Duración del tratamiento para la bacteriuria asintomática durante el embarazo
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Alternate allocation. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Informed consent was obtained. No description of sample size or power calculation was provided. | |
| Participants | 64 women enrolled in study. Setting: out‐patient clinic in United Kingdom. Inclusion criteria: pregnant women > 16 years; confirmed asymptomatic bacteriuria with 2 consecutive positive bacteriologic count of identical organisms; urine culture sensitive to amoxicillin. Exclusion criteria: allergic to penicillin or cephalosporins; inability to take oral medications; requires parenteral antibiotics. | |
| Interventions | Experimental group: amoxicillin 3 g x 2 doses. | |
| Outcomes | Clinical outcomes: medication side effects. | |
| Notes | Type of healthcare provider: unknown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The author used alternate allocation which is not a good practice. |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
| Methods | Randomized controlled trial. Envelopes containing group assignment were used (no information re: sealed/opaque). No further description was provided regarding allocation. Method of randomization not described. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Consent process not described. No description of sample size or power calculation. | |
| Participants | 44 women enrolled in study. Setting: out‐patient clinic in New Zealand. Inclusion criteria: pregnant women < 30 weeks estimated gestational age; confirmed asymptomatic bacteriuria with mid‐stream urine culture of bacterial count > 100,000, and second urine specimen by suprapubic bladder aspiration showing infection regardless of bacterial count; urine culture was sensitive to co‐trimoxazole. Exclusion criteria: allergic to sulphonamides or co‐trimoxazole. | |
| Interventions | Experimental group: co‐trimoxazole 1.92 g x 1 dose. | |
| Outcomes | Clinical outcomes: preterm delivery, pyelonephritis, medication side effects. | |
| Notes | Type of healthcare provider: unknown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | 2 of the women treated with a 5‐day course of cotrimoxazole dropped out of the study. |
| Methods | Randomized controlled trial. Envelopes containing group assignment were used (no information re: sealed/opaque). No further description was provided regarding allocation. Method of randomization not described. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Consent process not described. No description of sample size or power calculation. | |
| Participants | 60 women enrolled in study. Population race/ethnicity 28% 'Polynesian'. Setting: out‐patient clinic in New Zealand. Inclusion criteria: pregnant women 16‐30 weeks' estimated gestational age; confirmed asymptomatic bacteriuria with mid‐stream urine culture of bacterial count > 100,000, and second urine specimen by suprapubic bladder aspiration showing infection regardless of bacterial count. Exclusion criteria: not described. | |
| Interventions | Experimental group: trimethoprim 600 mg x 1 dose. | |
| Outcomes | Clinical outcomes: preterm delivery, pyelonephritis, medication side effects. | |
| Notes | Type of healthcare provider: unknown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Envelopes were used but the allocation was not defined |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | 2 of the women treated with a 5‐day course of trimethoprim moved to another city after initial bacteriological follow‐up. |
| Methods | Randomized controlled trial. 90 pregnant women were randomized to receive either a single dose fosfomycin trometamol or a 5‐day course of cefuroxime axetyl. Pregnant women were not blinded to treatment assignment. It was unclear whether the following criteria were met: measurement of contamination of control group, assessments of co‐interventions. Power calculation described, sample size calculation conducted. | |
| Participants | 90 women were enrolled in the trial. 1 patient in the fosfomycin trometamol group and 5 patients in the cefuroxime axetyl group were lost to follow‐up and excluded from the trial. Inclusion criteria: pregnant women in the second trimester of gestation, confirmed asymptomatic bacteriuria with 2 consecutive clean‐catch urine specimens yielding positive cultures of the same uropathogen. Exclusion criteria: gravidas presenting leukocytosis, fever, urolithiasis, lower back pain, previous urologic surgery, anomalies of the urinary tract. | |
| Interventions | Experimental group: single dose of 3 g fosfomycin trometamol. Control group: cefuroxime axetyil 250 mg twice a day for 5 days. | |
| Outcomes | Clinical outcome: side effects. Laboratory outcome: bacteriological eradication of uropathogens. | |
| Notes | Cure rates are informed only as percentages. No ratios are informed. Side effects are informed in percentages. No risk ratios nor confidence intervals are informed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization method was used to ensure an equal number of patients in each group. |
