بررسی تاثیر استفاده زودهنگام (< 8 روزه) از کورتیکواستروئیدهای سیستمیک پس از زایمان برای پیشگیری از دیسپلازی برونکوپولمونری در نوزادان پرهترم
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multi‐centre double‐blind placebo‐controlled randomised trial | |
| Participants | 109 infants with birth weight 500 grams to 999 grams, gestation < 32 weeks, need for mechanical ventilation and supplemental oxygen by 4 hours of age. Stratified by weight (500 grams to 749 grams vs 750 grams to 999 grams) | |
| Interventions | 4 doses of dexamethasone 0.25 mg/kg each at 12‐hourly intervals or normal saline as placebo. First dose was given before 6 hours. Open‐label dexamethasone was allowed when deemed necessary by attending physician, but its use was discouraged. | |
| Outcomes | Survival to 36 weeks without IVH (grade III to IV), PVL (echodensities after first week or periventricular cysts on ultrasound), or BPD (oxygen at 36 weeks); growth, duration of assisted ventilation and oxygen, late corticosteroid treatment, infection, hyperglycaemia, hypertension, ROP, PDA, GI bleeding and perforation, NEC | |
| Notes | This paper also reported a meta‐analysis of early short vs early prolonged dexamethasone treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation by coded vials prepared in the pharmacy at each centre |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified primary and secondary outcomes reported |
| Methods | Double‐blind placebo‐controlled randomised trial | |
| Participants | 44 preterm infants < 24 hours old with respiratory distress confirmed both clinically and radiologically | |
| Interventions | Hydrocortisone 25 mg/kg on admission and 12 hours later intravenously | |
| Outcomes | Death, FiO2, cortisol levels, and blood gases | |
| Notes | The oldest study, carried out in 1972. Used hydrocortisone in a very short course of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via random numbers and sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre randomised placebo‐controlled trial | |
| Participants | Infants at 23 to 26 completed weeks' gestation with study‐defined low blood pressure | |
| Interventions | Hydrocortisone 1 mg/kg loading, then 0.5 mg/kg at 12‐hourly intervals for 6 doses | |
| Outcomes | Short‐term outcomes during primary hospitalisation of death, BPD (not defined), IVH grade III or IV, PVL, and NEC requiring surgery | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled infants were randomised from a prespecified sequence, allocated by centre, and received treatment from an investigational pharmacist. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome of the study was to determine the feasibility of a randomised trial of blood pressure management, rather than effects on bronchopulmonary dysplasia. |
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 523 inborn infants at 24 to 27 weeks’ gestational age in the first 24 hours after birth recruited from 21 French centres with NICU facilities between 25 May 2008 and 31 January 2014. Exclusions: rupture of membranes at < 22 weeks’ gestation; birth weight < third centile according to French sex‐customised curves; severe perinatal asphyxia (Apgar score = 0–3 for longer than 5 minutes, cord blood pH < 7·00, or both) and expected to die shortly after birth; congenital malformations (birth defects or major structural abnormalities detectable prenatally); known chromosomal aberrations | |
| Interventions | Hydrocortisone hemisuccinate 1 mg/kg/d divided into 2 doses for 7 days, then 0.5 mg/kg/d once per day for 3 days (total dose 8.5 mg/kg) Control infants were given an equivalent volume of 5% glucose placebo. Open‐label corticosteroids were not allowed during first 10 days of treatment. | |
| Outcomes | Short‐term primary outcome: survival free of bronchopulmonary dysplasia (BPD) at 36 weeks’ postmenstrual age. BPD was diagnosed at 36 weeks (± 3 days) without additional testing if an infant required mechanical ventilation, non‐invasive ventilation with continuous positive airway pressure, or 30% or more supplemental oxygen concentration. BPD was diagnosed in infants requiring only 22% to 29% oxygen if the oxygen requirement was confirmed by a standardised oxygen‐reduction test, which was completed by neonatologists masked to treatment groups. Secondary outcomes: bronchopulmonary dysplasia at 36 weeks’ postmenstrual age; death; surgical ligation of patent ductus arteriosus; air leaks; pulmonary haemorrhage; insulin requirement; late‐onset sepsis (positive blood culture or symptomatic pneumonia); necrotising enterocolitis, gastrointestinal perforation; grade 3 or 4 IVH; cystic PVL; death before discharge; severe retinopathy of prematurity (requiring laser treatment or surgery) Longer term: Children were assessed at approximately 22 months’ corrected age. Children underwent a French‐based developmental assessment that was standardised in the mid‐1990s, and a standardised neurodevelopmental assessment based on the Amiel‐Tison and Denver scales. Neurodevelopmental impairment (NDI) was defined as any disability on the standardised neurodevelopmental assessment, cerebral palsy, blindness, deafness, or a formal developmental assessment score < ‐1 SD (< 85). | |
| Notes | Study was stopped early because of lack of funding, rather than because any predetermined threshold had been reached, at approximately 2/3 of projected sample size of 786. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (1:1) via a secure study website Strata for 24 to 25 weeks and 26 to 27 weeks |
| Allocation concealment (selection bias) | Low risk | Remote electronic allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding maintained by identical placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to knowledge of treatment group at both primary hospitalisation phase and 22‐month follow‐up phase |
| Incomplete outcome data (attrition bias) | Low risk | Low risk for short‐term outcomes, as all but 2 randomised participants have short‐term outcomes reported. However, moderate risk at follow‐up phase because although 93% (379/406) of long‐term survivors were assessed at 22 months’ corrected age, only 75% (304/406) had full neurological and developmental assessment. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported |
| Methods | Multi‐centre placebo‐controlled randomised trial | |
| Participants | 253 infants < 30 weeks' gestation, within 9 hours of birth at entry; all mechanically ventilated | |
| Interventions | Hydrocortisone 1 mg/kg/d as continuous infusion for 5 days, then 0.5 mg/kg/d for 2 days. Also given tri‐iodothyronine 6 µg/kg/d for 5 days, halving to 3 µg/kg/d for 2 days | |
| Outcomes | Primary outcome was death or ventilator dependence at 7 days, or death or oxygen dependence at 14 days. | |
| Notes | Hydrocortisone combined with T3 infusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by Oxford Perinatal Trials Unit |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Two‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 70 infants with birth weight < 1000 grams or < 28 weeks' gestation, ventilator‐dependent after 7 days of age, and considered to be a candidate for corticosteroids | |
| Interventions | Active treatment – total dose of hydrocortisone 10.5 mg/kg over 10 days | |
| Outcomes | Primary outcomes: survival free of disability at 2 years of age, mortality up to 2 years of age, and neurological outcome after discharge | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation centrally |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment blind: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up reporting: yes for outcomes during primary hospital stay ‐ 98% of surviving infants traced to 2 years of age |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | 34 infants of gestation > 23 weeks and < 29 weeks, and birth weight > 500 grams and < 1000 grams enrolled by 2 hours of age | |
| Interventions | Hydrocortisone intravenously at dose of 1 mg/kg every 12 hours for 2 days, followed by 0.3 mg/kg every 12 hours for 3 days | |
| Outcomes | Blood pressure, urine output, hyperglycaemia, mortality, durations of mechanical ventilation and hospital stay, BPD (oxygen at 36 weeks), infection, NEC, intestinal perforation, PDA, IVH, PVL, cortisol levels | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via sequentially numbered, preassigned treatment designations in sealed, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre placebo‐controlled randomised trial | |
| Participants | 241 infants weighing between 500 grams and 1500 grams, received surfactant, at significant risk for BPD or death using a model to predict at 24 hours | |
| Interventions | 3‐Day course of dexamethasone beginning at 24 to 48 hours. First 2 doses were 0.4 mg/kg, third and fourth doses 0.2 mg/kg, and fifth and sixth doses 0.1 mg/kg and 0.05 mg/kg, respectively. Dexamethasone dose reduced slightly after first interim analysis (see Notes) | |
| Outcomes | Primary outcomes were survival without BPD defined as oxygen therapy at 36 weeks to maintain SaO2 above 91% and mortality. | |
| Notes | At first interim analysis (n = 75), increased risk of GI perforation was noted in the dexamethasone group. Data Monitoring Committee recommended reducing the dexamethasone dose to 4 doses of 0.25 mg/kg/dose every 12 hours begun at 24 to 48 hours, followed by doses of 0.125 mg/kg and 0.05 mg/kg at the next two 12‐hour periods, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by study pharmacists at each centre |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Placebo‐controlled randomised trial | |
| Participants | 248 infants, birth weight ≤ 1500 grams, gestation < 34 weeks, with evidence of "birth asphyxia" (1‐minute Apgar score < 5, prolonged resuscitation, and metabolic acidosis (HCO3 < 15 mmol/L within 1 hour of birth)) | |
| Interventions | 7‐Day course of dexamethasone 1 mg/kg 12‐hourly beginning on first day of life | |
| Outcomes | Neonatal mortality, mortality to discharge, NEC, PDA, sepsis, severe IVH | |
| Notes | Possible exclusion of 5 deaths after randomisation, but not clear which group they came from | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via list of random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Primary prespecified outcome of NEC was reported, as were a large number of other outcomes. |
| Methods | Placebo‐controlled randomised trial | |
| Participants | 22 infants, gestational age ≤ 30 weeks or birth weight ≤ 1250 grams, and < 48 hours after birth, with an arterial catheter in place, invasive mean blood pressure < gestational age on 3 consecutive measurements 10 minutes apart, and after treatment with 1 or 2 boluses of 10 mL of 0.9% saline. Excluded if blood loss, hydrops, or major cardiac lesions | |
| Interventions | Hydrocortisone 7 mg/kg total over 48 hours, or equal volume of 0.9% saline placebo | |
| Outcomes | Mortality (presumably to discharge), NEC, BPD, positive blood culture, insulin treatment | |
| Notes | Major outcome was to determine whether hydrocortisone reduced vasopressor doses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Short‐term outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Only short‐term outcomes reported, but major outcome of effects on vasopressor doses not reported |
| Methods | Two‐centre randomised placebo‐controlled trial | |
| Participants | 70 infants < 28 weeks' gestation requiring intermittent mandatory ventilation and arterial catheterisation | |
| Interventions | Dexamethasone 0.2 mg/kg within 2 hours of delivery | |
| Outcomes | Ventilation Index (VI), IMV rate, mean blood pressure, incidence of PDA, need for indomethacin, number extubated during first week, usual complications of RDS | |
| Notes | After an interim analysis showed that the incidence of IVH was much lower than expected, enrolment was stopped and analysis was limited to a comparison of ventilator settings, blood pressure, and pressor use during first 7 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in the hospital pharmacy stratified by use of antenatal corticosteroids; exact method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, but others reported too |
| Methods | Three‐armed randomised controlled trial: (1) nebulised pentoxifylline, (2) intravenous dexamethasone, (3) nebulised water placebo | |
| Participants | 150 infants < 1500 grams birth weight who needed oxygen on fourth day of life, regardless of the need for assisted ventilation. Major malformations and grade 3 or 4 IVH led to exclusions. | |
| Interventions | Dexamethasone 0.25 mg/kg/dose every 12 hours for 3 days | |
| Outcomes | Primary endpoint BPD (oxygen dependency at 36 weeks). Secondary endpoints included PDA, IVH and PVL, | |
| Notes | All prespecified outcomes reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind treatment groups for comparison of dexamethasone vs nebulised water placebo |
| Blinding of outcome assessment (detection bias) | High risk | Unable to blind treatment groups for comparison of dexamethasone vs nebulised water placebo |
| Incomplete outcome data (attrition bias) | Low risk | Short‐term outcomes reported for all participants |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Placebo‐controlled randomised trial | |
| Participants | 40 infants of 500 grams to 1999 grams with severe RDS, needing IPPV within 6 hours of birth | |
| Interventions | Dexamethasone 0.25 mg/kg 12‐hourly from 1 to 7 days, 0.12 mg/kg 12‐hourly from 8 to 14 days, 0.05 mg/kg 12‐hourly from 15 to 21 days, 0.02 mg/kg 12‐hourly from 22 to 28 days | |
| Outcomes | Mortality at 28 days; discharge, failure to extubate (during study), death or BPD (36 weeks), BPD (28 days and 36 weeks), infection (clinical), severe IVH, plasma glucose, mean blood pressure on days 2, 5, 7, and 16; weight at 2 weeks | |
| Notes | Sequential analysis for 12 pairs. Data given for 40 infants as randomised into the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in a paired sequential trial. Assignment determined by pharmacist and groups stratified by birth weight: 500 grams to 999 grams, 1000 grams to 1500 grams, and 1501 grams to 1999 grams. Allocation by drawing lots |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Single‐centre randomised controlled trial | |
| Participants | 19 infants < 34 weeks and < 2000 grams who could be provided with ventilation. Clinical and radiographic evidence of RDS; IPPV with oxygen > 30% | |
| Interventions | Dexamethasone 0.5 mg/kg/dose 12‐hourly for 3 days starting within 6 hours of birth | |
| Outcomes | Changes in oxygen requirements, mean duration of ventilation, culture‐positive sepsis, PDA, BPD (not defined), pneumothorax, mortality | |
| Notes | Infants were entered into the trial only if a ventilator was available. Surfactant was not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment: not sure |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Double‐blind randomised controlled trial | |
| Participants | 48 infants of gestation < 32 weeks and birth weight < 1500 grams who had systemic hypotension despite treatment with volume expanders and dopamine within the first 7 days of life Infants also had to have an indwelling arterial catheter for continuous BP monitoring. | |
| Interventions | Hydrocortisone 1 mg/kg every 8 hours for 5 days | |
| Outcomes | BP, use of vasopressors, duration of ventilation, oxygen and hospital stay, PIE, pulmonary haemorrhage, pneumothorax, hyperglycaemia, glycosuria, IVH (grade III or IV), PVL, NEC, GI perforation, sepsis, ROP (> stage II), mortality | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation in blocks of 6 by computer‐generated random numbers and opening numbered, sealed, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Primary outcome was blood pressure, which was reported. |
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 51 infants with birth weight 501 grams to 1250 grams, gestation 23 to 30 weeks, needing mechanical ventilation before the age of 24 hours. The subgroup 1000 grams to 1250 grams had to need supplemental oxygen and mechanical ventilation > 24 hours despite surfactant. Exclusions: lethal malformations, suspected chromosomal abnormalities | |
| Interventions | Hydrocortisone 2.0 mg/kg/d intravenously 8‐hourly for 2 days, 1.5 mg/kg/d 8‐hourly for 2 days, 0.75 mg/kg/d 12‐hourly for 6 days | |
| Outcomes | Survival without BPD (oxygen at 36 weeks), IVH (grades III or IV), cystic PVL, durations of ventilation, oxygen and hospital stay, sepsis, hyperglycaemia, hypertension, PDA, GI bleeding, GI perforation, NEC, ROP, and cortisol levels Long‐term outcomes: At 2 years ‐ neurosensory impairments (blindness, deafness, developmental delay assessed by MDI on Bayley Scales, cerebral palsy) and disabilities (severe ‐ any of severe cerebral palsy (not likely to walk), blindness, or severe developmental delay (MDI < 55, moderate‐moderate cerebral palsy (not walking at 2 years but likely to do so), deafness, moderate developmental delay (MDI 55 to < 70), mild‐mild cerebral palsy (walking at 2 years), or mild developmental delay (MDI 70 to < 85). Follow‐up rate was 87% (40/46). At 6 years ‐ IQ (Wechsler Preschool and Primary Scale of Intelligence ‐ Revised) and language (Reynell Developmental Language Scale III) were assessed, as were diagnoses of cerebral palsy, blindness, and deafness. Follow‐up rate was 80% (37 of the 46 survivors). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation at each centre via identical coded syringes. Exact method of randomisation not stated. Stratified by birth weight (501 grams to 750 grams vs 750 grams to 999 grams vs 1000 grams to 1250 grams) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes (for primary hospital outcomes). Follow‐up rates at 2 and 6 years listed above |
| Selective reporting (reporting bias) | Low risk | Primary outcome was reported as specified. |
| Methods | Double‐blind randomised controlled trial | |
| Participants | 70 preterm infants < 12 hours old, weighing 700 grams to 1500 grams with respiratory distress syndrome (RDS) confirmed clinically and radiologically; infants needed mechanical ventilation > 30% O2 and/or MAP 7 cmH2O a/A < 0.25 after surfactant treatment. | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.15 mg/kg/d for 3 days, 0.05 mg/kg/d for 3 days | |
| Outcomes | FiO2, MAP, BPD (28 days and CXR), severe BPD (36 weeks), duration O2, infections, deaths, pneumothorax, pulmonary haemorrhage, PDA, IVH, NEC, hyperglycaemia, insulin use, hypertension, ROP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation: via a pharmacy list; stratified for birth weight |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Randomised non‐blinded controlled trial | |
| Participants | 50 infants < 1251 grams or < 33 weeks, oxygen‐dependent at 72 hours, and at high risk of BPD according to a scoring system predicting 90% risk of BPD | |
| Interventions | Dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, and 0.125 mg/kg/d for 1 day | |
| Outcomes | Survival to 28 days, survival to discharge, PDA, IVH (grades 3 and 4), PVL, sepsis, NEC, ROP (stages III and above), requiring ventilation at 28 days, BPD at 28 days and 36 weeks, hyperglycaemia, hypertension, needed late corticosteroids, growth failure, left ventricular hypertrophy | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via random numbers, concealed in numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome measurements: no |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Randomised double‐blind controlled trial | |
| Participants | 40 infants < 30 weeks' gestation and 12 to 18 hours old with RDS, both clinical and radiological. Infants were treated with mechanical ventilation and surfactant | |
| Interventions | Dexamethasone 0.5 mg/kg twice, 12 hours apart | |
| Outcomes | MAP, FiO2, mortality, extubation < 7 days, pulmonary function tests, duration IPPV, O2, hospital, mortality, BPD (36 weeks O2), late corticosteroids | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in the pharmacy via sealed envelopes. Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported, but definitions vague |
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 248 preterm infants with birth weight 500 grams to 2000 grams, 1 to 3 days old, requiring mechanical ventilation with more than 40% oxygen | |
| Interventions | Intravenous dexamethasone 0.25 mg/kg every 12 hours 6 times | |
| Outcomes | Mortality, survival with no O2, mechanical ventilation at 3 and 7 days, BPD, duration in hospital, IVH, PVL, pneumothorax, PIE, PDA, sepsis, hypertension, hyperglycaemia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, stratified by centre and birth weight, from random numbers list in the pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes for short‐term; 84% for long‐term |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre randomised double‐blind trial | |
| Participants | 384 infants < 30 weeks' gestation with RDS by clinical and radiographic signs, needing IPPV at 12 to 18 hours of age; had received at least 1 dose of surfactant | |
| Interventions | Dexamethasone 0.5 mg/kg at 12 to 18 hours of age, second dose 12 hours later | |
| Outcomes | Primary outcomes were survival, survival without oxygen at 28 days or 36 weeks, and survival without oxygen at 28 days or 36 weeks and without late corticosteroids | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in the pharmacy via labelled syringes. Stratification by centre. Exact method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre randomised double‐blind trial | |
| Participants | 542 infants weighing 501 grams to 1000 grams who required assisted ventilation < 12 hours, had received surfactant by 12 hours, were physiologically stable, and had no life‐threatening congenital anomalies | |
| Interventions | Dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.10 mg/kg/d for 3 days, and 0.05 mg/kg/d for 3 days. Control infants received a similar volume of normal saline. | |
| Outcomes | Primary outcome was BPD or death at 36 weeks' adjusted age. | |
| Notes | Published as an extended abstract and presented at a clinical meeting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in hospital pharmacies by opening opaque, sealed envelopes. Precise method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre randomised double‐blind trial | |
| Participants | 220 infants with birth weight 501 grams to 1000 grams, mechanically ventilated < 12 hours. Infants > 750 grams also needed to receive surfactant and have FiO2 > 0.29. | |
| Interventions | Dexamethasone 0.15 mg/kg/d for 3 days, then tapered over 7 days | |
| Outcomes | Death or BPD, oxygen at 28 days, PIE, late corticosteroid treatment, hypertension, hyperglycaemia, GI perforation | |
| Notes | Factorial design; infants also randomised to routine ventilator management or a strategy of minimal ventilator support to reduce mechanical lung injury. After enrolling 220 infants (sample size estimate was 1200), the trial was halted owing to unanticipated adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via numbers generated by a random, permuted block algorithm, stratified by birth weight |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Randomised controlled trial ‐ factorial design | |
| Participants | 42 preterm infants, entry at 96 hours if gestation < 32 weeks, mechanical ventilation from birth, surfactant treatment, and high risk of developing BPD based on score (Ryan 1996) | |
| Interventions | Intravenous dexamethasone at 12‐hourly intervals for 6 days; 0.5 mg/kg/dose for 6 doses and 0.25 mg/kg/dose for a further 6 doses. Inhaled NO 5 to 20 ppm for 72 hours | |
| Outcomes | Mortality, BPD at 28 days and > 36 weeks with abnormal chest radiograph | |
| Notes | Note factorial design, which means that half of treated infants and half of control infants also received 72 hours of inhaled NO | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation by computer‐generated random numbers and sealed envelopes. Factorial design provided 4 groups: early dexamethasone, inhaled NO, both drugs together, and neither drug. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome measurements: no |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | 26 preterm infants < 2 hours old, with birth weight < 1500 grams if FiO2 > 0.50, or > 1500 grams birth weight with FiO2 > 0.70 Exclusions: known sepsis, cardiac anomalies, malformations of lung or CNS | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 5 days | |
| Outcomes | Blood gases, ventilator settings, mortality IVH, BPD (O2 28 days), NEC, late sepsis, PDA, ROP, air leak, duration in hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation via sealed envelopes. Randomisation achieved by drawing lots |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre double‐blind placebo‐controlled randomised trial | |
| Participants | 113 (4 exclusions for congenital abnormality, early sepsis, and failure to obtain follow‐up data) infants with birth weight between 700 and 1600 grams, clinical and radiological diagnosis of RDS, needing mechanical ventilation, and < 36 hours of age | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.12 mg/kg/d for 3 days, and 0.06 mg/kg/d for 3 days | |
| Outcomes | Primary outcomes were death before hospital discharge, BPD (oxygen need at 28 days and x‐ray changes), death or BPD, and oxygen need at 36 weeks. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation via ampoules of dexamethasone and saline prepared in the hospital pharmacy. Exact method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: almost (109/113) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | 20 infants with birth weight < 1251 grams and gestation < 33 weeks who were oxygen‐ and ventilator‐dependent on fourth day of life and were at high risk of BPD by study authors' own scoring system | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, and 0.125 mg/kg/d for 1 day (total dose 2.375 mg/kg) | |
| Outcomes | Tracheal aspirates for cell counts, pulmonary mechanics, PDA, IVH (grades III and IV), extubation during study period | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment: uncertain |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of intervention: uncertain |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: uncertain |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Double‐blind randomised controlled trial | |
| Participants | 63 infants with birth weight from 1000 grams to 1999 grams, AGA, clinical and radiographic RDS, IPPV (0 to 12, age after birth) | |
| Interventions | Dexamethasone 0.25 mg/kg 12‐hourly from 1 to 7 days, 0.125 mg/kg 12‐hourly from 8 to 14 days, 0.05 mg/kg 12‐hourly from 15 to 21 days. First dose administered at < 12 hours | |
| Outcomes | Oxygen requirements; PCO2; MAP; SP‐A and SP‐D in tracheal aspirate; failure to extubate by third day, 7th day, 14th day, and 28th day; mortality before discharge; sepsis; BPD at 28 days | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in a double‐blind fashion; method not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Two‐centre double‐blind randomised controlled trial | |
| Participants | 40 infants weighing between 500 grams and 999 grams who were AGA and needed mechanical ventilation < 48 hours of age | |
| Interventions | Hydrocortisone 1.0 mg/kg/d every 12 hours for 9 days, 0.5 mg/kg/d for 3 days | |
| Outcomes | Primary outcome was survival without supplemental oxygen at 36 weeks' post conception. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation at each centre by constant block design with 4 participants per block to minimise bias over time. Separate randomisation tables were used for infants exposed to antenatal corticosteroids. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 360 infants of 500 grams to 999 grams birth weight, needing mechanical ventilation, aged 12 to 48 hours | |
