Derivaciones portosistémicas versus tratamiento endoscópico para las nuevas hemorragias por varices en pacientes con cirrosis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised trial. Inclusion of all randomised participants at evaluation: 1 patient from each group died before treatment and not included in the analysis. Time from bleeding episode to randomisation: Three days after bleeding was controlled. | |
| Participants | Inclusion criteria: all cirrhotic patients admitted with an episode of acute oesophageal variceal bleeding. Exclusions (one or more of the following): presence of gastric varices with active bleeding, episodes of chronic encephalopathy, severe acute alcoholic hepatitis, end‐stage cirrhosis, neoplastic disease, septicaemia and portal vein thrombosis. The two groups comparable in‐terms of age, Child's status and number of alcoholic patients. | |
| Interventions | ET: Shunt: | |
| Outcomes | Incidence of rebleeding. | |
| Notes | Long‐term follow‐up published as abstract in Hepato‐Gastroenterology 1998, Third International Congress of Hepato‐Pancreato‐Biliary Association. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Duplicate publication of Cello 1987. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation, not mentioned. | |
| Participants | Inclusion criteria: Child's C patients with actively bleeding varices confirmed on endoscopy, requiring six or more units of blood transfusion. Exclusions: moribund patients. Pre‐treatment variables were comparable across the two groups other than active alcoholics which were significantly greater in the ES group. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | Only 16 patients in the shunt group and 14 in the ET group discharged after the index hospitalisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation (mean, SD) = TIPS (35.4,5.6 hours), ET (37.4, 4.7 hours). Time from randomisation to treatment (mean, SD) = TIPS ( 59.5, 6.7 hours). | |
| Participants | Inclusion criteria: all patients admitted with massive or submassive acute gastrointestinal tract haemorrhage from large oesophageal varices. Exclusions (one or more of the following): prisoners, <18 or >75 years of age, Cerebrovascular accident three months before the onset of bleeding, refusal to accept blood products, gastric variceal haemorrhage, ECG changes compatible with myocardial infarction, specified limits of PO2, creatinine, bilirubin, prothrombin time and platelet count measurements, Grade IV encephalopathy, cancer other than skin cancer, AIDS, sepsis, pneumonia, peritonitis, alcoholic hepatitis (clinical evidence only), thrombosis of portal, hepatic or inferior vena cava. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from variceal bleeding to therapy in days, mean (SD): TIPS 5.4 (2.1), ET 5.6 (2.2). | |
| Participants | Inclusion criteria: endoscopically proven oesophageal variceal bleeding, diagnosis of cirrhosis based on clinical history and laboratory, ultrasonography, and/ or liver biopsy findings, age between 18 to 75 years and informed consent from the patient. Exclusions (one or more of the following): history of chronic encephalopathy, portal vein thrombosis, hepatocellular carcinoma and end‐stage liver disease. Comparable with respect to age, gender, etiology. Endoscopic group had a significantly greater proportion of patients with pre‐existing encephalopathy but were comparable in‐terms of Child‐Pugh class. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial (Abstract). Randomised to TIPS: 32; ET: 33. | |
| Participants | Child's C cirrhotic patients presenting with variceal bleeding. Treated with sclerotherapy prior to randomisation. | |
| Interventions | ET: Shunt: | |
| Outcomes | Variceal rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation (mean, SD) = TIPS 13, 3(days), ET 14, 3 (days). Time from randomisation to treatment = unclear. | |
| Participants | Inclusion criteria: age > 18 years, endoscopic evidence of variceal bleeding within 2 months before randomisation, stable heamodynamic condition. Exclusion criteria: isolated gastric varices, isolated bleeding from gastric varices, large or diffuse liver tumour, liver transplantation intended in six months, hepatic encephalopathy > grade 2, Child Pugh >13, extra hepatic cholestasis, heart failure, sepsis, multi‐organ failure. | |
| Interventions | ET: TIPS: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation, not mentioned. | |
| Participants | Inclusion criteria: biopsy proven cirrhosis, endoscopic evidence of varices and suitability for a DSRS shunt established with angiography. Exclusions (one or more of the following): living more than 200 miles from the base hospital, referred for specific therapy, previous chronic sclerotherapy, emergent or urgent surgery, noncirrhotic variceal bleed. | |
