Profilaxis antibiótica para la cesárea
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | 'Divided randomly into two groups'; not placebo‐controlled. | |
| Participants | Both elective and non‐elective cesarean deliveries. | |
| Interventions | Ampicillin 500mg before operation and 250mg 6 hourly for at least 7 days (intramuscularly until able to take orally) (n = 58) versus no antibiotics unless temperature 38 degrees C after the third postoperative day (n = 48). Both groups received curative doses of chloroquine. | |
| Outcomes | Wound infection; urinary tract infection (not defined further); 'genital sepsis' (not defined further). | |
| Notes | Episodes of 'genital sepsis' classified as endometritis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized list of placebo or drug, kept in hospital pharmacy; code not broken until after patient classified as 'morbid' or 'non‐morbid'; placebo‐controlled. | |
| Participants | Women undergoing cesarean section (criteria not specified). | |
| Interventions | Cephalothin 1g IV on call to operating room, further 2g IV intra‐operatively and every 6 hours for 48 hours, then 500mg IM for additional 72 hours (n = 5) versus placebo (n = 7). | |
| Outcomes | Morbidity (temperature > 100.9 degrees fahrenheit twice, 6 hours apart after first 48 hours or other clinical signs of infection); not separated. For this review, the authors' definition of morbidity has been classified as fever. | |
| Notes | Part of a larger randomized trial of prophylactic antibiotics in gynecologic surgery; most patients (87%) were undergoing hysterectomy; only 12/300 patients enrolled underwent cesarean section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Double blind, placebo controlled. 'Randomly divided into 2 groups'. | |
| Participants | Both elective and non‐elective cesarean deliveries. | |
| Interventions | Ticarcillin 6g intravenously within 15 minutes of cord clamping (n = 139) versus saline placebo (n = 120). Subset of 22 in each group received ticarcillin 3g/saline 6‐8 hours postoperatively (results similar so authors combined results with single dose group). | |
| Outcomes | Endomyometritis (pyrexia, uterine tenderness and no evidence of other infection). | |
| Notes | Authors' definition of low and high risk not comparable to definitions for elective/non‐elective used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized (computer‐based allocation), double‐blind, placebo controlled. | |
| Participants | Women undergoing elective cesarean delivery. Exclusion: prior antibiotics within 2 weeks, allergy to penicillin or cephalosporin, rupture of membranes. | |
| Interventions | Cefoxitin (2g IV after cord clamping) (n = 237) vs matching placebo (n = 238). | |
| Outcomes | Febrile morbidity (oral temperature >38 degrees C twice 6 hours apart after first 24 hours); wound infection (wound cellulitis, erythema, discharge with or without fever); endometritis (fever, uterine tenderness, malodorous lochia); urinary tract infection (fever and positive urine culture); pneumonia; duration of hospital stay. | |
| Notes | 11% were HIV positive; Staphylococcus aureus most common pathogen (43%) isolated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Allocation using random number table; not placebo controlled. | |
| Participants | Women undergoing elective cesarean section or labour <12 hours. | |
| Interventions | Cephapirine 1g IV with induction of anaesthesia and 6 hours after operation, gentamycin 80mg IM with induction, metronidazole 500mg IV with induction (n = 133), versus no treatment (n = 136). | |
| Outcomes | Endometritis; wound infection; pyrexia only (>38 degrees C 48 hours after surgery): antibiotic 4/133 vs control 9/136; septicemia (0/133 vs 3/136, included as serious morbidity); duration of hospital stay (antibiotic 5.36 days vs control 6.21, p = 0.03, variance not given). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocated using last digit of patient's file number to treatment or no treatment. | |
| Participants | Women undergoing cesarean section due to acute fetal distress. | |
| Interventions | Ceftriaxone 1g (n = 25) vs mezlocillin 2g (n = 23) vs clindamycin 600mg and amikacin 500mg (n = 18) vs sulbactam ampicillin 1g (n = 25) intravenously after clamping of the cord vs no treatment (n = 28). | |
| Outcomes | Wound infection (redness, tenderness, pain and purulent discharge); urinary tract infection (renal angle tenderness, fever, dysuria and pyuria); endometritis (vaginal spotting, purulent discharge with fever and pain) plus positive cultures. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized (computer‐generated), partially double blind placebo‐controlled (3 groups: antibiotic irrigation, saline placebo irrigation, no irrigation). As the objective of this review is to compare antibiotic with no antibiotic, rather than the effect of irrigation, only the first 2 groups are compared (double blind comparison). | |
| Participants | Both 'low risk' (labor < 6 hours) and 'high risk' (>6 hours) women undergoing cesarean section. | |
| Interventions | Irrigation of the uterus and peritoneal cavity with 2g cefamandole in 1000ml normal saline (n = 73), versus saline placebo (n = 75). | |
| Outcomes | Metritis (pyrexia >38 degrees C twice 8 hours apart, after 24 hours plus abnormal uterine tenderness, without another apparent source); duration of maternal stay (treatment 5.29 days vs placebo 6.32 days, variance could not be calculated). | |
| Notes | Authors' definition of low and high risk do not correspond to those used for elective/non‐elective in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomly allocated (abstract only; no further details). | |
| Participants | Women undergoing high‐risk cesarean section. | |
| Interventions | Cefazolin 2g in 1000ml irrigation (n = 20) vs normal saline 1000ml irrigation (n = 20). | |
| Outcomes | Wound infection, endometritis, urinary tract infection. | |
| Notes | Follow up 4‐6 weeks post‐operatively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Double blind' randomized trial (the anaesthetist was not blind); list of random numbers consulted by nurse. | |
| Participants | All women undergoing cesarean section. | |
| Interventions | Intravenous therapy at time of induction of anaesthesia: ampicillin 1g (n = 96); ampicillin 1g and metronidazole 500mg (n = 104); ampicillin 1g and salbactam 500mg (n = 99), versus placebo (normal saline) (n = 101). Results of the three treatment groups combined. | |
| Outcomes | Febrile morbidity (oral temperature of more than 38 degrees C at least twice after day 1); wound infection (induration, serosanguinous discharge or dehiscence with purulent discharge); urinary tract infection (positive culture); genital tract infection (pain and uterine tenderness, purulent uterine discharge with microbiological confirmation); any infection anywhere (antibiotic 75/299 vs placebo 28/101); post‐operative antibiotic use (22/299 vs 9/101). | |
| Notes | Only moderate or prolonged febrile morbidity (as defined) included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled, computer generated sequence. Allocation to irrigation or intravenous route according to social security number. | |
| Participants | Women at increased risk of post‐cesarean section endometritis (in labor or with ruptured membranes). Classified as non‐elective for this review. | |
| Interventions | Administration by irrigation of uterus and peritoneal cavity with 2g cefoxitin in 500ml saline (n = 37), versus 500ml normal saline (n = 23), or intravenously after clamping of the umbilical cord, cefoxitin 2g (n = 31) versus saline (n = 33). Irrigation and intravenous groups combined for this review. | |
| Outcomes | Endometritis (febrile morbidity and uterine tenderness); total infection‐related morbidity (cefoxitin 10/68 vs saline 14/56); fever index; duration of intravenous antibiotics; additional antibiotics; days in hospital (no difference, variance not given). | |
| Notes | One woman developed an allergic reaction to cefoxitin (acute pruritic rash). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Allocated by sealed envelopes; not blinded or placebo controlled. | |
| Participants | Women undergoing cesarean section; both elective and non‐elective deliveries. | |
| Interventions | Cefotetan 2g after clamping of umbilical cord (n = 55) versus no antibiotic (n = 55). | |
| Outcomes | Endometritis; urinary infection; local complications; fever only (cefotetan 0/55 vs control 6/55); antibiotic therapy (10/55 vs 25/55); mean days in hospital (10.0 vs 10.2, no variance given). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomly assigned', no details given; not placebo‐controlled. | |
| Participants | Women in labour with ruptured membranes requiring internal monitoring (non‐elective delivery). | |
| Interventions | Short course kefzol (1g intravenously 6 hourly for 24 hours, n = 24); long course (kefzol 1g intravenously for 8 or more doses and keflex 500mg orally 6 hourly for 5 days, n = 25); versus no prophylactic antibiotics. Short and long courses combined for this review. | |
| Outcomes | Endometritis and/or wound infection (antibiotic 12/49 vs control 20/31). | |
| Notes | It was possible to deduce the rate of endometritis alone, but not wound infection, for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled trial. Computer‐generated numbers using the mixed congruential method. | |
| Participants | All women undergoing cesarean section. | |
| Interventions | Irrigation during cesarean section with 2g of either cephapirin sodium (n = 79), cefamandole nafate (n = 70), moxalactam disodium (n = 64) or ampicillin sodium (n = 70), versus saline (n = 77). A vitamin was added to each solution for disguise. The antibiotic groups have been considered together in this review. | |
| Outcomes | Fever (>38 degrees C twice 6 hours apart, excluding the first 24 hours); endomyometritis (pyrexia >37.8 degrees C, uterine tenderness and pelvic peritoneal irritation without other localising signs of irritation; urinary tract infection (positive culture); wound infection; fever index; all infection‐related morbidity; therapeutic antibiotics; mean postoperative days (variance not given). | |
| Notes | Three episodes of pelvic thrombophlebitis (all in treated groups). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized, double blind, placebo‐controlled. | |
| Participants | All patients undergoing cesarean section. | |
| Interventions | Metronidazole 1g rectal suppository 10‐45 minutes before and 8 hours after procedure (n = 91) versus placebo suppository (n = 91). | |
| Outcomes | Fever (>37.9 degrees C on at least one occasion); wound infection; mean febrile days (0.56 for treatment vs 1.23 for control), hospital days, any antibiotic use (18/91 vs 23/91). | |
