Óxido nítrico inhalado para la insuficiencia respiratoria en recién nacidos prematuros
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multi‐centre trial | |
| Participants | 582 infants < 1250 grams on assisted ventilation at 7‐21 days (or, if < 800 grams, on CPAP) | |
| Interventions | Inhaled NO at 20 ppm initial dose for 48‐96 hours; the dose was subsequently decreased to 10, 5 and 2 ppm at weekly intervals, with a minimum treatment duration of 24 days | |
| Outcomes | Survival without BPD at 36 weeks' postmenstrual age Secondary outcomes included duration of oxygen therapy and duration of hospitalisation. In addition, investigators prospectively evaluated the need for hospitalisation and respiratory support, including mechanical ventilation, continuous positive airway pressure and oxygen supplementation at 40, 44, 52 and 60 weeks' postmenstrual age. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in permuted blocks at study centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Masking of intervention: yes |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Funding Source | Low risk | Funded by a government agency (NICHD); study gas provided by industry (Ikaria) |
| Methods | Single‐centre trial | |
| Participants | 40 preterm infants ventilated with severe RDS with FiO2 > 0.5 and arterial‐alveolar oxygen ratio < 0.15, despite surfactant treatment | |
| Interventions | iNO at 10 ppm for 4 hours followed by 6 ppm compared with no treatment. Weaning started at 72 hours or when the infant was extubated, or when FiO2 was < 0.3 with mean airway pressure < 8 cmH2O | |
| Outcomes | The primary endpoint was death or BPD. Bronchopulmonary dysplasia was defined as oxygen requirement at 36 weeks' postmenstrual age. Secondary endpoints were evaluation of ventilation changes during iNO therapy, duration of oxygen treatment, NCPAP and mechanical ventilation, incidence of patent ductus arteriosus (PDA), pulmonary hypertension, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), necrotising enterocolitis (NEC), sepsis and length of stay in the intensive care unit and in hospital. | |
| Notes | Study terminated after 40 infants enrolled. Initially planned to include 26 per group. Unplanned interim analysis was performed because of an impression that the results were significant. No evidence indicated that the analysis was adjusted to account for potential multiple looks at the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting, but protocol not available |
| Other bias | High risk | Early termination of trial due to impression of an effect |
| Funding Source | Unclear risk | Funding source unclear |
| Methods | Multi‐centre trial | |
| Participants | 800 infants between 24 weeks' and 28 weeks' gestation and 6 days enrolled at less than 24 hours of age. If intubated, they had to have received surfactant and could be enrolled if on CPAP requiring > 30% oxygen. Patients were ineligible if they required more than 50% O2 to maintain saturation over 85% on a mean airway pressure ≥ 8 cmH2O. | |
| Interventions | Inhaled NO at 5 ppm for at least 7 and a maximum of 21 days | |
| Outcomes | Primary outcome was survival without BPD at 36 weeks' postmenstrual age. Secondary outcome was survival without severe brain injury on head ultrasonography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralised interactive Web‐based enrolment and randomisation system |
| Blinding (performance bias and detection bias) | Low risk | Masking of intervention: yes Masking of outcome assessment: yes |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | High risk | Funded by industry (Ikaria), initiated by investigators |
| Methods | Multi‐centre trial | |
| Participants | 860 infants < 32 weeks enrolled but not eligible for study gas unless they developed hypoxic respiratory failure (i.e. need for mechanical ventilation, FiO2 > .40, and arterial alveolar O2 ratio < 0.22 at 6 to 48 hours of age; n = 145 | |
| Interventions | Inhaled NO was started at 5 ppm, with adjustments allowed depending on response up to a maximum of 10 ppm. Eventually, 61 were treated with iNO and 84 were given the control intervention. Participants were allowed to receive iNO in either group if they developed refractory hypoxaemia. | |
| Outcomes | Primary outcome was survival to 28 days without death, need for oxygen, IVH > grade 1 or refractory hypoxaemia defined as need for 100% oxygen with PaO2 < 50. Secondary outcomes included incidence and severity of IVH and periventricular leukomalacia (PVL), BPD or steroid treatment and pulmonary haemorrhage, patent ductus arteriosus (PDA), necrotising enterocolitis and nosocomial infection. | |
| Notes | Open‐label iNO provided to all infants when they met refractory hypoxaemia criteria ‐ 20 infants received treatment before 6 hours and were not included; 28 control infants received open‐label iNO after the randomised intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, blocked central randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | High risk | Masking of intervention: no Masking of outcome assessment: no |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | Registration documents or protocol not found |
| Funding Source | Low risk | Supported by local sources (University of Nantes) and industry (Air Liquide) |
| Methods | Multi‐centre trial | |
| Participants | 108 preterm infants (< 34 weeks) less than 28 days of age, with "severe respiratory failure" | |
| Interventions | Inhaled NO usually at 5 ppm up to 40 ppm (n = 55) or no supplemental gas (n = 53) | |
| Outcomes | Primary outcomes were (1) death or severe disability at 1 year corrected postnatal age; and (2) death or continued oxygen need at expected date of birth. Secondary outcomes included length of stay in hospital; length of time on supplemental oxygen; length of time on ventilatory support; pneumothorax; other pulmonary air leak; pulmonary haemorrhage; major cerebral abnormality; necrotising enterocolitis; patent ductus arteriosus needing medical treatment; treatment of retinopathy of prematurity; infection; and age at which full oral feeding was established. | |
