بررسی تاثیر استفاده زودهنگام (< 7 روز) از کورتیکواستروئیدهای سیستمیک پس از زایمان برای پیشگیری از بروز دیسپلازی برونکوپولمونری در نوزادان نارس
چکیده
پیشینه
دیسپلازی برونکوپولمونری (bronchopulmonary dysplasia; BPD) یک مشکل اساسی برای نوزادانی است که بسیار نارس (preterm) متولد میشوند. التهاب پایدار در ریهها مهمترین پاتوژنز این وضعیت محسوب میشود. به دلیل تاثیرات ضد التهابی قوی کورتیکواستروئیدهای سیستمیک، آنها برای پیشگیری یا درمان BPD مورد استفاده قرار میگیرند.
اهداف
بررسی مزایای نسبی و عوارض جانبی آغاز تجویز کورتیکواستروئیدهای سیستمیک پس از زایمان طی شش روز نخست زندگی برای نوزادان نارس که در معرض خطر پیشرفت BPD قرار دارند.
روشهای جستوجو
در 25 سپتامبر 2020 جستوجوی بهروزشدهای را در بانکهای اطلاعاتی زیر انجام دادیم: CENTRAL از طریق CRS Web و MEDLINE از طریق Ovid. همچنین بانکهای اطلاعاتی کارآزماییهای بالینی و فهرست منابع مقالات بازیابی شده را برای یافتن کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) جستوجو کردیم. کارآزماییهای خوشهای تصادفیسازی شده، ککارآزماییهای متقاطع (cross‐over)، یا شبه‐RCTها را وارد مرور نکردیم.
معیارهای انتخاب
برای این مرور، RCTهایی را انتخاب کردیم که به بررسی درمان با کورتیکواستروئید سیستمیک (داخل وریدی یا خوراکی) پس از زایمان طی شش روز نخست زندگی (زودهنگام) در نوزادان نارس پُر خطر پرداختند. مطالعاتی را وارد کردیم که استفاده از دگزامتازون (dexamethasone) را ارزیابی کردند، همچنین مطالعاتی که به بررسی مصرف هیدروکورتیزون (hydrocortisone) پرداختند، حتی زمانی که دومین دارو عمدتا برای مدیریت هیپوتانسیون استفاده میشد، نه برای درمان مشکلات ریوی. کارآزماییهای انجام شده را با کورتیکواستروئیدهای استنشاقی وارد مرور نکردیم.
گردآوری و تجزیهوتحلیل دادهها
از روشهای استاندارد کاکرین بهره گرفتیم. دادههای مربوط به پیامدهای بالینی را از جمله مورتالیتی، BPD، مورتالیتی یا BPD، عدم موفقیت در خارج کردن لوله تراشه، بروز عوارض حین بستری اولیه در بیمارستان، و پیامدهای طولانیمدت سلامت و تکامل سیستم عصبی را استخراج و تجزیهوتحلیل کردیم. از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (Grading of Recommendations Assessment, Development and Evaluation; GRADE) برای ارزیابی قطعیت شواهد استفاده شد.
نتایج اصلی
استفاده از رویکرد GRADE نشان داد که قطعیت شواهد برای پیامدهای اصلی در نظر گرفته شده در سطح بالا قرار داشت، به جز برای BPD در 36 هفته برای همه مطالعات ترکیب شده، که به دلیل وجود شواهدی از سوگیری (bias) انتشار، یک سطح به حد متوسط کاهش یافت.
تعداد 32 RCT (4395 نوزاد) را وارد کردیم. خطر کلی سوگیری در مطالعات وارد شده پائین بود؛ همه مطالعات از نوع RCT بوده، و بیشتر کارآزماییها از روشهای دقیقی استفاده کردند.
تجویز زودهنگام کورتیکواستروئیدهای سیستمیک بهطور کلی تاثیری اندک یا عدم تاثیر بر مورتالیتی تا آخرین سن گزارش شده دارند (خطر نسبی (RR): 0.95؛ 95% فاصله اطمینان (CI): 0.85 تا 1.06؛ 31 مطالعه، 4373 نوزاد، شواهد با قطعیت بالا)، اما هیدروکورتیزون تنها، مورتالیتی را کاهش میدهد (RR: 0.80؛ 95% CI؛ 0.65 تا 0.99؛ 11 مطالعه، 1433 نوزاد؛ شواهد با قطعیت بالا).
تجویز زودهنگام کورتیکواستروئیدهای سیستمیک بهطور کلی بروز BPD را در هفته 36 پس از قاعدگی (postmenstrual age; PMA) احتمالا کاهش میدهند (RR: 0.80؛ 95% CI؛ 0.73 تا 0.88؛ 26 مطالعه، 4167 نوزاد؛ شواهد با قطعیت متوسط)، دگزامتازون نیز چنین تاثیری را نشان میدهد (RR: 0.72؛ 95% CI؛ 0.63 تا 0.82؛ 17 مطالعه، 2791 نوزاد؛ شواهد با قطعیت بالا)، اما هیدروکورتیزون تاثیری اندک تا عدم تاثیر بر جای میگذارد (RR: 0.92؛ 95% CI؛ 0.81 تا 1.06؛ 9 مطالعه، 1376 نوزاد؛ شواهد با قطعیت بالا).
تجویز زودهنگام کورتیکواستروئیدهای سیستمیک بهطور کلی پیامد ترکیبی مورتالیتی یا BPD را در 36 هفته PMA کاهش میدهند (RR: 0.89؛ 95% CI؛ 0.84 تا 0.94؛ 26 مطالعه، 4167 نوزاد؛ شواهد با قطعیت بالا)، دگزامتازون (RR: 0.88؛ 95% CI؛ 0.81 تا 0.95؛ 17 مطالعه، 2791 نوزاد؛ شواهد با قطعیت بالا)، و هیدروکورتیزون (RR: 0.90؛ 95% CI؛ 0.82 تا 0.99؛ 9 مطالعه، 1376 نوزاد؛ شواهد با قطعیت بالا) نیز این تاثیر را نشان دادند.
تجویز زودهنگام کورتیکواستروئیدهای سیستمیک بهطور کلی خطر پرفوراسیون دستگاه گوارش را افزایش میدهند (RR: 1.84؛ 95% CI؛ 1.36 تا 2.49؛ 16 مطالعه، 3040 نوزاد؛ شواهد با قطعیت بالا)، دگزامتازون (RR: 1.73؛ 95% CI؛ 1.20 تا 2.51؛ 9 مطالعه، 1936 مطالعه؛ شواهد با قطعیت بالا)، و هیدروکورتیزون (RR: 2.05؛ 95% CI؛ 1.21 تا 3.47؛ 7 مطالعه، 1104 نوزاد؛ شواهد با قطعیت بالا) نیز این تاثیر را بر جای میگذارند.
تجویز زودهنگام کورتیکواستروئیدهای سیستمیک بهطور کلی خطر وقوع فلج مغزی را افزایش میدهند (RR: 1.43؛ 95% CI؛ 1.07 تا 1.92؛ 13 مطالعه، 1973 نوزاد؛ شواهد با قطعیت بالا)، دگزامتازون نیز همین اثر را نشان میدهد (RR: 1.77؛ 95% CI؛ 1.21 تا 2.58؛ 7 مطالعه، 921 مطالعه؛ شواهد با قطعیت بالا)، اما هیدروکورتیزون چنین تاثیری ندارد (RR: 1.05؛ 95% CI؛ 0.66 تا 1.66؛ 6 مطالعه، 1052 نوزاد؛ شواهد با قطعیت بالا).
تجویز زودهنگام کورتیکواستروئیدهای سیستمیک بهطور کلی تاثیری اندک تا عدم تاثیر بر پیامد ترکیبی مورتالیتی یا فلج مغزی دارند (RR: 1.03؛ 95% CI؛ 0.91 تا 1.16؛ 13 مطالعه، 1973 نوزاد؛ شواهد با قطعیت بالا)، اما چنین تاثیری از هیدروکورتیزون دیده نشد (RR: 0.86؛ 95% CI؛ 0.71 تا 1.05؛ 6 مطالعه، 1052 نوزاد؛ شواهد با قطعیت بالا). با این حال، تجویز زودهنگام دگزامتازون احتمالا خطر پیامد ترکیبی مورتالیتی یا فلج مغزی را افزایش میدهد (RR: 1.18؛ 95% CI؛ 1.01 تا 1.37؛ 7 مطالعه، 921 نوزاد؛ شواهد با قطعیت بالا).
در تجزیهوتحلیل حساسیت (sensitivity) بر اساس قصد اولیه برای درمان با هیدروکورتیزون (مشکلات ریوی در برابر هیپوتانسیون) در مقایسه با انجام آنالیز بر اساس اندیکاسیون تجویز دارو، شواهد کمی از وجود تفاوت در تاثیرات درمان بر پیامدهای اصلی مورتالیتی، BPD، یا ترکیب مورتالیتی یا BPD به دست آمد.
نتیجهگیریهای نویسندگان
درمان زودهنگام با کورتیکواستروئید سیستمیک پس از زایمان (که طی شش روز نخست پس از تولد نوزاد شروع شود) از بروز BPD و پیامد ترکیبی مورتالیتی یا BPD پیشگیری میکند. با این حال، خطر پرفوراسیون دستگاه گوارش، فلج مغزی، و پیامد ترکیبی مورتالیتی یا فلج مغزی را افزایش مییابد. اکثر تاثیرات مفید و مضر مربوط میشود به درمان زودهنگام با دگزامتازون تا درمان زودهنگام با هیدروکورتیزون، اما تجویز زودهنگام هیدروکورتیزون ممکن است از وقوع مورتالیتی پیشگیری کند، در حالی که تجویز زودهنگام دگزامتازون این تاثیر را ندارد. پیگیری طولانیمدتتر نوزادان تا اواخر دوران کودکی برای ارزیابی پیامدهای مهم که در اوایل دوران کودکی قابل ارزیابی نیستند، مانند تاثیرات درمان زودهنگام با کورتیکواستروئید بر عملکردهای نورولوژیک در مراحل بالاتر، از جمله عملکرد شناختی، عملکرد اجرایی، عملکرد تحصیلی، عملکرد رفتاری، سلامت روان، عملکرد حرکتی، و عملکرد ریوی، حیاتی است. انجام RCTهای بیشتر از تجویز زودهنگام کورتیکواستروئیدها به ویژه هیدروکورتیزون، باید شامل بررسی بقای طولانیمدتتر نوزاد بدون ناتوانی در تکامل سیستم عصبی به عنوان پیامد اولیه باشند.
PICO
خلاصه به زبان ساده
تجویز زودهنگام (شروع طی شش روز) کورتیکواستروئیدهای سیستمیک پس از زایمان برای پیشگیری از بروز دیسپلازی برونکوپولمونری در نوزادان نارس
هدف مرور:تعیین مزایا و آسیبهای نسبی درمان با داروهایی که التهاب را سرکوب میکنند، به نام کورتیکواستروئیدها، که برای نوزادانی که زود به دنیا آمدهاند، در طول هفته اول پس از تولد، به منظور پیشگیری از بروز آسیب ریوی، به نام دیسپلازی برونکوپولمونری (bronchopulmonary dysplasia) (که گاهی اوقات «بیماری مزمن ریوی» نیز نامیده میشود) مصرف میشود.
پیشینه:این وضعیت یک مشکل عمده برای نوزادان تازه متولد شده در بخش مراقبتهای ویژه نوزادان است. التهاب پایدار ریهها علت اصلی آن برشمرده میشود. به دلیل تاثیرات ضد التهابی قوی داروهای کورتیکواستروئیدی، از آنها برای پیشگیری یا درمان دیسپلازی برونکوپولمونری استفاده شده، اما ممکن است عوارض جانبی عمدهای بر جای بگذارند.
ویژگیهای مطالعه: همه کارآزماییهای بالینی را با حضور نوزادان نارس مرور کردیم که کورتیکواستروئیدها را به صورت سیستمیک، یعنی تزریقی یا خوراکی، طی هفته اول پس از تولد تجویز کرده، و دادههایی را در مورد نرخ دیسپلازی برونکوپولمونری در اواخر دوره نوزادی آنها ارائه دادند. تعداد 32 مطالعه (4395 نوزاد) را وارد کردیم. این جستوجو تا 25 سپتامبر 2020 بهروز است.
نتایج کلیدی:این مرور از کارآزماییها نشان داد که مزایای آغاز مصرف کورتیکواستروئیدهای سیستمیک در نوزادان طی شش روز پس از تولد، ممکن است نسبت به عوارض جانبی شناخته شده آن بیشتر نباشد. با این حال، یک کورتیکواستروئید خاص به نام هیدروکورتیزون (hydrocortisone)، امیدی را در بهبود پیامدهای کوتاهمدت، بدون تاثیر منفی بر تکامل سیستم عصبی در طولانیمدت، نشان میدهد، اگرچه دادههای مربوط به پیامدهای طولانیمدت محدود هستند. تاثیرات مفید کورتیکواستروئیدهای سیستمیک بهطور کلی شامل زمان کوتاهتر استفاده از ونتیلاتور و کمتر بودن دیسپلازی برونکوپولمونری بود؛ عوارض جانبی عبارت بودند از فشار خون بالاتر، خونریزی معده یا روده، پرفوراسیون روده، گلوکز بیش از اندازه در جریان خون، و افزایش خطر فلج مغزی در دوره پیگیری، به ویژه در افراد درمان شده با دگزامتازون (dexamethasone) ‐ نوع دیگری از کورتیکواستروئید. تا زمان انجام پژوهشهای بیشتر، باید استفاده زودهنگام از کورتیکواستروئیدها، به ویژه دگزامتازون (dexamethasone)، برای درمان یا پیشگیری از بروز دیسپلازی برونکوپولمونری، کاهش یابد.
قطعیت شواهد: در کل، قطعیت شواهدی که از نتیجهگیریهای ما حمایت میکنند، در سطح بالایی قرار دارد.
Authors' conclusions
Summary of findings
| Early systemic postnatal corticosteroids (dexamethasone and hydrocortisone) compared with placebo or no treatment for preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo or no treatment | Risk with early systemic postnatal corticosteroids | |||||
| Mortality at latest reported age
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 0.95 | 4373 | ⊕⊕⊕⊕ | critical P = 0.05 for subgroup differences | |
| 232 per 1000 | 221 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.02 | 2940 | ⊕⊕⊕⊕ | critical | ||
| 236 per 1000 | 241 per 1000 (212 to 274) | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.80 | 1433 | ⊕⊕⊕⊕ | critical | ||
| 225 per 1000 | 180 per 1000 | |||||
| BPD (36 weeks' PMA)
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 0.80 | 4167 | ⊕⊕⊕⊝ | important P = 0.01 for subgroup differences | |
| 308 per 1000 | 247 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 0.72 | 2791 | ⊕⊕⊕⊕ | important | ||
| 269 per 1000 | 194 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.92 | 1376 | ⊕⊕⊕⊕ | important | ||
| 385 per 1000 | 354 per 1000 | |||||
| Mortality or BPD at 36 weeks' PMA
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 0.89 | 4167 | ⊕⊕⊕⊕ | critical | |
| 515 per 1000 | 458 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 0.88 | 2791 | ⊕⊕⊕⊕ | critical | ||
| 487 per 1000 | 429 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.90 | 1376 | ⊕⊕⊕⊕ | critical | ||
| 569 per 1000 | 512 per 1000 | |||||
| Gastrointestinal perforation during primary hospitalisation
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 1.84 | 3040 | ⊕⊕⊕⊕ | important | |
| 39 per 1000 | 71 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.73 | 1936 | ⊕⊕⊕⊕ | important | ||
| 41 per 1000 | 71 per 1000 | |||||
| Study population (subgroup of infants treated with hydrocortisone | RR 2.05 | 1104 | ⊕⊕⊕⊕ | important | ||
| 34 per 1000 | 70 per 1000 | |||||
| Cerebral palsy at latest reported age
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 1.42 | 1973 | ⊕⊕⊕⊕ | critical P = 0.09 for subgroup differences | |
| 74 per 1000 | 106 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.77 | 921 | ⊕⊕⊕⊕ | critical | ||
| 89 per 1000 | 158 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 1.05 | 1052 | ⊕⊕⊕⊕ | critical | ||
| 62 per 1000 | 65 per 1000 | |||||
| Mortality or cerebral palsy at latest reported age
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 1.03 | 1973 | ⊕⊕⊕⊕ | critical P = 0.02 for subgroup differences | |
| 335 per 1000 | 345 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.18 | 921 | ⊕⊕⊕⊕ | critical
| ||
| 383 per 1000 | 452 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.86 | 1052 | ⊕⊕⊕⊕ | critical | ||
| 295 per 1000 | 254 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
| aDowngraded one level for serious study limitations owing to evidence of publication bias for studies overall, but not within subgroups. | ||||||
Background
Description of the condition
Advances in neonatal care, including use of antenatal corticosteroids and surfactant therapy, have improved the outcomes of preterm infants with respiratory distress syndrome (also called hyaline membrane disease), but risk of chronic lung disease or bronchopulmonary dysplasia (BPD) has been only modestly reduced (Egberts 1997). More recent data suggest approximately 50% of infants born at < 28 weeks' gestation who survive to 36 weeks' gestation have BPD, with rates remaining stubbornly high, even though exogenous surfactant and more non‐invasive ventilation have been introduced into clinical care over the past 30 years (Cheong 2020). The terms 'chronic lung disease' and 'bronchopulmonary dysplasia' are often used interchangeably; for the purposes of this review, we have decided to use 'bronchopulmonary dysplasia' to describe the condition of infants with oxygen dependency at 28 days after birth or at 36 weeks' postmenstrual age. More infants with BPD are now cared for in neonatal intensive care units (NICUs), and management of their condition is both time‐consuming and costly. BPD refers to injury with maldevelopment of the lung that follows preterm birth and is a major problem in NICUs. Persistent inflammation in the lungs is a major feature in its pathogenesis.
Description of the intervention
Postnatal corticosteroid treatment has been shown to have some beneficial acute effects on lung function in infants with established BPD, especially among those who are ventilator‐dependent (CDTG 1991; Mammel 1983). However, clinicians have been concerned that the benefits of corticosteroids might not outweigh associated adverse effects, which include hypertension, hyperglycaemia, intestinal perforation, and extreme catabolism (Anonymous 1991; Ng 1993).
Systemic (enteral or parenteral) corticosteroids have been used to try to prevent BPD by treating at‐risk preterm infants, starting within the first four days after birth. It is not clear whether early use of systemic corticosteroids provides long‐term benefits, neither is it clear if adverse neurological outcomes observed in some animal studies apply to the immature human newborn infant.
How the intervention might work
Systemic corticosteroids might prevent BPD through their potent anti‐inflammatory effects.
Why it is important to do this review
Multiple systematic reviews have examined the use of postnatal corticosteroids in infants with or at risk of BPD (Arias‐Camison 1999; Bhuta 1998; Doyle 2000; Doyle 2010a; Doyle 2010b; Doyle 2010c; Doyle 2014a; Doyle 2014b; Doyle 2017a; Halliday 1997; Halliday 1999; Tarnow‐Mordi 1999). Other systematic reviews have explored early versus late use of inhaled corticosteroids and comparisons of systemic versus inhaled steroids for prevention or treatment of BPD (Onland 2017; Shah 2007b; Shah 2012a; Shah 2012b; Shah 2017).
Two existing Cochrane Reviews have reviewed separately trials in which systemic postnatal corticosteroids were started before eight days of birth or after the first seven days following birth (Doyle 2017a; Doyle 2017b). This review examines the outcomes of trials in which preterm infants were treated with corticosteroids starting within six days after birth. Several trials that started on Day 7 after birth have been included in the late review (Doyle 2017b), which is an update of previous Cochrane Reviews and includes long‐term outcome data from 13 trials.
Objectives
To examine the relative benefits and adverse effects of systemic postnatal corticosteroids commenced within the first six days after birth for preterm infants at risk of developing BPD.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify randomised controlled trials (RCTs) of systemic postnatal corticosteroid therapy for preterm infants at risk of developing BPD, who were enrolled within the first six days after birth (early postnatal corticosteroids). We included trials of hydrocortisone in the first six days after birth when BPD and mortality were reported, even if hydrocortisone had been used primarily to treat or prevent hypotension.
Types of participants
We included preterm infants at risk of developing BPD, including those who are ventilator‐dependent.
Types of interventions
We included trials of Intravenous or oral corticosteroids versus control (placebo or no treatment). We did not include in this review trials of inhaled corticosteroids.
Types of outcome measures
Outcome measures are divided into primary and secondary outcomes.
Primary outcomes
-
Mortality (at 28 days after birth, at 36 weeks' postmenstrual age, at discharge home, and at the latest reported age)
-
Bronchopulmonary dysplasia (at 28 days after birth, at 36 weeks' postmenstrual age, and at 36 weeks' postmenstrual age in survivors)
-
Mortality or bronchopulmonary dysplasia (at 28 days after birth and at 36 weeks' postmenstrual age)
-
Long‐term outcomes (including blindness, deafness, cerebral palsy, and major neurodevelopmental disability)
Secondary outcomes
-
Failure to extubate (at 3, 7, 14, and 28 days)
-
Late rescue with corticosteroids (for all infants and for survivors)
-
Need for home oxygen therapy
-
Complications during the primary hospitalisation (including infection, hyperglycaemia, hypertension, pulmonary air leak, patent ductus arteriosus, severe intraventricular haemorrhage, cystic periventricular leukomalacia, necrotising enterocolitis, gastrointestinal bleeding, intestinal perforation, and severe retinopathy of prematurity)
Search methods for identification of studies
Electronic searches
We conducted an update search in September 2020 of the following: Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9), in the Cochrane Library; and OVID MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1 January 2016 to 25 September 2020). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing and recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), along with the US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the International Standard Randomized Controlled Trials Number Registry (ISRCTN) for any unique trials not found through the Cochrane CENTRAL search (http://www.isrctn.com/).
Although we searched Embase in 2017, we did not search Embase for this update. Although Embase records are included in CENTRAL, we acknowledge that its omission for this update may have reduced the sensitivity of our search.
This is the fifth update of this review. Our previous search details are listed in Appendix 2 and Appendix 3.
Searching other resources
We also searched the reference lists of all published trials to identify trials overlooked during the electronic literature search.
Data collection and analysis
We used the methods of Cochrane Neonatal for data collection and analysis.
Selection of studies
We included all RCTs that fulfilled the selection criteria described in the previous section. We did not include cluster randomised, cross‐over, or quasi‐randomised trials Two review authors (LWD and JC) independently reviewed results of the updated search and selected studies for inclusion. We resolved disagreements by discussion.
Data extraction and management
For each trial, we sought information regarding methods of randomisation, blinding, stratification, and reporting of outcomes for all infants enrolled, and whether the trial used a single‐centre or multi‐centre setting. Information on trial participants included birth weight, gestational age, severity of respiratory distress syndrome, need for mechanical ventilation via an endotracheal tube or other respiratory support not requiring an endotracheal tube and surfactant, and sex. We analysed information on clinical outcomes for mortality, survival without BPD, BPD defined at 28 days of life and at 36 weeks' postmenstrual age, failure to extubate, pneumothorax, infection, hyperglycaemia, hypertension, severe retinopathy of prematurity, patent ductus arteriosus, severe intraventricular haemorrhage, cystic periventricular leukomalacia, necrotising enterocolitis, gastrointestinal bleeding, intestinal perforation, and need for late corticosteroid treatment, as well as long‐term outcomes such as developmental delay, blindness, deafness, cerebral palsy, and major neurosensory disability.
For each study, one review author (LD) entered final data into Review Manager (RevMan) 5 software (Review Manager 2020); a second review author (JC or SH) checked the data for accuracy. We resolved discrepancies through discussion or by consultation with a third assessor (HH).
We attempted to contact authors of the original reports to request further details when information regarding any of the above was unclear.
Assessment of risk of bias in included studies
Two review authors (LD and JC) independently assessed risk of bias (low, high, or unclear) for all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 4 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used standard methods of Cochrane Neonatal when analysing data.
We performed statistical analyses using Review Manager 5 (RevMan) 5 software (Review Manager 2020). We analysed dichotomous data using risk ratio (RR), risk difference (RD), and the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH). We reported 95% confidence intervals (CIs) for all estimates.
We included no continuous outcomes in this review. If included, we planned to analyse continuous data using the mean difference (MD) or the standardised mean difference (SMD) to combine trials that measure the same outcome using different methods.
Unit of analysis issues
For clinical outcomes such as episodes of sepsis, we analysed the data as proportions of neonates having one or more episodes.
Dealing with missing data
For included studies, we noted levels of attrition. If we had concerns regarding the impact of including studies with high levels of missing data in the overall assessment of treatment effect, we planned to explore this concern via sensitivity analysis.
We conducted all outcome analyses on an intention‐to‐treat basis, that is, we included in the analyses all participants randomised to each group. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting forest plots and by quantifying the impact of heterogeneity using the I² statistic. If noted, we planned to explore possible causes of statistical heterogeneity by conducting prespecified subgroup analyses (e.g. differences in study quality, participants, intervention regimens, outcome assessments).
Assessment of reporting biases
We assessed possible publication bias and other biases by examining symmetry/asymmetry of funnel plots. In addition, we computed Egger's statistic on funnel plots to assess the strength of the evidence for publication bias.
For included trials that were recently performed (and therefore were prospectively registered), we explored possible selective reporting of study outcomes by comparing primary and secondary outcomes described in the reports against primary and secondary outcomes proposed at trial registration, using the websites www.clinicaltrials.gov and www.controlled-trials.com. If we found such discrepancies, we planned to contact the primary investigators to request missing outcome data on outcomes prespecified at trial registration.
Data synthesis
When we judged meta‐analysis to be appropriate, we carried out the analysis using Review Manager (RevMan) 5 (Review Manager 2020), as supplied by Cochrane. We used the Mantel‐Haenszel method to obtain estimates of typical RR and RD. We included no continuous outcomes in this review. We planned to use the inverse variance method to analyse continuous measures, if included.
We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses by type of corticosteroid used (dexamethasone or hydrocortisone) when we identified sufficient numbers of trials to make such subgroup analyses meaningful.
Sensitivity analysis
We planned to perform sensitivity analyses for situations that might affect interpretation of significant results (e.g. when risk of bias is associated with the quality of some included trials).
Because the indication for early hydrocortisone treatment might be primarily to treat lung problems or low blood pressure, we performed a sensitivity analysis by indication for hydrocortisone for major outcomes of mortality at the latest age, BPD at 36 weeks, or mortality or BPD at 36 weeks.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following clinically relevant outcomes: mortality, BPD, the combined outcome of mortality or BPD, intestinal perforation, cerebral palsy, and the combined outcome of mortality or cerebral palsy.
Two review authors (LD and JC) independently assessed the certainty of evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create summary of findings Table 1 to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
-
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
We have provided results of the search for this review update in the study flow diagram (Figure 1).