| Notes | Elective cesarean section not defined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double‐blind, placebo‐controlled; numbered packages randomized by pharmacy; 9/110 'packages' not included (either damaged or patients failed to meet inclusion criteria); imbalance in group size (46‐placebo vs 55‐cefoxitin) not explained. | |
| Participants | All women undergoing cesarean section (one third elective). | |
| Interventions | Cefoxitin 2g IV (n = 46) versus saline placebo (n = 55) after clamping the umbilical cord and at 4 and 10 hours post‐operatively. | |
| Outcomes | Febrile morbidity (temperature >38 degrees C twice 6 hours apart after first 24 hours); endometritis (fever, uterine tenderness, leukocytosis); wound infection (fever, cellulitis, exudate); maternal length of stay. | |
| Notes | No serious life‐threatening infection in either group; no drug‐related side‐effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Double‐blind, randomized, prepared by hospital pharmacy; placebo‐controlled. | |
| Participants | All women undergoing either primary or repeat cesarean section (44% elective). | |
| Interventions | Ampicillin 1g IV prior to surgery and 6 and 12 hours post‐operatively (n = 26) versus placebo (n = 31). | |
| Outcomes | Febrile morbidity (>100.3 degrees fahrenheit twice 6 hours apart after first 24 hours); endomyometritis (fever, uterine and abdominal tenderness, purulent lochia); urinary tract infection (positive culture); wound infection (induration, erythema and warmth with purulent drainage); need for antibiotics (treatment 3/26 vs placebo 13/31); maternal hospital stay (6.03 vs 6.9; no variance given). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized in double‐blind fashion; placebo‐controlled. | |
| Participants | Women undergoing cesarean section who were not in labor and did not have ruptured membranes (elective). | |
| Interventions | Ampicillin 1g 30 min prior to surgery and at 4 and 8 hours post‐operatively (n = 42) versus placebo solution (n = 40). | |
| Outcomes | Febrile morbidity (>100.4 degrees fahrenheit twice 6 hours apart after the first 24 hours); endomyometritis (fever, uterine and adnexal tenderness, purulent lochia); urinary tract infection; wound infection (induration, erythema and warmth with purulent drainage); need for antibiotics (treatment 1/42 vs placebo 6/40); maternal hospital stay (4.3 vs 4.6; no variance given). | |
| Notes | No life‐threatening infection related complications nor bacteremic episodes in either group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, using a table of random numbers; not placebo‐controlled. Allocated to either intravenous antibiotic, antibiotic irrigation, both routes or no treatment. | |
| Participants | Women in active labor or ruptured membranes and at least one digital vaginal examination (categorized as non‐elective in this review although duration of membrane rupture not stated). | |
| Interventions | Cefoxitin 2g IV after clamping the cord, repeated every six hours for 48 hours (n = 39) versus uterine and peritoneal lavage with 2g cefoxitin after delivery of the placenta (n = 42) versus irrigation plus intravenous therapy (n = 38) versus no therapy (n = 39). The three treatment groups have been combined in this review. | |
| Outcomes | Febrile morbidity (>37.9 degrees C twice 6 hours apart after first 24 hours); endometritis (fever and uterine tenderness); urinary tract infection (positive culture); wound infection (including fever, cellulitis and exudate); hospital stay (treatment 4.86 vs control 5.2; variance could not be calculated). | |
| Notes | 3 episodes of septicemia reported in control group vs none in treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Assigned at random to either a control group or the study group by computer‐generated list of random numbers; not placebo‐controlled. | |
| Participants | Women undergoing cesarean section. Exclusion criteria: severe penicillin allergy, renal insufficiency, antibiotic use, amniotic infection. | |
| Interventions | Mezlocillin 4g and oxacillin 2g every 8 hours after clamping of the cord for three doses (n = 50) vs no treatment (n = 50). | |
| Outcomes | Endometritis, urinary tract infections, wound infections. | |
| Notes | Detailed pre‐ and post‐ antibiotic microbiological cultures were performed; there were fewer gram positive cocci and more gram negative rods in cervical cultures of the treated group; more break‐through infections in the treated group were with mezlocillin‐resistant organisms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Double blind, randomized by computerized tables; matching placebo doses; 3 patients excluded for inadequate follow‐up (group allocation not provided). | |
| Participants | Women undergoing cesarean section (labor <12 hours, membrane rupture <12 hours, <7 vaginal exams). | |
| Interventions | Ampicillin 1g intravenously every six hours x 3 then 1g every 6 hours x 7 days (n = 23) vs ampicillin 1g every 6 hours x 3 doses then placebo (n = 37) vs placebo (n = 31). | |
| Outcomes | Fever >38 degrees C x 2 at least 6 hours apart after first 24 hours; endometritis (temperature >38 degrees C, purulent lochia, pain on internal examination); wound infection (increased warmth, size or colour of wound, or purulent secretions); urine infection (dysuria and positive culture). | |
| Notes | No explanation provided for unequal size groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized (number allocated randomly to each of 90 boxes) placebo‐controlled (coloured vitamin added to placebo solution), double blind. | |
| Participants | Women undergoing non‐elective cesarean section. | |
| Interventions | Cefoxitin 2g IV (n = 30) versus cefazolin 1g IV (n = 30) versus placebo (n = 30) at clamping of the cord and at 6 and 12 hours later. Both treatment groups have been combined. | |
| Outcomes | Endometritis, wound infection, urinary tract infection (symptoms or two successive positive cultures) septicemia, pelvic abscess, pelvic thrombophlebitis. Follow‐up at 6 weeks. No side effects observed. | |
| Notes | There were no serious infections in any of the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled. | |
| Participants | All women undergoing either a repeat cesarean section or in labor. | |
| Interventions | Cefazolin 1g intramuscularly pre‐operatively and cephalothin 2g intravenously at 6, 12, and 24 hours after first dose (n = 46) versus placebo (n = 49). | |
| Outcomes | Wound infection (cellulitis, purulent exudate, intraperitoneal abscess or peritonitis); endometritis; urinary tract infection; maternal hospital stay. | |
| Notes | No minor side‐effects (rash or pruritus) or major reactions (anaphylaxis) observed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomly divided'; no further details; placebo‐controlled. | |
| Participants | Women < 21 years old undergoing cesarean section. | |
| Interventions | Trimethoprim 240mg and sulfamethoxazole 1200mg intravenously after clamping of cord (n = 29) versus placebo (n = 28). | |
| Outcomes | Endomyometritis (fever [>100.3 degrees fahrenheit twice within 24 hours after first day], uterine tenderness, absence of another focus); urinary tract infection (fever and positive culture); wound infection (fever, abnormal appearing wound with cellulitis or a wound draining purulent material). | |
| Notes | Authors' definition of high risk not comparable with that used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Women randomized (no further details provided in translation); not placebo‐controlled. | |
| Participants | Women undergoing cesarean section. | |
| Interventions | Metronidazole (n = 53) versus no treatment (n = 50). | |
| Outcomes | Fever (>38 degrees C on two subsequent days); wound infection; endometritis; additional use of antibiotics (treatment 13/53 vs control 22/50); maternal hospital days. | |
| Notes | Full translation pending. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Random allocation is presumed although method not described; placebo‐controlled; antibiotics prepared in coded identical vials by pharmacy; 17 patients initially randomized not included in outcome (? all in the treatment group). | |
| Participants | Women undergoing primary cesarean section or repeat section. | |
| Interventions | Ampicillin 1g, methicillin 1g and kanamycin 0.5g intramuscularly 15 ‐ 30 minutes before, and at 2 and 8 hours after delivery (n = 33) versus placebo (n = 28). | |
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathogenic organism without other cause); urinary tract infection; wound infection (fever, cellulitis and exudate); morbidity [fever >100 degrees fahrenheit in two separate 24 hour periods after first post‐partum day or positive post‐operative urine culture of >100,000 colonies/ml] (treatment 9/33 vs placebo 17/28); maternal hospital stay (6.5 vs 6.9 days; no variance given). | |
| Notes | Two serious infections: one pelvic abscess in treatment group, one septicemia in placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized (although method not described); placebo‐controlled; antibiotics prepared in coded identical vials by pharmacy. | |
| Participants | Women undergoing primary cesarean section or repeat section. | |
| Interventions | Ampicillin 1g and kanamycin 0.5g intramuscularly 15 to 30 minutes before, and at 2 and 8 hours after delivery (n = 34) versus placebo (n = 34). | |
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathogenic organism without other cause); urinary tract infection; wound infection (fever, cellulitis and exudate; any grade); morbidity [fever >100 degrees fahrenheit in two separate 24 hour periods after first post‐partum day or positive post‐operative urine culture of >100,000 colonies/ml] (treatment 8/34 vs placebo 22/34). | |
| Notes | One pelvic abscess in placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomized, double‐blind', details not specified; placebo‐controlled. | |
| Participants | Women in labor with rupture of membranes (non‐elective). | |
| Interventions | Cefamandole 2 g IV after cord clamping, and at 4 and 8 hours post‐operatively (n = 50) versus identical appearing placebo (n = 50). | |
| Outcomes | Endomyo(para)metritis; wound infection; maternal hospital stay; records reviewed 6 weeks to 6 months after discharge. Four episodes of bacteremia (1 in treatment group, 3 in placebo) have been categorized as serious outcomes. | |
| Notes | No incidence of pelvic abscess or septic thrombophlebitis in either group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'At random'; not placebo‐controlled, not double‐blind; investigator not intimately involved with post‐operative care. | |
| Participants | Women undergoing cesarean section. | |