| Allocation concealment (selection bias) | Low risk | The blocks were numbered, placed into a bag, and a staff member blinded to the research protocol selected the patients into the treatment groups. |
| Blinding (performance bias and detection bias) | Low risk | The staff member was blinded but the patient not. |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient in the fosfomycin trometamol group and 5 patients in the cefuroxime axetyl group did not come to the follow‐up visit; therefore, they were excluded from the study. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Methods | Randomized controlled trial. Randomization tables were used to allocate participants. Unclear measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. No description of sample size or power calculation. | |
| Participants | 54 women enrolled in a out‐patient antenatal clinic. | |
| Interventions | Experimental group: oral amoxicillin 3 g x 2 doses during 1 day. | |
| Outcomes | Clinical outcomes: birthweight, medication side effects. | |
| Notes | 24% were symptomatic in both groups, 65% were in the second trimester. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
| Methods | Randomized, prospective, longitudinal, unblinded trial. Allocation concealment done using random number tables. Method of randomization not described. | |
| Participants | 131 pregnant women enrolled in the study. Setting: out‐patient clinic in Spain. Inclusion criteria: pregnant women with asymptomatic bacteriuria (≧ 100,000 CFU/ml of the same microorganism in two consecutive cultures) without fever or symptoms of UTI. Exclusion criteria: having taken antibiotics 14 days prior to taking the culture for any reason other than having UTI; allergy to penicillins; high‐risk pregnancy; admitted to hospital; impossibility of performing follow‐up; anomalies in the urinary tract; infection due to microorganisms resistant to either of the two antibiotics and symptomatic UTI. | |
| Interventions | Experimental group: fosfomycin 3g x 1 dose Control group: amoxicillin‐clavulanate 500mg/125mg tablets every 8 hours for 7 days. | |
| Outcomes | Clinical outcomes: microbiological cure, recurrences, reinfection, persistences, secondary effects, and therapeutic compliance. | |
| Notes | There were no losses to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Random number tables |
| Blinding (performance bias and detection bias) | High risk | |
| Incomplete outcome data (attrition bias) | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomized controlled trial. Allocation concealment not described. Allocation to treatment done in blocks of 10‐5 per treatment group. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Informed consent was obtained. No description of sample size or power calculation was provided. | |
| Participants | 91 women (53 single dose, 38 longer course) enrolled in study. Setting: multicenter out‐patient clinic in Austria. Inclusion criteria: pregnant women; confirmed asymptomatic bacteriuria with mid‐stream urine culture of bacterial count > 100,000 and bladder catheterization with bacterial count > 10,000 diagnosed with the dip‐slide method; urine culture sensitive to amoxicillin. | |
| Interventions | Experimental group: amoxicillin 3 g x single dose. | |
| Outcomes | Clinical outcomes: medication side effects. | |
| Notes | Healthcare providers: physicians and nurses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization in groups of 10; 5 patients per regiment. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
| Methods | Randomized, double blind, placebo controlled non‐inferiority trial. Pregnant women were randomly allocated to receive either a 1‐day or a 7‐day course of nitrofurantoin. Compliance was of 98% in both groups. Power calculation described, sample size calculation conducted. Assessment of co‐interventions was done. Deviations from protocol were described. | |
| Participants | 778 were enrolled in the trial. 9 women in the 1‐day regimen and 10 women in the 7‐day regimen were lost to follow‐up. Sociodemographic characteristics of each group were similar at entry. Inclusion criteria: pregnant women at gestational age 12‐32 weeks with no symptoms of urinary tract infection. Exclusion criteria: history of urinary tract infection during current pregnancy, under steroids and/or antibiotic treatment, presence of any hematologic disease including glucose‐6‐phosphate dehydrogenase deficiency. | |
| Interventions | Experimental group: one‐day nitrofurantoin 100 mg twice a day. Control group: seven‐day nitrofurantoin 100 mg twice a day. | |
| Outcomes | Clinical outcomes: incidence of symptomatic urinary tract infection, pyelonephritis, preterm delivery, low birthweight, adverse effects. Laboratory outcomes: bacteriologic cure after antibiotic treatment assessed by a urine culture 14 days after the initiation of the treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | it was generated using computer‐generated random numbers with randomly varying blocks of 6‐8 (SAS software, SAS Institute, Inc., Cary, NC). |
| Allocation concealment (selection bias) | Low risk | The random allocation was concealed by using sealed, opaque treatment boxes numbering sequentially. |
