Interventions for promoting smoking cessation during pregnancy
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | A randomised pilot study including two different interventions and usual care provided to "pregnant teens" recruited through local prenatal clinics and public schools in Pittsburgh, USA. The hypothesis was that an intervention including peer support would be more effective than the intervention alone. The aim was to develop an effective intervention which could be implemented by clinics and schools. | |
| Participants | Inclusion criteria were: | |
| Interventions | Usual Care (UC) 30 minutes individual educational session with project nurse including information about the risks of smoking to the mother and the fetus + brochures on smoking and pregnancy. | |
| Outcomes | Smoking cessation at 4‐6 weeks post baseline, validated by exhaled CO. | |
| Notes | TFS and UC outcomes were combined in this preliminary paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Cluster‐randomised trial measuring (i) the short‐term effects of routine prenatal care provider (midwife) smoking cessation counselling and provision of smoking cessation materials. All midwives in a province were allocated to either intervention or control care. Only 24.2% of chairs of midwifery agreed to approach midwives in their region to participate. The first 40 practices (118 midwives) were selected, from 4 provinces, which were then matched (by location and level of urbanisation) into 2 pairs. (ii)measuring the longer‐term effects. | |
| Participants | Women using public health services, who smoke more than 1 cigarette per day, literate in Dutch, and gravidity less than or equal to 4. 80% eligible population approached. Participation rate 72% (n = 318). Mean cigarettes per day at intake I = 9.1, C = 7.7. Mean gest at intake I = 12.4, C = 13.5. (ii) included women from trial (i) and spontaneous quitters; n = 253 (I) and 303 (C); 80% approached. 72% participation. | |
| Interventions | Control group received routine smoking cessation counselling + a folder about smoking cessation in pregnancy, (Both trials i and ii) | |
| Outcomes | Self reported quit attempts at 6 weeks postpartum, with urine cotinine biochemical validation in a small proportion of participants (n = 14). | |
| Notes | inconsistent information on gravidity criteria. Good process evaluation documented poor implementation in some aspects. A separate detailed paper published on process evaluation issues. (ii) Only 16.7% of women received the post‐delivery booklet. No validation of longer‐term self‐reported smoking. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | A randomised pilot study of the effect of medical advice on smoking cessation in pregnancy, in two public antenatal clinics in Bolton and District General Hospital, England. No sample size or randomisation details. | |
| Participants | Women smokers or ex‐smokers, at their first antenatal visit, less than 20 weeks gestation. 110 women, mostly working‐class, mostly long‐term and heavy smokers. I n = 63 C: n = 47. | |
| Interventions | Control group received usual care, which was advice at the discretion of the doctor. | |
| Outcomes | Smoking cessation assessed by self report in a home interview 11 weeks after baseline visit. No biochemical validation of smoking status. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial of effectiveness of use of exhaled carbon monoxide feedback for promoting smoking cessation in pregnancy, in Guildford County, North Carolina. Trial over 6 months in 1981. Allocation by a computer‐generated random number table to experimental or control group. No randomisation details or sample size justification. | |
| Participants | Women currently or recently smoking, attending public clinics. No exclusion criteria details or characteristics of participants in each group. 47% were current smokers, 43% had completed high school education, 56% were black, 80% classified as having no pregnancy risks other than smoking. 38% in the first trimester and 46% in the second trimester of pregnancy. | |
| Interventions | Experimental group provided breath specimen in which carbon monoxide was measured, with feedback of the result, and a 135 word script describing the relationship between CO and cigarette smoking + harmful effects of smoking during pregnancy, by health educator. | |
| Outcomes | Smoking cessation 6 weeks after intervention confirmed by subsequent CO <= 9 ppm in breath specimen. Outcome measurement for 170/226 women. Attrition rate 24.8%, and allocation not reported. | |
| Notes | Not clear whether this was a group intervention ‐ in which case there was no adjustment for clustering. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial of psychosocial support in pregnancy in 4 hospitals in Latin America (Argentina, Brazil, Cuba, Mexico). January 1989 ‐ March 1991. | |
| Participants | High‐risk women whose antenatal care began at 15 ‐ 22 weeks gestation, singleton pregnancy, 1 or more of the following: prior LBW infant; preterm birth; perinatal/infant death; < 18 years; body weight <= 50 kg; height <= 150 cm; low family income (local definitions applied); < 3 years school; crowded household (4 or more persons/bedroom); smoking; not living with husband or partner. 2235 women recruited 1115 to intervention 1120 to control. | |
| Interventions | Control group received routine antenatal care. High intensity intervention involving flexible use of a standardised manual, based on site‐specific ethnographic studies of needs, fears, expectations, social support networks, including detailed descriptions of situations likely to occur during home visits. 4 to 6 home visits of 1 to 2 hours with emphasis on psychosocial support, education on health habits including better nutrition, reducing smoking alcohol and other drugs, reducing their physical workload, recognition of alarm signs and symptoms, improved access to hospital facilities, reinforcement of health service utilization. Additional components were a poster, a booklet, hot line to project office, guided tour of hospital, encouragement of family support and participation. Intervention was provided by specially trained female social workers or obstetric nurses with previous experience of childbirth. | |
| Outcomes | Self reported smoking cessation, no biochemical validation. Multiple perinatal and maternal health outcome data were collected. As there are many paths other than smoking reduction/cessation by which these outcomes might have been modified by the intervention, only smoking cessation has been abstracted in this review. | |
| Notes | Sample size was planned for the primary trial objective. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Trial of telephone support for improving outcomes in late pregnancy, in the outpatient department of a large maternity hospital in New Zealand, or its associated GP practices, or self‐referral, from March to December 1993. Computer‐generated random assignment to control or intervention in balanced blocks of 50. Caregiver blinded to allocation. No sample size justification. No sample size justification. | |
| Participants | Women with telephone access, who were either single or with an unemployed partner, were recruited before 20 weeks gestation. The eligible population was 221 women of whom 131 took part (103 OPD, 22 from GPs, 6 self‐referred). 49 were never located, 23 were not interested, 10 refused after explanation, 8 moved away, did not speak English or had a miscarriage. | |
| Interventions | Introductory letter, phone call, full discussion of "Healthy Mothers/Healthy Babies". | |
| Outcomes | Both perinatal and maternal health outcomes were assessed but as there were other intervention components which might have influenced these outcomes only smoking cessation data were abstracted for this review. No biochemical validation of smoking status. 9 women (of 131) were lost to follow up by late pregnancy, counted as still smoking. Attrition = 7%. | |
| Notes | No process evaluation is reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of CO assessment and brief directive feedback, in a large US municipal hospital antenatal clinic, over an 18 month study period. No description of randomisation. Caregivers blinded to allocation. | |
| Participants | All attending women screened for smoking by questionnaire + CO breath measurement (>= 9 ppm). Pregnant women, currently smoking, at any stage of gestation. Over 50% were current smokers; 40% of women were Black. | |
| Interventions | Control group (usual care): clinic nurse provided health education, including smoking. | |
| Outcomes | CO measurements (biochemical validation) and smoking data were collected at all subsequent visits. | |
| Notes | Simple intervention so no process evaluation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of provision of videotaped vignettes for promoting smoking cessation and relapse prevention in a community‐based university setting, Texas, US. No details of randomisation, caregiver blinded. | |
| Participants | Volunteers who were willing to quit within two weeks, were recruited through local media, such as newspaper, radio, subscriber letters, community business flyers, waiting room posters. Exclusion criteria: women smoking < 3 cigarettes per day; < 18 years; > 30 weeks pregnant; do not have a working VCR (approximately 12% Americans); not depressed. Participants n = 82. Mean cigarettes/day at first visit I = 17.3, C = 14.5. No significant difference in socioeconomic variables between groups. | |
| Interventions | The control group received a quit calendar and tip guide. | |
| Outcomes | Self reported smoking abstinence obtained within 2‐3 days of quit date, 4‐5 weeks after the quit date and one month postpartum. Biochemically validated with salivary cotinine. | |
| Notes | Authors say women in this study tend to be heavier smokers than described in previous studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of "Significant Other Supporter" (SOS) program, of bolstered social support and direct financial rewards, for low income high‐risk women in 4 Oregon WIC program sites, US. Conducted between June 1996‐June 1997. No randomisation details. Quality score = moderate‐risk of bias. | |
| Participants | Women smoking (even a puff in the last 7 days); less than 28 weeks gestation; over 15 years of age; literate in English. Participation rate 71%. Mean salivary cotinine at baseline: I: 45.4 (n = 112); | |
| Interventions | Control group received verbal and written information on the importance of smoking cessation, a pregnancy specific smoking cessation self help kit, and were telephoned monthly for self reports on their smoking status. | |
| Outcomes | Smoking cessation biochemically validated with salivary cotinine at 34 weeks gestation and 2 months postpartum. Attrition rate I = 32%; C = 51.5%. | |
| Notes | Data in outcome tables is inconsistent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial of advice to stop smoking in pregnancy, provided by a (public health) doctor, reinforced by the woman's own GP and other providers involved in shared antenatal care, in 3 UK maternity units. Randomisation details unclear. Caregivers not blinded (asked to reinforce information). Quality score = high‐risk of bias. | |
| Participants | Pregnant women < 35; currently smoking >= 5 cigarettes/day and had been smoking >= 1/day at the onset of pregnancy; < 30 weeks gestation at first visit; no prior perinatal death; not seeking, nor sought termination. Other exclusions: not pregnant; refused consent; miscarriage or termination of pregnancy; moved to another care provider; twin pregnancy or birth before 28 weeks. | |
| Interventions | Control group received ANC usually provided by the hospital, including any anti‐smoking advice which may have been given routinely. Intervention: individualised medical advice | |
| Outcomes | Self reported smoking in cigarettes/day at four stages of pregnancy; mean birthweight; low birthweight; preterm birth (< 36 weeks); perinatal deaths. No data on smoking cessation. | |
| Notes | Details of the intervention are in Donovan et al 1975 [see Donovan 1977]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of midwifery counselling around the "stages of change" model", in a large UK maternity. No details of randomisation and caregivers aware of allocation. Quality score = high‐risk of bias. | |
| Participants | 100 women; pregnant and booked for maternity care; < 18 weeks gestation; currently smoking 1 or more cigarettes/day. 13 midwives selected for the intervention group and 13 for the control group. | |
| Interventions | Intervention midwives were trained to assess the stages of change and provide a behavioural intervention, using the Health Education Authority material "Helping pregnant smokers quit: training for health professionals", 1994. | |