| Interventions | Ampicillin 1g IV 15‐30 minutes before surgery and at 2 and 8 hours post‐operatively (n = 38) versus ampicillin 1g IV immediately after cord clamping and at 2 and 8 hours post‐operatively (n = 40) versus no antibiotic (n = 36); results for both treatment groups combined. | |
| Outcomes | Endometritis; wound infection; urinary tract infection; maternal hospital stay (5.1 and 4.7 for pre‐ and post‐ administration of antibiotics respectively vs 6.0 for no treatment, variance not given). | |
| Notes | Although emergency cesarean sections were excluded, the women enrolled did not conform to our definition of an elective section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomly divided' (no details provided); placebo‐controlled. | |
| Participants | Women undergoing cesarean section; elective cesarean sections not included but definition not provided. | |
| Interventions | Metronidazole 500mg intravenously after cutting of cord (n = 109) vs placebo (n = 110). | |
| Outcomes | Wound infection, endometritis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blind manner, according to prenumbered envelopes maintained in the central pharmacy; placebo‐controlled. | |
| Participants | Women undergoing primary, nonelective cesarean section (while it appears most women were in labour and/or had ruptured membranes it is unclear whether all patients fulfilled our criteria for non‐elective). | |
| Interventions | Cefamandole 500mg IV immediately after the cord was clamped, again in the recovery room and two more doses 6 hours apart (n = 43) versus identical‐appearing placebo (n = 47). | |
| Outcomes | Infectious morbidity (fever >100.3 degrees fahrenheit twice 6 hours apart after first 24 hours); endomyometritis (fever, uterine tenderness, and positive culture from endometrium); wound infection, urinary tract infection; maternal duration of stay (treatment 5.1 days vs placebo 5.4; not significant, no variance given). | |
| Notes | There was one episode of bacteremia in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double‐blind, randomized (method not described); placebo‐controlled. | |
| Participants | Women undergoing emergency cesarean section (during labor and/or after rupture of membranes). | |
| Interventions | Cefuroxime 1.5g IV at the start of the operation and 12 hours later (n = 80) versus saline placebo (n = 80). | |
| Outcomes | Endometritis (fever >38 degrees C twice at least 1 hour apart, after the first post‐operative day, and increased tenderness of the uterus); wound infection (redness, tenderness, increased heat and edema of wound); urinary tract infection. | |
| Notes | There were no cases of septicemia or abscess formation observed in either group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled; 'according to a random schedule'. | |
| Participants | Women undergoing cesarean section after labor or rupture of membranes (method section unclear as to duration of ruptured membranes; it has been assumed that all women were in labor). | |
| Interventions | Cefoxitin 2g intravenously after cord clamping, and at 6 and 12 hours after initial dose (n = 196) versus matching mannitol and riboflavin placebo (n = 196). | |
| Outcomes | Febrile morbidity (fever >37.9 degrees C twice at least 4 hours apart after first post‐operative day); endomyometritis (fever >38 degrees C with uterine tenderness, maternal white blood cell count >15000/cu mm, malodorous lochia and no apparent cause for fever); urinary tract infection; incision infection (purulent drainage with induration and tenderness); additional antibiotic therapy (treatment 26/196 vs placebo 68/190). | |
| Notes | Increase in enterococci and decrease in Staphylococcus aureus, various streptococci, E. coli and a variety of anaerobes from infected sites in prophylactic group compared with placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blinded, placebo‐controlled. | |
| Participants | Women undergoing cesarean section (at 'high' risk because of ruptured membranes in active labor); classified as 'non‐elective'. | |
| Interventions | Cefoxitin 2g intravenously at time of cord clamping (n = 64) versus cefoxitin 2g at time of cord clamping and at 4 and 8 hours post‐operatively (n = 60) versus identical‐appearing placebo; both treatment groups combined in this analysis. | |
| Outcomes | Febrile morbidity (>38 degrees C twice at least 8 hours apart, after first post‐operative day); endometritis (fever, foul, excessive lochia or uterine tenderness); urinary tract infection (fever and positive culture); wound infection (fever, cellulitis or exudate with positive cultures). | |
| Notes | No adverse drug reactions in cefoxitin groups, no septicemia in any group; four patients in placebo group were considered seriously ill (although do not fit the criteria for serious morbidity in this review) compared to none in treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Double‐blind, randomized, placebo‐controlled. | |
| Participants | Undergoing cesarean section. | |
| Interventions | Cefoxitin 2g after cord clamped and at 4 and 8 hours (n = 74) versus placebo (n = 78). | |
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathologic organism without other focus); wound infection (fever, cellulitis and exudate); urinary tract infection (fever and symptoms or positive culture). | |
| Notes | In the placebo group there were 8 episodes of serious morbidity (6 cases of sepsis; one pelvic abscess; one episode of pelvic thrombophlebitis) compared with one in the treated group (one episode of sepsis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly assigned (method not stated); placebo controlled. | |
| Participants | Women undergoing cesarean section. | |
| Interventions | Mezlocillin 5g intravenously during 30 minutes prior to surgery (n = 38) vs placebo (n = 40). | |
| Outcomes | Febrile morbidity (>38 degrees C twice at least 4 hours apart after first 24 hours post‐operative); endometritis (fever and uterine tenderness); urinary tract infection (single culture of >100,000 bacteria/ml); wound infection (redness, cellulitis, tenderness and exudate from incision). | |
| Notes | Authors' definition of emergency not consistent with definitions used in this review (classified as 'both/undefined'). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized by computer program to one of two groups at time of their first antenatal visit; not placebo‐controlled. | |
| Participants | Low risk women requiring cesarean delivery (elective procedure, duration of membrane rupture <3 hours, no more than two vaginal examinations). | |
| Interventions | Cefazolin 1g after clamping of the cord (n = 167) versus no treatment (n = 140). | |
| Outcomes | Febrile morbidity (fever >37.7 degrees C twice at least 4 hours apart after first 24 hours); endometritis (fever, uterine tenderness and abnormal lochia); urinary tract infection (fever and positive culture); wound infection (fever, cellulitis or exudate with positive culture); therapeutic antibiotic use (treatment group 6.5% versus 20% in control group, p <0.001). | |
| Notes | Although some women were in labour at the time of the procedure (mean duration of labour 53 and 44 minutes in the two groups), the study population so closely resembles the criteria for elective cesarean section used in this review that the results have been included in the 'elective' category. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomized according to a code'; placebo‐controlled. 8 women excluded: 4 because they were febrile before the operation, four because of mistakes in administration; data not provided to perform intent to treat analysis. | |
| Participants | Initially all women undergoing cesarean section (n = 80); thereafter women undergoing non‐elective (ruptured membranes) section (n = 72). | |
| Interventions | Tinidazole 500mg IV at cord clamping (n = 75) versus identical placebo (n = 77). | |
| Outcomes | Febrile morbidity (>38 degrees C on 2 postoperative days, excluding the first); endometritis (fever, foul lochia or uterine tenderness); wound infection (fever, cellulitis or exudate); urinary tract infection (fever and positive culture). | |
| Notes | Authors' definition of non‐elective (ruptured membranes) and elective (unruptured membranes) not consistent with the definitions used in this review; classified in this review as 'both'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized by last digit of hospital admission number to no irrigation, antibiotic irrigation or saline irrigation (placebo‐controlled). As the objective of this review is to compare antibiotic with no antibiotic, rather than the effect of irrigation, the two irrigation groups are compared. | |
| Participants | Women undergoing nonelective cesarean section (including prolonged ruptured membranes and prolonged labor, as well as general risk factors such as poor nutrition and poverty). | |
| Interventions | Cefamandole 2g in 800ml saline irrigation during the procedure (n = 84) versus saline irrigation (n = 86) versus no treatment (n = 92); only first two groups included. | |
| Outcomes | Febrile morbidity (>100.6 degrees fahrenheit twice 6 hours apart after first post‐operative day); serious morbidity (fever and endomyometritis or abscess requiring IV antibiotics for resolution). | |
| Notes | Authors' definition of high risk does not correspond to that used for non‐elective in this review, classified as 'both'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | 'Random allocation', placebo controlled. | |
| Participants | All women undergoing cesarean section (51/97 not in labor; 61/97 without ruptured membranes). | |
| Interventions | Cefazolin 1g IV pre‐operatively and at 2 and 8 hours post‐operatively (n = 48) versus similar volume of placebo (n = 49). | |
| Outcomes | Febrile morbidity (>100.3 degrees fahrenheit twice on any of first 10 post‐partum days after the first); endometritis (fever and uterine tenderness, or fever and pathogen from endometrium without other cause); urinary tract infection (fever or positive culture and symptoms); wound infection (fever, cellulitis and/or exudate). | |
| Notes | Aerobic isolates unchanged, fewer anaerobes in patients given placebo; most pathogens isolated were resistant to cefazolin whether treatment or placebo given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomly allocated' using envelope containing empty vial or vial containing treatment; not placebo‐controlled; women, attending physicians and study coordinators were 'blind'. | |
| Participants | Women undergoing nonelective cesarean section (58/201 without labour; 65/201 without ruptured membranes). | |
| Interventions | Cefuroxime 750mg IV after cord clamping (n = 102) versus no treatment (n = 99). | |
