Anestesia para la cirugía por fractura de cadera en adultos
Resumen
Antecedentes
La mayoría de los pacientes con fractura de cadera reciben tratamiento quirúrgico, por lo que se requiere anestesia.
Objetivos
El centro de interés principal de esta revisión es la comparación de anestesia regional versus general para la reparación de la fractura de cadera (fractura femoral proximal) en adultos. En esta revisión no se consideraron los bloqueos regionales complementarios porque se han estudiado en otra revisión.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; the Cochrane Library; 2014, número 3), MEDLINE (Ovid SP, 2003 hasta marzo de 2014) y EMBASE (Ovid SP, 2003 hasta marzo de 2014). Se volvió a realizar la búsqueda en febrero de 2017. Se añadieron posibles nuevos estudios de interés a una lista de "Estudios en espera de clasificación" y se incorporarán a los hallazgos de la revisión oficial durante la actualización de la misma.
Criterios de selección
Se incluyeron los ensayos aleatorizados que compararon diferentes métodos de anestesia para la cirugía por fractura de cadera en adultos. El objetivo primario de esta revisión fue la comparación de la anestesia regional versus la anestesia general. En otra revisión se evalúa el uso de bloqueos nerviosos preoperatorios junto con anestesia general. Los resultados principales fueron mortalidad, neumonía, infarto de miocardio, accidente cerebrovascular, estado de confusión agudo, trombosis venosa profunda y retorno del paciente al domicilio.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos. Los datos se analizaron con los modelos de efectos fijos (I2 < 25%) o de efectos aleatorios. La calidad de la evidencia se evaluó según los criterios desarrollados por el grupo de trabajo GRADE.
Resultados principales
En total, se incluyeron 31 estudios (con 3231 participantes) en la revisión. De esos 31 estudios, 28 (2976 participantes) proporcionaron datos para los metanálisis. De los 28 estudios, 24 se utilizaron para la comparación de bloqueo neuroaxial versus anestesia general. En base a 11 estudios que incluyeron a 2152 participantes, no se encontró una diferencia entre las dos técnicas anestésicas para la mortalidad al mes: riesgos relativos (RR) 0,78; intervalo de confianza (IC) del 95%: 0,57 a 1,06; I2 = 24% (modelo de efectos fijos). Sobre la base de seis estudios que incluyeron a 761 participantes, no se encontró una diferencia en el riesgo de neumonía: RR 0,77; IC del 95%: 0,45 a 1,31; I2 = 0%). Sobre la base de cuatro estudios que incluyeron a 559 participantes, no se encontró una diferencia en el riesgo de infarto de miocardio: RR 0,89; IC del 95%: 0,22 a 3,65; I2 = 0%). Sobre la base de seis estudios que incluyeron a 729 participantes, no se encontró una diferencia en el riesgo de accidente cerebrovascular: RR 1,48; IC del 95%: 0,46 a 4,83; I2 = 0%). Sobre la base de seis estudios que incluyeron a 624 participantes, no se encontró una diferencia en el riesgo de estado de confusión agudo: RR 0,85; IC del 95%: 0,51 a 1,40; I2 = 49%). Sobre la base de las pruebas de laboratorio, el riesgo de trombosis venosa profunda se redujo cuando no se utilizaron precauciones específicas o cuando sólo se utilizó la movilización temprana: RR 0,57, IC del 95%: 0,41 a 0,78; I2 = 0%; (número necesario a tratar para un resultado beneficioso adicional (NNTB) = 3, IC del 95%: 2 a 7, basado en un riesgo basal del 76%) pero no cuando se administró heparina de bajo peso molecular: CR 0,98; IC del 95%: 0,52 a 1,84; I2 para la heterogeneidad entre los dos subgrupos = 58%. Para los bloqueos neuroaxiales comparados con anestesia general, la calidad de la evidencia se calificó como muy baja para la mortalidad (a los 0 a 30 días), la neumonía, el infarto de miocardio, el accidente cerebrovascular, el estado de confusión agudo, la tasa reducida de trombosis venosa profunda a falta de tromboprofilaxis potente y el retorno del paciente al domicilio. El número de estudios que compararon otras técnicas anestésicas fue limitado.
Conclusiones de los autores
No se encontró una diferencia entre las dos técnicas, excepto en la trombosis venosa profunda a falta de tromboprofilaxis potente. Los estudios incluyeron una variedad amplia de prácticas clínicas. El número de participantes incluidos en la revisión no es suficiente para eliminar una diferencia entre las dos técnicas en la mayoría de los resultados estudiados. Por lo tanto, se requieren ensayos aleatorizados grandes que reflejen la práctica clínica real antes de establecer conclusiones finales.
PICOs
Resumen en términos sencillos
Anestesia regional o general para la cirugía por fractura de cadera en adultos
Antecedentes: La mayoría de los pacientes con fractura de cadera son de edad avanzada y se tratan con cirugía, que requiere anestesia. Generalmente la fractura se debe a una caída sencilla. Estos pacientes a menudo tienen muchos otros problemas médicos asociados con el envejecimiento que los coloca en alto riesgo de mortalidad después de la anestesia. Los tipos más frecuentes de anestesia son "general" y "regional". La anestesia general incluye pérdida de la conciencia (sueño inducido). La anestesia regional incluye la inyección de una solución que contiene un anestésico local dentro de la columna (bloqueo neuroaxial) o alrededor de los nervios fuera de la columna (bloqueo nervioso periférico) para prevenir el dolor en la pierna debido a la fractura de cadera. Se revisó la evidencia acerca del efecto de la anestesia regional sobre los pacientes a los que se les realiza cirugía por fractura de cadera.
Características de los estudios: La evidencia está actualizada hasta marzo 2014. En total, se incluyeron 31 estudios (con 3231 participantes) en la revisión. De esos 31 estudios, 28 (2976 participantes) proporcionaron datos para los metanálisis. La media de edad de los participantes varió de 75 a 86 años. Esos estudios fueron publicados entre 1977 y 2013, por lo que abarcan una variedad amplia de prácticas clínicas y mejorías en las técnicas con el transcurso del tiempo. Dos estudios fueron financiados por el fabricante del fármaco anestésico o por un organismo con un interés comercial, uno recibió financiación de una organización benéfica y otro fue financiado por una dependencia gubernamental. Se volvió a realizar la búsqueda en febrero de 2017. Se añadieron posibles nuevos estudios de interés a una lista de "Estudios en espera de clasificación" y se incorporarán a los hallazgos de la revisión oficial durante la actualización de la misma.
Resultados clave: Los informes de ensayo de muchos de los estudios indicaron un nivel subsubóptimo de rigor metodológico y el número de participantes incluidos a menudo no fue suficiente para poder establecer una conclusión definitiva sobre muchos de los resultados estudiados. No se encontraron diferencias en la mortalidad al mes (11 ensayos con 2152 participantes) entre los bloqueos neuroaxiales y la anestesia general. Tampoco se encontraron diferencias en la neumonía, el infarto de miocardio, el accidente cerebrovascular, el estado de confusión agudo, la insuficiencia cardíaca congestiva, la lesión renal aguda, la embolia pulmonar, el número de pacientes transfundidos con eritrocitos, la duración de la cirugía y la duración de la estancia hospitalaria entre estas dos técnicas anestésicas en dos a 12 estudios. Asimismo, cuando se administraron fármacos profilácticos potentes (como la heparina de bajo peso molecular) contra la formación de coágulos posoperatorios, no se encontró una diferencia en el riesgo de trombosis venosa profunda. Sin la profilaxis con fármacos anticoagulantes potentes el riesgo de trombosis venosa profunda fue menor con el bloqueo neuroaxial.
Calidad de la evidencia: El nivel de la evidencia fue muy bajo para la mortalidad, la neumonía, el infarto de miocardio, el accidente cerebrovascular, el estado de confusión agudo, la disminución en la incidencia de trombosis venosa profunda a falta de profilaxis potente y el retorno del paciente al domicilio. Lo anterior significa que cualquier estimación del efecto es muy incierta.
Authors' conclusions
Summary of findings
| Neuraxial block compared to general anaesthesia for hip fracture repair | ||||||
| Patient or population: Patients with hip fracture repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| General anaesthesia | Neuraxial block | |||||
| Mortality | Study population | RR 0.78 | 2152 | ⊕⊝⊝⊝ | ||
| 80 per 1000 | 62 per 1000 | |||||
| Low | ||||||
| 35 per 1000 | 27 per 1000 | |||||
| High | ||||||
| 95 per 1000 | 74 per 1000 | |||||
| Pneumonia | Study population | RR 0.77 | 761 | ⊕⊝⊝⊝ | ||
| 64 per 1000 | 50 per 1000 | |||||
| Low | ||||||
| 30 per 1000 | 23 per 1000 | |||||
| High | ||||||
| 80 per 1000 | 62 per 1000 | |||||
| Myocardial infarction | Study population | RR 0.89 | 559 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Low | ||||||
| 5 per 1000 | 4 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 44 per 1000 | |||||
| Cerebrovascular accident | Study population | RR 1.48 | 729 | ⊕⊝⊝⊝ | ||
| 8 per 1000 | 12 per 1000 | |||||
| Low | ||||||
| 10 per 1000 | 15 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 74 per 1000 | |||||
| Acute confusional state | Study population | RR 0.85 | 624 | ⊕⊝⊝⊝ | ||
| 177 per 1000 | 150 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 212 per 1000 | |||||
| Deep vein thrombosis | Study population | RR 0.57 | 116 | ⊕⊝⊝⊝ | For this outcome, we retained | |
| 780 per 1000 | 444 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 114 per 1000 | |||||
| High | ||||||
| 900 per 1000 | 513 per 1000 | |||||
| Return of patient to their own home | Study population | RR 0.84 | 130 | ⊕⊝⊝⊝ | ||
| 578 per 1000 | 486 per 1000 | |||||
| Low | ||||||
| 400 per 1000 | 336 per 1000 | |||||
| High | ||||||
| 800 per 1000 | 672 per 1000 | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment and/or blinding of outcome assessors unclear/inadequate in 75% or more of the included studies. | ||||||
Background
Description of the condition
The term 'proximal femoral fracture', or 'hip fracture', refers to a fracture of the femur in the area of bone immediately distal to the articular cartilage of the hip, to a level of about five centimetres below the lower border of the lesser trochanter. The majority of these fractures occur in the elderly population, and more than 30% of the patients are 85 years or older (Brauer 2009). In the United States, while the age‐adjusted incidence of hip fracture increased from 1986 to 1995, a steady decline from 1995 to 2005 has been reported. In women, the incidence increased by 9.0% in 1995 compared to 1986, with a subsequent decline of 24.5% in 2005. In men, the increase in incidence from 1986 to 1995 was 16.4%, and the subsequent decrease to 2005 was 19.2% (Brauer 2009). Despite this improvement, hip fractures in the elderly are still quite common, and this improvement may not apply to developing countries. Between 1986 and 2005, in the United States, the annual mean number of hip fractures was 957.3 per 100,000 (95% confidence interval (CI), 921.7 to 992.9) for women and 414.4 per 100,000 (95% CI, 401.6 to 427.3) for men (Brauer 2009). The injury is usually the result of a simple fall.
The majority of these fractures are treated surgically; thus hip fracture surgery represents one of the most common emergency orthopaedic procedures. Surgical treatment may be either fixation of the fracture or replacement of the femoral head with an arthroplasty. Internal fixation involves using screws or pins, either alone, or in combination with a side plate applied to the femur, or by the use of an intramedullary nail with a cross screw inserted into the femoral head. Arthroplasty involves excision of the fractured area of bone and replacement with a partial or total hip replacement, which may be cemented in place.