| Outcomes | Smoking cessation; cigarettes/day; "stage of change" at 11 to 18 weeks vs 37 weeks. No biochemical validation of smoking status. | |
| Notes | 3700 births/year at the hospital, all women who smoked were eligible to take part so it is not clear why only 100 took part (described as "all 100"). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Prospective randomised controlled trial in 5 health centres of the same HMO in Los Angeles, 1985 ‐ 87. Educator turned over a pre‐assigned card after a brief smoking related interview to determine allocation. | |
| Participants | English‐speaking women < 18 weeks gestation; still smoking >= 7 cigarettes a week (n = 323, 165 + 158, with losses due to termination (7 + 11); miscarriage (12 + 13); disenrollment or transfer to another HMO (20 + 18); leaving 126 + 116. | |
| Interventions | Control group: 2 page pamphlet on hazards of smoking and on the need to quit; 2 minutes discussion with a health educator (within a 45 minutes individual conference); advised of free 5 session smoking cessation program available through the HMO. Coverage in antenatal classes remained unchanged. | |
| Outcomes | Smoking cessation validated with urine cotinine; birthweight; low birthweight; preterm birth (< 37 weeks); stillbirths. Attrition I = 51%, C = 49%. | |
| Notes | Process evaluation showed good implementation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Ershoff 1989 trial data of relapse prevention in the women who had spontaneously quit smoking in early pregnancy. | |
| Participants | The pre‐pregnancy smokers who had quit spontaneously before the first antenatal contact: 110+ 108, with losses due to termination (5); miscarriage (17) and transfer to alternative prenatal care (25) leaving 87 + 84. | |
| Interventions | See Ershoff 1989 except that the intervention group received the first 4 booklets at the first interview with booklets 5 to 8 mailed weekly thereafter; control group were congratulated on quitting and given a tip sheet on "staying quit". | |
| Outcomes | Smoking data validated with urine cotinine only collected, no perinatal data. | |
| Notes | Detailed process evaluation and analysis of factors promoting or inhibiting cessation and maintenance of non‐smoking. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial of three alternative methods of smoking cessation interventions, in a large group model managed care organization in California, US. No details of randomisation. Caregivers blinded to allocation. | |
| Participants | Smokers were identified at first visit as women who self report "smoking now", "smoke but have cut down since pregnancy", or "smoke from time to time". Researchers attempted to phone all women over 18 years and less than 26 weeks gestation (n = 931). 150 could not be contacted and 90 refused to be interviewed. 233 were excluded as they did not speak English (n = 44), smoked less than 7 cigarettes per week pre‐pregnancy (n = 114) or experienced miscarriage (n = 34). 380/458 women (82%) agreed to participate. 60% white, approximately 50% college educated, with a mean age of 29.4. Mean cigarette/day at first visit = 6.6. | |
| Interventions | 3 interventions, based on stages of change model. | |
| Outcomes | Smoking cessation in the third trimester "not even a puff in the last 7 days", biochemically validated with urine cotinine. Baseline mental health index and Cohen's perceived stress scale. | |
| Notes | Data from group one and group three only compared in outcome tables. Good process evaluation of each of the methods. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial of a smoking cessation and relapse prevention intervention in an urban, prenatal clinic in Baltimore, US. Nov 1996 ‐ June 1997. No details of randomisation and caregivers not blinded to allocation. | |
| Participants | Pregnant women currently smoking (even 1 puff in the past 7 days); < 28 weeks gestation; African‐American or white; 85% of whom were on medical assistance, attending the Outpatient Department at Johns Hopkins. No other exclusions specified. 2319 women assessed, 32% currently smoking by above definition, ‐1585 non‐smokers, ‐72 (gestation, ethnicity, not interviewed at their first visit or changing to another care provider) leaving 662 eligible of whom 510 agreed to take part. 25 quit prior to first visit, 18 did not wish to quit, leaving 467 (232 + 235) reduced by withdrawals, miscarriage, termination and change of care provider to (193 + 193). Mean cigarettes/day at intake I = 9.7, C = 7.5 (P = 0.01). | |
| Interventions | Control: a brief discussion with a nurse about the risks of smoking; a recommendation to quit and pamphlets from the areas's voluntary agencies. | |
| Outcomes | Smoking cessation in third trimester, validated by salivary cotinine. | |
| Notes | Guide developed through needs assessment with pregnant women, constructs from the PRECEDE/PROCEED diagnosis and social learning theory, tested with focus groups, additional section on relapse prevention, and on passive smoking postpartum. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial in physicians offices and clinic sites within Maine, 1984‐7, of providing feedback on cotinine measured in maternal serum screening programme (for the identification of open neural tube defects) as part of an smoking cessation intervention. Random allocation through computer‐generated number on maternal serum screening request form. Caregiver not blinded. | |
| Participants | Pregnant women with a singleton live pregnancy; having maternal serum AFP screening at 15‐20 weeks gestation; who smoked >= 10 cigarettes a day. 25,628 screened, 97% answered question on smoking, about 3,000 met smoking criteria (17%). 1423 intervention and 1425 control with 41 + 39 lost to follow up. | |
| Interventions | Control: standard medical care not otherwise specified. | |
| Outcomes | No smoking cessation data. Smoking data limited to comparability at first assessment and serum cotinine levels; mean birthweight; low and very low birthweight; preterm birth (< 37 weeks); fetal deaths; neonatal deaths; postneonatal deaths. 695/1343 women provided repeat serum cotinine for comparison. | |
| Notes | Physician consent only sought. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Cluster randomised trial of a brief midwife‐delivered smoking cessation intervention in 9 hospital and community trusts in the UK. 290 midwives randomised to provide intervention or control care. Sample size justification. | |
| Participants | Women recruited at first visit (approximately 12 weeks gestation) and considered eligible if they reported current smoking or having stopped within the last 3 months (n = 1287). 189 current smokers not motivated to stop, therefore received no intervention. | |
| Interventions | Control group midwives received 1 hour of training to discuss the study and were asked to provide usual care and any usual pamphlets. Intervention midwives received 2 hours training which included using the CO monitor and providing "stage of change" based advice, CO assessments. Intervention group also received written advice and motivational materials for current and recent smokers, including designating a "quit date", a "quiz" and the offer of "buddying" to another pregnant smoker for support. | |
| Outcomes | Smoking cessation biochemically validated with exhaled CO in the early postnatal period and at 6 months postpartum. | |
| Notes | Data not adjusted for clustering, so they were not included in outcome tables. Good process evaluation showed poor implementation in some areas, with only 61% of midwives actually recruiting any women for the study. Financial incentives paid to service to improve recruitment. Discussion of barriers includes 65% of midwives reporting the intervention could not be undertaken in the time they had available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial of medical smoking cessation counselling and peer support, in a teaching hospital (academic) clinic in North Carolina 1991‐1993. Randomised by computer‐generated random number table and charts "flagged" to identify those in the intervention group. | |
| Participants | All women receiving prenatal care at the University of North Carolina residents clinic were surveyed: 842/846 completed survey; 793/846 provided a carbon monoxide breath sample; 2 were excluded as > 36 weeks gestation; 1 for psychiatric diagnosis; leaving 266 eligible smokers (smoked at least once in the prior week) of whom 12 refused, 4 were missed, 2 were not pregnant and 1 was a private patient; 247 recruited, losses were 40 (‐4 miscarriage first trimester, ‐3 miscarriage second trimester, ‐ 3 terminations, ‐15 moved to alternative care , ‐12 lost to follow up) leaving 107 intervention and 100 control. | |
| Interventions | All 1‐4 year residents given didactic and role play training for smoking cessation counselling, including self‐assessment of current techniques and skills, which they were asked to continue with for the control group. | |
| Outcomes | Smoking cessation biochemically validated by exhaled CO at each visit. Attrition rate 16%. | |
| Notes | Concerns about residents having to treat similar/consecutive patients differently, and self‐help manuals accidentally given to some controls. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial of multimodel intervention to promote smoking cessation in pregnancy in a large midwifery centre in the Netherlands, 1996 ‐ 1998. Quasi‐randomised, allocation of even/uneven birth dates to designated clinic days. Usual caregivers provided intervention, so not blinded to allocation. Sample size justification. | |
| Participants | Pregnant women attending first antenatal visit (approximately 16 weeks gestation) who identified as "daily smokers" were invited (n = 905). Exclusion criteria: inability to speak Danish; age > 18 years; gestation > 22 weeks; verified psychiatric disease, and alcohol or drug abuse. Participation rate 77% (n = 696). I = 348, C = 347. 87 in the intervention group accepted intensive smoking program (81 group & 6 individual). 75 opted to use NRT. Withdrawals = 48 (miscarriage, moving and premature birth) excluded from the smoking cessation outcomes. Mean cigarettes/day = 11 in both groups. Significant difference in partner smoking I = 67%, C = 77% (p = 0.03). | |
| Interventions | Control group received standard smoking cessation counselling from their midwife about risk of smoking and general advice on cessation or reduction, within the standard 30 minute booking consultation. | |
| Outcomes | Self reported smoking cessation at 37 weeks gestation, biochemically validated in 51% participants. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Quasi‐randomised (allocation by birth date) trial of smoking cessation intervention ‐ based on RA Windsor self help manual ‐ in 13/14 public health maternity clinics in Gothenburg, Sweden 1987‐1988. | |
| Participants | Women who spoke Swedish, smoking >= 1 cigarette/day, gestational age < 12 weeks at first antenatal visit, (no other exclusion criteria specified), leaving n = 745 of whom 22 had quit by the second antenatal visit. 15% refused to take part (‐75) leaving 417 in the intervention and 231 in the control group. | |
| Interventions | All women were advised to quit by the midwife at the first antenatal clinic; pre‐intervention. | |
| Outcomes | Smoking cessation data; biochemically validated (blood thiocyanate < 100 ng/ml) at first and second antenatal visit and in late pregnancy, and postpartum; mean birthweight; low birthweight; preterm birth (< 36 weeks). | |
| Notes | Same data published by Svanberg 1992. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial of tailored, scripted "stage‐of‐change" intervention and fact booklet, for infertile and pregnant women, in 3 university teaching hospitals in Ontario, Canada. Randomisation of consenting participants using a computer‐generated, blocked schedule, administered through numbered opaque envelopes. Caregivers not blinded to allocation. | |
| Participants | Pregnant women smoking 3 or more cigarettes per day in the past 6 months. Mean gest at enrolment I = 18.91, C = 20.55. 110 recruited. Mean number of cigarettes/day I = 13.43, C = 12. | |
| Interventions | Control group completed a questionnaire and self identified current smoking "stage of change" and received standard information about the negative effects of smoking in pregnancy, reinforced with whatever literature was available and CO measurements. The intervention group received the same as the control group + (i) scripted advice prompted by sets of cards, which are tailored to each stage; (ii) stage specific information booklets; (iii) referral for more in depth counselling. | |
| Outcomes | Self reported smoking levels, validated by exhaled CO, and movement in the stages of change measured at enrolment, 6 and 12 months. | |
| Notes | Data from both infertile and pregnant women combined, so not included in tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Canadian double‐blind, placebo controlled trial of nicotine replacement therapy (patches) in pregnancy. | |
