study of 30 participants who underwent partial hip replacement surgery. 15 received general anaesthesia plus epidural block with 15 mL of 0.5% bupivacaine, and 15 received general anaesthesia plus psoas compartment block with 30 mL of 0.5% bupivacaine. Both groups had similar pain scores, but the epidural group showed greater drops in mean arterial blood pressure from baseline and more complications. We excluded the study from this review because it did not include a control group that did not receive nerve block | |
| Different intervention. This was a randomized study of 3 different combinations of doses of local anaesthetics given to produce a 'three in one' femoral nerve block. We excluded this study from the review because it did not include a 'control' group that did not receive nerve block |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Ultrasound‐guided femoral nerve block using 1% ropivacaine as a method of pain control in patients who present to emergency with a fractured hip |
| Methods | Parallel RCT Open label Approved by the ethics committee |
| Participants | Inclusion criteria: 18 years or older with radiological proof of fractured neck of femur Exclusion criteria: women lactating, pregnant or of childbearing potential who are not willing to avoid becoming pregnant during the study, < 18 years old, allergy to ropivacaine, allergy to paracetamol and morphine, anticoagulated patients and those with significant coagulation abnormalities that increase their risk of bleeding. localized injection site infection. neurological deficits in the distribution of the femoral nerve noted, consent denied, documented severe hepatic disease, unable to give consent themselves, history of heart block or on amiodarone, acute cardiac event in the last 3 months |
| Interventions | Treatment group: An ultrasound‐guided femoral nerve block will be placed with 1% ropivacaine. An ultrasound vascular probe is placed to locate structures anatomically: The main structures were the femoral nerve itself, the femoral artery and vein and the fascia iliaca. Then under real‐time ultrasound guidance via an out‐of‐plane approach, 15 mL of 1% ropivacaine is injected around the femoral nerve as visualized. A 2‐person technique is employed with the needle attached to the syringe of ropivacaine via a 90 cm minimum volume extension set. The probe and needle operator is present along with an assistant who injects the anaesthetic. Digital pressure is then placed for 30 seconds just distal to the injection site. The entire procedure takes about 15 to 20 minutes. Objective measure of the nerve block is assessed at 30 minutes by testing sensation over the anterolateral aspect of the thigh Control group: Both study and control groups will receive regular oral tablet paracetamol 1 G every 4 to 6 hours to a maximum dose of 4 G in 24 hours and parenteral (intravenous) morphine as required for pain control |
| Outcomes | Primary outcome: morphine use in patients for both groups at 24 hours Secondary outcomes: pain scores and subsequent pain scores to be looked at in each group and compared over the first 12 hours. Scores will range from 0 to 10, with 10 indicating the worst pain. Pain scores in both groups will be assessed at enrolment into the study, 30 minutes after enrolment or after nerve block has been given, then at 4 then 8 then 12 hours after enrolment |
| Starting date | 04/04/2009 |
| Contact information | Dr Edmond Park, Emergency Department, St Vincents Hospital, Victoria Street, Darlinghurst 2010 New South Wales +61 2 8382 2040, [email protected] |
| Notes | Recruiting |
| Trial name or title | A randomized controlled trial of fascia iliaca compartment block versus morphine for pain in fractured neck of femur in the emergency department: a pilot study ‐ fascia Iliaca compartment block versus parenteral morphine sulphate |
| Methods | RCT Open label |
| Participants | Inclusion criteria: suspected isolated hip fracture (clinical suspicion of hip fracture defined as the presence of painful, unilateral shortening and external rotation of the lower limb, preventing weight‐bearing), alert and oriented, willing to be randomized for pain relief as described, > 18 years of age Exclusion criteria: other significant injury, clinical suspicion of fracture other than to the hip, sensitivity to any study preparation, local infection at the injection site, symptoms/Injury > 8 hours, prehospital parenteral analgesia, unable to give informed consent as a result of current or pre‐existing physical or mental condition, known coagulopathy, taking warfarin or clopidogrel within 2 weeks, body mass index > 40 |
| Interventions | Treatment group: fascia iliaca compartment block with bupivacaine 0.25% Control group: morphine |
| Outcomes | Primary outcome measure: comparison between experimental and control groups of the difference in maximum tolerable passive hip flexion from supine (verbal pain score ≤ 3), expressed in degrees measured with a goniometer at baseline (on arrival after recruitment but before randomization) vs 45 minutes post intervention Secondary objective: investigation of duration of effect, ease of use, failure rates and morphine‐sparing effect of using fascia iliaca block in this participant group |
| Starting date | |
| Contact information | Nottingham University Hospitals NHS Trust |
| Notes |
| Trial name or title | Blocking the femoral nerve in patients with suspected hip fracture ‐ does it work in clinical practice? |
| Methods | Parallel RCT Double‐blind |
| Participants | Inclusion criteria: 132 participants with suspected hip fracture, ≥ 65 years old Exclusion criteria: more fractures incurred in the context of an accident, at home for more than 12 hours after the accident, hypersensitivity to local analgesics, infection, neurovascular problems, blockade not available within a specific period, patients for whom the attending physician considers that blockade could be harmful (e.g. atrioventricular block type II or III, elderly, severe liver disease, greatly reduced renal function) |
| Interventions | Treatment group: femoral nerve block with ropivacaine Control group: placebo, subcutaneous injection |
| Outcomes | Primary objective: pain scores Secondary outcomes: complications (decubitus ulcers), rehabilitation time, analgesic use |
| Starting date | 24/10/2008 |
| Contact information | |
| Notes |
| Trial name or title | The FINOF (Femoral Nerve‐Block Intervention in Neck Of Femur Fracture) study ‐ FINOF |
| Methods | RCT Single‐blind |
| Participants | Inclusion criteria: ≥ 70 years of age, resident in own home or in warden‐aided flat, cognitively intact (as defined by a score ≥ 7 on the Abbreviated 10 point Mental Test Score (AMTS), prior fracture New Mobility Score ≥ 3, indicating independent indoor ambulation) Exclusion criteria: pre‐fracture hospitalization, contraindications to femoral nerve block analgesia, regular pre‐fracture opioid or glucocorticoid therapy, alcohol or substance abuse, morphine intolerance, postoperative surgical restrictions for ambulation |
| Interventions | Treatment group: femoral nerve block with ropivacaine 0.2% Control group: standard care |
| Outcomes | Primary endpoint(s): from day 1 to day 3 postoperatively: cumulative ambulation score, cumulative dynamic pain score postoperatively Secondary objective: to estimate the cost‐effectiveness of femoral nerve blockade vs usual care, to examine issues of compliance, acceptability to staff and participants |
| Starting date | 20/04/2011 |
| Contact information | Nottingham University Hospitals NHS Trust, United Kingdom; no contact provided |