Description of the intervention
The term 'regional anaesthesia' may include neuraxial blocks or peripheral nerve blocks. Neuraxial blockade refers to placement of a solution containing local anaesthetics close to the spinal cord. Neuraxial blocks may be executed by spinal or epidural or combined spinal/epidural blockade. For a spinal block, the solution containing the local anaesthetics is placed in the cerebrospinal fluid. For an epidural block, the solution containing the local anaesthetic is placed in the epidural space, outside the dura matter (membrane surrounding the cord and the cerebrospinal fluid). Peripheral nerve blocks refer to placement of local anaesthetics around peripheral nerves or plexus outside the spine. Peripheral nerve blocks for this indication are usually performed by a posterior lumbar plexus block (psoas compartment block), with or without additional blocks (sacral plexus block, iliac crest infiltration). For posterior lumbar plexus blocks and for sacral plexus blocks, the local anaesthetics are placed around the roots of the nerves, immediately after their exit from the spine at the lumbar level (posterior lumbar plexus block) or at the sacral level (sacral plexus block). An iliac crest infiltration is the deposition of local anaesthetics under the skin above the iliac crest along its superior border. Neuraxial or peripheral nerve blocks may be performed by a single shot injection or by incremental doses, with or without a continuous infusion thereafter. When a patient is having an operation under regional anaesthesia alone, he/she remains conscious, but insensitive to pain. The muscle relaxation obtained is also usually sufficient to allow the surgeon to repair the fracture. General anaesthesia refers to the use of a variety of intravenous and or inhalation drugs to render the patient unconscious, amnesic of the procedure and insensitive to pain. Neuromuscular blocking agents are often added for a hip fracture repair under general anaesthesia.
How the intervention might work
Whilst the hip fracture is usually the only injury, the patients frequently have many other medical problems associated with ageing. Indeed, an increase in all comorbidities (except paralysis) in patients with hip fracture was recorded from 1986 to 2005 (Brauer 2009). Advanced age and frequent multiple associated comorbidities put these patients at high risk of mortality after anaesthesia. The 10‐year probability of survival of patients with comorbidities (American Society of Anesthesiologists) physical status (ASA) III or IV undergoing major surgery is lower than that of patients without significant comorbidities (ASA I or II) (Kennedy 2010). A recent Cochrane Overview, found that the 0 to 30‐day mortality of patients undergoing high or moderate cardiac risk procedures is lower in patients having an operation under neuraxial blocks compared to those having an operation under general anaesthesia: risk ratio (RR) 0.71, 95% CI 0.53 to 0.94; moderate quality of evidence (Guay 2014). Major orthopaedic surgeries such as hip fracture repairs are considered moderate cardiac risk procedures (Fleisher 2007).
Why it is important to do this review
In a previous version of this review, we concluded that regional anaesthesia was associated with a borderline decreased mortality at one month: RR 0.69, 95% CI 0.50 to 0.95 (Parker 2004). This finding however, is not corroborated with a recent large retrospective study, where the authors concluded that the use of regional anaesthesia compared with general anaesthesia was not associated with lower 30‐day mortality, but only with a modestly shorter length of hospital stay (Neuman 2014).
This review is an update of previous versions (Parker 2001; Parker 2004; Urwin 2000). We undertook this update to search for new studies and adjust the methodology to the latest Cochrane requirements.
Objectives
The main focus of this review is the comparison of regional versus general anaesthesia for hip (proximal femoral) fracture repair in adults. The scope of this review, originally published in 2000 (Urwin 2000), was expanded in the second update (Parker 2001) to also cover other methods of anaesthesia. We did not consider supplementary regional blocks in this review as they have been studied in another review (Parker 2002).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs). We excluded cluster and cross‐over trials. We included all RCTs regardless of language of publication or publication status.
Types of participants
We considered studies that included participants ≥ 16 years old undergoing hip fracture surgery (an emergency surgery).
Types of interventions
We included studies that compared any combination of the following interventions.
-
Neuraxial blocks: epidural (single shots or continuous), spinals (single shots or continuous), or combined spinal/epidural (single shots or continuous), with or without intravenous sedation.
-
Peripheral nerve blocks: posterior lumbar (psoas) plexus blocks, with or without sacral plexus blocks, or any other peripheral nerve blocks, with or without sedation.
-
General anaesthesia based on inhalational agents (with or without opioids and/or neuromuscular blocking agents), or on total intravenous anaesthesia (ketamine‐based technique or other). Any technique where an endotracheal tube or a laryngeal mask airway was used was considered as general anaesthesia.
Types of outcome measures
Primary outcomes
-
Mortality from any cause at 30 days, three months, six months, and one year (cumulative).
-
Pneumonia (author's definition).
-
Myocardial infarction (author's definition).
Secondary outcomes
-
Cerebrovascular accident (author's definition).
-
Acute confusional state (author's definition).
-
Deep vein thrombosis.
-
Return of patient to their own home.
-
Congestive cardiac failure (author's definition).
-
Acute kidney injury (author's definition).
-
Pulmonary embolism.
-
Unsatisfactory surgical results.
-
Number of patients transfused.
-
Length of hospital stay.
-
Length of surgery (in minutes).
-
Operative hypotension (author's definition).
-
Urine retention.
-
Incomplete or unsatisfactory analgesia.
Please see Table 1 for the definitions and time points of these outcomes.
| Outcome | Study | Definition | Time point |
| Pneumonia | "treated for" | "during the postoperative period" | |
| Unspecified | "postoperatively" | ||
| Chest X‐Ray in clinical suspicion | Up to four weeks | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| "The clinical criteria adopted as indicating respiratory problems were productive cough, the presence of rhonchi or crepitations on auscultation or abnormalities on chest X‐ray." However the criteria adopted for the diagnosis of a pneumonia ("respiratory infection") are not clearly mentioned | Up to four weeks | ||
| "clinical and radiological criteria" | "in hospital" | ||
| Unspecified | Within four weeks | ||
| Myocardial infarction | EKG and troponin measurement daily for three days, no definition provided | Within one month | |
| Serial preprogrammed EKGs up to postoperative day 10 interpreted by a blinded cardiologist. Q wave | In hospital | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| World Health Organization criteria applied by a blinded investigator | Within one month | ||
| Congestive cardiac failure | "treated for" | "during the postoperative period" | |
| "acute heart failure" | Within one month | ||
| "cardiac decompensation" | "postoperatively" | ||
| "life‐threatening complications" "congestive heart failure" | Within four weeks | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| "episode of congestive heart failure" | In hospital | ||
| Cerebrovascular accident | "stroke" | All patients developed their stroke on postoperative day one | |
| "stroke" | Within one month | ||
| "Neurological sequelae", apoplexy for the sole event reported | "postoperatively" | ||
| "cerebrovascular accident" | Within four weeks | ||
| "cerebrovascular accident" "diagnosed by the consultant specialist" | "postoperative" | ||
| "cerebrovascular accident" | In hospital | ||
| Acute confusional state | Diagnostic and Statistical Manual of Mental Disorders (DSM‐Ill) as criteria for acute confusional state | Within seven days (the period 0‐8 hours after the surgery was excluded) | |
| "Mental confusion" | "postoperatively" | ||
| Mini Mental State Examination test decreased 2 points from baseline | Within seven days | ||
| Mini Mental Status Examination lower than 5 | Between the third and fifth postoperative day | ||
| Cognitive dysfunction based on time, person, and place disorientation | Up to postoperative day two | ||
| Delirium was judged by floor nurse, using the Inoue's confusion assessment method diagnosis algorithm | Up to postoperative day four | ||
| Confusion with agitation | In hospital | ||
| Unspecified | Within four weeks | ||
| Renal failure (or acute kidney injury) | "acute renal failure" | Within four weeks | |
| Blood creatinine > 135 micromol/Liter | In hospital | ||
| Deep vein thrombosis | Systematic bilateral contrast venography | Postoperative day ten | |
| 125‐iodine fibrinogen uptake test performed daily for seven days | Within seven days | ||
| "deep veins thrombosis" "diagnosed by the consultant specialist" | "postoperative" | ||
| "thrombosis" | Within seven days | ||
| Systematic venography | Between postoperative day seven and ten | ||
| "deep vein thrombosis" | Within four weeks | ||
| Pulmonary embolism | "pulmonary embolism" | "postoperatively" | |
| "pulmonary embolus" | "postoperatively" | ||
| Pulmonary venous angiogram or ventilation‐perfusion lung scanning on clinical suspicion | Unclear | ||
| "pulmonary emboli" "diagnosed by the consultant specialist" | "postoperatively" | ||
| Clinical suspicion confirmed with angiography | In hospital | ||
| Unsatisfactory surgical results | Either an unstable fixation of the fracture by nail and plate or the dislocation of a prosthesis, which required bedrest on traction and prevented early mobilization | In hospital | |
| Operative hypotension | Decrease > 30% from baseline for systolic arterial blood pressure | Intraoperative | |
| Decrease of 30% from baseline for arterial blood pressure | Intraoperative | ||
| Requiring the administration of a sympathomimetic | Intraoperative | ||
| Decrease in systolic arterial pressure 20% from baseline | Intraoperative | ||
| Decrease of 40 mmHG in systolic arterial blood pressure | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 33% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 50% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure of 25% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure of 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 30% from baseline | Intraoperative | ||
| Urine retention | "urinary retention" | "postoperative" | |
| "that required catheterization" | In hospital |
EKG: electrocardiogram
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2014, Issue 3, see Appendix 1), MEDLINE (Ovid SP, 2003 to March 2014, see Appendix 2) and EMBASE (Ovid SP, 2003 to March 2014, see Appendix 3). We also screened the reference lists of all studies retained. We reran the search in February 2017. We will deal with the two studies of interest when we update the review.’
We imposed no language restriction. We considered articles of all languages and translated them if necessary
Searching other resources
We looked at: http://www.clinicaltrials.gov, http://isrctn.org, http://www.umin.ac.jp/ctr/index.htm, http://www.anzctr.org.au/, http://www.trialregister.nl/, and https://eudract.ema.europa.eu/ for trials in progress in 2014.
Data collection and analysis
Selection of studies
Two review authors (JG and SK) independently screened abstract/titles. We retrieved all potentially relevant studies.We excluded duplicate publications based on the sites and dates of data collection. We noted reasons for exclusion. We resolved disagreements by discussion; involvement of a third review author was never required. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (see Figure 1; Moher 2009), and 'Characteristics of excluded studies' table.’
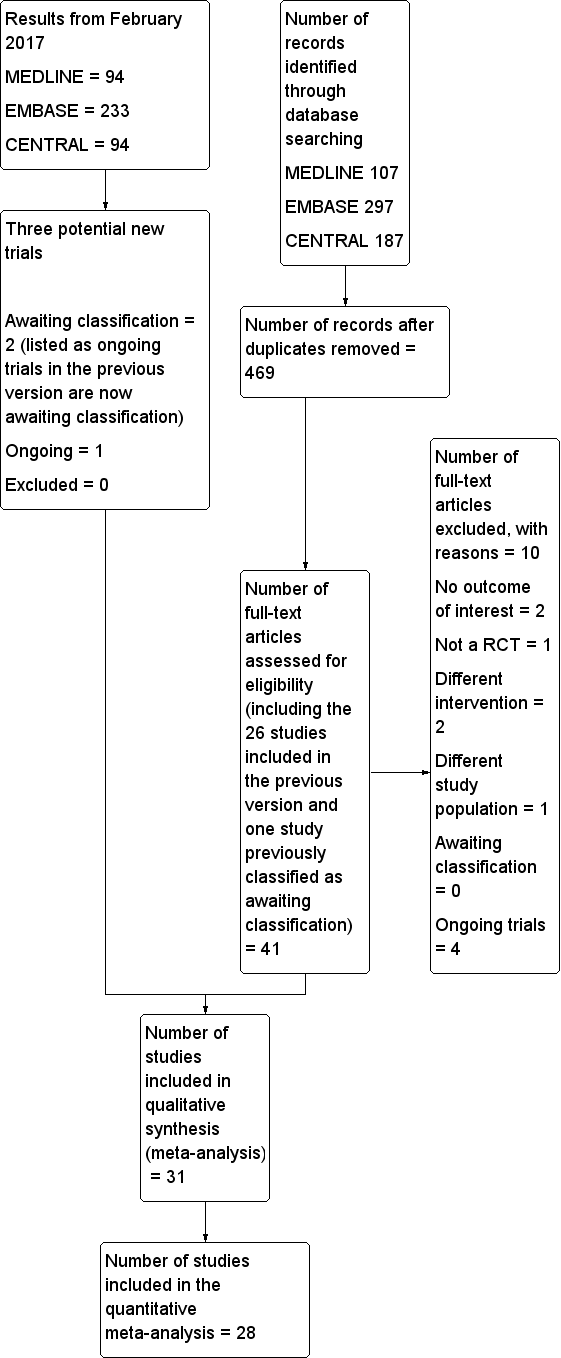
Study flow diagram.
