Vacunas para la prevención de la gripe en pacientes asmáticos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomisation: no details | |
| Participants | Location: Houston, TX | |
| Interventions | Vaccine type: intranasal bivalent (H3N2+H1N1) influenza A vaccine. 0.25 mL per nostril | |
| Outcomes | Early: lung function tests on days 0, 3 or 4, and 7; performed in the mornings (no bronchodilators taken before testing). The authors regarded a reduction in forced expiratory volume in 1 second (FEV1) of 13% (or greater) from baseline to be clinically significant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by statistical group in General Clinical Research Centre |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind but no details of similarity between placebo and active vaccine |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | No drop‐outs reported |
| Methods | Randomisation: by hospital number | |
| Participants | Location: Denver, CO. Residential asthma care centre | |
| Interventions | Vaccination type: bivalent (A/Port Chalmers/1/73 and B/Hong Kong/5/72) vaccine containing inactivated influenza virus. 0.25 mL or 0.5 mL given | |
| Outcomes | Early: change in peak flow and mean number of nebulised treatments given | |
| Notes | First arm of cross‐over trial included. Data expressed as mean difference in % change in predicted peak flow, and nebuliser usage, between vaccinated and non‐vaccinated groups. Standard deviation calculated from published standard error of the mean CAUTION: no baseline comparability of the 2 groups was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Hospital number |
| Allocation concealment (selection bias) | High risk | Allocation based on last digit of patient's chart number |
| Blinding of participants and personnel (performance bias) | High risk | No placebo |
| Blinding of outcome assessment (detection bias) | High risk | No placebo |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Methods | Randomisation took place by the manufacturer when packing vaccine and placebo, from a computer‐generated list | |
| Participants | Location: Rotterdam, Netherlands, community‐based study | |
| Interventions | Vaccination type: inactivated influenza vaccine intramuscular injection. The vaccine composition for 1999 to 2000 was a combination of A/Sydney/5/97 H3N2‐like, A/Beijing/262/95‐like and B/Beijing/184/93‐like strains and for 2000 to 2001 A/Moscow/10/99 H3N2‐like, A/New Caledonia/20/99 H1N1‐like and B/Beijing/184/93‐like strains as advised by the World Health Organization Placebo group: buffered phosphate solution with the same pH value and similar appearance as the inactivated influenza vaccine | |
| Outcomes | Primary outcome: influenza‐related asthma exacerbations (number, duration and severity) | |
| Notes | Power calculations suggested 600 patients needed to be enrolled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Randomisation, packing and labelling took place by the manufacturer |
| Blinding of participants and personnel (performance bias) | Low risk | All those involved, i.e. patients and parents, GPs and investigators, were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All those involved, i.e. patients and parents, GPs and investigators, were blinded |
| Incomplete outcome data (attrition bias) | Low risk | 344/347 and 344/349 participants provided diary data |
| Methods | Cross‐over design | |
| Participants | Location: 19 centres in the US | |
| Interventions | Vaccination type: heat‐inactivated trivalent split‐virus influenza type A and B vaccine (Fluzone, Aventis‐Pasteur) | |
| Outcomes | Primary outcome: exacerbation of asthma within 14 days of vaccination | |
| Notes | Bubble sizes were noted to be larger in the placebo syringes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design |
| Allocation concealment (selection bias) | Low risk | Assignment list prepared by data co‐ordinating centre |
| Blinding of participants and personnel (performance bias) | Low risk | Identical looking placebo syringes containing saline |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | 96% received both injections and completed both 14‐day post‐injection diaries |
| Methods | Design: parallel, open‐label study designed to test non‐inferiority | |
| Participants | Location: 145 study sites in Europe | |
| Interventions | Arm 1: live attenuated influenza vaccine (CAIV‐T) | |
| Outcomes | Primary outcome of the study: culture‐confirmed influenza caused by a subtype that was antigenically similar to the vaccine. The primary safety end point was the incidence of asthma exacerbation, defined as acute wheezing illness associated with hospitalisation, any unscheduled clinical visit or any new prescription (including rescue medication) | |
| Notes | Sequence generation adequate: automated interactive voice response system | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Randomisation was accomplished using an automated interactive voice response system |
| Blinding of participants and personnel (performance bias) | High risk | Open design |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors reported |
| Incomplete outcome data (attrition bias) | Low risk | Only 7 patients failed to complete the study |
| Methods | Randomisation: stratified by 4 morbidity categories | |
| Participants | Location: Netherlands | |
| Interventions | Vaccination type: purified split vaccine H1N1, H3N2, B45/90, B1/87 given intramuscularly | |
| Outcomes | Early: adverse reactions (recalled by the patients after 4 weeks) | |
| Notes | No serologically confirmed influenza was seen in either the immunised or the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Next consecutive numbered syringe used |
| Blinding of participants and personnel (performance bias) | Unclear risk | Saline placebo but no further details |
| Blinding of outcome assessment (detection bias) | Low risk | Questionnaires analysed by researchers blind to vaccination status |
| Incomplete outcome data (attrition bias) | Low risk | 1791/1838 completed |
| Methods | Randomisation: stratified by baseline forced expiratory volume in 1 second (FEV1) (no details of allocation concealment) | |
| Participants | Location: Wurzburg, Germany | |
| Interventions | Vaccination types: | |
| Outcomes | Lung function measurements in clinic (2 weeks before and after treatment). Home measurement of peak flow (best of 3, twice daily) and symptoms recorded by patients (including breathing difficulty) | |
| Notes | No lung function measurements documented, only "no significant change in lung function following either vaccination or placebo" (even in the patients on systemic corticosteroids) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (communication from the authors) |
| Allocation concealment (selection bias) | Low risk | The physician always had to pick the next available vial and was not allowed to change sequence |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details of any differences in appearance between placebo and active injections |
| Blinding of outcome assessment (detection bias) | Low risk | The code was opened at the end |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Methods | Randomised, placebo‐controlled, double‐blind cross‐over trial | |