| Interventions | Hydrocortisone 1 mg/kg/d 12‐hourly for 12 days, then 0.5 mg/kg/d for 3 days | |
| Outcomes | Survival without BPD (oxygen at 36 weeks), physiological BPD, death before 36 weeks, death before discharge, BPD in survivors, durations of mechanical ventilation and oxygen, hospital stay, weight and OFC at 36 weeks, PDA, infection, NEC, GI perforation, major IVH (grade 3 or 4), cystic PVL, ROP, and open‐label corticosteroid therapy | |
| Notes | Sample size estimate was 712, but the study was stopped early because of increased incidence of apparently spontaneous GI perforation in the hydrocortisone group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, stratified by centre and birth weight (500 grams to 749 grams vs 750 grams to 999 grams), via a permuted block scheme with blocks of 6 in each stratum. Randomisation lists in each pharmacy in a sealed envelope |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Double‐blind randomised controlled trial | |
| Participants | 57 preterm infants weighing between 700 grams and 1999 grams, < 13 hours old, with severe RDS both clinically and radiologically. They needed mechanical ventilation < 4 hours and were excluded if they had infection. | |
| Interventions | Intravenous dexamethasone 0.50 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.12 mg/kg/d for 3 days, 0.05 mg/kg/d for 3 days | |
| Outcomes | MAP, FiO2, pulmonary function tests, BP, glucose, mortality, BPD, duration O2, hospital, weight loss, sepsis, PDA, IVH (> grade I), ROP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in blocks of 10 via a pharmacy list. Exact method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: almost |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 262 infants of birth weight < 2000 grams with RDS and requiring mechanical ventilation after birth | |
| Interventions | Dexamethasone 0.25 mg/kg/dose every 12 hours intravenously on days 1 to 7; 0.12 mg/kg/dose every 12 hours intravenously from days 8 to 14; 0.05 mg/kg/dose every 12 hours intravenously from days 15 to 21; and 0.02 mg/kg/dose every 12 hours intravenously from days 22 to 28 | |
| Outcomes | BPD judged at 28 days or at 36 weeks | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation via central pharmacy random number list; exact method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: almost for short‐term; 81% for long‐term |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
ACTH: adrenocorticotrophic hormone.
ABA: appropriate for gestational age.
BP: blood pressure.
BPD: bronchopulmonary dysplasia.
CLD: chronic lung disease.
CNS: central nervous system.
CXR: chest x‐ray.
FiO2: fraction of inspired oxygen.
GI: gastrointestinal.
HCO3: bicarbonate.
IMV: intermittent mandatory ventilation.
IPPV: intermittent positive‐pressure ventilation.
IVH: intraventricular haemorrhage.
MAP: mean airway pressure.
MDI: Mental Developmental Index.
NDI: neurodevelopmental impairment.
NEC: necrotising enterocolitis.
NO: nitric oxide.
NRN: Neonatal Research Network.
O2: oxygen.
OFC: occipito‐frontal circumference.
PDA: patent ductus arteriosus.
PDI: Psychomotor Developmental Index.
PIE: pulmonary interstitial emphysema.
ppm: parts per million.
PVL: periventricular leukomalacia.
RDS: respiratory distress syndrome.
ROP: retinopathy of prematurity.
SaO2: oxygen saturation.
SGA: small for gestational age.
SP‐A: surfactant protein‐A.
SP‐D: surfactant protein‐D.
T3: triiodothyronine.
VI: Ventilation Index.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| 20 VLBW infants were randomised to both hydrocortisone and caffeine as active treatments, compared with "standard guidelines", which presumably meant no hydrocortisone or caffeine. Major outcomes reported included BPD and BPD combined with death. As caffeine reduces BPD (Schmidt 2006), the independent effect of hydrocortisone cannot be determined. | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Primary outcome was need for an epinephrine infusion 12 hours after treatment. No long‐term outcomes reported | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "'Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Recruited term infants only for a study of early hydrocortisone to treat hypotension | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Before after study only ‐ not an RCT | |
| Not an RCT; 26 study infants and 12 historical controls | |
| Study of late postnatal corticosteroids included in the review "'Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) | |
| Study of early dexamethasone, but no outcomes relevant to this review were reported |
BPD: bronchopulmonary dysplasia.
RCT: randomised controlled trial.
VLBW: very low birth weight.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality (up to 28 days) Show forest plot | 19 | 2950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.19] |
| Analysis 1.1  Comparison 1 Mortality, Outcome 1 Neonatal mortality (up to 28 days). | ||||
| 1.1 Dexamethasone | 16 | 2603 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.24] |
| 1.2 Hydrocortisone | 3 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 2 Mortality at 36 weeks Show forest plot | 20 | 3733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.14] |
| Analysis 1.2 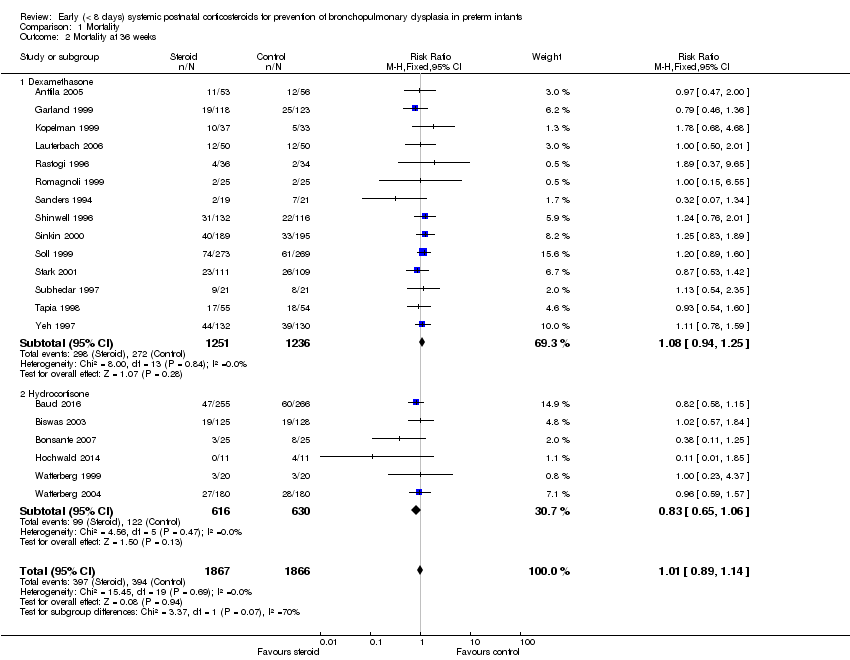 Comparison 1 Mortality, Outcome 2 Mortality at 36 weeks. | ||||
| 2.1 Dexamethasone | 14 | 2487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 2.2 Hydrocortisone | 6 | 1246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.06] |
| 3 Mortality to hospital discharge Show forest plot | 30 | 4273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| Analysis 1.3  Comparison 1 Mortality, Outcome 3 Mortality to hospital discharge. | ||||
| 3.1 Dexamethasone | 19 | 2840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 3.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 4 Mortality at latest reported age Show forest plot | 31 | 4373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| Analysis 1.4 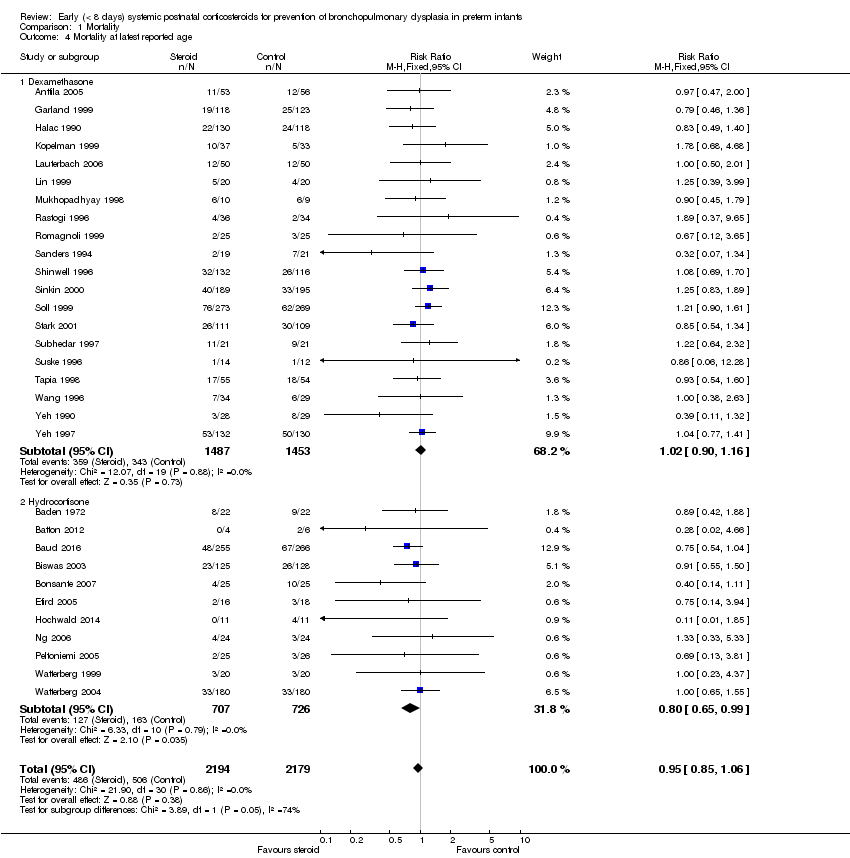 Comparison 1 Mortality, Outcome 4 Mortality at latest reported age. | ||||
| 4.1 Dexamethasone | 20 | 2940 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.16] |
| 4.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD (28 days of life) Show forest plot | 17 | 2874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| Analysis 2.1  Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 1 BPD (28 days of life). | ||||
| 1.1 Dexamethasone | 16 | 2621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.79, 0.92] |
| 1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 2 BPD (36 weeks' postmenstrual age) Show forest plot | 24 | 3929 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.87] |
| Analysis 2.2 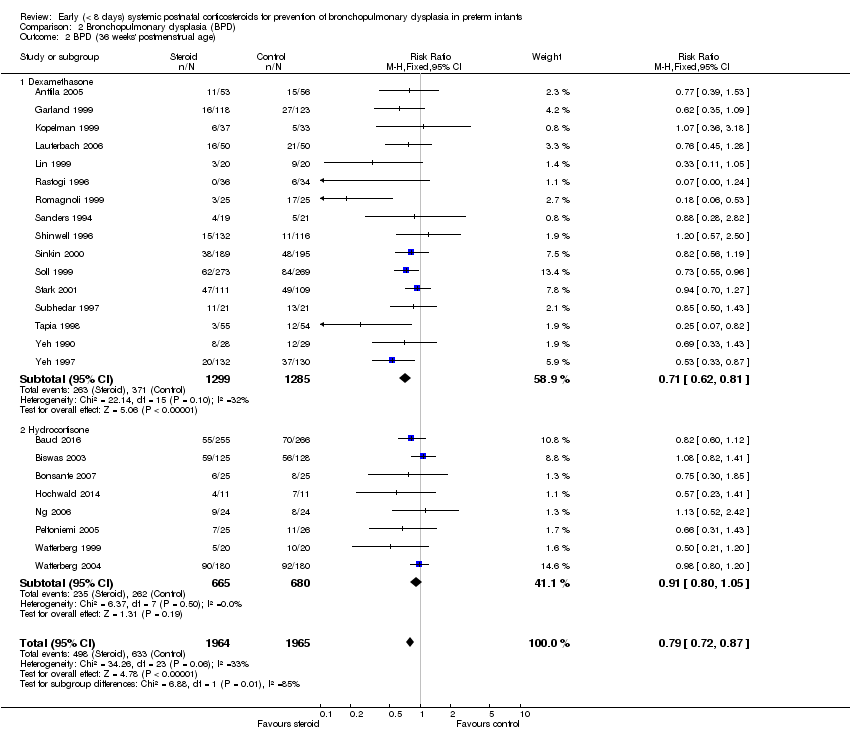 Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 2 BPD (36 weeks' postmenstrual age). | ||||
| 2.1 Dexamethasone | 16 | 2584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.62, 0.81] |
| 2.2 Hydrocortisone | 8 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.05] |
| 3 BPD at 36 weeks' postmenstrual age in survivors Show forest plot | 21 | 2970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.88] |
| Analysis 2.3 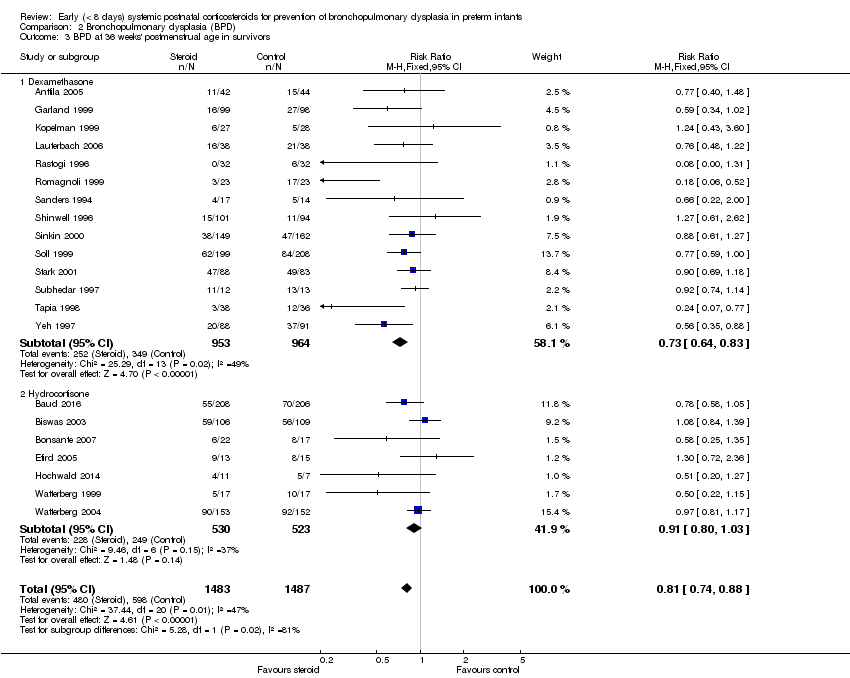 Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 3 BPD at 36 weeks' postmenstrual age in survivors. | ||||
| 3.1 Dexamethasone | 14 | 1917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 3.2 Hydrocortisone | 7 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4 Late rescue with corticosteroids Show forest plot | 14 | 2483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.82] |
| Analysis 2.4  Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 4 Late rescue with corticosteroids. | ||||
| 4.1 Dexamethasone | 10 | 1974 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| 4.2 Hydrocortisone | 4 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 5 Survivors who had late rescue with corticosteroids Show forest plot | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.89] |
| Analysis 2.5 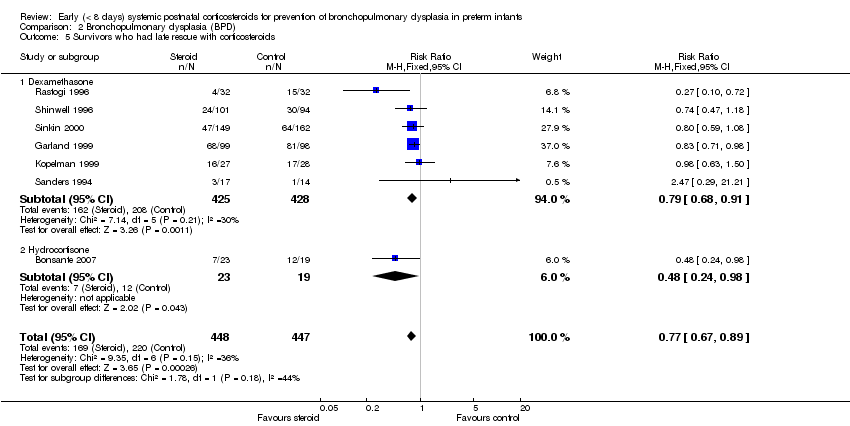 Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 5 Survivors who had late rescue with corticosteroids. | ||||
| 5.1 Dexamethasone | 6 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| 5.2 Hydrocortisone | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.98] |
| 6 Survivors discharged home on oxygen Show forest plot | 6 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.03] |
| Analysis 2.6  Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 6 Survivors discharged home on oxygen. | ||||
| 6.1 Dexamethasone | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.48, 1.26] |
| 6.2 Hydrocortisone | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 28 days of life Show forest plot | 15 | 2546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.88, 0.96] |
| Analysis 3.1  Comparison 3 Death or bronchopulmonary dysplasia (BPD), Outcome 1 Death or BPD at 28 days of life. | ||||
| 1.1 Dexamethasone | 14 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.96] |
| 1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 2 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 25 | 3960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.83, 0.93] |
| Analysis 3.2  Comparison 3 Death or bronchopulmonary dysplasia (BPD), Outcome 2 Death or BPD at 36 weeks' postmenstrual age. | ||||
| 2.1 Dexamethasone | 16 | 2581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.94] |
| 2.2 Hydrocortisone | 9 | 1379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to extubate by third day Show forest plot | 4 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.95] |
| Analysis 4.1  Comparison 4 Failure to extubate, Outcome 1 Failure to extubate by third day. | ||||
| 1.1 Dexamethasone | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.86] |
| 1.2 Hydrocortisone | 1 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 2 Failure to extubate by seventh day Show forest plot | 8 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.68, 0.85] |
| Analysis 4.2 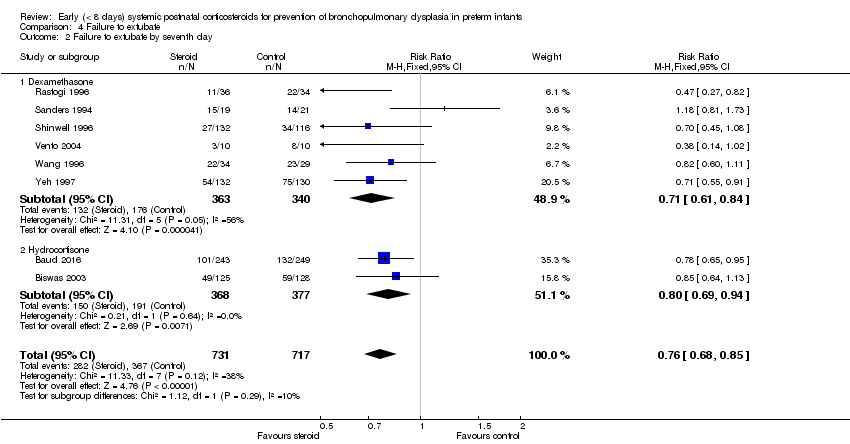 Comparison 4 Failure to extubate, Outcome 2 Failure to extubate by seventh day. | ||||
| 2.1 Dexamethasone | 6 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.61, 0.84] |
| 2.2 Hydrocortisone | 2 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.94] |
| 3 Failure to extubate by 14th day Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.97] |
| Analysis 4.3  Comparison 4 Failure to extubate, Outcome 3 Failure to extubate by 14th day. | ||||
| 4 Failure to extubate by 28th day Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.98] |
| Analysis 4.4 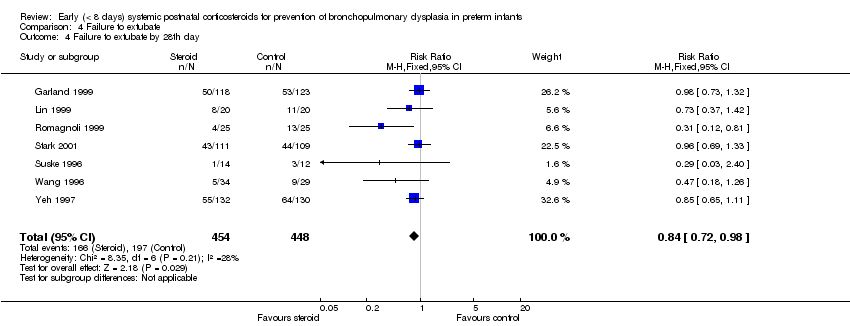 Comparison 4 Failure to extubate, Outcome 4 Failure to extubate by 28th day. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 25 | 4101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| Analysis 5.1  Comparison 5 Complications during primary hospitalisation, Outcome 1 Infection. | ||||
| 1.1 Dexamethasone | 18 | 2821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 1.2 Hydrocortisone | 7 | 1280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 2 Hyperglycaemia Show forest plot | 13 | 2167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.20, 1.47] |
| Analysis 5.2  Comparison 5 Complications during primary hospitalisation, Outcome 2 Hyperglycaemia. | ||||
| 2.1 Dexamethasone | 12 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.21, 1.49] |
| 2.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.67] |
| 3 Hypertension Show forest plot | 11 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.54, 2.22] |
| Analysis 5.3  Comparison 5 Complications during primary hospitalisation, Outcome 3 Hypertension. | ||||
| 3.1 Dexamethasone | 10 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.53, 2.21] |
| 3.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
| 4 Hypertrophic cardiomyopathy Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [1.40, 13.37] |
| Analysis 5.4 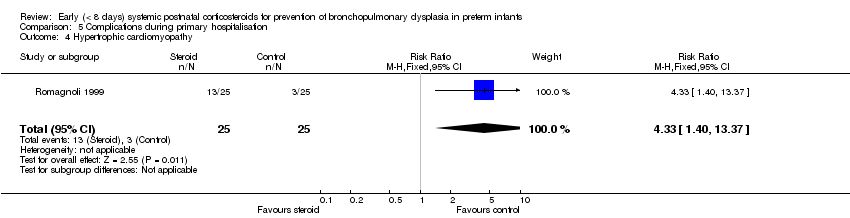 Comparison 5 Complications during primary hospitalisation, Outcome 4 Hypertrophic cardiomyopathy. | ||||
| 5 Growth failure Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.27, 19.62] |
| Analysis 5.5  Comparison 5 Complications during primary hospitalisation, Outcome 5 Growth failure. | ||||
| 6 Pulmonary air leak Show forest plot | 16 | 3225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| Analysis 5.6 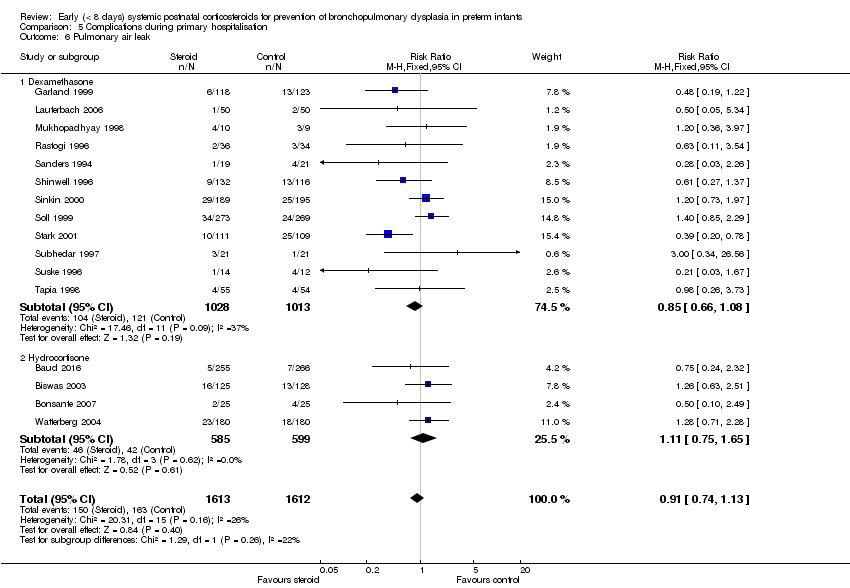 Comparison 5 Complications during primary hospitalisation, Outcome 6 Pulmonary air leak. | ||||
| 6.1 Dexamethasone | 12 | 2041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 6.2 Hydrocortisone | 4 | 1184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.65] |
| 7 Patent ductus arteriosus (PDA) Show forest plot | 24 | 4013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.72, 0.85] |
| Analysis 5.7 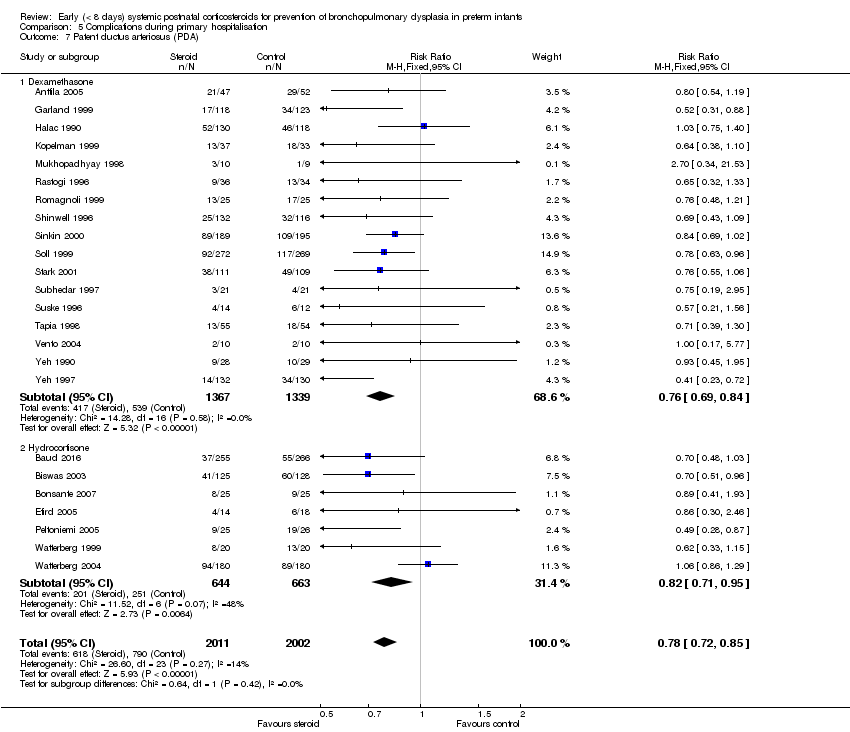 Comparison 5 Complications during primary hospitalisation, Outcome 7 Patent ductus arteriosus (PDA). | ||||
| 7.1 Dexamethasone | 17 | 2706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 7.2 Hydrocortisone | 7 | 1307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.95] |
| 8 Severe IVH Show forest plot | 26 | 4103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| Analysis 5.8  Comparison 5 Complications during primary hospitalisation, Outcome 8 Severe IVH. | ||||
| 8.1 Dexamethasone | 17 | 2736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 8.2 Hydrocortisone | 9 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 9 Severe intraventricular haemorrhage (IVH) in infants examined Show forest plot | 7 | 1909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| Analysis 5.9  Comparison 5 Complications during primary hospitalisation, Outcome 9 Severe intraventricular haemorrhage (IVH) in infants examined. | ||||
| 10 Periventricular leukomalacia (PVL) Show forest plot | 15 | 2807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.78, 1.46] |
| Analysis 5.10  Comparison 5 Complications during primary hospitalisation, Outcome 10 Periventricular leukomalacia (PVL). | ||||
| 10.1 Dexamethasone | 8 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.81] |
| 10.2 Hydrocortisone | 7 | 1293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.46, 1.40] |
| 11 PVL in infants with cranial ultrasound scans Show forest plot | 7 | 1841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.60] |
| Analysis 5.11  Comparison 5 Complications during primary hospitalisation, Outcome 11 PVL in infants with cranial ultrasound scans. | ||||
| 12 PVL in survivors seen at follow‐up Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.60, 2.48] |
| Analysis 5.12  Comparison 5 Complications during primary hospitalisation, Outcome 12 PVL in survivors seen at follow‐up. | ||||
| 13 Necrotising enterocolitis (NEC) Show forest plot | 25 | 4050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| Analysis 5.13  Comparison 5 Complications during primary hospitalisation, Outcome 13 Necrotising enterocolitis (NEC). | ||||
| 13.1 Dexamethasone | 15 | 2661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 13.2 Hydrocortisone | 10 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 14 Gastrointestinal bleeding Show forest plot | 12 | 1816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.35, 2.55] |
| Analysis 5.14 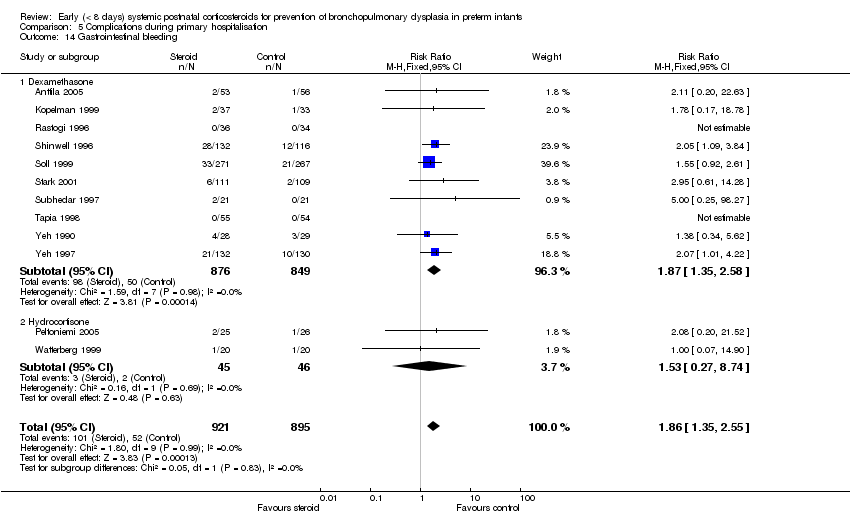 Comparison 5 Complications during primary hospitalisation, Outcome 14 Gastrointestinal bleeding. | ||||
| 14.1 Dexamethasone | 10 | 1725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.35, 2.58] |
| 14.2 Hydrocortisone | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.27, 8.74] |
| 15 Gastrointestinal perforation Show forest plot | 16 | 3040 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.02, 0.05] |
| Analysis 5.15  Comparison 5 Complications during primary hospitalisation, Outcome 15 Gastrointestinal perforation. | ||||
| 15.1 Dexamethasone | 9 | 1936 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 15.2 Hydrocortisone | 7 | 1104 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 16 Pulmonary haemorrhage Show forest plot | 10 | 1820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.54] |
| Analysis 5.16  Comparison 5 Complications during primary hospitalisation, Outcome 16 Pulmonary haemorrhage. | ||||
| 16.1 Dexamethasone | 7 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 16.2 Hydrocortisone | 3 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.92, 2.03] |
| 17 Any retinopathy of prematurity (ROP) Show forest plot | 9 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.97] |
| Analysis 5.17 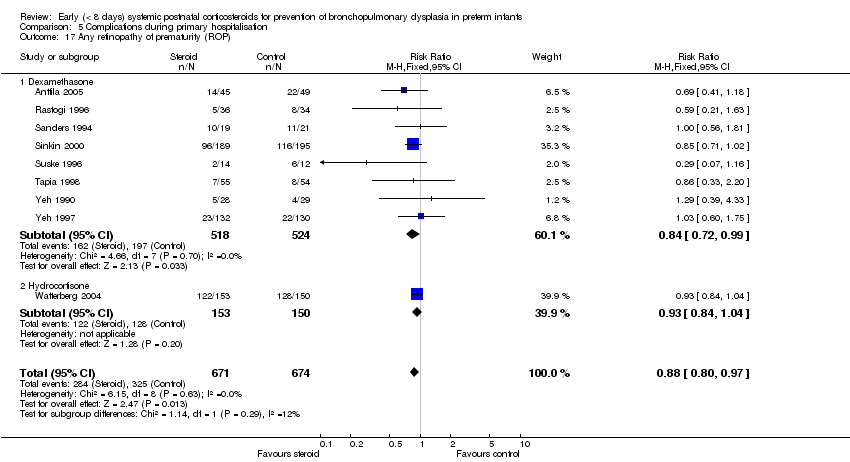 Comparison 5 Complications during primary hospitalisation, Outcome 17 Any retinopathy of prematurity (ROP). | ||||
| 17.1 Dexamethasone | 8 | 1042 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 17.2 Hydrocortisone | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 18 Severe ROP Show forest plot | 14 | 2577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.98] |
| Analysis 5.18  Comparison 5 Complications during primary hospitalisation, Outcome 18 Severe ROP. | ||||
| 18.1 Dexamethasone | 8 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 18.2 Hydrocortisone | 6 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 19 Severe ROP in survivors Show forest plot | 12 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.94] |
| Analysis 5.19  Comparison 5 Complications during primary hospitalisation, Outcome 19 Severe ROP in survivors. | ||||
| 19.1 Dexamethasone | 10 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 19.2 Hydrocortisone | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Bayley Mental Developmental Index (MDI) <‐2 SD Show forest plot | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.29] |
| Analysis 6.1 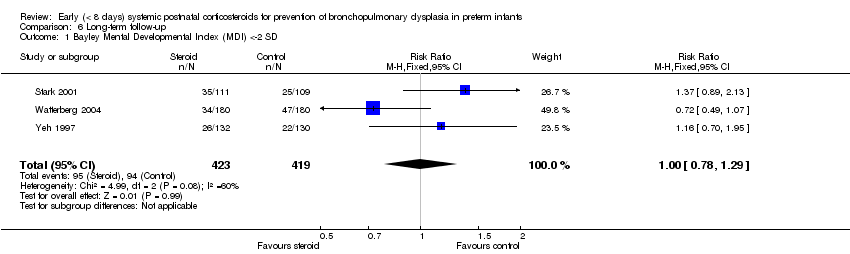 Comparison 6 Long‐term follow‐up, Outcome 1 Bayley Mental Developmental Index (MDI) <‐2 SD. | ||||
| 2 Bayley MDI <‐2 SD in tested survivors Show forest plot | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.25] |
| Analysis 6.2  Comparison 6 Long‐term follow‐up, Outcome 2 Bayley MDI <‐2 SD in tested survivors. | ||||
| 3 Bayley Psychomotor Developmental Index (PDI) <‐2 SD Show forest plot | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.85, 1.60] |
| Analysis 6.3  Comparison 6 Long‐term follow‐up, Outcome 3 Bayley Psychomotor Developmental Index (PDI) <‐2 SD. | ||||
| 4 Bayley PDI <‐2 SD in tested survivors Show forest plot | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| Analysis 6.4 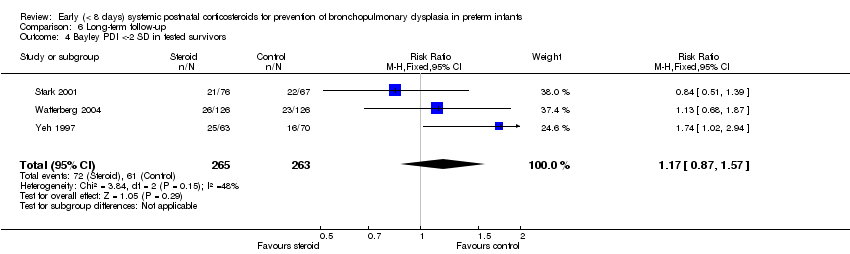 Comparison 6 Long‐term follow‐up, Outcome 4 Bayley PDI <‐2 SD in tested survivors. | ||||
| 5 Developmental delay (criteria not specified) Show forest plot | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.08, 2.61] |
| Analysis 6.5  Comparison 6 Long‐term follow‐up, Outcome 5 Developmental delay (criteria not specified). | ||||
| 6 Developmental delay (criteria not specified) in tested survivors Show forest plot | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.30, 2.88] |
| Analysis 6.6 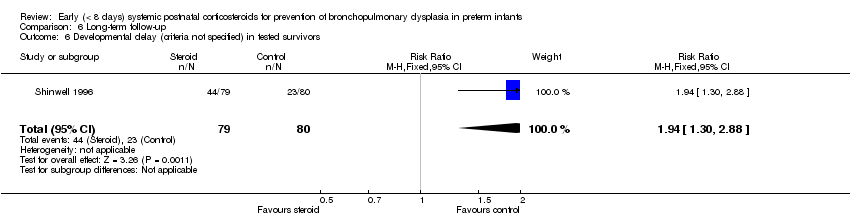 Comparison 6 Long‐term follow‐up, Outcome 6 Developmental delay (criteria not specified) in tested survivors. | ||||
| 7 Blindness Show forest plot | 8 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.74, 5.50] |
| Analysis 6.7 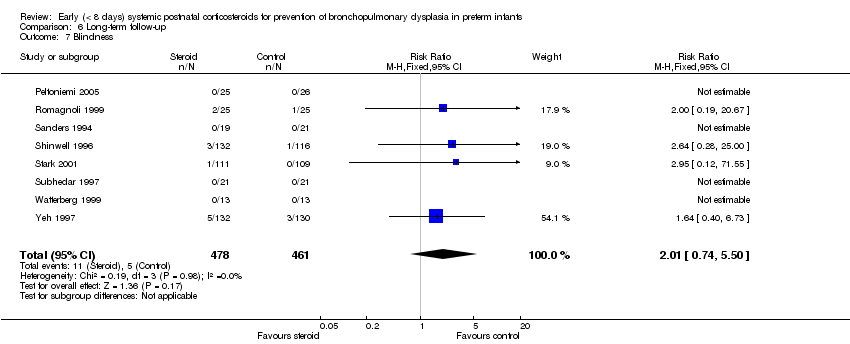 Comparison 6 Long‐term follow‐up, Outcome 7 Blindness. | ||||
| 8 Blindness in survivors assessed Show forest plot | 8 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.80, 5.86] |
| Analysis 6.8 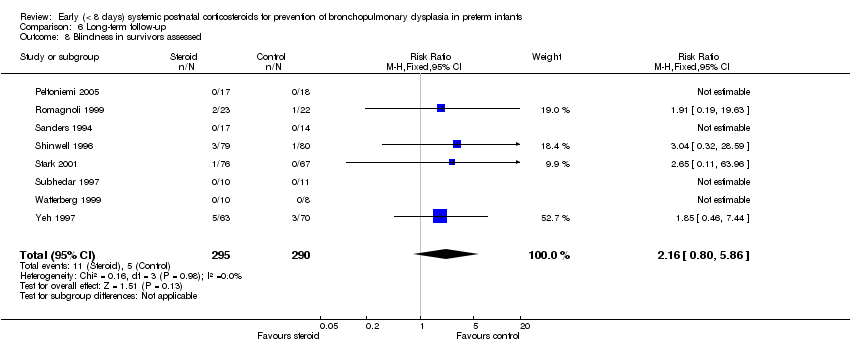 Comparison 6 Long‐term follow‐up, Outcome 8 Blindness in survivors assessed. | ||||
| 9 Deafness Show forest plot | 8 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.39, 3.37] |
| Analysis 6.9  Comparison 6 Long‐term follow‐up, Outcome 9 Deafness. | ||||
| 10 Deafness in survivors assessed Show forest plot | 8 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.29] |
| Analysis 6.10  Comparison 6 Long‐term follow‐up, Outcome 10 Deafness in survivors assessed. | ||||
| 11 Cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (IV, Fixed, 95% CI) | 1.42 [1.06, 1.91] |
| Analysis 6.11  Comparison 6 Long‐term follow‐up, Outcome 11 Cerebral palsy. | ||||
| 11.1 Dexamethasone | 7 | 921 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [1.20, 2.55] |
| 11.2 Hydrocortisone | 6 | 1052 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.66, 1.66] |
| 12 Death before follow‐up in trials assessing cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.05] |
| Analysis 6.12 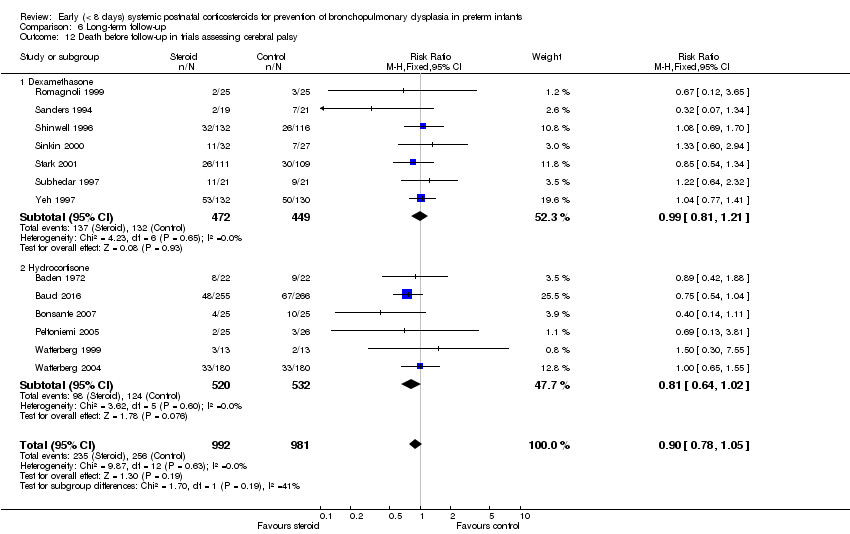 Comparison 6 Long‐term follow‐up, Outcome 12 Death before follow‐up in trials assessing cerebral palsy. | ||||
| 12.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 12.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 13 Death or cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.16] |
| Analysis 6.13 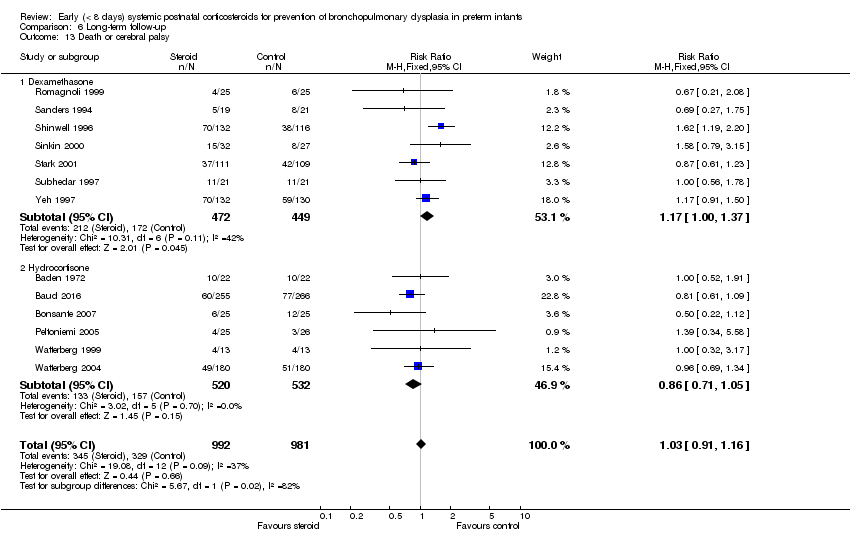 Comparison 6 Long‐term follow‐up, Outcome 13 Death or cerebral palsy. | ||||
| 13.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.37] |
| 13.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 14 Cerebral palsy in survivors assessed Show forest plot | 13 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.11, 1.90] |
| Analysis 6.14 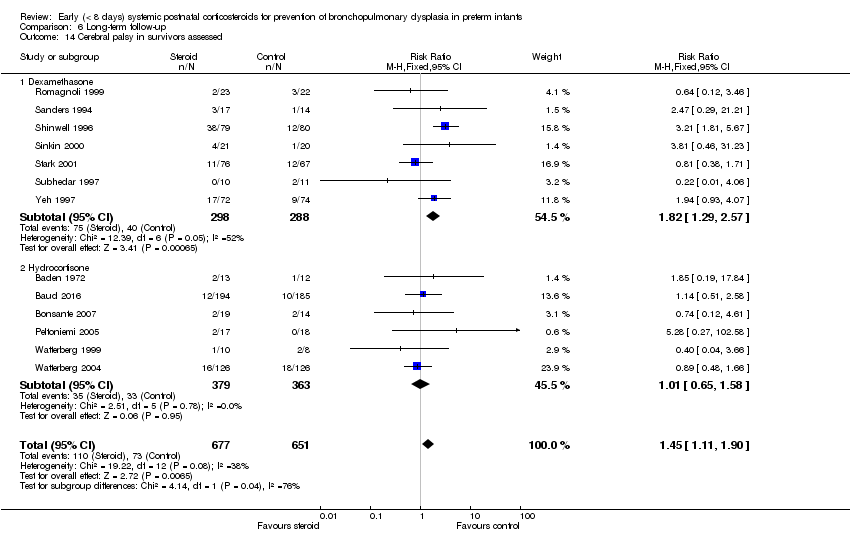 Comparison 6 Long‐term follow‐up, Outcome 14 Cerebral palsy in survivors assessed. | ||||
| 14.1 Dexamethasone | 7 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.29, 2.57] |
| 14.2 Hydrocortisone | 6 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 15 Major neurosensory disability (variable criteria ‐ see individual studies) Show forest plot | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.33] |
| Analysis 6.15  Comparison 6 Long‐term follow‐up, Outcome 15 Major neurosensory disability (variable criteria ‐ see individual studies). | ||||
| 15.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.03, 1.83] |
| 15.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.14] |
| 16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria) Show forest plot | 6 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.17] |
| Analysis 6.16 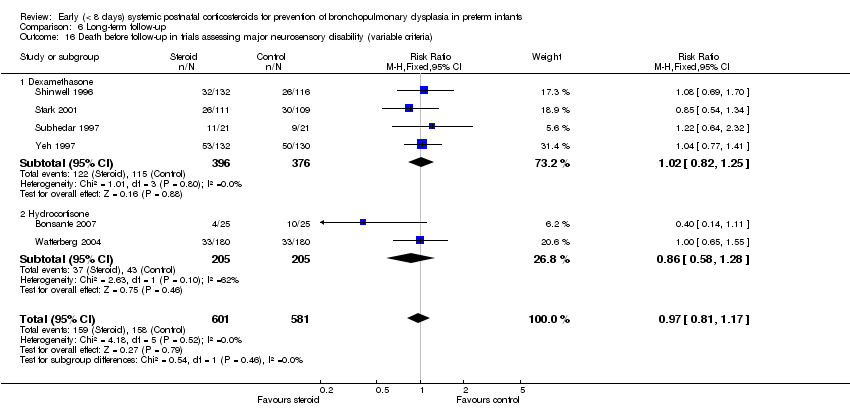 Comparison 6 Long‐term follow‐up, Outcome 16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria). | ||||
| 16.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.25] |
| 16.2 Hydrocortisone | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| 17 Death or major neurosensory disability (variable criteria) Show forest plot | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| Analysis 6.17  Comparison 6 Long‐term follow‐up, Outcome 17 Death or major neurosensory disability (variable criteria). | ||||
| 17.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.30] |
| 17.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 18 Major neurosensory disability in survivors examined (variable criteria) Show forest plot | 8 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| Analysis 6.18 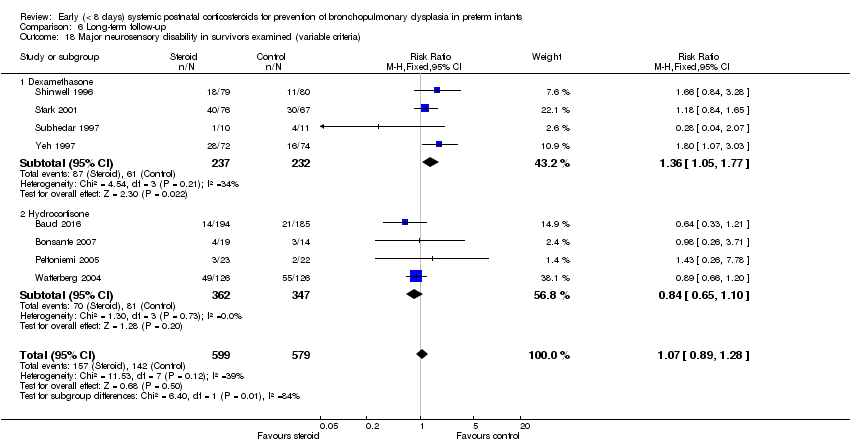 Comparison 6 Long‐term follow‐up, Outcome 18 Major neurosensory disability in survivors examined (variable criteria). | ||||
| 18.1 Dexamethasone | 4 | 469 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.77] |
| 18.2 Hydrocortisone | 4 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.10] |
| 19 Abnormal neurological exam (variable criteria ‐ see individual studies) Show forest plot | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.33, 2.47] |
| Analysis 6.19  Comparison 6 Long‐term follow‐up, Outcome 19 Abnormal neurological exam (variable criteria ‐ see individual studies). | ||||
| 20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria) Show forest plot | 6 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.07] |
| Analysis 6.20 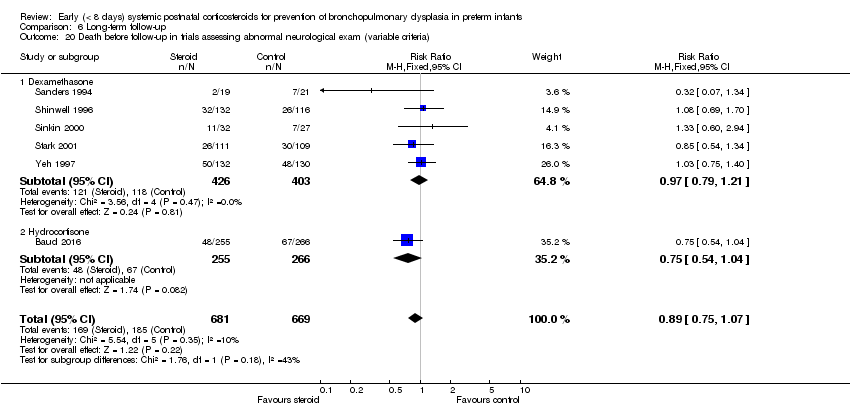 Comparison 6 Long‐term follow‐up, Outcome 20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria). | ||||
| 20.1 Dexamethasone | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.21] |
| 20.2 Hydrocortisone | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 21 Death or abnormal neurological exam (variable criteria) Show forest plot | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.06, 1.42] |
| Analysis 6.21 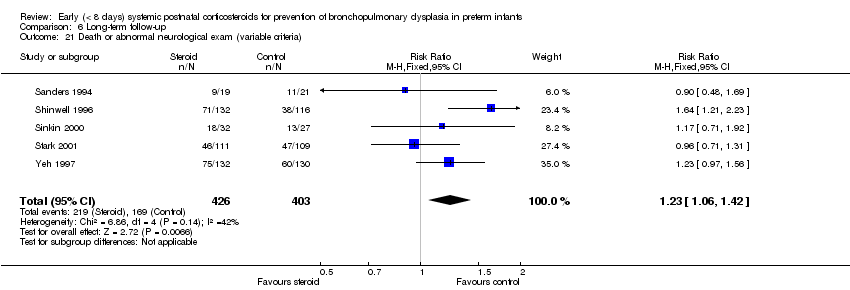 Comparison 6 Long‐term follow‐up, Outcome 21 Death or abnormal neurological exam (variable criteria). | ||||
| 22 Abnormal neurological exam in tested survivors (variable criteria) Show forest plot | 5 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.41, 2.52] |
| Analysis 6.22 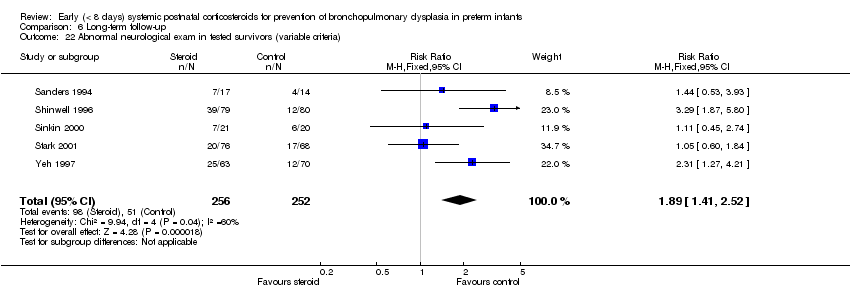 Comparison 6 Long‐term follow‐up, Outcome 22 Abnormal neurological exam in tested survivors (variable criteria). | ||||
| 23 Intellectual impairment (IQ < 70) Show forest plot | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.57, 3.31] |
| Analysis 6.23 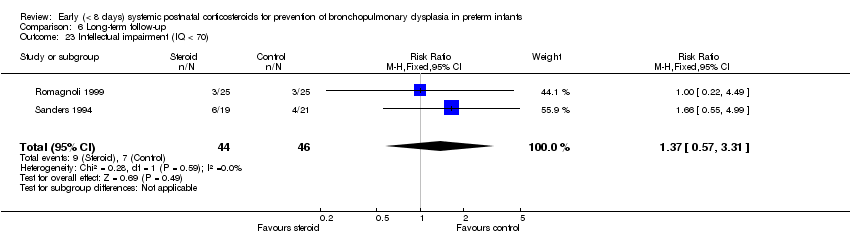 Comparison 6 Long‐term follow‐up, Outcome 23 Intellectual impairment (IQ < 70). | ||||
| 24 Intellectual impairment (IQ < 70) in survivors assessed Show forest plot | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.47, 2.65] |
| Analysis 6.24 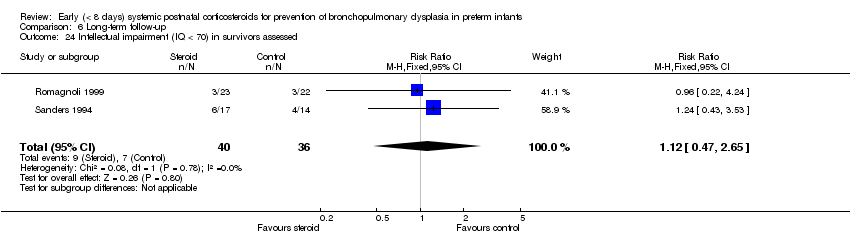 Comparison 6 Long‐term follow‐up, Outcome 24 Intellectual impairment (IQ < 70) in survivors assessed. | ||||
| 25 "Major neurosensory impairment" ‐ blindness or deafness Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| Analysis 6.25 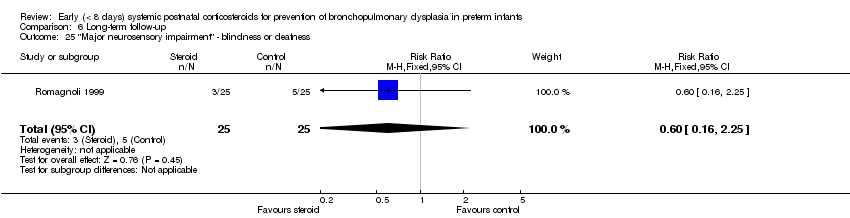 Comparison 6 Long‐term follow‐up, Outcome 25 "Major neurosensory impairment" ‐ blindness or deafness. | ||||
| 26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.12] |
| Analysis 6.26 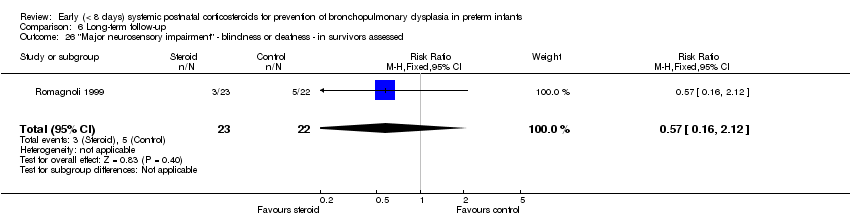 Comparison 6 Long‐term follow‐up, Outcome 26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed. | ||||
| 27 Behaviour abnormalities Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| Analysis 6.27  Comparison 6 Long‐term follow‐up, Outcome 27 Behaviour abnormalities. | ||||
| 28 Behaviour abnormalities in 3‐year‐old survivors assessed Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.22] |
| Analysis 6.28 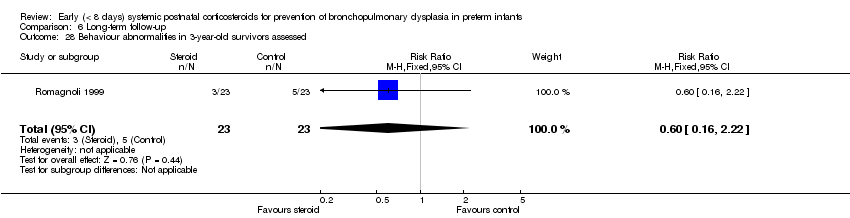 Comparison 6 Long‐term follow‐up, Outcome 28 Behaviour abnormalities in 3‐year‐old survivors assessed. | ||||
| 29 Abnormal EEG Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.66, 2.33] |
| Analysis 6.29  Comparison 6 Long‐term follow‐up, Outcome 29 Abnormal EEG. | ||||
| 30 Abnormal EEG in tested survivors Show forest plot | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.08] |
| Analysis 6.30 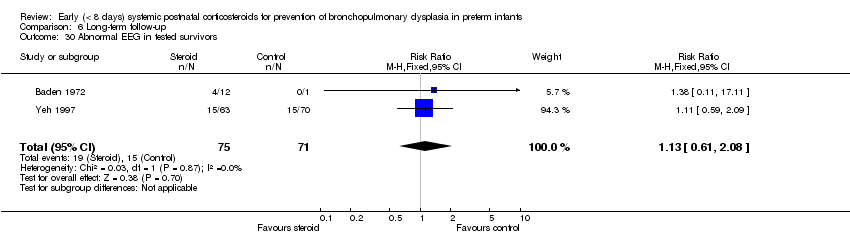 Comparison 6 Long‐term follow‐up, Outcome 30 Abnormal EEG in tested survivors. | ||||
| 31 Rehospitalisation in infancy Show forest plot | 3 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| Analysis 6.31  Comparison 6 Long‐term follow‐up, Outcome 31 Rehospitalisation in infancy. | ||||
| 32 Rehospitalisation in infancy in survivors Show forest plot | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| Analysis 6.32 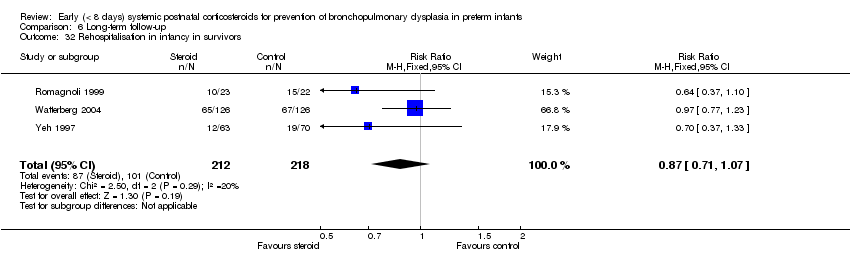 Comparison 6 Long‐term follow‐up, Outcome 32 Rehospitalisation in infancy in survivors. | ||||
Study flow diagram: review update.
Funnel plot of comparison: 1 Mortality, outcome: 1.4 Mortality at latest reported age.
Funnel plot of comparison: 2 Bronchopulmonary dysplasia (BPD), outcome: 2.2 BPD (36 weeks' postmenstrual age).
Funnel plot of comparison: 3 Death or bronchopulmonary dysplasia (BPD), outcome: 3.2 Death or BPD at 36 weeks' postmenstrual age.