Study flow diagram: review update.
Results of the search
We included 32 studies (4395 infants) in this review (Figure 1). We identified no new studies compared with the previous version of the review (Doyle 2017a). Most of the included studies enrolled low birth weight infants with respiratory distress syndrome who were receiving mechanical ventilation.
Included studies
See Characteristics of included studies.
Twenty‐one studies used primarily dexamethasone (Anttila 2005; Garland 1999: Halac 1990; Kopelman 1999; Lauterbach 2006; Lin 1999; Mukhopadhyay 1998; Rastogi 1996: Romagnoli 1999: Sanders 1994; Shinwell 1996: Sinkin 2000: Soll 1999: Stark 2001: Subhedar 1997: Suske 1996: Tapia 1998: Vento 2004: Wang 1996: Yeh 1990: Yeh 1997), The most common treatment regimen consisted of 0.50 mg/kg/d of dexamethasone for three days followed by 0.25 mg/kg/d for three days, then 0.12 mg/kg/d for three days followed by 0.05 mg/kg/d for three days. However, trialists described considerable variation in treatment regimens, including short courses of one to two days, and longer courses of up to four weeks.
Eleven studies used hydrocortisone (Baden 1972; Batton 2012; Baud 2016; Biswas 2003; Bonsante 2007; Efird 2005; Hochwald 2014; Ng 2006; Peltoniemi 2005; Watterberg 1999; Watterberg 2004). In some cases, when low (almost physiological) doses were used, the indication was management of hypotension (see under Description of studies).
Anttila 2005 was a multi‐centre, double‐blind, placebo‐controlled trial of infants with birth weight of 500 grams to 999 grams, gestation less than 32 weeks, and respiratory failure by four hours of age. Investigators randomised 53 infants to receive four doses of dexamethasone (0.25 mg/kg at 12‐hour intervals) and 56 infants to receive saline placebo. Country: Finland. Participants were recruited between June 1998 and February 2001. Supported by grants from the Foundation for Pediatric Research, the Foundation of Alma and K.A. Snellman, and the Sigrid Juselius Foundation (Finland).
Baden 1972 included 44 infants with respiratory distress syndrome, mild hypoxia and hypercapnia, and a chest radiograph compatible with respiratory distress syndrome. Researchers randomised infants to receive hydrocortisone 15 mg/kg on admission and 12 hours later intravenously (total dose 30 mg/kg hydrocortisone) (n = 22), or placebo (n = 22). Birth weight ranged from 800 grams to 2805 grams, and gestational age from 26 to 36 weeks. Country: Canada. Participants were recruited between August 1971 and August 1972. Upjohn and Company supplied the hydrocortisone and placebo.
Batton 2012 was a pilot study of infants at 23 to 26 weeks' gestation with low blood pressure in the first 24 hours of life. Investigators compared dopamine and hydrocortisone versus placebo using a factorial design. The dose of hydrocortisone was 1 mg/kg loading, then 0.5 mg/kg 12‐hourly for six doses (total dose, 4.0 mg/kg hydrocortisone over three days). The trial was stopped early because of slow recruitment after only 10 infants were enrolled; four received hydrocortisone and six received placebo. Country: USA. Participants were recruited between 3 December 2009 and 3 December 2010. The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development provided grant support, including funding from the Best Pharmaceuticals for Children Act, for the Neonatal Research Network’s Early Blood Pressure Pilot Study.
Baud 2016 was a multi‐centre double‐blind RCT of 523 infants at 24 to 27 weeks’ gestational age who were recruited from 21 French centres with NICU facilities in the first 24 hours after birth between 25 May 2008 and 31 January 2014. Parents of one infant in each group withdrew consent after randomisation, hence results are reported for 421 infants overall. The treatment group received hydrocortisone hemisuccinate 1 mg/kg/d divided into two doses for seven days, then 0.5 mg/kg/d once per day for three days (total dose, 8.5 mg/kg hydrocortisone over 10 days) (n = 255). Control infants were given an equivalent volume of 5% glucose placebo (n = 266). The trial was halted early because of lack of funding, with 523 of a planned total of 786 infants recruited. Country: France. Funded by Assistance Publique‐Hôpitaux de Paris.
Biswas 2003 was a multi‐centre randomised trial of 253 infants at less than 30 weeks' gestational age. Investigators mechanically ventilated infants and entered them into the study within nine hours of birth. They gave all infants surfactant during the first 24 hours of life. Those randomised to the treatment group (n = 125) received an infusion of hydrocortisone 1 mg/kg/d and tri‐iodothyronine (T3) 6 µg/kg/d for five days, then hydrocortisone 0.5 mg/kg/d and T3 3 µg/kg/d for two days (total dose 6 mg/kg hydrocortisone over 7 days). The placebo group (n = 128) received an equal volume of 5% dextrose. Country: England. Participants were recruited between January 1996 and April 1998.
Bonsante 2007 enrolled a total of 50 infants of birth weight less than 1250 grams or at 24 to 30 weeks' gestation who were less than 48 hours old and were ventilator‐dependent after surfactant treatment. Exclusion criteria were cardiopulmonary malformations, perinatal asphyxia, mortality within 12 hours after recruitment, or use of steroids for any reason within 12 days after birth. Researchers excluded no infants for these latter two reasons. They stratified infants by birth weight (not specified), gestational age (not specified), and antenatal steroid exposure, then randomly allocated infants to a 12‐day course of hydrocortisone (1.0 mg/kg for nine days, then 0.5 mg/kg/d for three days) (total dose 10.5 mg/kg hydrocortisone over 12 days) (n = 25), or an equivalent volume of 0.9% saline placebo (n = 25). Study authors based the sample size calculation on the results of Watterberg 1999, resulting in an estimate of 138 infants to be recruited. The study was stopped early when 50 infants had been enrolled because of reports from other trials of spontaneous intestinal perforation with early hydrocortisone treatment. Country: Italy. Participants were recruited between April 2003 and September 2005. Supported by the University of Bari, Bari, Italy.
Efird 2005 was an RCT of hydrocortisone to prevent hypotension in infants of birth weight less than 1000 grams at gestation of 24 to 28 weeks. Trialists randomised 16 infants to receive 1 mg/kg of intravenous hydrocortisone 12‐hourly for two days, followed by 0.3 mg/kg 12‐hourly for three days (total dose 5.8 mg/kg hydrocortisone over five days), or a normal saline placebo (n=18). Country: USA. Participants were recruited between May 2000 and May 2002. Supported by Forest Pharmaceuticals, Inc.
Garland 1999 reported a prospective, multi‐centre, randomised trial comparing a three‐day course of dexamethasone therapy, beginning at 24 to 48 hours of life, versus placebo. Researchers enrolled 241 preterm infants (dexamethasone n = 118, placebo n = 123) who weighed between 500 grams and 1500 grams, had received surfactant therapy, and were at significant risk for BPD or mortality, using a predictive model at 24 hours. Trial authors gave dexamethasone to infants in a three‐day tapering course at 12‐hour intervals. The first two doses were 0.4 mg/kg, the third and fourth doses were 0.2 mg/kg, and the fifth and sixth doses were 0.1 mg/kg and 0.05 mg/kg, respectively (total dose 1.35 mg/kg dexamethasone over three days). They gave a similar volume of normal saline to placebo‐treated infants at similar time intervals. Country: USA. Participants were recruited between December 1992 and November 1997. Supported by the Perinatal Foundation, Milwaukee, WIsconsin.
Halac 1990 was a randomised trial undertaken to determine if prenatal corticosteroid therapy would reduce the incidence of necrotising enterocolitis. Investigators randomised women to prenatal betamethasone or placebo when they were admitted in preterm labour and were expected to deliver within 24 hours. They then randomised infants of mothers who had received placebo to postnatal dexamethasone or placebo; we included in this review only infants who were randomised to postnatal therapy. Study infants weighed less than 1501 grams at birth or were born at less than 34 weeks' gestation and had evidence of "birth asphyxia" (one‐minute Apgar score < 5, prolonged resuscitation, and metabolic acidosis (bicarbonate < 15 mmol/L within one hour of birth)). Study groups were assigned via a table of random numbers. The treatment group (n = 130) received 2 mg/kg/d of dexamethasone phosphate intravenously for seven days (total dose 14 mg/kg dexamethasone over seven days); the control group (n = 118) received an equal volume of 10% dextrose. The major endpoint of this study was necrotising enterocolitis. Country: Argentina. Participants were recruited between January 1985 and December 1987.
Hochwald 2014 reported a single‐centre randomised trial conducted to determine the effects of hydrocortisone on vasopressor dosing in hypotensive infants at < 31 weeks' gestation or with birth weight < 1251 grams during the first 48 hours after birth. Researchers randomly allocated 11 infants to hydrocortisone 2 mg/kg for one dose and 1 mg/kg for three doses, six hours apart, then 0.5 mg/kg for four doses, six hours apart (total dose 7 mg/kg hydrocortisone over two days), or an equal volume of saline placebo (n = 11). Country: Canada. Participants were recruited between January 2007 and December 2009.
Kopelman 1999 was a prospective blinded RCT of 70 infants who required mechanical ventilation at less than 28 weeks' gestation. Thirty‐seven infants received dexamethasone 0.20 mg/kg at delivery (total dose 0.2 mg/kg dexamethasone as one dose), and 33 infants received placebo consisting of an equal volume of saline. Country: USA. Participants were recruited between August 1994 and November 1995.
Lauterbach 2006 presented a single‐centre randomised trial to determine the effects of two active drugs on occurrence of BPD at 36 weeks. The two active drugs were nebulised pentoxifylline diluted in distilled water and intravenous dexamethasone. Infants weighing < 1251 grams at birth who were receiving supplemental oxygen on the fourth day after birth were eligible if they did not have a grade 3 or 4 intraventricular haemorrhage. Study authors randomly allocated a total of 150 infants to nebulised pentoxifylline every six hours for three days (n = 50), intravenous dexamethasone 0.25 mg/kg/12‐hourly for three days (total dose 1.5 mg/kg dexamethasone over three days, minimum) (n = 50), or nebulised saline placebo every six hours for three days (total dose 1.5 mg/kg dexamethasone over three days, minimum) (n = 50). Study drugs could be repeated every seven days if the infant was still ventilator‐ or oxygen‐dependent and a diagnosis of BPD had not been established. The number of repeat doses for any group.was not reported. Only data from the dexamethasone group and the control group were entered into the current meta‐analysis. Country: Poland. Participants were recruited between 1 January 2000 and 30 September 2003.
Lin 1999 was a randomised trial with a sequential design involving infants weighing 500 grams to 1999 grams. Investigators stratified infants by birth weight into three groups: 500 grams to 999 grams, 1000 grams to 1500 grams, and 1501 grams to 1999 grams. Within each group, equal numbers of dexamethasone‐treated or control cards were placed into envelopes for random selection of the first infant of each pair. The next infant of the appropriate birth weight stratum was enrolled for the match. A pharmacist opened the envelope, and investigators administered dexamethasone or saline placebo blind. Entry criteria included the presence of severe radiographic respiratory distress syndrome, the need for assisted ventilation within six hours of birth, and receipt of one dose of surfactant. Treated infants were given dexamethasone starting within 12 hours of birth at 0.25 mg/kg/dose 12‐hourly for seven days, 0.12 mg/kg/dose 12‐hourly for seven days, 0.05 mg/kg/dose 12‐hourly for seven days, and 0.02 mg/kg/dose 12‐hourly for seven days, resulting in a total of four weeks of treatment (total dose 6.16 mg/kg dexamethasone over four weeks). Results were reported for 20 treated and 20 control infants. Country: Taiwan. Supported by the National Health Research Institute and Department of Health, Taiwan.
Mukhopadhyay 1998 reported a randomised trial that included untreated controls. Study authors did not describe the method of randomisation used. Treated infants received dexamethasone 0.5 mg/kg/dose 12‐hourly for three days (total dose 3 mg/kg dexamethasone over three days), beginning within six hours of birth. Researchers included 19 infants (10 treated with dexamethasone; 9 control) at less than 34 weeks' gestation and weighing less than 2000 grams who could be provided with mechanical ventilation. These infants had severe respiratory distress syndrome but were not given surfactant. Country: India. Participants were recruited between February 1996 and July 1996.
Ng 2006 was a double‐blind RCT of a “stress dose” of hydrocortisone for treatment of refractory hypotension. Investigators randomised 48 infants of birth weight less than 1500 grams to receive hydrocortisone 1 mg/kg eight‐hourly for five days (total dose 15 mg/kg hydrocortisone over five days) (n = 24), or an equivalent volume of isotonic saline (n = 24). Country: China (Hong Kong). Participants were recruited between June 2001 and November 2004. Supported by Research Grants Council of the Hong Kong Special Administrative Region.
Peltoniemi 2005 enrolled a total of 51 infants weighing less than 1251 grams at birth or born at less than 31 weeks' gestation, who were under 36 hours old and were ventilator‐dependent. Investigators conducted this trial at three collaborating centres in Finland. They stratified infants by centre and by birth weight (501 grams to 749 grams, 750 grams to 999 grams, and 1000 grams to 1250 grams) and randomly allocated them to a 10‐day tapering course of hydrocortisone (2 mg/kg/d for two days, 1.5 mg/kg/d for two days, 0.75 mg/kg/d for six days) (total dose 11.5 mg/kg hydrocortisone over 10 days) (n = 25), or an equivalent volume of 0.9% saline placebo (n = 26). Researchers based the sample size calculation on detecting an increase in survival without BPD from 50% to 70% and required inclusion of 160 participants per study arm (alpha and beta error 0.05 and 0.20, respectively). This study was stopped early at 51 infants because four of the hydrocortisone‐treated infants had intestinal perforation and other RCTs of early hydrocortisone had reported the same complication. Children were followed up at two years and at five to seven years of age. Long‐term outcomes included in the meta‐analysis pertain to the five‐ to seven‐year follow‐up study only. Country: Finland. Participants were recruited between 12 August 2002 and 4 March 2004. Supported by grants from Foundation for Pediatric Research, The Alma and K.A. Snellman Foundation (Oulu, Finland), and the Sigrid Juselius Foundation (Finland).
Rastogi 1996 recruited 70 infants with birth weight of 700 grams to 1500 grams who had severe respiratory distress syndrome (assisted ventilation with ≥ 40% oxygen and/or 7 cmH₂O mean airway pressure and alveolar/arterial (a/A) partial pressure of oxygen (PO₂) ratio ≤ 0.24) who had been treated with surfactant before entry. Infants were less than 12 hours old, and trialists excluded them if they had major malformations, chromosome abnormalities, five‐minute Apgar scores < 3, or severe infection. The intervention group received dexamethasone intravenously every 12 hours according to the following schedule: 0.50 mg/kg/d on Days 1 to 3, 0.30 mg/kg/d on Days 4 to 6, 0.20 mg/kg/d on Days 7 to 9, and finally 0.10 mg/kg/d on Days 10 to 12 (total dose 3.3 mg/kg dexamethasone over 12 days) (n = 36). The control group received a saline placebo intravenously (n = 34). Country: USA. Participants were recruited between July 1992 and August 1993.
Romagnoli 1999 was a randomised trial that used numbered, sealed envelopes involving 25 dexamethasone‐treated infants and 25 untreated controls. Entry criteria were birth weight < 1251 grams, gestational age < 33 weeks, ventilator‐ and oxygen‐dependent at 72 hours, and high risk of BPD based on a local scoring system that predicted 90% risk. Treated infants were given dexamethasone beginning on the fourth day at a dose of 0.5 mg/kg/d for three days, 0.25 mg/kg/d for three days, and 0.125 mg/kg/d for one day (total dose 2.375 mg/kg dexamethasone over seven days). Country: Italy. Participants were recruited between November 1996 and October 1998.
Sanders 1994 enrolled 40 infants at less than 30 weeks' gestation who had respiratory distress syndrome diagnosed by clinical and radiographic signs, required mechanical ventilation at 12 to 18 hours of age, and had received at least one dose of surfactant. Exclusion criteria at entry included a strong suspicion of sepsis or pneumonia, congenital heart disease, chromosome abnormalities, and receipt of an exchange transfusion. Infants were randomised to receive dexamethasone 0.50 mg/kg at between 12 and 18 hours of age and a second dose 12 hours later (total dose 1 mg/kg dexamethasone over one day) (n = 190), or a saline placebo (n = 21). They received both treatments intravenously. Country: USA. Participants were recruited between December 1989 and January 1991. Supported by a Pulmonary Specialized Center of Research (SCOR) grant from the NIH (HL‐36543), a clinical research grant from the March of Dimes (6‐0785), and a General Clinical Research Center grant (RR00044).
Shinwell 1996 reported a multi‐centre trial that randomised 248 infants of birth weight 500 grams to 2000 grams who had clinical and radiographic evidence of respiratory distress syndrome, required mechanical ventilation with more than 40% oxygen, were less than 12 hours old, and had no contraindications to corticosteroid treatment, such as a bleeding tendency, hypertension, hyperglycaemia, or active infection. Investigators excluded infants with lethal congenital malformations. The intervention group received dexamethasone 0.25 mg/kg intravenously every 12 hours for a total of six doses (total dose 1.5 mg/kg dexamethasone over three days) (n = 132). The control group received intravenous saline (n = 116). Country: Israel. Participants were recruited between April 1993 and January 1994. Supported by CTS Industries, Israel. Surfactant TA supplied by Tokyo Tanabe, Japan.
Sinkin 2000 was a multi‐centre randomised double‐blind trial that included 384 infants at less than 30 weeks' gestation with respiratory distress syndrome. A total of 189 infants received dexamethasone 0.50 mg/kg at 12 to 18 hours of age and a second dose 12 hours later (total dose 1 mg/kg dexamethasone over one day), and 195 infants received an equal volume of saline placebo. Country: USA. Participants were recruited between March 1992 and February 1997. Supported by a Pulmonary SCOR grant from the NIH (HL‐36543), General Clinical Research Center Grant 5 MO1 RR00044, and a clinical research grant from the March of Dimes (6‐0785),
Soll 1999 described a multi‐centre randomised double‐blind controlled trial that compared dexamethasone given at 12 hours of age versus selective late dexamethasone therapy for preterm infants weighing 501 grams to 1000 grams (early dexamethasone n = 272, late selective therapy n = 270). Infants required assisted ventilation, had received surfactant therapy, were physiologically stable, had no obvious life‐threatening congenital anomaly, had blood cultures obtained, and had started antibiotic therapy. Infants were randomly assigned to early dexamethasone therapy or saline placebo. Intravenous dexamethasone was administered for 12 days according to the following schedule: 0.5 mg/kg/d for three days, 0.25 mg/kg/d for three days, 0.1 mg/kg/d for three days, and 0.05 mg/kg/d for three days (total dose 2.7 mg/kg dexamethasone over 12 days). Infants in either group could receive late postnatal corticosteroids beginning on Day 14 if they needed assisted ventilation, with supplemental oxygen greater than 30%. The trial was halted early because of concern about serious side effects in the early steroid treatment group and the unlikelihood that additional subject enrolment would yield a significant result regarding the primary outcome measure, with 542 of a planned total of 822 infants recruited. Countries: USA, Canada. Supported in part by a grant from the Children’s Miracle Network and the University of Vermont General Clinical Research Center Grant MO1 RR00109.
Stark 2001 was a randomised multi‐centre controlled trial conducted to compare a tapering course of stress‐dose corticosteroid started on the first day versus placebo. Infants with birth weight 501 grams to 1000 grams needing mechanical ventilation before 12 hours of age were eligible for the study. Infants with birth weight over 750 grams also needed to have received surfactant and required an oxygen concentration of 30% or greater. The initial dose of dexamethasone was 0.15 mg/kg/d for three days, tapered over seven days (total dose 0.89 mg/kg dexamethasone over 10 days). After enrolling 220 infants (sample size was 1200), the trial was halted because of an excess of intestinal perforations in the dexamethasone‐treated group. Researchers randomised 111 infants to receive dexamethasone and 109 to receive placebo. Country: USA. Participants were recruited between February 1998 and September 1999. Supported by cooperative agreements with the National Institute of Child Health and Human Development (U10 HD34167, U10 HD34216, U10 HD21373, U10 HD27881, U10 HD21385, U10 HD27853, U10 HD27904, U01 HD21397, U01 HD36790, U10 HD27851, U10 HD21364, U10 HD27871, and U10 HD21415) and by grants from the General Clinical Research Centers Program (M01 RR 02635, M01 RR 02172, M01 RR 00997, M01 RR 08084, M01 RR 06022, M01 RR 08084, and M01 RR 00070).
Subhedar 1997 reported a randomised trial that enrolled infants into one of four treatment groups using a factorial design. Investigators compared both inhaled nitric oxide (iNO) and early dexamethasone separately versus controls. They randomised 42 infants: 10 to receive iNO alone, 11 dexamethasone alone, 10 both treatments, and 11 neither treatment. Researchers compared 21 infants receiving dexamethasone versus 21 controls. Infants were eligible for entry into the trial at 96 hours of age if they met the following criteria: gestational age less than 32 weeks, mechanical ventilation from birth, had received surfactant therapy, and were thought to be at high risk of developing BPD based on a scoring system (Ryan 1996). Exclusion criteria were major congenital anomaly, structural cardiac defect, significant ductus shunting, culture‐positive sepsis, intraventricular haemorrhage with parenchymal involvement, pulmonary or gastrointestinal haemorrhage, disordered coagulation, and platelet count < 50,000. Infants received dexamethasone intravenously at 12‐hourly intervals for six days: 0.50 mg/kg/dose for six doses and 0.25 mg/kg/dose for a further six doses (total dose 4.5 mg/kg dexamethasone over six days). Control infants did not receive a placebo. Country: England. Participants were recruited between August 1996 and September 1997. NVS was supported by the British Heart Foundation (R.F.Martin Junior Research Fellowship). This study was also supported by an equipment grant from the North West Regional Health Authority Research and Development Executive, and by Micro Medical Ltd., which supplied some of the gas monitoring equipment.
Suske 1996 randomised 26 infants with gestational age of 24 to 34 weeks who had respiratory distress syndrome and had been treated with surfactant. Infants with known septicaemia during the first week of life, haemodynamically relevant cardiac anomalies except for patent ductus arteriosus, or malformations of the lung or central nervous system (CNS) were excluded. Randomisation was performed by drawing lots before the age of two hours. The intervention group (n = 14) received dexamethasone 0.50 mg/kg intravenously in two divided doses for five days (total dose 2.5 mg/kg dexamethasone over five days), and controls (n = 12) received no placebo. Country: Germany. Participants were recruited between March 1991 and June 1993.
Tapia 1998 was a multi‐centre double‐blind placebo‐controlled trial of 109 preterm infants with respiratory distress syndrome and birth weight between 700 grams and 1600 grams who were treated with mechanical ventilation and surfactant. Researchers randomised 55 infants to receive dexamethasone 0.50 mg/kg/d for three days, followed by 0.25 mg/kg/d for three days, followed by 0.12 mg/kg/d for three days, then 0.06 mg/kg/d for three days (total dose 2.79 mg/kg dexamethasone over 12 days). A total of 54 control infants received an equal volume of saline. Country: Chile. Participants were recruited between 1 December 1992 and 30 June 1995. Supported by The Wellcome Foundation and Laboratorios Saval.
Vento 2004 enrolled 20 neonates with birth weight less than 1251 grams and gestation less than 33 weeks who were oxygen‐ and ventilator‐dependent on the fourth day of life and randomised them to receive dexamethasone 0.50 mg/kg/d for three days, 0.25 mg/kg/d for three days, and 0.125 mg/kg/d for one day (total dose 2.375 mg/kg dexamethasone over seven days) (n = 10), or no corticosteroid treatment (n = 10). Country: Italy. Participants were recruited between August 1998 and July 2000.
Wang 1996 reported a randomised trial of a 21‐day course of dexamethasone or saline placebo given in a double‐blind fashion. Study authors did not state the method of randomisation used. Entry criteria were birth weight 1000 grams to 1999 grams, appropriate for gestational age, clinical and radiological severe respiratory distress syndrome, mechanical ventilation, and age less than 12 hours. Surfactant was not given, as it was not commercially available in Taiwan at the time of the study. Treated infants were given dexamethasone 0.25 mg/kg/dose 12‐hourly for seven days, 0.125 mg/kg/dose 12‐hourly for seven days, and 0.05 mg/kg/dose 12‐hourly for seven days (total dose 5.95 mg/kg dexamethasone over 21 days). The first dose of dexamethasone was given during the first 12 hours of life. Participants included 34 infants in the dexamethasone group and 29 in the placebo control group. Country: Taiwan. Participants were recruited between October 1992 and September 1993. Supported in part by grants NSC 80‐0412‐B006‐27 and NSC 80‐0412‐B006‐47 from National Science Councils, and by grant DOH 82‐HR‐C17 from the National Institute of Health Research, Department of Health, Taiwan, Republic of China.
Watterberg 1999 described a randomised double‐masked placebo‐controlled pilot study conducted to compare early treatment with low‐dose hydrocortisone (1.0 mg/kg/d for nine days, then 0.5 mg/kg/d for three days) (total dose 10.5 mg/kg hydrocortisone over 12 days), begun before 48 hours of age, versus placebo. Researchers enrolled at two centres 40 infants weighing between 500 grams and 999 grams who were mechanically ventilated: 20 hydrocortisone‐treated infants and 20 placebo controls. Country: USA. Participants were recruited between June 1996 and May 1998. Supported by Grant MCJ‐420633 from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services.
Watterberg 2004 was a multi‐centre masked randomised trial of hydrocortisone to prevent early adrenal insufficiency. Investigators randomised 360 infants with birth weight of 500 grams to 999 grams who were mechanically ventilated to receive hydrocortisone 1 mg/kg/d for 12 days, then 0.5 mg/kg/d for three days (total dose 13.5 mg/kg hydrocortisone over 15 days) (n = 180), or saline placebo (n = 180). They enrolled infants at between 12 and 48 hours of life. The trial was stopped because of an increase in spontaneous gastrointestinal perforation in the hydrocortisone group. Country: USA. Participants were recruited between 1 November 2001 and 30 April 2003. Supported by National Institute of Child Health and Human Development grant R01‐HD38540, grant MO1 RROOO54 from the General Clinical Research Centers Programs at the University of New Mexico, Tufts‐New England Medical Center grant 5MO1 RROO997, and University of Colorado grant MO1‐RROOO69.
Yeh 1990 enrolled 57 infants whose birth weight was < 2000 grams and who had severe respiratory distress syndrome diagnosed on the basis of a chest radiograph and the need for mechanical ventilation within four hours after birth. Absence of infection was required for inclusion. Infants were randomly assigned to receive dexamethasone 0.50 mg/kg/dose 12‐hourly from Days 1 to 3, then 0.25 mg/kg/dose 12‐hourly from Days 4 to 6, then 0.12 mg/kg/dose 12‐hourly from Days 7 to 9, and finally 0.05 mg/kg/dose 12‐hourly from Days 10 to 12 (total dose 5.52 mg/kg dexamethasone over 12 days) (n = 28). Researchers administered all doses intravenously and gave a saline solution to infants in the placebo group (n = 29). Country: USA. Participants were recruited between June and November 1988. Supported in part (grant No. 052) by Washington Square Health Foundation, Inc., Chicago, Illinois.
Yeh 1997 reported a multi‐centre randomised double‐blind clinical trial of 262 preterm infants (< 2000 grams) who had respiratory distress syndrome and required mechanical ventilation from shortly after birth. The treated group received dexamethasone 0.25 mg/kg/dose 12‐hourly intravenously from Day 1 to Day 7; 0.12 mg/kg/dose 12‐hourly intravenously from Day 8 to Day 14; 0.05 mg/kg/dose 12‐hourly intravenously from Day 15 to Day 21; and 0.02 mg/kg/dose 12‐hourly intravenously from Day 22 to Day 28 (total dose 6.16 mg/kg dexamethasone over 28 days) (n = 132). Control infants received a saline placebo (n = 130). Country: Taiwan. Participants were recruited between October 1992 and April 1995. Supported by grants DOH 82‐HR‐C17, DOH 83‐HR‐217, and DOH 84‐HR‐217 from the National Health Research Institute and Department of Health, Taiwan, Republic of China.
Excluded studies
We excluded 30 studies. See Characteristics of excluded studies.
We excluded studies for a variety of reasons. In one study, the primary outcome was the need for an epinephrine infusion 12 hours after treatment (Gaissmaier 1999). Study authors reported no long‐term outcomes. Two studies were not RCTs; one Tsukahara 1999 comprised 26 study infants and 12 historical controls; Smolkin 2014 comprised 35 infants treated with betamethasone with no controls. Two studies were RCTS of hydrocortisone to treat low blood pressure. In one such study (Bouchier 1997), hydrocortisone (n = 21) was compared with dopamine (n = 19) in very low birth weight infants. Although this was an RCT that did report some in‐hospital outcomes relevant to the current review, there was no comparison of hydrocortisone with either placebo or nothing. In the other such study (Salas 2014), hydrocortisone was compared with placebo but no important outcomes relevant to the current review were reported. Investigators in one trial randomised 120 very low birth weight infants to both hydrocortisone and caffeine as active treatments, compared with treatment described in “standard guidelines”, which presumably meant no hydrocortisone or caffeine (Dobryansky 2012). Major outcomes reported were BPD and BPD combined with mortality. As caffeine alone reduces BPD (Schmidt 2006), the independent effect of hydrocortisone cannot be determined. Although researchers in another trial randomly allocated 29 very low birth weight infants to dexamethasone or placebo before six hours of age, they reported none of the outcomes that are applicable to this review (Yaseen 1999). Outcomes reported comprised only changes in mean values over the first five days for oxygenation, blood pressure, and serum creatinine, urea, and glucose ‐ not rates of BPD, hypertension, or hypoglycaemia, for example. Gross 2005 was reporting outcomes at 15 years of age for survivors from an RCT that is included in the “Late” review under a different name (Cummings 1989); relevant outcomes at 15 years are included in the “Late” review.
We excluded 23 studies in which treatment was started after the first week of life that are included in the review titled “Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants” (Ariagno 1987; Avery 1985; Brozanski 1995; CDTG 1991; Cummings 1989; Doyle 2006; Durand 1995; Harkavy 1989; Kari 1993; Kazzi 1990; Kothadia 1999; Kovacs 1998; Noble‐Jamieson 1989; Ohlsson 1992; Onland 2019: Papile 1998; Parikh 2013; Romagnoli 1997; Scott 1997; Vento 2004; Vincer 1998; Walther 2003; Yates 2019). One of the studies listed as excluded had data for two separate cohorts of infants ‐ the first cohort included those 10 days of age when randomised (it is these data that are excluded from this “Early” review), whereas the second cohort included those four days of age when randomised (hence they are included in this “Early” review) (Vento 2004).
We found no studies that are currently awaiting further assessment.
Risk of bias in included studies
The overall risk of bias was low (Figure 2; Figure 3). All studies were RCTs, although the method of random allocation is not always clear. Allocation concealment applied to most studies. Blinding of investigators and others was achieved most often through the use of placebo, usually saline solution. Follow‐up reporting for short‐term outcomes most often was complete but was more variable for long‐term outcomes beyond discharge and later into childhood. Most studies reported primary outcomes as specified in their methods.