| Participants | 286 adults aged 18 to 64 years, in Poland, with 12‐month history of asthma with perennial symptoms and a positive spirometry reversibility test, or a positive methacholine or histamine provocation test Exclusion criteria: allergy to egg or chicken protein, neomycin, formaldehyde and octoxinol‐9; known or suspected disease of the immune system; acute febrile illness (temperature > 37.0 °C) in the 72 hours preceding inclusion; autoimmune disease; prior immunisation against influenza for the 2004/2005 season; and having received another vaccine within 2 weeks preceding inclusion or planning to receive another vaccine within 6 weeks after inclusion | |
| Interventions | Vaccination type: intramuscular injection of trivalent inactivated influenza vaccine, Vaxigrip (Sanofi Pasteur), to right deltoid A/New Caledonia/20/99(H1N1)‐like strain derived from IVR‐116_ A/Fujian/41/2002(H3N2)‐like strain A/Wyoming/3/2003_ B/Shanghai/361/2002‐like strain B/Jiangsu/10/2003 Placebo: saline vaccine 2 vaccinations given in random order on day 1 and day 14. Assessed on day 14 and day 28 | |
| Outcomes | Primary outcome: asthma exacerbations. Mild defined by emergency visit due to asthma, or doubling of inhaled maintenance treatment, or peak expiratory flow (PEF) 60% to 80% of personal best, or increased rescue inhaler > 2 per day above baseline. Severe defined by hospital/emergency department visit, oral corticosteroids or PEF > 60% personal best Secondary outcomes: adverse events | |
| Notes | Sponsored by Sanofi Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, placebo injections (but no details in relation to how similar the saline injections were to active injections) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Only 5 of 291 participants dropped out |
| Methods | Randomisation: no details | |
| Participants | Location: Istanbul, Turkey | |
| Interventions | Vaccination type: inactivated influenza vaccine given subcutaneously | |
| Outcomes | PC20 for methacholine challenge before vaccine and after 24 hours | |
| Notes | PC20 (standard deviation) in the placebo group was 7.02 (9.3) before challenge and 7.3 (3.6) after 24 hours. In the vaccine group, PC20 was 9.5 (10.6) before challenge and 9.8 (9.3) after 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Placebo saline injection (no details of how the placebo matched the active injection) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Methods | Randomisation: no details | |
| Participants | Location: Minami‐Fukuoka chest hospital, Japan. Inpatients on asthma ward | |
| Interventions | Vaccination type: intranasal cold‐adapted recombinant trivalent influenza vaccine (H1N1, H3N2, B). Dose 0.3 mL by nasal spray | |
| Outcomes | Early: asthma attacks | |
| Notes | Serology at the start was NOT comparable with 17/19 in the vaccinated group having a starting titre over 1:64 whereas only 8/25 in the non‐vaccinated group had a starting titre over 1:64 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No placebo |
| Blinding of outcome assessment (detection bias) | High risk | No placebo |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Methods | Randomisation: sealed envelopes, computer‐generated randomisation code provided by vaccine manufacturer | |
| Participants | Location: 9 respiratory centres and 2 asthma clinics in the UK | |
| Interventions | Cross‐over design with 2 intramuscular injections given 2 weeks apart in random order | |
| Outcomes | Outcome measures: primary clinical outcome was an asthma exacerbation within 72 hours of injection (defined as 20% fall in PEF compared to lowest of the 3 days before vaccination). Also measured were change in mean PEF, inhaled beta‐agonist use (72 hours before and after injection), antibiotic and oral corticosteroid use for 7 days after injection, unscheduled medical attendance and hospital admission for 7 days after each injection. Symptom scores were also analysed for 72 hours before and after injection of vaccine or placebo | |
| Notes | PEF was examined using percentage change for individuals of the worst test for 3 days before and after injection and also using the mean test result over the same periods. On all occasions only the best of 3 blows was used for the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Identical saline placebo syringes |
| Blinding of outcome assessment (detection bias) | Low risk | Allocation concealed until data had been entered on the computer and all analytical programs had been tested |
| Incomplete outcome data (attrition bias) | Low risk | 255/287 had complete paired data |
| Methods | Randomisation: stratified by lung function results | |
| Participants | Location: Germany Exclusion criteria: no details | |
| Interventions | Vaccination type: whole virus, split virus and subunit vaccines (A/Texas, A/USSR, B/Hong Kong). Patients were re‐vaccinated at 6 weeks | |
| Outcomes | Pulmonary function measured for 7 days before vaccination and compared with 3 days after vaccination | |
| Notes | No placebo group and results stated as "no significant change in lung function for individual or for the combined vaccines" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Open study? |
| Blinding of outcome assessment (detection bias) | High risk | Open study? |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐arm trial for 56 days following first vaccination | |
| Participants | Location: Mexico Number and age of participants: 163 (31 placebo, 132 influenza vaccine) children aged 5 to 9 years with mild intermittent and moderate persistent asthma Exclusion criteria: history of allergy to egg protein or thimerosal No details of past vaccination against influenza | |
| Interventions | Vaccination type: intramuscular injection of trivalent inactivated influenza vaccine, 2 doses (28 days apart) Fluzone1 (Sanofi Pasteur) A/New Caledonia/20/99 (H1N1), A/Panama/2007/99 (H3N2), B/Victoria/504/2000. Placebo: injection used | |
| Outcomes | Primary outcome: adverse events (systemic and local) Secondary outcomes: pulmonary function tests (force expiratory volume in 1 second (FEV1) 5 days after each vaccination) and immunogenicity | |
| Notes | Sponsored by Sanofi Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Single blind, the contents of the syringe were shielded from the participants and administered by a clinician who was not involved in assessment |
| Blinding of outcome assessment (detection bias) | Low risk | "All the data were recorded by research nurses and physicians who were not aware of the product administered to individuals in the study" |
| Incomplete outcome data (attrition bias) | Unclear risk | No details of drop‐outs |
| Methods | Randomisation: computer‐generated random numbers | |
| Participants | Location: 2 paediatric allergy practices in Seattle (WA) and 1 in Stockton | |
| Interventions | Vaccination type: intranasal influenza virus trivalent, types A and B, live, cold‐adapted (CAIV‐T) | |
| Outcomes | The primary outcome index was the % change in % predicted FEV1 before and after vaccination. Peak flows, clinical asthma symptom scores and night‐time awakening scores were measured daily from 7 days pre‐ to 28 days post‐vaccination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind (intra‐nasal placebo contents described) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
| Methods | Randomisation: no details | |
| Participants | Location: Newcastle, UK | |
| Interventions | Parallel design double blind | |
| Outcomes | Spirometry (FEV1) and airways responsiveness (methacholine dose that caused a 20% fall in the forced expiratory volume in 1 second (FEV1) (PD20)). Both were measured twice at an interval of 2 weeks before vaccination and compared with measurements at 48 and 96 hours post‐vaccination | |
| Notes | Data presented without standard deviations. The study was powered to detect a halving of the geometric mean PD20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Patients were assigned in double blind fashion |