Funnel plot of comparison: 6 Long‐term follow‐up, outcome: 6.11 Cerebral palsy.

Comparison 1 Mortality, Outcome 1 Neonatal mortality (up to 28 days).
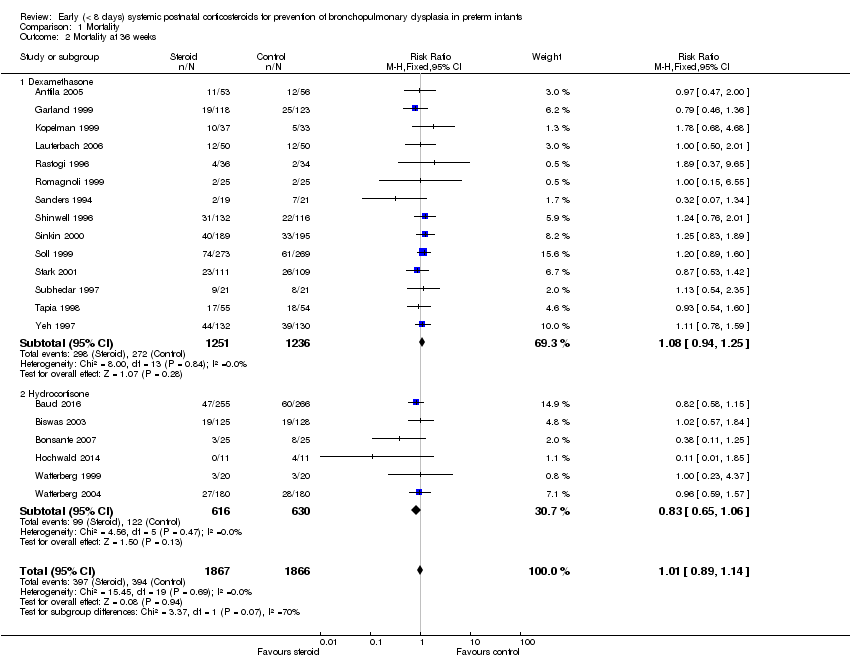
Comparison 1 Mortality, Outcome 2 Mortality at 36 weeks.
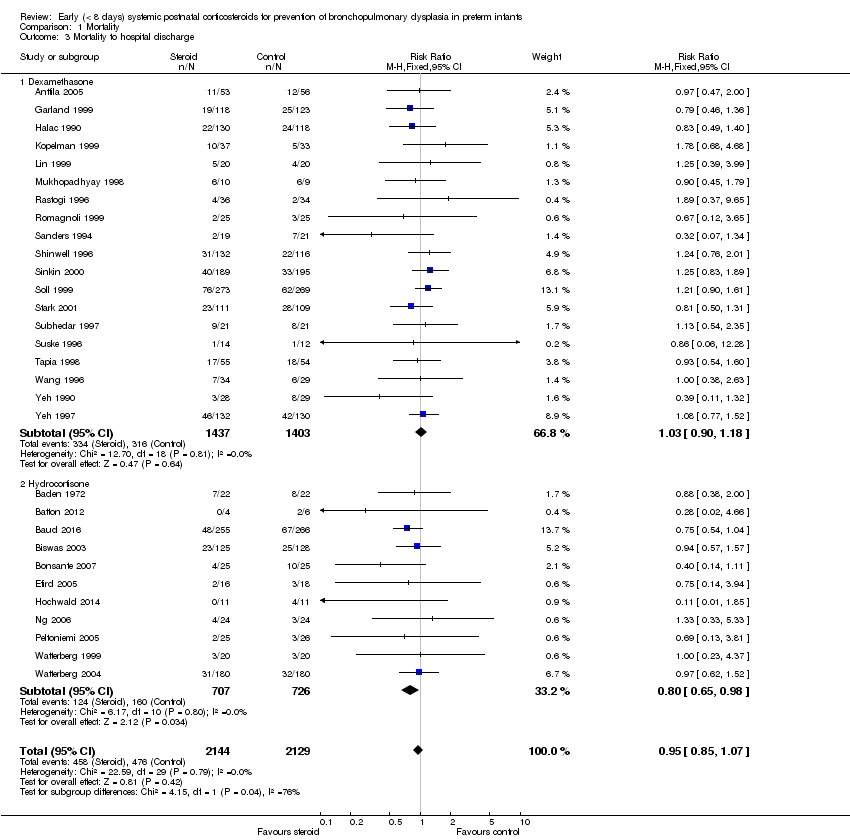
Comparison 1 Mortality, Outcome 3 Mortality to hospital discharge.
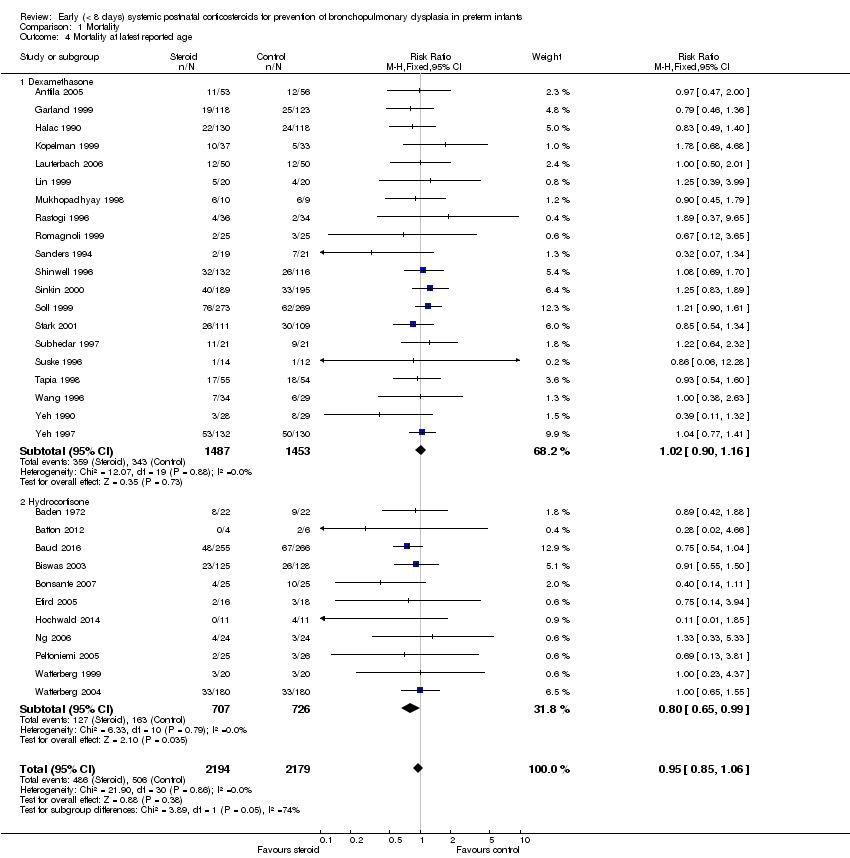
Comparison 1 Mortality, Outcome 4 Mortality at latest reported age.

Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 1 BPD (28 days of life).
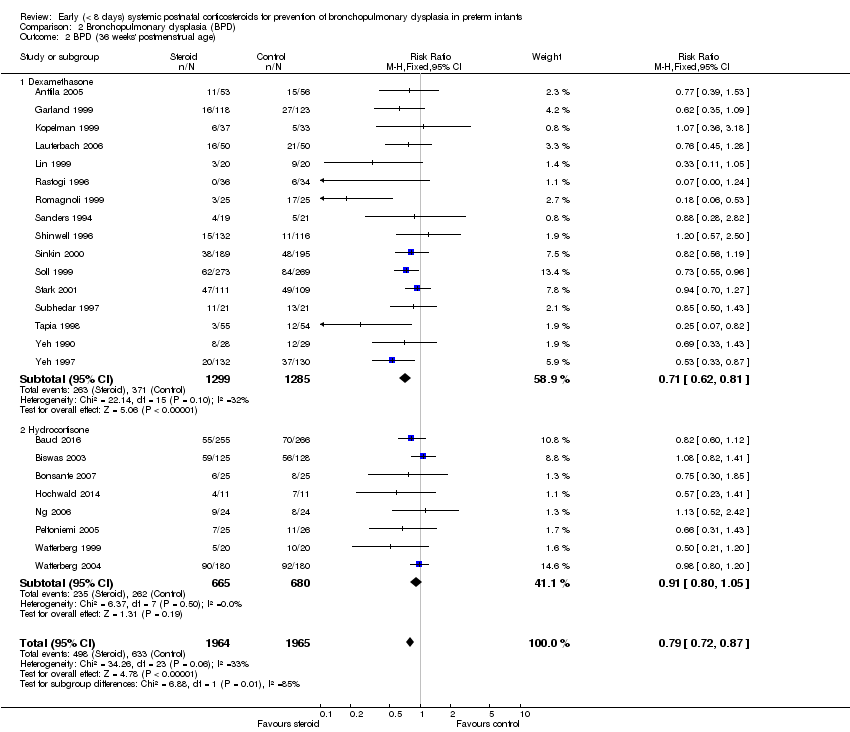
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 2 BPD (36 weeks' postmenstrual age).
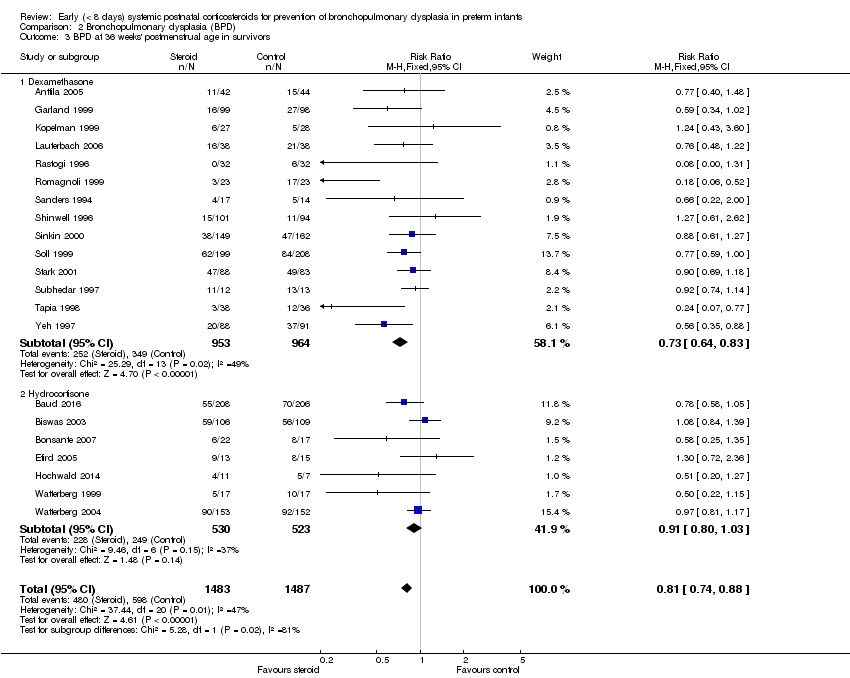
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 3 BPD at 36 weeks' postmenstrual age in survivors.
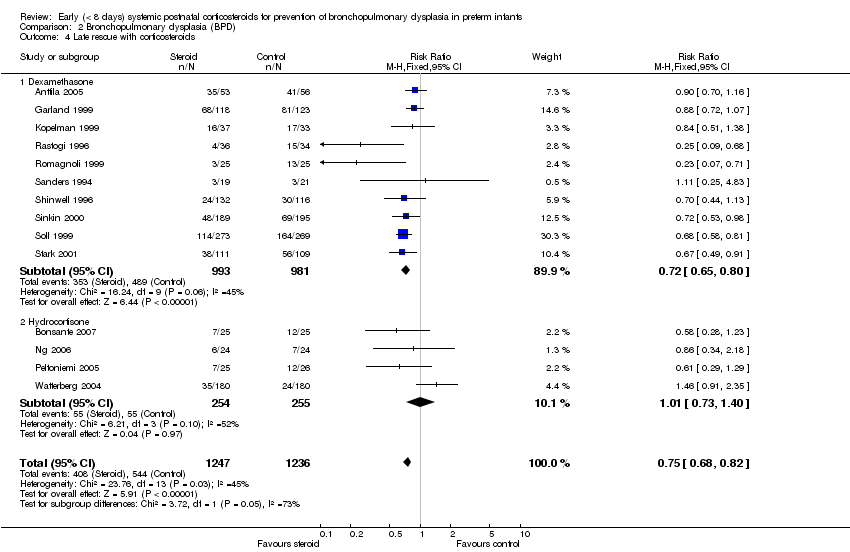
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 4 Late rescue with corticosteroids.
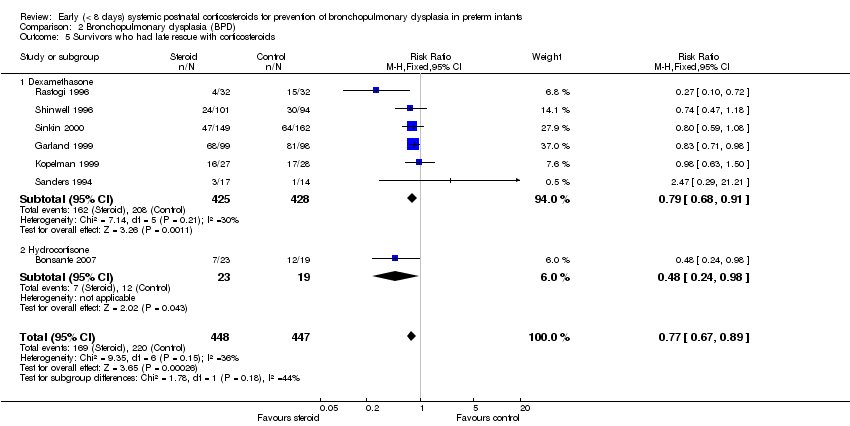
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 5 Survivors who had late rescue with corticosteroids.
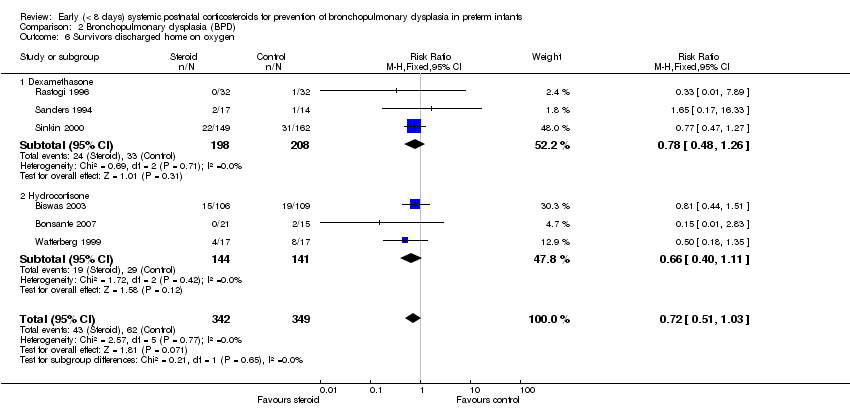
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 6 Survivors discharged home on oxygen.

