Buflomedil for intermittent claudication
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: States randomized, double‐blinded. Method of randomization: Not stated. Blinding: Double‐blinded. Dropouts: 2 patients from the buflomedil group, because they felt better, and 4 from the placebo group; 1 for no apparent reason, 1 fatal AMI, 1 underwent surgery and 1 failed to report. | |
| Participants | Country: Greece. Setting: Clinic. No. 40 patients; 21 buflomedil, 19 placebo). Gender: 36 males (19 buflomedil, 17 placebo), 4 females (2 bufomedil, 2 placebo). Age: mean 60.1 years (range 39 to 77). Inclusion criteria: All had maturity onset diabetes mellitus (11 insulin‐dependent, At the start of a 4‐week observation period before entry into the study, patients were given a full physical examination and an ECG at rest and after exercise on a treadmill (3.6 km/h at 12.5°C). Exclusion criteria: Vascular surgery or specific physical training, participation in another study, history of cerebrovascular accident or coronary heart disease, concomitant therapy that could influence any of the parameters to be studied, evidence of excessive Monckeberg's sclerosis, diabetic retinopathy, severe renal, hepatic or cardiopulmonary impairment, uncontrolled arterial hypertension, previous AMI, angina pectoris, severe cardiac arrhythmias, evidence of peripheral sensory neuropathy, arterial bleeding, pregnancy or lactation, allergy to any medication, history of convulsions or other central nervous system diseases, grossly over‐ or under‐weight diabetics based on tables size‐weight, musculoskeletal disorders limiting walking ability. | |
| Interventions | Treatment: During 6 month treatment period patients received 600 mg buflomedil per day, 2 x 150 mg tablets taken twice a day. Control: Matching placebo tablets. Compliance checked every 3 to 4 weeks by patients returning their bottles to the outpatient clinic where remaining tablets were counted. Duration: Six months. | |
| Outcomes | Primary: Distances at 6 months constituted the primary response variables. PFWD and MWD measured at entry (baseline) and after 3 and 6 months' treatment using a standardized exercise test on the treadmill (3.6 km/h at 12.5°C). Secondary: Not stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Study design: States randomized, double‐blinded. Method of randomization: Not stated. Blinding: Double‐blinded. Dropouts: 8 in the placebo group and 12 in the buflomedil group, leaving 93 for analysis (46 in placebo group, 47 in buflomedil group). | |
| Participants | Country: Germany. Setting: Clinic. No.: 113 patients. Age: 50 to 70 years old. Gender: Not stated. Inclusion criteria: Symptoms of intermittent claudication for 6 months to 5 years and PFWD of 50 to 400 m. After a four‐week run‐in period during which all patients received placebo and other vasoactive drugs were stopped, patients were checked to test whether their PFWD differed by not more than 30% from the value at the beginning of the run‐in period (mean of two consecutive measurements). Patients were then allocated randomly to treatment groups. Exclusion criteria: Special physical training for at least six months prior to study start; systolic blood pressure at the ankle og less than 70 mmHg, diabetic microangiopathy or necrosis, infalmmatory vascular disease such as endarteritis obliterans, coronoary heart disease with angina pectoris, myocardial insufficiency, respiratory insufficiency, arthropathy, symptoms of severe liver or kidney disease, laboratory values of more than 10% outside normal range, pregnancy, beta‐blockers and any other vasoactive drugs. | |
| Interventions | All patients received placebo tablets (four daily ‐ 2 in the morning, one at midday and one in the evening) for four weeks run‐in. Treatment: Buflomedil 600 mg daily (2 x 150 mg in the morning, 1 x 150 mg at midday and 1 x 150mg in the evening.) Control: Matching placebo. Duration: 12 weeks treatment. | |
| Outcomes | Primary: PFWD and MWD were measured by treadmill (5 km/h at 10° elevation). Ankle‐arm pressures were measured by Doppler at rest and after exercise and pressure differences (brachial artery‐posterior tibial artery) were calculated. Measurements were performed at days 28, 56, and 84. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
AMI: acute myocardial infarction
ECG: electrocardiogram