| Participants | Women recruited from the Motherisk Program at 12‐24 weeks gestation, smoked > 15 cigarettes/day, and who reported they wanted to quit, but could not do so, in the first trimester. | |
| Interventions | Intervention group received a 12 week NRT patch regimen: 18 hour 15 mg patch for 8 weeks; 10 mg patch for 2 weeks, and 5 mg patch for 2 weeks + counselling with a video presentation at baseline, 1, 4 and 8 weeks. | |
| Outcomes | Smoking cessation during second trimester, biochemically validated with serum and salivary cotinine levels. | |
| Notes | Study ceased after only 30 women recruited due to severe withdrawal symptoms in the 30th recruit (allocated to placebo). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Cluster randomised trial of smoking cessation in public prenatal and WIC clinics in Maryland, Colorado and Missouri, USA, 1987‐89. Clinics stratified by size of clinic and also by prior low birthweight programme (Colorado) or % minority clients (Maryland), and randomly assigned to deliver either intervention or continue with standard care. | |
| Participants | 5262, 6087 and 4943 pregnant women screened in Colorado, Missouri and Maryland respectively, with nearly 50% of women in each State smoking. Smoking defined as "even a puff within the last 7 days before the women knew she was pregnant" (includes recent quitters). Consent for data collection ranged from 66% to 79%. High proportions were young, < 12 years education, White, unmarried and poor. Mean gest at enrolment = 15.2 ‐ 16.6 weeks. Mean cigarettes/day at enrolment combined for smokers = 12 cigarettes/day. | |
| Interventions | Control: usual care not otherwise specified. | |
| Outcomes | Smoking cessation biochemically validated with urine cotinine. The necessary adjustment for clustering means that the data cannot be put into the standard table of comparisons. Adjusted data showed no differences in verified quitting, mean birthweight or low birthweight. | |
| Notes | Substantial misclassification of self report as non‐smoking: 28% at enrollment; 35% at 8th month; 49% of self reported quitters at intervention clinics; 32% of self reported quitters at control clinics. Process evaluation suggested less difference between I and C clinics than might have been expected. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Cluster‐randomised trial of two different interventions, in community midwife clinics in the West Midlands region of the UK. A computerised minimisation programme was used to allocate 72 eligible practices into 3 equal groups from 101 available practices. Caregivers not blinded (implementing intervention). Sample size calculation given, but unable to recruit sufficient numbers. 17 practices added to arm A, 12 to arm B and 0 to arm c to increase recruitment. | |
| Participants | Inclusion criteria were all women seen in routine antenatal appointments who were aged 16 years or over, a current smoker at booking. Women not fluent in English were excluded. Initial target of 1440 participants was reduced to 900 due to slow recruitment (particularly in standard care arm). Eligible smokers approached A = 34%, B = 47%, C = 75%. Refusal rate A = 13.4%, B = 7.2%, C = 22.5%. Mean cigarettes per day at baseline were similar between groups. | |
| Interventions | Control group (A) received standard care. Midwives received a half day training on research protocol, and asked all midwives to give women the Health Education Authority booklet "Thinking about stopping". Group B midwives received two and a half days training on theory of transtheoretical model. Participants received a set of 6 stage based self help manuals "Pro‐Change programme for a healthy pregnancy". The midwife assessed participants stage of change and pointed the woman to the appropriate manual. No more than 15 minutes was spent on the intervention. Group C midwives received the same training as for Group B, and participants received the same self help manual and intervention as group B. Additionally the participants used a computer programme on the occasions, which consisted of questions to stage the woman with auto feedback of what stage they were in and what this meant, and a range of other concepts. It took about 20 minutes for the woman to complete. Printed information of the feedback was sent to the participant within a week of the intervention. | |
| Outcomes | Biochemically validated smoking cessation at 28 ‐ 30 weeks gestation and 10 days post birth. Point prevalence and sustained abstinence of 10 weeks or more were calculated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | A randomised trial in Newcastle Hospital antenatal clinic (UK) and with other shared antenatal care providers of individual counselling to promote smoking cessation over 3 months in 1982. | |
| Participants | All pregnant women currently smoking >= 1 cigarette a day at the time of the first antenatal clinic, and < 28 weeks gestation. 156 contacted, ‐5 > 28 weeks leaving 151, 5 exclusions (not pregnant, guilt over previous stillbirth, and 3 miscarriages), leaving 72 (I) + 73 (C). | |
| Interventions | Control: usual antenatal care + possible exposure to a concurrent television series (6 x 10 minute programme on stopping smoking in pregnancy). | |
| Outcomes | Smoking status and smoking/day assessed 6 weeks later. Not biochemically validated. | |
| Notes | Short interval between intervention and assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial of anti‐smoking interventions (individual and group) based on the MRFIT trial, carried out in Oregon where 95% of pregnant women attending one of the two hospitals were enrolled in the Kaiser Permanente HMO, 1979‐1980. No details of randomisation or whether caregivers masked to allocation. | |
| Participants | , questionnaire response rate 25%. Pregnant women contacted at first antenatal visit: 3856 asked about smoking; 963 self reported current smokers (25%). 21% of them in receipt of public assistance but only 7% of non‐smokers. Poor participation in the study: 83.6% contacted; refusal rate 37%. | |
| Interventions | Planned intervention: (i) letter of invitation with sae, reminder letter; | |
| Outcomes | Smoking cessation by late pregnancy, biochemically validated with cord blood thiocyanate in a subsample, but no misclassification of self reported non‐smoking. | |
| Notes | Very poor response to group sessions so intervention changed over the course of the trial to individual counselling, which also had very low participation overall: 18% active; 25.2% dropped out; 38% did not participate; 18% could not be contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | A randomised trial of relapse prevention among women who had stopped smoking since the beginning of pregnancy, in the public maternity clinics of a large hospital in Birmingham, Alabama 1987‐1989, USA. | |
| Participants | Pregnant women recruited at their first prenatal visit reporting as having quit since conception, no exclusions mentioned, n = 115, 9 refused to participate leaving 106 of whom 3 had a miscarriage, 4 moved and 2 had babies for adoption, leaving 54 (I) and 45 (C), Follow up data were available on 80%. | |
| Interventions | Control: nurses' advice to all women not to smoke. | |
| Outcomes | Smoking cessation in late pregnancy, biochemically validated with salivary thiocyanate. Included in relapse prevention outcome tables only. | |
| Notes | Concurrent trial with Windsor 1993. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Quasi‐randomised study using alternate allocation within antenatal clinic of a large metropolitan public hospital in Brisbane to assess the effectiveness of a self‐help booklet developed by Windsor (for women of low socioeconomic status ‐ mostly black women ‐ in Alabama), in urban Australian women. This first trial (i) was followed by a second one (ii) with a modified intervention, but no other change to the methods | |
| Participants | All pregnant women attending for a first antenatal clinic, who identified themselves as current smokers, had no current complications of pregnancy and were not planning to have the child adopted, were approached at their first antenatal clinic appointment (n = 244 ‐ 27 who declined = 217).(ii) Participation rate of 91%, 108 women recruited, 8 had a miscarriage or fetal death or discontinued care at the hospital; 2 withdrew from the study and 19 were lost to follow up (LTFU) by 20 weeks. All those LTFU were counted as continuing smokers | |
| Interventions | Control: given the self help booklet and a midwife caution against smoking. | |
| Outcomes | Smoking reduction and cessation assessed at the 20 week visit. Biochemical validation of smoking status in self reported non‐smokers, same for (i) and (ii). | |
| Notes | Process evaluation showed poor response to the booklet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | See Lowe 1998a for setting as this trial followed immediately after the first one. Quasi‐randomised trial with alternate weeks allocated to control and intervention. | |
| Participants | See Lowe 1998a. The participation rate was 91% with 108 women recruited of whom 8 had a miscarriage, or a fetal death or discontinued care at the hospital. Two more withdrew and 19 were lost to follow up by 20 weeks. All those lost to follow up were counted as continuing smokers. | |
| Interventions | Booklet modified from the one used in Lowe 1998a, through focus group discussions with input from health promotion specialists, medical specialists and GPs to a glossy format with coverage of other topics (growth and development of the fetus, enjoyment of certain foods and sex during pregnancy, emotional and physical aspects of pregnancy and stopping smoking). | |
| Outcomes | Smoking behaviour and smoking cessation at 20 weeks, biochemically validated. | |
| Notes | Process evaluation of materials was positive, though staff identified a range of barriers to implementing smoking cessation counselling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Quasi‐randomised trial with alternation of 4 week blocks to intervention or control in a large English city maternity hospital to identify effects on fetal size at birth mediated by an anti‐smoking intervention, 1981‐1982. | |
| Participants | Pregnant women smoking at booking: 29% had been pre‐pregnancy smokers, 23% were smoking at booking. 1008/1156 women identified as smokers interviewed, 48 lost (early discharge, infection/isolation, changed surname); Exclusions were multiple births (6 (I) + 8 (C); records not linked to hospital data 8 (I) + 4 (C)) leaving 493 (I) and 489 (C). Mean cigarettes/day at booking I = 14.4, C = 13.7. | |
| Interventions | Intervention: advice to stop smoking + information or discussion of the effects of smoking on the fetus offered by the obstetrician at the first antenatal (booking) visit, supported by giving her a leaflet to be shared with the partner, family and friends. If leaflet not given by obstetrician, the midwife was asked to give it to the woman and advise her to stop smoking. | |
| Outcomes | Smoking cessation and reduction ‐ biochemical validation commenced, but abandoned when it became clear it did not distinguish levels of smoking. Birthweight, length and head circumference; | |
| Notes | Consent not sought from individual women, implementation of the trial across all clinics routinely. Process evaluation shows poor implementation, with only 10% receiving "full intervention". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial of effects of peer counselling on smoking cessation and reduction in a large urban clinic. Hartford Hospital, US, Jan 1998‐Feb 2000. Computer‐generated random allocation, with usual care providers masked to allocation. | |
| Participants | Low income, uninsured women, who smoke "at least one cigarette per day before pregnancy, less than 20 weeks gestation, literate in English or Spanish, and intending to carry to term. High smoking prevalence in pregnancy (29%). Recruited n = 142 (I = 67, C = 75). Mean cigarettes/day at baseline significantly higher in intervention group. I = 13.3, C = 11.2. | |
| Interventions | The control group received routine care, which included the program of "Ask, Advise, Arrange and Assist", based on cognitive behaviour, described by Windsor et al, 2000. The intervention received as for the control group + peer counselling from lay community health outreach workers (telephone or home visits). Peer counsellors received 2 x 3 hours of training. | |
| Outcomes | Smoking cessation and reduction at 36 weeks gestation, biochemically validated with urine cotinine and exhaled CO. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Cluster randomised trial of a smoking cessation program in 10 public clinics in Chicago, US, 1994‐6. Randomisation to study group within matched pairs of clinics. | |