Comparison 3 Death or bronchopulmonary dysplasia (BPD), Outcome 1 Death or BPD at 28 days of life.
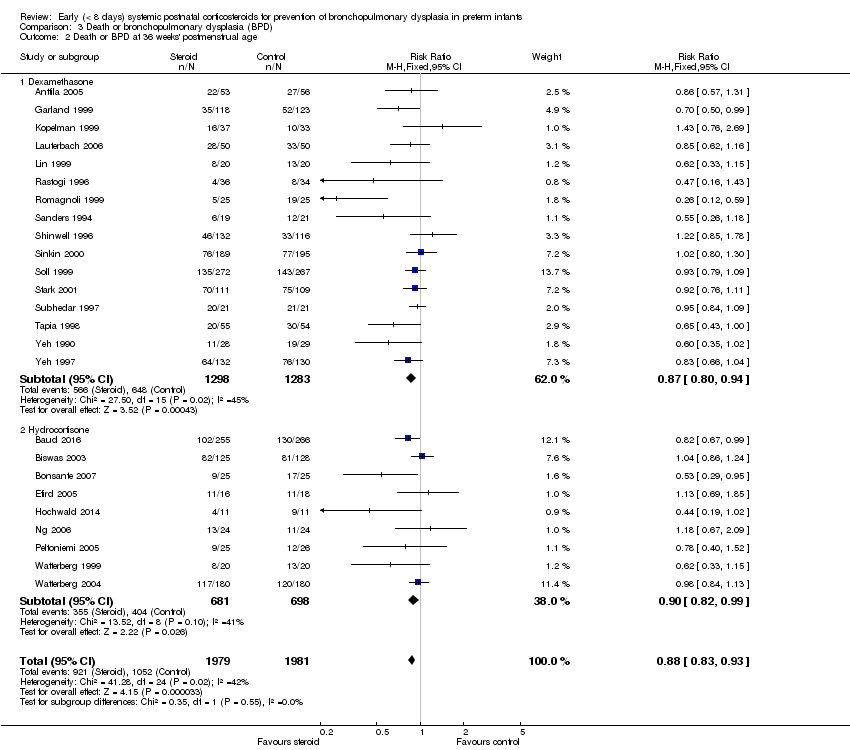
Comparison 3 Death or bronchopulmonary dysplasia (BPD), Outcome 2 Death or BPD at 36 weeks' postmenstrual age.
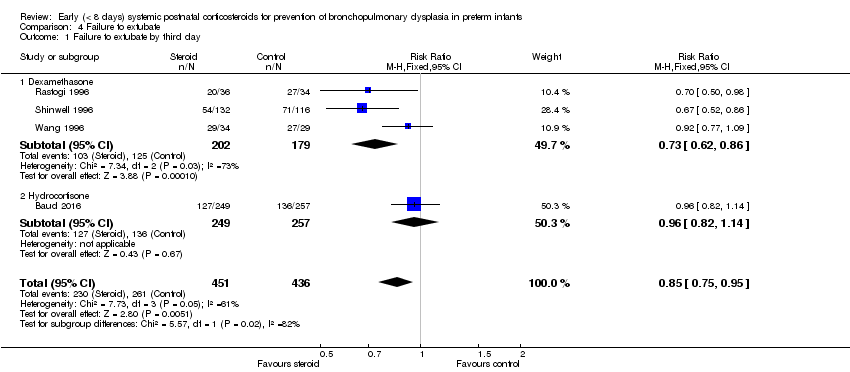
Comparison 4 Failure to extubate, Outcome 1 Failure to extubate by third day.