| Notes |
| Trial name or title | Analgesic effect of a supplemental nerve block in patients with hip fracture |
| Methods | Parallel RCT Double‐blind |
| Participants | Inclusion criteria: clinical suspicion of hip fracture, successful sensory effect of femoral nerve block, mentally capable of comprehending and using verbal pain score and distinguishing between pain from fractured hip and pain from other location, arrival in the emergency department at times when one of the doctors who do the nerve blocks for this investigation are on call, possible visualization of necessary structures with ultrasound, verbal pain score (0‐10) > 3 at rest or > 5 with passive leg raise 30 minutes after femoral nerve block, informed consent, ≥ 18 years of age Exclusion criteria: hip fracture not confirmed by x‐ray, weight < 45 kg, previously included in this trial If participant wishes to be excluded, allergy to local anaesthetics or adrenocortical hormone, visible infection in the area of the point of needle injection |
| Interventions | Treatment group: femoral nerve block with bupivacaine 0.25% Control group: placebo |
| Outcomes | Primary outcome: frequency of sufficient analgesia 20 minutes after a supplemental obturator nerve block vs placebo Secondary outcomes: success rate, time to perform the block, onset time |
| Starting date | 17/03/2015 |
| Contact information | Department of Anaesthesia and Intensive Care, Aarhus University Hospital, Nørrebrogade 44, 8000 Aarhus, Denmark +4528782877 |
| Notes |
| Trial name or title | The effect of the use of fascia iliaca nerve blockade on patient positioning for spinal anaesthesia and the effect of continuous nerve blockade on postoperative pain and mobility outcomes in patients with hip fractures |
| Methods | RCT Double‐blind Approved by the ethics committee: Health and Social Care Research Ethics Committee (HSC REC 1) (Northern Ireland) approved on 18th of April 2008 (ref: 08/NIR01/20) Funding: governmental: Belfast Health and Social Care Trust (UK) (ref: RGHT 000559) |
| Participants | Target number of participants 100 participants ‐ 40 in first part of study and 60 in second part of study Inclusion criteria: ASA physical status class I‐IV, able to give written informed consent, requiring operative repair of fractured neck of femur, aged 18 years and over, either sex Exclusion criteria: history of dementia or difficulty in obtaining consent, history of allergy to any of the medications used in the study |
| Interventions | Treatment group: fascia iliaca block for positioning before spinal anaesthesia and continuous infusion after surgery Part 1: Participants randomized to receive fascia iliaca compartment block with 2 mg/kg 1% lignocaine or conventional sedation with 0.2 mg/kg IV ketamine and 0.025 mg/kg IV midazolam. At completion of surgery, a fascia iliaca block with 1 mg/kg 0.25% levobupivacaine will be performed in all participants Control group: no block |
| Outcomes | Primary outcome measures: ‐ Part 1: comparison of pain score at rest and positioning for spinal anaesthesia in participants who have received a fascia iliaca compartment block or conventional sedation Secondary outcome measures: length of time to first request of additional analgesia, level of assistance required for transfer from sitting to standing position, incidence and severity of motor blockade, time to mobilization with walking aid, measurement of oxygen saturations without supplemental oxygen in both groups, incidence of all complications associated with analgesic techniques in both groups, incidence of nausea and/or vomiting within first 48 hours after surgery in both groups, use of blood products in all groups |
| Starting date | 01/07/2009 |
| Contact information | Ms Rosemary Hogg Department of Anaesthetics & Intensive Care Medicine, Queen's University Belfast, 2nd Floor, Mulhouse Building, Grosvenor Road |
| Notes | Completed Protocol/serial number: RGHT000559 |
| Trial name or title | Femoral nerve blockade in hip fracture patients |
| Methods | RCT Approved by the ethics committee Funding: governmental: Umeå University (Sweden) |
| Participants | Target number of participants: 250 Inclusion criteria: both males and females, aged 70 years and above, all hip fracture patients admitted to the orthopaedic department Exclusion criteria: local infection, allergy to local anaesthesia, dying patients, pathologic hip fractures |
| Interventions | Treatment group: Participants in the intervention group will receive a femoral nerve blockade as soon as they arrive. Participants with pain scores > 4 will be given morphine IV according to the standard protocol (morphine 10 mg/mL, 1 to 5 mg when necessary) Control group: regular use of opioids Both groups will receive 1 G of paracetamol 4 times/d. Postoperative pain treatment will be given according to the standard protocol in both arms of the study. Total follow‐up for both arms will end at the time of discharge |
| Outcomes | Primary outcome measures: postoperative delirium, assessed 3 times a day; postoperative complications, such as decubital ulcers, infections, thrombosis, heart failure and pain. A cognitive test will be assessed in the ambulance pre‐hospital arrival and at 24 +/‐ 6 hours postoperatively. At days three to five, a more thorough assessment will be done including delirium, depression, cognitive status, quality of life and more Secondary outcome measures: mortality, orthopaedic recovery recorded at the time of discharge from the hospital, EQ‐5D, economics |
| Starting date | 30/03/2009 |
| Contact information | Prof Ola Winso Operationscentrum |
| Notes | Completed |
| Trial name or title | Intra‐ and post‐operative analgesia for patients undergoing surgery for hip fracture ‐ role of fascia iliaca compartment block |
| Methods | RCT Double‐blind (participants and caregivers) Funding: governmental: Department of Health, Richmond House, 79 Whitehall, London, SW1A 2NL, United Kingdom |
| Participants | 40 adult participants of ASA I‐III admitted to Selly Oak Hospital with hip fracture and scheduled for fixation will be recruited after consent is obtained inclusion criteria: scheduled for hip fracture surgery Exclusion criteria: dementia/confusion, preoperative chest infection and/or poor respiratory function, temperature ≥ 38°C, white cell count > 11,000 mm3, respiratory rate > 25 per minute, auscultation and/or chest x‐ray evidence, SpO2 < 90% on air, congestive cardiac failure, bed‐bound or use of ≥ 2 aids for mobilization pre‐fracture, malignancy, coagulopathy, known or suspected allergy to ropivacaine and/or morphine, local infection at site where the block is to be performed, refusal of permission to approach general practitioner |
| Interventions | Treatment group: fascia iliaca compartment block (n = 20) Control group: morphine (n = 20) Each participant will have a standard preoperative assessment, standard monitoring and recovery care and will be given assessments at 1, 3, 6 and 24 hours postop. Thereafter, data will be collected on a daily basis until 30 days postop/discharge/death, whichever is earlier. Our hospital already has a framework for data collection (Integrated Care Pathway). For our study, we will be using the same data |
| Outcomes | Primary outcome measures: total dose of morphine required during first 24 hours post‐op Secondary outcome measures: time to first dose of morphine postop, pain scores in recovery room and at 13, 16 and 24 hours after surgery, time to first appropriate response to verbal commands, time to discharge from recovery room, occurrence of nausea and vomiting in recovery and number of episodes during the first 24 hours postop, total dose of cyclizine required in the first 24 hours, need for granisetron during the first 24 hours, sedation scores at 1, 3, 6 and 24 hours, mental test scores at 1, 3, 6 and 24 hours, complications, rehabilitation outcomes, mortality |