| Participants | Clinics matched on size, type, location, and racial mix of clientele. Smokers in intervention group more likely to be African‐American. Participation rate I = 76% (n = 1025), C = 86% (n = 784). Mean cigarettes/day at intake. | |
| Interventions | Control group received smoking cessation advice and available brochures, dependant on the clinician. The intervention group received brief advice to quit (from a variety of clinicians), a written agreement on a quit date, a take home motivational self‐help booklet "Its Time", a reminder letter, and a 15 minute telephone motivational interview. High intensity intervention based on stages of change theory and Millers brief motivational interviewing approach. | |
| Outcomes | Self reported smoking cessation, not biochemically validated. Movement in stages of change. Attrition rate I = 38%, C = 41%. | |
| Notes | Data not included in outcome tables due to inconsistent data reporting (baseline and control groups combined) and data not adjusted for clustering. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Trial comparing three smoking cessation interventions in WIC clinics in Grand Rapids, Michigan, USA, 1985‐86. Not details of randomisation or whether caregivers masked to allocation. | |
| Participants | Women currently smoking (>= 1 cigarette/day) comprised 271/641 attending the clinics (42%), 219 agreed to take part, data on 186. Losses to follow up were that a quarter refused, and the rest either moved, changed their source of antenatal care or had a miscarriage (no details of numbers). Mean cigarettes/day prior to pregnancy I = 19.9, C = 20.3. | |
| Interventions | Control: printed information about the risks of smoking in pregnancy. | |
| Outcomes | Smoking cessation in late pregnancy and postpartum, biochemically validated with salivary thiocyanate in approximately a third of participants, but no adjustment for misclassification. | |
| Notes | No process evaluation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial of relapse prevention at the Group Health Cooperative of Puget Sound (Seattle, USA) (HMO), and Park‐Nicollet of Minnesota (USA), a multispecialty group practice. No details of randomisation. Caregivers masked to allocation. | |
| Participants | Women booked for a first prenatal visit were offered, by letter, study participation and unless they opted out were given a baseline telephone interview. Women who had completed the baseline survey, were < 20 weeks of pregnancy, were currently smoking or had smoked in the 30 days before pregnancy but had quit at the time of the baseline survey. | |
| Interventions | There were 3 stage of change based interventions, all delivered by mail or telephone without involving prenatal care providers. | |
| Outcomes | Smoking cessation; relapse prevention and patterns of smoking; biochemically validated with salivary cotinine at 28 weeks gestation; 8 weeks PP; 6 months PP; and 12 months PP. Response rates were 92% at 28 weeks; 91% at 8 weeks postpartum; 89% at 6 months postpartum; 87% at 12 months postpartum. | |
| Notes | Process evaluation describes participation in specific intervention components, including relapse prevention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Cluster randomised trial of provision of self help in 3 UK NHS hospital trusts, 1998‐2000. 118 midwives stratified according to workload and randomly allocated to provide intervention or control care. Computer‐generated randomisation, caregivers not masked to allocation. Sample size justification. | |
| Participants | Women attending first visit; > 16 years; < 17 weeks gestation; literate in English. Smokers counted as those who reported "I smoke now", "I smoke now but have cut down since I thought I might be pregnant", or "I have stopped smoking since I thought I might be pregnant". Mean number of cigarettes per day at baseline I = 16, C = 15.1. | |
| Interventions | Control group midwives continued to give routine advice according to usual practice. | |
| Outcomes | Self reported smoking cessation validated by urine cotinine (94%). | |
| Notes | Data not included in outcome tables as it was not adjusted for clustering. Good qualitative and quantitative process analysis of participants and midwives views of the intervention, which suggested poor implementation in some areas. Some concerns about contamination of control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomised, factorial design to identify the best way of encouraging the disclosure of smoking in pregnant women, in a HMO, Texas, 1988‐1990. No randomisation details. | |
| Participants | Pregnant women enrolled in an HMO; >= 18 years; able to speak and read English; free of mental or sensory handicap; mental retardation or mental illness. 1078/1206 recruited. 121 refused others were < 18 or non‐English speaking. | |
| Interventions | The 4 options compared were: (1) Format (i) a single yes/no question vs (ii) a multiple choice. (2) Channel (iii) oral vs (iv) written forms of the two questions. Oral vs written forms of the two questions. | |
| Outcomes | Proportion of women smokers who disclosed smoking, biochemically validated with urine cotinine cutoff >= 50 ng/ml. No smoking cessation data. | |
| Notes | Those who refused urine testing were classified as "smoking disclosed". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quasi‐randomised (allocation by alternate days) trial of a new smoking cessation programme provided by public health nurses in the antenatal clinic of an Ontario (Canada) teaching hospital, compared with previous standard care. | |
| Participants | 1028 women screened, 267 daily smokers (673 non‐smokers, 88 spontaneous quitters). Ineligible (39) late gestation; miscarriage; missed abortion; termination; malformation; mental illness; mental retardation. Refusal (4). 224 at baseline; 202 at 1 month follow up; 174 at 36 weeks; 190 at 4 weeks postpartum. Reasons for dropout: miscarriage (17), no further clinic visit (3), subsequent refusal (2), and preterm birth (16 ‐ all of these seen postpartum), and 12 lost to follow up. Mean cigarettes/day at intake I = 13, C = 12.8. | |
| Interventions | Control: 3‐5 minutes explanation of the risks of smoking during pregnancy + pamphlet inviting women to a 2 hour cessation class in the evenings where the Windsor self help manual would be taught/provided. | |
| Outcomes | Smoking cessation biochemically validated by urine cotinine. | |
| Notes | No one attended the evening group class which was offered and was free. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Randomised trial with 4 arms whose aims were to improve the uptake of prenatal care and pregnancy outcomes, especially low birthweight, in a semi‐rural county of New York State, USA, 1978‐1980. No details of randomisation and unclear whether caregivers were masked to allocation. | |
| Participants | Active recruitment of pregnant women with no prior live births + any of the following: < 19 years; single; low socioeconomic status, and any other women with no prior live births who wished to participate in the program. Exclusions were > 25 weeks gestation (though some were enrolled at 25 ‐ 29 weeks). Recruitment was through private obstetricians' offices, planned parenthood, public schools health department antenatal clinics and other health and human service agencies. 10% of target population entered prenatal care too late, 10% were not referred from private care, 500 interviewed, 400 participated; 47% < 19, 62% single, 61% low ses. Non‐Whites (46) excluded because too few; serious maternal or fetal conditions (20) excluded. Mean cigarettes per day at intake: C = 6.94, I = 7.65. | |
| Interventions | Control (i) health and developmental screening of the baby at 12 and 24 months | |
| Outcomes | Smoking cessation with biochemical cotinine validation in a subsample (n = 116). Data not included in high intensity outcome tables, as smoking was not the focus of the intervention. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Trial of personalised smoking cessation interventions in a low socioeconomic population in Australia. No details of randomisation methods or whether caregivers were masked to allocation. | |
| Participants | Women who identified as "current smokers" at their first antenatal visit at approximately 12 weeks gestation ("even a puff in the last 7 days"). Exclusion criteria: > 20 weeks gestation; twin pregnancy; not literate in English; drug dependency. | |
| Interventions | Control group received usual care, which included advice at the discretion of the caregiver, a group counselling session, and a pamphlet "Smoking & Pregnancy". The intervention group received as for the control group + 4 counselling sessions by a midwife specifically trained and employed to provide smoking cessation counselling, using cognitive behaviour therapy. Sessions included video presentation, interactive discussion and strong verbal messages. These were followed up with a 5 ‐ 10 minute personalised counselling session. High intensity intervention. | |
| Outcomes | Self reported smoking cessation biochemically validated with urine cotinine at 36 weeks gestation, 6 weeks postpartum, and 6 months postpartum. Breastfeeding at 6 weeks and 6 months postpartum. General health assessment at first visit and 36 weeks. Preterm delivery rate, mean birth weight, proportion LBW (< 2500 g). Attrition rate = 15%. | |
| Notes | Process evaluation showed 71% women in the intervention group received the full intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | A randomised trial comparing the impact on smoking cessation of two different packages of material mailed to current smokers and recent quitters at a large Boston HMO, USA, 1986‐1988. Randomisation using table of random numbers for one intervention. Clinic staff were not aware of the allocation. Allocation to intervention 2 was not randomised but offered to all eligible enrollees at one clinic: data on this intervention is not included in the review. | |
| Participants | English‐speaking women enrolling in prenatal care; >= 18 years; < 24 weeks gestation who reported themselves as currently occasional or regular smokers or who had quit smoking in the previous 3 months. 1439/1442 screened (3 refused), 317 current/ recent smokers, 93 dropped out because of miscarriage, termination, moved away or left the HMO; 274 at second assessment and 224 at 8 weeks postpartum. 78 control and 71 intervention at baseline. | |
| Interventions | Usual care: routine obstetric care, mailed list of community‐based smoking cessation resources other pregnancy‐related health education materials. | |
| Outcomes | Smoking cessation for smokers and spontaneous quitters at mid‐pregnancy and 6 months, postpartum. Biochemical validation in 50% women. | |
| Notes | Refusal of urine test = coded as smoking. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | A randomised comparison of two different minimal contact interventions to encourage smoking cessation and reduction during pregnancy, in women of low ses and low education, compared with usual care in an inner urban setting, Toledo, Ohio, USA, 1987‐89. Randomisation by dice, which did not work well (no allocation to usual care some of the time). Unclear whether allocation masked to caregivers. | |
| Participants | "Typically low income, single and poor". 1164 approached, 486 (42%) were current smokers: 60% not enrolled (exclusion criteria not listed, though includes gestation > 28 weeks and refusal); 193 entered the study. Relatively low participation and 57% dropout from enrolment to completion. | |
| Interventions | Control: usual care not specified or assessed but "usual for physicians to address this issue with participants at least one prenatal visit". | |
| Outcomes | Smoking reduction and cessation, validated by exhaled CO monitoring. | |
| Notes | Program was developed with input from a questionnaire and open‐ended questions about the advantages and disadvantages of smoking when pregnant from local population to inform Health Belief Model used in program. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | An analysis within a subset of births in the RADIUS trial (births in Missouri, USA) to see whether ultrasound of the fetus at 18 ‐ 21 weeks and 31 ‐ 33 weeks promoted maternal smoking cessation during pregnancy. Randomisation by microcomputer based sequencing. Not clear whether caregivers were blinded to allocation. | |
| Participants | 53,367 pregnant women; ‐32,317 ineligible or excluded; leaving 21,050 ‐3,163 refused; ‐2,357 had miscarriage or change of provider; leaving 15,530 (7,812 intervention + 7,718 controls). subsequently ‐ 64 + 63 miscarriage, ‐131+121 records lost or women moved, leaving 7,617 + 7,534; 1,768 smoking (I) and 1,803 smoking (C). Smoking defined as any smoking within the year before their enrolment. Inclusion criteria = last menstrual period known within one week, gest age < 18 weeks, no plans to change providers. Exclusion criteria include medical or obstetric complications, planning an ultrasound for other reasons, twin pregnancy, not intending to continue pregnancy. | |