| Blinding of participants and personnel (performance bias) | Unclear risk | Placebo vaccine but no further details |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Very short follow‐up |
| Methods | Randomisation: no details | |
| Participants | Location: Ankara, Turkey | |
| Interventions | Cross‐over design, single blind | |
| Outcomes | Asthma symptoms, morning and evening peak expiratory flow (PEF), bronchodilator use all for 1 week following vaccination. Spirometry with methacholine challenge at baseline and 2 weeks after vaccination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details (we employed a randomised cross‐over design) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Single‐blind (but not clear which group was blinded, presumably participants) |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No details of drop‐outs |
| Methods | Randomisation: stratified into 3 age groups (15 to 29 years, 30 to 49 years, 50 years or more) Patients selected themselves by choosing a folded piece of paper marked A or B inside | |
| Participants | Location: 9 centres in Finland, asthmatic patients living in the community | |
| Interventions | Vaccination type: split influenza vaccine (H3N2, B) with subviron component (H1N1) 0.5 mL intramuscular injection | |
| Outcomes | Early: daily PEF readings, symptom score, daily medication for first week | |
| Notes | The incidence of influenza was very low in Finland in the follow‐up period. Subgroup analysis was performed on the early outcomes to investigate the change in PEF in different asthma types | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Folded pieced of paper marked A or B were placed in a bowl and selected by the participants |
| Allocation concealment (selection bias) | Low risk | Ampoules were labelled in the laboratory and known only to the packer |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo saline injection |
| Blinding of outcome assessment (detection bias) | Low risk | Contents of ampoules unknown |
| Incomplete outcome data (attrition bias) | Low risk | 318/322 completed the first 3 weeks of the study |
| Methods | Randomisation: no details | |
| Participants | Location: Minami‐Fukuoka Chest Hospital, Japan. In‐patients on asthma ward. | |
| Interventions | Vaccination type: intranasal cold‐adapted recombinant trivalent influenza vaccine (H1N1, H3N2, B). Dose 0.3 mL both nostrils by nasal spray | |
| Outcomes | Early: "asthma attacks", school absence | |
| Notes | Baseline serology was similar in vaccinated and placebo groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | High risk | High proportion of withdrawals (14/45) |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants were not randomised to active treatment or control (age/sex‐matched controls were selected for the control group) | |
| Non‐randomised before and after study | |
| Mixed population of patients with rhinitis and asthma with no separate data for asthmatic patients | |
| Not randomised | |
| Not on children with asthma | |
| No randomisation. No separate asthma data, mixed group of allergic patients | |
| Not on children with asthma | |
| Comment on Bueving 2003 study | |
| Patients with mild and severe asthma randomised to 15 µg or 30 µg H1N1 influenza vaccination, but no placebo arm used in the trial | |
| Not clearly stated as being randomised and no response from authors | |
| Quasi‐randomised as patients were alternately allocated to treatment groups | |
| Not randomised | |
| Cohort study | |
| Not stated as randomised and no response from authors | |
| Not stated as randomised | |
| Not randomised | |
| No asthma outcomes measured | |
| No randomisation of vaccination in asthmatics (no control intervention) | |
| No separate data on asthmatic patients (study of children in 7 chronic disease categories) | |
| No randomisation of vaccination (comparison of influenza vaccination in asthmatics without asthma symptoms or with acute asthma) | |
| Non‐randomised study | |
| Case control study (not randomised) | |
| No asthma outcomes measured | |
| Self‐selected treatment group (no randomisation) | |
| Not randomised | |
| No randomisation of asthmatic patients | |
| Not randomised |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐related asthma exacerbations Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 1 Influenza‐related asthma exacerbations. | ||||
| 1.1 Number of participants with influenza‐related exacerbations | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Number of patients with any asthma exacerbation | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration of influenza‐related asthma exacerbation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 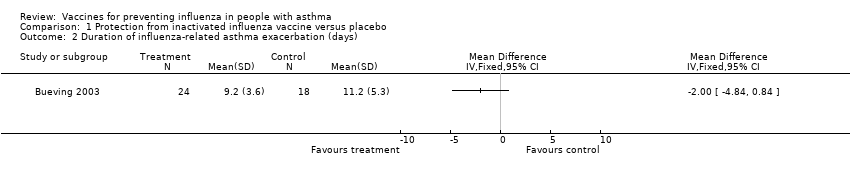 Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 2 Duration of influenza‐related asthma exacerbation (days). | ||||
| 3 Severity of influenza‐related asthma exacerbation (symptom score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 3 Severity of influenza‐related asthma exacerbation (symptom score). | ||||
| 4 Difference in symptom score during influenza positive weeks Show forest plot | 1 | Mean difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 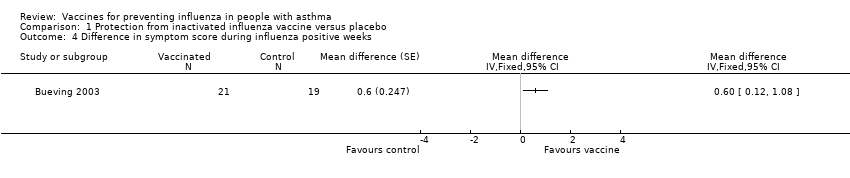 Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 4 Difference in symptom score during influenza positive weeks. | ||||
| 5 Proportion of patients with minimum important difference in total symptom score (influenza‐positive weeks) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 5 Proportion of patients with minimum important difference in total symptom score (influenza‐positive weeks). | ||||
| 6 FEV1 (% predicted) during influenza positive weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6 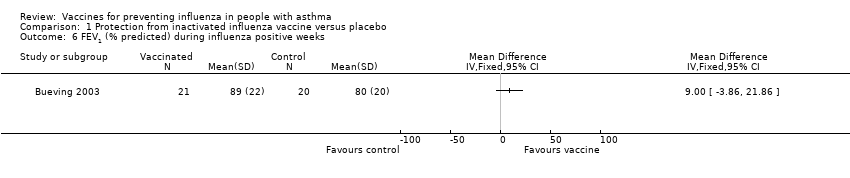 Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 6 FEV1 (% predicted) during influenza positive weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Asthma exacerbation within 2 weeks Show forest plot | 2 | 2238 | Risk Difference (Random, 95% CI) | 0.01 [‐0.01, 0.04] | ||||||||||||
| Analysis 2.1  Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 1 Asthma exacerbation within 2 weeks. | ||||||||||||||||
| 1.1 Adults | 2 | 1526 | Risk Difference (Random, 95% CI) | 0.02 [‐0.01, 0.05] | ||||||||||||
| 1.2 Children | 1 | 712 | Risk Difference (Random, 95% CI) | 0.01 [‐0.04, 0.05] | ||||||||||||
| 2 Asthma exacerbation within 3 days Show forest plot | 2 | 2212 | Risk Difference (Random, 95% CI) | 0.01 [‐0.03, 0.05] | ||||||||||||
| Analysis 2.2  Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 2 Asthma exacerbation within 3 days. | ||||||||||||||||
| 3 Asthma exacerbation within 2 weeks (subgrouped by previous vaccination status) Show forest plot | 2 | 2206 | Risk Difference (Random, 95% CI) | 0.01 [‐0.02, 0.04] | ||||||||||||
| Analysis 2.3 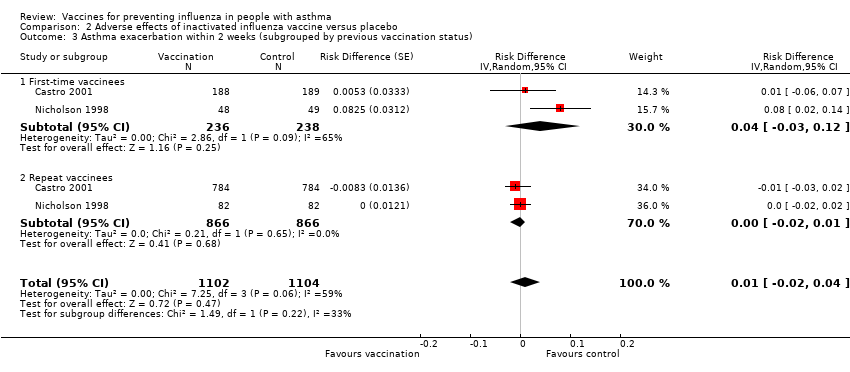 Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 3 Asthma exacerbation within 2 weeks (subgrouped by previous vaccination status). | ||||||||||||||||
| 3.1 First‐time vaccinees | 2 | 474 | Risk Difference (Random, 95% CI) | 0.04 [‐0.03, 0.12] | ||||||||||||
| 3.2 Repeat vaccinees | 2 | 1732 | Risk Difference (Random, 95% CI) | ‐0.00 [‐0.02, 0.01] | ||||||||||||
| 4 Hospital admission (0 to 14 days post vaccination) Show forest plot | 1 | Risk Difference (Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.4  Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 4 Hospital admission (0 to 14 days post vaccination). | ||||||||||||||||