| Blinding (performance bias and detection bias) | Low risk | Both the women and the health providers were blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Delivery outcomes were available in 91.7% and 89.0% of the women in the 1‐day and 7‐day regimen respectively. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Methods | Randomized controlled trial. Allocation by computer‐generated randomization. There was blinding of outcome assessment and providers (treatment allocation by clinic secretary). Pregnant women were not blinded. It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Informed consent was obtained. Power calculation described; sample size calculation conducted, however, sample size achieved inadequate due to limited trial period. | |
| Participants | 102 women were enrolled in study, 90 completed the protocol, and 62 women were analyzed as the subgroup 'antenatal asymptomatic bacteriuria'. Wives (British nationality) of servicemen from United Kingdom stationed in Germany. Setting: out‐patient clinic. Inclusion criteria: pregnant women 28‐36 weeks' estimated gestational age; confirmed asymptomatic bacteriuria with 2 consecutive urine cultures of bacterial count ≧ 100,000 colonies/ml urine; urine culture was ampicillin sensitive. Exclusion criteria: pyelonephritis; history of drug sensitivity to beta‐lactam agents; or use of antibiotics within last 2 weeks. | |
| Interventions | Experimental group: amoxicillin 3 g single dose. | |
| Outcomes | Laboratory outcomes: 'no cure', recurrent asymptomatic bacteriuria. Re‐infection assessed at 1 week and 6 weeks after treatment. | |
| Notes | Type of healthcare provider: general practitioners and obstetricians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Computer randomization. |
| Blinding (performance bias and detection bias) | Low risk | Treatment allocation done by secretary. Obstetrician and microbiologist were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 90 out of 102 patients enrolled completed the protocol. |
| Methods | Randomized controlled trial. No description of method for generation and concealment of treatment. Providers and pregnant women were not blinded. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Informed consent was obtained. No description of sample size or power calculation. | |
| Participants | 41 women enrolled in study. Setting: out‐patient clinic in Denmark. Inclusion criteria: pregnant women < 36 weeks estimated gestational age; confirmed asymptomatic bacteriuria with 2 consecutive urine cultures of bacterial count ≧ 100,000 colonies/ml urine; urine culture was sensitive to sulfamethizole. Exclusion criteria: signs of urinary tract infection; chronic disease of the genitourinary tract; history of more than two urinary tract infections in previous 12 months; threatening preterm labour > 26 weeks estimated gestational age; allergy to sulphonamides; antibiotic therapy for any reason within 3 weeks prior to study. | |
| Interventions | Experimental group: sulfamethizole 2 g x single dose. | |
| Outcomes | Clinical outcomes: medication side effects. | |
| Notes | Type of healthcare provider: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | 2 dropouts in the longer treatment arm. |
| Methods | Randomized controlled trial. 44 pregnant women were divided into 2 groups: 1 treated traditionally and the other with single dose. Allocation to treatment is not described. Pregnant women were not blinded. Consent process is not described. It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. The traditional protocol produced an immediate response in 86.4% of the cases demonstrating its superiority over the single‐dose treatment that brought success in 54.5% of the cases. | |
| Participants | 44 women enrolled in the study. Setting: out‐patient clinic in Trieste University. October 1980 to December 1985. Inclusion criteria: pregnant women aged 17‐35 years old, confirmed asymptomatic bacteriuria with positive bacteriologic count of > 100,000 CFU/ml. Exclusion criteria: 1st bacteriological count < 100,000 CFU/ml or 2nd bacteriological count > 100,000 CFU/ml but different microorganism comparing with the first one. | |
| Interventions | Experimental group: single dose of amoxicillin 3 g, or ampicillin 3.5 g, or trimethoprim 320 mg, or sulfamethoxazole 1600 mg, or cephalexin 3 g. Control group: 2‐4 times daily x 1‐2 weeks of the antibiotics named in the experiment group. | |
| Outcomes | Clinical outcomes: medication side effects. Laboratory outcomes: no cure rate, recurrent asymptomatic bacteriuria. | |
| Notes | Type of health provider: not described. Attrition bias: no description of loss to follow‐up from experimental group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used. |
| Methods | Generation of allocation sequence was by alternation. It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Blinding of outcome assessment, providers and pregnant women was not done. Consent process not described. No description of sample size or power calculation. | |