Data extraction and management
When the study was already included in the previous version, one review author (JG) rechecked all entries from the manuscripts. For the five new studies (Biboulet 2012; Cao 2008; Heidari 2011; Hoppenstein 2005; Messina 2013), two review authors independently extracted the data for our selected outcomes. We entered the number of participants with events and total number of patients included in each treatment group in an Excel sheet for mortality from any cause at 30 days, three months, six months, and one year (cumulative), pneumonia, myocardial infarction, congestive heart failure, cerebrovascular accidents, acute confusional state, acute kidney injury, deep venous thrombosis, pulmonary embolism, return of patient to their own home, unsatisfactory surgical results, number of patients transfused, operative hypotension and urine retention. For continuous data (length of surgery and of hospital stay) we entered mean, standard deviation and total number of participants in each group, or P values and number of participants in each group when the former were not available.
We resolved all disagreements by discussion, involvement of a third review author was never required. One review author (JG) also entered in Comprehensive Meta‐Analysis software the information required for heterogeneity exploration (http://www.meta‐analysis.com): year when the study was published, mean age of participants, percentage of participants undergoing arthroplasty, cut‐off point for operative hypotension definition, preinduction administration of fluids, regional anaesthetic technique in the treatment group (type of block [spinal versus epidural versus peripheral nerve block], single shot versus continuous technique, uni‐ versus bilateral spinal), inhalational agent used in the control group, mean ASA physical status, delay before the surgery, thromboprophylaxis, and use of neuromuscular blocking agent in the control group.
Assessment of risk of bias in included studies
Two review authors (JG and SK) independently assessed the quality of the studies with the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed the risk of bias based on the information presented in the reports, with no assumptions: low risk, high risk or unclear risk of bias. When there was not enough information in the report to make an assessment, we judged the item as high risk for blinding (blinding of participants and personnel and blinding of outcome assessment) and as unclear for all other items. We resolved any disagreements by discussion; involvement of a third review author was never required.
Measures of treatment effect
Data are expressed as risk ratios (RRs) (dichotomous), mean difference (MDs) (continuous data) or standardized mean difference (SMDs) and their 95% CI.
Unit of analysis issues
We included only parallel RCTs. When the study contained more than two groups, in order to avoid including duplicate data, we either selected only the groups relevant to the review, or fused two subgroups, or split the control group in half. The choice between the two latter options was made according to the solution that best fitted our criteria for heterogeneity exploration.
Dealing with missing data
We only analysed the available data; we made no imputation.
Assessment of heterogeneity
We measured statistical heterogeneity using the I2 statistic.
Assessment of reporting biases
We judged a study to have used selective reporting when data were collected as stated in the methods section, but not reported in the results section. We mentioned data that were provided as per the protocol (not on an intention‐to‐treat basis) as other risk of bias.
Data synthesis
We analysed data with Comprehensive Meta‐analysis software (http://www.meta‐analysis.com). In addition, we used Review Manager 5 with fixed‐effect (I2 < 25%; Higgins 2003) or random‐effects models (RevMan 2014). We expressed data as RRs (dichotomous), MDs (continuous data) or SMDs and their 95% CI. When we found an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) from the odds ratio (http://www.nntonline.net/visualrx/). When we did not find any effect, we calculated the optimal information size according to Pogue 1997 from http://stat.ubc.ca/˜rollin/stats/ssize/b2.html and power with Power and Precision V3.2 for our major outcomes (http://power‐analysis.com/).
Subgroup analysis and investigation of heterogeneity
We explored any moderate amount of heterogeneity (I2 > 25%; Higgins 2003) by visual inspection of the forest plots, with studies placed in order according to a specific moderator, subgroupings (categorical moderators) or meta‐regressions (continuous moderators). We considered the following factors in the heterogeneity exploration: year when the study was published; fixation versus joint replacement; single shot versus incremental dose for neuraxial block; uni‐ versus bilateral spinal; inhalational agents or not (type); neuromuscular blocking agents or not; ASA physical status; mean age of participants; administration of an intravenous bolus fluid before the neuraxial block; and thromboprophylaxis. We used the Egger's regression intercept to assess the possibility of a small‐study effect (Rucker 2011).
Sensitivity analysis
A sensitivity analysis could be performed when the results of one single study appeared as an outlier on the forest plot or on the basis of the 'Risk of bias' assessment.
Grading the body of evidence
We judged the quality of the body of evidence using GRADEProGDT (http://ims.cochrane.org/revman/gradepro) (Guyatt 2011a), and presented in a 'Summary of findings' table each major outcome: mortality at one month, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, deep venous thrombosis, and return of patient to their own home. For risk of bias, we judged the quality of evidence as low risk of bias when most information came from studies at low risk of bias, we downgraded by one level when most information came from studies at low or unclear risk of bias and downgraded by two levels when the proportion of information from studies at high risk of bias was sufficient to affect the interpretation of results.
For inconsistency, we downgraded the quality of evidence by one when the I2 statistic was 50% or higher without satisfactory explanation and by two levels when the I2 statistic was 75% or higher without an explanation. We did not downgrade the quality of evidence for indirectness as all outcomes were based on direct comparisons, were performed on the population at interest and were not surrogate markers (Guyatt 2011b), except for deep venous thrombosis. In the included studies, the latter was evaluated by systematic venographies performed within 10 days after surgery. This was considered as a surrogate marker for clinically relevant events.
For imprecision (Guyatt 2011c), we downgraded the quality of evidence by one when: the CI around the effect size was large or overlapped an absence of effect, and failed to exclude an important benefit or harm; and the number of participants was lower than the optimal information size. We downgraded the quality by two levels when the CI was very wide and included both appreciable benefit and harm.
For publication bias, we downgraded the quality of evidence by one when correcting for the possibility of publication as assessed by the Duval and Tweedie’s fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one when the effect size was large (< 0.5 or > 2.0) and by two when the effect size was very large (RR < 0.2 or > 5) (Guyatt 2011d). We applied the same rules for OR when the basal risk was lower than 20%. For SMD, we used 0.8 as the cut‐off point for a large effect (Pace 2011). We also upgraded the quality by one when evidence of a dose related response was found.
We upgraded the quality by one when the possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect when results showed no effect. When the quality of the body of evidence is high quality, further research is very unlikely to change our confidence in the estimate of effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When the quality is low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
The flow diagram of the study selection is included in Figure 1 (Moher 2009). From the previous version, we reassessed 26 included studies and one study awaiting classification. We also assessed 14 new studies from the electronic search or from the reference lists of studies. We excluded ten studies for the following reasons: no outcome of interest for this review (Darling 1994; Messaoudi 2009), quasi‐randomized trial (Adams 1990), different intervention (Ungemach 1987; Yao 1997), different study population (Lattermann 2005), and ongoing trials (ISRCTN36381516; NCT00590707; NCT02190903; NCT02213380). We reran the search in February 2017. The search terms were slightly modified from those of the previous searches. The modified strategy used for 2014 and onwards can be found in Appendix 4. Three hundred and eighty‐two new citations were found: 55 in CENTRAL, 233 in Embase and 94 in Medline.
Included studies
In total, we included 31 studies (with 3231 participants) in our review. Of those 31 studies, 28 (2976 participants) provided data for the meta‐analyses. The reasons for not including three studies in the meta‐analysis were: the time point at which mortality was measured was unclear (Tasker 1983); the time point did not correspond to the time points chosen for this review (mortality data provided at two weeks only; Ungemach 1993); and results provided for our study population were mental tests (Wajima 1995). Therefore, we included 28 studies with 2976 participants published between 1977 and 2013 for the quantitative meta‐analysis.
Two studies were funded by the drug manufacturer or by an agency with a commercial interest (Davis 1987; Valentin 1986), one received charitable funding (Davis 1981), and one was funded by a government agency (Berggren 1987). The source of funding was unspecified for the other studies. The mean age of the participants varied from 74.8 to 86 years. Their mean ASA physical status varied from 2.0 to 3.3. The mean delay before the surgery varied from 24 to 240 hours. The percentage of participants undergoing arthroplasty varied from 0% to 100%. The thromboprophylaxis used was: early mobilization (Davis 1981); socks (Valentin 1986); dextran (Berggren 1987); antivitamine K drugs (Couderc 1977); unfractionated heparin (Heidari 2011; Racle 1986); or low molecular heparin (Brichant 1995). The neuraxial blocks used were: spinal (Biboulet 2012; Biffoli 1998; Bigler 1985; Bredahl 1991; Casati 2003; Davis 1981; Davis 1987; de Visme 2000; Eyrolle 1998; Hoppenstein 2005; Ibanez 1993; Juelsgaard 1998; Kamitani 2003; Maurette 1988; McKenzie 1984; McLaren 1978; Messina 2013; Racle 1986; Svarting 1986; Valentin 1986; White 1980); epidural (Berggren 1987; Cao 2008; Couderc 1977; Wajima 1995); or any of these two techniques (Brichant 1995; Heidari 2011). Inhalational agents used for general anaesthesia were: nitrous oxide alone (Bigler 1985; Bredahl 1991; Davis 1981; Davis 1987; McLaren 1978; Spreadbury 1980; Svarting 1986); methoxyflurane (Couderc 1977); halothane (Berggren 1987; Heidari 2011; McKenzie 1984; White 1980); enflurane (Juelsgaard 1998; Maurette 1988; Racle 1986); enflurane or nitrous oxide (Valentin 1986); isoflurane (Biffoli 1998; Hoppenstein 2005); or sevoflurane (Casati 2003; Kamitani 2003; Messina 2013; Wajima 1995).
