Óxido nítrico inhalado para la insuficiencia respiratoria en recién nacidos prematuros
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000509.pub5Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KJB and NF were involved in development and writing of the review protocol, the literature search and appraisal, data extraction and completion of the final review. TP assisted in revision of the article, analysis of publications and completion of the latest update.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
Dr Barrington was Chair of the Canadian Medical Advisory Committee for iNO therapeutics for one meeting in 2004.
Dr Barrington and Dr Finer were participants in a research project funded by Ikaria, an individual patient data meta‐analysis of iNO in the preterm, from which neither received any income.
Dr Barrington received an honorarium for speaking at a symposium funded by Mallinckrodt on the topic of probiotics.
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 03 | Inhaled nitric oxide for respiratory failure in preterm infants | Review | Keith J Barrington, Neil Finer, Thomas Pennaforte | |
| 2010 Dec 08 | Inhaled nitric oxide for respiratory failure in preterm infants | Review | Keith J Barrington, Neil Finer | |
| 2007 Jul 18 | Inhaled nitric oxide for respiratory failure in preterm infants | Review | Keith J Barrington, Neil Finer | |
| 2006 Jan 25 | Inhaled nitric oxide for respiratory failure in preterm infants | Review | Keith J Barrington, N N Finer | |
| 2001 Oct 23 | Inhaled nitric oxide for respiratory failure in preterm infants | Review | Keith J Barrington, Neil N Finer | |
Differences between protocol and review
The initial protocol did not foresee dividing included trials into three groups according to indications for enrolment. We added methods, the plan for Summary of findings tables and GRADE recommendations, which were not included in the original protocol.
We added the following outcomes post hoc (as reported in trials but not prespecified): oxygenation within two hours of therapy, pulmonary artery pressure, duration of assisted ventilation.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Inhalation;
- Bronchopulmonary Dysplasia [mortality, prevention & control];
- Cerebral Hemorrhage [epidemiology, prevention & control];
- Incidence;
- Infant, Premature;
- Infant, Premature, Diseases [prevention & control, *therapy];
- Nitric Oxide [*administration & dosage];
- Randomized Controlled Trials as Topic;
- Respiratory Insufficiency [*therapy];
- Salvage Therapy;
- Vasodilator Agents [*administration & dosage];
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO

Study flow diagram: review update.
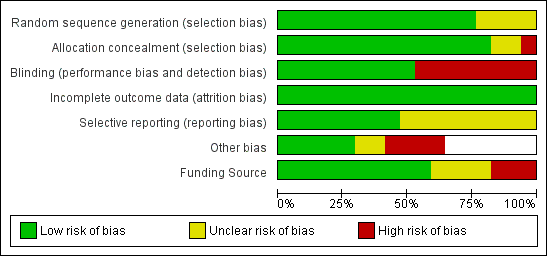
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inhaled NO compared with control, Outcome 1 Death before discharge.

Comparison 1 Inhaled NO compared with control, Outcome 2 Death before 36 weeks' postmenstrual age.

Comparison 1 Inhaled NO compared with control, Outcome 3 Bronchopulmonary dysplasia among survivors at 36 weeks.

Comparison 1 Inhaled NO compared with control, Outcome 4 Death or bronchopulmonary dysplasia at 36 weeks.

Comparison 1 Inhaled NO compared with control, Outcome 5 Intraventricular haemorrhage (all grades).
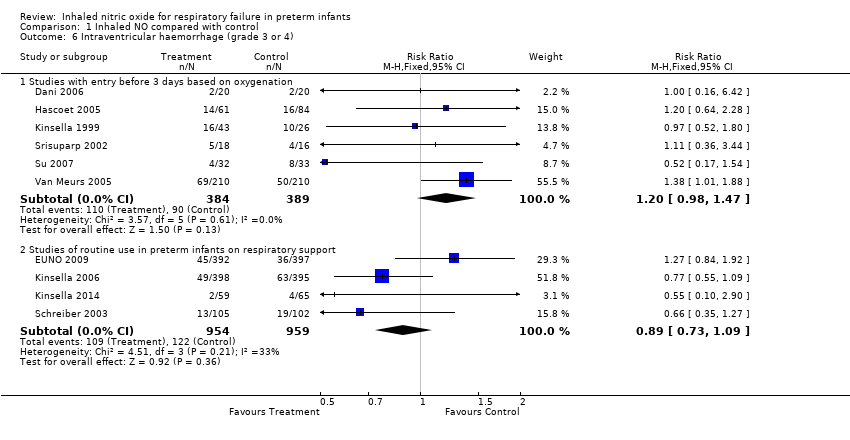
Comparison 1 Inhaled NO compared with control, Outcome 6 Intraventricular haemorrhage (grade 3 or 4).