| 5 Number of symptom‐free days in 2 weeks after vaccination Show forest plot | 1 | Mean Difference (Random, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.5  Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 5 Number of symptom‐free days in 2 weeks after vaccination. | ||||||||||||||||
| 6 ≥ 1 day off school or work Show forest plot | 2 | 2648 | Risk Difference (Random, 95% CI) | ‐0.00 [‐0.02, 0.01] | ||||||||||||
| Analysis 2.6  Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 6 ≥ 1 day off school or work. | ||||||||||||||||
| 7 Medical consultation (0 to 14 days after immunisation) Show forest plot | 3 | 2894 | Risk Difference (Random, 95% CI) | 0.00 [‐0.01, 0.01] | ||||||||||||
| Analysis 2.7 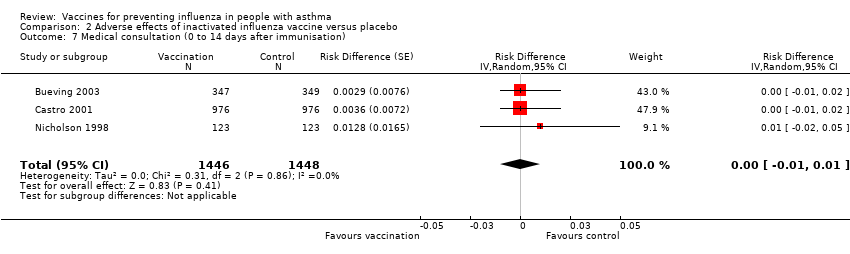 Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 7 Medical consultation (0 to 14 days after immunisation). | ||||||||||||||||
| 8 Patients at least 15% fall in FEV1 within 5 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.8 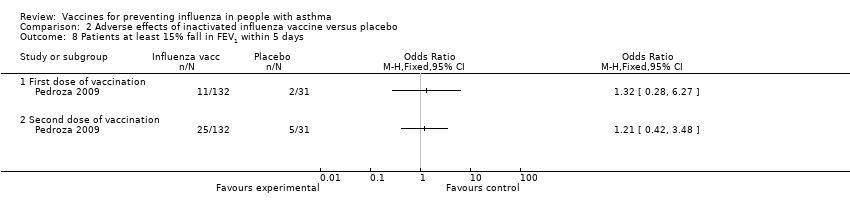 Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 8 Patients at least 15% fall in FEV1 within 5 days. | ||||||||||||||||
| 8.1 First dose of vaccination | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 8.2 Second dose of vaccination | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 9 Fall in mean peak flow (% baseline) days 2 to 4 Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 2.9 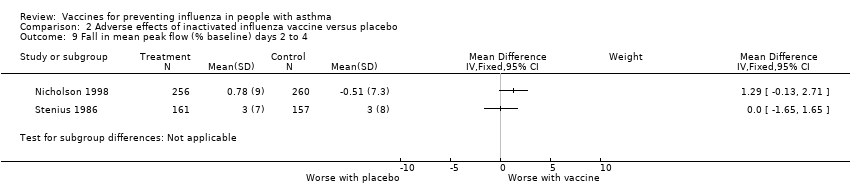 Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 9 Fall in mean peak flow (% baseline) days 2 to 4. | ||||||||||||||||
| 10 New or increased oral corticosteroid use (0 to 14 days after immunisation) Show forest plot | 2 | 2209 | Risk Difference (Random, 95% CI) | 0.00 [‐0.01, 0.02] | ||||||||||||
| Analysis 2.10 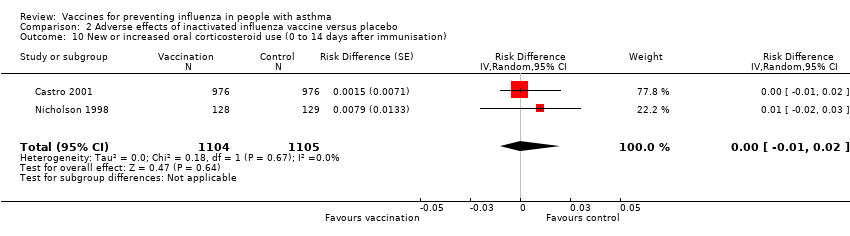 Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 10 New or increased oral corticosteroid use (0 to 14 days after immunisation). | ||||||||||||||||
| 11 Increased nebuliser usage within 3 days Show forest plot | 1 | Risk Difference (Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.11 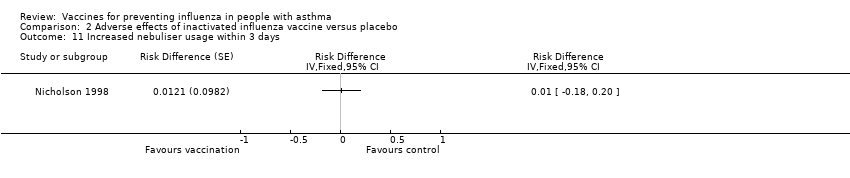 Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 11 Increased nebuliser usage within 3 days. | ||||||||||||||||
| 12 Increased use of rescue medication following vaccination (days 1 to 3) Show forest plot | 4 | 2810 | Risk Difference (Random, 95% CI) | ‐0.00 [‐0.02, 0.01] | ||||||||||||
| Analysis 2.12  Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 12 Increased use of rescue medication following vaccination (days 1 to 3). | ||||||||||||||||
| 13 Change in airways responsiveness Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.13
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 13 Change in airways responsiveness. | ||||||||||||||||
| 14 Change in asthma symptoms in the week following vaccination Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.14
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 14 Change in asthma symptoms in the week following vaccination. | ||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||
| 1 Hospital admission for asthma exacerbation Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.1  Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 1 Hospital admission for asthma exacerbation. | ||||||||||||
| 2 Asthma exacerbations in the month after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.2 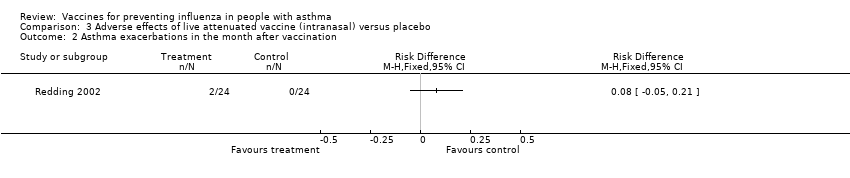 Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 2 Asthma exacerbations in the month after vaccination. | ||||||||||||
| 3 Asthma exacerbations in the week following vaccination Show forest plot | Other data | No numeric data | ||||||||||
| Analysis 3.3
Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 3 Asthma exacerbations in the week following vaccination. | ||||||||||||
| 4 Mean FEV1 at 2 to 5 days post vaccination (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.4  Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 4 Mean FEV1 at 2 to 5 days post vaccination (% predicted). | ||||||||||||
| 5 Number of patients with significant fall in FEV1 (over 12% to 15% or 50 mL) on day 2 to 4 Show forest plot | 2 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.12, 0.15] | ||||||||
| Analysis 3.5  Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 5 Number of patients with significant fall in FEV1 (over 12% to 15% or 50 mL) on day 2 to 4. | ||||||||||||
| 6 Fall in mean FEV1 (L) (day 2 to 4) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.6  Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 6 Fall in mean FEV1 (L) (day 2 to 4). | ||||||||||||
| 7 Number of puffs of beta2‐agonist per day (in month following vaccination) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.7 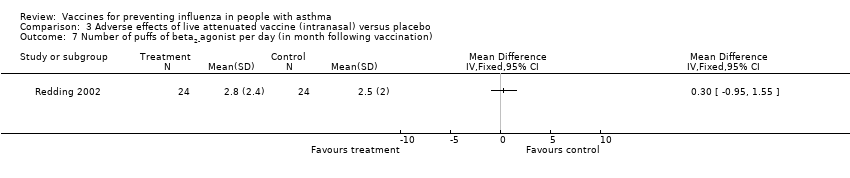 Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 7 Number of puffs of beta2‐agonist per day (in month following vaccination). | ||||||||||||
| 8 Morning peak flow of greater than 30% below baseline at least once in the 4 weeks after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.8 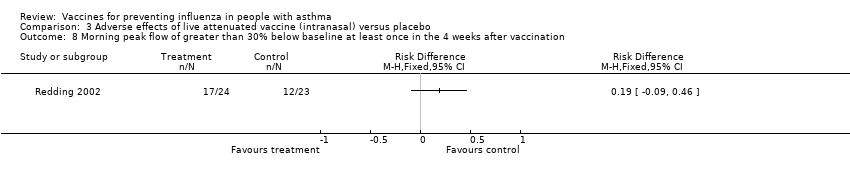 Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 8 Morning peak flow of greater than 30% below baseline at least once in the 4 weeks after vaccination. | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in incidence of asthma exacerbation over total study period Show forest plot | 1 | % Rate difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1 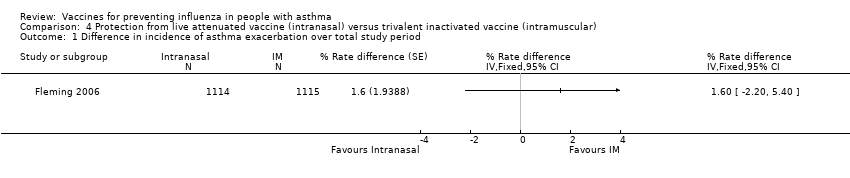 Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 1 Difference in incidence of asthma exacerbation over total study period. | ||||
| 2 Hospitalisations due to respiratory illness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 2 Hospitalisations due to respiratory illness. | ||||
| 3 Days off school or work (incidence rates) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3 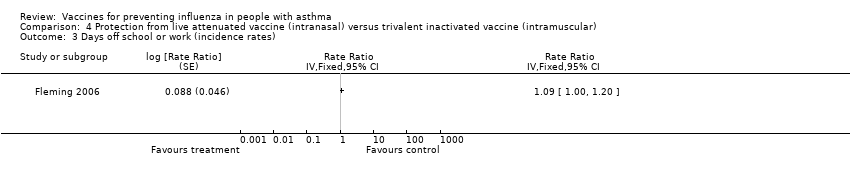 Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 3 Days off school or work (incidence rates). | ||||
| 4 Unscheduled healthcare visits (incidence rates) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4 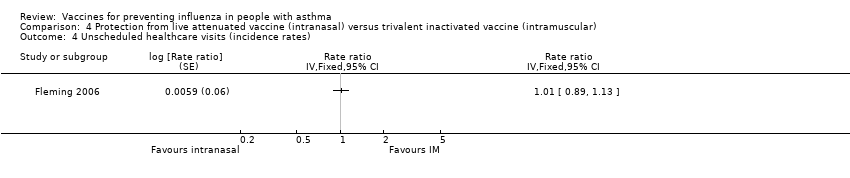 Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 4 Unscheduled healthcare visits (incidence rates). | ||||
| 5 Children with serious adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.5  Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 5 Children with serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subjects reporting wheeze in the first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 1 Subjects reporting wheeze in the first 15 days. | ||||
| 2 Subjects reporting runny nose or nasal congestion in the first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2 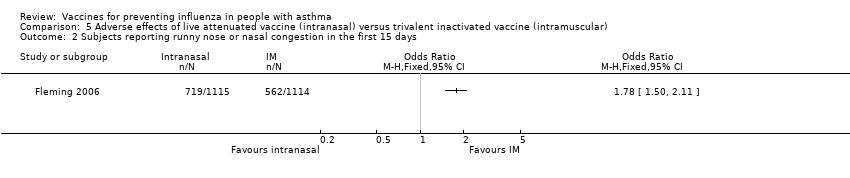 Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 2 Subjects reporting runny nose or nasal congestion in the first 15 days. | ||||
| 3 Subjects reporting bronchospasm as an adverse event in first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 3 Subjects reporting bronchospasm as an adverse event in first 15 days. | ||||
| 4 Subjects reporting rhinitis as an adverse event in the first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 4 Subjects reporting rhinitis as an adverse event in the first 15 days. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Protection from inactivated influenza vaccine versus placebo, outcome: 1.1 Influenza‐related asthma exacerbations.