Risk of bias table.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Anttila 2005 carried out randomisation in the pharmacy of the coordinating centre using coded vials, with blinding of study investigators. Open‐label dexamethasone was allowed when deemed necessary by the attending neonatologist, but its use was discouraged. Trialists performed intention‐to‐treat analysis and reported no follow‐up component.
Baden 1972 performed randomisation by using vials and a table of random numbers. Clinical personnel were not aware of the content of any vial. Study authors reported outcomes for all enrolled infants. Follow‐up consisted of the following: one paediatrician and one psychologist saw survivors at 12 months of age, corrected for prematurity. A neurologist saw all children with abnormal neurological signs. Observers were blinded to treatment group allocation. The follow‐up rate of survivors was 93% (25/27). Study authors did not specify criteria for the diagnosis of cerebral palsy, nor did they provide specific criteria for blindness or deafness (children were tested by free‐field pure‐tone audiometry). Psychological assessment consisted of the Griffiths Scales. Study authors did not report major neurosensory disability (Fitzhardinge 1974).
Batton 2012 did not state the method of randomisation used. Trialists administered an identical placebo and reported no follow‐up component.
Baud 2016 generated the randomisation sequence electronically using nQuery. After enrolment, researchers assigned treatment through a secure study website after verifying eligibility and consent status. They electronically randomised all infants before they reached 24 completed hours after birth and reported short‐term outcomes for all but two participants who were randomised. They followed up on 93% of survivors at 22 months' corrected age, although only 75% were given the full neurodevelopmental assessment battery. Investigators maintained double‐blinding through all aspects of the study.
Biswas 2003 conducted randomisation as performed by the Perinatal Trials Unit in Oxford, with stratification for centre and gender, and the study pharmacist held the code. Controls received an equal infusion rate of 5% dextrose. One pharmacy made the syringes and transported them to individual study centres. Short‐term outcomes were reported for all enrolled infants. Study authors reported no follow‐up component.
Bonsante 2007 conducted centralised randomisation using a computer‐generated random number sequence. Researchers stratified infants into six risk groups to ensure a homogeneous number of infants with regard to birth weight, gestation, and antenatal corticosteroid administration. They prepared drugs each day in the pharmacy, and the care team, parents, and personnel collecting data had no knowledge of the random assignment at any time. Study authors reported results of follow‐up at two years of age (follow‐up component) in conjunction with data from another study but did not describe clinical criteria for various outcomes (Peltoniemi 2009). Study authors reported follow‐up data for 92% (33/36) of survivors up to hospital discharge.
Efird 2005 performed randomisation by opening sequentially numbered, opaque envelopes containing pre‐assigned treatment designations. Investigators randomised infants of multiple gestations as separate participants and blinded clinicians to treatment identity. If hypotension persisted, the randomisation assignment could be unblinded and hydrocortisone administered if the infant had been assigned to the placebo group. Study authors reported no follow‐up component.
Garland 1999 randomised infants at each centre within each of four strata on the basis of birth weight (≤ 1000 grams, > 1000 grams) and a/A ratio before surfactant (≤ 0.15, > 0.15). Study pharmacists at each centre maintained randomisation codes. Investigators, caregivers, and parents were blinded to treatment allocation. The first interim analysis (n = 75) showed increased risk of gastrointestinal perforation in the dexamethasone group. After adjustment for severity of illness, the difference was not sufficiently statistically significant to stop enrolment. However, to ensure participant safety, the Data Monitoring Committee recommended that the dexamethasone dose should be reduced. Investigators changed the dosing schedule to four doses of 0.25 mg/kg/dose every 12 hours, begun at 24 to 48 hours, followed by doses of 0.125 mg/kg and 0.05 mg/kg at the next two 12‐hour periods, respectively. After the first interim analysis, all enrolled infants received ranitidine therapy during the first three days of the study. It appears that study authors reported outcome measures for all 241 infants enrolled in the study and included no follow‐up component.
Halac 1990 used a table of random numbers for randomisation, along with placebo blinding. Study authors stated that they had excluded from the study deaths before 10 days of age; they reported a total of five early deaths from sepsis, but it was not clear how often this occurred in each group. Apart from these infants, investigators provided outcome data for all remaining enrolled infants. They reported limited follow‐up to six months of age but provided no follow‐up results (apart from a statement that "growth and development were not hampered in any of these patients").
Hochwald 2014 did not state methods used for random sequence generation, allocation concealment, blinding of personnel and families, and blinding of outcomes, apart from use of a placebo, and reported no follow‐up component.
Kopelman 1999 performed randomisation in the pharmacy after stratifying infants for treatment with antenatal corticosteroids. The blinded clinical team provided care. Study authors provided outcome data for all enrolled infants and reported no follow‐up component.
Lauterbach 2006 used a computer‐generated random number table for randomisation. Investigators allocated infants to groups by opening numbered containers on the fourth day of life. They provided no placebo for the dexamethasone arm and hence reported no blinding of dexamethasone treatment. Study authors reported no follow‐up component.
Lin 1999 performed randomisation by opening sealed envelopes in the pharmacy. This study used a sequential analysis design and paired 12 infants successfully. Study authors reported outcome measures for all 40 enrolled infants, including those who remained unpaired. They described no follow‐up component.
Mukhopadhyay 1998 did not state the method of randomisation used. Investigators were able to provide ventilation for only 28 of 43 eligible infants and subsequently excluded eight infants owing to non‐availability of blood gases due to a technical fault, and excluded one baby because of congenital heart block. This left 19 infants included in the study: 10 received intravenous dexamethasone, and nine received no drug treatment. Study authors did not mention placebo. They reported outcome measures for these 19 infants and described no follow‐up component.
Ng 2006 performed randomisation by using computer‐generated random numbers and by opening sequentially numbered, sealed, opaque envelopes in the pharmacy. They assigned infants in blocks of six, and once an envelope was opened, an infant would be irrevocably entered into the trial. To ensure effective blinding of medications, both types of trial drug were colourless and odourless and were filled to the same volume before they were sent to the ward. Study authors reported no follow‐up component.
In the Peltoniemi 2005 study, non‐clinical staff achieved randomisation centrally, independent of the chief investigators, using random variation in block sizes of two to eight, separately for each centre. Study authors did not specify the method used for randomisation. Researchers had syringes prepared and labelled identically in the pharmacy department of the centre, thereby concealing allocation from study site investigators and caregivers of the infant. Open‐label corticosteroids were discouraged after randomisation but were not prohibited; some infants may have received both a second course of their initially allocated study drug and open‐label corticosteroids. No one apart from the pharmacist at study sites had access to the treatment codes. Study authors reported short‐term outcomes for all enrolled infants. Follow‐up consisted of the following: investigators assessed surviving children at 24 months of age, corrected for prematurity, and at five to seven years of age, when it was not stated that age was corrected for prematurity. Paediatricians, paediatric neurologists, speech therapists, and psychologists at individual study sites were blinded to treatment group allocation. At two years, children were considered to have a major neurosensory impairment if they had cerebral palsy, blindness (inability to see any objects, with the exception of light), deafness (failure to pass an evoked otoacoustic emission test during the neonatal period and no response in brainstem auditory evoked potentials), or developmental delay (defined as a Mental Developmental Index (MDI) on the Bayley Scales of Infant Development < 70 (< ‐2 standard deviations (SDs)) or a developmental quotient < 70 on the Griffiths Cognitive Scales). Researchers assessed cognitive development of children at five to seven years of age by using the Wechsler Presechool and Primary Scale of Intelligence ‐ Revised (WPPSI‐R). They diagnosed minor neurological dysfunction on the basis of the number of dysfunctional domains. Speech assessment included the Reynell Developmental Language Scale III (RDLS III). Study authors did not provide the criteria for blindness or deafness and reported the follow‐up rate of survivors at two years (98%; 45/46) and at five to seven years of age (80%; 37/46) (Peltoniemi 2009; Peltoniemi 2016).
Rastogi 1996 performed randomisation in the pharmacy, using a random number list after stratifying infants for birth weight into three groups: 700 grams to 999 grams, 1000 grams to 1249 grams, and 1250 grams to 1500 grams. The clinical team and other study personnel were blinded to assignments until the study was completed, and they recorded all outcome variables for all infants. Study authors reported no follow‐up component.
Romagnoli 1999 achieved randomisation through random number allocation by opening numbered, sealed envelopes. Trialists excluded infants with prenatal infections, congenital malformations, and evidence of sepsis at randomisation. They did not mention placebo and reported outcome measures for all 50 enrolled infants. Follow‐up consisted of the following: one paediatrician and one neurologist saw survivors at 34 to 42 months of age, corrected for prematurity, and observers were blinded to treatment group allocation. The follow‐up rate of survivors was 100% (45/45). The neurologist diagnosed cerebral palsy, but study authors did not specify the criteria used for this, nor for the diagnosis of blindness or deafness. Psychological assessment included the Stanford‐Binet 3rd Revision, and intellectual impairment comprised an intelligence quotient (IQ) < 70. Major neurosensory impairment consisted of either blindness or deafness (Romagnoli 2002).
Sanders 1994 randomised participants in the pharmacy after opening sealed envelopes. Dexamethasone or placebo was dispensed via labelled syringes. Clinical personnel were not aware of assignment of the intervention. Study authors reported outcomes for all 40 enrolled infants. Follow‐up consisted of the following: a paediatrician, a neurologist, and a psychologist saw survivors at mean ages of 64 (SD 8) months (dexamethasone) and 61 (SD 4) months (controls), not corrected for prematurity, with observers blinded to treatment group allocation. Researchers sought additional data from parents and teachers. The follow‐up rate of survivors was 100% (31/31). The criterion for the diagnosis of cerebral palsy was a fixed motor deficit diagnosed by the neurologist. Blindness comprised visual acuity < 6/60 in the better eye, and study authors defined deafness as the need for a hearing aid. Psychological assessment was based on the Wechsler Scales (Wechsler Intelligence Scale for Children (WISC) and WPPSI‐R) ‐ intellectual impairment comprised a full‐scale IQ < 70. Study authors did not specify major neurosensory disability and planned further follow‐up at 15 years of age (Sinkin 2002 (personal communication follow‐up to Sanders 1994)).
Shinwell 1996 supplied each participating unit with numbered sets of syringes containing dexamethasone or physiological saline. Syringes containing dexamethasone were not distinguishable from those containing saline. Syringe sets were numbered according to a random number list and stratified randomisation by centre and by two birth weight groups: 500 grams to 1000 grams, and 1001 grams to 2000 grams. No investigators knew the drug assignment until after the three‐month observation period of the last enrolled infant. Study authors reported outcomes for 248 of 255 enrolled infants. The seven infants subsequently excluded from analysis included three with major congenital abnormalities (two with myotonic dystrophy and one with cyanotic congenital heart disease), three with errors in drug administration, and one randomised after the age of 12 hours. Follow‐up consisted of the following: survivors were seen at a mean age of 53 (SD 18; range 24 to 71) months, presumably not corrected for prematurity. Multiple paediatricians saw these children at multiple follow‐up clinics, with observers blinded to treatment group allocation. The follow‐up rate of survivors was 83% (159/190). Trialists did not specify criteria for the diagnosis of cerebral palsy, but neurologists made the diagnosis in all cases. Study authors did not specify criteria for blindness but defined deafness as the need for hearing aids. Study personnel performed no formal psychological assessments, and multiple assessors assigned the judgement of developmental delay. Major neurosensory disability comprised any of non‐ambulant cerebral palsy, global retardation (not specified), blindness, or deafness. Researchers planned further follow‐up at school age (Shinwell 2002).
Sinkin 2000 performed randomisation with stratification by centre, using a set of sealed envelopes in the pharmacy. It appears that study authors provided outcome data for all enrolled infants. Follow‐up consisted of the following (Sinkin 2002 (personal communication follow‐up to Sinkin 2000)): researchers obtained data from one of the four original centres in the study, from follow‐up clinic appointments, and from questionnaires completed by parents and paediatricians. A paediatrician, a neurologist, and a psychologist saw survivors at approximately 12 months of age, corrected for prematurity, with observers blinded to treatment group allocation. The follow‐up rate of survivors was 13% (41/311) at 36 weeks' postmenstrual age overall but was confined to one of four individual study centres, within which the follow‐up rate was 100% (41/41). The criterion for the diagnosis of cerebral palsy was a fixed motor deficit diagnosed by the neurologist. Blindness comprised visual acuity < 6/60 in the better eye, and study authors defined deafness as the need for a hearing aid. Psychological assessment included the Bayley Scales of Infant Development. Investigators did not specify major neurosensory disability.
Soll 1999 performed randomisation in hospital pharmacies after opening opaque, sealed envelopes supplied by the Vermont Oxford Neonatal Network. The study was stopped before sample size goals were met owing to concern regarding adverse effects in the early corticosteroid therapy group. It appears that outcome measures were reported for most of the 542 enrolled infants. Study authors reported no follow‐up component.
Stark 2001 performed random allocation in hospital pharmacies using a random number scheme. This study used a factorial design, so that infants were also randomised to routine ventilator management or to a strategy of minimal ventilator support aimed at reducing mechanical lung injury. After 220 infants were enrolled from a sample estimated to include 1200, the trial was halted. It appears that study authors have reported outcome measures for all 220 participants enrolled in the trial. Follow‐up consisted of the following: trained developmental observers blinded to treatment group allocation saw survivors at 18 to 22 months of age, corrected for prematurity. The follow‐up rate of survivors was 88% (144/164). Criteria for the diagnosis of cerebral palsy included non‐progressive abnormalities of tone in at least one limb and abnormal control of movement and posture. Study authors defined blindness as no useful vision in either eye, and deafness as disability with bilateral hearing amplification. Psychological assessment included the MDI and the Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant Development‐II (Bayley 1993). Major neurosensory disability comprised any of moderate or severe cerebral palsy (sitting independently with support or worse), blindness, deafness, or an MDI or PDI < ‐2 SD (Stark 2014).
Subhedar 1997 performed block randomisation by using computer‐generated random numbers and sealed envelopes. Researchers used no placebo and provided no evidence of blinding of clinicians. Study authors reported outcome measures for all enrolled infants. Follow‐up consisted of the following (Subhedar 2002 (personal communication follow‐up to Subhedar 1997)): one developmental paediatrician who was blinded to treatment group allocation saw survivors at 30 months of age, corrected for prematurity. The follow‐up rate of survivors was 95% (21/22). Study authors specified criteria for the diagnosis of cerebral palsy but not for deafness; an ophthalmologist diagnosed blindness. Psychological assessment included the MDI and the PDI of the Bayley Scales of Infant Development. Major neurosensory disability comprised any of cerebral palsy, MDI or PDI < 71, blindness, or deafness.
Suske 1996 performed randomisation by drawing lots; lot numbers corresponded to numbers on non‐transparent envelopes. A neutral, uninvolved person drew random lots and envelopes. This was considered a pilot trial conducted before a multi‐centre study was begun, and researchers planned that the trial would be stopped if they found a statistically significant difference between groups. A total of 41 infants met the inclusion criteria. Owing to lack of co‐operation and co‐ordination at the beginning of the study, investigators did not randomise nine infants. They excluded four infants after randomisation because they showed definitive signs of septicaemia. Study authors reported results for 26 of the 28 remaining infants and described no follow‐up component.
Tapia 1998 achieved random assignment at each centre using ampoules of dexamethasone and saline prepared in the hospital pharmacy at one of the centres. Researchers reported outcomes for 109 of 113 enrolled infants. They excluded two infants from the dexamethasone group ‐ one because of congenital cystic adenomatoid malformation, and the other because of early sepsis. Investigators also excluded two patients from the placebo group ‐ one because of early sepsis, and the other because of transfer to another hospital at two weeks of age. Study authors did not provide further data on outcomes and reported no follow‐up component.
Vento 2004 did not state the method of randomisation used. Whether clinicians caring for infants were blinded to treatment allocation remains unclear. Control infants did not receive a placebo, and study authors reported no follow‐up component.
Wang 1996 reported that random allocation was double‐blind but did not describe the exact method used. Study authors reported outcome measures for all 63 infants enrolled in the study and reported no follow‐up component.
Watterberg 1999 randomised Infants at each centre by using a constant block design with four participants per block to minimise imbalance over time. Investigators used separate randomisation tables for infants exposed to antenatal corticosteroids. Hospital pharmacies prepared hydrocortisone doses and the placebo of normal saline. Study authors reported outcome measures for all of the 40 infants enrolled in the trial. Follow‐up consisted of the following (Watterberg 2002 (personal communication follow‐up to Watterberg 1999)): a neonatologist and a physiotherapist saw survivors at a regular follow‐up clinic for one of the two study sites at a mean age of 11 (SD 2) months, corrected for prematurity, with observers blinded to treatment group allocation. The follow‐up rate of survivors was 53% (18/34) for the study overall, but 86% (18/21) for the study centre with follow‐up data. Researchers specified criteria for the diagnosis of cerebral palsy, which comprised abnormal tone and movement. An ophthalmologist diagnosed blindness, and investigators screened participants for deafness in early infancy and at follow‐up. They performed no formal psychological testing and did not define major neurosensory disability.
Watterberg 2004 performed randomisation centrally, stratifying infants for birth weight (500 grams to 749 grams, and 750 grams to 999 grams) and by centre, using permuted block sizes of six within each stratum. Only pharmacists at individual sites who prepared the drug were aware of group assignment. All other personnel were masked. Twins were randomised together to the same study arm. Researchers reported mortality for all enrolled infants but described other short‐term outcomes for all but three infants who were withdrawn from the study. Follow‐up consisted of the following: assessors at individual study sites who were blinded to treatment group allocation assessed surviving children at 18 to 22 months of age, corrected for prematurity. They considered children to have a neurodevelopmental (neurosensory) impairment if they had cerebral palsy (criteria included abnormalities of tone, movement, and posture), functional blindness (inability to complete the Bayley Scales of Infant Development ‐ Second Edition (BSID‐II) because of visual impairment), functional deafness (inability to complete BSID‐II because of hearing impairment), developmental delay (defined as MDI on the BSID‐II < 70 (< ‐2 SD)), or motor delay (defined as a PDI on the BSID‐II < 70 (< ‐2 SD)) (Bayley 1993). The follow‐up rate of survivors at 18 to 22 months was 86% (252/294), or 87% (252/291) when three children whose families had withdrawn consent were excluded (Watterberg 2007).
Yeh 1990 performed randomisation in the pharmacy using balanced blocks of 10. Personnel working in the pharmacy labelled vials, and clinical staff were unaware of assignment. Trialists included 60 infants in the study and subsequently withdrew three: one because of death from Haemophilus influenzae septicaemia six hours after enrolment, and two because of an error in measurement of birth weight (581 grams and 2200 grams). Study authors did not report outcomes for these three infants and described no follow‐up component.
Yeh 1997 completed randomisation in the central pharmacy using an assignment list. Investigators calculated sample size on the basis of an expected 50% reduction in the incidence of BPD with early dexamethasone, allowing a 5% chance of a type I error, and a 10% chance of a type II error. Study authors reported short‐term outcome data for all 262 enrolled infants and described the study as double‐blind. Follow‐up consisted of the following: in 1998, researchers reported that one neurologist and one psychologist saw survivors at a mean age of 25 months, corrected for prematurity, with observers blinded to treatment group allocation (Yeh 1998). The follow‐up rate of survivors was 81% (133/164). Study authors did not specify criteria for the diagnosis of cerebral palsy, blindness, or deafness. Psychological assessment included the MDI and the PDI of the Bayley Scales of Infant Development. Major neurosensory disability comprised severe motor dysfunction (child non‐ambulant) or MDI or PDI < ‐2 SD. In 2004, investigators in the Yeh trial reported that trial personnel re‐assessed survivors at seven to nine years of age (Yeh 2004). The follow‐up rate of survivors was 92% (146/159). Assessors were blind to treatment allocation. A paediatric neurologist evaluated children for cerebral palsy, assessing motor skills using the Movement ABC, and IQ using the WISC‐III. Trial personnel formally evaluated vision and hearing. Major neurological disability comprised any of cerebral palsy, vision worse than 20/60, deafness requiring hearing aids, or an IQ < 5th centile. Whether age was corrected for prematurity remained unclear. We used data for cerebral palsy at eight years in the meta‐analysis, as the diagnosis of cerebral palsy is more certain at eight years than at two years of age, and because the follow‐up rate was higher when participants were eight years of age. Trialists measured blood pressure, height, weight, and head circumference at eight years of age but did not report these as standardised scores (SD or Z‐scores), to enable pooling of data for meta‐analysis.
Allocation
We found little evidence of allocation bias overall; most studies had no evidence of allocation bias, and in a small minority the risk was unclear.
Blinding
We found little evidence of blinding bias overall; most studies had no evidence of blinding bias, but small minorities had unclear or high risk of blinding bias.
Incomplete outcome data
We found little evidence of attrition bias overall; most studies had no evidence of attrition bias, and a small minority had unclear risk.
Selective reporting
Just over one‐half of studies had no evidence of selective reporting bias; the remainder had unclear risk of selective reporting bias.
Other potential sources of bias
A majority of studies used a valid method of random sequence generation, but in approximately 40% of studies, the methods used for randomisation were unclear.
Effects of interventions
Results of meta‐analysis
Meta‐analysis of these 32 studies of early postnatal corticosteroid treatment yielded the following results.
Mortality
No evidence suggests that early postnatal corticosteroid treatment reduced mortality at 28 days of life (typical risk ratio (RR) 1.01, 95% confidence interval (CI) 0.87 to 1.18; typical risk difference (RD) 0.00, 95% CI ‐0.03 to 0.03; 20 studies, 2933 infants; Analysis 1.1), at 36 weeks' postmenstrual age (typical RR 1.01, 95% CI 0.90 to 1.13; typical RD 0.00, 95% CI ‐0.02 to 0.03; 27 studies, 4176 infants; Analysis 1.2), before discharge (typical RR 0.96, 95% CI 0.85 to 1.07; typical RD ‐0.01, 95% CI ‐0.03, 0.01; 29 studies, 4164 infants; Analysis 1.3), or at the latest age possible to determine the outcome (typical RR 0.95, 95% CI 0.85 to 1.06; typical RD ‐0.01, 95% CI ‐0.04 to 0.01; 31 studies, 4373 infants; Analysis 1.4). We found little evidence of publication bias for mortality at the latest age overall (Egger test, P = 0.20), or for studies examining treatment with dexamethasone (Egger test, P = 0.37) or hydrocortisone (Egger test, P = 0.40) separately (Figure 4).