| Outcomes | Febrile morbidity (>37.9 degrees C twice at least 6 hours apart after first post‐operative day); endometritis (fever, uterine tenderness and abnormal lochia); wound infection (fever, cellulitis and/or purulent discharge); urinary tract infection; cost of post‐operative antibiotics (treatment $US0.69 vs control $US7.47); maternal hospital stay (treatment 8.1 vs control 8.0, no variance given). | |
| Notes | No woman had a severe infection such as pelvic abscess or septic pelvic thrombophlebitis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, placebo controlled. | |
| Participants | Women undergoing elective or non‐elective cesarean section. Age range 17‐40 years. | |
| Interventions | Metronidazole 500mg intravenously 2 hours or immediately preoperatively, 500 mg intraoperatively, 1000mg 8 hours postoperatively (n = 50), versus placebo (n = 50). | |
| Outcomes | Wound infection; endometritis; inadequate wound healing (metronidazole 1/50 vs placebo 8/50); mean temperature (36.8 degrees C SD 1.02 vs 37.6, 1.03); duration of hospital stay. | |
| Notes | Language: Bulgarian. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomly divided'; blinded, placebo‐controlled. | |
| Participants | Women undergoing primary cesarean section after onset of labour (corresponds to the definition of non‐elective). | |
| Interventions | Pipericillin 4g peri‐operatively (n = 50) versus pipericillin 4g peri‐operatively and at 4 and 8 hours post‐operatively (n = 50) versus placebo (n = 50); both treatment groups combined in analysis. | |
| Outcomes | Febrile morbidity (> 38.0 degrees C twice at least 6 hours apart after first post‐operative day); endometritis (fever, tender uterus and purulent lochia); hospital stay (no significant difference, variance not given). | |
| Notes | Use of saline or antibiotic lavage not allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized (using lottery method), double‐blind, placebo‐controlled; four women excluded because of protocol deviations. | |
| Participants | All women undergoing cesarean section (39/128 repeat section). | |
| Interventions | Cefoxitin 2g in 1L saline irrigation (n = 41) versus cephapirin 2g in 1L saline irrigation (n = 44) versus identical appearing placebo saline irrigation (n = 43) after delivery of the placenta; both treatment groups combined in the analysis. | |
| Outcomes | Urinary tract infection (positive culture); wound infection (purulent wound discharge with or without wound separation); endometritis (fever >100.4 degrees fahrenheit after first post‐operative day, uterine tenderness, foul smelling lochia without other source). | |
| Notes | Follow‐up for 8 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Random, double‐blind, placebo‐controlled; results on 15/227 women initially randomized not included in analysis. | |
| Participants | Women undergoing elective and nonelective cesarean section. | |
| Interventions | Ticarcillin 5g in 1200ml saline irrigation (n = 112) versus saline irrigation (n = 100). | |
| Outcomes | Febrile morbidity (>100.3 degrees fahrenheit twice at least 4 hours apart after first post‐operative day); endomyometritis, wound infection, urinary tract infection, septicemia, maternal hospital stay (treatment 4.5 vs placebo 5.4, no variance given). | |
| Notes | Definition of elective and nonelective cesarean section not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly allocated (using randomized list of treatment numbers), double‐blind, placebo‐controlled. | |
| Participants | All women undergoing elective cesarean section (before onset of labour or rupture of membranes; corresponds to our definition of elective). | |
| Interventions | Crystalline penicillin 2MU and chloramphenicol 500mg pre‐operatively ( n = 115) versus matching placebo (n = 117). | |
| Outcomes | Fever (>37.9 degrees C twice at least 4 hours apart after first post‐operative day); wound sepsis (graded as abnormal erythema and/or induration, oozing wound without frank pus or pus formation); endomyometritis (fever, uterine tenderness and foul‐smelling lochia), pelvic abscess formation, bacteremia; maternal hospital stay (treatment 5.43 vs placebo 6.18, variance not given). | |
| Notes | No woman developed pelvic abscess nor required a laparotomy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized trial 'by drawing of lots', placebo‐controlled. | |
| Participants | 'Low risk' women, undergoing cesarean section (27% in labour). Exclusion: allergy to beta‐lactam antibiotics, receipt of antibiotics within 3 days; ruptured membranes >12 hours; fever, amniotic infection. | |
| Interventions | Cefotetan 1g IV at the time of cord clamping (n = 136) versus placebo injection (n = 130). | |
| Outcomes | Endometritis, wound infection, septicemia; additional antibiotic use (10/136 in treatment group vs 19/130 in placebo); antibiotic costs; maternal hospital stay. | |
| Notes | There was one episode of septicemia in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled. | |
| Participants | All women undergoing cesarean section (8/73 were repeat). | |
| Interventions | Metronidazole 500mg IV prior to incision and metronidazole 2g suppository at end of surgery (n = 35) versus matching placebo infusion and suppository (n = 38). | |
| Outcomes | Fever (>37.9 degrees C within 14 days of delivery); wound infection, endometritis, urinary tract infection, major complication (return to theatre or hospitalized >10 days because of post‐operative morbidity); need for antibiotic therapy (treatment 13 vs placebo 10); fever index (257 degree hours vs 165 hours). | |
| Notes | One major complication (not infectious) in each group (bleeding from lower segment in one, major deep vein thrombosis extending into iliac veins in another). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'On a random basis'; partly placebo‐controlled. | |
| Participants | All patients undergoing cesarean section. | |
| Interventions | Ampicillin 500mg IM pre‐operatively and 8 hourly for 48 hours followed by 500mg orally 8 hourly for 4 days (n = 150) versus no treatment for first 48 hours then oral placebo 8 hourly for 4 days (n = 150). | |
| Outcomes | Urinary tract infection (culture positive), intra‐uterine infection not defined further, classified as endometritis), wound infection. | |
| Notes | Fewer post‐partum urinary isolates in treated group were sensitive to ampicillin (8/17 vs 18/26). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled (using unmarked code‐numbered separate boxes). | |
| Participants | Women undergoing emergency cesarean section (ruptured membranes for >6 hours and <20 hours; corresponds to our definition of non‐elective). | |
| Interventions | Lincomycin 600mg (n = 20) versus metronidazole 500mg (n = 20) versus placebo (n = 20) intravenously 2 hours pre‐operatively and 8 hourly for 48 hours; both treatment groups are combined for the analysis. | |
| Outcomes | Wound discharge/abscess formation, puerperal sepsis (>37.9 degrees C twice in first 48 hours or >37.5 degrees C from 2nd post‐operative day), septicemia, urinary tract infection. | |
| Notes | Authors' definition of puerperal sepsis has been classified as fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized (code generated by pharmacy), double‐blind, placebo‐controlled; code broken when fever developed | |
| Participants | All women undergoing cesarean section (49/148 were repeat procedure; 57/148 were not in labour). | |
| Interventions | Cephalothin 2g IV 15‐30 minutes prior to surgery and 1g every 6 hours for 36 hours, then cephalexin 500mg orally every 6 hours until 5th post‐operative day (n = 74) versus identical appearing placebo (n = 74). | |
| Outcomes | Fever (>100.3 degrees fahrenheit twice after 48 hours); endometritis (fever, uterine tenderness, foul‐smelling or abnormal lochia and positive cultures); urinary tract infection, wound infection; maternal hospital stay (treatment 6.2 vs placebo 7.5, no variance given). | |
| Notes | All bacterial isolates in treatment group were sensitive to cephalothin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Alternate allocation to treatment or no treatment; not placebo‐controlled. | |
| Participants | All women undergoing cesarean section. | |
| Interventions | Aqueous penicillin 10 MU every 8 hours and kanamycin 500mg IM every 12 hours pre‐operatively and for 3 days post‐operatively (n = 115) versus no treatment (n = 115). | |
| Outcomes | Fever (>100.9 degrees fahrenheit after first post‐operative day), severe pelvic infection (treatment 27% vs control 7%); 'free of infectious morbidity' (3.6 vs 6.8 days); maternal hospital stay (5.4 vs 8.8 days, no variance given). | |
| Notes | No adverse drug reactions reported; no evidence of development of resistance reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized to treatment or no treatment (method not described). Two patients excluded (one from cefoperazone group, one from no treatment group); intent to treat analysis performed. | |
| Participants | Women undergoing cesarean section. | |
| Interventions | Cefoperazone 1g every 12 hours x 3 (n = 71) vs ampicillin 500mg every 6 hours x 4 (n = 74) vs no treatment (n = 77); both treatment groups combined for data analysis. | |
| Outcomes | Wound infection (inflammation over wound with serous or purulent discharge); any antibiotics post‐operatively (cefoperazone vs ampicillin vs no treatment: 6.6% vs 16.2% vs 25.7%). Hospital stay: ampicillin vs no treatment 5.57 days (SD 1.43) vs 6.5 days (SD 3.67). | |
| Notes | Author's definition of emergency not consistent with criteria used in this review; classified as both/undefined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly assigned, double‐blind, placebo‐controlled; medication code kept in pharmacy. | |
| Participants | All women undergoing cesarean section (35/71 were a repeat section). The authors definition of primary and repeat are different from those used in this review and have not been analysed separately; most women for repeat section were in early labour at the time the operation was performed. | |
| Interventions | Ampicillin 2g pre‐operatively (n = 34) versus similar‐appearing placebo (n = 37). | |
| Outcomes | Fever (>37.0 degrees C twice at least six hours apart after first post‐operative day); endometritis, urinary tract infection, wound infection, bacteremia, pelvic abscess, maternal hospital stay. | |
| Notes | There was one pelvic abscess in the placebo group; there were 3 episodes of bacteremia (1 Klebsiella spp. in treatment group, 2 group B streptococcal infections in placebo); combined for outcome of serious morbidity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomly assigned, case number known only by pharmacy; placebo‐controlled. | |
| Participants | All women undergoing cesarean section (46/122 were a repeat section). The authors' definition of primary and repeat do not correspond to definitions of elective and nonelective used in this review (repeat sections included women in labor with ruptured membranes). The results for these two categories have been combined in this review. | |
