Anestesia para la cirugía por fractura de cadera en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000521.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 febrero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving this update: Martyn Parker (MP), Joanne Guay (JG)
Co‐ordinating the review: JG, MP
Screening search results: JG and PRG
Organizing retrieval of papers: JG
Screening retrieved papers against inclusion criteria: JG and Sandra Kopp (SK)
Appraising quality of papers: JG and SK
Abstracting data from papers: JG and SK
Writing to authors of papers for additional information: JG
Data management for the review: JG
Entering data into Review Manager (RevMan 5.3): JG
RevMan statistical data: JG
Other statistical analysis not using RevMan: JG
Interpretation of data: JG, MP, PRG and SK
Statistical inferences: JG
Writing the review: JG, MP, PRG and SK
Securing funding for the review: Departmental resources only
Performing previous work that was the foundation of the present study: JG and MP
Guarantor for the review (one author): JG
Person responsible for reading and checking review before submission: JG, MP, PRG and SK
Sources of support
Internal sources
-
University of Teesside, Middlesbrough, UK.
-
Peterborough and Stamford Hospitals NHS Foundation Trust, Peterborough, UK.
External sources
-
No sources of support supplied
Declarations of interest
Joanne Guay: I am the editor of a multi authors textbook on anaesthesia (including notions on general and regional anaesthesia).
Martyn J Parker has received expenses and honorarium from a number of commercial companies and organizations for giving lectures on different aspects of hip fracture treatment. In addition he has received royalties from BBraun ltd related to the design and development of an implant used for the internal fixation of intracapsular hip fractures. This implant and fracture type is not considered in this review and none of these payments related directly to this review. He is the author of one ongoing trial (ISRCTN36381516).
Pushpaj R Gajendragadkar: none known.
Sandra Kopp: none known.
Acknowledgements
We thank Helen Handoll who worked on the first three versions of the review for giving us the opportunity to update this review (Parker 2001; Parker 2004; Urwin 2000), Karl Sales for the translation of Ibanez 1993, Jia Jiang for the translation of Cao 2008, Richard Griffiths who was also an author on the last published version (Parker 2004), and Karen Hovhannisyan for the search for this update.
We thank the Bone, Joint and Muscle Trauma Group for their help in the past with our reviews (Parker 2001; Parker 2004; Urwin 2000).
We thank Mark D Neuman (content editor), Marialena Trivella (statistical editor), Santosh Rath (peer reviewer), Arthur Atchabahian (peer reviewer) and Janet L Wale (consumer reviewer) for their help in this update.
We also thank Janne Vendt who redesigned the search reran in February 2017 for 2014 and onwards.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Feb 22 | Anaesthesia for hip fracture surgery in adults | Review | Joanne Guay, Martyn J Parker, Pushpaj R Gajendragadkar, Sandra Kopp | |
| 2004 Oct 18 | Anaesthesia for hip fracture surgery in adults | Review | Martyn J Parker, Helen HG Handoll, Richard Griffiths | |
| 2001 Oct 23 | Anaesthesia for hip fracture surgery in adults | Review | MJ Parker, HHG Handoll, R Griffiths, SC Urwin | |
Differences between protocol and review
We made the following changes to the published protocol (Parker 1997)
Change in title: The first review (Urwin 2000), and update (Parker 2001), were published under the title: "General versus spinal/epidural anaesthesia for surgery for hip fractures in adults". The title was changed in the second update to reflect an expansion in the scope of the review to include comparisons of all forms of anaesthesia (Parker 2004).
Changes made in 2016 updated version
Background was updated
Objectives were reformulated from:
The following null hypotheses were tested within the trials included so far in this review:
(1) There is no difference in outcome between regional anaesthesia (spinal or epidural) and general anaesthesia.
(2) There is no difference in outcome between regional anaesthesia (spinal or epidural) supplemented with a ’light’ general anaesthetic and general anaesthesia alone.
(3) There is no difference in outcome between regional anaesthesia (spinal or epidural) and regional nerve blocks alone.
(4) There is no difference in outcome between anaesthesia using ketamine (with or without a benzodiazepine) and inhalation general anaesthesia.
To:
The main focus of this review is the comparison of regional versus general anaesthesia for hip fracture repair. More precisely we tried to determine whether there are any major advantages in using regional anaesthesia compared to general anaesthesia for hip fracture repairs. The scope of this review, originally published in 2000 (Urwin 2000), was expanded in the second update to also cover other methods of anaesthesia (Parker 2001). We did not consider supplementary regional blocks in this review as they have been studied in another review (Parker 2002).
