Tratamiento oral con ácido 5‐aminosalicílico para mantener la remisión de la colitis ulcerosa
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000544.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 09 mayo 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud digestiva
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Sources of support
Internal sources
-
University of Calgary, Calgary, Alberta, Canada.
The University of Calgary provided support for the original version of this review which was published in 1997. The current version of the review was not supported by any internal sources.
External sources
-
Searle Mucosal Defense Unit, Oakville, Ontario, Canada.
Searle provided support for the original version of this review which was published in 1997. The current version of the review was not supported by any external sources.
Declarations of interest
Yongjun Wang: None known
Claire E Parker: None known
Brian G Feagan has received fees from Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics Inc., Bristol‐Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, TiGenix, Tillotts Pharma AG and UCB Pharma for Scientific Advisory Board membership; fees from Abbott/AbbVie, Actogenix, Akros, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics Inc., Avir Pharma, Axcan, Baxter Healthcare Corp., Biogen Idec, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, VHsquared Ltd., Warner‐Chilcott, Wyeth, Zealand, and Zyngenia for consultancy; payment for lectures from Abbott/AbbVie, JnJ/Janssen, Takeda, Warner‐Chilcott, UCB Pharma; his institution has received grants/grants pending from Abbott/AbbVie, Amgen, Astra Zeneca, Bristol‐Myers Squibb (BMS), Janssen Biotech (Centocor), JnJ/Janssen, Roche/Genentech, Millennium, Pfizer, Receptos, Santarus, Sanofi, Tillotts, and UCB Pharma
John K MacDonald: None known
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Aug 28 | Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis | Review | Alistair Murray, Tran M Nguyen, Claire E Parker, Brian G Feagan, John K MacDonald | |
| 2016 May 09 | Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis | Review | Yongjun Wang, Claire E Parker, Brian G Feagan, John K MacDonald | |
| 2012 Oct 17 | Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis | Review | Brian G Feagan, John K MacDonald | |
| 2006 Apr 19 | Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis | Review | Lloyd R Sutherland, John K MacDonald | |
| 2002 Oct 21 | Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis | Review | Lloyd R Sutherland, Daniel Roth, Paul Beck, Gary May, Kazuya Makiyama | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Oral;
- Aminosalicylic Acids [*administration & dosage];
- Anti‐Inflammatory Agents, Non‐Steroidal [*administration & dosage];
- Colitis, Ulcerative [*drug therapy, prevention & control];
- Maintenance Chemotherapy [*methods];
- Medication Adherence [statistics & numerical data];
- Mesalamine [*administration & dosage];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Remission Induction [methods];
- Sulfasalazine [administration & dosage];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
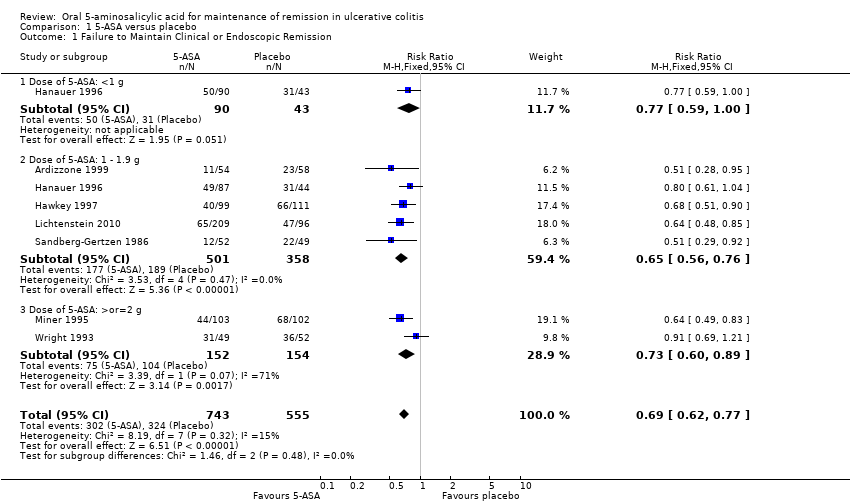
Comparison 1 5‐ASA versus placebo, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission.

Comparison 1 5‐ASA versus placebo, Outcome 2 Development of Any Adverse Event.

Comparison 1 5‐ASA versus placebo, Outcome 3 Development of Any Adverse Event (Sensitivity analysis).

Comparison 1 5‐ASA versus placebo, Outcome 4 Withdrawal from Study due to Adverse Event.

Comparison 1 5‐ASA versus placebo, Outcome 5 Withdrawal from Study due to Adverse Event (Sensitivity analysis).

Comparison 1 5‐ASA versus placebo, Outcome 6 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 2 5‐ASA versus sulfasalazine, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission.
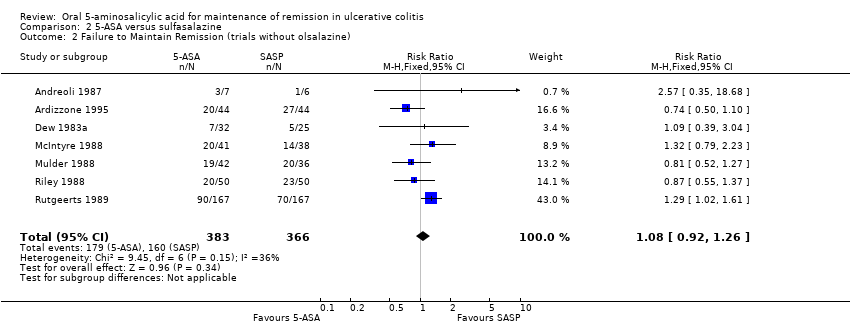
Comparison 2 5‐ASA versus sulfasalazine, Outcome 2 Failure to Maintain Remission (trials without olsalazine).
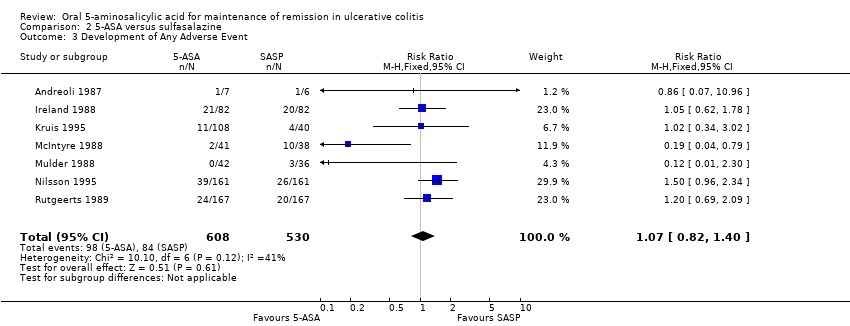
Comparison 2 5‐ASA versus sulfasalazine, Outcome 3 Development of Any Adverse Event.
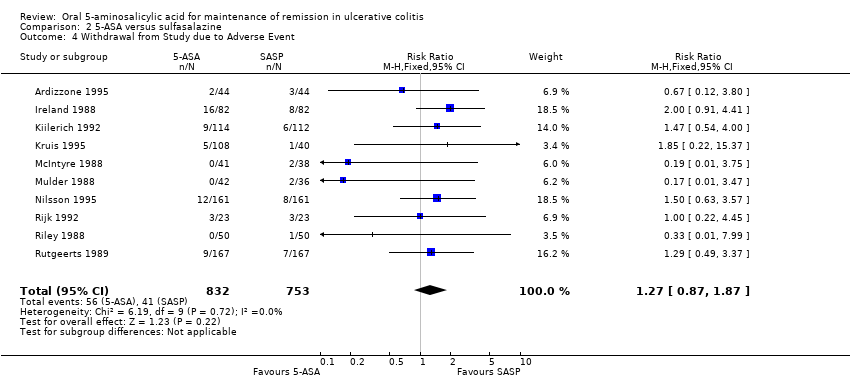
Comparison 2 5‐ASA versus sulfasalazine, Outcome 4 Withdrawal from Study due to Adverse Event.

Comparison 2 5‐ASA versus sulfasalazine, Outcome 5 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 3 Once daily versus conventional dosing, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission at 6 months.

Comparison 3 Once daily versus conventional dosing, Outcome 2 Failure to Maintain Clinical or Endoscopic Remission at 12 months.
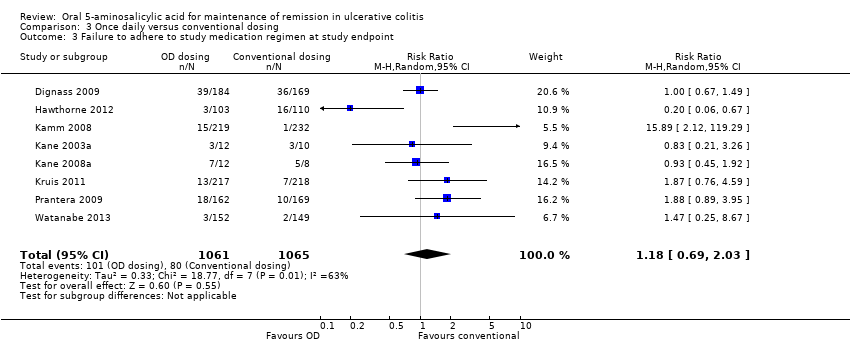
Comparison 3 Once daily versus conventional dosing, Outcome 3 Failure to adhere to study medication regimen at study endpoint.

Comparison 3 Once daily versus conventional dosing, Outcome 4 Failure to adhere to study medication regimen (Sensitivity analysis ‐ excluding outliers).

Comparison 3 Once daily versus conventional dosing, Outcome 5 Development of Any Adverse Event.
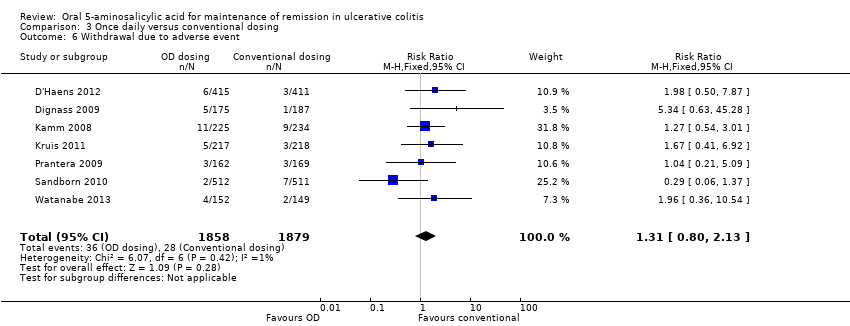
Comparison 3 Once daily versus conventional dosing, Outcome 6 Withdrawal due to adverse event.
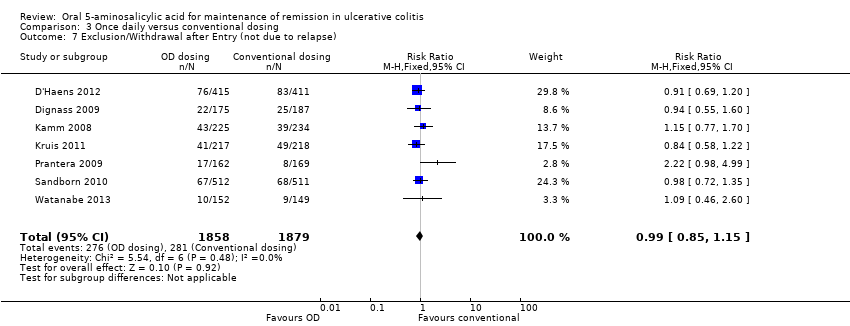
Comparison 3 Once daily versus conventional dosing, Outcome 7 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission at 12 months.

Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 2 Development of Any Adverse Event.

Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 3 Withdrawal from Study due to Adverse Event.
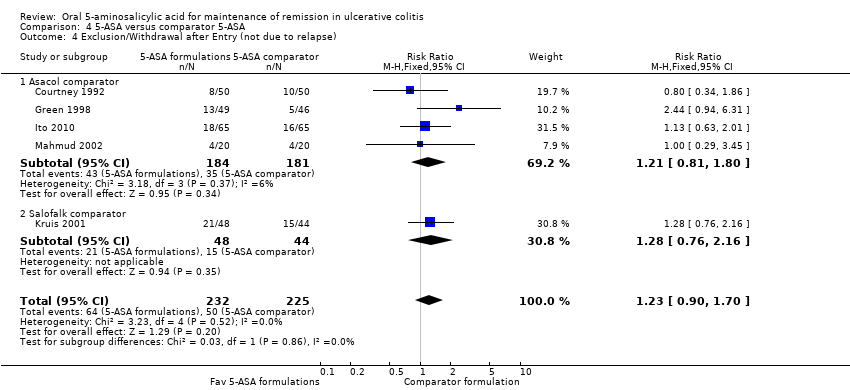
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 4 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 5 5‐ASA (dose ranging), Outcome 1 Failure to Maintain Clinical or Endoscopic Remission.

Comparison 5 5‐ASA (dose ranging), Outcome 2 Development of Any Adverse Event.

Comparison 5 5‐ASA (dose ranging), Outcome 3 Withdrawal from Study due to Adverse Event.
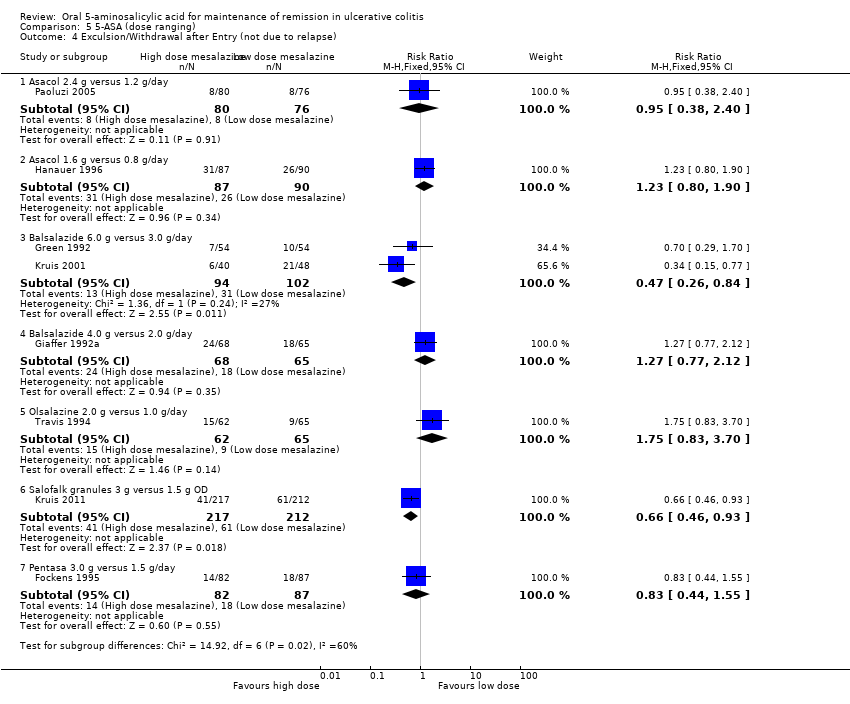
Comparison 5 5‐ASA (dose ranging), Outcome 4 Exculsion/Withdrawal after Entry (not due to relapse).
| Oral 5‐ASA versus placebo for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus placebo | |||||
| Failure to maintain clinical or endoscopic remission at study end‐point | 584 per 10001 | 403 per 1000 | RR 0.69 | 1,298 | ⊕⊕⊕⊕ | |
| Any adverse event | 393 per 10001 | 369 per 1000 | RR 0.94 | 774 | ⊕⊕⊕⊝ | |
| Withdrawal due to adverse event | 42 per 10001 | 36 per 1000 | RR 0.86 | 1096 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Oral 5‐ASA versus SASP for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus SASP | |||||
| Failure to maintain clinical or endoscopic remission at study end‐point | 429 per 10001 | 489 per 1000 | RR 1.14 | 1,655 | ⊕⊕⊕⊕ | |
| Any adverse event | 158 per 10001 | 169 per 1000 | RR 1.07 | 1,138 | ⊕⊕⊕⊝ | |
| Withdrawal due to adverse event | 54 per 10001 | 69 per 1000 | RR 1.27 | 1,585 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Once daily dosing versus conventional dosing for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | OD versus conventional | |||||
| Failure to maintain clinical or endoscopic remission at 6 months | 184 per 10001 | 188 per 1000 | RR 1.02 | 1,871 | ⊕⊕⊝⊝ | |
| Failure to maintain clinical or endoscopic remission at 12 months | 314 per 10001 | 286 per 1000 | RR 0.91 | 3,127 | ⊕⊕⊕⊝ | |
| Failure to adhere to study medication regimen | 87 per 10001 | 106 per 1000 | RR 1.22 | 1,462 | ⊕⊕⊕⊝ | |
| Development of any adverse event | 453 per 10001 | 453 per 1000 | RR 1.00 | 2,714 | ⊕⊕⊕⊕ | |
| Withdrawal due to adverse events | 15 per 10001 | 20 per 1000 | RR 1.31 | 3,737 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Oral 5‐ASA versus comparator 5‐ASA formulation for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus 5‐ASA | |||||
| Failure to maintain clinical or endoscopic remission at 12 months | 407 per 10001 | 440 per 1000 | RR 1.08 | 707 | ⊕⊕⊝⊝ | |
| Development of any adverse event | 686 per 10001 | 645 per 1000 | RR 0.94 | 357 | ⊕⊕⊝⊝ | |
| Withdrawal due to adverse events | 44 per 10001 | 55 per 1000 | RR 1.25 | 457 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| High dose oral 5‐ASA versus low dose 5‐ASA for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | High dose 5‐ASA versus low dose 5‐ASA | |||||
| Failure to maintain clinical or endoscopic remission at 12 months | 357 per 10001 | 286 per 1000 | RR 0.80 | 112 | ⊕⊝⊝⊝ | Asacol 4.8 g/day versus 2.4 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 330 per 10001 | 251 per 1000 | RR 0.76 | 216 | ⊕⊝⊝⊝ | Balsalazide 6.0 g/day versus 3.0 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 554 per 10001 | 366 per 1000 | RR 0.66 | 133 | ⊕⊕⊕⊝ | Balsalazide 4.0 g/day versus 2.0 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 392 per 10001 | 255 per 1000 | RR 0.65 | 429 | ⊕⊕⊕⊝ | Salofalk granules 3.0 g OD versus 1.5 g OD |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission Show forest plot | 7 | 1298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
| 1.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| 1.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 5 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.56, 0.76] |
| 1.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.89] |
| 2 Development of Any Adverse Event Show forest plot | 4 | 875 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.39] |
| 2.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.31] |
| 2.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.20] |
| 2.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.14, 20.58] |
| 3 Development of Any Adverse Event (Sensitivity analysis) Show forest plot | 3 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.77, 1.15] |
| 3.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.31] |
| 3.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.20] |
| 3.3 Dose of 5‐ASA: >or=2 g | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.12] |
| 4 Withdrawal from Study due to Adverse Event Show forest plot | 6 | 1197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.78, 2.30] |
| 4.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.15, 13.38] |
| 4.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 4 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.50, 2.23] |
| 4.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.78, 4.15] |
| 5 Withdrawal from Study due to Adverse Event (Sensitivity analysis) Show forest plot | 5 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.63] |
| 5.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.15, 13.38] |
| 5.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 4 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.50, 2.23] |
| 5.3 Dose of 5‐ASA: >or=2 g | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.60] |
| 6 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 5 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.44] |
| 6.1 Dose of 5‐ASA: <1 g | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.58, 1.40] |
| 6.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 3 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.71] |
| 6.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.69, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission Show forest plot | 12 | 1655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.03, 1.27] |
| 2 Failure to Maintain Remission (trials without olsalazine) Show forest plot | 7 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 3 Development of Any Adverse Event Show forest plot | 7 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 4 Withdrawal from Study due to Adverse Event Show forest plot | 10 | 1585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.87, 1.87] |
| 5 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 9 | 1497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission at 6 months Show forest plot | 3 | 1871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 1.1 Asacol (OD vs BID or TID) | 2 | 1045 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 1.2 MMX (OD) vs Asacol (BID) | 1 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| 2 Failure to Maintain Clinical or Endoscopic Remission at 12 months Show forest plot | 8 | 3127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 2.1 Asacol (OD vs BID or TID) | 3 | 1256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.15] |
| 2.2 MMX (OD) vs Asacol (BID) | 1 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.33] |
| 2.3 Pentasa (OD vs BID) | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.93] |
| 2.4 MMX (OD vs BID) | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.47] |
| 2.5 Salofalk granules (OD vs TID) | 1 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.10] |
| 3 Failure to adhere to study medication regimen at study endpoint Show forest plot | 8 | 2126 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.69, 2.03] |
| 4 Failure to adhere to study medication regimen (Sensitivity analysis ‐ excluding outliers) Show forest plot | 6 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.64] |
| 5 Development of Any Adverse Event Show forest plot | 6 | 2714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 6 Withdrawal due to adverse event Show forest plot | 7 | 3737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.13] |
| 7 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 7 | 3737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.85, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission at 12 months Show forest plot | 6 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 1.1 Asacol comparator | 5 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.26] |
| 1.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.86, 1.98] |
| 2 Development of Any Adverse Event Show forest plot | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 2.1 Asacol comparator | 3 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 2.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.34] |
| 3 Withdrawal from Study due to Adverse Event Show forest plot | 5 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.56, 2.78] |
| 3.1 Asacol comparator | 4 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.61, 4.42] |
| 3.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 2.90] |
| 4 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 5 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.70] |
| 4.1 Asacol comparator | 4 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.80] |
| 4.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.76, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Asacol 4.8 g versus 2.4 g/day | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.46, 1.38] |
| 1.2 Asacol 3.2 g versus 2 g/day | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.83, 1.37] |
| 1.3 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 1.4 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.32] |
| 1.5 Balsalazide 6.0 g versus 3.0 g/day | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.21, 2.79] |
| 1.6 Balsalazide 4.0 g versus 2.0 g/day | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.97] |
| 1.7 Olsalazine 2.0 g versus 1.0 g/day | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 1.8 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.49, 0.86] |
| 1.9 Pentasa 3.0 g versus 1.5 g/day | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.15] |
| 2 Development of Any Adverse Event Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 2.2 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.18, 2.95] |
| 2.3 Balsalazide 6.0 g versus 3.0 g/day | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.88, 2.24] |
| 2.4 Olsalazine 2.0 g versus 1.0 g/day | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.94, 1.99] |
| 2.5 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.91] |
| 3 Withdrawal from Study due to Adverse Event Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 3.2 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.25] |
| 3.3 Balsalazide 6.0 g versus 3.0 g/day | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.21, 1.70] |
| 3.4 Balsalazide 4.0 g versus 2.0 g/day | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.54, 3.80] |
| 3.5 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.29, 3.33] |
| 3.6 Pentasa 3.0 g versus 1.5 g/day | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.69] |
| 4 Exculsion/Withdrawal after Entry (not due to relapse) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.38, 2.40] |
| 4.2 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.80, 1.90] |
| 4.3 Balsalazide 6.0 g versus 3.0 g/day | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.26, 0.84] |
| 4.4 Balsalazide 4.0 g versus 2.0 g/day | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.77, 2.12] |
| 4.5 Olsalazine 2.0 g versus 1.0 g/day | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.83, 3.70] |
| 4.6 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.93] |
| 4.7 Pentasa 3.0 g versus 1.5 g/day | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.44, 1.55] |

