Buflomedil for intermittent claudication
Abstract
Background
Intermittent claudication (IC) is pain caused by chronic occlusive arterial disease, that develops in a limb during exercise and is relieved with rest. Buflomedil is a vasoactive agent used to treat peripheral vascular disease. However, its clinical efficacy for IC has not yet been critically examined. This is an update of a Cochrane review first published in 2000, and previously updated in 2007 and 2008.
Objectives
To evaluate the available evidence on the efficacy of buflomedil for IC.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched January 2013) and CENTRAL (2012, Issue 12).
Selection criteria
Double‐blinded, randomized controlled trials (RCTs) in patients with IC (Fontaine stage II) receiving oral buflomedil compared with placebo. Pain‐free walking distance (PFWD) and maximum walking distance (MWD) were analysed by standardized exercise test.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted study authors for additional information.
Main results
We included two RCTs with 127 participants. Both RCTs showed moderate improvements in PFWD for patients on buflomedil. This improvement was statistically significant for both trials (WMD 75.1 m, 95% confidence interval (CI) 20.6 to 129.6; WMD 80.6 m, 95% CI 3.0 to 158.2), the latter being a wholly diabetic population. For both RCTs, MWD gains were statistically significant with wide confidence intervals (WMD 80.7 m, 95% CI 9.4 to 152; WMD 171.4 m, 95% CI 51.3 to 291.5), respectively.
Authors' conclusions
There is little evidence available to evaluate the efficacy of buflomedil for IC. Most trials were excluded due to poor quality. The two included trials showed moderately positive results; these are undermined by publication bias since we know of at least another four unpublished, irretrievable, and inconclusive studies.
Buflomedil's benefit is small in relation to safety issues and its narrow therapeutic range.
Plain language summary
Buflomedil for intermittent claudication
Intermittent claudication (IC) is pain that develops in a limb (mostly calves and thighs) during exercise and is relieved with rest. It is caused by insufficient blood flow due to peripheral arterial occlusive disease.
Treatment should contain all measures of secondary prevention of cardiovascular diseases. Regular exercise and smoking cessation is the most effective therapy to improve the symptoms of claudication. Drug treatments include vasoactive agents to improve blood flow (such as vasodilators and other hemorheologic agents that reduce blood viscosity), anticoagulants, antiplatelet agents and lipid‐lowering agents. Only a minority of patients undergo angioplasty or vascular surgery.
Buflomedil is a vasoactive agent widely used to treat intermittent claudication. The review authors identified eleven trials but could not use nine of them because of the methodologies used and high risk of bias. The two remaining controlled trials randomised a total of 127 participants to receive buflomedil or placebo for at least three months. One of these trials involved 40 participants with diabetes. Taken together, the trials showed moderately positive results for improvements in pain‐free walking distance on a treadmill (76.9 m, 95% CI 32.3 to 121.5) and maximum walking distance (112.6 m, 95% 27.7 to 197.5) with buflomedil for 12 weeks, showing a wide variation in benefit between participants.
The excluded studies consisted of three small marginally positive studies and one larger negative study. At least another four unpublished studies could not be retrieved and were reported to have inconclusive results.
Recent safety concerns have been raised about buflomedil because of lethal and non‐lethal neurologic and cardiovascular advents events in cases of accidental and voluntary overdoses.
The benefit of buflomedil is small in light of relatively little evidence on efficacy and narrow therapeutic range along with recent safety issues.
Authors' conclusions
Background
Description of the condition
Chronic occlusive arterial disease of the lower extremities can present itself in different ways:
-
asymptomatic arterial insufficiency;
-
symptomatic disease presenting as intermittent claudication, i.e. pain that develops in the affected limb with exercise and is relieved with rest;
-
rest pain with, or without, atrophic skin disorders;
-
critical leg ischemia, in which the ischemic process endangers part or all of the lower extremity.
Atherosclerosis is the most common cause of chronic arterial occlusive disease of the lower extremities. It causes arterial narrowing, or occlusion, which reduces blood flow to the lower limb during exercise or at rest. In the clinical condition of intermittent claudication, the severity of the symptoms depends upon the extent of the narrowing and the collateral circulation.