| Interventions | ET: Shunt: | |
| Outcomes | Survival. | |
| Notes | Encephalopathy not considered as an outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation and treatment: not specified, but patients were included after their variceal bleeding was arrested with emergency endoscopic sclerosis and if they fulfilled the entry criteria. | |
| Participants | Inclusion criteria: age between 20 to 75 years, endoscopically verified varices as the source of bleeding, portal hypertension, biopsy confirmed cirrhosis. Exclusions: not specified. Patients comparable in‐terms of age and Child's status but alcoholics slightly greater in the ES group. | |
| Interventions | ET: Shunt: | |
| Outcomes | Survival. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation: 24 hours after cessation of bleeding. | |
| Participants | Inclusion criteria: all cirrhotic patients between 18 and 75 yrs of age who presented with a first (index) episode of variceal bleeding. Exclusions (one or more of the following): rebleeding from varices from varices within 24 hours of initial endoscopy, bleeding from ectopic varices, previous endoscopic treatment for varices, malignancy, portal vein thrombosis. | |
| Interventions | ET: Shunt: | |
| Outcomes | Variceal rebleeding. | |
| Notes | Only trial to employ variceal banding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation: not mentioned. | |
| Participants | Inclusion criteria: all patients with cirrhotic portal hypertension (Child‐Pugh A) with minimum of two variceal bleeding episodes who received less than one session of ET. Exclusions: not mentioned. | |
| Interventions | ET: Shunt: | |
| Outcomes | Variceal rebleeding. | |
| Notes | Only trial to employ variceal banding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial. Time from bleeding episode to randomisation: Three strata of time intervals used: I = acutely bleeding patients (one to seven days), II = patients referred from other centres following a bleed but without endoscopic treatment (one to six weeks), III = patients referred following various intervals after a variceal bleed for advice and treatment (seven weeks to six months). | |
| Participants | Inclusion criteria: patients with variceal bleeding (proven or presumed) according to pre‐specified criteria. Exclusions (one or more of the following): complete portal vein thrombosis, previous episode/s of chronic recurrent hepatic encephalopathy, advanced hepatocellular carcinoma, previous multiple sessions of sclerotherapy, ongoing pharmacological prophylaxis of rebleeding (one emergency session during the acute bleeding phase was permissible), severe cardio‐vascular contraindications; or concomitant morbid condition/s with a life expectancy of less than a year. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation (mean, S.D) = TIPS (19.1,2 days), ET (17.9,1.9). Time from randomisation to treatment (mean, SD) = Not clear. | |
| Participants | Inclusions: cirrhosis with recent variceal haemorrhage, clinical stability at randomisation, age between 20 to 69 years. Exclusion criteria: hepatocellular carcinoma, episodes of chronic encephalopathy, portal vein thrombosis, Child‐Pugh > 13, serum creatinine >2.5 milligram per decilitre, serum bilirubin > 5 milligram per decilitre, active infection and severe cardiopulmonary disease. | |
| Interventions | ET: Sclerotherapy using 5% ethanolamine | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial, reported as abstract only. Duplicate publication of P‐Layrargues 2001. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial. Time from bleeding to randomisation: After patients had been haemodynamically stabilised for 24 hours. | |
| Participants | Inclusion criteria: cirrhosis, Child‐Pugh grade B and C, age between 18 to 75 years, with an episode of endoscopically verified variceal bleeding. Exclusions: portal vein thrombosis, previous endoscopic therapy within three months, previous shunt, fundal varices, hepatocellular carcinoma, cardiac, renal or respiratory failure, non‐compliance, sepsis, and uncontrolled bleeding. | |
| Interventions | ET: Shunt: | |
| Outcomes | Survival. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Duplicate publication of Teres 1987. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Randomised trial. Time from bleeding episode to randomisation: Three days after stabilisation following a variceal bleed. | |
| Participants | Inclusion criteria: Child‐Campbell A and B cirrhotic patients who were considered following endoscopically proven variceal haemorrhage. Exclusions (one or more of the following): Child‐Campbell class C, uncontrollable haemorrhage, or early rebleeding between admission and randomisation. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Duplicate publication of Rikkers 1993. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial. Time from bleeding episode to randomisation: not specified. | |