| Interventions | Cefazolin 500mg IV 30 minutes before and 500mg at 2 and 1g at 8 hours after delivery (n = 61) versus matching placebo (n = 61). | |
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathogenic organism); urinary tract infection (fever and symptoms, or positive culture); wound infection (fever, cellulitis and exudate); maternal hospital stay (treatment 5.5 days versus placebo 5.7 days, no variance given). | |
| Notes | Two women developed serious complications as stated by the authors: one in treatment group developed septic pelvic thrombophlebitis; one given placebo developed pneumonia and endoparametritis (both included in outcome of serious morbidity). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 'Randomly allocated'; double blind; placebo‐controlled. | |
| Participants | All women undergoing cesarean section (other than repeat section); criteria do not correspond with our definition of non‐elective. | |
| Interventions | Cefazolin 2g after cord clamped (n = 146) and at 4 and 8 hours after first dose versus matching placebo (n = 132). | |
| Outcomes | Fever (oral temperature >100.3 degrees fahrenheit on any of 2 of first 10 postoperative days); urinary tract infection, wound infection (only pus‐draining included in outcome of wound infection); endometritis (fever, tenderness on pelvic examination, abnormal discharge); pelvic abscess; septic pelvic thrombophlebitis, bacteremia; subsequent antibiotic use (23% for placebo vs 12% for treatment). | |
| Notes | Outcome of fever and minor wound infection combined (11/146 for treatment vs 13/132 for placebo). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized to treatment or no treatment. One drop‐out (no treatment) included in intent to treat analysis. | |
| Participants | Women undergoing cesarean section. | |
| Interventions | Mezlocillin 2g intravenously half hour pre‐operatively then every eight hours x 4 (n = 70) vs no treatment (n = 70). | |
| Outcomes | Wound infection (inflammation with or without exudation); endometritis (fever and tenderness of the uterus or fever with pathogens from the cervical canal); urinary tract infection (>100,000 bacteria/ml). | |
| Notes | One episode of allergic skin reaction occurred with the injection of mezlocillin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Assigned at random' to one of three regimens; code kept secret; placebo‐controlled; data from a fourth group that consisted of patients allergic to one of the drugs or undergoing an emergency section have not been included. | |
| Participants | All women undergoing cesarean section. | |
| Interventions | 10 million units benzyl penicillin IV (n = 46) versus 500mg clindamycin IV and 80 mg gentamicin IM (n = 42) versus glucose solution placebo (n = 40) IV by infusion starting 30 minutes before operation and stopping 4 hours after. Results of both treatment groups combined. | |
| Outcomes | Endometritis (fever, uterine tenderness and foul‐smelling vaginal discharge); wound infection (all grades combined); hospital stay (treatment 7.7 vs 7.7 placebo; no variance given). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized using computer‐generated number screen; not placebo controlled, but patient and study co‐ordinators unaware of group allocation. | |
| Participants | Women undergoing elective cesarean section (absence of labor and before rupture of membranes). | |
| Interventions | Cefuroxime 1.5g after clamping of the cord vs no treatment. | |
| Outcomes | Febrile morbidity (temperature of >38 degrees C after first 48 hours); endometritis (uterine tenderness and offensive lochia with fever and no other source); wound infection (erythema, induration or purulent discharge); urinary tract infection (>100,000 bacteria/ml). | |
| Notes | Majority of patients were indigent; follow‐up at 6 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 'Randomly allocated'; placebo‐controlled. | |
| Participants | All women undergoing cesarean section (77/129 were elective sections). | |
| Interventions | Cefoxitin 2g (n = 64) versus matching placebo (n = 65) IV bolus immediately following clamping of the cord and at 6 and 12 hours later. | |
| Outcomes | Febrile morbidity (>38 degrees C for at least 24 hr after first 24 hr); endometritis (fever, fetid lochia and/or uterine tenderness on pelvic examination); wound infection (palpable induration, wound dehiscence and/or pus drained); urinary tract infection (positive culture), bacteremia. | |
| Notes | One episode of Staphylococcus aureus bacteremia (in cefoxitin group) not considered life‐threatening (included in outcome of serious morbidity). No serious antibiotic side‐effects reported in cefoxitin‐treated group; one patient in cefoxitin group developed diarrhoea. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomized, sequential basis'; placebo controlled. | |
| Participants | Women undergoing emergency cesarean section (in active labour with membrane rupture). | |
| Interventions | Metronidazole 500mg (n = 57) versus placebo (n = 58) IV infusion at start of procedure; postoperatively metronidazole or placebo suppository twice daily for 5 days. | |
| Outcomes | Pyrexia (>38 degrees C twice 4 hours apart after first 24 hours); wound infection; endometritis (heavy, offensive lochia and pyrexia); urinary tract infection; antibiotic use (15/57 in treatment group vs 20/58 in control group). | |
| Notes | One woman in the control group developed a pelvic abscess. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized using last digit of hospital chart: even to treatment, odd to no treatment (not placebo‐controlled). | |
| Participants | All women undergoing cesarean section (divided into "no labour" and "labor" groups which correspond to the definitions of elective/non‐elective used in this review. | |
| Interventions | Cephalothin 2g IV and kanamycin 1g IM at induction of anesthesia, then cephalothin 2g IV q6hrs x 8 doses and kanamycin 500mg IM q12hr x 4 doses (n = 47) versus no treatment (n = 53). | |
| Outcomes | Endometritis (fever, uterine tenderness and positive culture or fever and pathogenic organism); urinary tract infection, wound infection (fever and cellulitis or exudate). | |
| Notes | No difference in average duration of hospital stay between groups (data not shown). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized using computer‐generated code, kept confidential, to treatment or identical placebo. | |
| Participants | Women undergoing cesarean section (both elective and emergency). | |
| Interventions | Cefazolin 1g after clamping of the cord (n = 221) vs matching placebo (n = 220). | |
| Outcomes | Febrile morbidity (>38 degrees C twice 4 hours apart after first 24 hours); endometritis (fever, uterine tenderness and abnormal lochia); wound infection (fever, cellulitis or exudate with positive culture); urinary tract infection (fever and positive urine culture); pneumonia, bacteremia, pelvic abscess, unexplained fever, therapeutic antibiotics, length of post‐operative stay. | |
| Notes | Emergency cesarean section not defined, results reported in undefined category. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomly allocated using table of random numbers by pharmacy to one of three groups (antibiotic irrigation, placebo irrigation, no irrigation); vitamin solution added to make placebo visually identical; physicians and patients blinded to treatment. | |
| Participants | All women undergoing cesarean section (19/60 women had ruptured membranes >6 hours; 40/60 were in active labour). | |
| Interventions | Cefamandole 2g in 800ml normal saline irrigation (n = 30) versus irrigation with 800 ml normal saline (n = 30). Non‐irrigation control group (n = 30) not included. | |
| Outcomes | Endomyometritis (fever, unusual uterine and parametrial tenderness without evidence of other source of infection); maternal length of stay. | |
| Notes | Length of hospital stay for the control group included results from both the no irrigation group and the placebo irrigation group (5.37 days vs 4.53 for treatment group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized, placebo‐controlled. | |
| Participants | Women in active labor undergoing cesarean section. | |
| Interventions | Metronidazole 1g IV (n = 50) versus identical appearing placebo (n = 50) immediately after cord clamping. | |
| Outcomes | Endometritis (purulent and/or foul odor lochia); wound infection (wound edges tender, red and swollen, or frank pus or sanguino‐purulent material exuded); urinary tract infection (bacteria seen in sediment) ; maternal hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized, double‐blind, placebo controlled. | |
| Participants | High risk women undergoing cesarean section (in active labour and/or ruptured membranes >4hours); classified as non‐elective in this review. | |
| Interventions | Ceftizoxime 2g (n = 50) versus placebo (n = 49) IV at time of cord clamping. | |
| Outcomes | Febrile morbidity (oral temperature >37.9 degrees C twice at least 8 hr apart, after first 24 hr); endometritis (fever and foul lochia or uterine tenderness); urinary tract infection (fever and positive culture); wound infection (fever, abnormal‐looking wound, surrounded by cellulitis and/or draining purulent material). | |
| Notes | Ceftizoxime is a third generation cephalosporin with broad aerobic and anaerobic activity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Double‐blind, randomized, placebo‐controlled. | |
| Participants | Women undergoing cesarean deliveries for various indications. | |
| Interventions | Metronidazole gel 5g intravaginally (n = 31) vs matching placebo (n = 32). | |
| Outcomes | Endometritis. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly assigned; not placebo controlled. | |
| Participants | Women undergoing emergency cesarean section (58/60 women in spontaneous labor; classified as non‐elective). | |
| Interventions | Cefuroxime 750mg IM 30‐60 minutes before surgery and 8 and 16 hours after (short term)(n = 20) versus 750mg three times a day for five days (first dose being given post‐operatively after the woman had returned to the ward) (long term) (n = 20) versus no treatment (n = 20). The results of both treatment groups have been combined. | |
| Outcomes | Fever (>100.3 degrees fahrenheit twice 6 hr apart); endometritis (fever and uterine tenderness); maternal stay (treatment 7.1 vs control 7.9 days, no variance given). | |
| Notes | Note: the group given long‐term prophylaxis received the first dose after return to the ward. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Randomly referred'; not placebo controlled. | |