Description of the intervention
Patients with intermittent claudication are most often managed conservatively. Only a minority (10% to 25%) (Verhaeghe 1998) of the patients undergo angioplasty or vascular surgery. Regular exercise (Leng 2000) and smoking cessation can improve the symptoms of claudication and may be beneficial for the often associated coronary artery or cerebrovascular disease or both.
Conservative treatment should achieve:
-
improvement of functional capacity, i.e. an increase in walking distance;
-
inhibition of progression of atherosclerotic lesions;
-
reduction of cardiac and cerebrovascular morbidity and mortality.
Many types of drugs are used in the treatment of intermittent claudication: vasoactive agents (vasodilators and hemorheologic agents), antithrombotic agents, antiplatelet agents, lipid‐lowering agents. Trials have also been performed with vitamin E (Kleijnen 1998), garlic (Jepson 1997) and sex hormones (Price 2001) with varying results.
How the intervention might work
Vasodilators cause vasodilation in the arterioles of the lower limbs. However, they are thought to be ineffective because large vessel dimensions are fixed by the atherosclerotic process and collaterals are already maximally dilated in patients with intermittent claudication. Sometimes vasodilators may even worsen ischemia due to a 'steal' phenomenon. Hence, the interest has shifted from vasodilators to drugs that improve flow by altering viscosity. Decreased erythrocyte deformability and abnormal whole blood viscosity are present in patients with peripheral arterial disease and offer potential therapeutic targets for agents that affect viscosity (hemorheologic agents). One of these agents is buflomedil, for which beneficial effects on microcirculation of the legs and on cerebral blood flow in patients with cerebrovascular disease have been claimed. Pathophysiological research has shown that buflomedil inhibits platelet aggregation, may improve red cell deformability and reduces whole blood and plasma viscosity by reducing plasma fibrinogen (Bevan 1992).
Why it is important to do this review
Buflomedil was registered before the era of rigorous regulatory review and was accepted for peripheral arterial occlusive disease (PAOD) and for cerebrovascular disease. Its exact market for these two indications is not known but is predominantly for PAOD. The product is still widely used. Health insurers in many countries have become increasingly critical of the efficacy of this product and sometimes have restricted or abolished reimbursement. Therefore, a reassessment of the efficacy of this product is timely.
Objectives
To collect and to evaluate systematically the available evidence from placebo‐controlled, double‐blind randomized controlled trials (RCTs) on the efficacy of buflomedil, given orally, in intermittent claudication (Fontaine stage II) (Fontaine 1954), by measuring the pain‐free walking distance (PFWD) and the maximum walking distance (MWD), with a standardized treadmill test.
Methods
Criteria for considering studies for this review
Types of studies
Included studies were prospective, randomized, double blind, placebo‐controlled, parallel or cross‐over trials involving more than 30 participants. For cross‐over studies, only the first period of the cross over was considered (Cameron 1988).
Types of participants
Patients with intermittent claudication in Fontaine‐stage II (with the criteria explicitly described), regardless of duration of onset or smoking status. Studies including patients with inflammatory arteriopathy, thromboangiitis obliterans, acute ischemia, purely neuropathic ulceration or gangrene necessitating immediate amputation and attempted reconstruction or sympathectomy within the preceding three months or both were excluded.
Types of interventions
Interventions using buflomedil orally in doses of 300 mg to 900 mg per day, compared with a placebo control group.
Types of outcome measures
Primary outcomes
The primary outcome measure was the pain‐free walking distance (PFWD), assessed by treadmill exercise using prespecified criteria, with a minimum of three months duration between baseline and outcome assessment.
Secondary outcomes
The secondary outcome measure was the maximum walking distance (MWD).
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched January 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 12, part of The Cochrane Library (www.thecochranelibrary.com). See (Appendix 1) for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the (Specialised Register) section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
Authors' searches
For the original review the authors searched MEDLINE for the free text string 'buflomedil'. To identify randomized controlled trials (RCTs) we first used the systematic search strategy suggested by the Cochrane Collaboration (Dickersin 1994). However, this search was too restrictive. We then simplified our search strategy by crossing the active ingredient name with the MeSH term "intermittent claudication". This resulted in references to case reports, editorials, reviews, and original study reports. We obtained the full text of the publications pertaining to intermittent claudication and checked their reference lists for additional studies. We consulted the International Pharmaceutical Abstracts. We asked Abbott, the distributor of the drug, to provide reports of controlled clinical trials available to them. From this pool of studies we extracted the placebo‐controlled RCTs matching our criteria for patient selection, intervention and outcome (PIO‐criteria). We finally submitted a core of relevant articles to a Science Citation Index search.