Comparison 4 Failure to extubate, Outcome 2 Failure to extubate by seventh day.
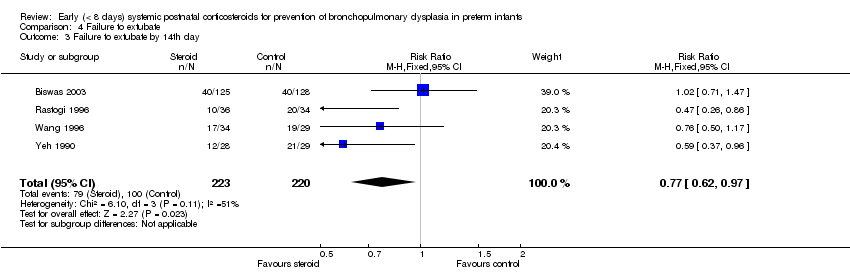
Comparison 4 Failure to extubate, Outcome 3 Failure to extubate by 14th day.

Comparison 4 Failure to extubate, Outcome 4 Failure to extubate by 28th day.

Comparison 5 Complications during primary hospitalisation, Outcome 1 Infection.

Comparison 5 Complications during primary hospitalisation, Outcome 2 Hyperglycaemia.
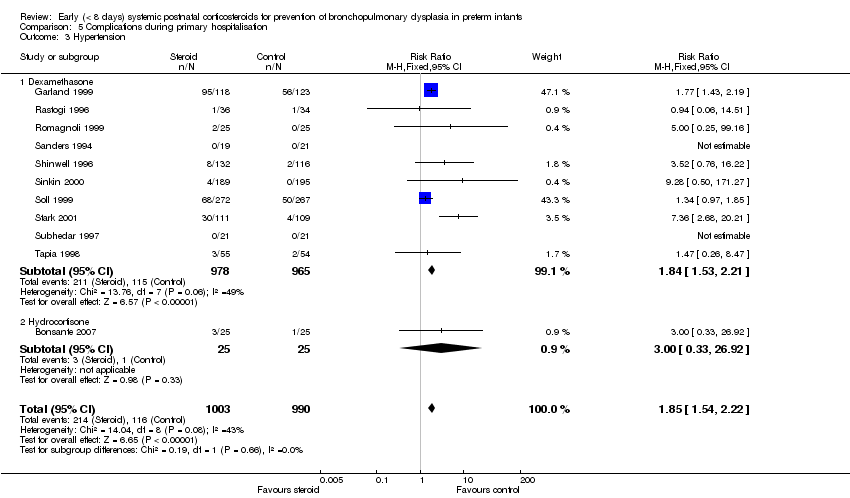
Comparison 5 Complications during primary hospitalisation, Outcome 3 Hypertension.

Comparison 5 Complications during primary hospitalisation, Outcome 4 Hypertrophic cardiomyopathy.

Comparison 5 Complications during primary hospitalisation, Outcome 5 Growth failure.

Comparison 5 Complications during primary hospitalisation, Outcome 6 Pulmonary air leak.

Comparison 5 Complications during primary hospitalisation, Outcome 7 Patent ductus arteriosus (PDA).

Comparison 5 Complications during primary hospitalisation, Outcome 8 Severe IVH.
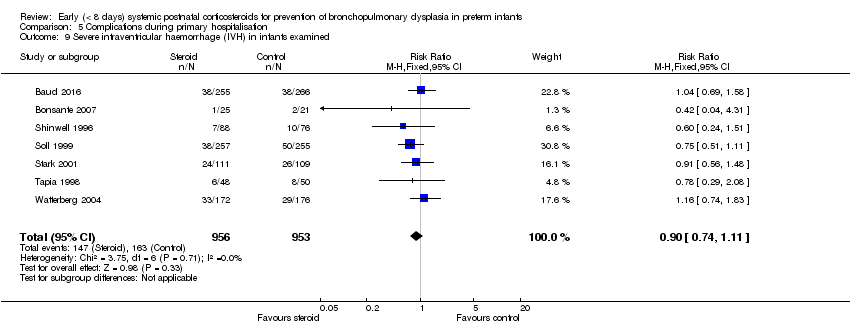
Comparison 5 Complications during primary hospitalisation, Outcome 9 Severe intraventricular haemorrhage (IVH) in infants examined.