| Participants | Women with rupture of membranes for >6 hours (equivalent to non‐elective group). | |
| Interventions | Cefuroxime 1.5g IV q8hr for 24 hours, starting immediately before or during the operation, followed by oral cefadroxil 500mg twice daily for 6 days (n = 26) versus no treatment (n = 27). | |
| Outcomes | Endometritis (marked uterine tenderness with or without a foul discharge with fever at least twice); wound infection (redness, tenderness, induration and pus in the wound); urinary tract infection (positive culture). | |
| Notes | Data provided (but not included) for a second control group eligible for inclusion but not randomized. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized using consecutively numbered. sealed envelopes to treatment groups or no treatment. 14 patients excluded from study (8/147 from treatment groups, 6/51 from control group); included in intent to treat analysis. | |
| Participants | Women undergoing elective caesarean section. Exclusion: hypersensitivity to penicillin or cephalosporin; prior antibiotic therapy within 3 days; hepatorenal insufficiency; positive cultures or definite evidence of infection. | |
| Interventions | Pipericillin 4g intravenously after the cord was clamped (n = 48) vs cephadrine 500mg plus metronidazole 500mg both intravenously after the cord was clamped and every 8 hours x 2 (n = 47) vs pipericillin 2g intravenously after clamping of the cord and 2g every 8 hours x 2 (n = 52) vs no treatment (n = 51). | |
| Outcomes | Febrile morbidity (fever >38 degrees C twice 4 hours apart after first day); endometritis (uterine and parametrial tenderness, foul smelling vaginal discharge); wound infection (local induration and tenderness with wound exudate). | |
| Notes | Three patients who developed drug reactions were excluded from study (one from each of the treatment groups). Late morbidity evaluated at 4‐6 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly allocated (individually randomized block ensuring two‐to‐one randomization at each centre); placebo‐controlled; women and investigators blind to allocation throughout the study. | |
| Participants | All women undergoing cesarean section (46% in labor). | |
| Interventions | Cephradine 1g IV (n = 133) versus placebo (n = 66) within 1 hour prior to surgery, repeated at 4 hours. | |
| Outcomes | Febrile morbidity (oral temperature >37.7 degrees C twice 4 hours apart, after first 48 hours); endometritis (uterine tenderness, fever and purulent discharge), wound infection (increased local tenderness, redness or swelling); urinary tract infection (positive culture); maternal length of stay (treatment 5.8 days vs placebo 7.57 days; p<0.05, variance not given). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly assigned, placebo‐controlled. | |
| Participants | All women in labour or with ruptured membranes (duration of ruptured membranes not stated; mean duration 9.97 hours; included in non‐elective category). | |
| Interventions | Cefoxitin 2g (n = 124) versus cefazolin 1g (n = 120) versus placebo (n = 117) infused intravenously immediately after cord clamped and 6 and 12 hours later. Results of both treatment groups combined. | |
| Outcomes | Febrile morbidity (oral temperature >37.9 degrees C twice at least 6 hours apart after first 24 hours); wound infection (redness, induration, tenderness and/or purulent discharge from the incision line); endometritis/parametritis (uterine and/or adnexal tenderness with fever) urinary tract infection (dysuria or pyuria and positive culture); need for antibiotic therapy (11% for treatment groups vs 27% for placebo); maternal length of stay (7.3 and 7.4 days for treatment groups vs 7.9 for placebo). | |
| Notes | Side‐effects documented: two infusion‐related hypotensive episodes (one with cefazolin, one with placebo that necessitated withdrawal from study); six episodes of phlebitis (five in treated, one in placebo group); one episode of angioedema (placebo patient). Data provided on antibiotic resistance in wound isolates and screening cervical cultures. One episode of bacteremia (in placebo group); one episode of septic shock (in cefazolin‐treated group); both outcomes included as serious morbidity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized as determined by table of random numbers; placebo‐controlled; double‐blind | |
| Participants | Women undergoing primary cesarean section (inclusion criteria not consistent with the definition of non‐elective cesarean section used in this review). | |
| Interventions | Cefoxitin 2g IV immediately after the cord was clamped and at 4 and 8 hours (n = 52) versus matched placebo (mannitol with riboflavin) (n = 61). | |
| Outcomes | Febrile morbidity (oral temperature >37.9 degrees C twice at least 6 hours apart after first 24 hours); urinary tract infection (positive culture); wound infection (purulence, cellulitis or dehiscence); endometritis (fever, uterine tenderness, abnormal lochia); septicemia (positive blood culture in a clinically septic patient); additional antibiotic use (8 in treatment group vs 12 in placebo). | |
| Notes | Both episodes of septicemia occurred in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Alternate patients undergoing caesarean section allocated to treatment or no treatment. | |
| Participants | Women undergoing cesarean section (both elective and emergency). | |
| Interventions | Cephradine 2g intravenously after induction of anesthesia and 1g 6 and 12 hours after the operation (n = 101) vs no treatment (n = 100). | |
| Outcomes | Puerperal infection (temperature >37.5 degrees C after 24 hours); endometritis (pyrexia with uterine or adnexal tenderness); wound infection (purulent discharge or erythema, induration and serous discharge with positive culture); urinary tract infection (>100,000 colony forming units in urine culture); length of hospital stay (7.63 for treatment group, 7.18 for control group [SD not provided]). | |
| Notes | Definitions of elective and emergency procedure, nor separate outcomes for each group, provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Selected in a random manner; double‐blind; placebo‐controlled. | |
| Participants | Women in labour (non‐elective). | |
| Interventions | Cefuroxime 750mg IV within 1 hour of surgery and every 8 hours for 72 hours (n = 46) versus matching placebo (comparable in appearance and viscosity) (n = 50). | |
| Outcomes | Febrile morbidity (oral temperature of >100.3 degrees fahrenheit twice 6 hours apart) and infection of endometrium, urinary tract and wound (not defined); results of duration of maternal stay only provided for febrile patients. | |
| Notes | No patients had any major complications from the use of cefuroxime. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocated 'in random form' using a random table; not placebo‐controlled. | |
| Participants | Women undergoing urgent cesarean section. | |
| Interventions | Ampicillin 2g intravenously every 4 hours x 3 after clamping of cord (n = 59) vs no treatment. | |
| Outcomes | Febrile syndrome; wound infection; abdominal wall abscess; endometritis. | |
| Notes | No definitions of outcomes provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 'Selected at random'; not placebo‐controlled. | |
| Participants | Women undergoing primary cesarean section after the onset of labor. | |
| Interventions | Penicillin G 2 million units IV every 4 hours and kanamycin 500mg IM every 12 hours as soon as it was decided to perform a cesarean section, at the time of operation or immediately post‐operatively and continued for a minimum of 3 days post‐operatively (n = 40) versus no treatment (n = 40). | |
| Outcomes | Febrile morbidity (temperature of >100.3 degrees fahrenheit on any two days after first 24 hours); urinary tract infection, endometritis and wound infection (not defined); maternal length of stay (treatment 5.8 days vs 8.7 days for control group, no variance given). | |
| Notes | One patient receiving penicillin had a drug rash on the third day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized in numbered packages by the pharmacy department; placebo‐controlled; blinded. | |
| Participants | Women with ruptured membranes who underwent internal fetal monitoring (not consistent with the definition of non‐elective used in this review). | |
| Interventions | Cefazolin 1g IV after the cord was clamped and at 4‐6 hours and 10‐12 hours post‐operatively (n = 48) versus placebo (n = 45). | |
| Outcomes | Standard temperature morbidity, endomyometritis, abdominal wound infection, urinary infections (no definitions provided for any outcomes). | |
| Notes | Two women were said to develop a serious infection: one (cefazolin group) developed septic thrombophlebitis and is included as a serious outcome; the other (placebo group) was treated with antibiotics for prolonged fever (judged not to be a serious outcome for this review). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Selected in random, double‐blind manner; placebo‐controlled. | |
| Participants | Women in labor. | |
| Interventions | Cephalothin 2g IV within one hour of operation and at 4 and 8 hours after (n = 40) versus comparable appearing placebo (n = 40). | |
| Outcomes | Febrile morbidity (oral temperature >100.3 degrees fahrenheit twice 6 hours apart); infection of endometrium, urinary tract and wound (definitions not provided); fever index (40 degree hours for treatment group vs 83 for placebo group). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized into three groups (irrigation vs systemic treatment vs no treatment). | |
| Participants | Women undergoing both elective (n = 112) and non‐elective (n = 105) cesarean section. Only women undergoing an elective cesarean section were randomized to treatment or no treatment and have been included in analysis. | |
| Interventions | Local irrigation with ampicillin 6g after delivery of the placenta (n = 39) vs penicillin 5.6 MU and gentamicin 240,000 U intravenously immediately after surgery and penicillin 1.6 MU and gentamicin 160,000 U per day intramuscularly x 3 days (n = 41) vs no treatment (n = 32). | |
| Outcomes | Endometritis (presence of any 2 of following: temperature above 37.5 degrees C, uterine tenderness, foul vaginal discharge); abdominal wound infection (cellulitis with small amount of exudate within 2 months of operation); uterine incision infection (associated with late post‐partum haemorrhage); fever index. | |
| Notes | Women undergoing non‐elective sections randomized to either treatment group (not included in this review). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Assigned by the anesthetist in a randomized, double‐blind manner; placebo‐controlled. | |
| Participants | Women undergoing cesarean section. | |