| Starting date | 04/04/2006 |
| Contact information | Dr FA Levins, Anaesthetics, Selly Oak Hospital, Birmingham, B29 6JD, United Kingdom |
| Notes | DOI 10.1186 Protocol/serial number: N0265178818 Completed |
| Trial name or title | The FINOF ‐ Femoral nerve‐block Intervention in Neck Of Femur fracture study Approved by Nottingham Research Ethics Committee 2, 28th January 2011, ref: 10/H0408/113 Funding: governmental; Funder name: National Institute of Health Research (NIHR) (UK) ‐ Research for Patient Benefit (RfPB) |
| Methods | RCT |
| Participants | 150 elderly patients admitted with an acute hip fracture Inclusion criteria: aged ≥ 70 years, resident in their own home or warden‐aided flat, cognitively intact (as defined by a score ≥ 7 on the Abbreviated 10 point Mental Test Score (AMTS), and prior fracture New Mobility Score ≥ 3 (indicating independent indoor ambulation) Exclusion criteria: prefracture hospitalization, contraindications to femoral nerve block analgesia, regular pre‐fracture opioid or glucocorticoid therapy, alcohol or substance abuse, morphine intolerance, postoperative surgical restrictions for ambulation |
| Interventions | Treatment group: femoral nerve block followed by continuous infusion Control group: standard analgesic care |
| Outcomes | Drug‐related adverse events, earlier recovery, shorter length of stay in hospital and overall, improved quality of life for patients suffering with an acute hip fracture Primary outcome measures: cumulative dynamic pain score, cumulative ambulation score measured from day 1 to day 3 postoperatively Secondary outcome measures: cumulative dynamic pain score preoperatively (at 30 minutes, 60 minutes, 12 hours after initial femoral nerve block), cumulative side effects (nausea, vomiting, constipation, delirium) (from admission to day 3 postoperatively), |
| Starting date | 02/01/2012 |
| Contact information | Dr Opinder Sahota, Queens Medical Centre, Derby Road, Nottingham, NG7 2UH, United Kingdom; [email protected] |
| Notes | Completed: DOI 10.1186/ISRCTN92946117 EudraCT number: 2010‐023871‐25 http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=10929 http://www.ncbi.nlm.nih.gov/pubmed/24885267
|
| Trial name or title | Improving pain and function in hip fracture |
| Methods | RCT Single‐blind (outcome assessor) |
| Participants | Inclusion criteria: adults (≥ 60 years) presenting to the MSMC ED from 8:00 to 20:00 with a radiographically confirmed hip fracture (femoral neck, intertrochanteric or pericapsular) Exclusion criteria: history of advanced dementia, presence of multiple trauma, pathological fractures, bilateral hip fractures, previous fracture or surgery at the currently fractured site, transferred from another hospital, cirrhosis or liver failure, able to self‐report pain intensity Other exclusion criterion: < age 60 because our focus is on treatment of pain in older adults |
| Interventions | Treatment group: femoral nerve block Control group: no intervention |
| Outcomes | Primary outcome measures: pain (11‐point numerical evaluated 3 times daily for the duration of hospital stay (average stay is 4 days)) Secondary outcome measures: delirium (confusion assessment method) evaluated 3 times daily for duration of hospital stay |
| Starting date | November 2008 |
| Contact information | R. Sean Morrison, MD, and Knox Todd, MD, Beth Israel, New York, USA 10003 |
| Notes | This study has been completed |
| Trial name or title | Perioperative analgesia by femoral perineural catheter for femoral neck fracture ‐ Study KTcol |
| Methods | Parallel RCT Double‐blind (participants, investigator) |
| Participants | Inclusion criteria: adults (≥ 18 years) with femoral neck fracture Exclusion criterion: contraindication with analgesia |
| Interventions | Treatment group: analgesic treatment with a femoral perineural catheter (inserted from hospital admission) with continuous infusion of ropivacaine Control group: placebo |
| Outcomes | Primary outcome measures: evaluation, in patients admitted in emergencies for suspicion of femoral neck fracture, the perioperative efficiency of an analgesic treatment provided with a femoral perineural catheter from hospital admission to 24 hours after surgical operation |
| Starting date | March 2009 |
| Contact information | Guillaume Bouhours UHAngers, Angers, France |
| Notes |
| Trial name or title | Postoperative pain control among intrathecal 0.1 mg morphine, femoral nerve block or periarticular infiltration of 20 mL of 0.25% bupivacaine in patients post intramedullary hip screw |
| Methods | Parallel RCT Single‐blind (outcome assessor) |
| Participants | Inclusion criteria: 18‐90 years old, good consciousness, well co‐operated, can use patient‐controlled analgesia machine, ASA physical status I‐III, no contraindication for spinal anaesthesia, accept spinal anaesthesia, body weight > 30 kg, body mass index 20‐35 kg/m2, no history of research drug allergy Exclusion criteria: previous history of hip surgery (the same side), pathological fracture, severe infection or bone cancer |
| Interventions | Treatment group 1: femoral nerve block, spinal anaesthesia plus femoral nerve block with 20 mL of 0.25% bupivacaine Treatment group 2: periarticular bupivacaine infiltration, spinal anaesthesia plus periarticular infiltration with 20 mL of 0.25% bupivacaine Control group 1: spinal anaesthesia with 0.5% bupivacaine alone Control group 2: spinal anaesthesia plus 0.1 mg of intrathecal morphine |
| Outcomes | Primary outcome measures: amount of morphine consumed during first 24 hours after surgery |
| Starting date | September 2010 |
| Contact information | Thitima Chinachoti, MD (Madihol University), Faculty of Medicine Siniraj Hospital, Bangok, Thailand, 10700 |
| Notes |
| Trial name or title | Does femoral nerve catheterization reduce the incidence of post‐operative delirium in patients presenting for hip fracture repair? |
| Methods | Parallel RCT Open label |
| Participants | 270 adults (≥ 50 years) presenting to Oschner Main Campus with a hip fracture Exclusion criteria: head trauma as reported in the medical record and/or participant response, high‐impact fracture as reported in the medical record, aphasia as reported in the medical record and/or participant response, deafness, blindness as reported in the medical record and/or participant response, true allergy (not sensitivity or side effects) to local anaesthetics or opiates, pregnant, inability to complete study activities preoperatively |
| Interventions | Treatment group: femoral nerve catheterization Control group: intravenous opioids |
| Outcomes | Primary outcome measure: number of participants developing delirium postoperatively during first 3 days after surgery Secondary outcome measures: hospital length of stay, pain score, consumption of pain medication for breakthrough pain relief |
| Starting date | March 2012 |
| Contact information | Leslie Thomas, MD, Oschner Clinic Foundation, New Orleans, Louisianna, USA 70121 |
| Notes | Recruiting |
| Trial name or title | Is regional anaesthesia of the hip preferable over traditional analgesia in the acute stage of the management of patients with a fracture of the hip |
| Methods | Parallel RCT Double‐blind (participants, caregivers, investigator, outcomes assessors) |