Table 2 contains the anaesthetic agents used to produce general anaesthesia or sedation. Twenty‐four studies were involved in the comparison neuraxial block versus general anaesthesia (Berggren 1987; Biboulet 2012; Biffoli 1998; Bigler 1985; Bredahl 1991; Brichant 1995; Cao 2008; Casati 2003; Couderc 1977; Davis 1981; Davis 1987; Heidari 2011; Hoppenstein 2005; Ibanez 1993; Juelsgaard 1998; Kamitani 2003; Maurette 1988; McKenzie 1984; McLaren 1978; Messina 2013; Racle 1986; Svarting 1986; Valentin 1986; Wajima 1995). Three studies were involved in the comparison neuraxial block versus peripheral nerve block (Cao 2008; de Visme 2000; Eyrolle 1998). One study compared a neuraxial block added to general anaesthesia versus general anaesthesia alone (White 1980). The same study was also used for a comparison between peripheral nerve block added to general anaesthesia compared to general anaesthesia alone (White 1980), and one study compared intravenous ketamine to classic general anaesthesia (Spreadbury 1980).
| Study | Sedative drugs for participants of the regional blockade group | Anaesthetic agents for general anaesthesia |
| Premedication: Meperidine No other sedative drugs mentioned as routinely administered for the surgery. | Premedication: Meperidine Induction: Thiopental and atropine Maintainance: Nitrous oxide, halothane and succinylcholine infusion | |
| None mentioned | Subgroup 1 Induction: Propofol Maintainance: Propofol infusion (for a bispectral index value of 50) and remifentanil Subgroup 2 Induction: Sevoflurane Maintainance: Sevoflurane (for a bispectral index value of 50) and remifentanil | |
| None mentioned | Induction: Propofol Maintainance: Nitrous oxide, isoflurane, fentanyl (intermittent injections) plus an atracurium infusion | |
| Premedication: Pethidine Small amounts of diazepam if needed | Premedication: Pethidine Induction: Diazepam and atropine Maintainance: Nitrous oxide, fentanyl and pancuronium | |
| Premedication: Pethidine Diazepam for mild sedation | Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, thiopental and pethidine | |
| Not mentioned | According to local practice | |
| Premedication: Tamazepam or pethidine No drug supplementation during the surgery | Premedication: Tamazepam or pethidine Induction: Thiopental or propofol Maintainance: Nitrous oxide, isoflurane or enflurane and atracurium 0.5 mg/kg one dose | |
| Midazolam and fentanyl if required | ||
| One dose of fentanyl before the block No other sedative drug routinely administered for the surgery. | Induction: Sevoflurane Maintainance: Nitrous oxide, sevoflurane | |
| Premedication: Hydroxyzine and atropine None mentioned for the surgery | Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide plus 1) Thiopental and dextromoramide or 2) methoxyflurane. One dose of pancuronium in some participants | |
| Ketamine 20‐25 mg (for eight participants) before the spinal Ketamine at unspecified total doses during the surgery (two participants) or 25 mg at skin closure (two participants) Diazepam (mean dose 9 mg; range 0‐35 mg) | Induction: Diazepam (mean dose 9.5 mg; range 2.5 to 30 mg) Maintainance: Nitrous oxide, fentanyl and pancuronium | |
| Benzodiazepine (optional) | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl and non‐depolarizing neuromuscular blocking agent | |
| Alfentanil before the block and as required during the surgery. No mandatory sedative drugs mentioned. One patient in the continuous peripheral nerve block received "sedation repeatedly". | ||
| Propofol as required | ||
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl, halothane and one dose of pancuronium | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, isoflurane, fentanyl and vecuronium | |
| Not reported | Not reported | |
| Premedication: Pethidine None mentioned for the surgery | Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of atracurium | |
| No sedative drug | Induction: Propofol Maintainance: Nitrous oxide, fentanyl, sevoflurane and one dose of vecuronium | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, dextromoramide and enflurane | |
| Small doses of diazepam | Induction: Althesin Maintainance: Nitrous oxide and halothane | |
| Althesin and nitrous oxide; arousable by ear lobe pressure | Induction: Althesin Maintainance: Nitrous oxide, fentanyl and pancuronium one dose | |
| None mentioned | Induction: Propofol Maintainance: Sevoflurane, remifentanil and one dose of cisatracurium | |
| Premedication: Hydroxyzine and atropine Flunitrazepam, verbal contact possible | Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of vecuronium | |
| No group with regional anaesthesia alone | Ketamine group Induction and maintenance: Ketamine and diazepam (2.5 to 10 mg) Relaxant group Technique at the discretion of the attending anaesthesiologist | |
| Premedication: Pethidine and atropine None mentioned for the surgery | Premedication: Pethidine and atropine Induction: Thiopental Maintainance: Nitrous oxide, fentanyl (repeated injections) and one dose of pancuronium | |
| Not reported | Not reported | |
| Not reported | Induction: Not reported Maintainance: Nitrous oxide, isoflurane and fentanyl | |
| Premedication: Pethidine and promethazine in some of the participants Small doses of diazepam and fentanyl | Premedication: Pethidine and promethazine in some of the participants Subgroup 1 Induction: Thiopental or not Maintainance: Nitrous oxide, enflurane and gallamine (not all participants) Subgroup 2 Induction: Not clearly mentioned Maintainance: Nitrous oxide, droperidol, fentanyl and gallamine | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide and sevoflurane | |
| Premedication: Diazepam Althesin, nitrous oxide and fentanyl (spontaneous breathing) for the two subgroups | Premedication: Diazepam Induction: Thiopental Maintainance: Nitrous oxide, halothane and fentanyl |
Excluded studies
We excluded 10 studies (see Characteristics of excluded studies for the reasons for their exclusion).
Awaiting classification
There are two studies awaiting classifications (Neuman 2016; Parker 2015). For further details see Characteristics of studies awaiting classification.
Ongoing studies
We found four ongoing trials (ISRCTN36381516; NCT00590707; NCT02190903; NCT02213380; see Characteristics of ongoing studies) in the 2014 search. When the search was reran in February 2017, two of the ongoing trials were now published and are classified as awaiting classification (ISRCTN36381516; NCT02190903). A new ongoing trial (NCT02507505) was found.
Risk of bias in included studies
The risk of bias of included studies can be found in Figure 2 and Figure 3.
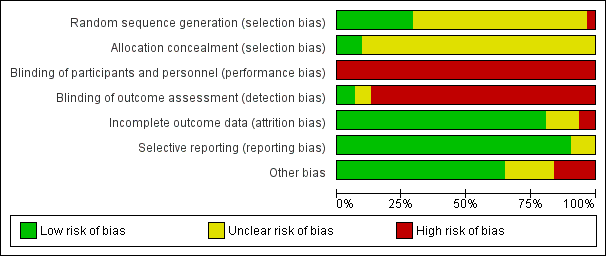
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
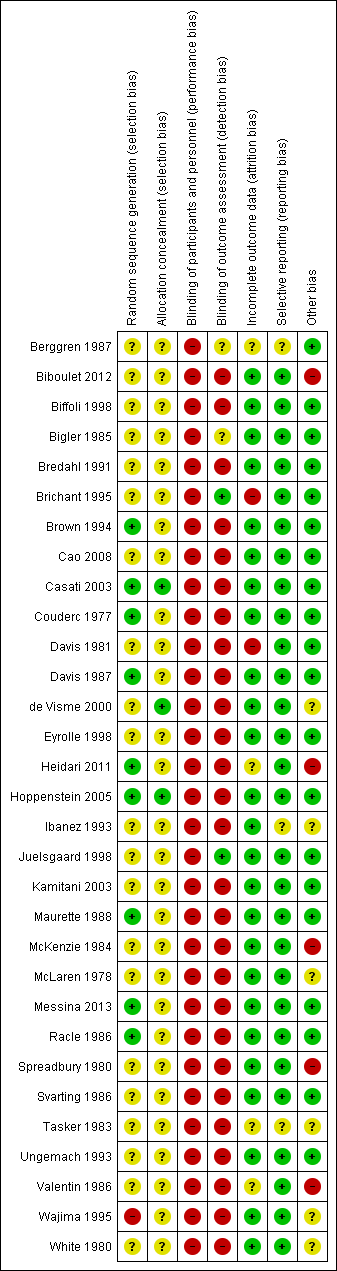
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation concealment to be at low risk of bias for three studies (Casati 2003; de Visme 2000; Hoppenstein 2005), and unclear for all other included studies (Figure 3).
Blinding
For a comparison between neuraxial and general anaesthesia, blinding of participants is not possible, and blinding of personnel taking care of the patient is probably an unrealistic expectation, at least for the personnel taking care of the patient during the first few hours after the surgery. We judged blinding of the outcome assessor to be at unclear risk of bias in Berggren 1987 and Bigler 1985 and at low risk of bias in Brichant 1995 and Juelsgaard 1998 (see Figure 2; Figure 3).
Incomplete outcome data
We classified six studies as unclear or at high risk of bias for this item (Berggren 1987; Brichant 1995; Davis 1981; Heidari 2011; Tasker 1983; Valentin 1986; seeFigure 2; Figure 3).
Selective reporting
We classified three studies as unclear for selective reporting (Berggren 1987; Ibanez 1993; Tasker 1983; see Figure 2; Figure 3).
Other potential sources of bias
We classified 11 studies as unclear or at high risk for other risks of bias (Biboulet 2012; de Visme 2000; Heidari 2011; Ibanez 1993; McKenzie 1984; McLaren 1978; Spreadbury 1980; Tasker 1983; Valentin 1986; Wajima 1995; White 1980; Figure 2; Figure 3).
Effects of interventions
Neuraxial block versus general anaesthesia
The definition and time points for all outcomes retained, can be found in Table 1.
Primary outcomes
1. Mortality
Mortality at one month
Based on 11 studies that included 2152 participants, we did not find a difference between the two techniques for mortality at one month: risk ratio (RR) 0.78, 95% confidence interval (CI) 0.57 to 1.06; I2 = 24% (fixed‐effect model) (Berggren 1987; Biboulet 2012; Bigler 1985; Davis 1981; Davis 1987; Heidari 2011; Juelsgaard 1998; McKenzie 1984; McLaren 1978; Racle 1986; Valentin 1986; Analysis 1.1). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that two studies might be missing to the left for an adjusted point of estimate: RR 0.70, 95% CI 0.51 to 0.97. Accepting that a publication bias occurred, and considering a basal rate of mortality of 8%, the number needed to treat for an additional beneficial outcome (NNTB) would be 42, 95% CI 26 to 339. A meta‐regression with the year where the study was published showed that effect size favouring neuraxial blockade compared to general anaesthesia might be higher in older studies (Figure 4). The number of participants included here allows to eliminate a difference of 25% in the risk of mortality at one month with a power of 0.57 (α 0.05; β 0.2; one‐sided test). A number of 4024 (2012 per group) would be required for a power of 0.8 (α 0.05; β 0.2; one‐sided test) if a large study would be done.
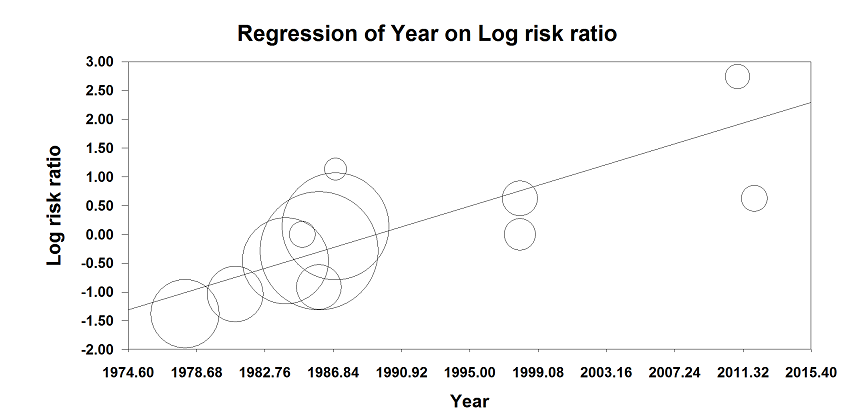
Meta‐regression of mortality at 0‐1 month versus the year when the study was published. The effect size decreases with time: P value = 0.002.
This meta regression plot was not produced in RevMan. The figure was generated automatically by the software, and cannot be amended. The software has expressed the years as decimals.
For mortality at one month, we downgraded the level of evidence by two for risk of bias based on the fact that we judged 75% or more of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade an absence of effect for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We downgraded the level by one for publication bias because correcting for this possibility would make the effect present instead of absent (RR after correction 0.70, 95% CI 0.51 to 0.97 versus RR 0.78, 95% CI 0.57 to 1.06 without correction). We did not change the level for amplitude of effect size (RR 0.78 and therefore > 0.5). We did not identify any confounding factors justifying upgrading. We upgraded the level for a dose response effect because we concluded to an absence of effect, and the meta‐regression showed that an effect was present only in older studies. We rated the quality of evidence as very low.
Mortality at three months
Based on five studies that included 953 participants (Berggren 1987; Couderc 1977; McKenzie 1984; Racle 1986; Valentin 1986), we did not find a difference in mortality at 3 months: RR 0.77, 95% CI 0.55 to 1.08; I2 = 0% (Analysis 1.2) . Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that one study might be missing to the left for an adjusted point of estimate: RR 0.78, 95% CI 0.56, 1.09. Considering a basal mortality rate of 13.8%, 2204 participants (1102 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done.
Mortality at six months
Based on two studies that included 726 participants (McKenzie 1984; Valentin 1986), we did not find a difference in mortality at six months: RR 1.00, 95% CI 0.73 to 1.37; I2 = 0% (Analysis 1.3). Considering a basal mortality rate of 17.5%, 1678 participants (839 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done.
Mortality at one year
Based on two studies that included 726 participants (McKenzie 1984; Valentin 1986), we did not find a difference in mortality at one year after the surgery: RR 1.06, 95% CI 0.81 to 1.39; I2 = 0% (Analysis 1.4). Considering a basal mortality rate of 21.8%, 1310 participants (655 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done.
2. Pneumonia
Based on six studies that included 761 participants (Berggren 1987; Bigler 1985; Davis 1981; Heidari 2011; McLaren 1978; Racle 1986), we did not find a difference in the risk of pneumonia: RR 0.77, 95% CI 0.45 to 1.31; I2 = 0% (Analysis 1.5). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that three studies might be missing to the right for an adjusted point of estimate: RR 1.15, 95% CI 0.71 to 1.86. Considering a pneumonia rate of 6.4%, 5106 participants (2553 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done. Likewise, the number of participants included here would give a power of 0.29 (α 0.05; β 0.2; one‐sided test).
For pneumonia, we downgraded the level of evidence by two for risk of bias based on the fact that we judged 75% or more of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We did not downgrade the level for publication bias because correcting for this possibility would not change the conclusion (RR after correction 1.15, 95% CI 0.71 to 1.86 versus RR 0.77, 95% CI 0.45 to 1.31 without correction). We did not change the level for amplitude of effect size (RR 0.77 and therefore > 0.5). We did not identify any confounding factors or dose response effect justifying upgrading. We rated the quality of evidence as very low.
3. Myocardial infarction
Based on four studies that included 559 participants (Biboulet 2012; Couderc 1977; Heidari 2011; Juelsgaard 1998), we did not find a difference in the risk of myocardial infarction: RR 0.89, 95% CI 0.22 to 3.65; I2 = 0% (Analysis 1.6). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Considering a basal myocardial infarction rate of 1%, 34318 participants (17159 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done. Likewise, the number of participants included here would give a power of 0.09 (α 0.05; one‐sided test).
For myocardial infarction, we downgraded the level of evidence by two for risk of bias based on the fact that we judged 75% or more of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
Secondary outcomes
1. Cerebrovascular accident (stroke)
Based on six studies that included 729 participants (Berggren 1987; Biboulet 2012; Bigler 1985; Davis 1981; Heidari 2011; Racle 1986), we did not find a difference in the risk of cerebrovascular accident: RR 1.48, 95% CI 0.46 to 4.83; I2 = 0% (Analysis 1.7). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Considering a basal cerebrovascular accident rate of 2%, 17,008 participants (8504 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.13 (α 0.05; β 0.2; one‐sided test).
For cerebrovascular accident, we downgraded the level of evidence by two for risk of bias based on the fact that we judged more than 75% of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
2. Acute confusional state
Based on six studies that included 624 participants (Berggren 1987; Bigler 1985; Casati 2003; Heidari 2011; Kamitani 2003; Racle 1986), we did not find a difference in the risk of acute confusional state (see Table 1 for exact definition): RR 0.85, 95% CI 0.51 to 1.40; I2 = 49% (Analysis 1.8). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Excluding the Heidari 2011 study, the RR would be 1.08,95% CI 0.75, 1.55); I2 = 0%. We classified Heidari 2011 at low risk of bias for two items only (randomization and selective reporting). The study enrolled 400 participants and randomized them to either general anaesthesia, maintained with nitrous oxide and halothane, or to a neuraxial block (spinal or epidural (5.7% continuous)). Intravenous morphine was given at participants' request for postoperative analgesia in both groups. Results are provided as a per‐protocol basis with 10 participants excluded from the neuraxial group and three excluded from the general anaesthesia group. Cognitive dysfunctions were noted before discharge from recovery room, and at 24 and 48 hours after the end of the surgery based on time, person, and place disorientation (unclear who assessed the participants and whether or not outcome assessors were blinded for this specific item). In the general anaesthesia group the number of participants who were classified as disorientated were 22 at the recovery room, six on first postoperative day, and three on the second postoperative day. For the neuraxial group, the number of participants classified as disorientated were seven, six, and one for the same periods. Halothane, an inhalational agent slowly eliminated from the body, is no longer in use in the vast majority of developed countries, and the choice of inhalational agent may influence mental function after general anaesthesia, particularly in the elderly (Rortgen 2010). Furthermore, the choice of morphine as the primary mode of analgesia after a major hip surgery may also contribute to postoperative cognitive dysfunction (Hebl 2005). These two factors may have contributed to a higher effect size of regional anaesthesia compared with general anaesthesia in the study of Heidari 2011, especially considering that the main difference was found in the postanaesthesia care unit. Considering a basal rate of 17.7%, 1652 participants (826 per group) would be required to eliminate a 25% difference (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.46 (α 0.05; β 0.2; one‐sided test).
For acute confusional state, we downgraded the level of evidence by two for risk of bias based on the fact that we judged more than 75% of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We downgraded the level by one for inconsistency because the I2 statistic was 49%%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
3. Deep vein thrombosis
The diagnosis of deep venous thrombosis was done by injection of 125‐iodine fibrinogen (Davis 1981), or venography (Brichant 1995; McKenzie 1984), or as diagnosed by the consultant specialist (Heidari 2011). Two studies including 116 participants did not use any potent thromboprophylaxis (Davis 1981; McKenzie 1984). The risk of deep vein thrombosis decreased when no specific precautions except just use of early mobilization was used (RR 0.57, 95% CI 0.41 to 0.78; I2 = 0%; NNTB = 3, 95% CI 2 to 7; based on a basal risk of 76%), but not when low molecular weight heparin was administered (RR 0.98, 95% CI 0.52 to 1.84; I2 = 58% for heterogeneity between the two subgroups) (Analysis 1.9). On the same basal risk the optimal information size for a large trial would be 152 participants (76 per group) for a 25% decrease (α 0.05; β 0.2; one‐sided test).
For deep venous thrombosis in the absence of potent thromboprophylactic agents, we downgraded the level of evidence by two for risk of bias based on the fact that we judged more than 75% of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. There was no evidence of inconsistency. We downgraded the level by one based on indirectness due to the fact that systematic venographies and injection of marked fibrinogen are surrogate markers for clinically relevant events. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
4. Return of patient to their own home
Data were available for one study only: RR 0.84, 95% CI 0.61 to 1.16 (McKenzie 1984; Table 3). Considering a basal rate of 5.3% (Brauer 2009), 7898 (3949 per group) would be required to eliminate a 25% difference (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.1 (α 0.05; one‐sided test).
| Comparison: Neuraxial block versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Patient returned to their own home | 130 | RR | 0.84 | 0.61 | 1.16 | |
| Urine retention | 57 | RR | 0.86 | 0.30 | 2.51 | |
| Comparison: Neuraxial block added to general anaesthesia compared to general anaesthesia alone | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Pneumonia | 30 | RR | 0.80 | 0.20 | 3.20 | |
| Acute confusional state | 30 | RR | 1.00 | 0.16 | 6.09 | |
| Deep vein thrombosis | 30 | RR | 0.17 | 0.01 | 3.94 | |
| Length of surgery (minutes) | 30 | MD | 0.00 | ‐17.96 | 17.96. | |
| Comparison: Neuraxial block versus peripheral nerve block | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Acute confusional state | 29 | RR | 0.89 | 0.35 | 2.28 | |
| Operative hypotension | 50 | RR | 6.00 | 2.02 | 17.83 | |
| Length of surgery (minutes) | 29 | MD | 17.00 | ‐0.76 | 34.76 | |
| Comparison: Intravenous ketamine alone (without neuromuscular blocking agent) versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Unsatisfactory surgical results defined as unstable fixation or prosthesis dislocation | 60 | RR | 2.33 | 0.67 | 8.18 | |
| Mortality | 60 | RR | 1.00 | 0.46 | 2.17 | |
| Patient returned home | 60 | RR | 0.95 | 0.66 | 1.38 | |
| Length of hospital stay | 39* | MD | 12.00 | 5.63 | 18.37 | |
CI: confidence interval; MD: mean difference; RR: risk ratio
*: Mean duration of admission refers only to those patients who were discharged home
For this outcome, we downgraded the level by two for risk of bias based on the fact that we judged the sole study included as unclear or inadequate for allocation concealment and blinding of outcome assessors. Inconsistency could not be evaluated. The included study was a direct comparison. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. Publication bias could not be evaluated. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
5. Congestive cardiac failure
Based on six studies that included 729 participants (Berggren 1987; Biboulet 2012; Bigler 1985; Davis 1981; Racle 1986), we did not find a difference in the risk of congestive heart failure: RR 0.78, 95% CI 0.31 to 1.96; I2 = 0% (Analysis 1.10). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Considering a basal congestive heart failure of 6%, 5466 participants (2733 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.23 (α 0.05; β 0.2; one‐sided test).
6. Acute kidney injury
Data were available only for two small studies that included 202 participants: RR 1.02, 95% CI 0.18 to 5.83; I2 = 0% (Davis 1981; Racle 1986; Analysis 1.11).
7. Pulmonary embolism
Based on five studies that included 642 participants (Berggren 1987; Bigler 1985; Brichant 1995; Heidari 2011; Racle 1986), we did not find a difference in the risk of pulmonary embolism: RR 3.35, 95% CI 0.82 to 13.57; I2 = 0% when data were analysed as risk ratio. However, if the Peto odds ratio method was used (Analysis 1.12), the difference became statistically significant in favour of general anaesthesia: Peto odds ratio 7.51, 95% CI 1.51 to 37.38; I2 = 0%. Egger's regression intercept did not show a small‐study effect. Duvall and Tweedie's trim and fill analysis did not show any evidence of a publication bias. The classical fail‐safe number was three. Because there was no event on the side of general anaesthesia a NNTH could not be calculated. Based on a rate of 1.9% in the treatment group, the NNTB would be 61, 95% CI 55 to 157, when general anaesthesia is used.
8. Unsatisfactory surgical results
There were no data for this outcome for this comparison.
9. Number of patients transfused
Based on two studies that included 172 participants (Bigler 1985; Davis 1981), we did not find a difference in the number of transfused (red blood cells) participants for femur fixation: RR 0.93, 95% CI 0.76 to 1.15; I2 = 0%. Based on one study that included 30 participants (Svarting 1986), we could not demonstrate a difference in the number of transfused patients for arthroplasty: RR 0.20, 95% CI 0.03 to 1.51. Heterogenity between the two subgroups = 55% (Analysis 1.13).
10. Length of hospital stay
Based on four studies that included 1143 participants (Davis 1987; Heidari 2011; McKenzie 1984; Racle 1986), we did not find a difference in length of hospital stay: mean difference (MD) ‐ 0.20, 95% CI ‐ 1.05 to 0.65; I2 = 0% (Analysis 1.14). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that one study might be missing to the left for an adjusted point of estimate of ‐ 0.21, 95% CI ‐ 1.05 to 0.63.
11. Length of surgery (in minutes)
Based on 12 studies that included 973 participants (Berggren 1987; Biffoli 1998; Bigler 1985; Bredahl 1991; Heidari 2011; Hoppenstein 2005; Kamitani 2003; Maurette 1988; McKenzie 1984; Messina 2013; Racle 1986; Svarting 1986), a neuraxial block would not reduce the surgical time: MD ‐ 2.73, 95% CI ‐ 8.50 to 3.04; I2 = 62% (Analysis 1.15). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that two studies might be missing to the right, for an adjusted point of estimate: ‐0.85, 95% CI ‐6.70 to 5.00.
12. Operative hypotension
The definition for operative hypotension varied from the number of participants who had a decrease of systolic arterial blood pressure of 20% (Casati 2003; Davis 1987; Racle 1986), mean arterial blood pressure of 20% (Maurette 1988), mean arterial blood pressure of 25% (Messina 2013), systolic arterial blood pressure of 30% (Berggren 1987; Svarting 1986), arterial blood pressure of 30% (Biffoli 1998), systolic arterial blood pressure of 33% (Juelsgaard 1998), arterial blood pressure ≥ 40 mm HG (Couderc 1977), systolic arterial blood pressure of 50% (McLaren 1978), or undefined (Brown 1994). The risk of operative hypotension was lower with a neuraxial block when low dose unilateral (RR 0.57, 95% CI 0.37 to 0.89; I2 = 0%) (Casati 2003; Messina 2013), or incremental spinals were performed (RR 0.20, 95% CI 0.05 to 0.78) (Juelsgaard 1998); but not with bilateral sensory/motor blockade single shot spinal anaesthesia (RR 1.31, 95% CI 0.87 to 1.95; I2 = 36%) (Biffoli 1998; Brown 1994; Davis 1987; Juelsgaard 1998; Maurette 1988; McLaren 1978; Racle 1986; Svarting 1986), or epidural anaesthesia (RR 1.01, 95% CI 0.50 to 2.07; I2 = 73%) (Berggren 1987; Couderc 1977; Analysis 1.16). A bolus of fluid was administered before the neuraxial block for six studies (Biffoli 1998; Couderc 1977; Davis 1987; Juelsgaard 1998; Racle 1986; Svarting 1986).
13. Urine retention
Based on one small study we did not find a difference in the risk of urine retention: RR 0.86, 95% CI 0.30 to 2.51 (Berggren 1987; Table 3).
Neuraxial block added to general anaesthesia compared to general anaesthesia alone
We found only one small study for this comparison (White 1980; Table 3). The study contains three groups including one where a psoas compartment block was added to general anaesthesia. This group was not retained because it was considered outside the scope of the present review: RR 0.80, 95% CI 0.20 to 3.20 for pneumonia, RR 1.00, 95% CI 0.16 to 6.09 for acute confusional state, and RR 0.17, 95% CI 0.01 to 3.94 for deep vein thrombosis. The MD for length of surgery was 0.00, 95% CI ‐17.96 to 17.96 minutes.
Neuraxial block versus peripheral nerve block
Three studies that included 139 participants compared a neuraxial block (spinal or epidural) to peripheral nerve blocks (Cao 2008; de Visme 2000; Eyrolle 1998). For the peripheral nerve blocks, two studies used posterior lumbar (psoas compartment) block alone (Cao 2008; Eyrolle 1998), and one study added sacral plexus block and iliac crest infiltration to the lumbar plexus block (de Visme 2000). Neuraxial blocks reduce the risk of a failed block: RR 0.24, 95% CI 0.12 to 0.49; I2 = 0 %) (Analysis 2.1). However, when the block combination was used (psoas compartment block plus sacral plexus block plus iliac crest infiltration), three out of the four participants (where the block was judged as incomplete), required only one bolus of 250 mcg of alfentanil at skin incision as supplemental analgesia (de Visme 2000).
We did not find a difference for the risk of acute confusional state: RR 0.89, 95% CI 0.35 to 2.28 (de Visme 2000; Table 3). Operative hypotension was more common with neuraxial blocks: RR 6.00, 95% CI 2.02 to 17.83 (Eyrolle 1998; Table 3) with a NNTH = 2 (95% CI 5 to 2) (basal rate of 12% in the peripheral nerve block group). This was also true for urine retention: RR 14.00, 95% CI 1.90 to 103.00; I2 = 0% (basal rate of 0% in the peripheral nerve block groups) (Cao 2008; Eyrolle 1998; Analysis 2.2). We found no difference in the surgical time based on one study: MD 17.00, 95% CI ‐ 0.76 to 34.76 minutes (de Visme 2000; Table 3).
Intravenous ketamine versus general anaesthesia
We found only one study for this comparison (Spreadbury 1980; Table 3). We could not demonstrate a difference in the risk for unsatisfactory surgical results defined as unstable fixation or prosthesis dislocation by ketamine alone without neuromuscular blocking agents: RR 2.33, 95% CI 0.67 to 8.18; in mortality during hospital stay: RR 1.00, 95% CI 0.46 to 2.17; or the chances of the participant returning home: RR 0.95, 95% CI 0.66 to 1.38. Length of hospital stay for the survivors was longer in the ketamine group: MD 12.00, 95% CI 5.63 to 18.37 days.
Discussion
Neuraxial block versus general anaesthesia
Many of the studies within this review involved small numbers of participants and reported only a few outcome measures. From the actual available RCTs, for adults undergoing hip fracture surgery, regional anaesthesia decreases the risk of deep venous thrombosis only in the absence of potent thromboprophylaxis (very low quality of evidence; summary of findings Table for the main comparison). A unilateral or incremental spinal anaesthesia decreases the incidence of operative hypotension. The optimal information size for an alpha error of 0.05 and a beta error of 0.2 (one‐sided test) to eliminate a difference of 25% was not achieved for: mortality at one month, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, and return of patient to their own home. Data were available from only one study for return of patient to their own home. The trial reports of many studies indicated a suboptimal level of methodological rigour, in particular regarding the exact method used for randomization, concealment of allocation, assessor blinding and intention‐to‐treat analysis (Figure 2; Figure 3). Therefore, an absence of difference cannot be stated with certainty for the vast majority of the outcomes included in the present review.
The type of anaesthetic techniques used in many of the studies may not reflect current clinical practice, and this may have prevented us in finding clinically relevant differences between general anaesthesia and regional anaesthesia. For instance, for acute confusional state, one study used diazepam as the induction agent in the general anaesthesia group and as a sedative in the regional anaesthesia group (Bigler 1985). Residual blood concentrations of benzodiazepines have not been shown to correlate with postoperative cognitive dysfunction (Rasmussen 1999). However, some authors reported a clear association between the risk of hip fractures and recently introduced benzodiazepines in the drug regimen of the elderly (Wang 2001), suggesting that newly introduced benzodiazepines will affect the elderly in someway. Therefore it is difficult to be certain that benzodiazepine administration in both groups did not mask an actual difference between general anaesthesia and regional anaesthesia. Likewise, halothane, an inhalational agent slowly eliminated from the body, that is no longer in use in the vast majority of developed countries, was administered in two of the studies and this may have increased the difference between the two anaesthetic techniques, this time favouring regional anaesthesia (Berggren 1987; Heidari 2011). The choice of an inhalational agent may influence mental functions after general anaesthesia, particularly in the elderly (Rortgen 2010).
The definitions of outcomes and the exact time points at which the participants were evaluated varied widely or were unclear (Table 1). For example, we took as "acute confusional state" all various authors' definitions without any discrimination. Although statistical heterogeneity disappeared (I2 = 0%) when Heidari 2011 was withdrawn from the analysis (Analysis 1.8), one can see from Table 3, that the definition of "acute confusional state" varied widely, and included various level of a possible transient decrease in mental function (minus 2 points out of 30 on the Mini Mental Status for Casati 2003 or a Mini Mental Status score lower than 5/30 for de Visme 2000), actual confusion (Berggren 1987; Bigler 1985; Heidari 2011), delirium with or without agitation (Kamitani 2003; Racle 1986), or was unspecified (White 1980). A transient decline in the ability to perform a mathematical test may not be as relevant as mental deterioration preventing the person from participating in their rehabilitation (confusion/delirium/agitation). Finally, the exact time point where the outcome was taken also varies widely (up to four weeks: White 1980). Although, there may not be any clinically relevant difference between general anaesthesia and regional anaesthesia after seven days, when any type of mental evaluation is accepted (Guay 2011), a transient decline in mental function sufficient to affect adequate communication between the patient and personnel taking care of her/him, may affect rehabilitation, or even put her/him at risk of further injury (Wang 2001). Further studies performed with short acting drugs may need to differentiate between the various levels of transient decline in mental function (enough to prevent adequate participation in own care and rehabilitation programme or not) and from delirium, with or without agitation (requiring restraining procedures or potent psychotropic drugs).
Studies included were published between 1977 and 2013 and clinical practice has changed during this period. The vast majority of centres will now preferentially use shorter acting drugs in the hope of decreasing the length of time during which patients may be under the residual influence of the anaesthetic agents. This may influence the person's ability to actively participate in their rehabilitation, and possibly their overall outcome. Therefore, we decided to explore the year when the study was published as a factor of heterogeneity and, indeed, we found a correlation between the effect size for mortality and the year when the study was published (Figure 4). This suggests that a lower mortality rate associated with regional anaesthesia compared with general anaesthesia might have been more pronounced in the oldest trials, before the widespread use of short acting anaesthetic drugs. Many of the included trials are relatively old and may not represent contemporary practice, nor account for the advances in safety in the field of anaesthesia. From 1986 to 2005, the one‐year mortality rate after a hip fracture decreased by 8.8% for women and 20.0% for men (Brauer 2009). This overall decrease in mortality associated with hip fractures probably reflects advances in the global care of this population. Among other factors, a reduction of the delay before surgery, improved surgical devices and movement toward replacement arthroplasty, combined with a push for earlier weight bearing exercise are all possible factors that may have contributed to this higher rate of survival. If the overall rate of mortality is lower, then the number of participants that will be needed to be included to eliminate a difference between regional and general anaesthesia will be higher.
Neuraxial block decreases the incidence of deep venous thrombosis compared to general anaesthesia only when potent antithrombotic agents such as low molecular weight heparin are not used (Analysis 1.9). It is important to note however that none of the three studies included in the present review used clinical symptoms of deep venous thrombosis as an outcome (Brichant 1995; Davis 1981; McKenzie 1984).
In the present review, regional anaesthesia was associated with a higher risk of pulmonary embolism (its statistical significance depended on the technique of analysis used). It is important to note that an absence of event in the general anaesthesia group, such as found here, is quite exceptional. Particluarly considering the fact that the thromboprophylaxis used in these studies was below actual standards for at least four of them: dextran (Berggren 1987), unfractionated heparin (Heidari 2011; Racle 1986), or unspecified (Bigler 1985). The rate of pulmonary embolism found here with regional anaesthesia (1.9%) is consistent with the rate found after lower limb arthoplasty with modern potent thromboprophylaxis: incidence 1.1%, 95% CI 0.3 to 1.9% (Samama 2007). A meta‐analysis performed on the efficacy of various regimen of thromboprophylaxis after hip arthroplasty found that compared with the risk obtained after a placebo (1.51%), only warfarin (0.16%), pneumatic compression (0.26%), and low molecular weight heparin (0.36%) were associated with a significantly lower risk of symptomatic pulmonary embolism (Freedman 2000). Therefore, this finding cannot be considered a clear demonstrated effect of an intervention because the 0% rate of pulmonary embolism found with general anaesthesia and suboptimal prophylaxis is not consistent with the medical literature. We have to attribute this exceptionally low rate to an absence of systematic screening, unclear outcome definitions and/or inadequate period of follow‐up.
Length of hospital stay can be considered as an indirect marker for cost. We did not find a difference in the mean length of hospital stay for hip fracture between these two anaesthetic techniques (Analysis 1.14). The length of hospital stay of patients operated for hip fracture has decreased from a median of 12 days (interquartile range (IQR), 8.0 to 16.0) between 1986 and 1988 to five days (IQR, 4.0 to 12.0) between 2003 and 2005 (Brauer 2009). This reduced length of hospital stay may however be falsely reassuring because it is associated with a decreased percentage of patients returning home after a hip fracture. In the Brauer 2009 study, 34.3% (95% CI 34.0% to 34.6%) of patients were going home with self care after a hip fracture between 1986 and 1988, while only 5.3% (95% CI 5.2% to 5.4%) were doing so between 2003 and 2005. Between 2003 and 2005, 52.8% of patients with hip fracture (95% CI 52.5% to 53.2%) were discharged to a skilled nursing facility. From limited data, we did not find a difference between the two techniques in the percentage of patients returning home after a fracture hip repair (Analysis 1.13).
In the past, operative hypotension associated with neuraxial blocks has been considered a major draw back to the use of neuraxial blocks in the aged population. One can see however, that this problem can be overcome with the use of a small dose unilateral spinal or incremental dose spinal anaesthesia (Analysis 1.16). Epidurals will not affect the incidence of operative hypotension compared to general anaesthesia (Analysis 1.16).
Neuraxial block added to general anaesthesia compared to general anaesthesia alone
The sole study to address this question involved only 20 participants in each group (White 1980). There was no statistically significant difference between techniques for any of the outcome measures reported. Because of the small numbers of participants involved, no conclusions about the lack of difference between the two techniques can be made.
Neuraxial block versus peripheral nerve block
The three included trials involved only 139 participants in total (Cao 2008; de Visme 2000; Eyrolle 1998). The limited results available suggest that the neuraxial anaesthesia is associated with a lower risk of incomplete or unsatisfactory analgesia (RR 0.24, 95% CI 0.12 to 0.47), and this would be particularly true if a posterior lumbar plexus block is used alone, without the addition of a sacral plexus block plus an iliac crest infiltration. Altogether, the information available would suggest that peripheral nerve blocks could be used as the sole anaesthetic technique, but only when both neuraxial block and general anaesthesia pose special risks.
Intravenous ketamine versus general anaesthesia
The sole trial comparing ketamine with general anaesthesia involved only 60 participants (Spreadbury 1980). The numbers of participants were too small to show if the increase in 'unsatisfactory surgical results' in the ketamine group was a significant factor of ketamine use.
Summary of main results
For adults undergoing hip fracture surgery, regional anaesthesia is associated with a decreased incidence of deep venous thrombosis only in the absence of potent thromboprophylaxis. Due to the inclusion of old trials that may not reflect actual clinical practice, an absence of clear definitions for many outcomes, and the small number of participants included for the vast majority of outcomes, we cannot make a clear statement on the presence/absence of difference in outcomes between regional and general anaesthesia for hip fracture repair for any other outcome.
Overall completeness and applicability of evidence
A substantial part of the evidence is derived from old small studies with suboptimal methodology. Rare adverse events related to the anaesthetic techniques cannot be evaluated from these small studies. These studies however, suggest that results obtained with general anaesthesia or neuraxial blocks would be relatively similar.
Quality of the evidence
Details of reasons for upgrading of downgrading the quality of evidence are given in the section effects of interventions (Results).
For neuraxial blocks compared to general anaesthesia, we rated the quality of evidence as very low for mortality at 0 to 30 days, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, decreased rate of deep venous thrombosis in the absence of potent thromboprophylaxis, and return of patient to their own home.
Potential biases in the review process
We included all available RCTs. Those studies were published between 1977 and 2013, thus covering a wide range of clinical practices.
Agreements and disagreements with other studies or reviews
The absence of difference in the mortality rate between neuraxial blockade and general anaesthesia is in agreement with a recent retrospective study (Neuman 2014).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Meta‐regression of mortality at 0‐1 month versus the year when the study was published. The effect size decreases with time: P value = 0.002.
This meta regression plot was not produced in RevMan. The figure was generated automatically by the software, and cannot be amended. The software has expressed the years as decimals.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 1 Mortality ‐ 1 month.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 2 Mortality ‐ 3 months.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 3 Mortality ‐ 6 months.
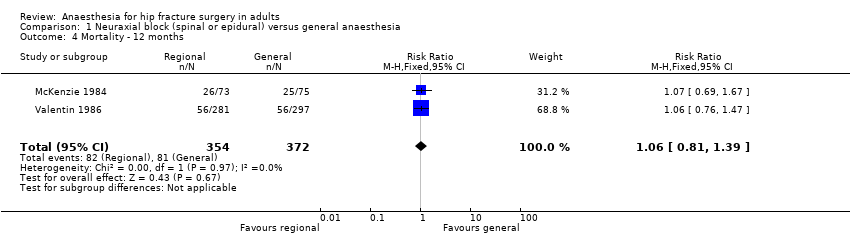
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 4 Mortality ‐ 12 months.
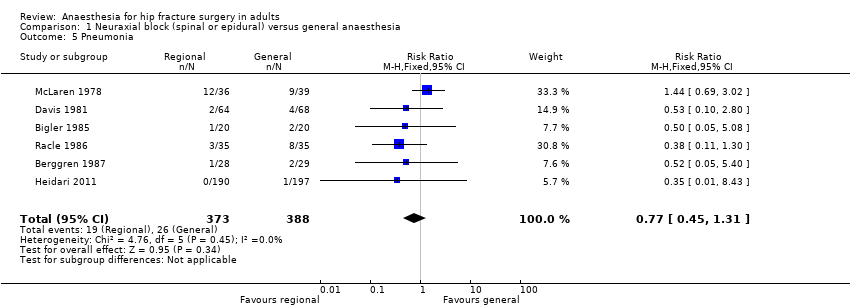
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 5 Pneumonia.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 6 Myocardial infarction.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 7 Cerebrovascular accident.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 8 Acute confusional state.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 9 Deep vein thrombosis.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 10 Congestive cardiac failure.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 11 Acute kidney injury.
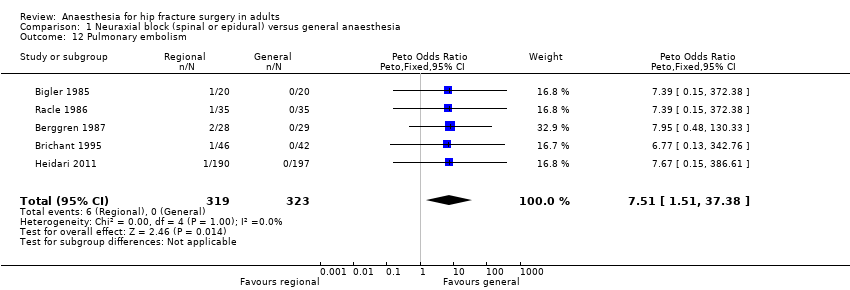
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 12 Pulmonary embolism.
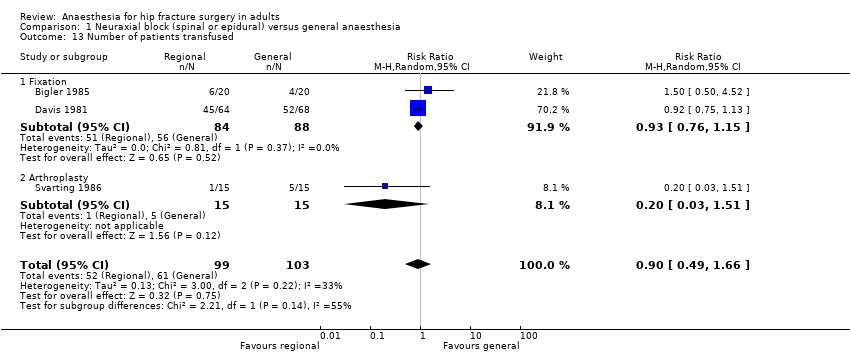
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 13 Number of patients transfused.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 14 Length of hospital stay.

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 15 Length of surgery (minutes).

Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 16 Operative hypotension.

Comparison 2 Regional (spinal or epidural) versus lumbar plexus nerve blocks, Outcome 1 Incomplete or unsatisfactory analgesia.

Comparison 2 Regional (spinal or epidural) versus lumbar plexus nerve blocks, Outcome 2 Urine retention.
| Neuraxial block compared to general anaesthesia for hip fracture repair | ||||||
| Patient or population: Patients with hip fracture repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| General anaesthesia | Neuraxial block | |||||
| Mortality | Study population | RR 0.78 | 2152 | ⊕⊝⊝⊝ | ||
| 80 per 1000 | 62 per 1000 | |||||
| Low | ||||||
| 35 per 1000 | 27 per 1000 | |||||
| High | ||||||
| 95 per 1000 | 74 per 1000 | |||||
| Pneumonia | Study population | RR 0.77 | 761 | ⊕⊝⊝⊝ | ||
| 64 per 1000 | 50 per 1000 | |||||
| Low | ||||||
| 30 per 1000 | 23 per 1000 | |||||
| High | ||||||
| 80 per 1000 | 62 per 1000 | |||||
| Myocardial infarction | Study population | RR 0.89 | 559 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Low | ||||||
| 5 per 1000 | 4 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 44 per 1000 | |||||
| Cerebrovascular accident | Study population | RR 1.48 | 729 | ⊕⊝⊝⊝ | ||
| 8 per 1000 | 12 per 1000 | |||||
| Low | ||||||
| 10 per 1000 | 15 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 74 per 1000 | |||||
| Acute confusional state | Study population | RR 0.85 | 624 | ⊕⊝⊝⊝ | ||
| 177 per 1000 | 150 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 212 per 1000 | |||||
| Deep vein thrombosis | Study population | RR 0.57 | 116 | ⊕⊝⊝⊝ | For this outcome, we retained | |
| 780 per 1000 | 444 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 114 per 1000 | |||||
| High | ||||||
| 900 per 1000 | 513 per 1000 | |||||
| Return of patient to their own home | Study population | RR 0.84 | 130 | ⊕⊝⊝⊝ | ||
| 578 per 1000 | 486 per 1000 | |||||
| Low | ||||||
| 400 per 1000 | 336 per 1000 | |||||
| High | ||||||
| 800 per 1000 | 672 per 1000 | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment and/or blinding of outcome assessors unclear/inadequate in 75% or more of the included studies. | ||||||
| Outcome | Study | Definition | Time point |
| Pneumonia | "treated for" | "during the postoperative period" | |
| Unspecified | "postoperatively" | ||
| Chest X‐Ray in clinical suspicion | Up to four weeks | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| "The clinical criteria adopted as indicating respiratory problems were productive cough, the presence of rhonchi or crepitations on auscultation or abnormalities on chest X‐ray." However the criteria adopted for the diagnosis of a pneumonia ("respiratory infection") are not clearly mentioned | Up to four weeks | ||
| "clinical and radiological criteria" | "in hospital" | ||
| Unspecified | Within four weeks | ||
| Myocardial infarction | EKG and troponin measurement daily for three days, no definition provided | Within one month | |
| Serial preprogrammed EKGs up to postoperative day 10 interpreted by a blinded cardiologist. Q wave | In hospital | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| World Health Organization criteria applied by a blinded investigator | Within one month | ||
| Congestive cardiac failure | "treated for" | "during the postoperative period" | |
| "acute heart failure" | Within one month | ||
| "cardiac decompensation" | "postoperatively" | ||
| "life‐threatening complications" "congestive heart failure" | Within four weeks | ||
| "diagnosed by the consultant specialist" | "postoperative" | ||
| "episode of congestive heart failure" | In hospital | ||
| Cerebrovascular accident | "stroke" | All patients developed their stroke on postoperative day one | |
| "stroke" | Within one month | ||
| "Neurological sequelae", apoplexy for the sole event reported | "postoperatively" | ||
| "cerebrovascular accident" | Within four weeks | ||
| "cerebrovascular accident" "diagnosed by the consultant specialist" | "postoperative" | ||
| "cerebrovascular accident" | In hospital | ||
| Acute confusional state | Diagnostic and Statistical Manual of Mental Disorders (DSM‐Ill) as criteria for acute confusional state | Within seven days (the period 0‐8 hours after the surgery was excluded) | |
| "Mental confusion" | "postoperatively" | ||
| Mini Mental State Examination test decreased 2 points from baseline | Within seven days | ||
| Mini Mental Status Examination lower than 5 | Between the third and fifth postoperative day | ||
| Cognitive dysfunction based on time, person, and place disorientation | Up to postoperative day two | ||
| Delirium was judged by floor nurse, using the Inoue's confusion assessment method diagnosis algorithm | Up to postoperative day four | ||
| Confusion with agitation | In hospital | ||
| Unspecified | Within four weeks | ||
| Renal failure (or acute kidney injury) | "acute renal failure" | Within four weeks | |
| Blood creatinine > 135 micromol/Liter | In hospital | ||
| Deep vein thrombosis | Systematic bilateral contrast venography | Postoperative day ten | |
| 125‐iodine fibrinogen uptake test performed daily for seven days | Within seven days | ||
| "deep veins thrombosis" "diagnosed by the consultant specialist" | "postoperative" | ||
| "thrombosis" | Within seven days | ||
| Systematic venography | Between postoperative day seven and ten | ||
| "deep vein thrombosis" | Within four weeks | ||
| Pulmonary embolism | "pulmonary embolism" | "postoperatively" | |
| "pulmonary embolus" | "postoperatively" | ||
| Pulmonary venous angiogram or ventilation‐perfusion lung scanning on clinical suspicion | Unclear | ||
| "pulmonary emboli" "diagnosed by the consultant specialist" | "postoperatively" | ||
| Clinical suspicion confirmed with angiography | In hospital | ||
| Unsatisfactory surgical results | Either an unstable fixation of the fracture by nail and plate or the dislocation of a prosthesis, which required bedrest on traction and prevented early mobilization | In hospital | |
| Operative hypotension | Decrease > 30% from baseline for systolic arterial blood pressure | Intraoperative | |
| Decrease of 30% from baseline for arterial blood pressure | Intraoperative | ||
| Requiring the administration of a sympathomimetic | Intraoperative | ||
| Decrease in systolic arterial pressure 20% from baseline | Intraoperative | ||
| Decrease of 40 mmHG in systolic arterial blood pressure | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 33% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 50% from baseline | Intraoperative | ||
| Decrease in mean arterial blood pressure of 25% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure of 20% from baseline | Intraoperative | ||
| Decrease in systolic arterial blood pressure > 30% from baseline | Intraoperative | ||
| Urine retention | "urinary retention" | "postoperative" | |
| "that required catheterization" | In hospital | ||
| EKG: electrocardiogram | |||
| Study | Sedative drugs for participants of the regional blockade group | Anaesthetic agents for general anaesthesia |
| Premedication: Meperidine No other sedative drugs mentioned as routinely administered for the surgery. | Premedication: Meperidine Induction: Thiopental and atropine Maintainance: Nitrous oxide, halothane and succinylcholine infusion | |
| None mentioned | Subgroup 1 Induction: Propofol Maintainance: Propofol infusion (for a bispectral index value of 50) and remifentanil Subgroup 2 Induction: Sevoflurane Maintainance: Sevoflurane (for a bispectral index value of 50) and remifentanil | |
| None mentioned | Induction: Propofol Maintainance: Nitrous oxide, isoflurane, fentanyl (intermittent injections) plus an atracurium infusion | |
| Premedication: Pethidine Small amounts of diazepam if needed | Premedication: Pethidine Induction: Diazepam and atropine Maintainance: Nitrous oxide, fentanyl and pancuronium | |
| Premedication: Pethidine Diazepam for mild sedation | Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, thiopental and pethidine | |
| Not mentioned | According to local practice | |
| Premedication: Tamazepam or pethidine No drug supplementation during the surgery | Premedication: Tamazepam or pethidine Induction: Thiopental or propofol Maintainance: Nitrous oxide, isoflurane or enflurane and atracurium 0.5 mg/kg one dose | |
| Midazolam and fentanyl if required | ||
| One dose of fentanyl before the block No other sedative drug routinely administered for the surgery. | Induction: Sevoflurane Maintainance: Nitrous oxide, sevoflurane | |
| Premedication: Hydroxyzine and atropine None mentioned for the surgery | Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide plus 1) Thiopental and dextromoramide or 2) methoxyflurane. One dose of pancuronium in some participants | |
| Ketamine 20‐25 mg (for eight participants) before the spinal Ketamine at unspecified total doses during the surgery (two participants) or 25 mg at skin closure (two participants) Diazepam (mean dose 9 mg; range 0‐35 mg) | Induction: Diazepam (mean dose 9.5 mg; range 2.5 to 30 mg) Maintainance: Nitrous oxide, fentanyl and pancuronium | |
| Benzodiazepine (optional) | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl and non‐depolarizing neuromuscular blocking agent | |
| Alfentanil before the block and as required during the surgery. No mandatory sedative drugs mentioned. One patient in the continuous peripheral nerve block received "sedation repeatedly". | ||
| Propofol as required | ||
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl, halothane and one dose of pancuronium | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, isoflurane, fentanyl and vecuronium | |
| Not reported | Not reported | |
| Premedication: Pethidine None mentioned for the surgery | Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of atracurium | |
| No sedative drug | Induction: Propofol Maintainance: Nitrous oxide, fentanyl, sevoflurane and one dose of vecuronium | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, dextromoramide and enflurane | |
| Small doses of diazepam | Induction: Althesin Maintainance: Nitrous oxide and halothane | |
| Althesin and nitrous oxide; arousable by ear lobe pressure | Induction: Althesin Maintainance: Nitrous oxide, fentanyl and pancuronium one dose | |
| None mentioned | Induction: Propofol Maintainance: Sevoflurane, remifentanil and one dose of cisatracurium | |
| Premedication: Hydroxyzine and atropine Flunitrazepam, verbal contact possible | Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of vecuronium | |
| No group with regional anaesthesia alone | Ketamine group Induction and maintenance: Ketamine and diazepam (2.5 to 10 mg) Relaxant group Technique at the discretion of the attending anaesthesiologist | |
| Premedication: Pethidine and atropine None mentioned for the surgery | Premedication: Pethidine and atropine Induction: Thiopental Maintainance: Nitrous oxide, fentanyl (repeated injections) and one dose of pancuronium | |
| Not reported | Not reported | |
| Not reported | Induction: Not reported Maintainance: Nitrous oxide, isoflurane and fentanyl | |
| Premedication: Pethidine and promethazine in some of the participants Small doses of diazepam and fentanyl | Premedication: Pethidine and promethazine in some of the participants Subgroup 1 Induction: Thiopental or not Maintainance: Nitrous oxide, enflurane and gallamine (not all participants) Subgroup 2 Induction: Not clearly mentioned Maintainance: Nitrous oxide, droperidol, fentanyl and gallamine | |
| None mentioned | Induction: Thiopental Maintainance: Nitrous oxide and sevoflurane | |
| Premedication: Diazepam Althesin, nitrous oxide and fentanyl (spontaneous breathing) for the two subgroups | Premedication: Diazepam Induction: Thiopental Maintainance: Nitrous oxide, halothane and fentanyl |
| Comparison: Neuraxial block versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Patient returned to their own home | 130 | RR | 0.84 | 0.61 | 1.16 | |
| Urine retention | 57 | RR | 0.86 | 0.30 | 2.51 | |
| Comparison: Neuraxial block added to general anaesthesia compared to general anaesthesia alone | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Pneumonia | 30 | RR | 0.80 | 0.20 | 3.20 | |
| Acute confusional state | 30 | RR | 1.00 | 0.16 | 6.09 | |
| Deep vein thrombosis | 30 | RR | 0.17 | 0.01 | 3.94 | |
| Length of surgery (minutes) | 30 | MD | 0.00 | ‐17.96 | 17.96. | |
| Comparison: Neuraxial block versus peripheral nerve block | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Acute confusional state | 29 | RR | 0.89 | 0.35 | 2.28 | |
| Operative hypotension | 50 | RR | 6.00 | 2.02 | 17.83 | |
| Length of surgery (minutes) | 29 | MD | 17.00 | ‐0.76 | 34.76 | |
| Comparison: Intravenous ketamine alone (without neuromuscular blocking agent) versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Unsatisfactory surgical results defined as unstable fixation or prosthesis dislocation | 60 | RR | 2.33 | 0.67 | 8.18 | |
| Mortality | 60 | RR | 1.00 | 0.46 | 2.17 | |
| Patient returned home | 60 | RR | 0.95 | 0.66 | 1.38 | |
| Length of hospital stay | 39* | MD | 12.00 | 5.63 | 18.37 | |
| CI: confidence interval; MD: mean difference; RR: risk ratio *: Mean duration of admission refers only to those patients who were discharged home | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality ‐ 1 month Show forest plot | 11 | 2152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.06] |
| 2 Mortality ‐ 3 months Show forest plot | 5 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.55, 1.08] |
| 3 Mortality ‐ 6 months Show forest plot | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 4 Mortality ‐ 12 months Show forest plot | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 5 Pneumonia Show forest plot | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 6 Myocardial infarction Show forest plot | 4 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.22, 3.65] |
| 7 Cerebrovascular accident Show forest plot | 6 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.46, 4.83] |
| 8 Acute confusional state Show forest plot | 6 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 9 Deep vein thrombosis Show forest plot | 4 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.91] |
| 9.1 No prophylaxis or early mobilization only | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.78] |
| 9.2 Low molecular weight heparin | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.84] |
| 9.3 Standard heparin | 1 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Congestive cardiac failure Show forest plot | 6 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.96] |
| 11 Acute kidney injury Show forest plot | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.18, 5.83] |
| 12 Pulmonary embolism Show forest plot | 5 | 642 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.51 [1.51, 37.38] |
| 13 Number of patients transfused Show forest plot | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.49, 1.66] |
| 13.1 Fixation | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.15] |
| 13.2 Arthroplasty | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.51] |
| 14 Length of hospital stay Show forest plot | 4 | 1143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.05, 0.65] |
| 15 Length of surgery (minutes) Show forest plot | 12 | 973 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐8.50, 3.04] |
| 16 Operative hypotension Show forest plot | 12 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.35] |
| 16.1 Unilateral | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.37, 0.89] |
| 16.2 Bilateral, incremental doses | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.05, 0.78] |
| 16.3 Bilateral single shot | 8 | 828 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.87, 1.95] |
| 16.4 Epidural | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.50, 2.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incomplete or unsatisfactory analgesia Show forest plot | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.11, 0.45] |
| 1.1 Neuraxial (spinal/epidural) block versus lumbar plexus block | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.49] |
| 1.2 Neuraxial (spinal/epidural) block versus lumbar plexus, sacral and iliac crest block | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.02] |
| 2 Urine retention Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.0 [1.90, 103.00] |