| Interventions | Augmentin 1.2g (amoxycillin sodium 1000mg and clavulanate potassium 200mg) in 10ml saline (n = 160) versus saline placebo (n = 160). | |
| Outcomes | Febrile morbidity (2 oral temperatures >37.9 degrees C at least 6 hours apart after first 24 hours); bacteriuria at day 3 (classified in this review as urinary tract infection); wound infection (purulent discharge, cellulitis, tenderness and wound abscess requiring incision and drainage); endometritis (fever, pelvic pain, uterine tenderness, purulent vaginal discharge without signs of infection in the lower genital tract); duration of hospital stay. | |
| Notes | Sub‐rectus Redivac drain routinely inserted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomly assigned; placebo‐controlled; physician team blinded. | |
| Participants | Women in labour with an intrauterine pressure catheter and fetal scalp electrode (non‐elective). | |
| Interventions | Cefoxitin 1g IV at time of cord clamping and at 4 and 8 hours (n = 50) versus matching placebo (n = 50). | |
| Outcomes | Endomyometritis, abdominal wound infection, serious complications; duration of maternal hospital stay (treatment 5.1 days vs control 5.9 days, not statistically significant, no variance given). | |
| Notes | One case of septic pelvic thrombophlebitis occurred in the treatment group; there were 8 episodes of bacteremia in the control group vs one in the treatment group; both outcomes combined under serious morbidity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
C = centigrade
hr = hour/hours
IM = intramuscularly
IV = intravenously
MU = million units
q6hrs = every six hours
SD/sd = standard deviation
vs = versus
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Did not include women undergoing cesarean section. | |
| Not relevant to this review. Ampicillin or gentamycin given prior to cesarean section in women with intrauterine infection, to measure transplacental transfer. No control group, and no clinical outcomes given. | |
| High risk women (membranes ruptured for more than six hours) initially were randomized to early treatment (ie prophylactic therapy continued for four days) vs standard treatment (ie treatment only started when infection apparent). When the results were compared midway through the study, standard therapy was abandoned. The results for the two groups prior to abandoning the no treatment group could not be obtained from the paper. | |
| Only the first 42 women were randomized to placebo or active treatment; after that a significant difference was observed between the placebo and treated groups and the placebo arms were discontinued. Further women were randomized to two different active treatments. The data for the first part of the study (with only the first 42 women) are not available from the published paper. | |
| Not all women were randomly allocated to treatment or no treatment. 150 women were assigned at random to each of the two wings of the department according to the day of their admission, each wing receiving women on alternate days. In both wings, of the last 50 women every second woman served as a control. Fifty women in one wing received IV cephalothin or oral cephalexin, 50 women in the other wing received IV or oral ampicillin. The first 50 women enrolled were all treated; separate results for the last 100 women (who were alternately allocated therapy or no treatment) are not available. | |
| After approximately 70% of the planned study population had been randomized to placebo or one of two active treatment groups, an unacceptably high morbidity rate in the placebo group was confirmed and the placebo arm was discontinued. Further women were randomized to two different active treatments. The data for the first part of the study when women were randomized to treatment or placebo are not available from the published paper. | |
| Eligible women were in active labor with ruptured membranes. While this study initially included a placebo control group, this group was dropped after 30 women had been enrolled on the basis of ethical considerations about assigning women to a nontreatment group in which the likelihood of morbidity was high. Only seven women (out of a total of 195 women entered) were randomized to placebo, separate results on the initial part of the study not available. The placebo (7) and treatment groups (188) were very imbalanced making a meaningful comparison between groups impossible. | |
| Abstract only; unable to confirm random allocation and method of allocation to no treatment group; data for separate outcomes of endometritis and wound infection not provided. | |
| No numerical data. | |
| Women were randomized to receive intravaginal metronidazole or placebo gel during labour; most, but not all patients also received one prophylactic dose of cefazolin after cord clamping. | |
| No clinical outcomes. | |
| In this study, in which women were alternately allocated to antibiotic prophylaxis or no treatment, the women enrolled were undergoing both gynaecological and obstetrical surgery. Rates of infectious complications are given for all abdominal surgery (cesarean section, abdominal hysterectomy and laparotomy). Data specifically on the women who underwent cesarean section are, however, not available from the published study. | |
| Abstract only. Rates for all post‐operative infection morbidity and clinically significant genital tract‐related infections (wound infections, endometritis) and abscess formation (septicemia) combined; rates for individual outcomes not provided. | |
| Results combined from three time periods. In only one period did it appear women were randomized to antibiotic therapy or no treatment; results just for this period not available in published report. | |
| Abstract only; results given for post‐operative sepsis (38.9% in placebo group, 0% in treatment group), but data for separate outcomes not given. | |
| All women received antibiotics (randomized to cefoxitin after cord clamping and then every fours hours x 2 or oral ampicillin 2g daily x seven days); no clinical outcomes reported. | |
| This was not a randomized trial of antibiotic prophylaxis. Three distinct groups of women were studied: one group was part of randomized trial that compared extracorporeal cesarean section with prophylactic antibiotic; the second group received extracorporeal cesarean section and no antibiotics; the third group received extracorporeal cesarean section with antibiotics (the decision to administer antibiotics in the latter two groups was at the discretion of the physician). | |
| Absolute numbers cannot be calculated from data provided in abstract; no published version of this study identified. |
IV = intravenous
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fever Show forest plot | 40 | 7180 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.39, 0.52] |
| Analysis 1.1  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 1 Fever. | ||||
| 1.1 Elective cesarean delivery | 9 | 1814 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.75] |
| 1.2 Non‐elective cesarean delivery | 15 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.51] |
| 1.3 Both elective and non‐elective, or undefined cesarean delivery | 21 | 3074 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.38, 0.58] |
| 2 Wound infection Show forest plot | 70 | 11142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.35, 0.48] |
| Analysis 1.2  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 2 Wound infection. | ||||
| 2.1 Elective cesarean delivery | 12 | 2015 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 0.99] |
| 2.2 Non‐elective cesarean delivery | 20 | 2780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.51] |
| 2.3 Both elective and non‐elective, or undefined cesarean delivery | 43 | 6347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.29, 0.43] |
| 3 Endometritis Show forest plot | 76 | 11957 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.34, 0.43] |
| Analysis 1.3  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 3 Endometritis. | ||||
| 3.1 Elective cesarean delivery | 12 | 2037 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.22, 0.64] |
| 3.2 Non‐elective cesarean delivery | 23 | 3132 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.34, 0.46] |
| 3.3 Both elective and non‐elective, or undefined cesarean delivery | 48 | 6788 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.30, 0.44] |
| 4 Urinary tract infection Show forest plot | 57 | 8857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.46, 0.64] |
| Analysis 1.4  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 4 Urinary tract infection. | ||||
| 4.1 Elective cesarean delivery | 9 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.11] |
| 4.2 Non‐elective cesarean delivery | 18 | 2572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.30, 0.60] |
| 4.3 Both elective and non‐elective, or undefined cesarean delivery | 34 | 4847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.48, 0.71] |
| 4.4 Elective cesarean delivery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious infectious morbidity/death Show forest plot | 29 | 4760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.28, 0.65] |
| Analysis 1.5  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 5 Serious infectious morbidity/death. | ||||
| 5.1 Elective cesarean delivery | 3 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.04, 24.21] |
| 5.2 Non‐elective cesarean delivery | 8 | 1336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.61] |
| 5.3 Both elective and non‐elective, or undefined cesarean delivery | 20 | 2979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.84] |
| 6 Maternal side‐effects Show forest plot | 11 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.91, 4.50] |
| Analysis 1.6  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 6 Maternal side‐effects. | ||||
| 6.1 Elective cesarean delivery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Non‐elective cesarean delivery | 7 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.67, 4.43] |
| 6.3 Both elective and non‐elective, or undefined cesarean delivery | 5 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.63, 13.74] |
| 7 Days in hospital (mother) Show forest plot | 15 | 2964 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.68, ‐0.26] |
| Analysis 1.7  Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 7 Days in hospital (mother). | ||||
| 7.1 Elective cesarean delivery | 3 | 830 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 7.2 Non‐elective cesarean delivery | 3 | 586 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.77, ‐0.13] |
| 7.3 Both elective and non‐elective, or undefined cesarean delivery | 10 | 1548 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.88, ‐0.19] |

Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 1 Fever.