| Participants | Inclusion criteria: adults (≥ 65 years) with a hip fracture Exclusion criteria: multiple fractures, delay (> 12 hours) from the time of injury until admission to the hospital, local infection, hypersensitivity to local analgesics, cognitive impairment |
| Interventions | Treatment group: local injection (fascia iliaca block) of 150 mg ropivacaine Control group: saline injection |
| Outcomes | Primary outcome measure: pain during first 3 hours after surgery Secondary outcome measures: medical complications (number of participants who develop pressure ulcers, number of participants who develop pneumonia): under hospitalization (expected average of 10 days) |
| Starting date | January 2012 |
| Contact information | Landstinget i Värmland, Othopedikinken, Centralsjukhuset I Karlsad, Karlsad, Vamiand, Sweden, S‐65185 |
| Notes | Terminated |
| Trial name or title | Hip fracture and perineural catheter |
| Methods | Parallel RCT Open label |
| Participants | Inclusion criteria: age ≥ 60 years, written informed consent obtained, ASA physical status I‐III, undergoing surgery for hip fracture, time < 24 hours after hip fracture Exclusion criteria: contraindication to regional anaesthesia (constitutional or acquired disorder of coagulation, sepsis, local infection of puncture area, history of vascular surgery, prosthetic femoral neuropathy scalable, allergy to local anaesthetics), weight < 40 kg, receiving anticoagulant drugs or antiplatelet therapy (other than aspirin and clopidogrel), contraindication for standardized anaesthetic technique in this study, contraindication for analgesics used postoperatively (respiratory failure, severe liver failure, brain injury associated with intracranial hypertension, uncontrolled epilepsy, simultaneous treatment with monoamine oxidase inhibitors, hypersensitivity to opioids), unable to give informed consent, adults under guardianship or curator, persons not affiliated with a health insurance plan, deprived of liberty |
| Interventions | Treatment group: continuous perineural catheter Control group: no intervention |
| Outcomes | Primary outcome measure: number of participants with cardiovascular events during the preoperative period: 3 days |
| Starting date | June 2012 |
| Contact information | Vincent Compere, University Hospital, Rouen, France |
| Notes | Recruiting |
| Trial name or title | Ultrasound‐guided femoral (three‐in‐one) nerve block versus ultrasound guided fascia iliacus compartment block versus standard treatment for pain control in patients with hip fractures in the emergency department |
| Methods | Parallel RCT Open label |
| Participants | Inclusion criteria: English‐speaking patients; ≥ 18 years of age; radiographic evidence of hip fracture; awake, alert and oriented to time, place and person; pain score ≥ 5 on 10‐point scale Exclusion criteria: cognitive deficits, allergy to amide‐type local anaesthetic or morphine, more injuries than just hip fracture |
| Interventions | Treatment group 1 : ultrasound‐guided 3‐in‐1 femoral nerve block Treatment group 2: ultrasound‐guided fascia iliaca compartment block Control group: IV morphine |
| Outcomes | Primary outcome measure: pain score at 30 minutes Secondary outcome measures: pain score at 60, 120, 240 and 480 minutes |
| Starting date | October 2008 |
| Contact information | Eitan Dickman, MD, Maiminides Medical Center, Brooklyn, New York, USA 11219 |
| Notes | Has results |
| Trial name or title | Comparison of ultrasound guided femoral nerve blockade and standard parenteral opioid pain management alone in patients with hip fracture in the emergency department |
| Methods | Parallel RCT Open label |
| Participants | Inclusion criteria: > 18 years of age presenting with radiologically established intracapsular, extracapsular hip fracture, able to consent and participate in the study, moderate to severe pain (numerical pain score ≥ 3) at time of enrolment Exclusion criteria: previous history of hypersensitivity to local anaesthetics, signs of local infection at the site of planned needle placement |
| Interventions | Treatment group: ultrasound‐guided femoral nerve block with 0.5% bupivacaine (2 mg/kg) Control group: IV morphine |
| Outcomes | Primary outcome measure: pain intensity reduction at 4 hours after initiation of study procedure |
| Starting date | February 2015 |
| Contact information | Elena Skomorovsky, MD, Beth Israel Deaconess Medical Center |
| Notes | This study is not yet open for participant recruitment |
| Trial name or title | Contribution of anaesthesia technique for post‐operative mortality reduction after proximal femur fractures surgical treatment ‐ a randomized clinical trial |
| Methods | Parallel RCT Double‐blind (investigator, outcome assessor) |
| Participants | Inclusion criteria: adults (≥ 60 years) admitted with a diagnosis of proximal femur fracture (ICD‐9 codes 820.0 to 820.9) and submitted to surgical internal fixation of femur or hip prosthesis (ICD‐9 codes 7935, 8151 and 8152) Exclusion criteria: multiple fractures; polytrauma, active malignancy, ASA physical status V, antiplatelet drugs (other than aspirin) in previous 5 days, known allergies to local anaesthetics, contraindication to general or regional anaesthesia |
| Interventions | Treatment group: femoral, lateral cutaneous nerve of the thigh and anterior obturator nerve blocks with ropivacaine and inhalational general anaesthesia with sevoflurane or desflurane Control group: spinal anaesthesia with bupivacaine |
| Outcomes | Primary outcome measures: survival rate up to 1 year after surgery Secondary outcome measures: incidence of postoperative delirium (up to 1 week postoperatively and measured with the 3D‐CAM Questionnaire (Confusion Assessment Method)), quality of life recovery (measured by quality of life assessment tools (SF12v2; EQ‐5D (EuroQol) at 30 days and 1 year after surgery) |
| Starting date | April 2015 |
| Contact information | Raul Carvalho, MD, MSc, Centro Hospitalar do Porto |
| Notes | Enrolling |
| Trial name or title | Fascia iliaca block in the emergency department for analgesia after femoral neck fracture |
| Methods | Parallel RCT Double‐blind (caregiver, investigator, outcome assessor) |
| Participants | Inclusion criteria: adults (≥ 18 years) with femoral neck fracture in the emergency department Exclusion criteria: presence of dementia, body weight < 40 kg, presence of cancer or receiving chemotherapy, allergy to local anaesthetics |
| Interventions | Treatment group: fascia iliaca block (injection of 30 mL of bupivacaine 0.5% with epinephrine 5 mcg/mL below the fascia iliaca, Carbostesin®) Control group: sham injection = subcutaneous injection of 5 mL of normal saline |
| Outcomes | Primary outcome measures: pain scores at rest: 45 minutes after injection Secondary outcome measures: pain scores at rest at 60 minutes, 4 hours, 8 hours, 12 hours and 24 hours after injection; pain scores on movement:at 60 minutes, 4 hours, 8 hours, 12 hours and 24 hours after injection; morphine consumption at 60 minutes, 4 hours, 8 hours, 12 hours and 24 hours after injection and length of stay |
| Starting date | October 2014 |
| Contact information | Eric Albrecht, Program Director, Regional Aanaesthesia, CHU Vaudois, Switzerland |
| Notes | Recruiting |
AMTS: Abbreviated 10‐point Mental Test Score
ASA: American Society of Anaesthesiologists physical status
C: Celsius
CAM Questionnaire: Confusion Assessment Method
EQ‐5D or EUROQOL: score for measurement of health‐related quality of life
G: gram
ICD‐9: list of codes for International Statistical Classification of Diseases and Related Health Problems
IV: intravenous
kg: kilogram