| Notes | Initially planned 200 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | High risk | Masking of intervention: no Masking of outcome assessment: no |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Unclear risk | Recruited half of planned sample size in the 2‐year time frame |
| Funding Source | Low risk | Funded by government agency (MRC, UK) |
| Methods | Multi‐centre trial | |
| Participants | 80 preterm infants (≤ 34 weeks) < 7 days of age, with a/AO2 < 0.1 on 2 blood gases after surfactant treatment | |
| Interventions | Inhaled NO at 5 ppm (n = 48) or no supplemental gas (n = 32) for 7 days, after which "trials off" were allowed. Maximum treatment duration was 14 days. | |
| Outcomes | Primary outcome was survival. Bronchopulmonary dysplasia, intraventricular haemorrhage and duration of ventilation were secondary outcomes. | |
| Notes | Initially planned 210 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Masked intervention |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration document found |
| Other bias | High risk | Study terminated early after planned first interim analysis, as little difference in outcomes was apparent |
| Funding Source | Low risk | Funded by government agency (NIH) and in part by industry (iNOtherapeutics) |
| Methods | Multi‐centre trial | |
| Participants | 793 preterm infants < 34 weeks, respiratory failure needing assisted ventilation in first 48 hours | |
| Interventions | iNO at 5 ppm (n = 398) or no iNO (n = 395) for 21 days or until extubation | |
| Outcomes | Primary outcome was death or bronchopulmonary dysplasia. Secondary outcomes included severe intraventricular haemorrhage, periventricular leukomalacia and ventriculomegaly. | |
| Notes | Baseline and follow‐up cranial ultrasonography was required. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central stratified, blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | Low risk | Blinded trial |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | Low risk | Funded by government agency (NHLBI) and in part by industry (iNOtherapeutics) |
| Methods | Multi‐centre parallel‐group randomised trial | |
| Participants | 124 preterm infants with birth weight of 500 to 1250 grams, receiving oxygen by non‐invasive means at < 72 hours of age | |
| Interventions | iNO at 10 ppm (to give effective concentration ≥ 5 ppm) or placebo, for at least 2 weeks and until 30 weeks' postmenstrual age | |
| Outcomes | Death or BPD, IVH, retinopathy of prematurity, necrotising enterocolitis, treatment of infants with PDA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial |
| Blinding (performance bias and detection bias) | Low risk | Masked trial |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | Low risk | Funded by government agency (NHLBI), funded in part by industry (Ikaria supplied gases) |
| Methods | Multi‐centre parallel‐group randomised trial | |
| Participants | 85 preterm infants (< 33 weeks) with OI of 12.5 to 30 at < 7 days | |
| Interventions | 10 ppm inhaled NO (n = 40) or control (n = 45). Open‐label treatment with NO allowed in controls if OI > 30 | |
| Outcomes | Primary outcome was decrease in OI after 2 hours of therapy. | |
| Notes | Initially planned 360 infants across both gestational age strata | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised phone randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration found |
| Other bias | Unclear risk | Stopped early because of slowing enrolment |
| Funding Source | Low risk | Funded by local agency, supported in part by industry (Air Liquide) |
| Methods | Single‐centre trial | |
| Participants | 207 infants < 34 weeks, < 72 hours of age, intubated and ventilated for RDS, birth weight < 2000 grams | |
| Interventions | Randomised to iNO (N = 105) (starting at 10 ppm for 1 day, then 5 ppm for 6 days; thereafter weaned by 1 ppm, stopped if extubated) vs control (N = 102); HFOV (N= 102) vs CMV (N = 105) | |
| Outcomes | Primary outcome was a decrease in death or BPD at 36 weeks. | |
| Notes | Factorial 2 × 2 design comparing high‐frequency ventilation vs conventional treatment and iNO vs placebo gas | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Masked allocation |
| Blinding (performance bias and detection bias) | Low risk | Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration documents found |
| Funding Source | High risk | Funded by industry (Ikaria), investigator initiated |
| Methods | Single‐centre trial | |
| Participants | 34 infants weighing < 2000 grams, ventilated after surfactant with an arterial catheter at < 72 hours of age. Also required to satisfy a severity of illness criterion. OI > 4 for birth weight < 1000 grams, > 6 for birth weight 1001‐1250 grams, > 8 for 1251‐1500 grams, > 10 for 1501‐1750 grams and > 12 for 1751‐2000 grams | |
| Interventions | iNO at 20 ppm or standard care, trial of weaning after 72 hours, maximum duration 7 days | |
| Outcomes | Primary outcome was severe intraventricular haemorrhage (grade 3 or 4). | |
| Notes | Performed as a preliminary pilot study, before Schreiber 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Unclear risk | Masking of allocation: unclear |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol found |
| Funding Source | Unclear risk | Not stated |
| Methods | Single‐centre randomised trial | |
| Participants | 65 preterm infants with birth weight < 1500 grams or gestational age < 32 weeks, intubated with OI ≥ 25 | |
| Interventions | iNO initially at a dose of 5ppm, could be increased to 20 ppm in cases of poor response, or decreased if good response obtained; duration of therapy not clear according to protocol, but mean duration of receipt of iNO was 4.9 days | |