| Interventions | Ultrasound only, at 18 ‐ 20 and 31 ‐ 33 weeks, no details about feedback to the mother or others. The women in the control group only had ultrasounds if ordered by their physician for medical reasons. | |
| Outcomes | Self reporting smoking cessation, recorded on birth certificate, not biochemically validated (not included in outcome tables). Mean birthweight, preterm birth (< 36 weeks) and very preterm birth (< 33 weeks). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | A randomised comparison of the effects on health behaviours (including smoking) of providing specific verbal and visual feedback to the mother about fetal size, shape and movement during an ultrasound examination (or having the screen not visible and providing no specific feedback) at the first antenatal visit, in London, UK. No details of randomisation or whether caregivers blinded to allocation. | |
| Participants | Pregnant women at 10 ‐ 14 weeks gestation; 18 to 32 years; stable relationship; Caucasian; 85% had planned pregnancy, at low risk of complications; 86% nulliparous. Exclusions: prior miscarriage or extended infertility investigations. | |
| Interventions | Control: no/low feedback. | |
| Outcomes | Self reported smoking cessation at 16 weeks gestation, without biochemical validation. | |
| Notes | Not clear whether quitting was recent or not ‐ no time period specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Quasi‐randomised study (allocation by alternate weeks) of the effectiveness of a health education intervention provided by a psychologist from booking to birth, compared with standard care, at a large maternity hospital in England, 1978‐1979. Caregivers not masked to allocation. | |
| Participants | Pregnant women registering for maternity care: 371/1645 were currently smoking at least 1 cigarette/day, 25 refused participation and 27 were lost because of miscarriage, termination or transfer to another care provider, leaving 319. No exclusions were mentioned or mean cigarettes/day pre‐pregnancy. | |
| Interventions | Control: standard care not otherwise specified. | |
| Outcomes | Smoking cessation, biochemically validated with exhaled CO and serum thiocyanate. Mean birthweight in subgroup smoking >= 5 cigarettes at booking. | |
| Notes | Detailed account of the intervention in King and Eiser 1981. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Evaluation of a program to train obstetric and family practice residents to give smoking advice during antenatal care, using pre‐ and post‐ training evaluation of their skills with a simulated patient, and exit interviews with women participating in a randomised trial of individualised smoking cessation counselling. 1988‐1990, Vermont, USA. | |
| Participants | All residents providing (supervised) prenatal care, at the University of Vermont. | |
| Interventions | Description of training and copies of 4 papers on smoking cessation advice + small group training by physician and psychologist during 1 hour workshop. Workshop: review of the project; description of advice and rationale for each step; use of protocol prompt sheet; video of advice being offered by GP and Obstetrician; role play with corrective feedback; basic care description; (individual training for residents unable to attend) + 30 minute refresher session with counsellor before the rotation + counsellor discussed actual progress and adherence. | |
| Outcomes | Scores on video/simulated patient (blinded assessment, systematic scoring) significantly increased with no change in the time required to provide the advice; exit interviews showed good adherence to the protocol by 96/99 (intervention) and 66/67 (control) interviewees, as did women's proposed actions post‐intervention, also in exit interviews. | |
| Notes | Useful for dissemination trials of smoking cessation in hospitals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | A randomised trial comparing the effectiveness of individualised, but protocol‐based smoking cessation counselling provided by a specially trained health educator, compared with usual care, at the University of Vermont, USA, 1984‐1987. No details of randomisation and it is unclear whether caregivers were masked to allocation. | |
| Participants | Women receiving prenatal care from obstetricians + nurse‐midwives, or residents; private and public including Maternal, Infant & Child clinic for under‐insured or non‐insured women (23% Medicaid in study); < 25 weeks pregnant (mean gest 13/40), smoking at least 1 cigarette a day, no exclusions mentioned. 808 interviewed, 33 refused, 175 sp quitters went into separate study of relapse prevention, leaving 300 + 300; (‐49: 27 miscarriage, 7 fetal deaths, 5 infant deaths), further losses were 24 + 24 changed care provider, 37 (I) + 4 (C) withdrew and 31 + 28 were lost to follow up. Mean cigarettes/day pre‐pregnancy I = 24.4, C = 25.1. | |
| Interventions | Control: usual care, not otherwise specified. | |
| Outcomes | Smoking cessation at 36 weeks gestation, 75% biochemically validated with cotinine. Mean birthweight, low birthweight, other smoking‐related complications (pPROM, placental abruption and placenta praevia). | |
| Notes | Sample size calculated for 10% increase (from 10% to 20%) in quitting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of relapse prevention counselling for spontaneous quitters, Vermont USA.. See Secker‐Walker 1994 for methodology details. | |
| Participants | Those from Secker‐Walker 1994 who had stopped smoking spontaneously before their first prenatal clinic visit (n = 175, 89 (I) and 86 (C) among whom there were 5 miscarriages, 1 termination, 1 fetal death and 1 infant death leaving 85 (I) and 80 (C). Further losses were 15 transferred to other care, 9 dropped out and 8 lost to follow up. | |
| Interventions | Control: usual care by own provider. | |
| Outcomes | Smoking cessation, biochemically validated). | |
| Notes | Exclusion of fetal and infant deaths. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial comparing the added effectiveness for smoking cessation during pregnancy of a free videotape using peer role models, Vermont, USA, 1992‐1993. No details of randomisation. Caregivers not masked to allocation. | |
| Participants | Women in a state supported clinic for underinsured women, currently smoking at least 1 cigarette/day, 7/67 refused leaving 30 (I) + 30 (C), 4 had miscarriage leaving 26 + 30, 3 lost to follow‐up and 7 moved to another care‐provider leaving 17 + 27 seen at 36 weeks. Mean cigarettes per day pre‐pregnancy = 22.6. | |
| Interventions | Control: advice from obstetrician or nurse‐midwife + tip sheet on quitting. | |
| Outcomes | Smoking cessation in late pregnancy (36/40), biochemically validated with exhaled CO measurements.. | |
| Notes | Process evaluation included perceptions of the videotape contents and showed 53% viewed the videotape. 17% had no VCR, and 10% reported having no time. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | A trial of structured physician's advice supported by individual counselling (I) provided to pregnant women during prenatal care compared with usual care (UC), Vermont, USA, 1988‐92. Sample size justification. The study included a relapse prevention component, reported separately. No details of randomisation. Caregivers could not be masked to allocation. | |
| Participants | Woman attending the state‐supported (Maternal and Infant Care) prenatal clinic for underserved women or attending the Adolescent clinic for women 12 to 18 years. | |
| Interventions | All participants received: | |
| Outcomes | Smoking cessation maintained in late pregnancy (36/40) and 1 year postpartum, biochemically validated with exhaled CO and urine cotinine. | |
| Notes | Methods included a detailed process evaluation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | A randomised trial of an intervention to increase birthweight by changing maternal smoking, carried out in Baltimore, USA. No details of randomisation and it is unclear whether usual caregivers were masked to allocation. | |
| Participants | Pregnant women who were smoking >= 10 cigarettes/day immediately prior to pregnancy (71% of whom were spontaneous quitters), < 18 weeks gestation, attending 52 private physicians and the hospital antenatal clinic. Heterogeneous population, including large inner‐city and suburban. 89% of those eligible were recruited n = 935, 463 (I), 472 (C). Mean cigarettes/day pre‐pregnancy I = 20.9, C = 20.7. | |
| Interventions | Control: usual care, not further specified. | |
| Outcomes | Smoking in late pregnancy, 97% biochemically validated with salivary thiocynate. Miscarriage; fetal deaths; mean birthweight; low birthweight; very low birthweight; % Apgar scores < 7 at 1 minute and 5 minutes; length and head circumference. | |
| Notes | Change of criteria for enrollment after the first 185 as 35% of these had smoked < 10/day and 71% of that group had quit spontaneously with little relapse. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | A trial of a smoking cessation intervention on women's 'stages of change' (precontemplation, contemplation, preparation and action) was assessed. No details of randomisation process. | |
| Participants | Low income pregnant women enrolled in a state‐supported service for uninsured and under‐insured women, receiving care in a large obstetric group practice. 521 women smoking >= 1 cigarette/day at the onset of pregnancy enrolled, 349 (67%) completed assessments at 1st, 2nd and 36 week visits. Mean cigarettes/day pre‐pregnancy I = 22.8, C = 23.6. | |
| Interventions | Control: 3 minute physician‐delivered protocol at first visit, acknowledging her smoking, concerns re quitting or staying quit; strong recommendation to quit + cessation pamphlet designed for pregnant women. | |
| Outcomes | Shifts in 'Stage of change' at 2nd visit and 36 weeks gestation. | |
| Notes | Comment made that stages of change at the first visit are not sustained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of proactive telephone peer support in a large obstetric practice in Vermont, US, 1996‐7. No description of randomisation procedure. Caregivers not able to be masked to allocation. | |
| Participants | Women reporting smoking at least 1 cigarette in the past week at their first antenatal visit, were approached. Refusal rate = 19%. Women tended to be white, English speaking, and of lower income and education. No exclusion criteria specified. Control n = 74, Intervention n = 77. Mean cigarettes/day before pregnancy I = 22.6, C = 20.2. | |
| Interventions | Control group received brief smoking cessation advice from a MW/Obst at each of the 3 prenatal visits and stage appropriate printed materials. MWs/Obst were provided with a 45 minute training session. The intervention group received the same as the control group + offered telephone peer support (from a female ex‐smoker, who received 8 hours of training) for women with moderate or high intentions of quitting. who called the participant within several days to provide support for positive changes in smoking behaviour. | |
| Outcomes | Self reported abstinence at 28 ‐ 34/40 gestation, defined as no smoking for the past 7 days, biochemically validated with urine cotinine measurement. Movement in stages of change and proportion of smoking reduction by more than 50%. Attrition = 16 (10.6%). | |
| Notes | Process evaluation showed 53% received the peer intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of individualized stage of change, motivational smoking cessation intervention ("one‐to‐one"), with personalized feedback for "resistant" pregnant smokers, in 3 large multispecialty clinics in Texas, US. Random allocation determined by a computer generated list. Unclear whether caregivers masked to allocation. | |
| Participants | Women who continue to smoke at 28 week gestation, after having counselling and 8 self help booklets earlier in pregnancy care. Inclusion criteria were women fluent in English, over 18 yrs, over 20 weeks gestation at first an visit, and smoke more than 5 cigarettes per week prior to pregnancy. All women had group insurance. Eligibility interview participation rate 97%. All eligible included in randomised sample (n = 269), as data collection and implementation were adopted as routine procedures, and required to formal written consent. Women in the intervention group had significantly higher proportion of women smoking > 61 cigarettes/week before pregnancy (I = 57.9%, C = 43%) and a higher proportion of partners who smoke (I = 69.6%, C = 62.5%). | |