Funnel plot of comparison: 1 Mortality, outcome: 1.4 Mortality at latest reported age.
Bronchopulmonary dysplasia
Early systemic corticosteroids reduced the incidence of BPD, defined as needing oxygen supplementation at 28 days of life (typical RR 0.86, 95% CI 0.80 to 0.93; typical RD ‐0.07, 95% CI ‐0.11 to ‐0.04; 15 studies, 2580 infants; Analysis 2.1), and at 36 weeks' postmenstrual age (typical RR 0.80, 95% CI 0.73 to 0.88; typical RD ‐0.06, 95% CI ‐0.09 to ‐0.03; 26 studies, 4167 infants; Analysis 2.2). We found some evidence of publication bias for BPD at 36 weeks' postmenstrual age overall (Egger test, P = 0.046) but little evidence of publication bias in either subgroup (Egger test: dexamethasone, P = 0.10; hydrocortisone, P = 0.47; Figure 5). There was a reduction in BPD at 36 weeks' postmenstrual age among survivors (typical RR 0.79, 95% CI 0.72 to 0.87; typical RD ‐0.08, 95% CI ‐0.11 to ‐0.05; 24 studies, 3093 infants; Analysis 2.3). Early systemic corticosteroids reduced the need for later corticosteroid treatment overall (typical RR 0.79, 95% CI 0.73 to 0.86; typical RD ‐0.10, 95% CI ‐0.13 to ‐0.07; 15 studies, 3004 infants; Analysis 2.4), and among survivors (typical RR 0.77, 95% CI 0.67 to 0.89; typical RD ‐0.11, 95% CI ‐0.17 to ‐0.05; 7 studies, 895 infants; Analysis 2.5). Results of analysis show no significant reduction in the proportion of survivors discharged home on oxygen, although fewer studies were able to determine this outcome (typical RR 0.86, 95% CI 0.70 to 1.07; 9 studies, 1442 infants; Analysis 2.6).

