Buflomedil for intermittent claudication
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000988.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 marzo 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Dr TL De Backer: Evaluated retrieved trials and was primary author of the manuscript.
Dr RH Vander Stichele: Assisted in designing of the review, searching the literature, evaluating retrieved trials and the manuscript editing.
Sources of support
Internal sources
-
Heymans Institute of Pharmacology, Medical School, University of Gent, Belgium.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
None known
Acknowledgements
We would like to thank Prof MG Bogaert for his work on previous versions of this review. We would like to thank the Cochrane Consumer Network for providing an updated Plain Language Summary. We would also like to thank the Cochrane Peripheral Vascular Diseases Group for their extensive assistance with the most recent update.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Mar 28 | Buflomedil for intermittent claudication | Review | Tine LM de Backer, Robert Vander Stichele | |
| 2008 Jan 23 | Buflomedil for intermittent claudication | Review | Tine LM de Backer, Marc Bogaert, Robert Vander Stichele | |
| 2007 Oct 17 | Buflomedil for intermittent claudication | Review | Tine LM de Backer, Marc Bogaert, Robert Vander Stichele | |
| 2000 Apr 24 | Buflomedil for intermittent claudication | Review | T LM De Backer, Robert Vander Stichele, Marc Bogaert, Tine LM de Backer | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
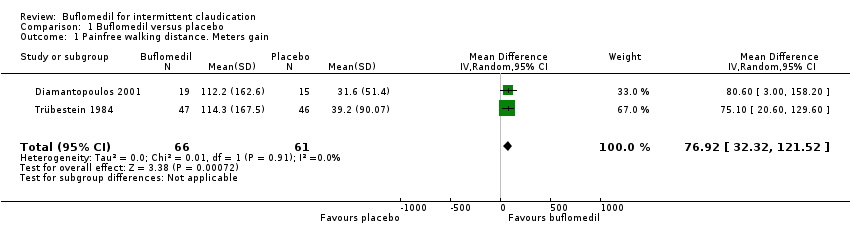
Comparison 1 Buflomedil versus placebo, Outcome 1 Painfree walking distance. Meters gain.

Comparison 1 Buflomedil versus placebo, Outcome 2 Maximal walking distance. Meters gain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painfree walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 76.92 [32.32, 121.52] |
| 2 Maximal walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 112.64 [27.73, 197.54] |
| Methods | Study design: international, multicenter, randomized, placebo‐controlled, double‐blinded. Participating centers: Czech Republic, France, Hungary, Russia Method of randomization:a centralized computer‐generated schedule (1:1 randomization in blocks of 6 and stratified by center and creatinine clearance) Blinding:double‐blind; buflomedil and placebo were indistinguishable in terms of shape, smell and appearance Statistical analysis: primary efficacy outcome performed on the intention‐to‐treat population; safety analysis performed on the as‐treated population; Dropouts:buflomedil: 223 patients permanently stopped treatment prior to study completion (including death) and 5 patients lost to follow‐up; placebo: 219 patients permanently stopped treatment prior to study completion (including death) and 10 patients lost to follow‐up |
| Participants | Patients > 40 y with documented peripheral arterial obstructive disease, intermittent claudication, and an ankle‐brachial index between 0,30 and 0,80 Patients were excluded from the study if they presented the following characteristics: ischemic rest pain (i.e., Fontaine's stage III PAD), ulceration or gangrene (i.e., Fontaine’s stage IV PAD), iliac or common femoral artery stenosis or occlusion, arterial occlusion of embolic origin, Buerger disease, or any nonatherosclerotic arterial n = 2078 patients randomized: 1043 to buflomedil, 1035 to placebo |
| Interventions | Long‐term administration (mean 33 months) of oral buflomedil or placebo |
| Outcomes | 1. The primary efficacy outcome was critical cardiovascular events, defined as the composite of cardiovascular death (including sudden death of presumed cardiac origin), nonfatal myocardial infarction, nonfatal stroke, symptomatic deterioration of peripheral arterial obstructive disease, or leg amputation 2. Secondary efficacy end points included the individual components of the primary efficacy outcome, death resulting from any cause, and all cardiovascular events. Other secondary efficacy endpoints were pain‐free and maximal walking distances (as assessed by patient questioning), ABI values, and quality of life (i.e. ability to perform daily life activities according to the patient using a 0‐ to 100‐mm subjective visual analogue scale) 3. The primary safety outcome was adverse events, with special attention paid to myoclonia and convulsion |