| Participants | 100 women enrolled in study. Setting: antenatal clinics in England. Inclusion criteria: pregnant women attending the bacteriuria clinic; confirmed bacteriuria with 2 consecutive urine cultures of bacterial count greater than or equal to 100,000 colonies/ml urine; urine culture was sensitive to sulphonamides. Exclusion criteria: not described. | |
| Interventions | Experiment group: sulphonamide sulfametopyrazine 2 g single dose, orally in 50 ml of water in the clinic. | |
| Outcomes | Clinical outcome: medication side effect. | |
| Notes | Type of healthcare provider: physicians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | 5/54 were lost to follow‐up from the experimental arm; 6/46 from the control one. |
| Methods | Randomized controlled study. Allocation to treatment not described. Pregnant women nor provider were blinded. Informed consent was taken. No description of sample size or power calculation was provided (the study did not conclude). It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. | |
| Participants | 23 women enrolled in the study. Setting: out‐patient clinic in Germany. Inclusion criteria: significant bacteriuria (10,000 CFU/ml or more) without symptoms. Exclusion criteria: not described. | |
| Interventions | Experimental group: single dose of fosfomycin trometamol 3 g. Control group: 7‐day course of nitrofurantoin 100 mg. | |
| Outcomes | Clinical outcome: medication side effect. | |
| Notes | Type of healthcare provider: not described. Attrition bias: no description of loss to follow‐up. from experimental group nor for control group. This study shows preliminary results. No final results were published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| In this study the 66% of the experimental group were determined to have symptomatic bacteriuria as compared to 33% with asymptomatic bacteriuria. Similarly, in the control group 63% of women were determined to have symptomatic bacteriuria as compared to 37% with asymptomatic bacteriuria. Both symptomatic and asymptomatic bacteriuria were combined when outcomes were assessed; therefore, the study is excluded because of the inability to assess asymptomatic bacteriuria separately. Clarification of data is being sought from authors. | |
| In this study 100 pregnant women were screened and randomly allocated in equal numbers to receive either 400 mg pivmecillinam 4 times daily for 7 days or 500 mg ampicillin 4 times daily for 7 days. The treatment did not differ in duration. No distinction was made between asymptomatic and symptomatic bacteriuria. Cure rates were 88% in the pivmecillinam group and 85% in the ampicillin group. Side effects were significantly more frequent in the pivmecillinam group. | |
| This paper includes 2 trials. The first trial compares a 7‐day course of treatment with trimethoprim‐sulfamethoxazole in 3 different combinations, but of the same duration. In the second trial, 4 drugs were administered at high doses to investigate the possibility that better results might be obtained with high blood levels of antibiotic than smaller dose. There was not a distinction between asymptomatic and symptomatic bacteriuria. | |
| This is not a randomized controlled trial. In this study bacteriuria was diagnosed from suprapubic aspiration specimens of urine. All participants received the same treatment: single dose of 3 g cephalexin. There was no distinction between symptomatic and asymptomatic pregnant women. The cure rate achieved was 70%. 30% of the participants who were not cured received a 7‐day course of antibacterial according to the sensitivity of the organism isolated. 36% continued with the infection and were given continuous antibacterial therapy. | |
| 4 drugs were compared but all were administered during a week. There were 53 women enrolled, 26 (49%) of whom had urinary symptoms at the first clinic attendance. | |
| In this study no distinction was made between asymptomatic bacteriuria and symptomatic bacteriuria. The study participants included pregnant women > 8 weeks estimated gestational age who had any bacteriuria > 100,000. The terminology used in the paper to refer to the exposure of interest was 'lower urinary tract infections'. Therefore, this study was excluded. In addition, it was unclear whether randomization was carried out: there was a marked imbalance in the study groups (52 versus 31). If one of the two antimicrobial treatment regimens failed, women were switched to the other treatment regimen; no description was provided on how many or which women crossed over. | |
| 86 pregnant women were sequentially assigned to 1 of 4 single‐dose, single‐antimicrobial treatment groups: ampicillin 2 g, plus probenimide 1 g; keflex 2 g, plus probenimide 1 g; macrodantin 200 mg; or, gantrisin 2 g. All participants were diagnosed as having asymptomatic bacteriuria if they had no symptoms of urinary tract infection and had 2 consecutive urine cultures > 100,000 colonies per ml of the same species of micro‐organism. The overall no cure rate was 31%, with a recurrence rate of 3.5%. | |