Funnel plot of comparison: 2 Bronchopulmonary dysplasia (BPD), outcome: 2.2 BPD (36 weeks' postmenstrual age).
Mortality or bronchopulmonary dysplasia
Early systemic corticosteroids reduced the incidence of mortality or BPD, defined as needing oxygen supplementation at 28 days of life (typical RR 0.92, 95% CI 0.87 to 0.96; typical RD ‐0.06, 95% CI ‐0.09 to ‐0.03; 14 studies, 2471 infants; Analysis 3.1), or at 36 weeks' postmenstrual age (typical RR 0.89, 95% CI 0.83 to 0.94; typical RD ‐0.06, 95% CI ‐0.09 to ‐0.03; 26 studies, 4167 infants; Analysis 3.2). We found little evidence of publication bias for mortality or BPD at 36 weeks overall (Egger test, P = 0.11), or for studies involving either dexamethasone (Egger test, P = 0.15) or hydrocortisone (Egger test, P = 0.41; Figure 6).

Funnel plot of comparison: 3 Death or bronchopulmonary dysplasia (BPD), outcome: 3.2 Death or BPD at 36 weeks' postmenstrual age.
Failure to extubate
Early systemic corticosteroids reduced rates of failure to extubate at three days (typical RR 0.85, 95% CI 0.75 to 0.95; typical RD ‐0.09, 95% CI ‐0.16 to ‐0.03; 4 studies, 887 infants; Analysis 4.1), seven days (typical RR 0.76, 95% CI 0.68 to 0.85; typical RD ‐0.12, 95% CI ‐ 0.17 to ‐0.07; 8 studies, 1448 infants; Analysis 4.2), 14 days (typical RR 0.77, 95% CI 0.62 to 0.97; typical RD ‐0.10, 95% CI ‐0.19 to ‐0.02; 4 studies, 443 infants; Analysis 4.3), and 28 days of life (typical RR 0.84, 95% CI 0.72 to 0.98; typical RD ‐0.07, 95% CI ‐0.13 to ‐0.01; 7 studies, 902 infants; Analysis 4.4).
Complications during primary hospitalisation
Metabolic complications
Early systemic corticosteroids increased risks of hyperglycaemia (typical RR 1.26, 95% CI 1.15 to 1.37; typical RD 0.09, 95% CI 0.05 to 0.12; 14 studies, 2688 infants; Analysis 5.2) and hypertension (typical RR 1.85, 95% CI 1.54 to 2.22; typical RD 0.10, 95% CI 0.07 to 0.13; 11 studies, 1993 infants; Analysis 5.3).
Gastrointestinal complications
Early systemic corticosteroids increased risks of gastrointestinal bleeding (typical RR 1.86, 95% CI 1.35 to 2.55; typical RD 0.05, 95% CI 0.03 to 0.08; 12 studies, 1816 infants; Analysis 5.14) and gastrointestinal perforation (typical RR 1.84, 95% CI 1.36 to 2.49; typical RD 0.03, 95% CI 0.02 to 0.05; 16 studies, 3040 infants; Analysis 5.15), but we found no evidence of an effect on the incidence of necrotising enterocolitis (typical RR 0.90, 95% CI 0.74 to 1.11; 25 studies, 4050 infants; Analysis 5.13). We found little evidence of publication bias on a funnel plot for the outcome of gastrointestinal perforation (Figure 7).

Funnel plot of comparison: 5 Complications during primary hospitalisation, outcome: 5.15 Gastrointestinal perforation.
Other effects
Early systemic corticosteroids increased risks of hypertrophic cardiomyopathy (RR 4.33, 95% CI 1.40 to 13.4; RD 0.40, 95% CI 0.17 to 0.63; 1 study, 50 infants; Analysis 5.4) and growth failure (RR 6.67, 95% CI 2.27 to 19.6; RD 0.68, 95% CI 0.48 to 0.88; 1 study, 50 infants; Analysis 5.5) in the only study in which these were reported. Early systemic corticosteroids reduced the risk of patent ductus arteriosus (typical RR 0.78, 95% CI 0.72 to 0.85; typical RD ‐0.09, 95% CI ‐0.12 to ‐0.06; 24 studies, 4013 infants; Analysis 5.7). Results show no significant effects on infection (typical RR 1.05, 95% CI 0.96 to 1.15; 25 studies, 4101 infants; Analysis 5.1), pulmonary air leaks (typical RR 0.90, 95% CI 0.73 to 1.11; 17 studies, 3276 infants; Analysis 5.6), severe intraventricular haemorrhage (typical RR 0.97, 95% CI 0.84 to 1.12; 26 studies, 4103 infants; Analysis 5.8), periventricular leukomalacia (typical RR 1.12, 95% CI 0.83 to 1.53; 15 studies, 2807 infants; Analysis 5.10), or pulmonary haemorrhage (typical RR 1.16, 95% CI 0.87 to 1.54; 10 studies, 1820 infants; Analysis 5.16). Early systemic corticosteroids reduced any retinopathy of prematurity (typical RR 0.88, 95% CI 0.80 to 0.97; 9 studies, 1345 infants; Analysis 5.17) and both severe retinopathy of prematurity (typical RR 0.81, 95% CI 0.67 to 0.99; RD ‐0.03, 95% CI ‐0.05 to ‐0.00; 14 studies, 2577 infants; Analysis 5.18) and severe retinopathy of prematurity among survivors (typical RR 0.77, 95% CI 0.64 to 0.94; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; 12 studies, 1575 infants; Analysis 5.19).
Follow‐up data
Follow‐up studies are few compared with the total number of studies: of 32 studies, 13 provided some follow‐up data.
Developmental delay
No evidence suggests that corticosteroids increased developmental delay in three studies that assessed development on the Bayley Scales of Infant Development and defined developmental delay as either MDI or PDI more than two SD below the mean (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4), nor in two studies that defined developmental delay by other criteria (Analysis 6.5).
Cerebral palsy
Evidence indicates that early systemic corticosteroids increased cerebral palsy (typical RR 1.43, 95% CI 1.07 to 1.92; typical RD 0.02, 95% CI 0.00 to 0.05; 13 studies, 1973 infants; Analysis 6.11), but results show little difference in the combined outcome, mortality or cerebral palsy (typical RR 1.03, 95% CI 0.91 to 1.16; 13 studies, 1973 infants; Analysis 6.13). We noted little evidence of publication bias for the outcome of cerebral palsy (Egger test, P = 0.82; Figure 8) or for the combined outcome, mortality or cerebral palsy (Egger test, P = 0.67; Figure 9).

Funnel plot of comparison: 6 Long‐term follow‐up, outcome: 6.11 Cerebral palsy.