| Participants | Inclusion criteria: portal hypertension secondary to cirrhosis, endoscopic documentation of acute or recent oesophageal variceal haemorrhage requiring a minimum transfusion of 3 U of blood, residence within 500 miles of Salt Lake City or Omaha, non‐operative control of acute variceal haemorrhage, patency of splenic and portal veins documented by selective angiography Exclusions: not specified. | |
| Interventions | ET: Shunts: | |
| Outcomes | Survival. | |
| Notes | Portacaval shunts were performed on 3 patients with medically intractable ascites and on 1 with massive rebleeding. SPD was not used in any of the patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial. Time from bleeding episode to randomisation in hours (SD): TIPS 6.3 (5.5), ET 4.4 (5). | |
| Participants | Inclusion criteria: liver cirrhosis, variceal bleeding within 2 weeks before randomisation, and age over 18 years. Exclusions (one or more of the following): hepatic encephalopathy grade 3 and 4, liver insufficiency (bilirubin more than 5mg/dl), cavernomatous portal‐vein thrombosis, advanced malignancy, contra‐indications to propranolol (obstructive lung disease, severe hypotension) and bleeding emergency | |
| Interventions | ET: Shunts: | |
| Outcomes | Rebleeding. | |
| Notes | Propranolol used in‐addition to sclerotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation: Clinical stability for at least 72 hours following a variceal bleed. | |
| Participants | Inclusion criteria: clinical stability in the absence of re bleeding 72 hours following a variceal bleed. Exclusions (one or more of the following): portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease which would limit the patients life span to less than one year. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation in days (SD): 1.1 days (1.1). Median observation time in years | |
| Participants | Inclusion criteria: cirrhosis and acute oesophageal haemorrhage. Exclusions (one or more of the following): gastric varices, prior endoscopic or surgical treatment for varices, portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease which would limit the patients life span to less than six months and uncontrolled bleeding requiring an emergency TIPS procedure. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | Propranolol used in‐addition to sclerotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial (abstract). 85 patients randomised to TIPS (43) or EB 42). | |
| Participants | Patients with cirrhosis and portal hypertension. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial. Time from bleeding episode to randomisation in days : 1.2 ‐ 3.2. Total number of patients evaluated and found eligible: 112. Randomised to TIPS: 43, randomised to ET: 42. Rebleeding episodes endoscopically verified: yes. Mean observation time in years | |
| Participants | Inclusion criteria: cirrhosis and acute first oesophageal haemorrhage. Exclusions (one or more of the following): gastric varices, prior endoscopic or surgical treatment for varices, portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease which would limit the patients life span to less than six months and uncontrolled bleeding. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | Propranolol used in‐addition to variceal banding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised trial. Time from bleeding episode to randomisation: ? When the patient achieved haemodynamic stability. | |
| Participants | Inclusion criteria: biopsy confirmed cirrhosis, endoscopically verified variceal bleed requiring at least one unit of blood transfusion, arrest of variceal haemorrhage either spontaneously or by the use drugs and or tamponade and or sclerotherapy, good liver function as reflected by Child‐Pugh class A and B, patency of the portal venous system, eligible for either shunt or sclerotherapy, absence of life threatening diseases and willingness to return for regular follow‐up. Exclusions: not specified. | |
| Interventions | ET: Shunt: | |
| Outcomes | Rebleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised trial. Time from bleeding episode to randomisation: 10 to 15 days. | |
| Participants | Inclusion criteria: Child‐Campbell A and B cirrhotic patients with at least one episode of variceal haemorrhage. Exclusions (one or more of the following): continual variceal bleeding despite medical treatment and early rebleeding between admission and randomisation. | |
| Interventions | ET: Shunt: | |
| Outcomes | Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SD: standard deviation.
SE: standard error of the mean.
ITT: intention to treat.
ET: endoscopic therapy.
TS: total shunt.
DSRS: distal splenorenal shunt.