| Outcomes | Primary outcome variable was OI 24 hours after randomisation. Secondary outcomes included mortality, BPD, intracranial haemorrhage, patent ductus arteriosus and retinopathy of prematurity. | |
| Notes | Not all of the infants received surfactant: 23 of 32 iNO treated and 24 of 33 controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation procedures provided |
| Blinding (performance bias and detection bias) | High risk | Masking of intervention: no Masking of outcome assessment: no |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No registration documents or protocol found |
| Funding Source | Unclear risk | Unclear |
| Methods | Single‐centre randomised comparison of iNO, dexamethasone, combined therapy and control, using a 2 × 2 factorial design | |
| Participants | 42 preterm infants less than 32 weeks' gestation with "high risk" of developing BPD | |
| Interventions | iNO initially administered at 20 ppm and weaned if effective (n = 20) or control (n = 22). Dexamethasone at 1 mg/kg/d for 3 days, followed by 0.5 mg/kg/d for 3 days (n = 21) (3 infants received a lower dose), or no steroids (n = 21) | |
| Outcomes | Primary outcome was survival without bronchopulmonary dysplasia. Secondary outcomes included duration of ventilation, intraventricular haemorrhage and other neonatal complications. | |
| Notes | Initially planned 88 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | High risk | Masking of intervention: no Masking of outcome measurement: no |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol found |
| Other bias | High risk | Terminated early because frequency of adverse primary outcome was close to 100% in all groups |
| Funding Source | Low risk | Government agency and local funds, some support from industry |
| Methods | Multi‐centre trial | |
| Participants | 420 preterm infants, < 34 weeks, OI ≥ 10 on 2 blood gases 30 minutes to 12 hours apart. ≥ 4 hours after surfactant | |
| Interventions | iNO initially at 5 ppm to 10 ppm (210) or placebo (210) (if no response at 10 ppm, study gas was stopped). Weaning ≥ 10 hours after initiation. Maximum duration was 336 hours. | |
| Outcomes | Primary outcome was reduced death or BPD at 36 weeks. Secondary outcomes were grade 3 or 4 intraventricular haemorrhage or periventricular leukomalacia, number of days of assisted ventilation and oxygen use, length of hospitalisation and threshold retinopathy of prematurity. | |
| Notes | Initially planned 220 infants per arm; stopped by the data monitoring committee because of apparent increase in severe IVH with no evidence of benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked central randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) | Low risk | Masking of intervention: yes |
| Incomplete outcome data (attrition bias) | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | High risk | Study terminated a little early because of an increase in severe IVH in the intervention group |
| Funding Source | Low risk | Support from government agency (NICHHD), partial support from industry (Ikaria) |
| Methods | Multi‐centre trial | |
| Participants | 29 infants at less than 34 weeks' gestation with birth weight > 1500 grams; ventilated with OI > 15 on 2 consecutive blood gases between 30 minutes and 12 hours apart | |
| Interventions | iNO initially at 5 ppm to 10 ppm (210) or placebo (210) (if no response at 10 ppm, study gas was stopped). Weaning ≥ 10 hours after initiation. Maximum duration was 14 days. | |
| Outcomes | Primary outcome was reduced death or BPD at 36 weeks. Secondary outcomes were grade 3 or 4 intraventricular haemorrhage or periventricular leukomalacia, number of days of assisted ventilation and oxygen use, length of hospitalisation and threshold retinopathy of prematurity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation, stratified and blocked |
| Allocation concealment (selection bias) | Low risk | Masked allocation |
| Blinding (performance bias and detection bias) | Low risk | Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Funding Source | Low risk | Government agency (NICHHD), partial support from industry (Ikaria) |
| Methods | Single‐centre randomised trial of iNO vs no treatment gas | |
| Participants | 60 preterm infants at less than 34 weeks, receiving mechanical ventilation or CPAP, with OI ≥ 11 2 hours after surfactant therapy | |
| Interventions | iNO initially at 5 ppm or placebo. Duration of therapy 7 days or until ventilation withdrawal | |
| Outcomes | Primary outcome was change in OI status over different times during first 3 days of therapy. Secondary outcomes included mortality, BPD, intraventricular haemorrhage and other neonatal complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | High risk | No details of randomisation procedures provided |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol found |
| Funding Source | Unclear risk | Not stated |
| Methods | Multi‐centre parallel‐group randomised trial | |
| Participants | 451 preterm infants, < 1250 grams and < 30 weeks, at 5 to 14 days of age, on invasive ventilation, or on non‐invasive support if they weighed < 800 grams | |
| Interventions | iNO at 20 ppm for 3 to 4 days, then 10 ppm for 10 days, then 5 ppm until 24 days | |
| Outcomes | Survival without BPD, duration of respiratory support and hospitalisation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial |
| Blinding (performance bias and detection bias) | Low risk | Masked trial |
| Incomplete outcome data (attrition bias) | Low risk | No significant attrition |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | High risk | Industry initiated and funded (Ikaria) |
a/AO2: arterial‐alveolar oxygen ratio.
BPD: bronchopulmonary dysplasia.
CMV: cytomegalovirus.
CPAP: continuous positive airway pressure.
FiO2: fraction of inspired oxygen.