| 50 asymptomatic pregnant women were treated with 3 g of amoxicillin or 2 g of cephalexin in accordance with the isolated micro‐organism disk sensitivity. The study was not based on randomization. There was no difference in duration of treatment. The immediate cure rate was 84% and the recurrence rate was 12%. The failure of treatment with single‐dose treatment was 16%, these participants were treated with the same drug administered for 7 days and were cured. It is possible that these participants had upper UTI or urinary tract malformations. | |
| The main comparison of interest to the present review, single dose versus 3‐day regimen, was not based on randomization. 2 3‐day regimens that compared cephalexin 1 g with pivmecillinam‐pivampicillin in an independent randomized trial (reported separately) were grouped together in this paper and compared with a non‐randomized series of pregnant women who received a single dose of cephalexin 3 g orally. | |
| In this study, participants were randomized to nitrofurantoin or placebo, all of them during a 3‐week period. | |
| In this study no distinction was made between asymptomatic bacteriuria and symptomatic one. Women were randomly allocated to receive either 1 tablet of amoxicillin‐clavulanic acid 3 times daily or 250 mg of cephalexin 3 times daily for 7 days. There was no difference in the duration of treatment. The study compared difference of choice of antimicrobial. Differences in cure rates were not statistically significant. No significant difference in the rate of side effects was found. No toxicity to the fetus was seen which could be ascribed to either drug. | |
| Women were alternately allocated into treatment with a 2‐week course of either cycloserine 250 mg 2 times daily or sulphadimidine 0.5 g 4 times daily. There was no difference in the duration of treatment; what was compared was the difference in choice of antimicrobial. | |
| This study aimed at reducing reinfection. Participants whose urine was found to be sterile (after 7‐day course of pivmecillinam 1 tablet thrice daily), received at random pivmecillinam sachets prophylactically, 100 mg in the evening on alternates days, or were allocated to a control group, who were given no treatment. | |
| Randomized and non‐randomized study participants were combined together. This study was excluded because it was not possible to analyze the results of randomized and non‐randomized groups separately. | |
| Pregnant women with symptomatic and asymptomatic bacteriuria were combined. The majority of women in the study population were described as having symptomatic bacteriuria; therefore, the study is excluded because of the inability to assess asymptomatic bacteriuria separately. An additional concern was the introduction of bias through the sampling method; the sample size of n = 291 was obtained from 25 different study sites in Italy. |
UTI: urinary tract infection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 No cure Show forest plot | 13 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.88, 2.01] |
| Analysis 1.1  Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 1 No cure. | ||||
| 1.1 Same antimicrobial agent | 10 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.87, 2.34] |
| 1.2 Different antimicrobial agents | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.49, 1.95] |
| 2 Preterm delivery Show forest plot | 3 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.52, 2.26] |
| Analysis 1.2  Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 2 Preterm delivery. | ||||
| 2.1 Same antimicrobial agent | 3 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.52, 2.26] |
| 3 Preterm delivery or low birthweight Show forest plot | 1 | 714 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.10] |
| Analysis 1.3  Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 3 Preterm delivery or low birthweight. | ||||
| 3.1 Same antimicrobial agent | 1 | 714 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.10] |
| 4 Pyelonephritis Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.51, 17.28] |
| Analysis 1.4  Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 4 Pyelonephritis. | ||||
| 4.1 Same antimicrobial agent | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.51, 17.28] |
| 5 Side effects Show forest plot | 12 | 1460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.88] |
| Analysis 1.5  Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 5 Side effects. | ||||
| 5.1 Same antimicrobial agent | 9 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.97] |
| 5.2 Different antimicrobial agents | 3 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.58] |
| 6 Recurrent asymptomatic bacteriuria Show forest plot | 8 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.75, 1.60] |
| Analysis 1.6  Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 6 Recurrent asymptomatic bacteriuria. | ||||
| 6.1 Same antimicrobial agent | 6 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.60] |
| 6.2 Different antimicrobial agents | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.23, 7.55] |
| 7 Need for repeat treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
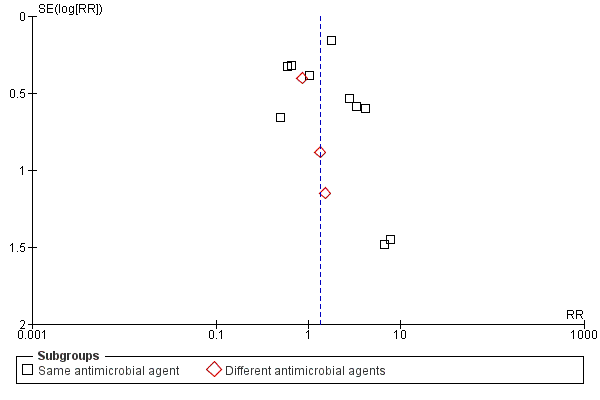
Funnel plot of comparison: 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, outcome: 1.6 No cure.