TIPS: transjugular intrahepatic porto‐systemic shunt.
yrs: years.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Randomisation was to two groups, sclerotherapy and oesophageal transection. The results were compared retrospectively to those of a separate group of patients who had received total shunts. | |
| Randomised groups included patients who received TIPS versus medical therapy. Endoscopic therapy was used acutely in after a variceal bleed. | |
| Patients were included who had not previously bled from varices. | |
| The study end‐points are not the subject of this review. | |
| Cost‐analysis study, possible overlap of previously published results. | |
| Variceal bleeding not controlled prior to randomisation. Endoscopic therapy not used in the medically treated group of patients. | |
| Non‐randomised study. | |
| Endoscopic therapy not employed in the medically treated group. | |
| Endoscopic therapy not employed in the medically treated group. | |
| The study outcome measures are not the subject of this review. Only seven patients randomised. | |
| The study outcome measures not a subject of this review. | |
| Non randomised study. Variceal bleeding not controlled prior to randomisation. | |
| Randomised groups included those who received TIPS compared to those who received TIPS and variceal banding. | |
| Child's C patients not randomised to the DSRS arm. Unable to extract data only for Child's A and B patients from the study. Unable to contact the authors. In addition, small study with unacceptably large attrition to follow‐up (more than 40%). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rebleeding (hazard ratio) Show forest plot | 11 | 835 | Peto Odds Ratio (95% CI) | 0.42 [0.32, 0.54] |
| Analysis 1.1  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 1 Rebleeding (hazard ratio). | ||||
| 1.1 Total shunts versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DSRS versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.3 TIPS versus endoscopic therapy | 11 | 835 | Peto Odds Ratio (95% CI) | 0.42 [0.32, 0.54] |
| 2 Rebleeding Show forest plot | 21 | 1487 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.18, 0.30] |
| Analysis 1.2  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 2 Rebleeding. | ||||
| 2.1 Total shunt versus endoscopic therapy | 3 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.23] |
| 2.2 DSRS versus endoscopic therapy | 4 | 262 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.07, 0.26] |
| 2.3 TIPS versus endoscopic therapy | 14 | 1034 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.22, 0.39] |
| 3 Development of hepatic encephalopathy (hazard ratio) Show forest plot | 10 | 725 | Peto Odds Ratio (95% CI) | 1.96 [1.47, 2.61] |
| Analysis 1.3  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 3 Development of hepatic encephalopathy (hazard ratio). | ||||
| 3.1 Total shunts versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 3.2 DSRS versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 3.3 TIPS versus endoscopic therapy | 10 | 725 | Peto Odds Ratio (95% CI) | 1.96 [1.47, 2.61] |
| 4 Hepatic encephaloapthy Show forest plot | 19 | 1338 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.59, 2.69] |
| Analysis 1.4  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 4 Hepatic encephaloapthy. | ||||
| 4.1 Total shunt versus endoscopic therapy | 3 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.67, 3.54] |
| 4.2 DSRS versus endoscopic therapy | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.83, 3.69] |
| 4.3 TIPS versus endoscopic therapy | 13 | 969 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.63, 2.98] |
| 5 Chronic hepatic encephalopathy Show forest plot | 14 | 991 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.20, 3.62] |
| Analysis 1.5  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 5 Chronic hepatic encephalopathy. | ||||
| 5.1 Total shunts versus endoscopic therapy | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.89 [0.39, 158.73] |
| 5.2 DSRS versus endoscopic therapy | 4 | 245 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.51, 3.29] |
| 5.3 TIPS versus endoscopic therapy | 9 | 677 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.19, 5.03] |
| 6 Duration of in‐patient stay (days) Show forest plot | 9 | 679 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐1.48, 3.05] |
| Analysis 1.6  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 6 Duration of in‐patient stay (days). | ||||
| 6.1 Total shunts versus endoscopic therapy | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐2.38, 8.58] |
| 6.2 DSRS versus endoscopic therapy | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐13.71, 6.91] |
| 6.3 TIPS versus endoscopic therapy | 7 | 507 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐1.95, 3.23] |
| 7 Mortality (hazard ratio) Show forest plot | 19 | 1358 | Peto Odds Ratio (95% CI) | 1.00 [0.82, 1.21] |
| Analysis 1.7  Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 7 Mortality (hazard ratio). | ||||
| 7.1 Total shunts versus endoscopic therapy | 3 | 191 | Peto Odds Ratio (95% CI) | 1.03 [0.68, 1.56] |
| 7.2 DSRS versus endoscopic therapy | 4 | 284 | Peto Odds Ratio (95% CI) | 0.88 [0.59, 1.31] |
| 7.3 TIPS versus endoscopic therapy | 12 | 883 | Peto Odds Ratio (95% CI) | 1.04 [0.80, 1.36] |

Funnel plot of shunt therapy versus endoscopic therapy, showing bias in favour of shunt therapy.