Funnel plot of comparison: 6 Long‐term follow‐up, outcome: 6.13 Death or cerebral palsy.
Major neurosensory disability
No evidence suggests effects of early systemic corticosteroids on major neurosensory disability (typical RR 1.08, 95% CI 0.89 to 1.33; 7 studies, 1703 infants; Analysis 6.15) nor on the combined outcome, mortality or major neurosensory disability (typical RR 0.97, 95% CI 0.87 to 1.08; 7 studies, 1703 infants; Analysis 6.17).
Abnormal neurological examination
Evidence indicates that early systemic corticosteroids increased the rate of abnormal neurological examination findings (typical RR 1.81, 95% CI 1.33 to 2.47; typical RD 0.10, 95% CI 0.05 to 0.15; 5 studies, 829 infants; Analysis 6.19) and the combined outcome, mortality or abnormal neurological examination (typical RR 1.23, 95% CI 1.06 to 1.42; typical RD 0.10, 95% CI 0.03 to 0.16; 5 studies, 829 infants; Analysis 6.21). Although criteria for this diagnosis were vague and varied between studies, the size of the difference in this outcome in trials for which data were available was similar to the size of the difference in cerebral palsy in the corresponding study. Yeh 1997 provided data for cerebral palsy obtained at age eight to nine years, whereas other investigators reported date for abnormal examination from earlier in childhood ‐ typically around two years of age.
Other long‐term outcomes
Results show no significant effects on other long‐term outcomes of blindness, deafness, formal psychometric testing, abnormal electroencephalogram (EEG), behaviour problems, or re‐hospitalisation in infancy.
Subgroup analysis by type of corticosteroid used
Mortality
Data show little difference in the effects of early dexamethasone or hydrocortisone on mortality at 28 days of life (typical RR dexamethasone 1.05, 95% CI 0.90 to 1.23; 16 studies, 2576 infants; typical RR hydrocortisone 0.77, 95% CI 0.49 to 1.21; 4 studies, 357 infants; P value for interaction = 0.20; Analysis 1.1) or of early dexamethasone on mortality at 36 weeks' postmenstrual age (typical RR dexamethasone 1.08, 95% CI 0.94 to 1.23; 17 studies, 2791 infants; Analysis 1.2) before discharge (typical RR dexamethasone 1.03, 95% CI 0.90 to 1.19; 18 studies, 2731 infants; Analysis 1.3), or at the latest age possible to determine the outcome (typical RR dexamethasone 1.02, 95% CI 0.90 to 1.16; 20 studies, 2940 infants; Analysis 1.4). However, some evidence shows that early hydrocortisone reduced mortality to discharge (typical RR hydrocortisone 0.80, 95% CI 0.65 to 0.99; 11 studies, 1433 infants; P value for interaction = 0.05; Analysis 1.3) and at the latest age possible to determine the outcome (typical RR hydrocortisone 0.80, 95% CI 0.65 to 0.99; 11 studies, 1433 infants; P value for interaction = 0.05; Analysis 1.4), but less evidence for an effect of early hydrocortisone on mortality at 36 weeks (typical RR hydrocortisone 0.85, 95% CI 0.67 to 1.06; 10 studies, 1385 infants; P value for interaction = 0.07; Analysis 1.2).
Bronchopulmonary dysplasia
Most of the benefit of early systemic corticosteroids in reducing the incidence of BPD was provided by dexamethasone, with little effect of hydrocortisone, regardless of the definition of BPD: needing oxygen supplementation at 28 days of life (typical RR dexamethasone 0.84, 95% CI 0.78 to 0.91; typical RD ‐0.08, 95% CI ‐0.12 to ‐0.05; 14 studies, 2327 infants; typical RR hydrocortisone 1.00, 95% CI 0.85 to 1.18; 1 study, 253 infants; P value for interaction = 0.07; Analysis 2.1), or needing oxygen at 36 weeks' postmenstrual age (typical RR dexamethasone 0.72, 95% CI 0.63 to 0.82; typical RD ‐0.08, 95% CI ‐0.12 to ‐0.05; 17 studies, 2791 infants; typical RR hydrocortisone 0.92, 95% CI 0.81 to 1.06; 9 studies, 1376 infants; P value for interaction = 0.01; Analysis 2.2). Benefits of reducing the need for oxygen at 36 weeks in survivors or of providing late rescue with postnatal corticosteroids were also largely confined to the dexamethasone group (oxygen at 36 weeks in survivors; typical RR dexamethasone 0.72, 95% CI 0.63 to 0.82; typical RD ‐0.10, 95% CI ‐0.14 to ‐0.06; 15 studies, 1948 infants; typical RR hydrocortisone 0.89, 95% CI 0.78 to 1.02; 9 studies, 1145 infants; P value for interaction = 0.02; Analysis 2.3) (late rescue with postnatal corticosteroids; typical RR dexamethasone 0.72, 95% CI 0.65 to 0.80; typical RD ‐0.14, 95% CI ‐0.18 to ‐0.10; 10 studies, 1974 infants; typical RR hydrocortisone 0.94, 95% CI 0.81 to 1.09; 5 studies, 1030 infants; P value for interaction = 0.003; Analysis 2.4). Strong evidence shows subgroup differences with low P values for interactions in Analysis 2.2, Analysis 2.3, and Analysis 2.4, but with lower power to detect subgroup differences for the first comparison (BPD at 28 days) because only one study provided data for hydrocortisone.
Mortality or bronchopulmonary dysplasia
Most of the benefit of early systemic corticosteroids in reducing the incidence of the combined outcome, mortality or bronchopulmonary dysplasia at 28 days of life, was provided by dexamethasone, with little effect of hydrocortisone (typical RR dexamethasone 0.88, 95% CI 0.85 to 0.95; typical RD ‐0.07, 95% CI ‐0.10 to ‐0.03; 13 studies, 2218 infants; typical RR hydrocortisone 1.00, 95% CI 0.90 to 1.12; 1 study, 253 infants; Analysis 3.1), but both drugs reduced the combined outcome of mortality or bronchopulmonary dysplasia at 36 weeks' postmenstrual age (typical RR dexamethasone 0.88, 95% CI 0.81 to 0.95; typical RD ‐0.06, 95% CI ‐0.09 to ‐0.02; 17 studies, 2791 infants; typical RR hydrocortisone 0.90, 95% CI 0.82 to 0.99; typical RD ‐0.06, 95% CI ‐0.11 to ‐0.00; 9 studies, 1376 infants; Analysis 3.2). Heterogeneity was substantial for the first comparison (Analysis 3.1), and there was low power to detect subgroup differences because only one study provided data for hydrocortisone.
Complications during primary hospitalisation
Of the short‐term complications observed with early systemic corticosteroids, only hyperglycaemia was related more to dexamethasone than to hydrocortisone (typical RR dexamethasone 1.35, 95% CI 1.21 to 1.49; typical RD 0.11, 95% CI 0.08 to 0.15; 12 studies, 2117 infants; typical RR hydrocortisone 1.01, 95% CI 0.84 to 1.22; 2 studies, 571 infants; P = 0.009 for subgroup interaction; Analysis 5.2), Both hypertension and gastrointestinal haemorrhage were more common with dexamethasone compared with control, but there were too few studies of hydrocortisone to ensure any effects of that drug on hypertension (typical RR dexamethasone 1.84, 95% CI 1.53 to 2.21; typical RD 0.10, 95% CI 0.07 to 0.13; 10 studies, 1943 infants; typical RR hydrocortisone 3.00, 95% CI 0.33 to 26.92; 1 study, 50 infants) or gastrointestinal haemorrhage (typical RR dexamethasone 1.87, 95% CI 1.35 to 2.58; typical RD 0.05, 95% CI 0.03 to 0.08; 10 studies, 1725 infants; typical RR hydrocortisone 1.53, 95% CI 0.27 to 8.74; 2 studies, 91 infants; Analysis 5.14). However, both types of corticosteroid were associated with greater gastrointestinal perforation (typical RR dexamethasone 1.73, 95% CI 1.20 to 2.51; typical RD 0.03, 95% CI 0.01 to 0.05; 9 studies, 1936 infants; typical RR hydrocortisone 2.05, 95% CI 1.21 to 3.47; typical RD 0.04, 95% CI 0.01 to 0.06; 7 studies, 1104 infants; Analysis 5.15) and lower rates of patent ductus arteriosus (typical RR dexamethasone 0.76, 95% CI 0.69 to 0.84; typical RD ‐0.10, 95% CI ‐0.13 to ‐0.06; 17 studies, 2706 infants; typical RR hydrocortisone 0.82, 95% CI 0.71 to 0,95; typical RD ‐0.07, 95% CI ‐0.12 to ‐0.02; 7 studies, 1307 infants; Analysis 5.7). Only dexamethasone was associated with reductions in rates of any retinopathy of prematurity (typical RR dexamethasone 0.84, 95% CI 0.72 to 0.99; 8 studies, 1042 infants; typical RR hydrocortisone 0.93, 95% CI 0.84 to 1.04; 1 study, 303 infants; Analysis 5.17), severe retinopathy of prematurity (typical RR dexamethasone 0.77, 95% CI 0.60 to 0.99; 8 studies, 1507 infants; typical RR hydrocortisone 0.89, 95% CI 0.65 to 1.23; 6 studies, 1070 infants; Analysis 5.18), and severe retinopathy of prematurity among survivors (typical RR dexamethasone 0.75, 95% CI 0.59 to 0.95; 10 studies, 1238 infants; typical RR hydrocortisone 0.83, 95% CI 0.60 to 1.17; 2 studies, 337 infants; Analysis 5.19), but power to detect subgroup differences was low.
Follow‐up data
Cerebral palsy and the combined outcome, mortality or cerebral palsy, were more common with dexamethasone than with hydrocortisone (cerebral palsy: typical RR dexamethasone 1.77, 95% CI 1.21 to 2.58; typical RD 0.05, 95% CI 0.01 to 0.09; 7 studies, 921 infants; typical RR hydrocortisone 1.05, 95% CI 0.66 to 1.66; 6 studies, 1052 infants; P = 0.09 for subgroup interaction; Analysis 6.11; mortality or cerebral palsy: typical RR dexamethasone 1.18, 95% CI 1.01 to 1.37; typical RD 0.07, 95% CI 0.01 to 0.13; 7 studies, 921 infants; typical RR hydrocortisone 0.86, 95% CI 0.71 to 1.05; typical RD ‐0.04, 95% CI ‐0.09 to 0.01; 6 studies, 1052 infants; P = 0.02 for subgroup interaction; Analysis 6.13).
Sensitivity analyses
Excluding studies with higher risk of bias
Five studies had higher risk of bias, largely because they included no control groups and hence blinding to knowledge of treatment allocation was not possible. All five studies involved dexamethasone, and none involved hydrocortisone (Lauterbach 2006; Mukhopadhyay 1998; Romagnoli 1999; Subhedar 1997; Suske 1996; Figure 3). Excluding these five studies from major outcomes of mortality at latest age, BPD at 36 weeks, combined mortality or BPD at 36 weeks, gastrointestinal perforation, cerebral palsy, or mortality or cerebral palsy had little effect on any RR nor on any CI, and no conclusions were altered (data not shown).
By indication for hydrocortisone
Mortality to latest age
No evidence suggests a differential effect of hydrocortisone on mortality to latest age by the main indication for the drug, whether given to treat lung problems or to treat low blood pressure (Analysis 7.1).
Bronchopulmonary dysplasia at 36 weeks
No evidence suggests a differential effect of hydrocortisone on BPD at 36 weeks by the main indication for the drug, whether given to treat lung problems or to treat low blood pressure (Analysis 7.2).
Mortality or bronchopulmonary dysplasia at 36 weeks
No evidence suggests a differential effect of hydrocortisone on the combined outcome, mortality or BPD at 36 weeks, by the main indication for the drug, whether given to treat lung problems or to treat low blood pressure (Analysis 7.3).
Results of individual trials
Anttila 2005: primary outcome was survival without BPD, intraventricular haemorrhage (grade 3 or 4), or periventricular leukomalacia, and although this tended to be greater in the dexamethasone group, differences compared with controls were not statistically significant. The RR for mortality or BPD at 36 weeks' postmenstrual age was 0.78 (95% CI 0.54 to 1.13) overall, and 0.61 (95% CI 0.33 to 1.11) in the subgroup with birth weight 750 grams to 999 grams. We noted no detectable trends in mortality, severe intraventricular haemorrhage, or periventricular leukomalacia. Rates of patent ductus arteriosus, retinopathy of prematurity, or sepsis did not differ between groups. Mean arterial blood pressures were increased in the dexamethasone group during the first week (P = 0.015), and the dexamethasone group tended to need more insulin therapy (49% versus 39%; P = 0.25).
Baden 1972: results of this study show no significant effects on blood gases, pH, oxygen requirement, need for assisted ventilation, or survival. Data indicate no significant differences in rates of cerebral palsy or deafness among survivors, in mean scores on Griffiths Scales, or in the combined rate of mortality or cerebral palsy (Fitzhardinge 1974).
Batton 2012: data show minimal effects on rates of mortality during primary hospitalisation, intraventricular haemorrhage, periventricular leukomalacia, or necrotising enterocolitis. BPD, which was undefined by both criteria and timing, occurred in two of four infants in the hydrocortisone group and in three of six in the control group; these data could not be added to 28‐day or 36‐week data for BPD because of lack of information about timing.
Baud 2016: mortality or BPD at 36 weeks' gestational age occurred in 40% (102/255) of the hydrocortisone group compared with 49% (130/266) of the placebo group (odds ratio (OR) 0.82, 95% CI 0.67 to 0.99). Rates of any neurodevelopmental impairment (NDI) among assessed survivors were similar in the two groups (hydrocortisone 27% (53/194); placebo 30% (55/185)), as were rates of moderate to severe impairment (hydrocortisone 7% (14/194); placebo 11% (21/185)). The combined outcome, mortality or moderate severe impairment in all randomised infants, was lower in the hydrocortisone group than in the control group (hydrocortisone 24% (62/255); placebo 33% (88/266); OR 0.73, 95% CI 0.56 to 0.97). Data were also reported by Shaffer and colleagues (Shaffer 2019).
Biswas 2003: results show no significant effects of infusion of hydrocortisone and T3 on the primary endpoint of mortality or failure to extubate by seven days, nor mortality or oxygen dependency at 14 days. Patent ductus arteriosus was significantly reduced in the treatment group (41/125 versus 60/128; RR 0.70, 95% CI 0.51 to 0.96), but data show no other significant differences in secondary outcomes.
Bonsante 2007: oxygen‐free survival was significantly greater in the hydrocortisone group than in the control group (64% versus 32%; P = 0.023). The effect of hydrocortisone was particularly evident in the subgroup not exposed to prenatal corticosteroids. Four infants in the hydrocortisone group died compared with 10 in the control group (16% versus 40%; P = 0.05). Duration of ventilation, patent ductus arteriosus, severe retinopathy of prematurity, severe intraventricular haemorrhage, and periventricular leukomalacia were not different between groups. Data were also reported by Shaffer and colleagues (Shaffer 2019).
Efird 2005: vasopressor was used less in the hydrocortisone‐treated group, significantly so on the second day of life. Results show no significant differences in cortisol levels between groups at any time point, and no significant differences in mortality, duration of ventilation, BPD (oxygen at 36 weeks' postmenstrual age), nosocomial infections, necrotising enterocolitis, spontaneous intestinal perforations, or intraventricular haemorrhage. No infants were treated or removed from the study as a result of hypertension. Data show no differences in the rate of glucose intolerance between groups, but two infants in the hydrocortisone group received insulin for five days.
Garland 1999: early dexamethasone‐treated infants were more likely than placebo‐treated controls to survive without BPD (83/118 versus 71/123; P = 0.03). They also were less likely to develop BPD if they survived to 28 days of life (16/99 versus 27/98; P = 0.042). Mortality rates were not significantly different. Subsequent dexamethasone therapy was used less often among early dexamethasone‐treated infants who survived (68/99 versus 81/98; P = 0.01). Intestinal perforation was more common, but not significantly so, among dexamethasone‐treated infants (12/118 versus 7/122; P = 0.20); during the first week of life, the difference was significant (9/118 versus 1/122; P = 0.009). Infants in the dexamethasone group also spent less time receiving oxygen (median days 43 versus 50; P = 0.04). Any grade of intraventricular haemorrhage (36% versus 52%; P = 0.02) and of patent ductus arteriosus ligation (14% versus 28%; P = 0.01) was also less common in the dexamethasone group. Hypertension (P < 0.001) and treatment with insulin (P = 0.007) occurred more often among dexamethasone‐treated infants than controls.
Halac 1990: investigators reported no substantial or statistically significant effects of dexamethasone on neonatal mortality, mortality to hospital discharge, necrotising enterocolitis, sepsis, patent ductus arteriosus, or severe intraventricular haemorrhage.
Hochwald 2014: data show no statistically significant effects of hydrocortisone on mortality to hospital discharge, BPD, mortality or BPD, necrotising enterocolitis, or sepsis.
Kopelman 1999: intermittent mandatory ventilation (IMV) rate and ventilation index improved more rapidly in the dexamethasone‐treated group. Mean blood pressure was higher in the dexamethasone group after the first day. Patent ductus arteriosus was less common in dexamethasone‐treated infants (13/37 versus 19/33; P = 0.08), and fewer received indomethacin (8/37 versus 15/33; P = 0.03). At the study hospital, where early extubation was practised, more dexamethasone‐treated infants were extubated during the first week (10/22 versus 2/16, P < 0.03). Results show no difference in intraventricular haemorrhage and no occurrence of adverse effects.
Lin 1999: for the endpoint of BPD at 28 days of life, statistical significance favouring dexamethasone was reached when analysis of 12 consecutive pairs in which one infant had BPD and the other did not showed that 10 pairs favoured dexamethasone and two pairs favoured control. Data presented for 40 infants (20 in each group) show a lower incidence of BPD at 28 days of life in the dexamethasone group (n = 4) than in the control group (n = 11; P < 0.05). Duration of oxygen therapy was also shorter in the dexamethasone group, at 7 ± 6 days versus 13 ± 12 days (P < 0.05). Among survivors, 12 of 15 in the dexamethasone group were extubated at the end of the study compared with 9 of 16 in the control group. Infants in the treated group had transient hyperglycaemia and hypertension, but data show no differences between groups for mortality, incidence of sepsis, or intraventricular haemorrhage.
Mukhopadhyay 1998: oxygen requirement was lower in the treated group than in the control group on Days 3, 4, and 5, although differences were not statistically significant. Mean duration of ventilation was shorter in the dexamethasone group (87 ± 42 hours) than in the control group (120 ± 46 hours; P value not given). Study authors reported one case of culture‐positive sepsis in the dexamethasone group, and two in the control group. None of the infants developed BPD (definition not given). Four infants in the dexamethasone group developed a pneumothorax versus three in the control group. Survival was 60% in the treated group and 55% in the control group.
Ng 2006: 19 infants (79%) in the hydrocortisone group were weaned from vasopressor support within 72 hours compared with eight controls (33%) (P < 0.001). Cumulative doses of dopamine and dobutamine after randomisation were significantly lower in the hydrocortisone group. Duration of ventilation, duration of oxygen, and incidence of BPD (oxygen at 36 weeks' postmenstrual age) were not significantly different between groups. Results show no differences between groups for highest serum glucose, culture‐proven sepsis, necrotising enterocolitis, intestinal perforation, duration of hospitalisation, and mortality. However, significantly more hydrocortisone‐treated infants had glycosuria (P = 0.029).
Peltoniemi 2005: hydrocortisone‐treated infants did not show a significant increase in survival without BPD (64% versus 54% placebo) nor a significant decrease in BPD among survivors (OR 0.53, 95% CI 0.17 to 1.71). However, the study enrolled only 16% of its intended sample size. Two infants in the hydrocortisone group and three in the placebo group died. During the first week of life, infants in the hydrocortisone group needed lower mean airway pressures than infants in the placebo group (P = 0.03). Patent ductus arteriosus (36% versus 73%; P = 0.01) and duration of oxygen therapy (34 versus 62 days; P = 0.02) occurred less often in the hydrocortisone group, but intraventricular haemorrhage, cystic periventricular leukomalacia, retinopathy of prematurity, sepsis, necrotising enterocolitis, gastrointestinal haemorrhage, open corticosteroid treatment, and duration of intubation and of hospitalisation were not different between groups. Risk of gastrointestinal perforation was increased in the hydrocortisone group (16% versus 0%; P = 0.05). Data show no differences in the rate of hyperglycaemia requiring insulin nor in blood pressures (diastolic and systolic). At six‐year follow‐up, data show no substantial differences between groups in rates of cerebral palsy, blindness, deafness, and intellectual impairment (IQ < 69; steroid group 8% (2/25) versus placebo group 4% (1/26)). However, scores for performance IQ on the WPPSI‐R were lower among those treated with hydrocortisone (mean difference ‐10.8, 95% CI ‐20.8 to ‐0.8). Data were also reported by Shaffer and colleagues (Shaffer 2019).
Rastogi 1996: ventilator variables at 5 to 14 days were significantly improved among infants who received dexamethasone compared with infants who received placebo. The effect seemed to be more marked among infants weighing less than 1250 grams at birth. Significantly more infants could be extubated by 14 days in the dexamethasone group (26/32 versus 14/32; P = 0.004). Dexamethasone therapy reduced the incidence of BPD at 28 days of life (OR 0.10, 95% CI 0.03 to 0.30) and eliminated BPD at 36 weeks' postmenstrual age. Dexamethasone‐treated infants were more likely to show weight loss at 14 days (12.9% versus 3.7%; P = 0.01) and higher blood pressure from Days 3 to 10. However, data show no differences in time to regain birth weight, hypertension (one infant in each group), nor incidence of intraventricular haemorrhage.
Romagnoli 1999: the incidence of BPD at 28 days of life and at 36 weeks' postmenstrual age was significantly lower in the dexamethasone group than in the control group (P < 0.001). Infants in the dexamethasone group remained intubated and required oxygen therapy for a shorter period than those in the control group (P < 0.001). Hyperglycaemia, hypertension, growth failure, and hypertrophy of the left ventricle were transient side effects of early corticosteroid administration. Data show no significant differences in rates of cerebral palsy, blindness, deafness, or intellectual impairment, nor in mean IQ, or in the combined rate of mortality or cerebral palsy (Romagnoli 2002).