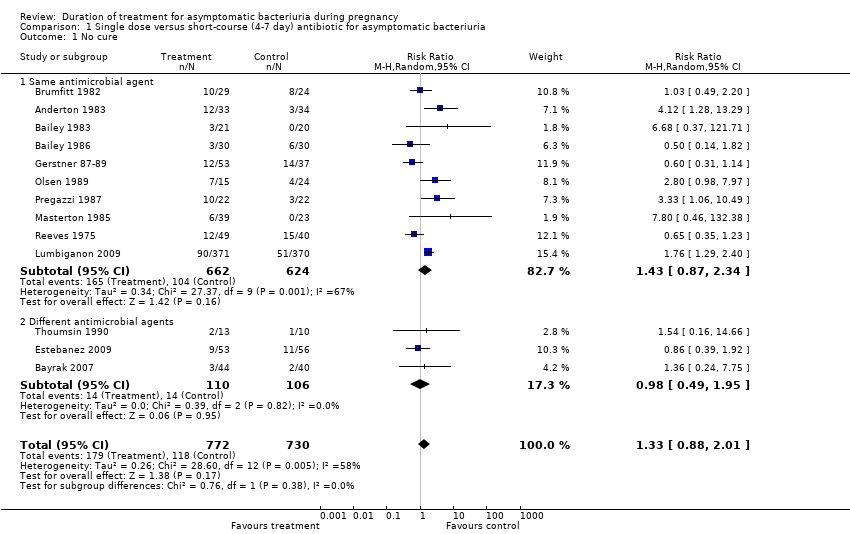
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 1 No cure.
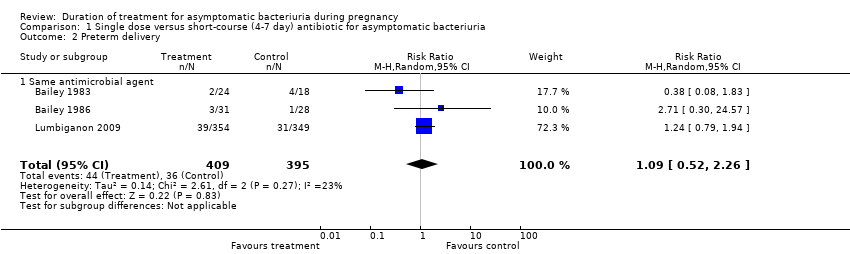
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 2 Preterm delivery.
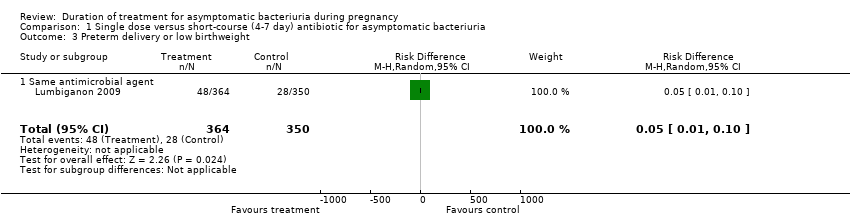
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 3 Preterm delivery or low birthweight.
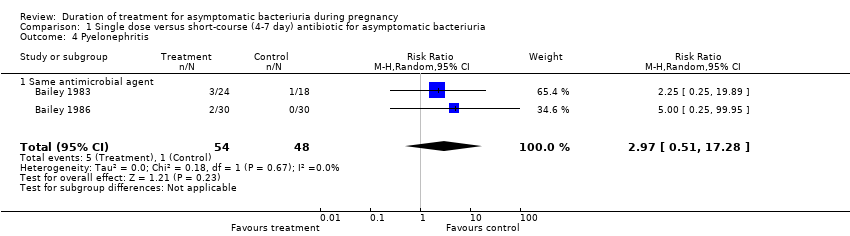
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 4 Pyelonephritis.