Funnel plot of shunt therapy versus endoscopic therapy, showing no bias in favour of shunt therapy on hepatic encephalopathy.
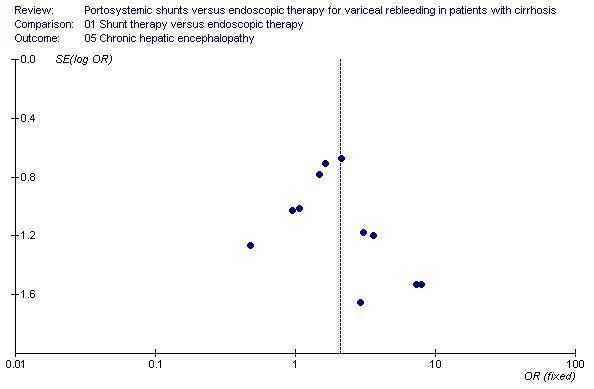
Funnel plot of shunt therapy versus endoscopic therapy, showing no bias in favour of shunt therapy on chronic hepatic encephalopathy.

Funnel plot of shunt therapy versus endoscopic therapy, showing no bias in favour of shunt therapy on mortality.
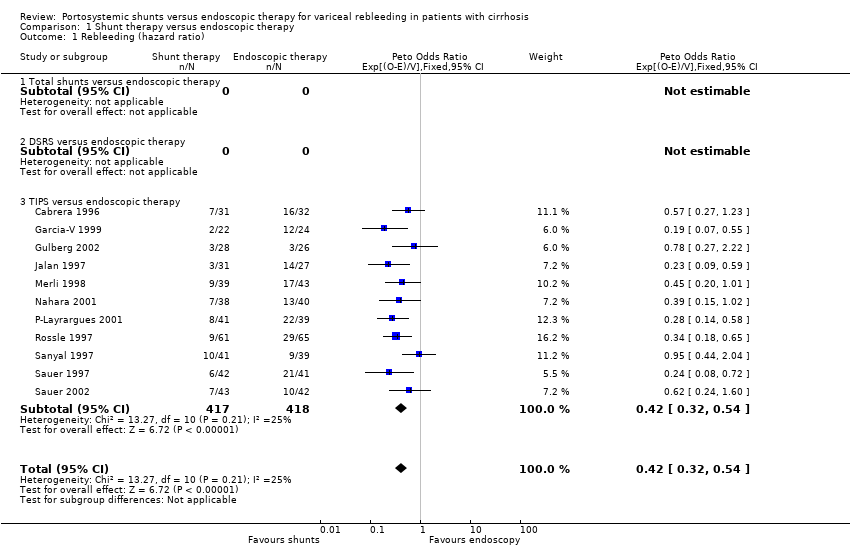
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 1 Rebleeding (hazard ratio).
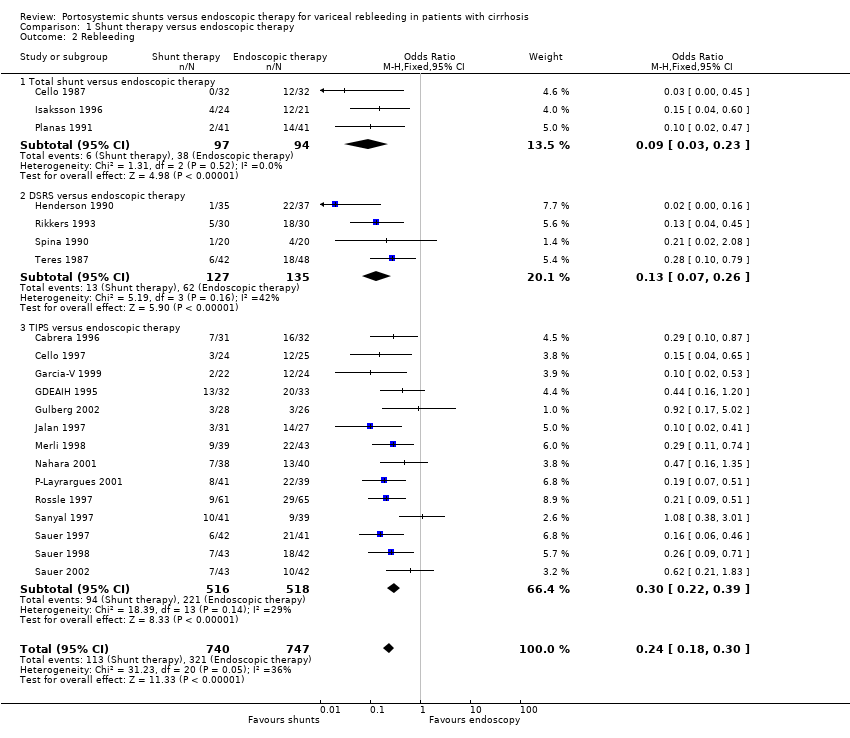
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 2 Rebleeding.

Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 3 Development of hepatic encephalopathy (hazard ratio).

Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 4 Hepatic encephaloapthy.

Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 5 Chronic hepatic encephalopathy.
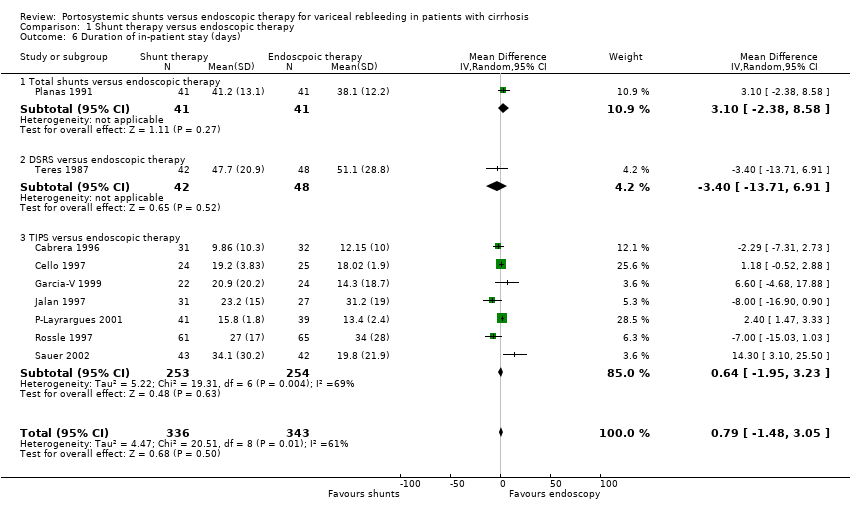
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 6 Duration of in‐patient stay (days).
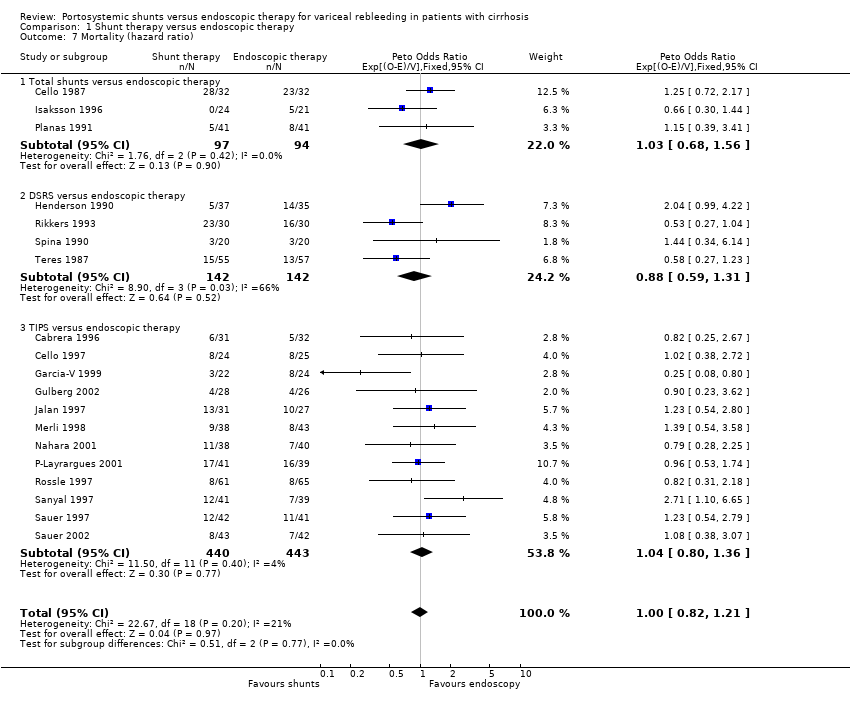
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 7 Mortality (hazard ratio).