kg/m2: kilogram per square metre
mm: millimetre
MSMC ED: Maimonides Medical Center emergency department
n: number
NHS: Nottingham University Hospitals
OMC: orientation‐memory‐concentration
RCT: randomized controlled trial
RfPB: Research for Patient Benefit
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain on movement within 30 minutes of block placement Show forest plot | 8 | 373 | Std. Mean Difference (Random, 95% CI) | ‐1.41 [‐2.14, ‐0.67] |
| Analysis 1.1  Comparison 1 Nerve block versus other modes of analgesia, Outcome 1 Pain on movement within 30 minutes of block placement. | ||||
| 2 Pain at rest within 30 minutes after block placement Show forest plot | 7 | 322 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.25, ‐0.35] |
| Analysis 1.2 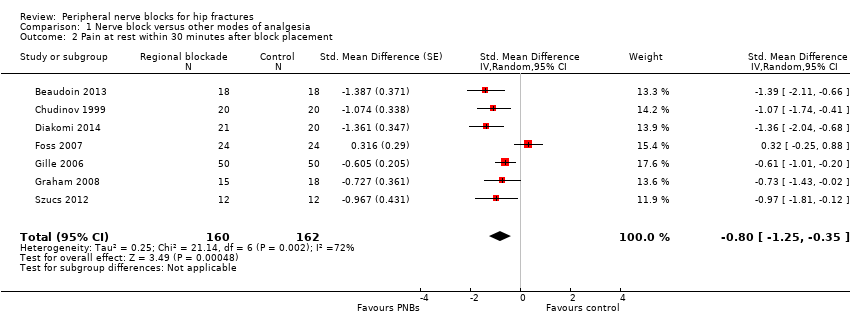 Comparison 1 Nerve block versus other modes of analgesia, Outcome 2 Pain at rest within 30 minutes after block placement. | ||||
| 3 Pain at rest at 6 to 8 hours after surgery Show forest plot | 5 | 286 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.70, ‐0.06] |
| Analysis 1.3  Comparison 1 Nerve block versus other modes of analgesia, Outcome 3 Pain at rest at 6 to 8 hours after surgery. | ||||
| 4 Pain on movement at 24 hours after surgery Show forest plot | 4 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| Analysis 1.4  Comparison 1 Nerve block versus other modes of analgesia, Outcome 4 Pain on movement at 24 hours after surgery. | ||||
| 4.1 Surgical technique unspecified | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.10, 0.29] |
| 4.2 Arthroplasty for 38.4% of participants | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐2.75, ‐1.13] |
| 5 Pain at rest at 24 hours after surgery Show forest plot | 8 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.23, ‐0.13] |
| Analysis 1.5  Comparison 1 Nerve block versus other modes of analgesia, Outcome 5 Pain at rest at 24 hours after surgery. | ||||
| 5.1 Single shot blocks | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.90, ‐0.10] |
| 5.2 Continuous blocks | 6 | 355 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.58, 0.03] |
| 6 Pain on movement at 48 hours Show forest plot | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.40] |
| Analysis 1.6  Comparison 1 Nerve block versus other modes of analgesia, Outcome 6 Pain on movement at 48 hours. | ||||
| 7 Pain at rest at 48 hours after surgery Show forest plot | 5 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| Analysis 1.7  Comparison 1 Nerve block versus other modes of analgesia, Outcome 7 Pain at rest at 48 hours after surgery. | ||||
| 7.1 Psoas compartment block | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.26, 0.06] |
| 7.2 Femoral nerve block | 3 | 191 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.16, 0.28] |
| 7.3 Fascia iliaca block | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.47, ‐0.36] |
| 8 Pain at rest at 72 hours after surgery Show forest plot | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.83, 0.87] |
| Analysis 1.8  Comparison 1 Nerve block versus other modes of analgesia, Outcome 8 Pain at rest at 72 hours after surgery. | ||||
| 8.1 Psoas compartment block | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.77, ‐0.63] |
| 8.2 Femoral nerve block | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.03, 0.33] |
| 9 Acute confusional state Show forest plot | 7 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.27] |
| Analysis 1.9  Comparison 1 Nerve block versus other modes of analgesia, Outcome 9 Acute confusional state. | ||||
| 9.1 Peripheral nerve block based on landmarks | 4 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.23, 2.93] |
| 9.2 Peripheral nerve block based on nerve stimulator | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.98] |
| 10 Pneumonia Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.89] |
| Analysis 1.10  Comparison 1 Nerve block versus other modes of analgesia, Outcome 10 Pneumonia. | ||||
| 11 Mortality Show forest plot | 7 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.52] |
| Analysis 1.11  Comparison 1 Nerve block versus other modes of analgesia, Outcome 11 Mortality. | ||||
| 12 Time to first mobilization Show forest plot | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐11.25 [‐14.34, ‐8.15] |
| Analysis 1.12 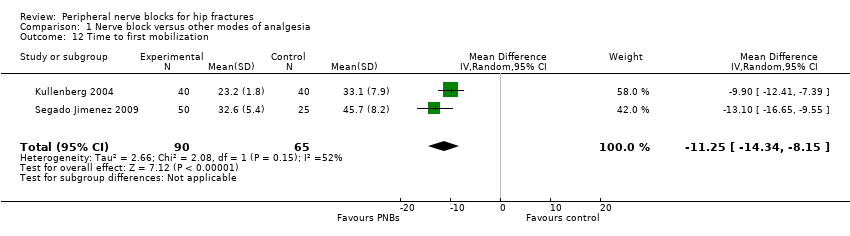 Comparison 1 Nerve block versus other modes of analgesia, Outcome 12 Time to first mobilization. | ||||
| 13 Costs of analgesic regimens Show forest plot | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Nerve block versus other modes of analgesia, Outcome 13 Costs of analgesic regimens. | ||||
| 13.1 Single shot blocks | 1 | 75 | Std. Mean Difference (Random, 95% CI) | ‐3.48 [‐4.23, ‐2.74] |
| 13.2 Continuous blocks | 1 | 62 | Std. Mean Difference (Random, 95% CI) | 0.93 [0.37, 1.48] |
| 14 Pressure sores Show forest plot | 3 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.53] |
| Analysis 1.14  Comparison 1 Nerve block versus other modes of analgesia, Outcome 14 Pressure sores. | ||||
| 14.1 Single shot femoral nerve block (on admission) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 14.2 Continous femoral nerve block (after surgery) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.19, 4.90] |
| 15 Opioid requirement Show forest plot | 7 | 285 | Std. Mean Difference (Random, 95% CI) | ‐0.70 [‐0.96, ‐0.44] |
| Analysis 1.15  Comparison 1 Nerve block versus other modes of analgesia, Outcome 15 Opioid requirement. | ||||
| 15.1 Single shot blocks | 5 | 245 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐1.01, ‐0.44] |
| 15.2 Continuous blocks | 2 | 40 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐1.18, 0.08] |
| 16 Participant satisfaction Show forest plot | 5 | 237 | Std. Mean Difference (Random, 95% CI) | 0.91 [0.62, 1.20] |
| Analysis 1.16  Comparison 1 Nerve block versus other modes of analgesia, Outcome 16 Participant satisfaction. | ||||
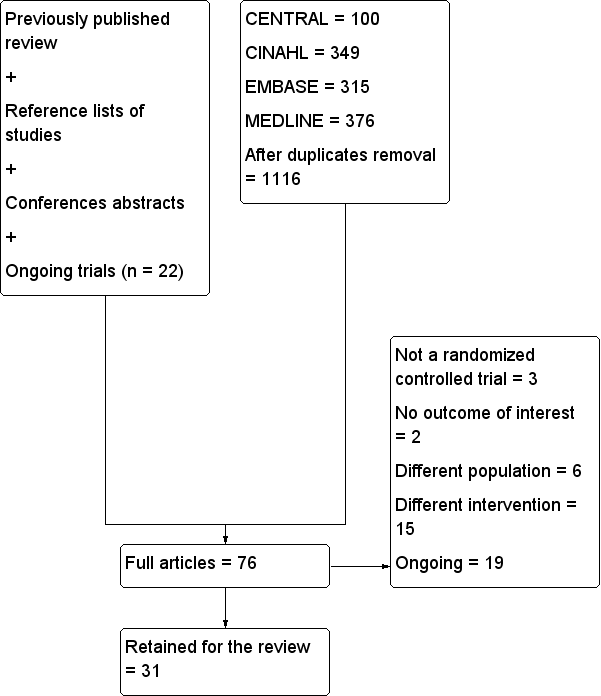
Flow diagram for this update.
n: number.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Pain on movement in participants with hip fracture between 20 and 30 minutes after block placement. The effect size is proportionate to the concentration of local anaesthetic (mg/mL) used in lidocaine equivalent (P < 0.00001).
Local anaesthetic concentration in lidocaine equivalent (calculated as follows: lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3 and tetracaine = 4).
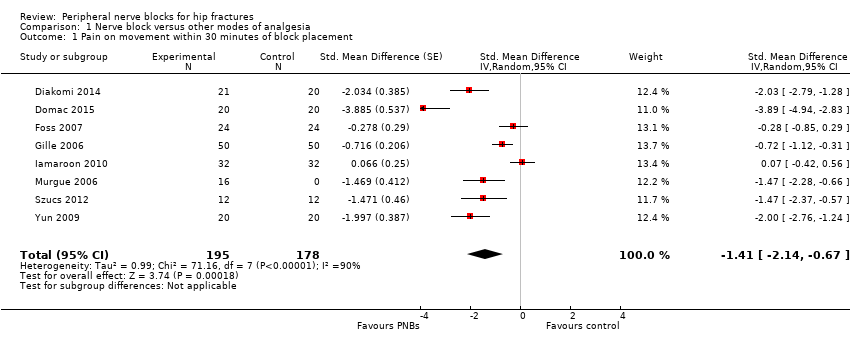
Comparison 1 Nerve block versus other modes of analgesia, Outcome 1 Pain on movement within 30 minutes of block placement.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 2 Pain at rest within 30 minutes after block placement.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 3 Pain at rest at 6 to 8 hours after surgery.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 4 Pain on movement at 24 hours after surgery.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 5 Pain at rest at 24 hours after surgery.
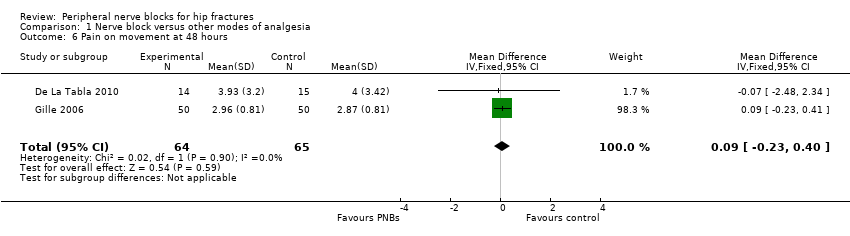
Comparison 1 Nerve block versus other modes of analgesia, Outcome 6 Pain on movement at 48 hours.
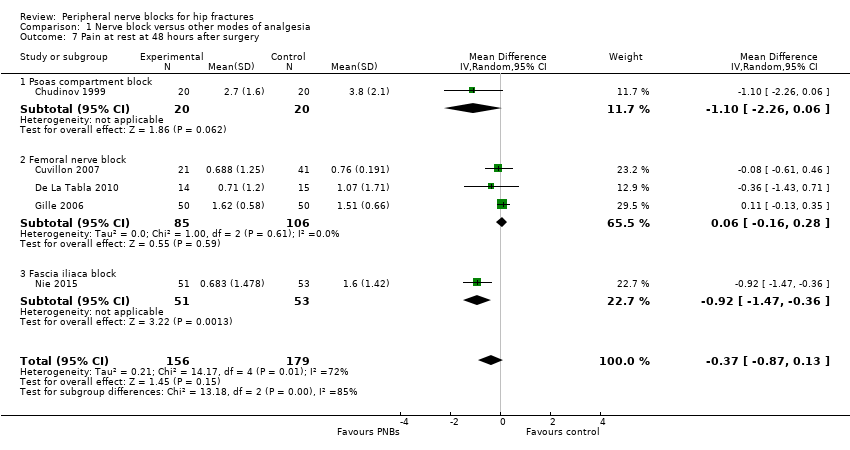
Comparison 1 Nerve block versus other modes of analgesia, Outcome 7 Pain at rest at 48 hours after surgery.
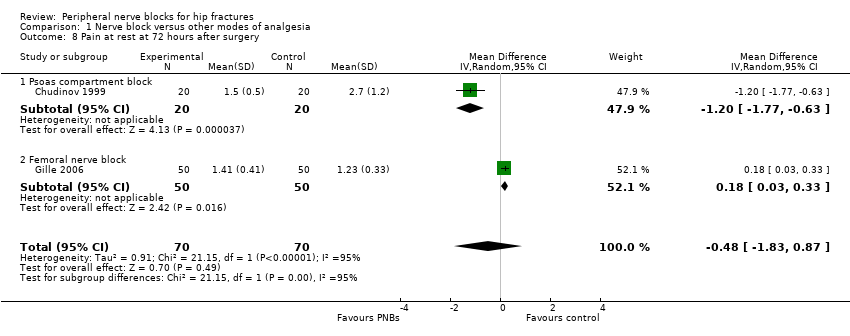
Comparison 1 Nerve block versus other modes of analgesia, Outcome 8 Pain at rest at 72 hours after surgery.
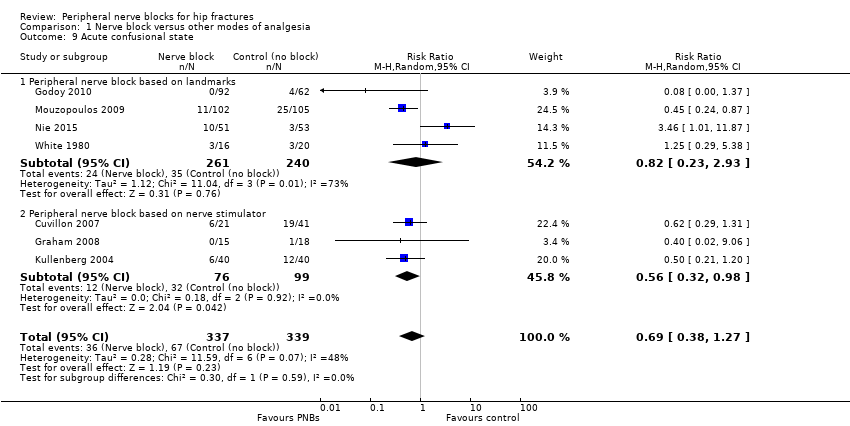
Comparison 1 Nerve block versus other modes of analgesia, Outcome 9 Acute confusional state.