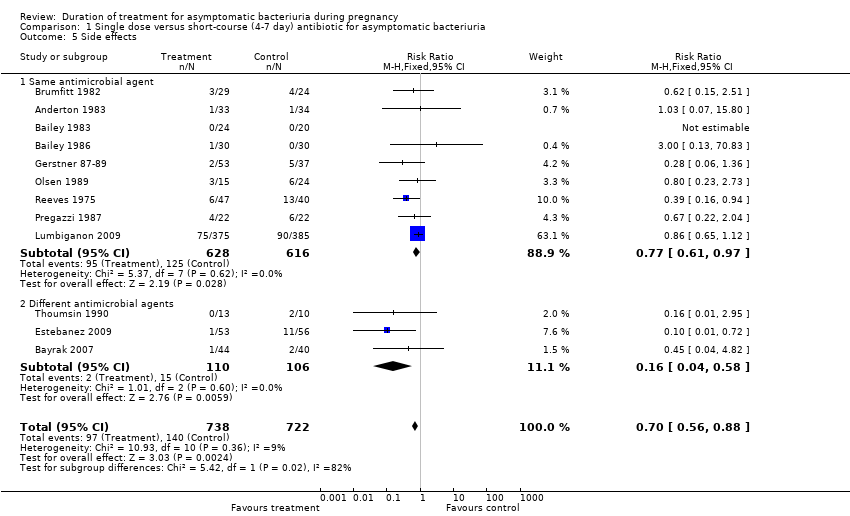
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 5 Side effects.
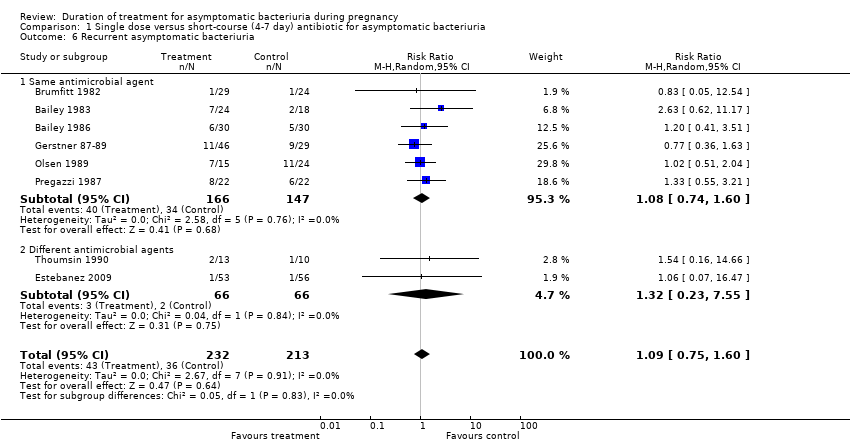
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 6 Recurrent asymptomatic bacteriuria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 No cure Show forest plot | 13 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.88, 2.01] |
| 1.1 Same antimicrobial agent | 10 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.87, 2.34] |
| 1.2 Different antimicrobial agents | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.49, 1.95] |
| 2 Preterm delivery Show forest plot | 3 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.52, 2.26] |
| 2.1 Same antimicrobial agent | 3 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.52, 2.26] |
| 3 Preterm delivery or low birthweight Show forest plot | 1 | 714 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.10] |
| 3.1 Same antimicrobial agent | 1 | 714 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.10] |
| 4 Pyelonephritis Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.51, 17.28] |
| 4.1 Same antimicrobial agent | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.51, 17.28] |
| 5 Side effects Show forest plot | 12 | 1460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.88] |
| 5.1 Same antimicrobial agent | 9 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.97] |
| 5.2 Different antimicrobial agents | 3 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.58] |
| 6 Recurrent asymptomatic bacteriuria Show forest plot | 8 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.75, 1.60] |
| 6.1 Same antimicrobial agent | 6 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.60] |
| 6.2 Different antimicrobial agents | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.23, 7.55] |
| 7 Need for repeat treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