Data collection and analysis
Selection of trials
Initially, we attempted to construct a data file of individual patients with a predefined list of variables. However this failed because we were unable to retrieve individual data, either by extraction from the published report or by seeking direct access to the original data from the authors. Hence, data extraction was performed on aggregated data for walking distances, as published in the study reports.
Assessment of methodological quality
The set of retrieved trials, complying with our inclusion criteria, was then subjected to a quality assessment.
Structured abstracts were made of every original article retrieved, in order to facilitate the collective quality evaluation process among the three authors. Three authors (TDB, RVS, MGB) independently assessed articles, structured abstracts and checklists. Discrepancies were discussed until consensus was reached. In a first round of quality assessment, we used the minimal quality criteria for PAOD trials described by Cameron et al (Cameron 1988): i.e. minimum of three months duration between baseline and outcome assessment, not less than thirty participants in total, and sufficient detail in reporting of variability of results (standard deviation or standard error of the mean given). The studies were also submitted to a more fundamental assessment of internal validity, according to the model of Schulz (Schulz 1995) and the model of Jadad (Jadad 1996). According to the methodology proposed by The Cochrane Collaboration (Mulrow 1999), studies were graded "A" if there was a low risk of bias (no flaws in design and internal validity), "B" if there was a moderate risk of bias and "C" if there was a high risk of bias (major flaws in design or internal validity or both or not conforming to the minimal criteria of Cameron) (Cameron 1988).
In some publications, results were not reported in sufficient detail. Attempts were made to contact the author(s) by phone, fax or e‐mail without success in all cases.
Extraction of data
We collected data on trial duration, participants' age, sex, smoking status, presence of diabetes comorbidity, dosage, the exclusion criteria, and the location of the arterial stenosis. Finally, the dropout rates in placebo and active limb were compared.
Statistical analysis
The evidence was summarized by extracting the mean and standard deviation of the initial and final walking distances for the active substance and for placebo. The differences in incremental gain between active and placebo groups for PFWD and MWD with their confidence intervals were calculated, using an approximate method, described by Gardner and Altman (Gardner 1989a; Gardner 1989b; Gardner 1989c; Gardner 1989d).
Results
Description of studies
Eleven trials were identified as described above, but nine were subsequently excluded.
Results of the search
Our search in the bibliographic databases resulted in the identification of four eligible trials (Bisler 1983; Fonseca 1988; Limbs 2008; Trübestein 1984). We also identified a meta‐analysis (Walker 1995), with references to ten trials, (Baitsch 1983; Bisler 1983; Trübestein 1984; Diamantopoulos 2001; Zinnagl 1986; Levien 1983; Lowe 1987; Lund 1988; Olsson 1986; Raithel 1985). We were able to retrieve the full text of seven eligible RCTs, six published (Bisler 1983; Diamantopoulos 2001; Fonseca 1988; Limbs 2008; Trübestein 1984; Zinnagl 1986) and one unpublished (Lowe 1987).The original text of one published trial was sent to us by the primary author (Diamantopoulos 1989). This trial was published again in 2001 (Diamantopoulos 2001). One other published (Zinnagl 1986) trial was sent to us in full text by one of the authors of the above mentioned meta‐analysis. We made multiple requests for the unpublished trials (Levien 1983; Lund 1988; Olsson 1986; Raithel 1985).
Included studies
Trübestein (published trial) (Trübestein 1984), conducted a well designed multicenter trial with 113 patients. Twenty dropouts were adequately described. The duration of the intervention period was 12 weeks. There was a statistically significant increase in PFWD and in MWD in the buflomedil group versus the placebo group.
Diamantopoulos (published trial) (Diamantopoulos 2001), conducted a rather small study (N = 40), with a wholly diabetic population of claudicants. There were six well described dropouts. The treatment period was six months.
Excluded studies
From the abstracts, we were able to determine that one trial (Baitsch 1983), was not eligible because the intervention was with intravenous treatment. Four trials were excluded because of high risk of bias due to problems of methodological quality: the duration of one trial was not long enough (Bisler 1983); the other three trials presented problems of internal validity and failed to give elementary details on variability of the results (Fonseca 1988; Lowe 1987; Zinnagl 1986). Four of the excluded studies consisted of three small marginally positive studies (Bisler 1983; Fonseca 1988; Zinnagl 1986) and one larger negative study (Lowe 1987).