Study selection was changed from:
Types of interventions
(1) Regional anaesthesia (if necessary supplemented by sedatives) achieved by injection of local anaesthetic into the epidural or subarachnoid spaces. This type of anaesthesia is also referred to as 'spinal' or 'epidural'
(2) General anaesthesia using intravenous or inhalation agents to render the patient unconscious. Unless otherwise stated, general anaesthesia refers to general anaesthesia using inhalation agents in this review.
(3) Intravenous ketamine.
(4) Local nerve blocks (if necessary supplemented by sedatives) when used as the primary method of anaesthesia.
Trials testing other methods of anaesthesia as the primary method of anaesthesia were considered for inclusion. Trials comparing the use of local nerve blocks in conjunction with general anaesthesia and the use of nerve blocks preoperatively, are evaluated in another Cochrane review (Parker 2001). Also not considered in this review were trials comparing different types of drugs or techniques of individual methods of anaesthesia.
To:
Types of interventions
We included studies that compared any combination of the following interventions:
-
Neuraxial blocks: epidural (single shots or continuous), spinals (single shots or continuous), or combined spinal/epidural (single shots or continuous) with or without intravenous sedation;
-
Peripheral nerve blocks: posterior lumbar (psoas) plexus blocks with or without sacral plexus blocks or any other peripheral nerve blocks with or without sedation;
-
General anaesthesia based on inhalational agents (with or without opioids and/or neuromuscular blocking agents), or on total intravenous anaesthesia (ketamine‐based technique or other). Any technique where an endotracheal tube or a laryngeal mask airway was used was considered as general anaesthesia.
Outcomes: the number was reduced.
Method: methods were brought up to date.
Notes
January 2014: This review has been transferred from the Bone, Joint and Muscle Trauma Group to the Cochrane Anaesthesia Group.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Female; Humans; Male;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
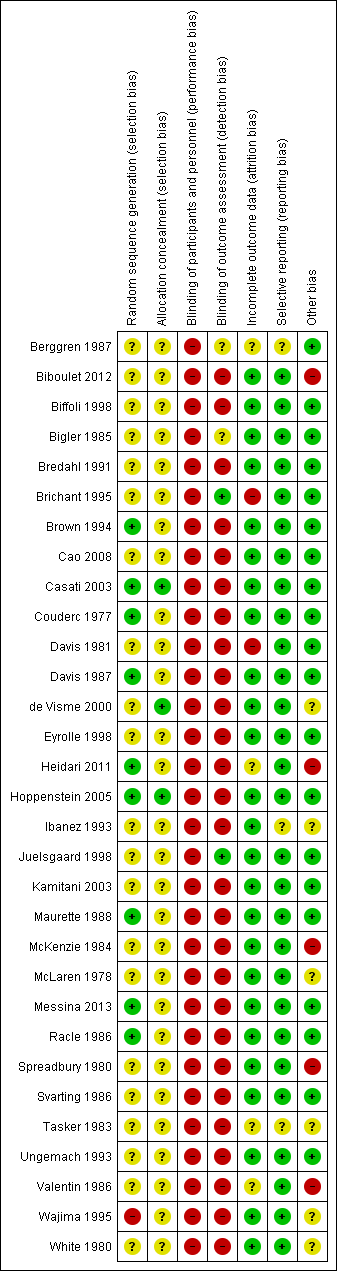
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Meta‐regression of mortality at 0‐1 month versus the year when the study was published. The effect size decreases with time: P value = 0.002.
This meta regression plot was not produced in RevMan. The figure was generated automatically by the software, and cannot be amended. The software has expressed the years as decimals.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 1 Mortality ‐ 1 month.