Comparison 1 Inhaled NO compared with control, Outcome 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia.
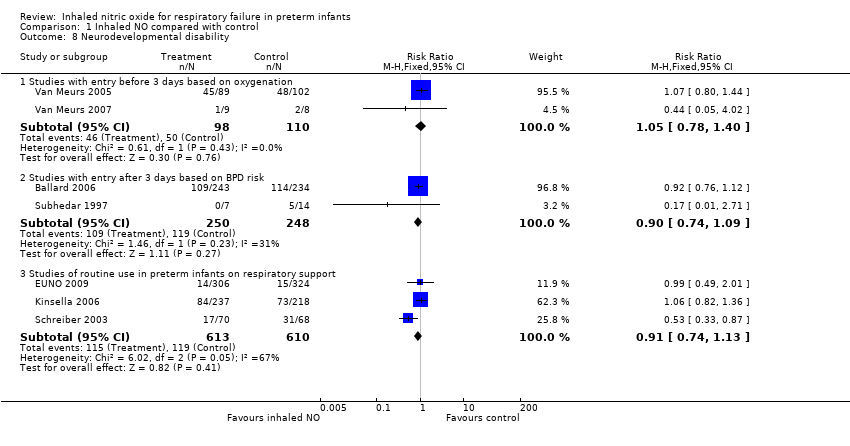
Comparison 1 Inhaled NO compared with control, Outcome 8 Neurodevelopmental disability.

Comparison 1 Inhaled NO compared with control, Outcome 9 Bayley MDI or PDI < ‐2 SD.
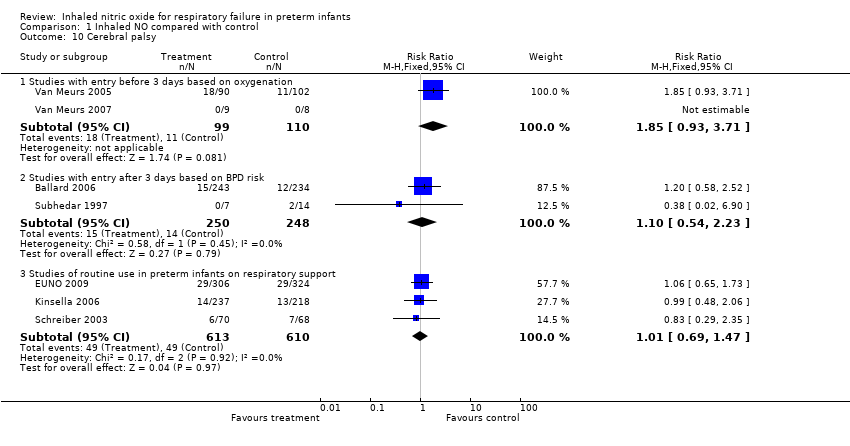
Comparison 1 Inhaled NO compared with control, Outcome 10 Cerebral palsy.