We were unable to retrieve the full texts of the other four unpublished trials (Levien 1983; Lund 1988; Olsson 1986; Raithel 1985). In the Excluded studies table we summarized the available information from these unpublished trials extracted from the tables in the meta‐analysis (Walker 1995).
The multicenter LIMB trial (Limbs 2008) randomized 2,078 Fontaine stage II PAOD patients with an ABI between 0.3 and 0.8 to buflomedil 300 mg BID (150 mg twice daily in case of Creatinine (Cr) Clearance < 40 ml/min) or placebo. Baseline characteristics in both groups were similar. After a median follow up of 2.75 years, patients on buflomedil (1,043 patients) had fewer lower limb amputations, cardiovascular death and total death compared to patients on placebo (1,035 patients) (9% versus 12%). Patients on buflomedil had significant improvement in pain‐free walking distance compared to controls (median increase of PFWD of 43% versus 0%). The drug was well tolerated. Dr Jeffrey W Olin of Mount Sinai School of Medicine, New York, NY, criticized the findings in the trial by pointing out potential confounds and sources of error: low to zero placebo effect; 62% of patients were Russian, with the rest distributed among the Czech Republic, France and Hungary; no information to assess internal validity; measurement of ABI questioned; WD clinically estimated (no treadmill) (Wood 2005). The study raises many questions on the performance, results and reporting of the study. Since the author did not respond, many questions still remain unanswered.
Risk of bias in included studies
The two included trials (Diamantopoulos 2001; Trübestein 1984) were both rated "B" (moderate risk of bias) because neither would stand up to modern standards of performing and reporting clinical trials.
Effects of interventions
Trübestein 1984: Patients assessed their walking ability as improved and the sensation after physical stress as diminished. The authors only report a statistically significant increase in PFWD (P < 0.001) and MWD (P < 0.01) in the buflomedil group versus placebo group was found between the first (pre‐trial) and fifth (after 84 days of treatment) examinations. The arithmetic means of the pressure difference (brachial artery‐posterior tibial artery) in the placebo and in the buflomedil group both showed a decrease when the first and fifth visits were compared.
Diamantopoulos 2001: The patients showed significant differences at the 5% level in mean increases in walking distances between the two groups at the three month examination. Mean increases over baseline in PFWD for the buflomedil and placebo groups were 52.8 m and 8.6 m respectively (P = 0.018) and for MWD 81.1 m and 8.8 m respectively (P = 0.022). The mean increases in both groups in PFWD at six months over baseline were not significantly different (P = 0.059) even though the actual difference was substantial (112.20 m for buflomedil; 31.6 m for placebo). The mean increases in MWD at six months showed a statistically significant difference (P = 0.011) (191.9 m for buflomedil; 20.5 m for placebo). The systolic blood pressure indices at rest and after exercise showed no statistically significant differences between the groups. No statistically or clinically significant changes were noted in any hematological or biochemical values. The drug was well tolerated by all patients and only minor side effects were observed during the six months of treatment. All participants in this trial were diabetic.
The two RCTs accepted after quality evaluation (Trübestein 1984; Diamantopoulos 2001) showed moderately positive results for the PFWD: (WMD 75.1 m, 95% CI 20.6 to 129.6; WMD 80.6 m, 95% CI 3.0 to 158.2) metres gain of active (buflomedil) over placebo, respectively. The gains in maximum walking distance (MWD) were (WMD 80.7 m, 95% CI 9.4 to 152; WMD 171.4 m, 95% CI 51.3 to 291.5), respectively. Both results were statistically significant but had wide confidence intervals. Pooling of data could not be performed because only two studies conducted in heterogeneous populations were included.