Sanders 1994: the dexamethasone group required less ventilatory support (mean airway, peak inspiratory and end‐expiratory pressures, and IMV) and supplemental oxygen after study Day 4 (all P < 0.05). Improved tidal volume in the dexamethasone group, as assessed by pulmonary function testing of infants who remained intubated, was seen on study Day 7 (P = 0.02). The dexamethasone group required a shorter time in hospital (median 95 days versus 106 days; P = 0.01). Survival in the dexamethasone group was 89% versus 67% in the placebo group (P = 0.08). Survival without BPD was 68% in the dexamethasone group versus 43% in the placebo group (P = 0.14). Mean blood pressure was elevated on study Days 4 to 7. Data show no differences in rates of hyperglycaemia, incidence or severity of intraventricular haemorrhage, or days to regain birth weight, and no significant differences in rates of cerebral palsy, blindness, deafness, or intellectual impairment, nor in the combined rate of mortality or cerebral palsy (Sinkin 2002 (personal communication follow‐up to Sanders 1994)).
Shinwell 1996: results show no differences in any outcome variable, except a reduction in the need for mechanical ventilation at three days in dexamethasone‐treated infants (54/122, 44% versus 71/106, 67%; P = 0.001). Gastrointestinal haemorrhage, hypertension, and hyperglycaemia were more common among treated infants, but no life‐threatening complications, such as gastrointestinal perforation, were encountered. Follow‐up of survivors at two to six years shows no significant differences in rates of blindness, deafness, or major neurosensory disability, nor in the combined rate of mortality or major neurosensory disability. However, data show significant increases in rates of abnormal neurological examination findings, developmental delay, and cerebral palsy, and a significant increase in the combined rate of mortality or cerebral palsy (Shinwell 2002).
Sinkin 2000: results show no differences between dexamethasone and placebo groups, respectively, for the primary outcomes of survival (79% versus 83%), survival without oxygen at 36 weeks' postmenstrual age (both 59%), and survival without oxygen at 36 weeks' postmenstrual age without late corticosteroids (46% versus 44%). We noted no significant differences between groups for median time in oxygen (50 versus 56 days), ventilation (20 versus 27 days), time to regain birth weight (15.5 versus 15.0 days), nor length of stay (88 versus 89 days). Infants given early dexamethasone were less likely to receive late corticosteroids for BPD during their hospital stay (25% versus 35%; P = 0.042). We noted no clinically significant side effects in the dexamethasone group, although transient elevations in blood glucose and blood pressure with return to baseline were evident by study Day 10. Among infants who died (40 versus 33), data show no differences in median days on oxygen, ventilation, or length of hospital stay. However, among survivors (149 versus 162), we observed the following: median days on oxygen 37 versus 45, ventilation 14 versus 19 days, and length of stay 79 versus 81 days, for dexamethasone versus placebo groups, respectively. Data show no significant differences in rates of cerebral palsy, in the combined rate of mortality or cerebral palsy, nor in mean Bayley scores (Sinkin 2002 (personal communication follow‐up to Sinkin 2000)).
Soll 1999: results show no differences in the primary outcome of BPD or mortality at 36 weeks' postmenstrual age (early therapy 135/272 versus 143/267; RR 0.93, 95% CI 0.79 to 1.09). Infants who received early corticosteroid therapy were less likely to need late treatment (114/270 versus 164/267; RR 0.69, 95% CI 0.58 to 0.81). They also had lower risk of patent ductus arteriosus (92/272 versus 117/269; RR 0.78, 95% 0.63 to 0.96) and were less likely to receive indomethacin therapy (132/273 versus 176/269; RR 0.74, 95% CI 0.64 to 0.86). However, infants who received early corticosteroid therapy were more likely to have complications such as hyperglycaemia (200/271 versus 151/263; RR 1.29, 95% CI 1.13 to 1.46) and to require insulin therapy (168/271 versus 102/267; RR 1.62, 95% CI 1.36 to 1.94). Data show trends towards increased gastrointestinal haemorrhage (33/271 versus 21/267; RR 2.55, 95% CI 0.92 to 2.61) and gastrointestinal perforation (31/271 versus 20/267; RR 1.53, 95% CI 0.89 to 2.61). Infants who had cranial ultrasound scans showed a trend towards an increase in periventricular leukomalacia in the early corticosteroid group (18/252 versus 8/250; RR 2.23, 95% CI 0.99 to 5.04). Infants who received early corticosteroid therapy received fewer days of supplemental oxygen but experienced poorer weight gain.
Stark 2001: corticosteroid‐treated infants had a lower incidence of the primary outcome, mortality or BPD at 36 weeks' postmenstrual age (63% versus 69%; P < 0.05). Fewer infants in the corticosteroid group had pulmonary interstitial emphysema (9% versus 23%; P < 0.05), required oxygen at 28 days of life (78% versus 94%; P < 0.05), or received subsequent corticosteroid treatment (34% versus 51%; P < 0.05). Rates of severe intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity, and nosocomial infection were similar. Hypertension and hyperglycaemia were more frequent in the corticosteroid group (27% versus 4% and 23% versus 12%, respectively; both with P < 0.05). During the first 14 days, 14/111 (13%) infants in the corticosteroid group and 3/109 (3%) in the placebo group had spontaneous gastrointestinal perforation without necrotising enterocolitis (P < 0.05). Spontaneous perforation was also associated with indomethacin treatment (P = 0.005), as was an interaction between indomethacin and corticosteroid therapy (P = 0.04). Data show no significant differences in rates of cerebral palsy, developmental delay, or major neurosensory disability, in the combined rate of mortality or cerebral palsy, nor in the combined rate of mortality or major neurosensory disability (Stark 2001).
Subhedar 1997: results show no differences in the combined incidence of BPD and/or mortality before discharge from hospital between infants treated with dexamethasone and control infants (RR 0.95, 95% CI 0.79 to ‐1.18) or between those treated with inhaled nitric oxide and controls (RR 1.05, 95% CI 0.84 to 1.25). Data show no significant differences in rates of cerebral palsy, blindness, deafness, developmental delay, the combined rate of mortality or cerebral palsy, nor the combined rate of mortality or major neurosensory disability (Subhedar 2002 (personal communication follow‐up to Subhedar 1997)).
Suske 1996: infants in the dexamethasone group were extubated earlier (6.6 days versus 14.2 days; P < 0.02) and required less time on supplemental oxygen (4.2 days versus 12.5 days; P < 0.02). Pulmonary complications tended to be fewer in the dexamethasone group (1/14 versus 4/12), and retinopathy of prematurity tended to occur less frequently (2/14 versus 6/12; P < 0.05).
Tapia 1998: results show no significant differences in mortality and/or BPD between groups. The number of infants requiring oxygen at 36 weeks' postmenstrual age was significantly reduced in the dexamethasone group (8% versus 33%; P < 0.05). Stepwise logistic regression analysis with oxygen dependency at 36 weeks as the dependent variable, and birth weight, gestational age, gender, prenatal corticosteroids, and study treatment as the independent variables, shows that study treatment was the only variable significantly associated with oxygen dependency at 36 weeks. Data show no differences between groups in the number of days of mechanical ventilation and oxygen treatment, and no differences in the incidence of major morbidity and possible complications related to corticosteroid administration, except a lower incidence of necrotising enterocolitis in the dexamethasone group.
Vento 2004: seven dexamethasone‐treated infants and two control infants were extubated during the study period of seven days. Data show no differences between groups for respiratory distress syndrome, patent ductus arteriosus, nor severe intraventricular haemorrhage (grade 3 or 4). Dexamethasone‐treated infants had lower absolute cell count and proportion of polymorphs in tracheal aspirate fluid compared with control infants as early as Day 1 of treatment. They also had significantly higher dynamic compliance values compared with control infants (P < 0.01) as early as Day 2 of treatment. Inspired oxygen concentrations were significantly lower on Day 2 (0.24 versus 0.31; P < 0.05), and mean airway pressure on Day 5 (4.8 versus 7.2 cmH₂O; P < 0.05).
Wang 1996: dexamethasone treatment decreased fractional inspired oxygen concentration, arterial carbon dioxide tension, and mean airway pressure, and facilitated successful weaning from mechanical ventilation. Surfactant protein (SP)‐A concentrations in tracheal aspirates were increased at Days 7 and 14, and SP‐D concentrations were increased from Days 3 to 14 in the dexamethasone‐treated group, compared with the control group.
Watterberg 1999: in this study, more infants treated with hydrocortisone survived without supplemental oxygen at 36 weeks' postmenstrual age (12/20 versus 7/20; P = 0.023). Hydrocortisone treatment was also associated with a reduction in duration of oxygen > 40% (7 versus 28 days; P = 0.06), duration of oxygen > 25% (48 versus 69 days; P = 0.02), and duration of mechanical ventilation (25 versus 32 days; P = 0.03). Data show no differences in rates of mortality, sepsis, patent ductus arteriosus, necrotising enterocolitis, gastrointestinal perforation, intraventricular haemorrhage, nor retinopathy of prematurity, and no significant differences in rates of cerebral palsy, blindness, or deafness, nor in the combined rate of mortality or cerebral palsy (Watterberg 2002 (personal communication follow‐up to Watterberg 1999)).
Watterberg 2004: results show no differences in primary outcomes between groups (hydrocortisone versus placebo): survival without BPD (35% versus 34%), mortality before 36 weeks' postmenstrual age (15% versus 16%), and mortality before discharge (16% versus 17%). In a subgroup of infants exposed to chorioamnionitis, the hydrocortisone‐treated group had significantly improved survival without BPD (38% versus 24%; P = 0.005) and lower mortality at 36 weeks' postmenstrual age (10% versus 18%; P = 0.02) and before discharge (12% versus 21%; P = 0.02). During treatment, rates of hyponatraemia, hypernatraemia, hyperkalaemia, hyperglycaemia, hypertension, and gastrointestinal bleeding were similar between groups. Seventy‐four infants (41%) in the hydrocortisone group and 62 (34%) in the placebo group were treated with insulin (P = 0.19). Serum sodium and mean arterial blood pressure were significantly higher in hydrocortisone‐treated infants (P < 0.001 and P = 0.022, respectively). Other outcomes included no differences in weight gain or head circumference, nor in duration of oxygen and ventilation, pulmonary air leaks, pulmonary haemorrhage, patent ductus arteriosus, sepsis, intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity, and necrotising enterocolitis. However, hydrocortisone‐treated infants were less likely to receive open‐label corticosteroids during the treatment period (18% versus 28%; P = 0.02) and were more likely to have a spontaneous gastrointestinal perforation (9% versus 2%; P = 0.01). Follow‐up data reveal no significant differences in rates of cerebral palsy, major neurological disability, developmental delay, or re‐hospitalisation, nor in combined rates of mortality or cerebral palsy, or mortality or major neurological disability (Watterberg 2007). Data were also reported by Shaffer and colleagues (Shaffer 2019).
Yeh 1990: infants in the dexamethasone group had significantly higher pulmonary compliance, tidal volume, and minute ventilation, and required lower mean airway pressure for ventilation than infants in the placebo group. The endotracheal tube was successfully removed from more infants in the dexamethasone group (16/28 versus 8/29; P < 0.025) at two weeks of age. Nineteen infants (65%) in the placebo group and 11 (39%) in the dexamethasone group (P < 0.05) had lung injury characterised by the following: surviving infants with BPD; infants who died of intractable respiratory failure and had evidence of BPD at autopsy; and infants who died of intractable respiratory failure with clinical evidence of BPD. Dexamethasone therapy was associated with a temporary increase in blood pressure and plasma glucose concentration and delayed somatic growth.
Yeh 1997: infants in the dexamethasone group had a significantly lower incidence of BPD than those in the placebo group, judged at 28 postnatal days (21/132 versus 40/130; P < 0.05) or at 36 weeks' postmenstrual age (20/132 versus 37/130; P < 0.05). More infants in the dexamethasone group were extubated during the study. Data show no difference in mortality between groups (39/130 versus 44/132); however, a higher proportion of infants in the dexamethasone group died during the late study period, probably as the result of infection. Results show no differences between groups in duration of oxygen therapy and hospitalisation. Significantly more infants in the dexamethasone group had bacteraemia or clinical sepsis (44/132 versus 27/130; P < 0.05). Other immediate but transient side effects observed in the dexamethasone group were hyperglycaemia, hypertension, cardiac hypertrophy, hyperparathyroidism, and delay in growth rate. At 25 months of age, data reveal no significant differences in rates of blindness, developmental delay, or major neurosensory disability, nor in the combined rate of mortality or cerebral palsy, or the combined rate of mortality or major neurosensory disability. However, we noted significant increases in rates of abnormal neurological examination and cerebral palsy among survivors (Yeh 1998). The follow‐up rate of survivors at eight years was 92% (146/159). Although rates of cerebral palsy were not significantly higher in the dexamethasone group, overall motor performance on the Movement ABC was worse than among controls. IQ and other cognitive performance were significantly worse in the dexamethasone group. Overall, survivors in the dexamethasone group had greater major neurological disability.
Discussion
Corticosteroids are potent drugs that may improve lung function in infants with bronchopulmonary dysplasia (BPD) through several different mechanisms. It has been suggested that corticosteroids might play a role in prevention of BPD by suppressing the inflammatory response in the lungs of infants at risk (Groneck 1995). It has been shown that infants who develop BPD have low cortisol levels following adrenocorticotrophic hormone (ACTH) stimulation during the first week of life (Watterberg 1999). To be effective in preventing BPD, corticosteroids may have to be given within the first few days of life.
This review has demonstrated that early corticosteroid treatment facilitates weaning from the ventilator. Additional advantages include increased survival without BPD at 28 days of life and at 36 weeks' postmenstrual age, reduced risks of BPD at 28 days of life and at 36 weeks' postmenstrual age, and reduced risks of late treatment with corticosteroids, patent ductus arteriosus, and retinopathy of prematurity. On the other hand, risks of gastrointestinal bleeding, intestinal perforation, hyperglycaemia, hypertension, hypertrophic cardiomyopathy, and growth failure may be increased.
Potential hazards of corticosteroid treatment for the neonate include growth restriction, protein breakdown, cardiac hypertrophy, and possible adverse effects on development of the central nervous system and lungs (Gibson 1993; Gramsbergen 1998; Tschanz 1995; van Goudoever 1994; Weichsel 1977; Werner 1992). One study showed a significant decline in the growth of head circumference with early corticosteroid treatment (Papile 1996). Long‐term follow‐up results show that early corticosteroid treatment is associated with a significant increase in risks of developmental delay and cerebral palsy, and has significant effects on the combined outcome of mortality or cerebral palsy in the subgroup of infants treated with dexamethasone. One study in which the rate of cerebral palsy was significantly higher at two years of age used a four‐week tapering course of dexamethasone, so is similar in duration to the six‐week tapering course of late systemic corticosteroids reported by Kothadia and colleagues, and is included in the systematic review of late systemic corticosteroids (Doyle 2014a; Kothadia 1999; Yeh 1997). However, in Yeh 1997, the numbers of surviving children with cerebral palsy declined between two and eight to nine years of age, and the difference became statistically non‐significant. In the 2002 follow‐up study to the 1996 Shinwell study, adverse long‐term neurological outcomes were reported in children treated with only a three‐day course of early dexamethasone starting within 12 hours of birth (Shinwell 1996; Shinwell 2002). This finding is of extreme importance and concern, as data show about a three‐fold increased risk of cerebral palsy among survivors, including children with spastic diplegia, spastic quadriplegia, and hemiplegia. Why dexamethasone given early for a short course should have such devastating effects remains unknown. Certainly some infants would have been treated with repeat courses of dexamethasone, but this would have been more likely among control infants. Periventricular leukomalacia is an obvious cause of cerebral palsy, but studies have shown no excess of this disorder in corticosteroid‐treated infants compared with controls. Despite an increase in the diagnosis of cerebral palsy, it is important to note that this does not necessarily translate into major functional disability for the children concerned.
Summary of main results
This systematic review found that early (≤ 7 days) systemic postnatal corticosteroids for prevention of BPD in preterm infants, in the regimens used, have had significant short‐term and long‐term effects ‐ both beneficial and harmful. A significant problem in interpreting late follow‐up data is that only 13 of 32 trials of early systemic postnatal corticosteroids have reported follow‐up results; therefore, the possibility of follow‐up bias and publication bias must be considered. Potential limitations of the study with a significant increase in the rate of cerebral palsy are that only 84% of surviving infants were examined, and children were assessed during early childhood (Shinwell 1996). It is important to remember that cerebral palsy had been diagnosed before children were five years of age in most studies; diagnosing cerebral palsy with certainty before five years of age is problematic (Stanley 1982). In another study, in which the rate of cerebral palsy was significantly worse at two years of age, with 81% follow‐up, the difference became non‐significant at eight to nine years ‐ an age when the diagnosis of cerebral palsy is more certain, and when the follow‐up rate was much better (92%), illustrating the importance of age of assessment and high follow‐up rates (Yeh 1997). No study was designed primarily to test effects of postnatal systemic corticosteroids on adverse long‐term neurosensory outcomes, and all studies were underpowered to detect clinically important differences in long‐term neurosensory outcomes.
Subgroup analyses by type of corticosteroid reveal that most beneficial and harmful effects were attributable to dexamethasone, and that hydrocortisone had little effect in most analyses, but the power to detect beneficial or harmful effects of hydrocortisone was low for most comparisons. However, the addition of data from the largest randomised controlled trial of early hydrocortisone reported by Baud and colleagues has revealed some advantages for early hydrocortisone in improving rates of mortality, extubation failure, and patent ductus arteriosus (Baud 2016).
Sensitivity analysis by indication for treatment with hydrocortisone (lung problems versus low blood pressure) revealed little difference in major outcomes of mortality, BPD or combined mortality, or BPD.
Overall completeness and applicability of evidence
Data on in‐hospital outcomes were relatively complete, but data on longer‐term outcomes were incomplete. Results are applicable largely to ventilator‐dependent preterm infants in the first week after birth.
Quality of the evidence
Review authors assessed the certainty of evidence for six major outcomes, not only overall but also in subgroups by type of corticosteroid investigated (dexamethasone or hydrocortisone) (summary of findings Table 1). We assessed the evidence as high certainty for mortality at latest age, mortality or BPD, gastrointestinal perforation, cerebral palsy, and mortality or cerebral palsy. We downgraded the outcome of BPD at 36 weeks by one level to moderate certainty because of evidence of publication bias in studies overall, but not within subgroups.
Although methods of random allocation were not fully described for all studies, we considered that the effect of this on overall results was not likely to be major, and hence we did not downgrade the level of evidence for any outcomes for this reason alone.
Excluding five studies at higher risk of bias from a sensitivity analysis altered no conclusions.
Potential biases in the review process
Although Embase was searched in 2017, it was not searched for this update. Although Embase records are included in CENTRAL, we acknowledge that its omission in this update may have reduced the sensitivity of our search.
Agreements and disagreements with other studies or reviews
The overall evidence on benefits and risks from systemic corticosteroids started before seven days of age for major outcomes is consistent with the previous published version of this review (Doyle 2017a).
In an observational study of infants born after antenatal corticosteroid therapy, an excess of periventricular leukomalacia was evident among those whose mothers had received dexamethasone rather than betamethasone (Baud 1999). Most studies of postnatal systemic corticosteroids used dexamethasone in high doses, at 0.5 to 1.0 mg/kg/d. Other systemic corticosteroids or lower doses of dexamethasone may prove to be safer, and emerging evidence supports the use of hydrocortisone as prophylaxis for BPD. However, the only study of hydrocortisone with school‐age outcomes reported lower scores on the performance scale of the Wechsler Presechool and Primary Scale of Intelligence ‐ Revised (WPPSI‐R) for children treated with hydrocortisone compared with placebo (Peltoniemi 2005). Further studies are needed to compare lower doses of corticosteroids, other corticosteroids, and alternative routes of administration (e.g. inhalation) (see Cochrane Review ‐ Shah 2017).
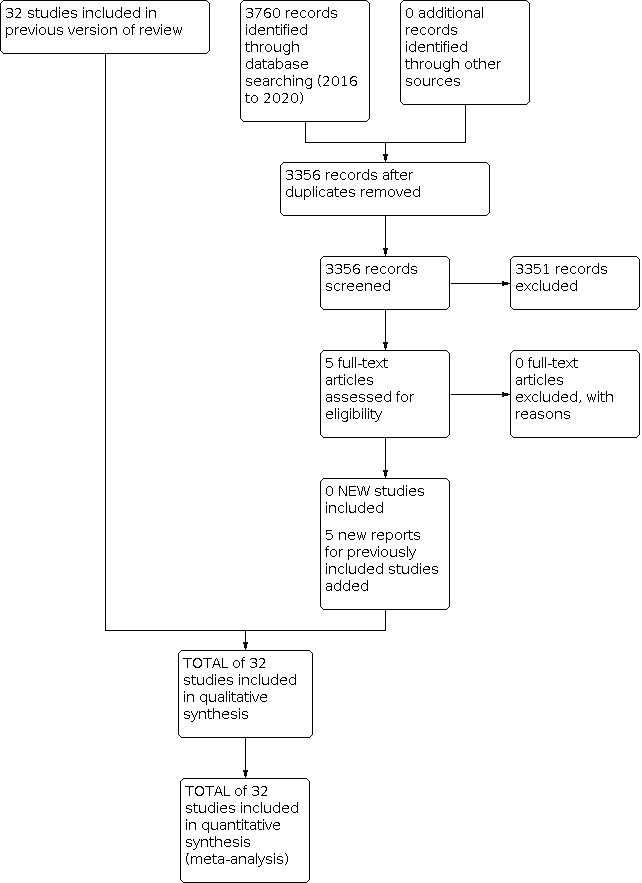
Study flow diagram: review update.