HVOF: high‐velocity oxygen fuel.
iNO: inhaled nitric oxide.
IVH: intraventricular haemorrhage.
MRC: Medical Research Council.
NEC: necrotising enterocolitis.
NICHD: Eunice Kennedy Shriver National Institute of Child Health and Human Development.
NO: nitric oxide.
PDA: patent ductus arteriosus.
PVL: periventricular leukomalacia.
RDS: respiratory distress syndrome.
ROP: retinopathy of prematurity.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This was a randomised controlled trial of 50 infants with pulmonary hypertension, 16 of whom were at less than 36 weeks' gestation. The definition of prematurity is not the same as that used in this review, and in any case, it is not possible to extract data that refer solely to preterm infants. | |
| No untreated control group was included. Short‐term randomised cross‐over trial of response to inhaled nitric oxide among infants on continuous positive airway pressure | |
| No untreated control group was included. This was a randomised comparison of 5 ppm and 20 ppm for 15 minutes in preterm infants. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death before discharge Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Inhaled NO compared with control, Outcome 1 Death before discharge. | ||||
| 1.1 Studies with entry before 3 days based on oxygenation | 10 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 1.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.71] |
| 1.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 2 Death before 36 weeks' postmenstrual age Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 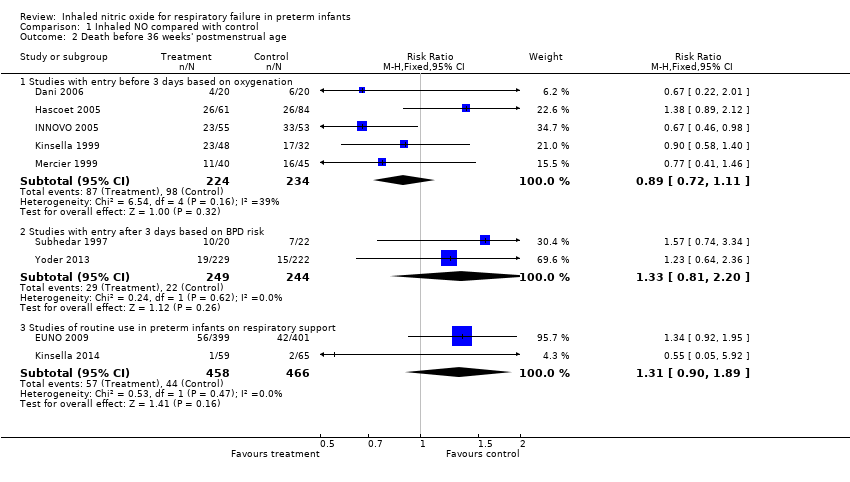 Comparison 1 Inhaled NO compared with control, Outcome 2 Death before 36 weeks' postmenstrual age. | ||||
| 2.1 Studies with entry before 3 days based on oxygenation | 5 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.11] |
| 2.2 Studies with entry after 3 days based on BPD risk | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.81, 2.20] |
| 2.3 Studies of routine use in preterm infants on respiratory support | 2 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.89] |
| 3 Bronchopulmonary dysplasia among survivors at 36 weeks Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 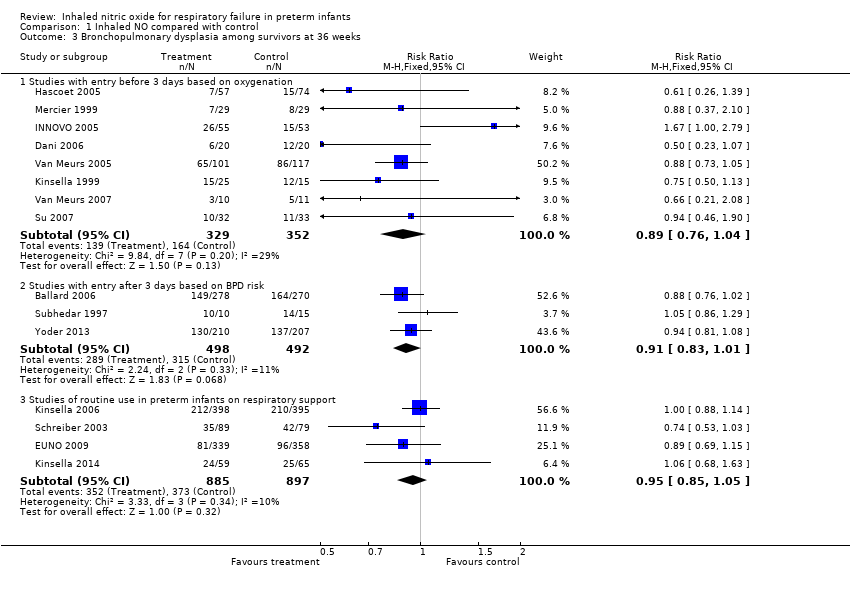 Comparison 1 Inhaled NO compared with control, Outcome 3 Bronchopulmonary dysplasia among survivors at 36 weeks. | ||||