MWD: maximal walking distance
PAOD: peripheral arterial occlusive disease
PFWD: painfree walking distance
SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| A small study (N = 38) with a mixed protocol of 14 days intravenous therapy (2 x 100 mg) followed by eight weeks oral therapy (daily dose of 600 mg in three doses. The duration of the study was too short (8 weeks) for inclusion. At randomization there were 20 participants in the active group versus 18 in the placebo group. Percentages of smokers and diabetics were not given. No mention of: dropouts, total number of patients screened, or how many of the original 38 patients completed the study. | |
| A small study (N = 34) with six poorly described dropouts (two in the active group, four in the placebo group). 23% of participants were diabetic. Daily dosage of 600 mg given in two doses. The primary aim of the study was to investigate whether buflomedil inhibits platelet aggregation in patients with PAOD. The secondary endpoints were PFWD, MWD and subjective improvement. The results of the primary endpoint were negative and so the researchers shifted attention to the secondary outcomes. Results of treadmill tests expressed in time units and not in distance units. Standard deviations of results not given. | |
| Study 08 in the meta‐analysis of Walker and Mac Hannaford, but not published. Twenty‐six participants with a mean age of 63 years, treated for six months with 600 mg of buflomedil or placebo. An effect size of ‐0.5 to 1.2 mentioned. We could not obtain this study. Example of publication bias. | |
| Unpublished multicenter study mentioned in the meta‐analysis of Walker and Mac Hannaford. We obtained this trial as confidential material with the help of the Cochrane PVD Group. The trial was rather short (12 weeks), daily dosage of 600 mg given in three doses, and results did not provide statistics on variation (SD or SEM). At randomization there were 97 participants in the active group versus 104 in the placebo group. Dropouts (29 in active group and 25 in placebo group) and protocol violations were mentioned but not well described. This study clearly showed negative results for the PFWD. | |
| Study 11 in the meta‐analysis of Walker and Mac Hannaford, but not published. One hundred and sixty‐eight participants were treated for three months with 600 mg of buflomedil or placebo. We could not retrieve this study. Example of publication bias. | |
| Study 10 in the meta‐analysis of Walker and Mac Hannaford, but not published. Sixty patients (mean age?) participated in this study and were treated for three months. An effect size of ‐0.3 to 07 was given. We could not retrieve this study. Example of publication bias. | |
| Study 09 in the meta‐analysis of Walker and Mac Hannaford, but not published. One hundred and five participants were treated for four months with a daily dose of 600 mg buflomedil or placebo. An effect size of ‐0.2 to 0.6 was given. We could not obtain this study. Example of publication bias. | |
| A small study (N = 40), with a short double blind phase of 60 days, inadequate reporting of trial procedures, and no data on variability of results. Total daily dosage was 600 mg given in four doses. Percentages of smokers and diabetics were not given. At randomization there were 20 participants in each group and there were no dropouts. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painfree walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 76.92 [32.32, 121.52] |
| Analysis 1.1  Comparison 1 Buflomedil versus placebo, Outcome 1 Painfree walking distance. Meters gain. | ||||
| 2 Maximal walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 112.64 [27.73, 197.54] |
| Analysis 1.2  Comparison 1 Buflomedil versus placebo, Outcome 2 Maximal walking distance. Meters gain. | ||||
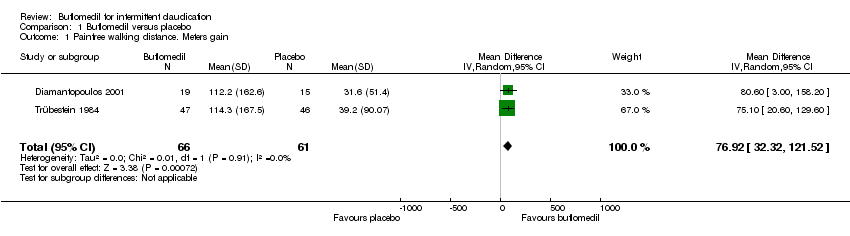
Comparison 1 Buflomedil versus placebo, Outcome 1 Painfree walking distance. Meters gain.

Comparison 1 Buflomedil versus placebo, Outcome 2 Maximal walking distance. Meters gain.
| Search strategy |
| #1 MeSH descriptor Intermittent Claudication explode all trees |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painfree walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 76.92 [32.32, 121.52] |
| 2 Maximal walking distance. Meters gain Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 112.64 [27.73, 197.54] |