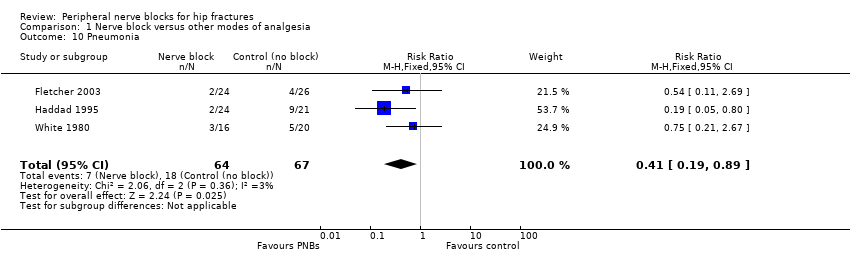
Comparison 1 Nerve block versus other modes of analgesia, Outcome 10 Pneumonia.
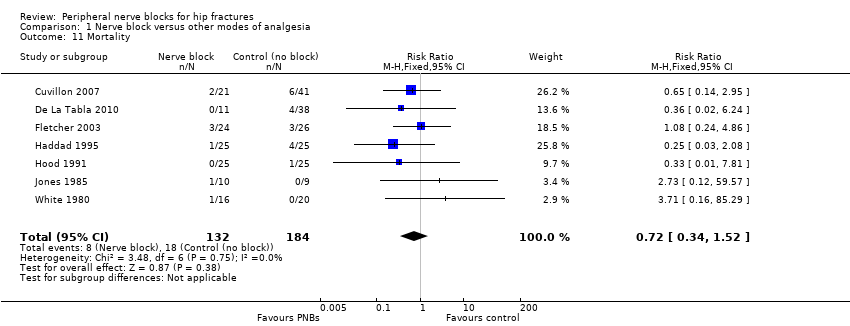
Comparison 1 Nerve block versus other modes of analgesia, Outcome 11 Mortality.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 12 Time to first mobilization.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 13 Costs of analgesic regimens.
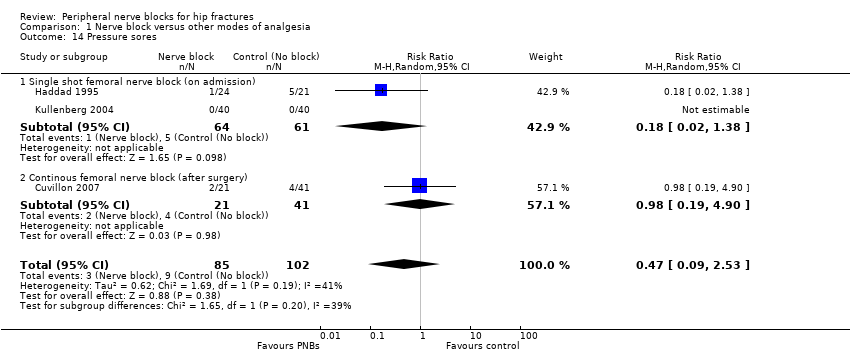
Comparison 1 Nerve block versus other modes of analgesia, Outcome 14 Pressure sores.
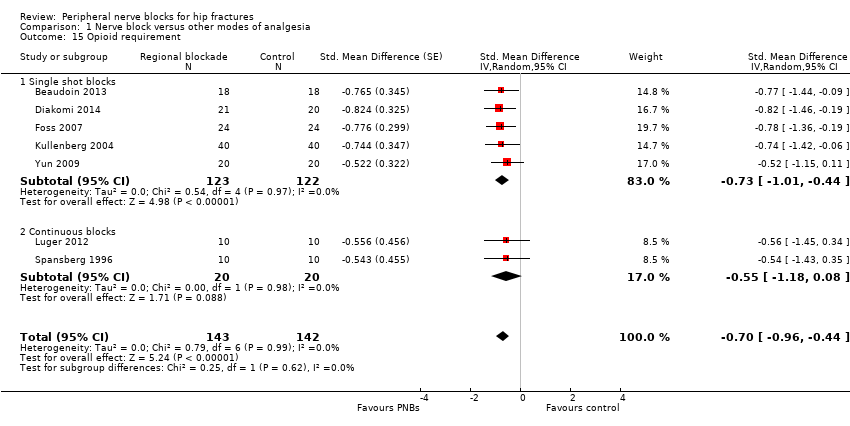
Comparison 1 Nerve block versus other modes of analgesia, Outcome 15 Opioid requirement.

Comparison 1 Nerve block versus other modes of analgesia, Outcome 16 Participant satisfaction.
| Peripheral nerve blocks for hip fracture | ||||||
| Patient or population: patients with hip fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain on movement at 30 minutes after block placement | Mean pain on movement at 30 minutes after block placement in the intervention groups was | 373 | ⊕⊕⊕⊕ | Equivalent to | ||
| Acute confusional state | Study population | RR 0.69 | 676 | ⊕⊝⊝⊝ | ||
| 198 per 1000 | 136 per 1000 | |||||
| Low | ||||||
| 150 per 1000 | 104 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 172 per 1000 | |||||
| Myocardial ischaemia | Study population | RR 0.2 | 20 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 100 per 1000 | |||||
| Low | ||||||
| 100 per 1000 | 20 per 1000 | |||||
| High | ||||||
| 500 per 1000 | 100 per 1000 | |||||
| Pneumonia | Study population | RR 0.41 | 131 | ⊕⊕⊕⊝ | ||
| 269 per 1000 | 110 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 20 per 1000 | |||||
| High | ||||||
| 200 per 1000 | 82 per 1000 | |||||
| Death | Study population | RR 0.72 | 316 | ⊕⊕⊝⊝ | ||
| 98 per 1000 | 70 per 1000 | |||||
| Low | ||||||
| 25 per 1000 | 18 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 108 per 1000 | |||||
| Time to first mobilisation | Mean time to first mobilisation in intervention groups was | 155 | ⊕⊕⊕⊝ | |||
| Cost of analgesic regimens for single shot blocks | Mean cost of analgesic regimens for single shot blocks in intervention groups was | 75 | ⊕⊕⊕⊝ | |||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| a50% or more of studies were rated as having unclear or high risk for allocation concealment or blinding of outcome assessor | ||||||
| Study | Purpose of blockade | Surgical anaesthesia | Block technique | Comparison |
| Preoperative and postoperative analgesia | Unspecified | Continuous lumbar plexus with 0.1% bupivacaine in a patient‐controlled analgesia mode | IV morphine | |
| Postoperative analgesia | Spinal anaesthesia | Continuous femoral nerve block, nerve stimulator, loaded with 18 mL of 0.25% bupivacaine followed by an infusion of 0.125% levobupivacaine at 3‐4 mL/h | IM pethidine | |
| Preoperative analgesia | Unspecified | Ultrasound‐guided femoral nerve block performed by an experienced operator with a 7.5 MHz linear probe, participant in Trendelenburg position, cross‐sectional view, 22 G Whitacre needle in‐plane and 25 mL of bupivacaine 0.5% and distal manual pressure for 5 minutes | IM morphine | |
| Preoperative and postoperative analgesia Surgery for some participants | Treatment group: psoas block alone (3/20) with a sciatic block (5/20), a spinal (11/20) or general anaesthesia (1/20) Control group: neuraxial block (19/20) or general anaesthesia (1/20). | Psoas, loss of resistance, Chayen's technique 0.8 mL/kg of bupivacaine 0.25%, operated side up (1 epidural spread) | IM meperidine and diclofenac | |
| Postoperative analgesia | General anaesthesia for all participants with etomidate, nitrous oxide, enflurane, fentanyl and vecuronium | Lateral cutaneous: 15 mL of 0.5% bupivacaine with epinephrine, Eriksson's technique Femoral (3‐in‐1): 15 mL of 0.5% bupivacaine with epinephrine, Winnie's technique | IM meperidine | |
| Postoperative analgesia | Spinal anaesthesia for all participants | Continuous femoral nerve block. Nerve stimulator 0.3 to 0.5 mA. Non‐stimulating catheter passed 10‐15 cm past the needle tip loaded with 30 mL of 1.5% lidocaine followed by ropivacaine 0.2% at 10 mL/h for 48 hours | SC morphine | |
| Preoperative analgesia | Unspecified | Femoral nerve block under dual guidance (ultrasound and nerve stimulator), catheter loaded with 15 mL of ropivacaine 0.2% followed by an infusion of the same solution at 5 mL/h and 10 mL every 30 minutes | IV metamizole and tramadol | |
| Preoperative analgesia (spinal positioning) | Spinal anaesthesia | Fascia iliaca block, Dalen's technique, landmarks, 40 mL of 0.5% ropivacaine | IV fentanyl | |
| Preoperative analgesia (spinal positioning) plus postoperative analgesia | Spinal anaesthesia | Fascia iliaca block with 15 mL of 0.5% bupivacaine and 15 mL of 2% lidocaine; 2‐3 cm below inguinal ligament at the junction of lateral 1/3 and medial 2/3 of a line from pubis tubercle to anterior iliac spine; 2 pops | IV morphine | |
| Preoperative analgesia | Unspecified | Fermoral (3‐in‐1 nerve block, Wiinie's technique with 20 mL of 0.5% bupivacaine and 5 minutes distal compression) | IV morphine | |
| Preoperative analgesia | Unspecified | Fascia iliaca block based on Dalen's landmarks with a 24 G blunted needle and 40 mL of 1% mepivacaine with epinephrine | IM morphine | |
| Preoperative and postoperative analgesia | Treatment group: spinal anaesthesia for 37/50 and general anaesthesia for 13/50 Control group: spinal anaesthesia for 38/50 and general anaesthesia for 12/50 | Femoral non‐stimulating catheter: needle 18 G, catheter 20 G (Brown‐Perifix‐Plexus Anaesthesia); 0.5 mA and 0.1 msec. Catheters were advanced about 10 cm past the needle tip and fixed. Loading dose was 40 mL of prilocaine 1% followed 2 hours later by ropivacaine 0.2% 30 mL, repeated every 6 hours. Amount (up to 40 mL; n = 5) and intervals (up to every 4 hours; n = 8) or both (n = 6) adjusted on pain scores | IV metamizole plus oral tilidine and naloxone | |
| Preoperative analgesia | Unspecified | Fascia iliaca compartment block, 21G long bevel needle, Dalen's technique with 0.3 mL/kg of 0.25% bupivacaine | IV non‐steroidal anti‐inflammatory drugs | |
| Preoperative analgesia | Unspecified | Femoral (3‐in‐1) nerve block, Winnie's technique, nerve stimulator and 30 mL of bupivacaine 0.5% (not exceeding 3 mg/kg) | IV morphine | |
| Preoperative analgesia | Unspecified | Femoral nerve block with 0.3 mL/kg of bupivacaine 0,25%. Paraesthesia technique with a short bevel needle | IM pethidine, oral co‐dydramol and IM voltarol | |
| Postoperative analgesia | General anaesthesia for all participants with etomidate, nitrous oxide, isoflurane and alfentanil | Femoral nerve block (triple nerve block) with nerve stimulator < 1.0 mA and 35 mL of prilocaine 0.75% and distal digital pressure plus infiltration above the iliac crest with 8 mL of the same solution | IM papaveratum | |
| Preoperative analgesia | Spinal anaesthesia | Femoral nerve block with nerve stimulator (0.2 to 0.4 mA) and 20 mL of bupivacaine 0.5% plus 10 mL of saline | IV fentanyl | |
| Preoperative analgesia | Spinal anaesthesia | Femoral nerve block with nerve stimulator (0.3 to 0.5 mA) and 15 mL of lidocaine 2% plus 5 mL of distilled water | IV fentanyl | |