| 3.1 Studies with entry before 3 days based on oxygenation | 8 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 3.2 Studies with entry after 3 days based on BPD risk | 3 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.01] |
| 3.3 Studies of routine use in preterm infants on respiratory support | 4 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.05] |
| 4 Death or bronchopulmonary dysplasia at 36 weeks Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 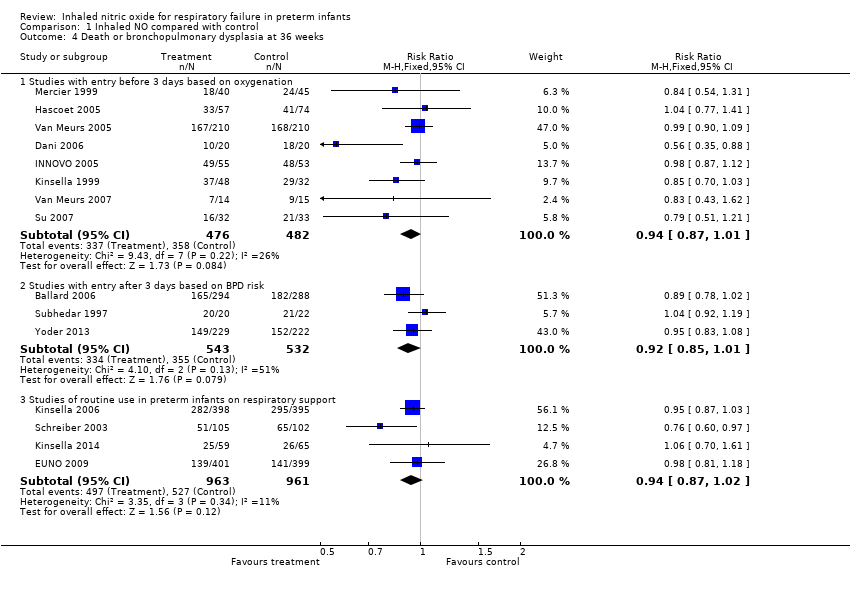 Comparison 1 Inhaled NO compared with control, Outcome 4 Death or bronchopulmonary dysplasia at 36 weeks. | ||||
| 4.1 Studies with entry before 3 days based on oxygenation | 8 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.01] |
| 4.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.01] |
| 4.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 5 Intraventricular haemorrhage (all grades) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 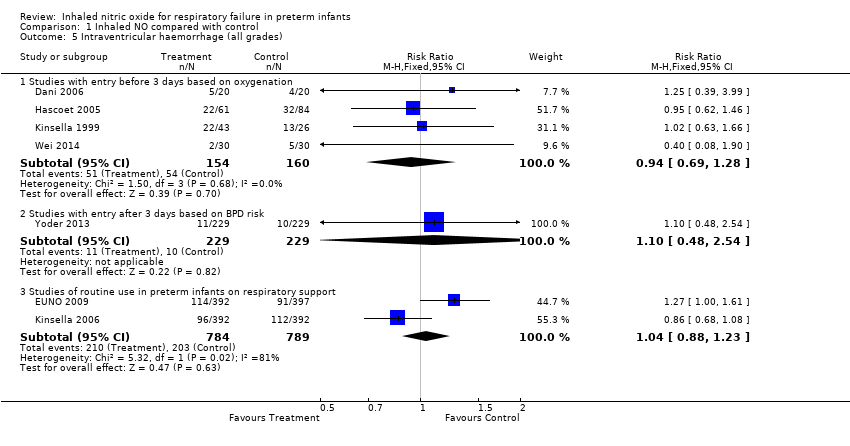 Comparison 1 Inhaled NO compared with control, Outcome 5 Intraventricular haemorrhage (all grades). | ||||
| 5.1 Studies with entry before 3 days based on oxygenation | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.28] |
| 5.2 Studies with entry after 3 days based on BPD risk | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.48, 2.54] |
| 5.3 Studies of routine use in preterm infants on respiratory support | 2 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 6 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Inhaled NO compared with control, Outcome 6 Intraventricular haemorrhage (grade 3 or 4). | ||||
| 6.1 Studies with entry before 3 days based on oxygenation | 6 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.98, 1.47] |
| 6.2 Studies of routine use in preterm infants on respiratory support | 4 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7 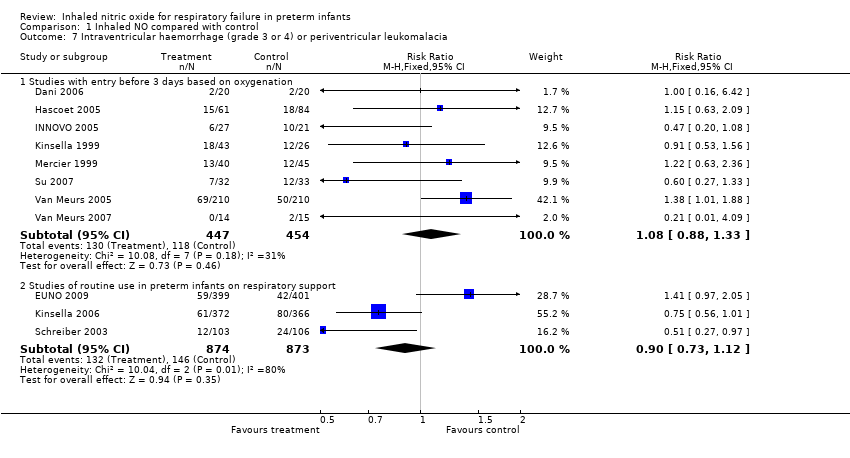 Comparison 1 Inhaled NO compared with control, Outcome 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia. | ||||
| 7.1 Studies with entry before 3 days based on oxygenation | 8 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 7.2 Studies of routine use in preterm infants on respiratory support | 3 | 1747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 8 Neurodevelopmental disability Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Inhaled NO compared with control, Outcome 8 Neurodevelopmental disability. | ||||
| 8.1 Studies with entry before 3 days based on oxygenation | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 8.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 8.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 9 Bayley MDI or PDI < ‐2 SD Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 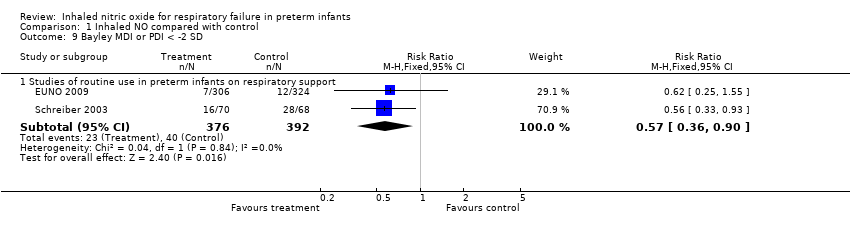 Comparison 1 Inhaled NO compared with control, Outcome 9 Bayley MDI or PDI < ‐2 SD. | ||||
| 9.1 Studies of routine use in preterm infants on respiratory support | 2 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.90] |
| 10 Cerebral palsy Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 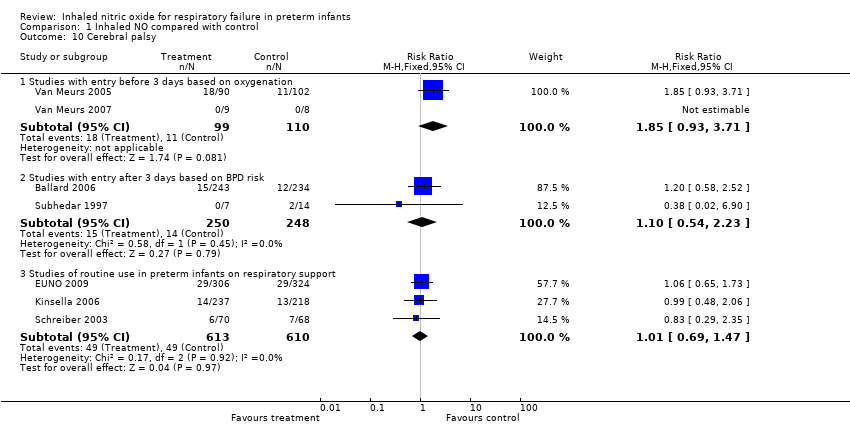 Comparison 1 Inhaled NO compared with control, Outcome 10 Cerebral palsy. | ||||
| 10.1 Studies with entry before 3 days based on oxygenation | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.93, 3.71] |
| 10.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.54, 2.23] |
| 10.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 11 Severe retinopathy of prematurity (≥stage 3) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11 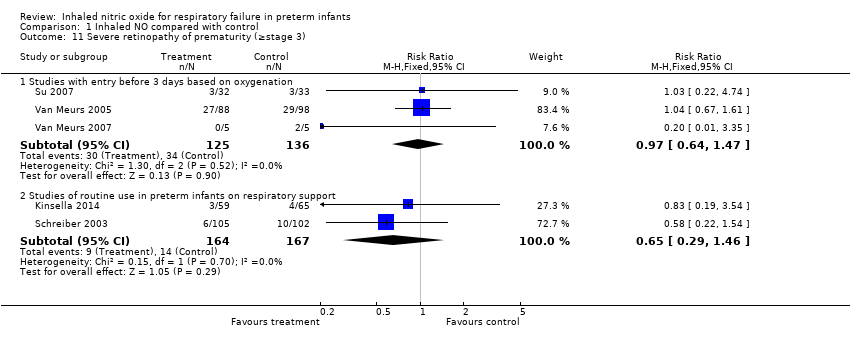 Comparison 1 Inhaled NO compared with control, Outcome 11 Severe retinopathy of prematurity (≥stage 3). | ||||
| 11.1 Studies with entry before 3 days based on oxygenation | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.47] |
| 11.2 Studies of routine use in preterm infants on respiratory support | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
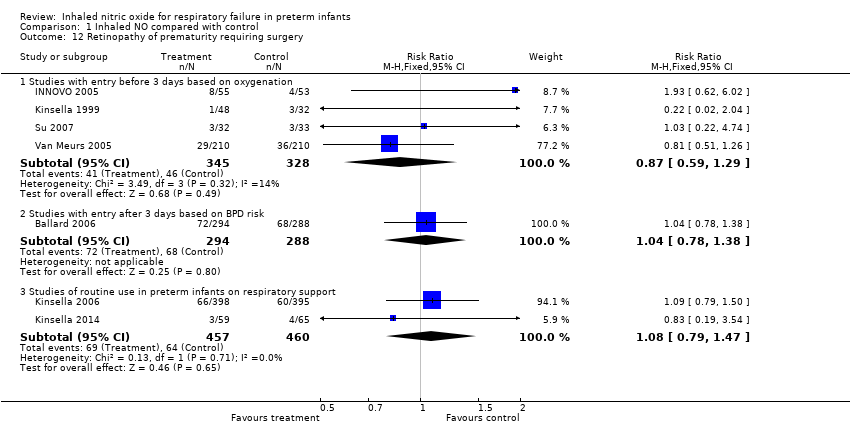
| 12 Retinopathy of prematurity requiring surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Inhaled NO compared with control, Outcome 12 Retinopathy of prematurity requiring surgery. | ||||
| 12.1 Studies with entry before 3 days based on oxygenation | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 12.2 Studies with entry after 3 days based on BPD risk | 1 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 12.3 Studies of routine use in preterm infants on respiratory support | 2 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.47] |

Study flow diagram: review update.