Discussion
Only randomized placebo‐controlled trials were considered for this systematic review because the course of intermittent claudication is highly variable and there is no gold standard for pharmacological treatment of this condition. We chose the standardized treadmill test as the common outcome measure because it is the best available method to measure relevant endpoints (pain‐free and/or maximum walking distances/times). Also, it is the most universal measure in studies in this research field although its value has been criticized (Wurzinger 1987). Studies on endpoints such as blood flow, blood viscosity, ankle/arm index, platelet aggregation, etc. are of interest, but the clinical relevance of changes of such endpoints by medication is not known and no correlation can be found between the clinical parameters and these surrogate endpoints (Wurzinger 1987). To the best of our knowledge, studies having mortality or amputation rate as endpoints do not exist. In any case, these would require a huge sample size because of the low frequency of these events in PAOD. Quality of life would be an interesting outcome measure. A disease‐specific questionnaire, the CLAU‐S scale, has been developed (Spengel 1997), and applied as an endpoint in the evaluation of naftidrofuryl versus placebo (Liard 1997). However, after careful scrutiny of the validity of the CLAU‐S scale, we did not consider it to be a convincing instrument for the measurement of quality of life because it has not yet been validated against the standardized treadmill test. The CLAU‐S scale has not been used in studies with buflomedil.
Our quality evaluation of the trials was pragmatic because most of the retrieved trials dated from before 1992 and did not conform to the modern quality criteria of conducting and reporting RCTs (Jadad 1996; Schulz 1995).
Only two more‐or‐less acceptable studies were included. There was large heterogeneity between patient groups in the included and excluded studies. The lack of information on the patients included, the small sample sizes and the small number of trials did not allow relevant subgroup analysis.
Pooling of the data could not be performed in that only two studies with heterogeneous populations were included.
In Walker and MacHannaford's meta‐analysis (Walker 1995), nine heterogeneous studies were pooled, of which two studies (the same two as included in our analysis) showed a statistically significant difference. The remaining seven studies in their meta‐analysis were not statistically significant; two were published trials excluded by us because of problems of internal validity; one trial was unpublished but retrieved by us from the author and subsequently excluded again because of problems of internal validity; four trials were unpublished, with only scant information about the quality of execution of the trial and on the variability of the results. The pooled effect size weighted for sample size of these nine studies was 0.27 (CI 0.11 to 0.42) for pain‐free walking distance and 0.28 (0.12 to 0.45) for maximum walking distance, which cannot be considered as a convincing indication of efficacy.
It is unlikely that we have missed studies with a positive result in our quest for evidence. We know of the existence of four unpublished buflomedil trials (Levien 1983; Lund 1988; Olsson 1986; Raithel 1985) which were cited in a meta‐analysis (Walker 1995). After examination of the effect sizes of these unpublished trials as reported in this meta‐analysis, their results can be considered to be inconclusive. This contrasts with the approach and optimistic conclusions of the meta‐analysis of Walker and Mac Hannaford (Walker 1995).
Hence, we concluded that under these circumstances statistical pooling is not appropriate and that the evidence on the efficacy of buflomedil is insufficient, and affected by publication bias.
Also recently the safety of buflomedil came into attention. In 2006, a safety crisis emerged in France concerning lethal and non‐lethal neurological and cardiovascular advents events in cases of accidental and voluntary overdoses with buflomedil, mainly with the 300 mg dosage form (Marimbert 2006).
As a consequence, a number of regulatory actions were taken in France (Marimbert 2006):
-
Change in toxicology information:
-
Minimal toxic dose: 3 gram
-
Lethal dose: 6 gram.
-
-
Withdrawal of the 300 mg dosage form, with only 150 mg tablets still available on the market.
-
Deletion of the indication Raynaud phenomenon. Only indication: symptomatic treatment of intermittent claudication.
-
Classification as drug with narrow therapeutic range.
-
Contra‐indication in severe renal insufficiency (Cr Clearance < 30 ml/min).
-
Posology: adaptation of the dose in mild to moderate renal insufficiency (Cr Clearance 30 to 80 ml/min): 1 tablet of 150 mg in the morning and evening, control of renal function before and at regular intervals during treatment, each time recall the adverse neurological and cardiovascular effects in case of no respect or no adaptation of dose in renal insufficiency.
The published trials on buflomedil do not mention numerical safety data, suitable for systematic appraisal. The absence of adverse event data from clinical trials might indicate that the drug is well tolerated within clinical trials, as a number of authors conclude. However, we consulted the international literature and overviews of reported adverse events for regulatory purposes with the following results.
a) The international literature
The terms which were used to search PUBMED are described in Appendix 2.
Forty‐ three articles were retrieved, of which 30 were relevant, 10 of which reported on fatal accidents.