Comparison 5 Complications during primary hospitalisation, Outcome 10 Periventricular leukomalacia (PVL).

Comparison 5 Complications during primary hospitalisation, Outcome 11 PVL in infants with cranial ultrasound scans.

Comparison 5 Complications during primary hospitalisation, Outcome 12 PVL in survivors seen at follow‐up.
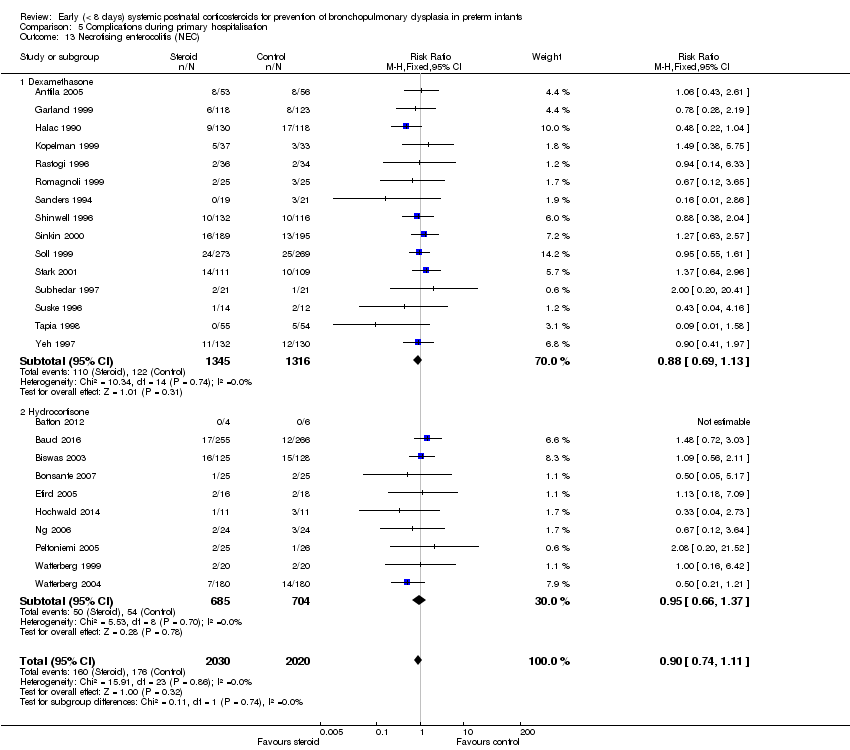
Comparison 5 Complications during primary hospitalisation, Outcome 13 Necrotising enterocolitis (NEC).

Comparison 5 Complications during primary hospitalisation, Outcome 14 Gastrointestinal bleeding.

Comparison 5 Complications during primary hospitalisation, Outcome 15 Gastrointestinal perforation.
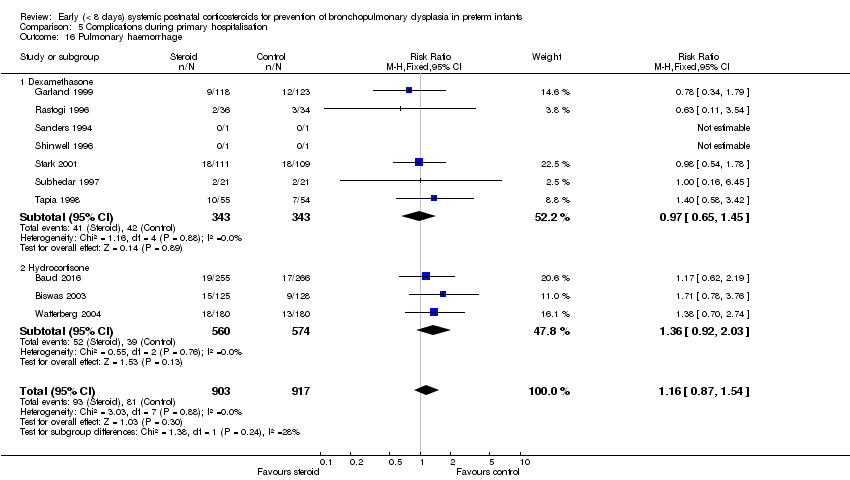
Comparison 5 Complications during primary hospitalisation, Outcome 16 Pulmonary haemorrhage.
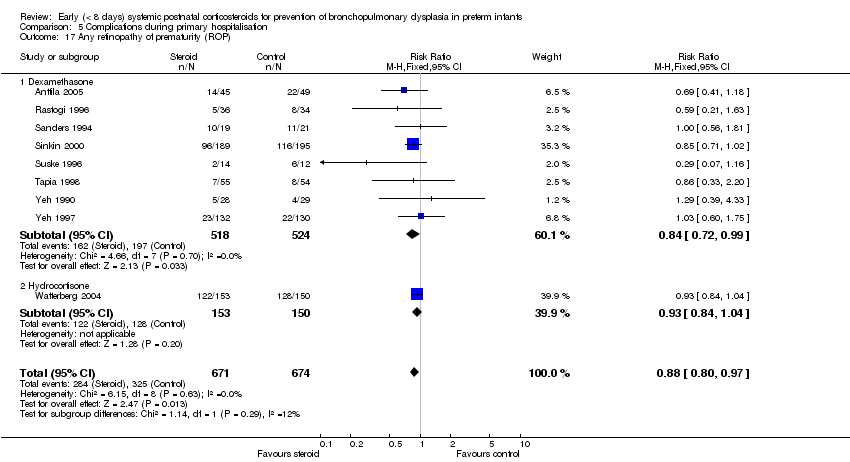
Comparison 5 Complications during primary hospitalisation, Outcome 17 Any retinopathy of prematurity (ROP).
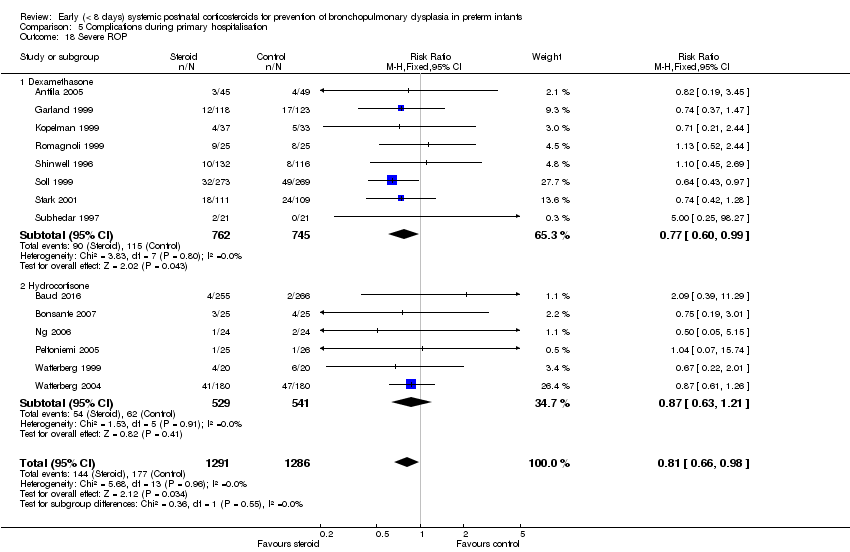
Comparison 5 Complications during primary hospitalisation, Outcome 18 Severe ROP.

Comparison 5 Complications during primary hospitalisation, Outcome 19 Severe ROP in survivors.

Comparison 6 Long‐term follow‐up, Outcome 1 Bayley Mental Developmental Index (MDI) <‐2 SD.
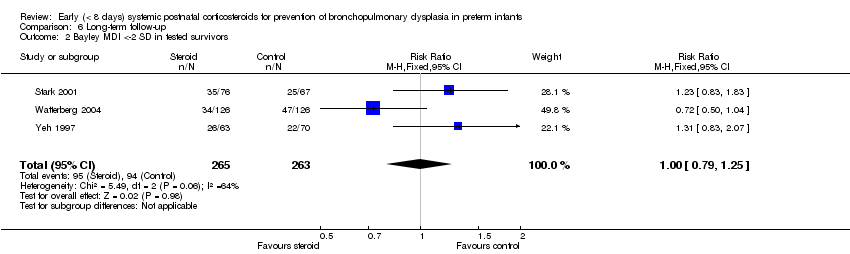
Comparison 6 Long‐term follow‐up, Outcome 2 Bayley MDI <‐2 SD in tested survivors.
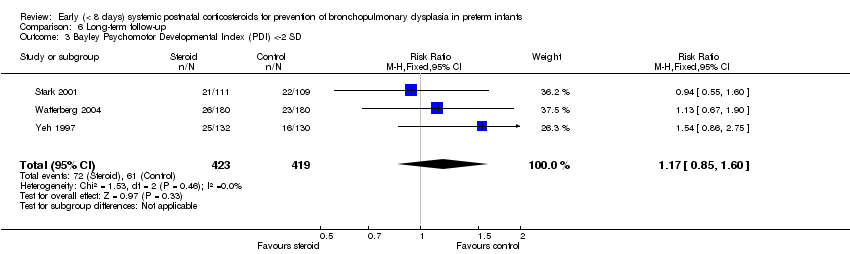
Comparison 6 Long‐term follow‐up, Outcome 3 Bayley Psychomotor Developmental Index (PDI) <‐2 SD.

Comparison 6 Long‐term follow‐up, Outcome 4 Bayley PDI <‐2 SD in tested survivors.

Comparison 6 Long‐term follow‐up, Outcome 5 Developmental delay (criteria not specified).
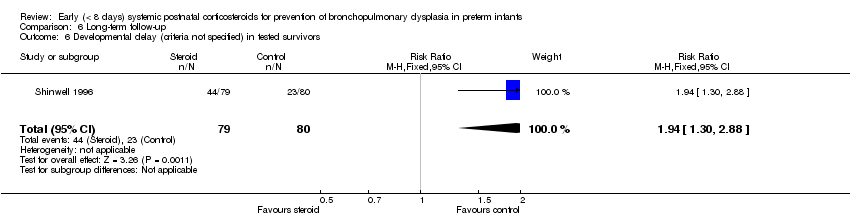
Comparison 6 Long‐term follow‐up, Outcome 6 Developmental delay (criteria not specified) in tested survivors.

Comparison 6 Long‐term follow‐up, Outcome 7 Blindness.

Comparison 6 Long‐term follow‐up, Outcome 8 Blindness in survivors assessed.
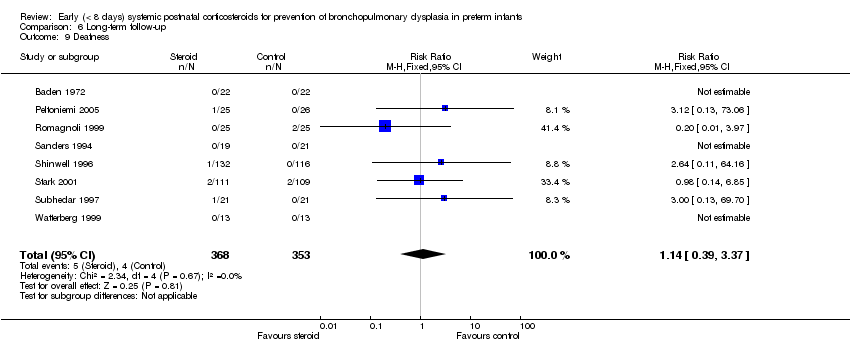
Comparison 6 Long‐term follow‐up, Outcome 9 Deafness.

Comparison 6 Long‐term follow‐up, Outcome 10 Deafness in survivors assessed.
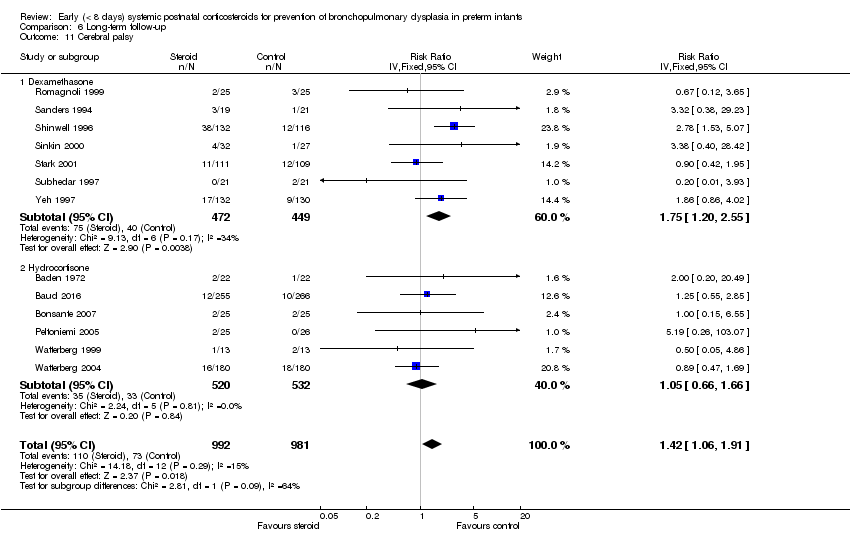
Comparison 6 Long‐term follow‐up, Outcome 11 Cerebral palsy.

Comparison 6 Long‐term follow‐up, Outcome 12 Death before follow‐up in trials assessing cerebral palsy.
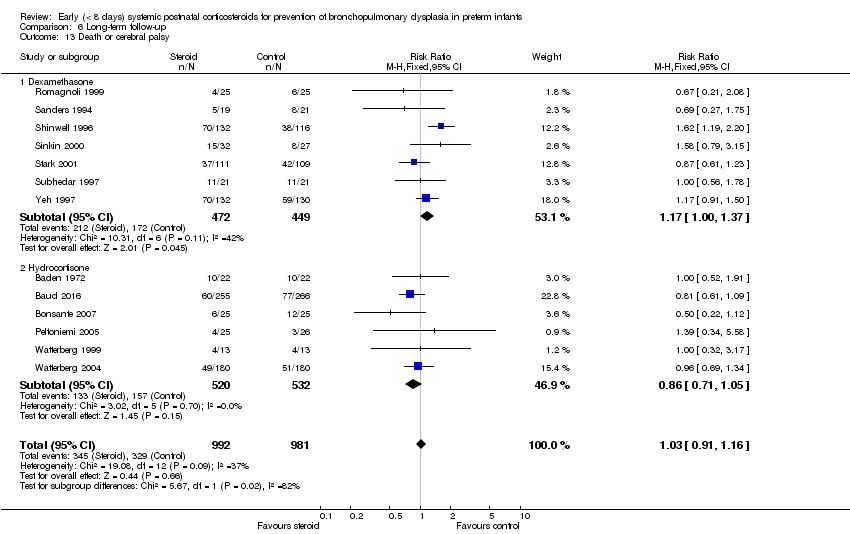
Comparison 6 Long‐term follow‐up, Outcome 13 Death or cerebral palsy.

Comparison 6 Long‐term follow‐up, Outcome 14 Cerebral palsy in survivors assessed.
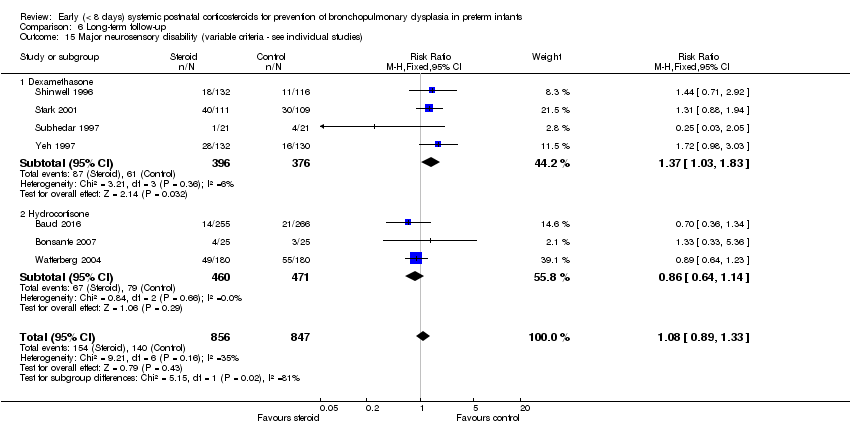
Comparison 6 Long‐term follow‐up, Outcome 15 Major neurosensory disability (variable criteria ‐ see individual studies).