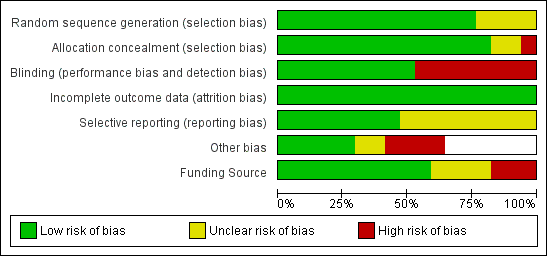
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inhaled NO compared with control, Outcome 1 Death before discharge.

Comparison 1 Inhaled NO compared with control, Outcome 2 Death before 36 weeks' postmenstrual age.

Comparison 1 Inhaled NO compared with control, Outcome 3 Bronchopulmonary dysplasia among survivors at 36 weeks.

Comparison 1 Inhaled NO compared with control, Outcome 4 Death or bronchopulmonary dysplasia at 36 weeks.

Comparison 1 Inhaled NO compared with control, Outcome 5 Intraventricular haemorrhage (all grades).
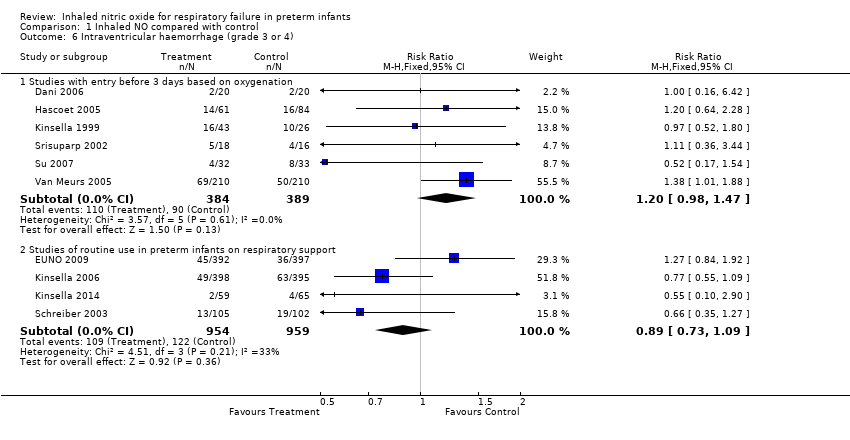
Comparison 1 Inhaled NO compared with control, Outcome 6 Intraventricular haemorrhage (grade 3 or 4).

Comparison 1 Inhaled NO compared with control, Outcome 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia.
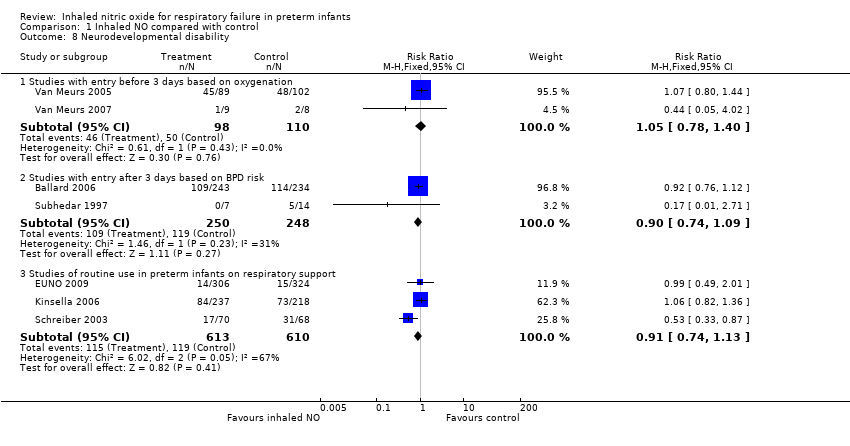
Comparison 1 Inhaled NO compared with control, Outcome 8 Neurodevelopmental disability.

Comparison 1 Inhaled NO compared with control, Outcome 9 Bayley MDI or PDI < ‐2 SD.