From this literature search it is obvious that accidental or voluntary overdose with buflomedil is dangerous. The drug is toxic at doses as low as 3 gram and potentially fatal at moderate doses of 6 gram, with kidney insufficiency as an aggravating factor.
b) Overview of reported adverse events for regulatory purposes (database from May 1979 through to December 2006).
Sixty‐five individual reports describing 102 adverse events of interest were identified. Eighty‐six percent of all reports came from European countries (in descending order of number of reports: France, Belgium, Germany, Italy, Spain, Greece, Portugal and Switzerland). Nineteen of the 102 events were fatal. Death occurred from cardiac rhythm disturbances and epileptic insults.
Of the 65 patients reported, 24 had taken an overdose (10 of which patients attempting suicide, and the others chronic users on normal dose but not adjusted to renal status), with serious cardiac and neurological complications. Forty‐one of the 65 patients were on normal doses and experienced non‐specific tachycardia and hypotension expected with vasodilator therapy.
The worldwide reporting rates per 100,000 PTY between 1 December 1994 and 30 November 2006 were 0.27 for suicide, 0.82 for overdose, and 0.48 for fatalities; this reflects a total of 2.9 million patient years.
Our statement on the negative balance between risk and benefit of this drug is based on the one hand on the disappointing results of a systematic review for efficacy data and a number of case reports of toxic effects. None of these elements were sufficient for meta‐analytic pooling.
The regulatory agencies of France and Belgium recently took regulatory actions with restrictions in indications and available dosage forms (only 150 mg forms available without extended release formulation). This makes it unpractical to use this drug in daily practice at the doses recommended in clinical trials (600 mg daily). Consequently, it is difficult to prescribe buflomedil, even to patients without renal insufficiency at the doses habitually used in the clinical trials (300 mg twice daily). Most pharmaceutical products on the market were 300 mg tablets. Hence, we conclude that the balance between benefit and risk for buflomedil in the symptomatic treatment of intermittent claudication is unfavourable.
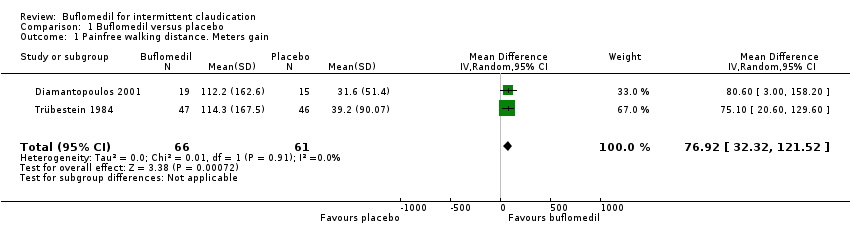
Comparison 1 Buflomedil versus placebo, Outcome 1 Painfree walking distance. Meters gain.

Comparison 1 Buflomedil versus placebo, Outcome 2 Maximal walking distance. Meters gain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painfree walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 76.92 [32.32, 121.52] |
| 2 Maximal walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 112.64 [27.73, 197.54] |
| Methods | Study design: international, multicenter, randomized, placebo‐controlled, double‐blinded. Participating centers: Czech Republic, France, Hungary, Russia Method of randomization:a centralized computer‐generated schedule (1:1 randomization in blocks of 6 and stratified by center and creatinine clearance) Blinding:double‐blind; buflomedil and placebo were indistinguishable in terms of shape, smell and appearance Statistical analysis: primary efficacy outcome performed on the intention‐to‐treat population; safety analysis performed on the as‐treated population; Dropouts:buflomedil: 223 patients permanently stopped treatment prior to study completion (including death) and 5 patients lost to follow‐up; placebo: 219 patients permanently stopped treatment prior to study completion (including death) and 10 patients lost to follow‐up |
| Participants | Patients > 40 y with documented peripheral arterial obstructive disease, intermittent claudication, and an ankle‐brachial index between 0,30 and 0,80 Patients were excluded from the study if they presented the following characteristics: ischemic rest pain (i.e., Fontaine's stage III PAD), ulceration or gangrene (i.e., Fontaine’s stage IV PAD), iliac or common femoral artery stenosis or occlusion, arterial occlusion of embolic origin, Buerger disease, or any nonatherosclerotic arterial n = 2078 patients randomized: 1043 to buflomedil, 1035 to placebo |
| Interventions | Long‐term administration (mean 33 months) of oral buflomedil or placebo |