| Interventions | All women smoking at intake (< 20 weeks), were provided with MI counselling and motivational self help books, based on "stage of change" program shown to be effective by Ershoff et al. Women still smoking at 28 weeks were randomised to this study. The high intensity intervention group (and their partners) then received: a 20‐30 min MI telephone counselling call (conducted by trained counsellors and nurse health educators), a personalised, stages of change based feedback letter, and a final MI‐base telephone call conducted 4 ‐ 5 days after the feedback letter was sent. | |
| Outcomes | Self reported smoking cessation at 34 weeks gestation, validated by an anonymous urine cotinine subsample. Postpartum follow up (6w, 3m, 6m) interview response rate 61% (data collected from a separate survey, with financial incentives). Movement in "Stages of Change". Breastfeeding rates and general health behaviours obtained but not reported. | |
| Notes | Only 55% of the experimental group received the full intervention (32% were never able to be reached). Implementation analysis suggested an effect in women who received full implementation: 43% vs 34% control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of personalised, computer generated, smoking cessation messages, in 2 university hospitals in North Carolina & Michigan, USA, Dec 1996‐97. Randomisation by computer algorithm. Unclear whether caregivers masked to allocation. | |
| Participants | Women who have "smoked 100 cigarettes in their lifetime and still smoking" or "had quit since becoming pregnant", completed a self administered computer screening program to determine eligibility (no details of inclusion or exclusion criteria). 173 women participated. Mean cigarettes/day smoked before pregnancy I = 20.3, C = 18.7 (ns). | |
| Interventions | Control group received "a pregnant woman's guide to quit smoking" at the first visit. The intervention group entered personal data into a hand‐held computer at antenatal visits, which subsequently generated personalized tailored messages, which were posted to the woman. | |
| Outcomes | Self reported smoking cessation validated by urine cotinine at first visit, 24/40 and 6 weeks postpartum. Attrition rate 14% in control group, and 15.2% in experimental group. | |
| Notes | Numbers in paper inconsistent: I = 88, C = 85 in methods section, I = 104, C = 87 in results section. No justification for change of denominators ‐ assumption was ITFV were smokers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Pilot study of home based motivational interviewing for smoking cessation in a Glasgow Hospital, Scotland, March‐May 1997. Consenting women stratified and randomly allocated to 2 equal groups using blinded telephone allocation. Unclear whether caregivers masked to | |
| Participants | Self reported women who identified as smokers on a questionnaire at antenatal clinic booking. Participation rate 75%, 27 refused. (n = 100). Mean cigarettes/day pre‐pregnancy I = 19.6, C = 18.1. | |
| Interventions | The control group received usual advice from their prenatal providers, which should include information about smoking. The intervention group received 2 ‐ 5 motivational interviewing sessions, based on stages of change, in the clients home conducted by a midwife trained in smoking cessation counselling. High intensity intervention. | |
| Outcomes | Self reported smoking cessation, at 27/40 or more, with urine cotinine validation in 93%. Mean birthweight, preterm births. Ranking interviews measured movement around the "cycle of change". Detailed evaluation of participant and midwifery views of interventions. Attrition rate 2%. | |
| Notes | Good process evaluation of implementation quality according to Millers rating tool, showed 79% of women in the intervention group received at least 2 counselling sessions, and less than 20% of the control group recalled being given smoking information at the time of booking. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Trial of smoking cessation counselling and information packs in a large public antenatal clinic, in Rotunda Ireland, during 3 months in 1995. Randomisation by random number tables, allocation concealed by opaque sealed envelopes and restricted in groups of ten. Intervention provided by trained facilitator, with staff unaware of allocation. | |
| Participants | Inclusion criteria: women who currently smoke or had spontaneously quit since becoming pregnant; have a viable pregnancy; and intend to deliver in the hospital. Participation rate = 81% (n = 418). Intervention group were less likely to have spontaneously quit, or be employed. Mean gest at first visit I = 15.5, C = 15.3. Number of daily cigarettes at intake: 1‐9 I = 61, C = 54; 10‐19 I = 74, C = 73; 20+ I = 68, C = 65. | |
| Interventions | The control group completed a questionnaire at first visit, followed by routine prenatal advice on a range of health issues, from midwives and obstetricians. The intervention group received as for the control group + structured one to one counselling by a trained facilitator (based on stages of change theory); partners invited to be involved in the program; an information pack; and invited to join a stop smoking support group. A carbon monoxide monitor was available for the intervention group, to quantify smoking habit and act as a motivational tool. High intensity intervention. | |
| Outcomes | Smoking cessation at delivery, biochemically validated by exhaled CO. Reduction in mean cigarettes/day, quit attempts, comparisons of quitters and non quitters at various stages. | |
| Notes | Good process analysis and participant feedback of program implementation. A high baseline smoking prevalence rate (58.7%). Limited exhaled CO measurement on postnatal ward. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Quasi‐randomised trial of smoking cessation interventions (allocation to 1 of 4 arms, 3 intervention and 1 control, by date of enrollment for care, with the four time blocks assigned randomly) in women smoking at the time of the 18 week ultrasound scan, at a regional hospital in Norway, 1988. | |
| Participants | 283 women reported current smoking and wanted to quit. (mean 9‐11 cigarettes/day) at the 18 weeks scan: 200 recruited, 50 in each arm. 1/3 receiving private obstetric care. | |
| Interventions | Control: not specified. | |
| Outcomes | Smoking cessation assessed immediately after the intervention, biochemically validated but not reported. intervention arms. | |
| Notes | Biochemical validation of smoking status using salivary thiocyanate was carried out but not reported in the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Quasi‐randomised trial of cognitive‐behavioural modification, (using RA Windsor's self‐help manual translated into Norwegian) to promote smoking cessation in women smoking heavily at the time of the 18 week ultrasound scan, in Oslo 1990‐1991. No details of randomisation and caregivers not masked to allocation. | |
| Participants | Pregnant women attending the National University Hospital Oslo at 18 weeks for ultrasound, and smoking 10 cigarettes/day. No exclusion criteria mentioned and no refusals. 112 women recruited (1800 births/year, study over 15 months). Pre‐pregnancy mean cigarettes/day: I = 8, C = 11. | |
| Interventions | Control: information on the negative effects of smoking + encouragement to quit, reinforced by a pamphlet, provided at the time of the ultrasound examination. | |
| Outcomes | Smoking cessation in late pregnancy. No biochemical validation. | |
| Notes | Evidence is provided for an increase in smoking compared with 18 weeks, especially in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomised trial of hypnosis for smoking cessation and reduction among women still smoking at the time of the 18 week ultrasound scan in a Norwegian hospital, 1990‐1993. Randomised by random number tables, with usual caregivers masked to allocation. | |
| Participants | Women were offered participation if still smoking at 18 week ultrasound visit, (after explanation including potential allocation to control) and then randomised after signing. Expected numbers of women in the recruitment period were 630, 158 (25%) agreed to participate. Of 80 allocated to intervention 13 did not receive an appointment in time, 15 did not attend leaving 52. Mean cigarettes/day prior to pregnancy I = 15.6, C = 15.0. | |
| Interventions | Control: "routine pregnancy health care". | |
| Outcomes | Self reported smoking cessation, reduction and increase at end of pregnancy, not biochemically validated. | |
| Notes | Process evaluation did not rate the intervention highly: mean score of 2.05 on a 7 point scale. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Trial of a structured, cognitive‐behavioural, smoking cessation program for pregnant women delivered by usual care providers in a public hospital antenatal clinic in Newcastle, Australia, 1990‐1991. Randomised after consent by precoded questionnaires in opaque envelopes, with a computer generated sequence. | |
| Participants | 1909 pregnant women were screened at the first visit (approximately 12 weeks gestation). Classified as a smoker if they answered yes to the question "Are you a smoker?": 725 smokers (38%), ‐ 187 ineligible > 26 weeks, ‐ 47 too ill or disturbed, ‐11 other reasons left 538. 293 agreed to take part. 7 (I) + 7 (C) withdrew, 10 + 10 had a miscarriage or termination, 4 + 3 gave birth preterm, leaving 125 + 127. Baseline smoking data not specified. | |
| Interventions | Control: Doctor and Midwife both informed women that smoking was an important cause of pregnancy problems and they should stop; Midwife provided a package (sticker, pamphlet on risks of smoking and 2 page cessation guide), none of which were specifically tailored to pregnant women. | |
| Outcomes | Smoking status at mid and late pregnancy and postpartum, biochemically validated with salivary cotinine (I = 86%, C = 78%). | |
| Notes | Midwives involved in recruitment to the trial had variable 'success'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | A randomised trial, comparing the effectiveness of 2 smoking cessation interventions with standard care, in public health clinics in Birmingham, Alabama, USA 1983‐1984. No details of randomisation. Usual caregivers masked to allocation. Sample size justification. | |
| Participants | 1838 pregnant women were screened, 460 current smokers (">= 1 cigarette in the last 7 days"), ‐30 antenatal care entry >= 32 weeks, ‐9 left system or moved, ‐10 miscarriage or termination ‐10 went to group discussions (this intervention abandoned), leaving 102 I1), 103 (I2) and 104 (SC). No baseline data on cigarettes/day. | |
| Interventions | Control: 2‐3 minutes within a group prenatal education session at the 1st visit, when maternity clinic staff recommend quitting. | |
| Outcomes | Smoking cessation or reduction, biochemically validated by salivary thiocynate, at mid‐pregnancy and within 48 hours of birth. | |
| Notes | "Multiple attempts were made to bring pregnant smokers together for a peer‐led, focused group discussion: not feasible in this setting". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Trial of an enhanced cognitive behaviour therapy intervention, to assist in smoking cessation and smoking reduction during pregnancy in women attending public maternity clinics at a large hospital in Birmingham, Alabama, USA, 1986‐91. Randomisation by a computer‐generated system. Caregivers not masked to allocation. | |
| Participants | 4352 pregnant women screened at approximately 4 weeks gestation, 1381 (31.7%) reported smoking at conception, 1171 current smokers (smoked 1 cigarette even a puff in the last 7 days), ‐110 ineligible by entry to care > 32 weeks, did not complete first visit, did not return, in earlier trial, prisoner, reading level too poor, leaving 1061 of whom 67 refused leaving 493 (I) and 501 (C), ‐93 + 87 miscarriage, termination or withdrawal from public care, leaving 400 (I) + 414 (C). NS difference in baseline cotinine. | |
| Interventions | Control: 2 minute talk in 30 minute group session at first antenatal visit in which women were urged to quit and given 2 pamphlets: "Smoking and the two of you'"+ "Where to find help if you want to stop" including the name, contact phone number and cost of their local program. | |
| Outcomes | Smoking cessation at 32 weeks gestation, biochemically validated with salivary thiocyanate. | |
| Notes | Separate paper on spontaneous quitters (Lowe et al, 1997). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Evaluation trial of behavioural impact of new patient education methods ("SCRIPT"), provided by trained medicaid maternity care staff members, in Alabama, USA, 1997‐2001. 17 eligible counties (> 50 pregnant smokers per year) stratified (% black: white pop; % pregnant smokers) into 8 clusters and 50% randomly selected (no details). Usual caregivers not able to be masked to allocation. | |
| Participants | Women screened at first visit (9 ‐ 12 weeks gestation) for self‐reported smoking, validated by salivary cotinine. 2 separate phases: participation rate phase one (1997) = 95% (n = 93), phase 2 (1998) participation rate = 60% (n = 172). Phase one and 20% phase 2 group combined to form control group (n = 126), 80% phase 2 group (n = 139) formed intervention group. Both groups smoked approximately 10 cigarettes/d at baseline. | |
| Interventions | Nurses, social workers and WIC administrators received orientation sessions. Partnerships were developed for program implementation. Control group patients had self report smoking status ("Ask", and a saliva sample, and counselling ("advise"). Intervention (based on cognitive behaviour therapy) group were provided with 2 further components "assist and arrange", which included a motivational video to take home to show partners, "A pregnant woman's guide to quit smoking", and < 5 min counselling session. | |
| Outcomes | Self reported smoking status at 60 days after first visit, validated by salivary cotinine. Significant (> 50%) reduction in baseline cotinine (harm reduction measures). No quit attempts. Attrition rate 13% (n = 34), counted as smokers. | |
| Notes | Mixture of RCT/sequential study with main control group being recruited in phase one of the study to identify representative sample, and small additional control group recruited in phase 2 with the intervention group. Good process evaluation showed nearly 100% experimental group received the intervention, confirming the feasibility of routine delivery by regular staff. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Double‐blind, placebo controlled trial of nicotine replacement therapy (patches) in pregnant women in a Danish obstetric hospital. Women consenting to participate were randomly assigned in blocks of 6, to nicotine or placebo patches. The investigation team were blinded to allocation. | |
| Participants | Healthy women less than 22 weeks gestation who smoked more than 10 cigarettes per day after the first trimester, were invited to participate n = 611. Participation rate 41% (n = 250).Mean cigarettes per day at intake I:n = 13.4, C: n = 14.2. | |
| Interventions | Both groups received strong smoking cessation advice and counselling from a midwife, reinforced with printed materials. The control group received a placebo patch. The intervention group received 16 hour 15 mg nicotine patches for 8 weeks and 10 mg for 3 weeks. | |
| Outcomes | Self reported abstinence of at least 7 days at 2nd, 3rd, and 4th prenatal visits, validated by salivary cotinine measurement. Telephone follow up at 3 and 12 months postpartum (self report). | |
| Notes | Very low recruitment, with non‐participants smoking more cigarettes per day. Compliance lower for placebo group, who may have guessed allocation. Limited details on 3 months and 1 year follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
AFP: alpha fetoprotein
BP: blood pressure
CO: carbon monoxide
GP: general practitioner
HMO: Health Maintenance Organisation
LBW: low birth weight
min: minutes
MRFIT: randomised trial of health promotion carried out in the US
OPD: out‐patient department
Pls: principal investigators
ppm: parts per million
pPROM: preterm, prelabour rupture of the membranes
sae: stamped addressed envelope
ses: socioeconomic status
SHO: senior house officer
TFS: teen fresh start
TFSB: teen fresh start + peer support
UC: usual care
WIC: Food program for Women, Infants and Children in the US
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| There are no data provided by trial allocation. | |
| Data are available on uptake of programs at a hospital level but not at present on smoking cessation effectiveness or perinatal outcomes. | |
| Quasi‐experimental evaluation study of the "Healthy Baby Second Hand Smoke Study" uses historical controls. Good documentation of implementation problems. | |
| The intervention took place in one HMO clinic with historical controls from the same clinic and concurrent controls from a second clinic. There was no randomisation of clinics and no adjustment of the data for clustering. | |
| Study of effect of one 15 minute counselling session and a follow‐up telephone call, performed 1994‐95, using historical controls from 1993‐1994. | |
| In this quasi‐randomised study the intervention was carried out in one hospital with another hospital in the same city acting as a control, after a prior descriptive study which showed the similarity between the two in terms of social and demographic factors including smoking. There was no randomisation and recruitment differed substantially across the two sites. Data for smoking reduction and smoking cessation are combined in the paper with no separate data on cessation and no adjustment for clustering. | |
| Although the multicomponent intervention included a smoking change component there are no smoking data in the paper. | |
| General practitioners, rather than individual women, were randomly allocated to provide the intervention or not. There was no adjustment for cluster randomisation in the analysis of the study findings. | |
| Controlled study, not randomised, of effects of a population based smoking cessation program and its impact on smoking in pregnancy. Controls were matched on inclusion criteria from another district. | |
| Prenatal classes, rather than individual women, were randomly allocated to provide the intervention or not. The intervention was provided in late pregnancy with no outcome data collected during pregnancy but only data four months after birth. There was no adjustment for cluster randomisation in the analysis of the study findings. | |
| Four WIC clinics in Los Angeles were matched and randomised within pairs to intervention or control status. There was no adjustment for clustered data. All those not contacted at postpartum visit (28%) were excluded even though they should be counted as smokers; their allocation is not stated so adjustment cannot be made for this. There was significant misclassification of self‐reported non‐smoking status and 44% did not provide a sample for cotinine analysis so that verified non‐smoking cannot be calculated. | |
| Data are available on uptake of programs at a hospital level but not at present on smoking cessation effectiveness or perinatal outcomes. | |
| Primary care practices, rather than individual women, were randomly allocated to provide the intervention or not. There was no adjustment for cluster randomisation in the analysis of the study findings. | |
| Data are provided on those who stopped smoking only, not data by trial allocation. | |
| Data are provided on those who stopped smoking only, not data by trial allocation. | |
| Outcome data on child development in this paper have been excluded because the multicomponent interventions being compared might have had effects on child development other than by a change in maternal smoking. | |
| This 3 armed randomised controlled trial of home visiting by paraprofessionals and nurses was excluded as it did not contain any quitting data, only urine cotinine measurements. | |
| The intervention in this trial was unusual in that the focus was on anticipated benefits of smoking cessation to women themselves (not on harm to the fetus and infant), and on alternative coping strategies, with a designated midwife‐facilitator to answer queries and provide friendly advice and encouragement. The intervention was carried out in one hospital with another being a comparison setting, after a prior study which showed the similarity between the two in social and demographic factors including smoking rates. There was no randomisation. Recruitment differed significantly across the two hospitals. Data for smoking cessation and smoking reduction are combined with no separate data on cessation and no adjustment for clustering. | |
| This quasi‐experimental study of the impact of using interactive software to promote smoking cessation, was excluded as it used historical controls. | |
| Data on smoking reduction and smoking cessation are combined with no separate data on smoking cessation. | |
| This prospective quasi‐experimental study design to test the effect of a low intensity intervention, used historical controls. | |
| This quasi‐randomised (clinic day allocation) study of the effect of midwifery training on smoking cessation intervention implementation and pregnancy outcomes, was excluded due to concerns about allocation concealment. |
HMO: Health Maintenance Organisation
WIC: Food program for Women, Infants and Children in the US
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 47 | 13882 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.96] |
| Analysis 1.1  Comparison 1 All trials, Outcome 1 Continued smoking in late pregnancy. | ||||
| 2 Mean birthweight Show forest plot | 16 | 13618 | Mean Difference (IV, Random, 95% CI) | 33.03 [11.32, 54.74] |
| Analysis 1.2  Comparison 1 All trials, Outcome 2 Mean birthweight. | ||||
| 3 Low birthweight (under 2500 g) Show forest plot | 13 | 8930 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| Analysis 1.3  Comparison 1 All trials, Outcome 3 Low birthweight (under 2500 g). | ||||
| 4 Very low birthweight (under 1500 g) Show forest plot | 3 | 4765 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.69, 2.32] |
| Analysis 1.4  Comparison 1 All trials, Outcome 4 Very low birthweight (under 1500 g). | ||||
| 5 Preterm birth (under 37 or under 36 weeks) Show forest plot | 11 | 10932 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.98] |
| Analysis 1.5  Comparison 1 All trials, Outcome 5 Preterm birth (under 37 or under 36 weeks). | ||||
| 7 Stillbirths Show forest plot | 5 | 4525 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.71, 1.88] |
| Analysis 1.7  Comparison 1 All trials, Outcome 7 Stillbirths. | ||||
| 8 Neonatal deaths Show forest plot | 3 | 4143 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.34, 4.01] |
| Analysis 1.8  Comparison 1 All trials, Outcome 8 Neonatal deaths. | ||||
| 9 Perinatal deaths Show forest plot | 3 | 4335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| Analysis 1.9  Comparison 1 All trials, Outcome 9 Perinatal deaths. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 35 | 10362 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.97] |
| Analysis 2.1  Comparison 2 Trials with biochemically validated smoking cessation, Outcome 1 Continued smoking in late pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 25 | 7819 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.93, 0.97] |
| Analysis 3.1  Comparison 3 Interventions with high quality scores, Outcome 1 Continued smoking in late pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 17 | 5252 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.94, 0.98] |
| Analysis 4.1  Comparison 4 Interventions of high intensity with high quality scores and biochemically validated smoking cessation, Outcome 1 Continued smoking in late pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 48 | 13884 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.96] |
| Analysis 5.1  Comparison 5 Trials subgrouped by intervention intensity, Outcome 1 Continued smoking in late pregnancy. | ||||
| 1.1 High intensity | 35 | 10503 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.97] |
| 1.2 Medium intensity | 10 | 2246 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.97] |
| 1.3 Low intensity | 3 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 46 | 13603 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.96] |
| Analysis 6.1  Comparison 6 Trials subgrouped by intervention strategy, Outcome 1 Continued smoking in late pregnancy. | ||||
| 1.1 Cognitive behaviour strategies | 20 | 6155 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.92, 0.97] |
| 1.2 Stages of Change | 7 | 1465 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.02] |
| 1.3 Feedback | 3 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.11] |
| 1.4 Rewards | 2 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.72, 0.82] |
| 1.5 Nicotine Replacement Therapy | 3 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.92, 0.98] |
| 1.6 Other | 11 | 3609 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking in late pregnancy Show forest plot | 5 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| Analysis 7.1  Comparison 7 Trials to prevent smoking relapse in women who stopped smoking in early pregnancy, Outcome 1 Smoking in late pregnancy. | ||||
Median intensity score over time

Comparison 1 All trials, Outcome 1 Continued smoking in late pregnancy.
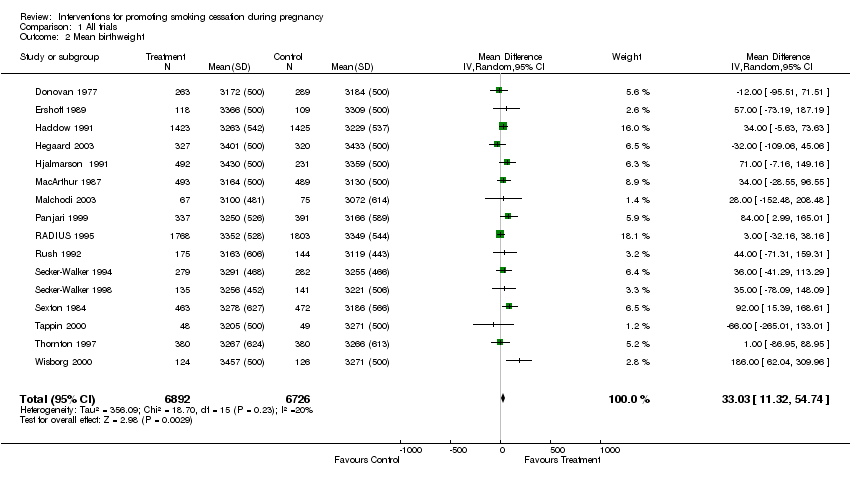
Comparison 1 All trials, Outcome 2 Mean birthweight.
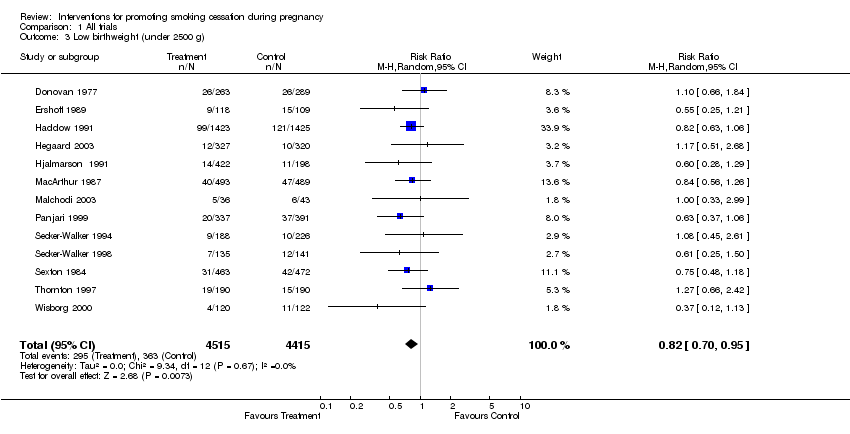
Comparison 1 All trials, Outcome 3 Low birthweight (under 2500 g).
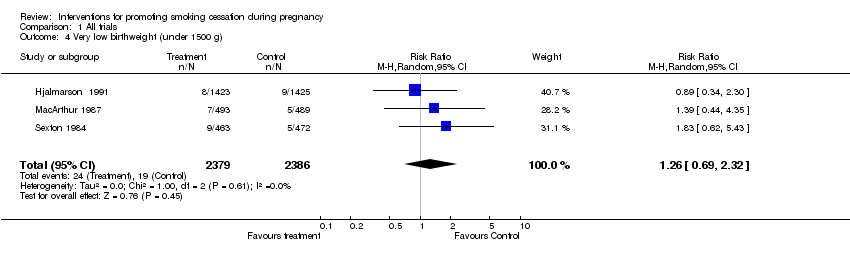
Comparison 1 All trials, Outcome 4 Very low birthweight (under 1500 g).

Comparison 1 All trials, Outcome 5 Preterm birth (under 37 or under 36 weeks).

Comparison 1 All trials, Outcome 7 Stillbirths.