Comparison 6 Long‐term follow‐up, Outcome 16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria).
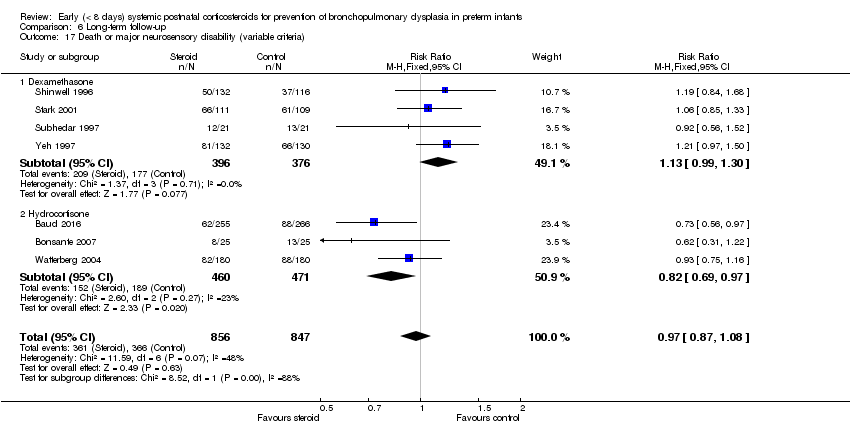
Comparison 6 Long‐term follow‐up, Outcome 17 Death or major neurosensory disability (variable criteria).
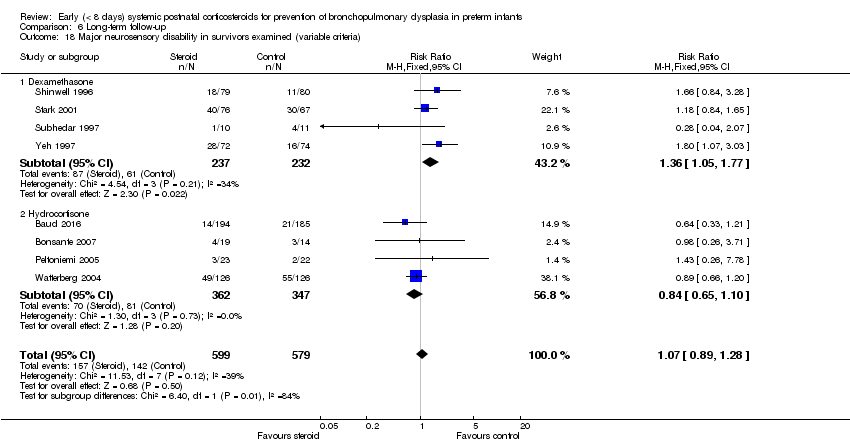
Comparison 6 Long‐term follow‐up, Outcome 18 Major neurosensory disability in survivors examined (variable criteria).
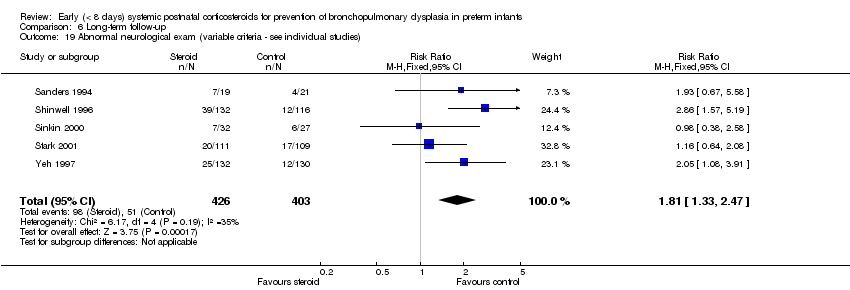
Comparison 6 Long‐term follow‐up, Outcome 19 Abnormal neurological exam (variable criteria ‐ see individual studies).
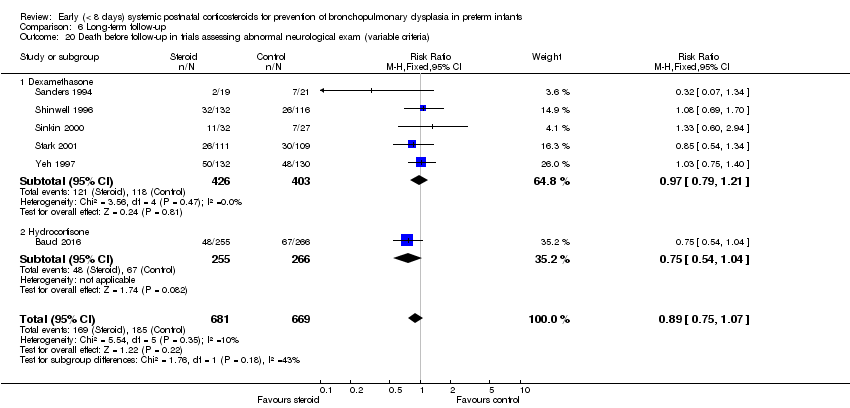
Comparison 6 Long‐term follow‐up, Outcome 20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria).
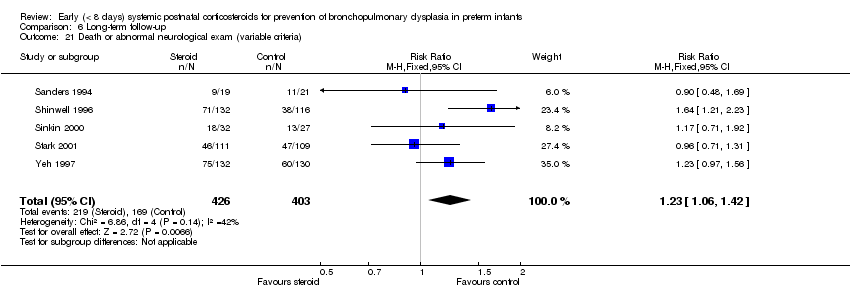
Comparison 6 Long‐term follow‐up, Outcome 21 Death or abnormal neurological exam (variable criteria).

Comparison 6 Long‐term follow‐up, Outcome 22 Abnormal neurological exam in tested survivors (variable criteria).

Comparison 6 Long‐term follow‐up, Outcome 23 Intellectual impairment (IQ < 70).

Comparison 6 Long‐term follow‐up, Outcome 24 Intellectual impairment (IQ < 70) in survivors assessed.

Comparison 6 Long‐term follow‐up, Outcome 25 "Major neurosensory impairment" ‐ blindness or deafness.

Comparison 6 Long‐term follow‐up, Outcome 26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed.
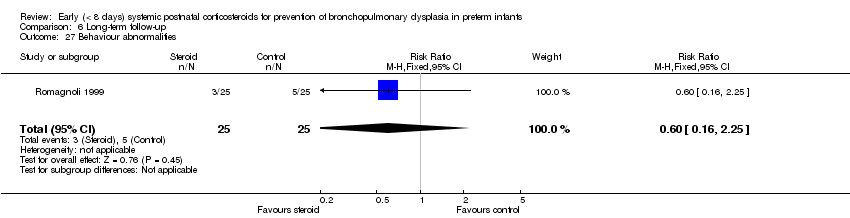
Comparison 6 Long‐term follow‐up, Outcome 27 Behaviour abnormalities.

Comparison 6 Long‐term follow‐up, Outcome 28 Behaviour abnormalities in 3‐year‐old survivors assessed.

Comparison 6 Long‐term follow‐up, Outcome 29 Abnormal EEG.

Comparison 6 Long‐term follow‐up, Outcome 30 Abnormal EEG in tested survivors.
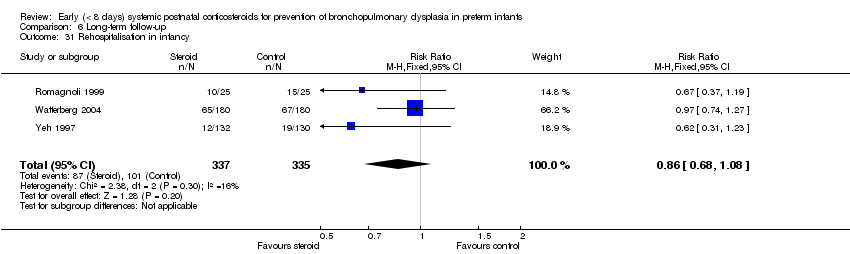
Comparison 6 Long‐term follow‐up, Outcome 31 Rehospitalisation in infancy.

Comparison 6 Long‐term follow‐up, Outcome 32 Rehospitalisation in infancy in survivors.
| Early systemic postnatal corticosteroids compared with placebo or no treatment for preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with early systemic postnatal corticosteroids | |||||
| Mortality at 36 weeks | Study population | RR 1.01 | 3733 | ⊕⊕⊕⊕ | ||
| 211 per 1000 | 213 per 1000 | |||||
| Mortality at latest reported age | Study population | RR 0.95 | 4373 | ⊕⊕⊕⊝ | ||
| 232 per 1000 | 221 per 1000 | |||||
| BPD (36 weeks) | Study population | RR 0.79 | 3929 | ⊕⊕⊕⊝ | ||
| 322 per 1000 | 254 per 1000 | |||||
| Death or BPD at 36 weeks | Study population | RR 0.88 | 3960 | ⊕⊕⊕⊝ | ||
| 531 per 1000 | 467 per 1000 | |||||
| Gastrointestinal perforation | Study population | RR 1.72 | 3040 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 74 per 1000 | |||||
| Cerebral palsy | Study population | RR 1.42 | 1973 | ⊕⊕⊕⊕ | ||
| 74 per 1000 | 106 per 1000 | |||||
| Death or cerebral palsy | Study population | RR 1.03 | 1973 | ⊕⊕⊕⊝ | ||
| 335 per 1000 | 345 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level for serious study limitations owing to weak evidence for publication bias, particularly for studies of hydrocortisone. bDowngraded one level for serious study limitations owing to weak evidence for publication bias, for both dexamethasone and hydrocortisone studies. cDowngraded one level for serious study limitations owing to moderate heterogeneity, particularly for studies of dexamethasone. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality (up to 28 days) Show forest plot | 19 | 2950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.19] |
| 1.1 Dexamethasone | 16 | 2603 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.24] |
| 1.2 Hydrocortisone | 3 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 2 Mortality at 36 weeks Show forest plot | 20 | 3733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.14] |
| 2.1 Dexamethasone | 14 | 2487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 2.2 Hydrocortisone | 6 | 1246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.06] |
| 3 Mortality to hospital discharge Show forest plot | 30 | 4273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 3.1 Dexamethasone | 19 | 2840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 3.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 4 Mortality at latest reported age Show forest plot | 31 | 4373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| 4.1 Dexamethasone | 20 | 2940 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.16] |
| 4.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD (28 days of life) Show forest plot | 17 | 2874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| 1.1 Dexamethasone | 16 | 2621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.79, 0.92] |
| 1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 2 BPD (36 weeks' postmenstrual age) Show forest plot | 24 | 3929 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.87] |
| 2.1 Dexamethasone | 16 | 2584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.62, 0.81] |
| 2.2 Hydrocortisone | 8 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.05] |
| 3 BPD at 36 weeks' postmenstrual age in survivors Show forest plot | 21 | 2970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.88] |
| 3.1 Dexamethasone | 14 | 1917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 3.2 Hydrocortisone | 7 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4 Late rescue with corticosteroids Show forest plot | 14 | 2483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.82] |
| 4.1 Dexamethasone | 10 | 1974 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| 4.2 Hydrocortisone | 4 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 5 Survivors who had late rescue with corticosteroids Show forest plot | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.89] |
| 5.1 Dexamethasone | 6 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| 5.2 Hydrocortisone | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.98] |
| 6 Survivors discharged home on oxygen Show forest plot | 6 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.03] |
| 6.1 Dexamethasone | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.48, 1.26] |
| 6.2 Hydrocortisone | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 28 days of life Show forest plot | 15 | 2546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.88, 0.96] |
| 1.1 Dexamethasone | 14 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.96] |
| 1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 2 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 25 | 3960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.83, 0.93] |
| 2.1 Dexamethasone | 16 | 2581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.94] |
| 2.2 Hydrocortisone | 9 | 1379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to extubate by third day Show forest plot | 4 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.95] |
| 1.1 Dexamethasone | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.86] |
| 1.2 Hydrocortisone | 1 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 2 Failure to extubate by seventh day Show forest plot | 8 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.68, 0.85] |
| 2.1 Dexamethasone | 6 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.61, 0.84] |
| 2.2 Hydrocortisone | 2 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.94] |
| 3 Failure to extubate by 14th day Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.97] |
| 4 Failure to extubate by 28th day Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 25 | 4101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 1.1 Dexamethasone | 18 | 2821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 1.2 Hydrocortisone | 7 | 1280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 2 Hyperglycaemia Show forest plot | 13 | 2167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.20, 1.47] |
| 2.1 Dexamethasone | 12 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.21, 1.49] |
| 2.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.67] |
| 3 Hypertension Show forest plot | 11 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.54, 2.22] |
| 3.1 Dexamethasone | 10 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.53, 2.21] |
| 3.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
| 4 Hypertrophic cardiomyopathy Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [1.40, 13.37] |
| 5 Growth failure Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.27, 19.62] |
| 6 Pulmonary air leak Show forest plot | 16 | 3225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 6.1 Dexamethasone | 12 | 2041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 6.2 Hydrocortisone | 4 | 1184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.65] |
| 7 Patent ductus arteriosus (PDA) Show forest plot | 24 | 4013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.72, 0.85] |
| 7.1 Dexamethasone | 17 | 2706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 7.2 Hydrocortisone | 7 | 1307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.95] |
| 8 Severe IVH Show forest plot | 26 | 4103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 8.1 Dexamethasone | 17 | 2736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 8.2 Hydrocortisone | 9 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 9 Severe intraventricular haemorrhage (IVH) in infants examined Show forest plot | 7 | 1909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 10 Periventricular leukomalacia (PVL) Show forest plot | 15 | 2807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.78, 1.46] |
| 10.1 Dexamethasone | 8 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.81] |
| 10.2 Hydrocortisone | 7 | 1293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.46, 1.40] |
| 11 PVL in infants with cranial ultrasound scans Show forest plot | 7 | 1841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.60] |
| 12 PVL in survivors seen at follow‐up Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.60, 2.48] |
| 13 Necrotising enterocolitis (NEC) Show forest plot | 25 | 4050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 13.1 Dexamethasone | 15 | 2661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 13.2 Hydrocortisone | 10 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 14 Gastrointestinal bleeding Show forest plot | 12 | 1816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.35, 2.55] |
| 14.1 Dexamethasone | 10 | 1725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.35, 2.58] |
| 14.2 Hydrocortisone | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.27, 8.74] |
| 15 Gastrointestinal perforation Show forest plot | 16 | 3040 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.02, 0.05] |
| 15.1 Dexamethasone | 9 | 1936 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 15.2 Hydrocortisone | 7 | 1104 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 16 Pulmonary haemorrhage Show forest plot | 10 | 1820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.54] |
| 16.1 Dexamethasone | 7 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 16.2 Hydrocortisone | 3 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.92, 2.03] |
| 17 Any retinopathy of prematurity (ROP) Show forest plot | 9 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.97] |
| 17.1 Dexamethasone | 8 | 1042 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 17.2 Hydrocortisone | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 18 Severe ROP Show forest plot | 14 | 2577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.98] |
| 18.1 Dexamethasone | 8 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 18.2 Hydrocortisone | 6 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 19 Severe ROP in survivors Show forest plot | 12 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.94] |
| 19.1 Dexamethasone | 10 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 19.2 Hydrocortisone | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Bayley Mental Developmental Index (MDI) <‐2 SD Show forest plot | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.29] |
| 2 Bayley MDI <‐2 SD in tested survivors Show forest plot | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.25] |
| 3 Bayley Psychomotor Developmental Index (PDI) <‐2 SD Show forest plot | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.85, 1.60] |
| 4 Bayley PDI <‐2 SD in tested survivors Show forest plot | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| 5 Developmental delay (criteria not specified) Show forest plot | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.08, 2.61] |
| 6 Developmental delay (criteria not specified) in tested survivors Show forest plot | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.30, 2.88] |
| 7 Blindness Show forest plot | 8 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.74, 5.50] |
| 8 Blindness in survivors assessed Show forest plot | 8 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.80, 5.86] |
| 9 Deafness Show forest plot | 8 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.39, 3.37] |
| 10 Deafness in survivors assessed Show forest plot | 8 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.29] |
| 11 Cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (IV, Fixed, 95% CI) | 1.42 [1.06, 1.91] |
| 11.1 Dexamethasone | 7 | 921 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [1.20, 2.55] |
| 11.2 Hydrocortisone | 6 | 1052 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.66, 1.66] |
| 12 Death before follow‐up in trials assessing cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.05] |
| 12.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 12.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 13 Death or cerebral palsy Show forest plot | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.16] |
| 13.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.37] |
| 13.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 14 Cerebral palsy in survivors assessed Show forest plot | 13 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.11, 1.90] |
| 14.1 Dexamethasone | 7 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.29, 2.57] |
| 14.2 Hydrocortisone | 6 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 15 Major neurosensory disability (variable criteria ‐ see individual studies) Show forest plot | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.33] |
| 15.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.03, 1.83] |
| 15.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.14] |
| 16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria) Show forest plot | 6 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.17] |
| 16.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.25] |
| 16.2 Hydrocortisone | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| 17 Death or major neurosensory disability (variable criteria) Show forest plot | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 17.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.30] |
| 17.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 18 Major neurosensory disability in survivors examined (variable criteria) Show forest plot | 8 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 18.1 Dexamethasone | 4 | 469 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.77] |
| 18.2 Hydrocortisone | 4 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.10] |
| 19 Abnormal neurological exam (variable criteria ‐ see individual studies) Show forest plot | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.33, 2.47] |
| 20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria) Show forest plot | 6 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.07] |
| 20.1 Dexamethasone | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.21] |
| 20.2 Hydrocortisone | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 21 Death or abnormal neurological exam (variable criteria) Show forest plot | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.06, 1.42] |
| 22 Abnormal neurological exam in tested survivors (variable criteria) Show forest plot | 5 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.41, 2.52] |
| 23 Intellectual impairment (IQ < 70) Show forest plot | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.57, 3.31] |
| 24 Intellectual impairment (IQ < 70) in survivors assessed Show forest plot | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.47, 2.65] |
| 25 "Major neurosensory impairment" ‐ blindness or deafness Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.12] |
| 27 Behaviour abnormalities Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 28 Behaviour abnormalities in 3‐year‐old survivors assessed Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.22] |
| 29 Abnormal EEG Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.66, 2.33] |
| 30 Abnormal EEG in tested survivors Show forest plot | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.08] |
| 31 Rehospitalisation in infancy Show forest plot | 3 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 32 Rehospitalisation in infancy in survivors Show forest plot | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |