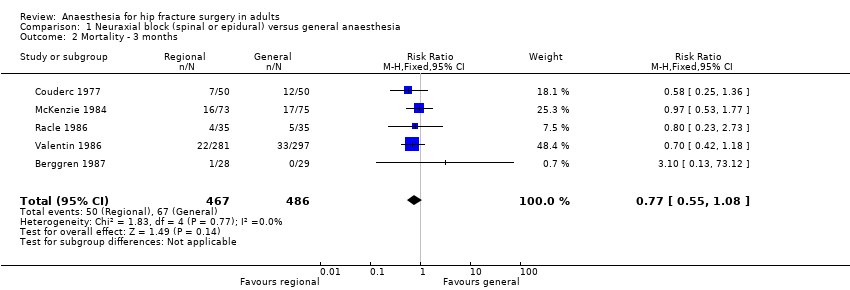
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 2 Mortality ‐ 3 months.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 3 Mortality ‐ 6 months.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 4 Mortality ‐ 12 months.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 5 Pneumonia.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 6 Myocardial infarction.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 7 Cerebrovascular accident.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 8 Acute confusional state.
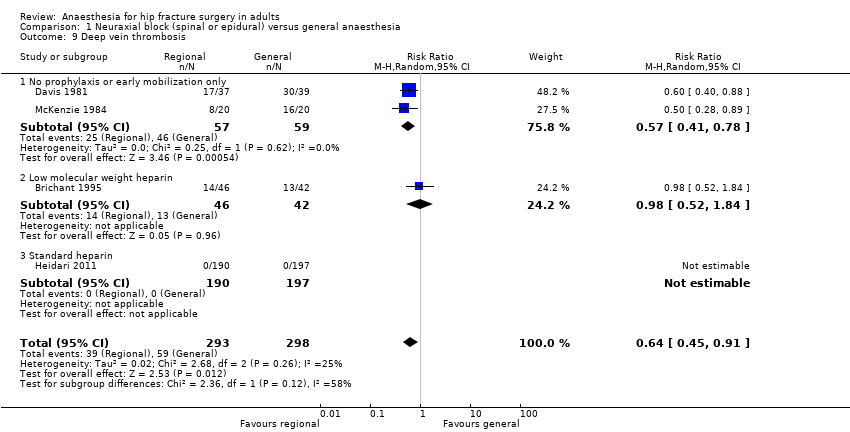
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 9 Deep vein thrombosis.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 10 Congestive cardiac failure.
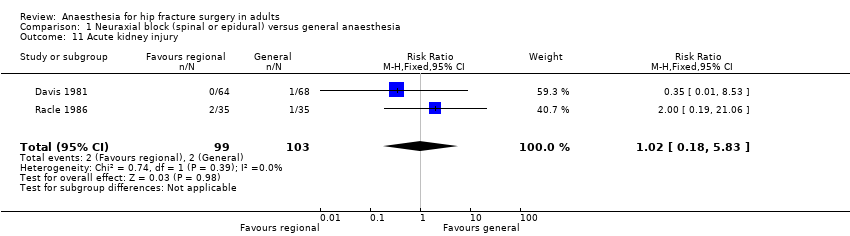
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 11 Acute kidney injury.
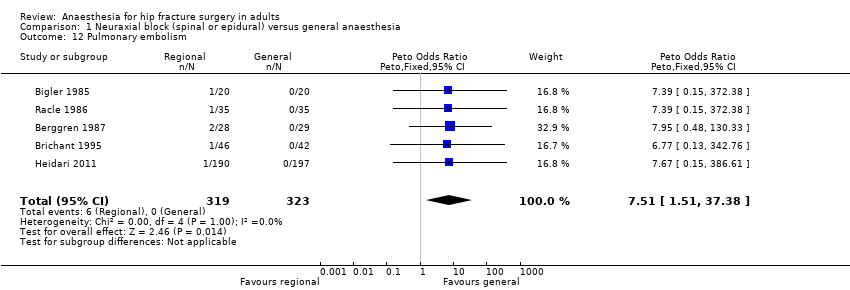
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 12 Pulmonary embolism.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 13 Number of patients transfused.
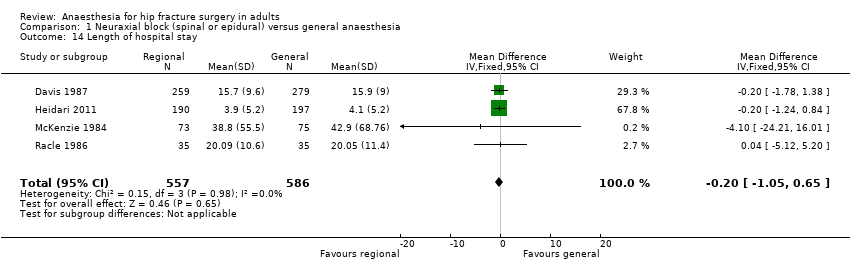
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 14 Length of hospital stay.