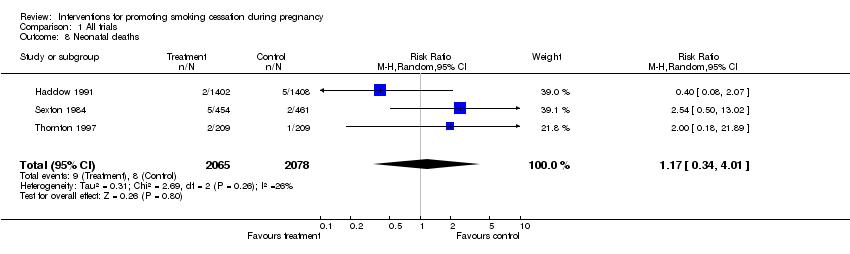
Comparison 1 All trials, Outcome 8 Neonatal deaths.

Comparison 1 All trials, Outcome 9 Perinatal deaths.

Comparison 2 Trials with biochemically validated smoking cessation, Outcome 1 Continued smoking in late pregnancy.

Comparison 3 Interventions with high quality scores, Outcome 1 Continued smoking in late pregnancy.

Comparison 4 Interventions of high intensity with high quality scores and biochemically validated smoking cessation, Outcome 1 Continued smoking in late pregnancy.
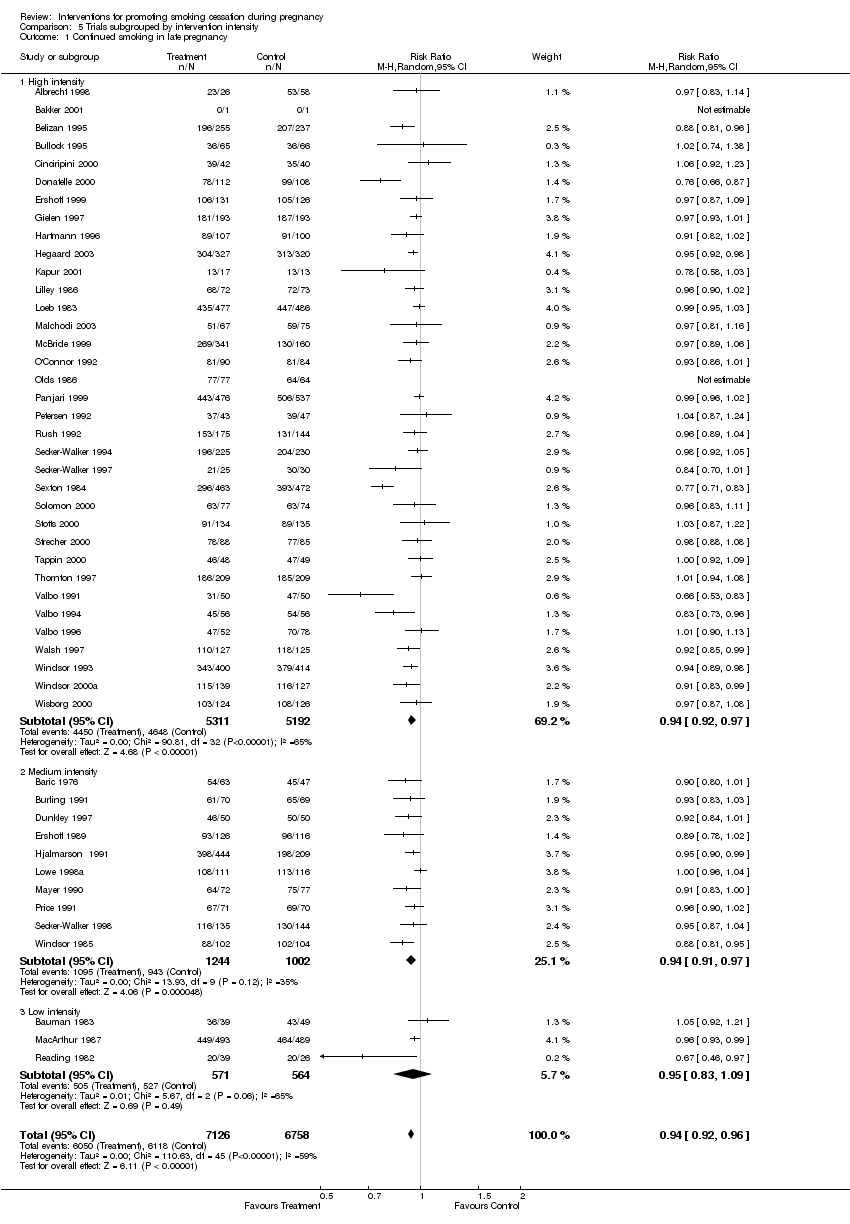
Comparison 5 Trials subgrouped by intervention intensity, Outcome 1 Continued smoking in late pregnancy.
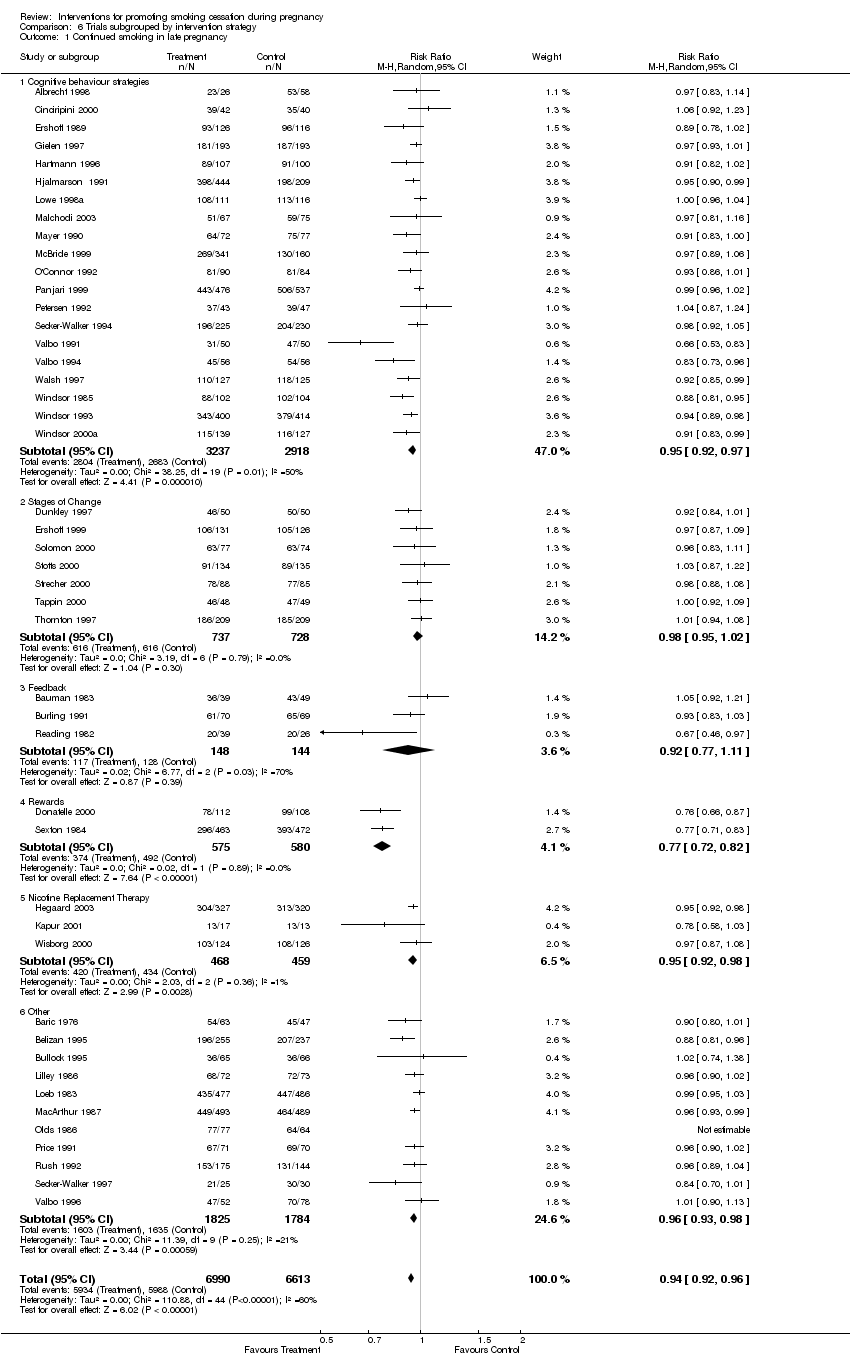
Comparison 6 Trials subgrouped by intervention strategy, Outcome 1 Continued smoking in late pregnancy.

Comparison 7 Trials to prevent smoking relapse in women who stopped smoking in early pregnancy, Outcome 1 Smoking in late pregnancy.
| Trial ID | Outcome measurement | Adjustment | Effect | 95% CI |
| Kendrick 1995 | Biochemically validated smoking cessation at 8 months of pregnancy | Odds ratio adjusted | 1.0 | 0.69‐1.6 |
| Manfredi 1999 | Self reported smoking cessation at 5‐8 weeks post birth | Odds ratio Z‐score adjusted | 0.67 | 0.38‐1.15 p = 0.006 |
| Bakker 2000b | Self reported smoking cessation at 6 weeks post birth | Odds ratio adjusted | 0.42 | 0.18‐0.97 |
| Hajek 2001 | Biochemically validated smoking cessation at birth | Odds ratio | 0.86 | 0.48‐1.57 |
| Moore 2002 | Biochemically validated smoking cessation at 6‐7 months of pregnancy | Odds ratio adjusted | 0.89 | 0.48‐1.57 |
| Lawrence 2003 | Biochemically validated smoking cessation at 10 days post birth | Odds ratio adjusted | 0.58 | 0.26‐1.33 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 47 | 13882 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.96] |
| 2 Mean birthweight Show forest plot | 16 | 13618 | Mean Difference (IV, Random, 95% CI) | 33.03 [11.32, 54.74] |
| 3 Low birthweight (under 2500 g) Show forest plot | 13 | 8930 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 4 Very low birthweight (under 1500 g) Show forest plot | 3 | 4765 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.69, 2.32] |
| 5 Preterm birth (under 37 or under 36 weeks) Show forest plot | 11 | 10932 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.98] |
| 7 Stillbirths Show forest plot | 5 | 4525 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.71, 1.88] |
| 8 Neonatal deaths Show forest plot | 3 | 4143 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.34, 4.01] |
| 9 Perinatal deaths Show forest plot | 3 | 4335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 35 | 10362 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 25 | 7819 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.93, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 17 | 5252 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.94, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 48 | 13884 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.96] |
| 1.1 High intensity | 35 | 10503 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.97] |
| 1.2 Medium intensity | 10 | 2246 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.97] |
| 1.3 Low intensity | 3 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continued smoking in late pregnancy Show forest plot | 46 | 13603 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.92, 0.96] |
| 1.1 Cognitive behaviour strategies | 20 | 6155 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.92, 0.97] |
| 1.2 Stages of Change | 7 | 1465 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.02] |
| 1.3 Feedback | 3 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.11] |
| 1.4 Rewards | 2 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.72, 0.82] |
| 1.5 Nicotine Replacement Therapy | 3 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.92, 0.98] |
| 1.6 Other | 11 | 3609 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking in late pregnancy Show forest plot | 5 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |