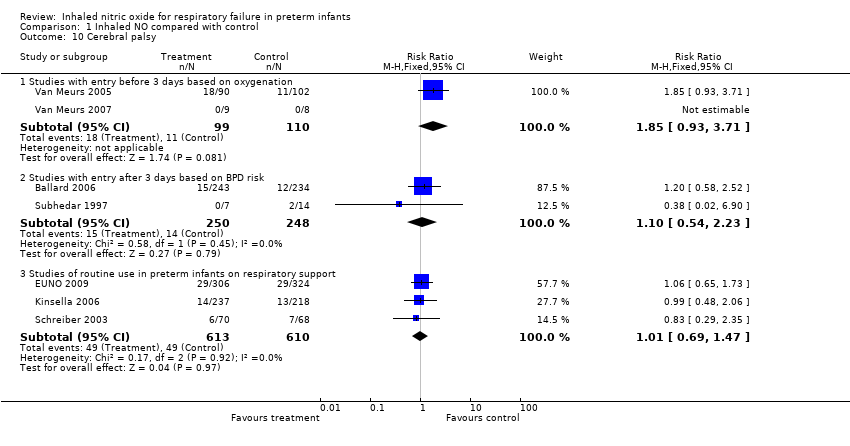
Comparison 1 Inhaled NO compared with control, Outcome 10 Cerebral palsy.

Comparison 1 Inhaled NO compared with control, Outcome 11 Severe retinopathy of prematurity (≥stage 3).
Comparison 1 Inhaled NO compared with control, Outcome 12 Retinopathy of prematurity requiring surgery.
| Inhaled NO compared with control for respiratory failure in preterm infants | ||||||
| Patient or population: respiratory failure in preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with Inhaled NO | |||||
| Death before discharge ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.02 | 1066 | ⊕⊕⊕⊕ | ||
| 394 per 1000 | 402 per 1000 | |||||
| Death before discharge ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 1.18 | 1075 | ⊕⊕⊕⊕ | ||
| 83 per 1000 | 98 per 1000 | |||||
| Death before discharge ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.90 | 1924 | ⊕⊕⊕⊝ | ||
| 170 per 1000 | 153 per 1000 | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 0.94 | 958 | ⊕⊕⊕⊕ | ||
| 743 per 1000 | 698 per 1000 | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 0.92 | 1075 | ⊕⊕⊕⊕ | ||
| 667 per 1000 | 614 per 1000 | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.94 | 1924 | ⊕⊕⊕⊕ | ||
| 548 per 1000 | 515 per 1000 | |||||
| Intraventricular haemorrhage (grade 3 or 4) ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.20 | 773 | ⊕⊕⊕⊕ | ||
| 231 per 1000 | 278 per 1000 | |||||
| Intraventricular haemorrhage (grade 3 or 4) ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.89 | 1913 | ⊕⊕⊕⊝ | ||
| 127 per 1000 | 113 per 1000 | |||||
| Neurodevelopmental disability ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.05 | 208 | ⊕⊕⊕⊝ | ||
| 455 per 1000 | 477 per 1000 | |||||
| Neurodevelopmental disability ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 0.90 | 498 | ⊕⊕⊕⊝ | ||
| 480 per 1000 | 432 per 1000 | |||||
| Neurodevelopmental disability ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.91 | 1223 | ⊕⊕⊕⊕ | ||
| 195 per 1000 | 178 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aHighly variable risk ratio in individual trials (I2 = 50%). bHighly variable risk ratio in individual trials (I2 = 33%). cBased on 2 studies, wide confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death before discharge Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Studies with entry before 3 days based on oxygenation | 10 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 1.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.71] |
| 1.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 2 Death before 36 weeks' postmenstrual age Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Studies with entry before 3 days based on oxygenation | 5 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.11] |
| 2.2 Studies with entry after 3 days based on BPD risk | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.81, 2.20] |
| 2.3 Studies of routine use in preterm infants on respiratory support | 2 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.89] |
| 3 Bronchopulmonary dysplasia among survivors at 36 weeks Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Studies with entry before 3 days based on oxygenation | 8 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 3.2 Studies with entry after 3 days based on BPD risk | 3 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.01] |
| 3.3 Studies of routine use in preterm infants on respiratory support | 4 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.05] |
| 4 Death or bronchopulmonary dysplasia at 36 weeks Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Studies with entry before 3 days based on oxygenation | 8 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.01] |
| 4.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.01] |
| 4.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 5 Intraventricular haemorrhage (all grades) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Studies with entry before 3 days based on oxygenation | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.28] |
| 5.2 Studies with entry after 3 days based on BPD risk | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.48, 2.54] |
| 5.3 Studies of routine use in preterm infants on respiratory support | 2 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 6 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Studies with entry before 3 days based on oxygenation | 6 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.98, 1.47] |
| 6.2 Studies of routine use in preterm infants on respiratory support | 4 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Studies with entry before 3 days based on oxygenation | 8 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 7.2 Studies of routine use in preterm infants on respiratory support | 3 | 1747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 8 Neurodevelopmental disability Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Studies with entry before 3 days based on oxygenation | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 8.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 8.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 9 Bayley MDI or PDI < ‐2 SD Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Studies of routine use in preterm infants on respiratory support | 2 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.90] |
| 10 Cerebral palsy Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Studies with entry before 3 days based on oxygenation | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.93, 3.71] |
| 10.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.54, 2.23] |
| 10.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 11 Severe retinopathy of prematurity (≥stage 3) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Studies with entry before 3 days based on oxygenation | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.47] |
| 11.2 Studies of routine use in preterm infants on respiratory support | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
| 12 Retinopathy of prematurity requiring surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Studies with entry before 3 days based on oxygenation | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 12.2 Studies with entry after 3 days based on BPD risk | 1 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 12.3 Studies of routine use in preterm infants on respiratory support | 2 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.47] |