| Notes | Although this is a large, double‐blind RCT, we excluded this study because PFWD and MWD were not measured by an objective standardized test. Because of safety reasons, oral buflomedil 300 mg tablets are not anymore on the market From certain experts in the vascular field (Olin J, NY, USA) criticism has been made on the reliability of the study data An associated editorial by MS Conte (Boston, MA, USA) (Circulation 2008;117:717‐9) There was also a letter to the Editor (Letter by De Backer et al regarding article, "Oral buflomedil in the prevention of cardiovascular events in patients with peripheral arterial obstructive disease: a randomized, placebo‐controlled, 4‐year study". Circulation 2008;118;e151) |
| Methods | Study design: international, multicenter, randomized, placebo‐controlled, double‐blinded. Participating centers: Czech Republic, France, Hungary, Russia Method of randomization:a centralized computer‐generated schedule (1:1 randomization in blocks of 6 and stratified by center and creatinine clearance) Blinding:double‐blind; buflomedil and placebo were indistinguishable in terms of shape, smell and appearance Statistical analysis: primary efficacy outcome performed on the intention‐to‐treat population; safety analysis performed on the as‐treated population; Dropouts:buflomedil: 223 patients permanently stopped treatment prior to study completion (including death) and 5 patients lost to follow‐up; placebo: 219 patients permanently stopped treatment prior to study completion (including death) and 10 patients lost to follow‐up |
| Participants | Patients > 40 y with documented peripheral arterial obstructive disease, intermittent claudication, and an ankle‐brachial index between 0,30 and 0,80 Patients were excluded from the study if they presented the following characteristics: ischemic rest pain (i.e., Fontaine's stage III PAD), ulceration or gangrene (i.e., Fontaine’s stage IV PAD), iliac or common femoral artery stenosis or occlusion, arterial occlusion of embolic origin, Buerger disease, or any nonatherosclerotic arterial n = 2078 patients randomized: 1043 to buflomedil, 1035 to placebo |
| Interventions | Long‐term administration (mean 33 months) of oral buflomedil or placebo |
| Outcomes | 1. The primary efficacy outcome was critical cardiovascular events, defined as the composite of cardiovascular death (including sudden death of presumed cardiac origin), nonfatal myocardial infarction, nonfatal stroke, symptomatic deterioration of peripheral arterial obstructive disease, or leg amputation 2. Secondary efficacy end points included the individual components of the primary efficacy outcome, death resulting from any cause, and all cardiovascular events. Other secondary efficacy endpoints were pain‐free and maximal walking distances (as assessed by patient questioning), ABI values, and quality of life (i.e. ability to perform daily life activities according to the patient using a 0‐ to 100‐mm subjective visual analogue scale) 3. The primary safety outcome was adverse events, with special attention paid to myoclonia and convulsion |
| Notes | Although this is a large, double‐blind RCT, we excluded this study because PFWD and MWD were not measured by an objective standardized test. Because of safety reasons, oral buflomedil 300 mg tablets are not anymore on the market From certain experts in the vascular field (Olin J, NY, USA) criticism has been made on the reliability of the study data An associated editorial by MS Conte (Boston, MA, USA) (Circulation 2008;117:717‐9) There was also a letter to the Editor (Letter by De Backer et al regarding article, "Oral buflomedil in the prevention of cardiovascular events in patients with peripheral arterial obstructive disease: a randomized, placebo‐controlled, 4‐year study". Circulation 2008;118;e151) |