| Postoperative analgesia | General anaesthesia for all participants with thiopental, nitrous oxide, halothane, fentanyl and alcuronium | Lateral cutaneous nerve block with 15 mL of 0.5% bupivacaine, Eriksson's technique | IM pethidine | |
| Preoperative analgesia | Unspecified | Femoral nerve block with 30 mL of 0.75% ropivacaine. Winnie's approach and nerve stimulator | IM ketobemidon plus tramadol and paracetamol | |
| Preoperative and postoperative analgesia | Spinal anaesthesia | Ultrasound‐guided femoral (3‐in‐1) nerve block (13‐6 MHz linear probe), catheter inserted ≥ 12‐15 cm past the needle tip) loaded with 30 mL of 0.25% bupivacaine followed by an infusion of 0.125% bupivacaine at 6 mL/h (motor blockade not evaluated) or Lumbar epidural analgesia with 0.125% bupivacaine at 8 mL/h | IV/SC piritramide or IV paracetamol | |
| Preoperative analgesia | Spinal anaesthesia | Fascia iliaca block with 20 mL of 1.5% lidocaine | IV fentanyl | |
| Preoperative and postoperative analgesia | Epidural anaesthesia | Fascia iliaca block daily, Dalen's technique with 0.3 mL/kg of bupivacaine (0.25%?) | IV and analgesics | |
| Preoperative analgesia | Unspecified | Femoral nerve block with nerve stimulator and 20 mL of mepivacaine | IV morphine or IV paracetamol and ketoprofen | |
| Postoperative analgesia | General anaesthesia with propofol, remifentanil and atracurium | Fascia iliaca block with landmarks (2‐3 cm below the inguinal ligament); catheter inserted at least 10 cm cranially and loaded with 20 to 30 mL (weight basis) of 0.5% ropivacaine followed by 0.25% bupivacaine at 0.1 mL/kg/h for 48 hours | IV patient‐controlled analgesia with fentanyl and tropisetron | |
| Postoperative analgesia | Spinal anaesthesia | Landmarks. Obturator nerve with 15 mL of bupivacaine with a vasoconstrictive agent, proximal to the obturator orifice. Femoral lateral cutaneous (Brown) with 10 mL of the same solution | IV morphine | |
| Postoperative analgesia | Spinal anaesthesia | Femoral nerve block with nerve stimulator, non‐stimulating catheter advanced 8‐15 cm past needle tip. Inserted just before surgery. Loaded with 0.4 L/kg of bupivacaine 0.5%; continuous infusion with 0.14 mL/kg/h of bupivacaine 0.25% for 16 hours after surgery | IM morphine | |
| Preoperative and postoperative analgesia | Spinal anaesthesia | Non‐stimulating catheter for femoral nerve block, inserted in the emergency department with a nerve stimulator, 0.4 mA and 0.1 msec, space dilated before catheter insertion with 10 mL of 2% lidocaine, catheter advanced 3 cm past the needle tip and 10 mL of 0.5% bupivacaine through the catheter followed by 0.25% bupivacaine infused at | IM morphine | |
| Postoperative analgesia | General anaesthesia for all participants with propofol, nitrous oxide, isoflurane, fentanyl, morphine and atracurium | Femoral (3‐in‐1) nerve block, nerve stimulator 0.1 mA, non‐stimulating catheter advanced 4‐5 cm past the needle tip. Loaded with 30 mL of 2% lidocaine with epinephrine followed by an infusion with bupivacaine 0.125% at 4 mL/h for 48 hours | IV morphine | |
| Intraoperative analgesia | General anaesthesia with thiopental, nitrous oxide, halothane and fentanyl or nitrous oxide and alfaxolone/alfadolone | Psoas block with 30 mL of 2% mepivacaine, side to be blocked uppermost, Chayen's technique or Spinal: 0.6 to 0.8 mL of hyperbaric cinchocaine | Conventional general anaesthesia | |
| Preoperative analgesia | Spinal anaesthesia | Fascia iliaca block, Dalen's technique with 30 mL of 0.375% ropivacaine | IV alfentanil | |
| G: gram h: hour IM: intramuscular mA: milliAmpere mcg/mL: microgram/millilitre mg/kg: milligram/kilogram MHz: megahertz mL: millilitre msec: millisecond n: number SC: subcutaneous | ||||
| Study | Study authors' definition |
| Clinical evaluation "somnolence‐confusion" | |
| "episodes of delirium" | |
| "acute confusional state" | |
| "transient confusion" | |
| "The primary outcome was perioperative delirium. "Daily patient assessments using the MMSE, DRS‐R‐ | |
| "Presurgery cognitive status was estimated using the | |
| "confused" |
| Study | Complications related to regional anaesthesia | Complications related to analgesic technique |
| Not reported | Not reported | |
| No complications such as motor block. local Five participants had accidental removal or the catheter: 4 during the procedure or while the catheter was secured and 1 while in the ward | Not reported | |
| No other adverse events were noted during the study period, and no other adverse events were reported to study investigators | Four‐hour oxygen saturation (%) 96 (93–99) vs (%) 98 (95–99) for regional blockade Adverse events: One participant had an episode of rapid atrial fibrillation requiring diltiazem, but the participant had a history of chronic atrial fibrillation | |
| No major complications were described in group regional blockade. Three participants developed local erythema at the catheter insertion site at the end of the study period No signs of local anaesthetic toxicity were documented One participant developed bilateral blockade (L1‐L3 on the opposite side) | Not reported | |
| No complications related to nerve blocks and no case of prolonged motor blockade | Not reported | |
| Four catheters were prematurely removed: 1 by a confused participant, 2 by nurses (unexplained fever) and 1 by a surgeon (unconfirmed suspicion of local anaesthetic toxicity (ropivacaine blood level < 2 ng/mL)) | More constipation (47% vs 19% for regional blockade) | |
| Not reported | Not reported | |
| Complications such as local anaesthetic toxicity recorded as well (none reported in results section) Nor did complication rates vary between groups | Complications such as hypoventilation (breathing rate < 8 breaths/min) were recorded as well Moreover, the 2 groups did not differ in these parameters at any time point until study completion at 24 hours after surgery. Nor did complication rates vary between groups | |
| Not reported | Not reported | |
| Among study participants, none experienced adverse effects as a result of nerve block administration | No clinically important differences between groups with respect to pulse rate, oxygen saturation or respiratory rate at any time interval. Oxygen saturation 94.87% | |
| No side effects attributable to femoral nerve block were noted in any participants during their hospital stay | More participants (P = 0.05) were sedated in the morphine group at 180 minutes after block placement No difference was noted between groups in nausea and vomiting, with 3 participants in each group having these side effects Tendency toward lower saturation was noted in the opioid group at 60 and 180 minutes after the block despite oxygen supplementation (P = 0.08) | |
| One inadvertent arterial puncture and blood aspiration positive for 3 participants Two transient paraesthesias No catheter site infection Ten catheters accidentally removed | No respiratory depression from systemic analgesia and no allergic reactions All complications were reversible | |
| The only complications were local bruises at the site of injection | Two participants with nausea, and 2 with nausea and vomiting | |