Risk of bias table.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Mortality, outcome: 1.4 Mortality at latest reported age.

Funnel plot of comparison: 2 Bronchopulmonary dysplasia (BPD), outcome: 2.2 BPD (36 weeks' postmenstrual age).

Funnel plot of comparison: 3 Death or bronchopulmonary dysplasia (BPD), outcome: 3.2 Death or BPD at 36 weeks' postmenstrual age.

Funnel plot of comparison: 5 Complications during primary hospitalisation, outcome: 5.15 Gastrointestinal perforation.

Funnel plot of comparison: 6 Long‐term follow‐up, outcome: 6.11 Cerebral palsy.

Funnel plot of comparison: 6 Long‐term follow‐up, outcome: 6.13 Death or cerebral palsy.

Comparison 1: Mortality at different ages, Outcome 1: Neonatal mortality (up to 28 days)

Comparison 1: Mortality at different ages, Outcome 2: Mortality at 36 weeks

Comparison 1: Mortality at different ages, Outcome 3: Mortality to hospital discharge

Comparison 1: Mortality at different ages, Outcome 4: Mortality at latest reported age

Comparison 2: Bronchopulmonary dysplasia (BPD) at different ages, Outcome 1: BPD (28 days of life)

Comparison 2: Bronchopulmonary dysplasia (BPD) at different ages, Outcome 2: BPD (36 weeks' postmenstrual age)

Comparison 2: Bronchopulmonary dysplasia (BPD) at different ages, Outcome 3: BPD at 36 weeks' postmenstrual age in survivors to 36 weeks

Comparison 2: Bronchopulmonary dysplasia (BPD) at different ages, Outcome 4: Late rescue with corticosteroids

Comparison 2: Bronchopulmonary dysplasia (BPD) at different ages, Outcome 5: Survivors who had late rescue with corticosteroids

Comparison 2: Bronchopulmonary dysplasia (BPD) at different ages, Outcome 6: Survivors discharged home on oxygen

Comparison 3: Mortality or bronchopulmonary dysplasia (BPD) at different ages, Outcome 1: Death or BPD at 28 days of life

Comparison 3: Mortality or bronchopulmonary dysplasia (BPD) at different ages, Outcome 2: Death or BPD at 36 weeks' postmenstrual age

Comparison 4: Failure to extubate at different ages, Outcome 1: Failure to extubate by third day

Comparison 4: Failure to extubate at different ages, Outcome 2: Failure to extubate by seventh day

Comparison 4: Failure to extubate at different ages, Outcome 3: Failure to extubate by 14th day

Comparison 4: Failure to extubate at different ages, Outcome 4: Failure to extubate by 28th day

Comparison 5: Complications during primary hospitalisation, Outcome 1: Infection

Comparison 5: Complications during primary hospitalisation, Outcome 2: Hyperglycaemia

Comparison 5: Complications during primary hospitalisation, Outcome 3: Hypertension

Comparison 5: Complications during primary hospitalisation, Outcome 4: Hypertrophic cardiomyopathy

Comparison 5: Complications during primary hospitalisation, Outcome 5: Growth failure

Comparison 5: Complications during primary hospitalisation, Outcome 6: Pulmonary air leak

Comparison 5: Complications during primary hospitalisation, Outcome 7: Patent ductus arteriosus (PDA)

Comparison 5: Complications during primary hospitalisation, Outcome 8: Severe IVH

Comparison 5: Complications during primary hospitalisation, Outcome 9: Severe intraventricular haemorrhage (IVH) in infants examined

Comparison 5: Complications during primary hospitalisation, Outcome 10: Periventricular leukomalacia (PVL)

Comparison 5: Complications during primary hospitalisation, Outcome 11: PVL in infants with cranial ultrasound scans

Comparison 5: Complications during primary hospitalisation, Outcome 12: PVL in survivors seen at follow‐up

Comparison 5: Complications during primary hospitalisation, Outcome 13: Necrotising enterocolitis (NEC)

Comparison 5: Complications during primary hospitalisation, Outcome 14: Gastrointestinal bleeding

Comparison 5: Complications during primary hospitalisation, Outcome 15: Gastrointestinal perforation

Comparison 5: Complications during primary hospitalisation, Outcome 16: Pulmonary haemorrhage

Comparison 5: Complications during primary hospitalisation, Outcome 17: Any retinopathy of prematurity (ROP)

Comparison 5: Complications during primary hospitalisation, Outcome 18: Severe ROP

Comparison 5: Complications during primary hospitalisation, Outcome 19: Severe ROP in survivors

Comparison 6: Long‐term follow‐up into later childhood, Outcome 1: Bayley Mental Developmental Index (MDI) < ‐2 SD

Comparison 6: Long‐term follow‐up into later childhood, Outcome 2: Bayley MDI < ‐2 SD in tested survivors

Comparison 6: Long‐term follow‐up into later childhood, Outcome 3: Bayley Psychomotor Developmental Index (PDI) < ‐2 SD

Comparison 6: Long‐term follow‐up into later childhood, Outcome 4: Bayley PDI < ‐2 SD in tested survivors

Comparison 6: Long‐term follow‐up into later childhood, Outcome 5: Developmental delay (other criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 6: Developmental delay (other criteria) in tested survivors

Comparison 6: Long‐term follow‐up into later childhood, Outcome 7: Blindness

Comparison 6: Long‐term follow‐up into later childhood, Outcome 8: Blindness in survivors assessed

Comparison 6: Long‐term follow‐up into later childhood, Outcome 9: Deafness

Comparison 6: Long‐term follow‐up into later childhood, Outcome 10: Deafness in survivors assessed

Comparison 6: Long‐term follow‐up into later childhood, Outcome 11: Cerebral palsy

Comparison 6: Long‐term follow‐up into later childhood, Outcome 12: Death before follow‐up in trials assessing cerebral palsy

Comparison 6: Long‐term follow‐up into later childhood, Outcome 13: Death or cerebral palsy

Comparison 6: Long‐term follow‐up into later childhood, Outcome 14: Cerebral palsy in survivors assessed

Comparison 6: Long‐term follow‐up into later childhood, Outcome 15: Major neurosensory disability (variable criteria ‐ see individual studies)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 16: Death before follow‐up in trials assessing major neurosensory disability (variable criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 17: Death or major neurosensory disability (variable criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 18: Major neurosensory disability in survivors examined (variable criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 19: Abnormal neurological exam (variable criteria ‐ see individual studies)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 20: Death before follow‐up in trials assessing abnormal neurological exam (variable criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 21: Death or abnormal neurological exam (variable criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 22: Abnormal neurological exam in tested survivors (variable criteria)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 23: Intellectual impairment (IQ < 70)

Comparison 6: Long‐term follow‐up into later childhood, Outcome 24: Intellectual impairment (IQ < 70) in survivors assessed

Comparison 6: Long‐term follow‐up into later childhood, Outcome 25: "Major neurosensory impairment" ‐ blindness or deafness

Comparison 6: Long‐term follow‐up into later childhood, Outcome 26: "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed

Comparison 6: Long‐term follow‐up into later childhood, Outcome 27: Behaviour abnormalities

Comparison 6: Long‐term follow‐up into later childhood, Outcome 28: Behaviour abnormalities in 3‐year‐old survivors assessed

Comparison 6: Long‐term follow‐up into later childhood, Outcome 29: Abnormal EEG

Comparison 6: Long‐term follow‐up into later childhood, Outcome 30: Abnormal EEG in tested survivors

Comparison 6: Long‐term follow‐up into later childhood, Outcome 31: Re‐hospitalisation in infancy

Comparison 6: Long‐term follow‐up into later childhood, Outcome 32: Re‐hospitalisation in infancy in survivors

Comparison 7: Sensitivity analyses by indication for hydrocortisone, Outcome 1: Mortality to latest age

Comparison 7: Sensitivity analyses by indication for hydrocortisone, Outcome 2: Bronchopulmonary dysplasia at 36 weeks

Comparison 7: Sensitivity analyses by indication for hydrocortisone, Outcome 3: Mortality or bronchopulmonary dysplasia at 36 weeks
| Early systemic postnatal corticosteroids (dexamethasone and hydrocortisone) compared with placebo or no treatment for preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo or no treatment | Risk with early systemic postnatal corticosteroids | |||||
| Mortality at latest reported age
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 0.95 | 4373 | ⊕⊕⊕⊕ | critical P = 0.05 for subgroup differences | |
| 232 per 1000 | 221 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.02 | 2940 | ⊕⊕⊕⊕ | critical | ||
| 236 per 1000 | 241 per 1000 (212 to 274) | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.80 | 1433 | ⊕⊕⊕⊕ | critical | ||
| 225 per 1000 | 180 per 1000 | |||||
| BPD (36 weeks' PMA)
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 0.80 | 4167 | ⊕⊕⊕⊝ | important P = 0.01 for subgroup differences | |
| 308 per 1000 | 247 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 0.72 | 2791 | ⊕⊕⊕⊕ | important | ||
| 269 per 1000 | 194 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.92 | 1376 | ⊕⊕⊕⊕ | important | ||
| 385 per 1000 | 354 per 1000 | |||||
| Mortality or BPD at 36 weeks' PMA
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 0.89 | 4167 | ⊕⊕⊕⊕ | critical | |
| 515 per 1000 | 458 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 0.88 | 2791 | ⊕⊕⊕⊕ | critical | ||
| 487 per 1000 | 429 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.90 | 1376 | ⊕⊕⊕⊕ | critical | ||
| 569 per 1000 | 512 per 1000 | |||||
| Gastrointestinal perforation during primary hospitalisation
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 1.84 | 3040 | ⊕⊕⊕⊕ | important | |
| 39 per 1000 | 71 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.73 | 1936 | ⊕⊕⊕⊕ | important | ||
| 41 per 1000 | 71 per 1000 | |||||
| Study population (subgroup of infants treated with hydrocortisone | RR 2.05 | 1104 | ⊕⊕⊕⊕ | important | ||
| 34 per 1000 | 70 per 1000 | |||||
| Cerebral palsy at latest reported age
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 1.42 | 1973 | ⊕⊕⊕⊕ | critical P = 0.09 for subgroup differences | |
| 74 per 1000 | 106 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.77 | 921 | ⊕⊕⊕⊕ | critical | ||
| 89 per 1000 | 158 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 1.05 | 1052 | ⊕⊕⊕⊕ | critical | ||
| 62 per 1000 | 65 per 1000 | |||||
| Mortality or cerebral palsy at latest reported age
| Study population (studies treating with dexamethasone or hydrocortisone) | RR 1.03 | 1973 | ⊕⊕⊕⊕ | critical P = 0.02 for subgroup differences | |
| 335 per 1000 | 345 per 1000 | |||||
| Study population (subgroup of studies treating with dexamethasone) | RR 1.18 | 921 | ⊕⊕⊕⊕ | critical
| ||
| 383 per 1000 | 452 per 1000 | |||||
| Study population (subgroup of studies treating with hydrocortisone) | RR 0.86 | 1052 | ⊕⊕⊕⊕ | critical | ||
| 295 per 1000 | 254 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
| aDowngraded one level for serious study limitations owing to evidence of publication bias for studies overall, but not within subgroups. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Neonatal mortality (up to 28 days) Show forest plot | 20 | 2933 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.18] |
| 1.1.1 Dexamethasone | 16 | 2576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.23] |
| 1.1.2 Hydrocortisone | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.49, 1.21] |
| 1.2 Mortality at 36 weeks Show forest plot | 27 | 4176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 1.2.1 Dexamethasone | 17 | 2791 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.23] |
| 1.2.2 Hydrocortisone | 10 | 1385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.06] |
| 1.3 Mortality to hospital discharge Show forest plot | 29 | 4164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.07] |
| 1.3.1 Dexamethasone | 18 | 2731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.19] |
| 1.3.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| 1.4 Mortality at latest reported age Show forest plot | 31 | 4373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| 1.4.1 Dexamethasone | 20 | 2940 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.16] |
| 1.4.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 BPD (28 days of life) Show forest plot | 15 | 2580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.80, 0.93] |
| 2.1.1 Dexamethasone | 14 | 2327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.78, 0.91] |
| 2.1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 2.2 BPD (36 weeks' postmenstrual age) Show forest plot | 26 | 4167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.73, 0.88] |
| 2.2.1 Dexamethasone | 17 | 2791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.63, 0.82] |
| 2.2.2 Hydrocortisone | 9 | 1376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.06] |
| 2.3 BPD at 36 weeks' postmenstrual age in survivors to 36 weeks Show forest plot | 24 | 3093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.87] |
| 2.3.1 Dexamethasone | 15 | 1948 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.63, 0.82] |
| 2.3.2 Hydrocortisone | 9 | 1145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.02] |
| 2.4 Late rescue with corticosteroids Show forest plot | 15 | 3004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.86] |
| 2.4.1 Dexamethasone | 10 | 1974 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| 2.4.2 Hydrocortisone | 5 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 2.5 Survivors who had late rescue with corticosteroids Show forest plot | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.89] |
| 2.5.1 Dexamethasone | 6 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| 2.5.2 Hydrocortisone | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.98] |
| 2.6 Survivors discharged home on oxygen Show forest plot | 9 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.07] |
| 2.6.1 Dexamethasone | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.48, 1.26] |
| 2.6.2 Hydrocortisone | 6 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Death or BPD at 28 days of life Show forest plot | 14 | 2471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.96] |
| 3.1.1 Dexamethasone | 13 | 2218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.86, 0.95] |
| 3.1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 3.2 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 26 | 4167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.83, 0.94] |
| 3.2.1 Dexamethasone | 17 | 2791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.95] |
| 3.2.2 Hydrocortisone | 9 | 1376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Failure to extubate by third day Show forest plot | 4 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.95] |
| 4.1.1 Dexamethasone | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.86] |
| 4.1.2 Hydrocortisone | 1 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 4.2 Failure to extubate by seventh day Show forest plot | 8 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.68, 0.85] |
| 4.2.1 Dexamethasone | 6 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.61, 0.84] |
| 4.2.2 Hydrocortisone | 2 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.94] |
| 4.3 Failure to extubate by 14th day Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.97] |
| 4.4 Failure to extubate by 28th day Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Infection Show forest plot | 25 | 4101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 5.1.1 Dexamethasone | 18 | 2821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 5.1.2 Hydrocortisone | 7 | 1280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 5.2 Hyperglycaemia Show forest plot | 14 | 2688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.15, 1.37] |
| 5.2.1 Dexamethasone | 12 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.21, 1.49] |
| 5.2.2 Hydrocortisone | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
| 5.3 Hypertension Show forest plot | 11 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.54, 2.22] |
| 5.3.1 Dexamethasone | 10 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.53, 2.21] |
| 5.3.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.33, 26.92] |
| 5.4 Hypertrophic cardiomyopathy Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [1.40, 13.37] |
| 5.5 Growth failure Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.27, 19.62] |
| 5.6 Pulmonary air leak Show forest plot | 17 | 3276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.11] |
| 5.6.1 Dexamethasone | 12 | 2041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 5.6.2 Hydrocortisone | 5 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.56] |
| 5.7 Patent ductus arteriosus (PDA) Show forest plot | 24 | 4013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.72, 0.85] |
| 5.7.1 Dexamethasone | 17 | 2706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 5.7.2 Hydrocortisone | 7 | 1307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.95] |
| 5.8 Severe IVH Show forest plot | 26 | 4103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.12] |
| 5.8.1 Dexamethasone | 17 | 2736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 5.8.2 Hydrocortisone | 9 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 5.9 Severe intraventricular haemorrhage (IVH) in infants examined Show forest plot | 8 | 1964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.12] |
| 5.9.1 Dexamethasone | 4 | 994 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.03] |
| 5.9.2 Hydrocortisone | 4 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.82, 1.49] |
| 5.10 Periventricular leukomalacia (PVL) Show forest plot | 15 | 2807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.83, 1.53] |
| 5.10.1 Dexamethasone | 8 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.81] |
| 5.10.2 Hydrocortisone | 7 | 1293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.59] |
| 5.11 PVL in infants with cranial ultrasound scans Show forest plot | 7 | 1841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.60] |
| 5.12 PVL in survivors seen at follow‐up Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.60, 2.48] |
| 5.13 Necrotising enterocolitis (NEC) Show forest plot | 25 | 4050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 5.13.1 Dexamethasone | 15 | 2661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 5.13.2 Hydrocortisone | 10 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 5.14 Gastrointestinal bleeding Show forest plot | 12 | 1816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.35, 2.55] |
| 5.14.1 Dexamethasone | 10 | 1725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.35, 2.58] |
| 5.14.2 Hydrocortisone | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.27, 8.74] |
| 5.15 Gastrointestinal perforation Show forest plot | 16 | 3040 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.36, 2.49] |
| 5.15.1 Dexamethasone | 9 | 1936 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.20, 2.51] |
| 5.15.2 Hydrocortisone | 7 | 1104 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.21, 3.47] |
| 5.16 Pulmonary haemorrhage Show forest plot | 10 | 1820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.54] |
| 5.16.1 Dexamethasone | 7 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 5.16.2 Hydrocortisone | 3 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.92, 2.03] |
| 5.17 Any retinopathy of prematurity (ROP) Show forest plot | 9 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.97] |
| 5.17.1 Dexamethasone | 8 | 1042 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 5.17.2 Hydrocortisone | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 5.18 Severe ROP Show forest plot | 14 | 2577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.99] |
| 5.18.1 Dexamethasone | 8 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 5.18.2 Hydrocortisone | 6 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] |
| 5.19 Severe ROP in survivors Show forest plot | 12 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.94] |
| 5.19.1 Dexamethasone | 10 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 5.19.2 Hydrocortisone | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Bayley Mental Developmental Index (MDI) < ‐2 SD Show forest plot | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.29] |
| 6.2 Bayley MDI < ‐2 SD in tested survivors Show forest plot | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.25] |
| 6.3 Bayley Psychomotor Developmental Index (PDI) < ‐2 SD Show forest plot | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.85, 1.60] |
| 6.4 Bayley PDI < ‐2 SD in tested survivors Show forest plot | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| 6.5 Developmental delay (other criteria) Show forest plot | 2 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.93, 2.03] |
| 6.5.1 Dexamethasone | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.08, 2.61] |
| 6.5.2 Hydrocortisone | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.26, 1.69] |
| 6.6 Developmental delay (other criteria) in tested survivors Show forest plot | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.05, 2.15] |
| 6.6.1 Dexamethasone | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.30, 2.88] |
| 6.6.2 Hydrocortisone | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.24, 1.53] |
| 6.7 Blindness Show forest plot | 9 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.74, 5.50] |
| 6.7.1 Dexamethasone | 6 | 862 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.74, 5.50] |
| 6.7.2 Hydrocortisone | 3 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.8 Blindness in survivors assessed Show forest plot | 9 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.80, 5.86] |
| 6.8.1 Dexamethasone | 6 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.80, 5.86] |
| 6.8.2 Hydrocortisone | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.9 Deafness Show forest plot | 9 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.39, 3.37] |
| 6.9.1 Dexamethasone | 5 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.30, 3.14] |
| 6.9.2 Hydrocortisone | 4 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.06] |
| 6.10 Deafness in survivors assessed Show forest plot | 8 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.29] |
| 6.11 Cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (IV, Fixed, 95% CI) | 1.43 [1.07, 1.92] |
| 6.11.1 Dexamethasone | 7 | 921 | Risk Ratio (IV, Fixed, 95% CI) | 1.77 [1.21, 2.58] |
| 6.11.2 Hydrocortisone | 6 | 1052 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.66, 1.66] |
| 6.12 Death before follow‐up in trials assessing cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.05] |
| 6.12.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 6.12.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 6.13 Death or cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.16] |
| 6.13.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.37] |
| 6.13.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 6.14 Cerebral palsy in survivors assessed Show forest plot | 13 | 1329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.12, 1.92] |
| 6.14.1 Dexamethasone | 7 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.31, 2.61] |
| 6.14.2 Hydrocortisone | 6 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 6.15 Major neurosensory disability (variable criteria ‐ see individual studies) Show forest plot | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.33] |
| 6.15.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.03, 1.83] |
| 6.15.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.14] |
| 6.16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria) Show forest plot | 8 | 1754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.06] |
| 6.16.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.25] |
| 6.16.2 Hydrocortisone | 4 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 6.17 Death or major neurosensory disability (variable criteria) Show forest plot | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 6.17.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.30] |
| 6.17.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 6.18 Major neurosensory disability in survivors examined (variable criteria) Show forest plot | 8 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 6.18.1 Dexamethasone | 4 | 469 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.77] |
| 6.18.2 Hydrocortisone | 4 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.10] |
| 6.19 Abnormal neurological exam (variable criteria ‐ see individual studies) Show forest plot | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.33, 2.47] |
| 6.20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria) Show forest plot | 6 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.07] |
| 6.20.1 Dexamethasone | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.21] |
| 6.20.2 Hydrocortisone | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 6.21 Death or abnormal neurological exam (variable criteria) Show forest plot | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.06, 1.42] |
| 6.22 Abnormal neurological exam in tested survivors (variable criteria) Show forest plot | 5 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.41, 2.52] |
| 6.23 Intellectual impairment (IQ < 70) Show forest plot | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.64, 3.33] |
| 6.23.1 Dexamethasone | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.57, 3.31] |
| 6.23.2 Hydrocortisone | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.21, 21.27] |
| 6.24 Intellectual impairment (IQ < 70) in survivors assessed Show forest plot | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.47, 2.65] |
| 6.25 "Major neurosensory impairment" ‐ blindness or deafness Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.16, 2.25] |
| 6.26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.12] |
| 6.27 Behaviour abnormalities Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.16, 2.25] |
| 6.28 Behaviour abnormalities in 3‐year‐old survivors assessed Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.16, 2.22] |
| 6.29 Abnormal EEG Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.66, 2.33] |
| 6.30 Abnormal EEG in tested survivors Show forest plot | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.08] |
| 6.31 Re‐hospitalisation in infancy Show forest plot | 3 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 6.32 Re‐hospitalisation in infancy in survivors Show forest plot | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Mortality to latest age Show forest plot | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| 7.1.1 Lung | 7 | 1319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.01] |
| 7.1.2 Blood pressure | 4 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.24, 1.38] |
| 7.2 Bronchopulmonary dysplasia at 36 weeks Show forest plot | 9 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.02] |
| 7.2.1 Lung | 6 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.02] |
| 7.2.2 Blood pressure | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.66, 1.54] |
| 7.3 Mortality or bronchopulmonary dysplasia at 36 weeks Show forest plot | 7 | 1297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 0.98] |
| 7.3.1 Lung | 6 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 7.3.2 Blood pressure | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.19, 1.02] |