| Trial | SH surveillance | SH dysfunction | SH complications | ET complications |
| Planas 1991 | Angiography or ultrasound 3‐10 months later or at the time of rebleeding. | 1/41 [percentage and 95% CI: 2(0.4 to 13)%] | Wound abscess 2, Sepsis 1, pneumonia 2, chylous pleural effusion 1, cholestasis 1 | Ulcers 3, stenosis 1, pneumonia 1, dysphagia 4. |
| Isaksson 1996 | Angiography at 4 months then annual ultrasound. | 1/24 [percentage and 95% CI: 4(0.7 to 20)%] | Oesophagitis 8. | Oesophageal stenosis 2, oesophagitis 7. |
| Cello 1987 | Not mentioned. | Not mentioned. | Not mentioned. | Not mentioned. |
| Trial | SH surveillance | SH dysfunction | SH complications | ET complications |
| Henderson 1990 | Not mentioned. | 1/35 [percentage and 95% CI: 3(0.5 to 15)%] | Not mentioned. | Not mentioned. |
| Rikkers 1993 | Angiography 3 months and then 1, 3, and 6 years. | 3/30 [percentage and 95% CI: 10(4 to 26)%] | Not mentioned. | Stenosis 2. |
| Spina 1990 | Angiography 10th day, 1, 3, and 6 months and then 6 monthly for 2 years. | 0/20 [percentage and 95% CI: 0(0 to 16)%] | Intestinal obstruction (one death). | Ulcers 2, stenosis 2, dysphagia 5. |
| Teres 1987 | Angiography or ultrasound or splenoportography 7 to 10 months after surgery or when rebleeding. | 6/43 [percentage and 95% CI: 14(7 to 27)%] | Not mentioned. | Ulcers 2, stenosis 3, dysphagia 15. |
| Trial | SH surveillance | SH dysfunction | SH complications | ET complications |
| Cabrera 1996 | Angiography 6 monthly. | 15/26 [percentage and 95% CI: 58(39 to 75)%] | Portal thrombosis 2, spontaneous bacterial peritonitis 2, haemobilia 1, sepsis 1. | Ulcers 5, stenosis 4, pneumonia 2, sepsis 1, spontaneous bacterial peritonitis 2. |
| Cello 1997 | Duplex ultrasound. | 4/22 [percentage and 95% CI: 18(7 to 39)%] | Not mentioned. | Not mentioned. |
| Garcia‐V 1999 | Angiography at 1 and 6 months. | 13/18 [percentage and 95% CI: 72(49 to 88)%] | Not mentioned. | Ulcers 5, stenosis 1. |
| Jalan 1997 | Duplex ultrasound at 1 week, angiography at 1 and 6 months, and then 6 monthly. | 9/28 [percentage and 95% CI: 32(18 to 51)%] | Sepsis 3, perforation of the capscule 1 (death). | Ulcers 12, sepsis 4, pneumonia 2. |
| Merli 1998 | Duplex ultrasound 6 monthly and angiography 6 monthly. | 21/33 [percentage and 95% CI: 64(47 to 78)%] | Haemolysis 1, intra‐hepatic haematoma 1, cardiac arrest 1, pulmonary embolism 1. | Ulcers 2, stenosis 2, pneumonia 1, stroke 1. |
| Rossle 1997 | Duplex ultrasound at 1, 3, 6, 9, and 12 months and then 6 monthly. | 18/60 [percentage and 95% CI: 30(20 to 43)%] | Stent migration 1, haemobilia 3, haemoperitoneum 2, bleeding in the liver 1 and sepsis 1. | Ulcers 8, dysphagia 5, mediastinitis 1, hypopyon 1. |
| Sanyal 1997 | Duplex ultrasound at day 1, first week, 1 and 3 months and then 3 monthly. | 20/34 [percentage and 95% CI: 59(42 to 74)%] | Haemolysis 5. | Ulcers 22, stenosis 3, dysphagia 5. |
| Sauer 1997 | Duplex ultrasound every 3 months and angiography every 3 months. | 29/42 [percentage and 95% CI: 69(54 to 81)%] | Shunt dislocation 4. | Ulcers 19, haemorrhage 5. |
| G‐P Layrargues 2001 | Duplex ultrasound at 24 hours and then 3 monthly for two years. | 24/41 [percentage and 95% CI: 59(43 to 72)%] | Haemoperitoneum causing death 1, 30 episodes of shunt dysfunction in 24. patients. | Sepsis 2. |
| GDEAIH 1995 | Information not reported in abstract. | Information not reported. | Information not reported. | Information not reported. |
| Sauer 1998 | Information not reported in abstract. | Reported as 56% after one year'. | Information not reported. | Information not reported. |