| No immediate complications occurred in either group | No immediate complications were noted in either group | |
| No local or systemic complications of femoral nerve blocks were noted | Not reported | |
| No untoward sequelae were associated with nerve blocks All plasma prilocaine concentrations (maximum 3 pg/mL) were below the suggested threshold for toxicity for prilocaine of 6 pg/mL | Not reported | |
| No adverse systemic toxicity of bupivacaine, such as seizure, arrhythmia or cardiovascular collapse was noted in the femoral nerve block group Neither vascular puncture nor paraesthesia occurred No complications, such as haematoma, infection or persistent paraesthesia, were observed within 24 hours after the operation | No participant in either group had hypoventilation (ventilatory rate < 10/min) or oxygen saturation < 95% | |
| Not reported | In participants of fentanyl group, drowsiness was observed that required the presence of more persons for holding the participant during positioning SpO2 was significantly lower in the fentanyl group (P = 0.001). However, no participant in either group had SpO2 < 90% during the procedure Mean arterial blood pressure was significantly lower in the fentanyl group (P = 0.0019) | |
| No untoward sequelae associated with the nerve block were seen | Not reported | |
| No complications related to the nerve blockade were noted in this study | Not reported | |
| Not reported | Not reported | |
| Not reported | Not reported | |
| No complications of femoral nerve block administrations occurred, except 3 local haematomas developed at the injection site, which resolved spontaneously | Not reported | |
| Not reported | Not reported | |
| No adverse effects, such as pain at the insertion site or paraesthesia, were observed No positive cultures were observed with the fascia iliaca block catheter tip, nor were any signs of infection noted in the current study | Not reported | |
| We did not observe any complications in the realization of regional anaesthetic techniques during or subsequent to the regional anaesthetic techniques | The incidence of side effects (sleepiness, hypotension, constipation, pruritus) was greater in the group with no block than in groups with blocks (P < 0.01) | |
| No haematomas at the site of femoral catheters | Two participants in each group experienced nausea and vomiting | |
| For 1 participant, the elastomeric pump failed, resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, and another participant, suffering from acute confusional state, disconnected his pump after 12 hours | The incidence of nausea/vomiting, pruritus or excessive sedation was similar in the 2 groups | |
| Not reported | Side effects (vomiting and pruritus) were observed significantly more frequently with intravenous analgesia | |
| No participants showed any evidence of local anaesthetic toxicity | Not reported | |
| No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited No complications, such as haematoma or persistent paraesthesia, were observed in participants with a femoral nerve block within 24 hours after the operation | Hypoventilation (ventilatory rate 6–8/min) or pulse oximetric desaturation (oxygen saturation 88% or 89%) was encountered in 4 participants (20%) in the intravenous analgesia group. This was reverted with assisted manual mask ventilation All participants in the intravenous group experienced mild dizziness, and mild drowsiness was present in 12/20 of them | |
| %: percentage L: litre mg: milligram min: minute ng/mL: nanogram/millilitre pg/mL: picogram/millilitre | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain on movement within 30 minutes of block placement Show forest plot | 8 | 373 | Std. Mean Difference (Random, 95% CI) | ‐1.41 [‐2.14, ‐0.67] |
| 2 Pain at rest within 30 minutes after block placement Show forest plot | 7 | 322 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.25, ‐0.35] |
| 3 Pain at rest at 6 to 8 hours after surgery Show forest plot | 5 | 286 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.70, ‐0.06] |
| 4 Pain on movement at 24 hours after surgery Show forest plot | 4 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 4.1 Surgical technique unspecified | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.10, 0.29] |
| 4.2 Arthroplasty for 38.4% of participants | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐2.75, ‐1.13] |
| 5 Pain at rest at 24 hours after surgery Show forest plot | 8 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.23, ‐0.13] |
| 5.1 Single shot blocks | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.90, ‐0.10] |
| 5.2 Continuous blocks | 6 | 355 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.58, 0.03] |
| 6 Pain on movement at 48 hours Show forest plot | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.40] |
| 7 Pain at rest at 48 hours after surgery Show forest plot | 5 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| 7.1 Psoas compartment block | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.26, 0.06] |
| 7.2 Femoral nerve block | 3 | 191 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.16, 0.28] |
| 7.3 Fascia iliaca block | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.47, ‐0.36] |
| 8 Pain at rest at 72 hours after surgery Show forest plot | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.83, 0.87] |
| 8.1 Psoas compartment block | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.77, ‐0.63] |
| 8.2 Femoral nerve block | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.03, 0.33] |
| 9 Acute confusional state Show forest plot | 7 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.27] |
| 9.1 Peripheral nerve block based on landmarks | 4 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.23, 2.93] |
| 9.2 Peripheral nerve block based on nerve stimulator | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.98] |
| 10 Pneumonia Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.89] |
| 11 Mortality Show forest plot | 7 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.52] |
| 12 Time to first mobilization Show forest plot | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐11.25 [‐14.34, ‐8.15] |
| 13 Costs of analgesic regimens Show forest plot | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 13.1 Single shot blocks | 1 | 75 | Std. Mean Difference (Random, 95% CI) | ‐3.48 [‐4.23, ‐2.74] |
| 13.2 Continuous blocks | 1 | 62 | Std. Mean Difference (Random, 95% CI) | 0.93 [0.37, 1.48] |
| 14 Pressure sores Show forest plot | 3 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.53] |
| 14.1 Single shot femoral nerve block (on admission) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 14.2 Continous femoral nerve block (after surgery) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.19, 4.90] |
| 15 Opioid requirement Show forest plot | 7 | 285 | Std. Mean Difference (Random, 95% CI) | ‐0.70 [‐0.96, ‐0.44] |
| 15.1 Single shot blocks | 5 | 245 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐1.01, ‐0.44] |
| 15.2 Continuous blocks | 2 | 40 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐1.18, 0.08] |
| 16 Participant satisfaction Show forest plot | 5 | 237 | Std. Mean Difference (Random, 95% CI) | 0.91 [0.62, 1.20] |