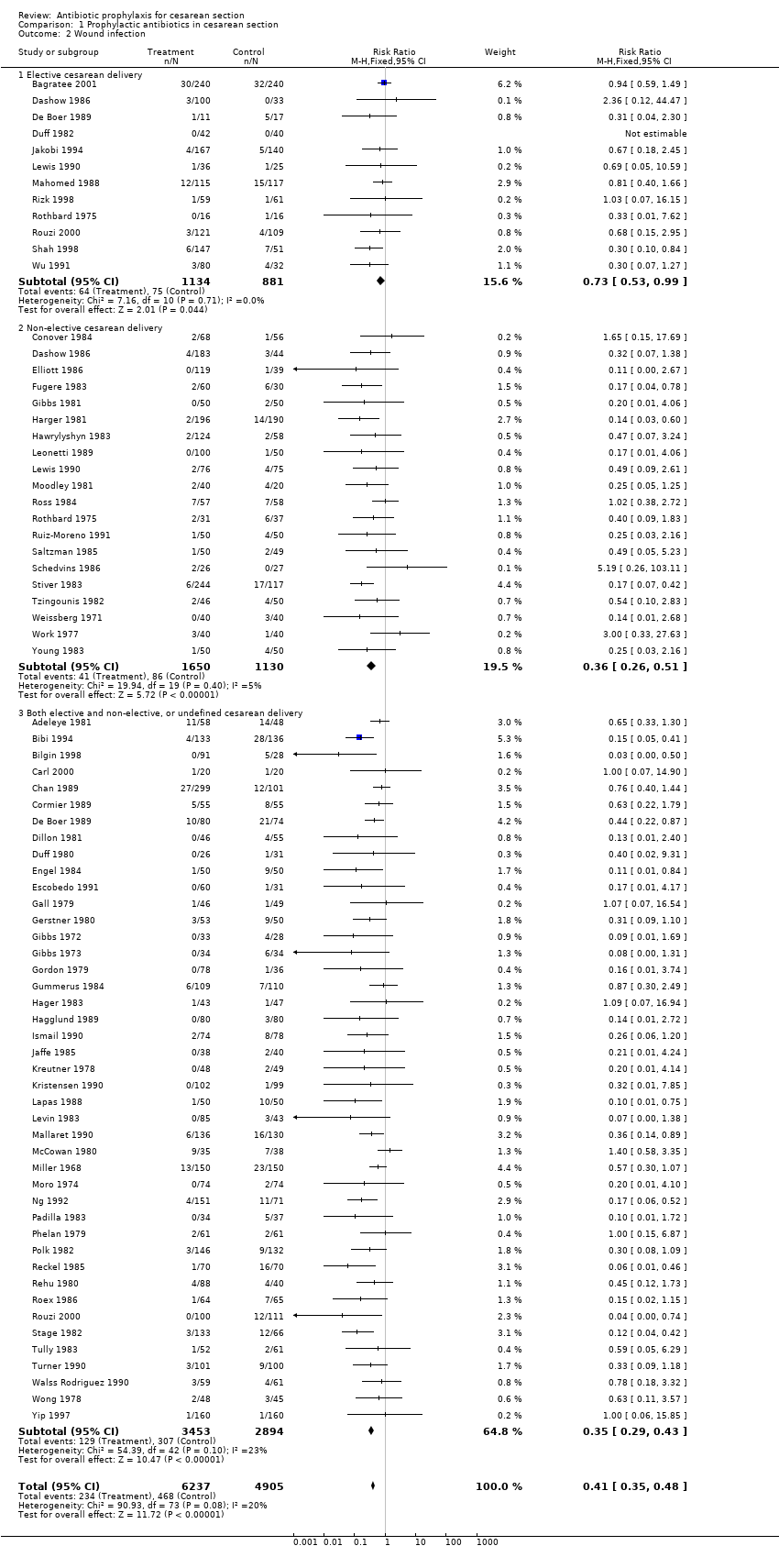
Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 2 Wound infection.

Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 3 Endometritis.
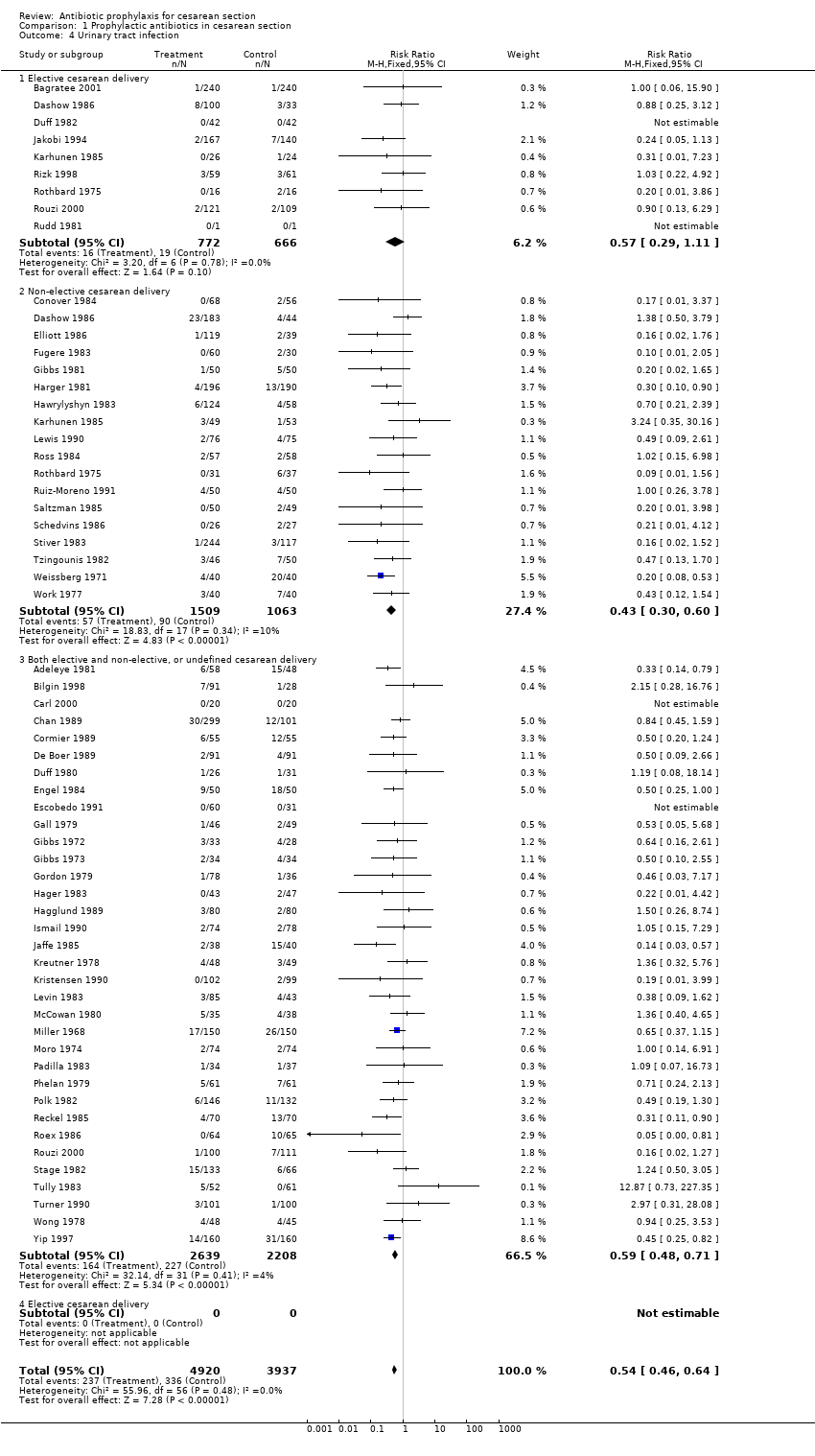
Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 4 Urinary tract infection.

Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 5 Serious infectious morbidity/death.

Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 6 Maternal side‐effects.

Comparison 1 Prophylactic antibiotics in cesarean section, Outcome 7 Days in hospital (mother).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fever Show forest plot | 40 | 7180 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.39, 0.52] |
| 1.1 Elective cesarean delivery | 9 | 1814 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.75] |
| 1.2 Non‐elective cesarean delivery | 15 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.51] |
| 1.3 Both elective and non‐elective, or undefined cesarean delivery | 21 | 3074 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.38, 0.58] |
| 2 Wound infection Show forest plot | 70 | 11142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.35, 0.48] |
| 2.1 Elective cesarean delivery | 12 | 2015 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 0.99] |
| 2.2 Non‐elective cesarean delivery | 20 | 2780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.51] |
| 2.3 Both elective and non‐elective, or undefined cesarean delivery | 43 | 6347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.29, 0.43] |
| 3 Endometritis Show forest plot | 76 | 11957 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.34, 0.43] |
| 3.1 Elective cesarean delivery | 12 | 2037 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.22, 0.64] |
| 3.2 Non‐elective cesarean delivery | 23 | 3132 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.34, 0.46] |
| 3.3 Both elective and non‐elective, or undefined cesarean delivery | 48 | 6788 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.30, 0.44] |
| 4 Urinary tract infection Show forest plot | 57 | 8857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.46, 0.64] |
| 4.1 Elective cesarean delivery | 9 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.11] |
| 4.2 Non‐elective cesarean delivery | 18 | 2572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.30, 0.60] |
| 4.3 Both elective and non‐elective, or undefined cesarean delivery | 34 | 4847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.48, 0.71] |
| 4.4 Elective cesarean delivery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious infectious morbidity/death Show forest plot | 29 | 4760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.28, 0.65] |
| 5.1 Elective cesarean delivery | 3 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.04, 24.21] |
| 5.2 Non‐elective cesarean delivery | 8 | 1336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.61] |
| 5.3 Both elective and non‐elective, or undefined cesarean delivery | 20 | 2979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.84] |
| 6 Maternal side‐effects Show forest plot | 11 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.91, 4.50] |
| 6.1 Elective cesarean delivery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Non‐elective cesarean delivery | 7 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.67, 4.43] |
| 6.3 Both elective and non‐elective, or undefined cesarean delivery | 5 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.63, 13.74] |
| 7 Days in hospital (mother) Show forest plot | 15 | 2964 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.68, ‐0.26] |
| 7.1 Elective cesarean delivery | 3 | 830 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 7.2 Non‐elective cesarean delivery | 3 | 586 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.77, ‐0.13] |
| 7.3 Both elective and non‐elective, or undefined cesarean delivery | 10 | 1548 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.88, ‐0.19] |