| Outcomes | 1. The primary efficacy outcome was critical cardiovascular events, defined as the composite of cardiovascular death (including sudden death of presumed cardiac origin), nonfatal myocardial infarction, nonfatal stroke, symptomatic deterioration of peripheral arterial obstructive disease, or leg amputation 2. Secondary efficacy end points included the individual components of the primary efficacy outcome, death resulting from any cause, and all cardiovascular events. Other secondary efficacy endpoints were pain‐free and maximal walking distances (as assessed by patient questioning), ABI values, and quality of life (i.e. ability to perform daily life activities according to the patient using a 0‐ to 100‐mm subjective visual analogue scale) 3. The primary safety outcome was adverse events, with special attention paid to myoclonia and convulsion |
| Notes | Although this is a large, double‐blind RCT, we excluded this study because PFWD and MWD were not measured by an objective standardized test. Because of safety reasons, oral buflomedil 300 mg tablets are not anymore on the market From certain experts in the vascular field (Olin J, NY, USA) criticism has been made on the reliability of the study data An associated editorial by MS Conte (Boston, MA, USA) (Circulation 2008;117:717‐9) There was also a letter to the Editor (Letter by De Backer et al regarding article, "Oral buflomedil in the prevention of cardiovascular events in patients with peripheral arterial obstructive disease: a randomized, placebo‐controlled, 4‐year study". Circulation 2008;118;e151) |
| Methods | Study design: international, multicenter, randomized, placebo‐controlled, double‐blinded. Participating centers: Czech Republic, France, Hungary, Russia Method of randomization:a centralized computer‐generated schedule (1:1 randomization in blocks of 6 and stratified by center and creatinine clearance) Blinding:double‐blind; buflomedil and placebo were indistinguishable in terms of shape, smell and appearance Statistical analysis: primary efficacy outcome performed on the intention‐to‐treat population; safety analysis performed on the as‐treated population; Dropouts:buflomedil: 223 patients permanently stopped treatment prior to study completion (including death) and 5 patients lost to follow‐up; placebo: 219 patients permanently stopped treatment prior to study completion (including death) and 10 patients lost to follow‐up |
| Participants | Patients > 40 y with documented peripheral arterial obstructive disease, intermittent claudication, and an ankle‐brachial index between 0,30 and 0,80 Patients were excluded from the study if they presented the following characteristics: ischemic rest pain (i.e., Fontaine's stage III PAD), ulceration or gangrene (i.e., Fontaine’s stage IV PAD), iliac or common femoral artery stenosis or occlusion, arterial occlusion of embolic origin, Buerger disease, or any nonatherosclerotic arterial n = 2078 patients randomized: 1043 to buflomedil, 1035 to placebo |
| Interventions | Long‐term administration (mean 33 months) of oral buflomedil or placebo |
| Outcomes | 1. The primary efficacy outcome was critical cardiovascular events, defined as the composite of cardiovascular death (including sudden death of presumed cardiac origin), nonfatal myocardial infarction, nonfatal stroke, symptomatic deterioration of peripheral arterial obstructive disease, or leg amputation 2. Secondary efficacy end points included the individual components of the primary efficacy outcome, death resulting from any cause, and all cardiovascular events. Other secondary efficacy endpoints were pain‐free and maximal walking distances (as assessed by patient questioning), ABI values, and quality of life (i.e. ability to perform daily life activities according to the patient using a 0‐ to 100‐mm subjective visual analogue scale) 3. The primary safety outcome was adverse events, with special attention paid to myoclonia and convulsion |
| Notes | Although this is a large, double‐blind RCT, we excluded this study because PFWD and MWD were not measured by an objective standardized test. Because of safety reasons, oral buflomedil 300 mg tablets are not anymore on the market From certain experts in the vascular field (Olin J, NY, USA) criticism has been made on the reliability of the study data An associated editorial by MS Conte (Boston, MA, USA) (Circulation 2008;117:717‐9) There was also a letter to the Editor (Letter by De Backer et al regarding article, "Oral buflomedil in the prevention of cardiovascular events in patients with peripheral arterial obstructive disease: a randomized, placebo‐controlled, 4‐year study". Circulation 2008;118;e151) |