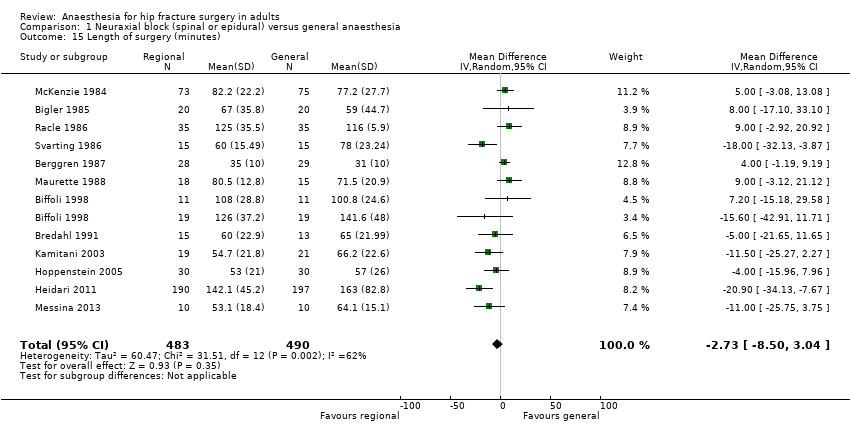
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 15 Length of surgery (minutes).

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 16 Operative hypotension.
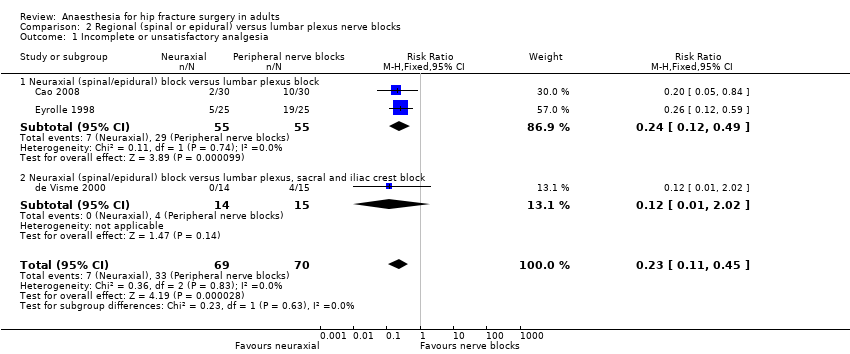
Comparison 2 Regional (spinal or epidural) versus lumbar plexus nerve blocks, Outcome 1 Incomplete or unsatisfactory analgesia.

Comparison 2 Regional (spinal or epidural) versus lumbar plexus nerve blocks, Outcome 2 Urine retention.
| Neuraxial block compared to general anaesthesia for hip fracture repair | ||||||
| Patient or population: Patients with hip fracture repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| General anaesthesia | Neuraxial block | |||||
| Mortality | Study population | RR 0.78 | 2152 | ⊕⊝⊝⊝ | ||
| 80 per 1000 | 62 per 1000 | |||||
| Low | ||||||
| 35 per 1000 | 27 per 1000 | |||||
| High | ||||||
| 95 per 1000 | 74 per 1000 | |||||
| Pneumonia | Study population | RR 0.77 | 761 | ⊕⊝⊝⊝ | ||
| 64 per 1000 | 50 per 1000 | |||||
| Low | ||||||
| 30 per 1000 | 23 per 1000 | |||||
| High | ||||||
| 80 per 1000 | 62 per 1000 | |||||
| Myocardial infarction | Study population | RR 0.89 | 559 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Low | ||||||
| 5 per 1000 | 4 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 44 per 1000 | |||||
| Cerebrovascular accident | Study population | RR 1.48 | 729 | ⊕⊝⊝⊝ | ||
| 8 per 1000 | 12 per 1000 | |||||
| Low | ||||||
| 10 per 1000 | 15 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 74 per 1000 | |||||
| Acute confusional state | Study population | RR 0.85 | 624 | ⊕⊝⊝⊝ | ||
| 177 per 1000 | 150 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 212 per 1000 | |||||
| Deep vein thrombosis | Study population | RR 0.57 | 116 | ⊕⊝⊝⊝ | For this outcome, we retained | |
| 780 per 1000 | 444 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 114 per 1000 | |||||
| High | ||||||
| 900 per 1000 | 513 per 1000 | |||||
| Return of patient to their own home | Study population | RR 0.84 | 130 | ⊕⊝⊝⊝ | ||
| 578 per 1000 | 486 per 1000 | |||||
| Low | ||||||
| 400 per 1000 | 336 per 1000 | |||||
| High | ||||||
| 800 per 1000 | 672 per 1000 | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment and/or blinding of outcome assessors unclear/inadequate in 75% or more of the included studies. | ||||||
| Outcome | Study | Definition | Time point |
| Pneumonia | "treated for" | "during the postoperative period" | |
| Unspecified | "postoperatively" | ||
| Chest X‐Ray in clinical suspicion | Up to four weeks | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| "The clinical criteria adopted as indicating respiratory problems were productive cough, the presence of rhonchi or crepitations on auscultation or abnormalities on chest X‐ray." However the criteria adopted for the diagnosis of a pneumonia ("respiratory infection") are not clearly mentioned | Up to four weeks | ||
| "clinical and radiological criteria" | "in hospital" | ||
| Unspecified | Within four weeks | ||
| Myocardial infarction | EKG and troponin measurement daily for three days, no definition provided | Within one month | |
| Serial preprogrammed EKGs up to postoperative day 10 interpreted by a blinded cardiologist. Q wave | In hospital | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| World Health Organization criteria applied by a blinded investigator | Within one month | ||
| Congestive cardiac failure | "treated for" | "during the postoperative period" | |
| "acute heart failure" | Within one month | ||
| "cardiac decompensation" | "postoperatively" | ||
| "life‐threatening complications" "congestive heart failure" | Within four weeks | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| "episode of congestive heart failure" | In hospital | ||
| Cerebrovascular accident | "stroke" | All patients developed their stroke on postoperative day one | |
| "stroke" | Within one month | ||
| "Neurological sequelae", apoplexy for the sole event reported | "postoperatively" | ||
| "cerebrovascular accident" | Within four weeks | ||
| "cerebrovascular accident" "diagnosed by the consultant specialist" | "postoperative" | ||