Comparison 1 Inhaled NO compared with control, Outcome 11 Severe retinopathy of prematurity (≥stage 3).
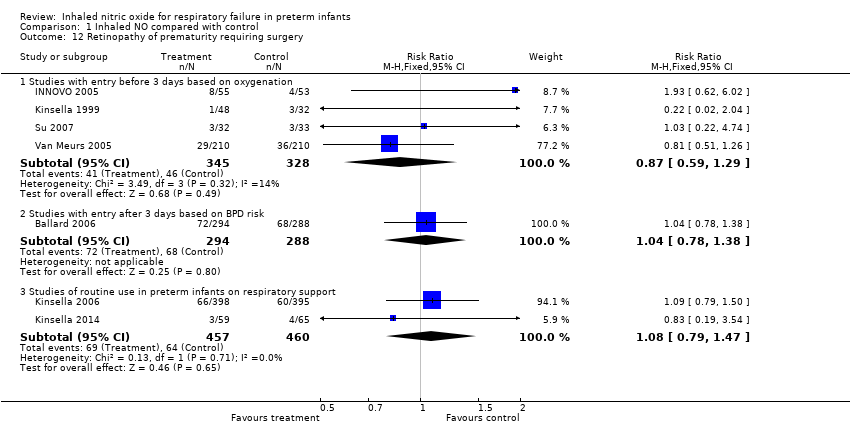
Comparison 1 Inhaled NO compared with control, Outcome 12 Retinopathy of prematurity requiring surgery.
| Inhaled NO compared with control for respiratory failure in preterm infants | ||||||
| Patient or population: respiratory failure in preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with Inhaled NO | |||||
| Death before discharge ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.02 | 1066 | ⊕⊕⊕⊕ | ||
| 394 per 1000 | 402 per 1000 | |||||
| Death before discharge ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 1.18 | 1075 | ⊕⊕⊕⊕ | ||
| 83 per 1000 | 98 per 1000 | |||||
| Death before discharge ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.90 | 1924 | ⊕⊕⊕⊝ | ||
| 170 per 1000 | 153 per 1000 | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 0.94 | 958 | ⊕⊕⊕⊕ | ||
| 743 per 1000 | 698 per 1000 | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 0.92 | 1075 | ⊕⊕⊕⊕ | ||
| 667 per 1000 | 614 per 1000 | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.94 | 1924 | ⊕⊕⊕⊕ | ||
| 548 per 1000 | 515 per 1000 | |||||
| Intraventricular haemorrhage (grade 3 or 4) ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.20 | 773 | ⊕⊕⊕⊕ | ||
| 231 per 1000 | 278 per 1000 | |||||
| Intraventricular haemorrhage (grade 3 or 4) ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.89 | 1913 | ⊕⊕⊕⊝ | ||
| 127 per 1000 | 113 per 1000 | |||||
| Neurodevelopmental disability ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.05 | 208 | ⊕⊕⊕⊝ | ||
| 455 per 1000 | 477 per 1000 | |||||
| Neurodevelopmental disability ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 0.90 | 498 | ⊕⊕⊕⊝ | ||
| 480 per 1000 | 432 per 1000 | |||||
| Neurodevelopmental disability ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.91 | 1223 | ⊕⊕⊕⊕ | ||
| 195 per 1000 | 178 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aHighly variable risk ratio in individual trials (I2 = 50%). bHighly variable risk ratio in individual trials (I2 = 33%). cBased on 2 studies, wide confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death before discharge Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Studies with entry before 3 days based on oxygenation | 10 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 1.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.71] |
| 1.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 2 Death before 36 weeks' postmenstrual age Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Studies with entry before 3 days based on oxygenation | 5 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.11] |
| 2.2 Studies with entry after 3 days based on BPD risk | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.81, 2.20] |
| 2.3 Studies of routine use in preterm infants on respiratory support | 2 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.89] |
| 3 Bronchopulmonary dysplasia among survivors at 36 weeks Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Studies with entry before 3 days based on oxygenation | 8 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 3.2 Studies with entry after 3 days based on BPD risk | 3 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.01] |
| 3.3 Studies of routine use in preterm infants on respiratory support | 4 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.05] |
| 4 Death or bronchopulmonary dysplasia at 36 weeks Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Studies with entry before 3 days based on oxygenation | 8 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.01] |
| 4.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.01] |
| 4.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 5 Intraventricular haemorrhage (all grades) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Studies with entry before 3 days based on oxygenation | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.28] |
| 5.2 Studies with entry after 3 days based on BPD risk | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.48, 2.54] |
| 5.3 Studies of routine use in preterm infants on respiratory support | 2 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 6 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Studies with entry before 3 days based on oxygenation | 6 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.98, 1.47] |
| 6.2 Studies of routine use in preterm infants on respiratory support | 4 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Studies with entry before 3 days based on oxygenation | 8 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 7.2 Studies of routine use in preterm infants on respiratory support | 3 | 1747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 8 Neurodevelopmental disability Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Studies with entry before 3 days based on oxygenation | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 8.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 8.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 9 Bayley MDI or PDI < ‐2 SD Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Studies of routine use in preterm infants on respiratory support | 2 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.90] |
| 10 Cerebral palsy Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Studies with entry before 3 days based on oxygenation | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.93, 3.71] |
| 10.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.54, 2.23] |
| 10.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 11 Severe retinopathy of prematurity (≥stage 3) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Studies with entry before 3 days based on oxygenation | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.47] |
| 11.2 Studies of routine use in preterm infants on respiratory support | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
| 12 Retinopathy of prematurity requiring surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Studies with entry before 3 days based on oxygenation | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 12.2 Studies with entry after 3 days based on BPD risk | 1 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 12.3 Studies of routine use in preterm infants on respiratory support | 2 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.47] |