| Sauer 2002 | Angiography or Duplex scanning every 3 months. | Cumulative dysfunction 89% during follow‐up, re‐intervention rate 62%. | pneumonia (4 patients), haemobilia (2 patients), stent dislocation (1 patient). | Dysphagia 3, pneumonia 3, septicaemia 2, post‐therapeutic haemorrhage 2. |
| Gulberg 2002 | Three monthly doppler sonography. | Not mentioned. | Perforation of liver capsule 1 (death). | Perforation of oesophagus 1. |
| Nahara 2001 | Three monthly duplex scanning. | Shunt dysfunction 71%. | Haemobilia 2, segmental hepatic infarction 1. | Dysphagia 2, pleural effusion 6, oesophageal stenosis requiring dilatation 1. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rebleeding (hazard ratio) Show forest plot | 11 | 835 | Peto Odds Ratio (95% CI) | 0.42 [0.32, 0.54] |
| 1.1 Total shunts versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DSRS versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.3 TIPS versus endoscopic therapy | 11 | 835 | Peto Odds Ratio (95% CI) | 0.42 [0.32, 0.54] |
| 2 Rebleeding Show forest plot | 21 | 1487 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.18, 0.30] |
| 2.1 Total shunt versus endoscopic therapy | 3 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.23] |
| 2.2 DSRS versus endoscopic therapy | 4 | 262 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.07, 0.26] |
| 2.3 TIPS versus endoscopic therapy | 14 | 1034 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.22, 0.39] |
| 3 Development of hepatic encephalopathy (hazard ratio) Show forest plot | 10 | 725 | Peto Odds Ratio (95% CI) | 1.96 [1.47, 2.61] |
| 3.1 Total shunts versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 3.2 DSRS versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 3.3 TIPS versus endoscopic therapy | 10 | 725 | Peto Odds Ratio (95% CI) | 1.96 [1.47, 2.61] |
| 4 Hepatic encephaloapthy Show forest plot | 19 | 1338 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.59, 2.69] |
| 4.1 Total shunt versus endoscopic therapy | 3 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.67, 3.54] |
| 4.2 DSRS versus endoscopic therapy | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.83, 3.69] |
| 4.3 TIPS versus endoscopic therapy | 13 | 969 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.63, 2.98] |
| 5 Chronic hepatic encephalopathy Show forest plot | 14 | 991 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.20, 3.62] |
| 5.1 Total shunts versus endoscopic therapy | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.89 [0.39, 158.73] |
| 5.2 DSRS versus endoscopic therapy | 4 | 245 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.51, 3.29] |
| 5.3 TIPS versus endoscopic therapy | 9 | 677 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.19, 5.03] |
| 6 Duration of in‐patient stay (days) Show forest plot | 9 | 679 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐1.48, 3.05] |
| 6.1 Total shunts versus endoscopic therapy | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐2.38, 8.58] |
| 6.2 DSRS versus endoscopic therapy | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐13.71, 6.91] |
| 6.3 TIPS versus endoscopic therapy | 7 | 507 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐1.95, 3.23] |
| 7 Mortality (hazard ratio) Show forest plot | 19 | 1358 | Peto Odds Ratio (95% CI) | 1.00 [0.82, 1.21] |
| 7.1 Total shunts versus endoscopic therapy | 3 | 191 | Peto Odds Ratio (95% CI) | 1.03 [0.68, 1.56] |
| 7.2 DSRS versus endoscopic therapy | 4 | 284 | Peto Odds Ratio (95% CI) | 0.88 [0.59, 1.31] |
| 7.3 TIPS versus endoscopic therapy | 12 | 883 | Peto Odds Ratio (95% CI) | 1.04 [0.80, 1.36] |