| "cerebrovascular accident" | In hospital | ||
| Acute confusional state | Diagnostic and Statistical Manual of Mental Disorders (DSM‐Ill) as criteria for acute confusional state | Within seven days (the period 0‐8 hours after the surgery was excluded) | |
| "Mental confusion" | "postoperatively" | ||
| Mini Mental State Examination test decreased 2 points from baseline | Within seven days | ||
| Mini Mental Status Examination lower than 5 | Between the third and fifth postoperative day | ||
| Cognitive dysfunction based on time, person, and place disorientation | Up to postoperative day two | ||
| Delirium was judged by floor nurse, using the Inoue's confusion assessment method diagnosis algorithm | Up to postoperative day four | ||
| Confusion with agitation | In hospital | ||
| Unspecified | Within four weeks | ||
| Renal failure (or acute kidney injury) | "acute renal failure" | Within four weeks | |
| Blood creatinine > 135 micromol/Liter | In hospital | ||
| Deep vein thrombosis | Systematic bilateral contrast venography | Postoperative day ten | |
| 125‐iodine fibrinogen uptake test performed daily for seven days | Within seven days | ||
| "deep veins thrombosis" "diagnosed by the consultant specialist" | "postoperative" | ||
| "thrombosis" | Within seven days | ||
| Systematic venography | Between postoperative day seven and ten | ||
| "deep vein thrombosis" | Within four weeks | ||
| Pulmonary embolism | "pulmonary embolism" | "postoperatively" | |
| "pulmonary embolus" | "postoperatively" | ||
| Pulmonary venous angiogram or ventilation‐perfusion lung scanning on clinical suspicion | Unclear | ||
| "pulmonary emboli" "diagnosed by the consultant specialist" | "postoperatively" | ||
| Clinical suspicion confirmed with angiography | In hospital | ||
| Unsatisfactory surgical results | Either an unstable fixation of the fracture by nail and plate or the dislocation of a prosthesis, which required bedrest on traction and prevented early mobilization | In hospital | |
| Operative hypotension | Decrease > 30% from baseline for systolic arterial blood pressure | Intraoperative | |
| Decrease of 30% from baseline for arterial blood pressure | Intraoperative | ||
| Requiring the administration of a sympathomimetic | Intraoperative | ||
| Decrease in systolic arterial pressure 20% from baseline | Intraoperative | ||
| Decrease of 40 mmHG in systolic arterial blood pressure | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 33% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 50% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure of 25% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure of 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 30% from baseline | Intraoperative | ||
| Urine retention | "urinary retention" | "postoperative" | |
| "that required catheterization" | In hospital | ||
| EKG: electrocardiogram | |||
| Study | Sedative drugs for participants of the regional blockade group | Anaesthetic agents for general anaesthesia |
| Premedication: Meperidine No other sedative drugs mentioned as routinely administered for the surgery. | Premedication: Meperidine Induction: Thiopental and atropine Maintainance: Nitrous oxide, halothane and succinylcholine infusion | |
| None mentioned | Subgroup 1 Induction: Propofol Maintainance: Propofol infusion (for a bispectral index value of 50) and remifentanil Subgroup 2 Induction: Sevoflurane Maintainance: Sevoflurane (for a bispectral index value of 50) and remifentanil | |
| None mentioned | Induction: Propofol Maintainance: Nitrous oxide, isoflurane, fentanyl (intermittent injections) plus an atracurium infusion | |
| Premedication: Pethidine Small amounts of diazepam if needed | Premedication: Pethidine Induction: Diazepam and atropine Maintainance: Nitrous oxide, fentanyl and pancuronium | |
| Premedication: Pethidine Diazepam for mild sedation | Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, thiopental and pethidine | |
| Not mentioned | According to local practice | |
| Premedication: Tamazepam or pethidine No drug supplementation during the surgery | Premedication: Tamazepam or pethidine Induction: Thiopental or propofol Maintainance: Nitrous oxide, isoflurane or enflurane and atracurium 0.5 mg/kg one dose | |
| Midazolam and fentanyl if required | ||
| One dose of fentanyl before the block No other sedative drug routinely administered for the surgery. | Induction: Sevoflurane Maintainance: Nitrous oxide, sevoflurane | |
| Premedication: Hydroxyzine and atropine None mentioned for the surgery | Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide plus 1) Thiopental and dextromoramide or 2) methoxyflurane. One dose of pancuronium in some participants | |
| Ketamine 20‐25 mg (for eight participants) before the spinal Ketamine at unspecified total doses during the surgery (two participants) or 25 mg at skin closure (two participants) Diazepam (mean dose 9 mg; range 0‐35 mg) | Induction: Diazepam (mean dose 9.5 mg; range 2.5 to 30 mg) Maintainance: Nitrous oxide, fentanyl and pancuronium | |
| Benzodiazepine (optional) | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl and non‐depolarizing neuromuscular blocking agent | |
| Alfentanil before the block and as required during the surgery. No mandatory sedative drugs mentioned. One patient in the continuous peripheral nerve block received "sedation repeatedly". | ||
| Propofol as required | ||
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl, halothane and one dose of pancuronium | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, isoflurane, fentanyl and vecuronium | |
| Not reported | Not reported | |
| Premedication: Pethidine None mentioned for the surgery | Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of atracurium | |
| No sedative drug | Induction: Propofol Maintainance: Nitrous oxide, fentanyl, sevoflurane and one dose of vecuronium | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, dextromoramide and enflurane | |
| Small doses of diazepam | Induction: Althesin Maintainance: Nitrous oxide and halothane | |
| Althesin and nitrous oxide; arousable by ear lobe pressure | Induction: Althesin Maintainance: Nitrous oxide, fentanyl and pancuronium one dose | |
| None mentioned | Induction: Propofol Maintainance: Sevoflurane, remifentanil and one dose of cisatracurium | |
| Premedication: Hydroxyzine and atropine Flunitrazepam, verbal contact possible | Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of vecuronium | |
| No group with regional anaesthesia alone | Ketamine group Induction and maintenance: Ketamine and diazepam (2.5 to 10 mg) Relaxant group Technique at the discretion of the attending anaesthesiologist | |
| Premedication: Pethidine and atropine None mentioned for the surgery | Premedication: Pethidine and atropine Induction: Thiopental Maintainance: Nitrous oxide, fentanyl (repeated injections) and one dose of pancuronium | |
| Not reported | Not reported | |
| Not reported | Induction: Not reported Maintainance: Nitrous oxide, isoflurane and fentanyl | |
| Premedication: Pethidine and promethazine in some of the participants Small doses of diazepam and fentanyl | Premedication: Pethidine and promethazine in some of the participants Subgroup 1 Induction: Thiopental or not Maintainance: Nitrous oxide, enflurane and gallamine (not all participants) Subgroup 2 Induction: Not clearly mentioned Maintainance: Nitrous oxide, droperidol, fentanyl and gallamine | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide and sevoflurane | |
| Premedication: Diazepam Althesin, nitrous oxide and fentanyl (spontaneous breathing) for the two subgroups | Premedication: Diazepam Induction: Thiopental Maintainance: Nitrous oxide, halothane and fentanyl |
| Comparison: Neuraxial block versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Patient returned to their own home | 130 | RR | 0.84 | 0.61 | 1.16 | |
| Urine retention | 57 | RR | 0.86 | 0.30 | 2.51 | |
| Comparison: Neuraxial block added to general anaesthesia compared to general anaesthesia alone | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Pneumonia | 30 | RR | 0.80 | 0.20 | 3.20 | |
| Acute confusional state | 30 | RR | 1.00 | 0.16 | 6.09 | |
| Deep vein thrombosis | 30 | RR | 0.17 | 0.01 | 3.94 | |
| Length of surgery (minutes) | 30 | MD | 0.00 | ‐17.96 | 17.96. | |
| Comparison: Neuraxial block versus peripheral nerve block | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Acute confusional state | 29 | RR | 0.89 | 0.35 | 2.28 | |
| Operative hypotension | 50 | RR | 6.00 | 2.02 | 17.83 | |
| Length of surgery (minutes) | 29 | MD | 17.00 | ‐0.76 | 34.76 | |
| Comparison: Intravenous ketamine alone (without neuromuscular blocking agent) versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Unsatisfactory surgical results defined as unstable fixation or prosthesis dislocation | 60 | RR | 2.33 | 0.67 | 8.18 | |
| Mortality | 60 | RR | 1.00 | 0.46 | 2.17 | |
| Patient returned home | 60 | RR | 0.95 | 0.66 | 1.38 | |
| Length of hospital stay | 39* | MD | 12.00 | 5.63 | 18.37 | |
| CI: confidence interval; MD: mean difference; RR: risk ratio *: Mean duration of admission refers only to those patients who were discharged home | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality ‐ 1 month Show forest plot | 11 | 2152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.06] |
| 2 Mortality ‐ 3 months Show forest plot | 5 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.55, 1.08] |
| 3 Mortality ‐ 6 months Show forest plot | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 4 Mortality ‐ 12 months Show forest plot | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 5 Pneumonia Show forest plot | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 6 Myocardial infarction Show forest plot | 4 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.22, 3.65] |
| 7 Cerebrovascular accident Show forest plot | 6 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.46, 4.83] |
| 8 Acute confusional state Show forest plot | 6 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 9 Deep vein thrombosis Show forest plot | 4 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.91] |
| 9.1 No prophylaxis or early mobilization only | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.78] |
| 9.2 Low molecular weight heparin | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.84] |
| 9.3 Standard heparin | 1 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Congestive cardiac failure Show forest plot | 6 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.96] |
| 11 Acute kidney injury Show forest plot | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.18, 5.83] |
| 12 Pulmonary embolism Show forest plot | 5 | 642 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.51 [1.51, 37.38] |
| 13 Number of patients transfused Show forest plot | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.49, 1.66] |
| 13.1 Fixation | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.15] |
| 13.2 Arthroplasty | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.51] |
| 14 Length of hospital stay Show forest plot | 4 | 1143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.05, 0.65] |
| 15 Length of surgery (minutes) Show forest plot | 12 | 973 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐8.50, 3.04] |
| 16 Operative hypotension Show forest plot | 12 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.35] |
| 16.1 Unilateral | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.37, 0.89] |
| 16.2 Bilateral, incremental doses | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.05, 0.78] |
| 16.3 Bilateral single shot | 8 | 828 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.87, 1.95] |
| 16.4 Epidural | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.50, 2.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incomplete or unsatisfactory analgesia Show forest plot | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.11, 0.45] |
| 1.1 Neuraxial (spinal/epidural) block versus lumbar plexus block | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.49] |
| 1.2 Neuraxial (spinal/epidural) block versus lumbar plexus, sacral and iliac crest block | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.02] |
| 2 Urine retention Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.0 [1.90, 103.00] |