Forest plot of comparison: 2 Split virus or surface antigen vaccine versus placebo (adverse events in first two weeks), outcome: 2.1 Asthma exacerbation within two weeks.
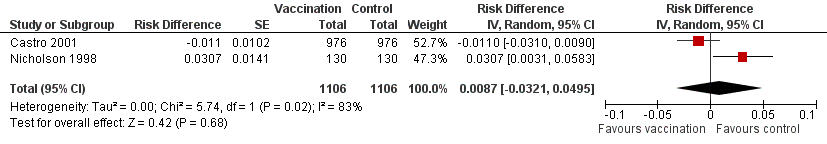
Forest plot of comparison: 2 Split virus or surface antigen vaccine versus placebo (adverse events in first two weeks), outcome: 2.2 Asthma exacerbation within three days.

Forest plot of comparison: 2 Split virus or surface antigen vaccine versus placebo (adverse events in first two weeks), outcome: 2.3 Asthma exacerbation within two weeks (subgrouped by previous vaccination status).

Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 1 Influenza‐related asthma exacerbations.
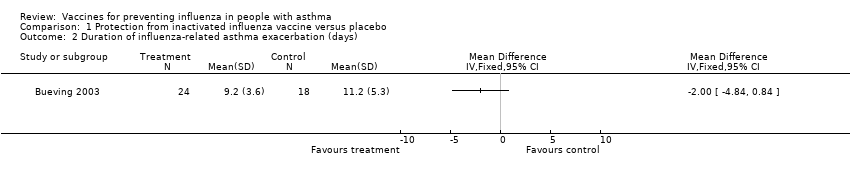
Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 2 Duration of influenza‐related asthma exacerbation (days).

Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 3 Severity of influenza‐related asthma exacerbation (symptom score).

Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 4 Difference in symptom score during influenza positive weeks.
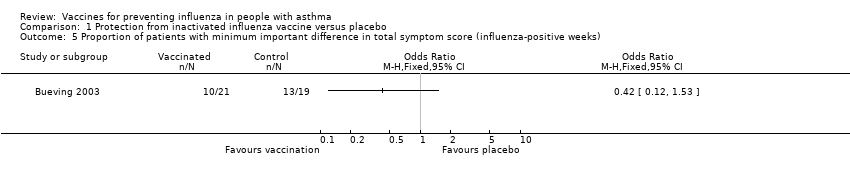
Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 5 Proportion of patients with minimum important difference in total symptom score (influenza‐positive weeks).

Comparison 1 Protection from inactivated influenza vaccine versus placebo, Outcome 6 FEV1 (% predicted) during influenza positive weeks.

Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 1 Asthma exacerbation within 2 weeks.
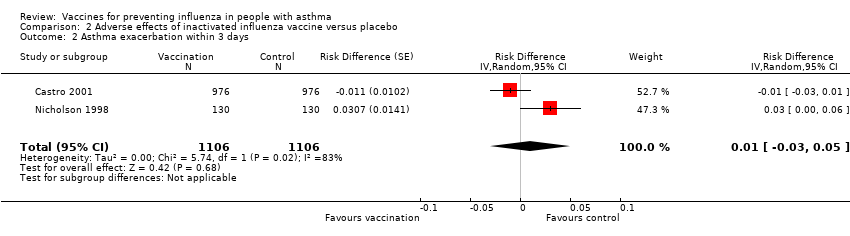
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 2 Asthma exacerbation within 3 days.

Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 3 Asthma exacerbation within 2 weeks (subgrouped by previous vaccination status).

Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 4 Hospital admission (0 to 14 days post vaccination).
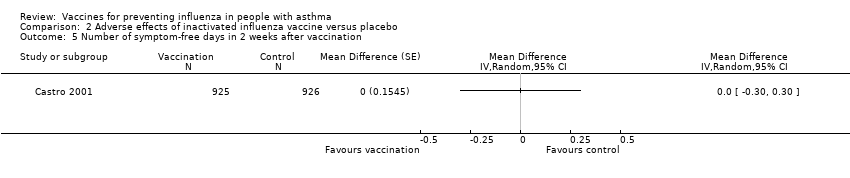
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 5 Number of symptom‐free days in 2 weeks after vaccination.

Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 6 ≥ 1 day off school or work.
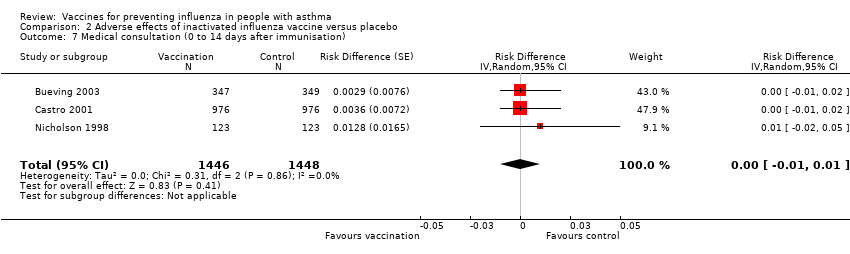
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 7 Medical consultation (0 to 14 days after immunisation).

Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 8 Patients at least 15% fall in FEV1 within 5 days.
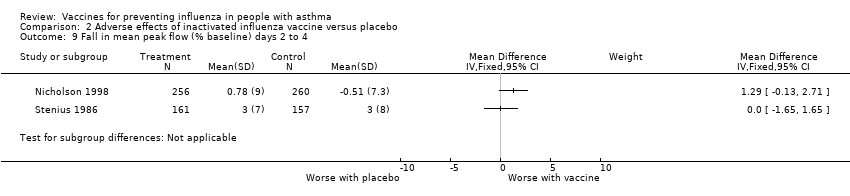
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 9 Fall in mean peak flow (% baseline) days 2 to 4.
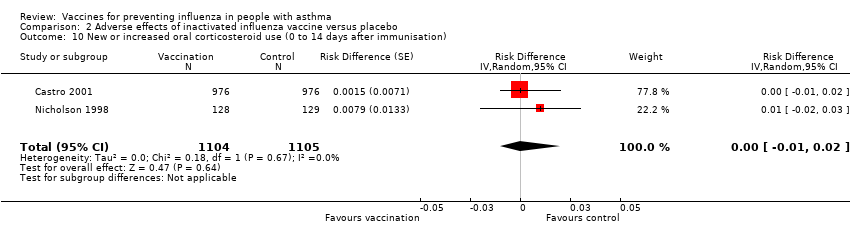
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 10 New or increased oral corticosteroid use (0 to 14 days after immunisation).
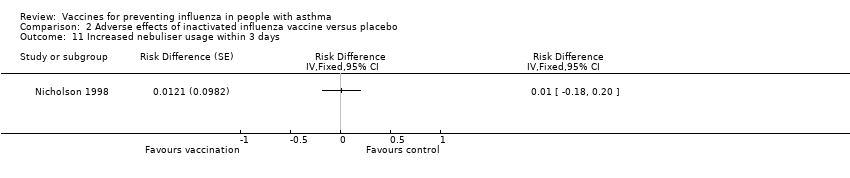
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 11 Increased nebuliser usage within 3 days.
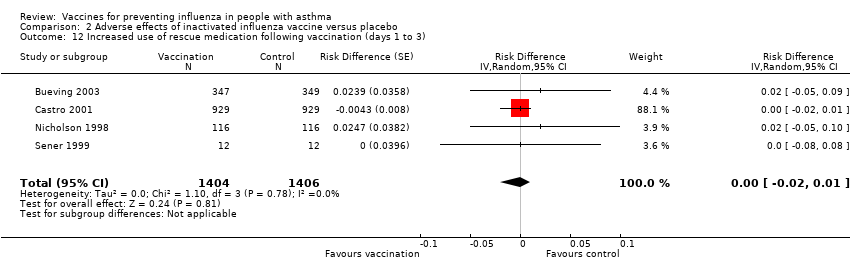
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 12 Increased use of rescue medication following vaccination (days 1 to 3).
| Study | |
| Kut 1999 | No significant change in PC20 following either placebo or vaccine. |
| Reid 1998 | No significant difference found in placebo group (n=5) or vaccination group (n=17) in either mean PD20 or mean FEV1 (tested by analysis of variance ANOVA). No individual patient in either group showed a change of PD20 of more than two‐fold. |
| Sener 1999 | No significant difference between placebo and vaccine in PD20 at 2 weeks. Vaccine 2.96(SD 3.2) and placebo 2.76 (SD 2.91) |
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 13 Change in airways responsiveness.
| Study | |
| Govaert 1992 | No adverse reactions on asthma symptoms reported from any of the 14 asthmatics immunised with split‐virus vaccine or the 11 astmatics given placebo. (Communication from author) |
| Hahn 1980 | No significant deterioration in home Peak Flow measurement in the split vaccine (25 patients), subunit vaccine (25 patients) or placebo group (16 patients) in the two weeks following vaccination. No numerical data given. |
| Sener 1999 | No significant difference in symptom scores in the week after vaccine. Placebo mean score 4.66 (SD 7.3), vaccine mean score 4.92 (SD 7.56) |
| Stenius 1986 | Similar in the vaccine and placebo groups. No numerical data provided. |
Comparison 2 Adverse effects of inactivated influenza vaccine versus placebo, Outcome 14 Change in asthma symptoms in the week following vaccination.
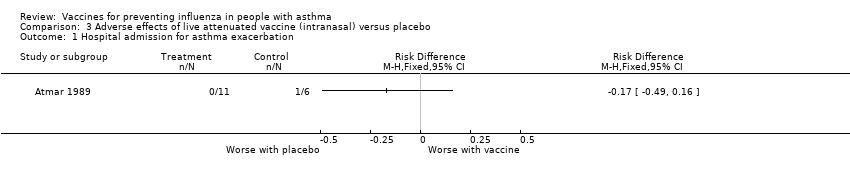
Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 1 Hospital admission for asthma exacerbation.

Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 2 Asthma exacerbations in the month after vaccination.
| Study | |
| Miyazaki 1993 | No asthma attacks were apparent following vaccination. Evaluation was made difficult by an Adenovirus outbreak during the study period. No defintion of asthma attack provided by the authors. |
| Tanaka 1993 | No asthma attacks were observed following vaccination (20 patients given CR vaccine and 25 given placebo). No defintion of asthma attack provided by the authors. |
Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 3 Asthma exacerbations in the week following vaccination.
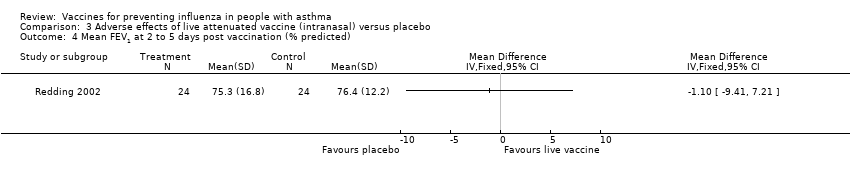
Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 4 Mean FEV1 at 2 to 5 days post vaccination (% predicted).
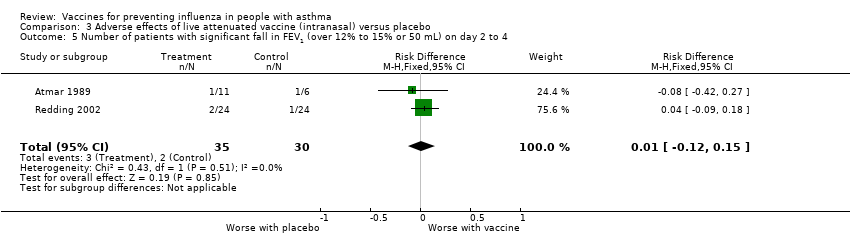
Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 5 Number of patients with significant fall in FEV1 (over 12% to 15% or 50 mL) on day 2 to 4.
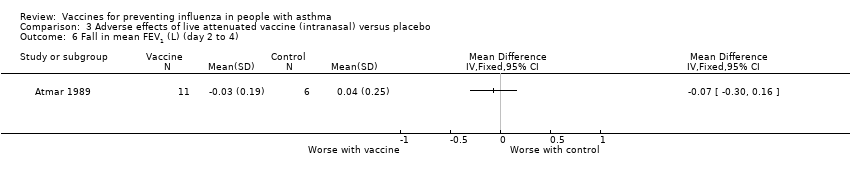
Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 6 Fall in mean FEV1 (L) (day 2 to 4).

Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 7 Number of puffs of beta2‐agonist per day (in month following vaccination).

Comparison 3 Adverse effects of live attenuated vaccine (intranasal) versus placebo, Outcome 8 Morning peak flow of greater than 30% below baseline at least once in the 4 weeks after vaccination.
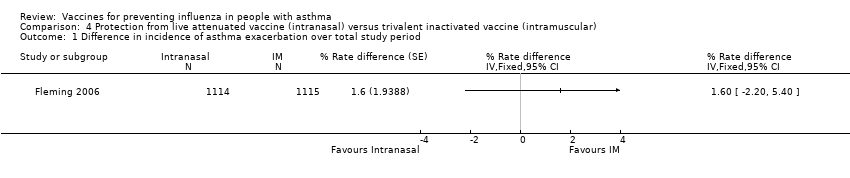
Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 1 Difference in incidence of asthma exacerbation over total study period.

Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 2 Hospitalisations due to respiratory illness.

Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 3 Days off school or work (incidence rates).

Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 4 Unscheduled healthcare visits (incidence rates).
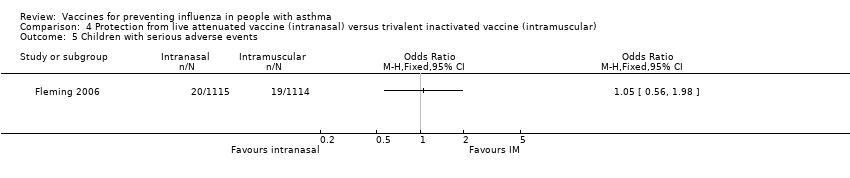
Comparison 4 Protection from live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 5 Children with serious adverse events.

Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 1 Subjects reporting wheeze in the first 15 days.
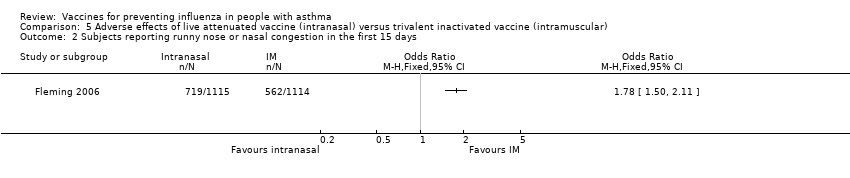
Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 2 Subjects reporting runny nose or nasal congestion in the first 15 days.
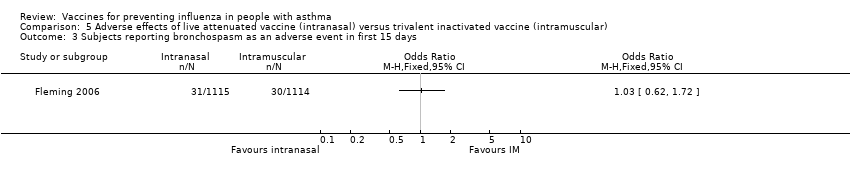
Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 3 Subjects reporting bronchospasm as an adverse event in first 15 days.
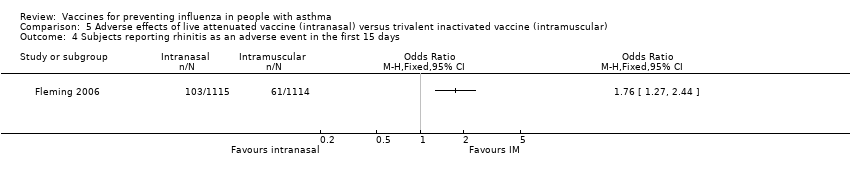
Comparison 5 Adverse effects of live attenuated vaccine (intranasal) versus trivalent inactivated vaccine (intramuscular), Outcome 4 Subjects reporting rhinitis as an adverse event in the first 15 days.
| Inactivated influenza vaccine versus placebo for people with asthma | ||||||
| Patient or population: children and adults with asthma | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adverse effects of inactivated influenza vaccine versus placebo | |||||
| Results from trials in children | ||||||
| Protection from experiencing an asthma exacerbation of any cause over the influenza season ‐ children (over 6 years of age) given inactivated influenza vaccine | 90 per 100 | 86 per 100 (81 to 90) | See comment | 696 | ⊕⊕⊕⊝ | Risks were calculated from risk difference in a single study (at low risk of bias) |
| Protection from experiencing an influenza‐related asthma exacerbation over the influenza season ‐ children (over 6 years of age) given inactivated influenza vaccine | 5 per 100 | 6 per 100 (3 to 9) | See comment | 696 | ⊕⊕⊕⊝ | Risks were calculated from risk difference in a single study (at low risk of bias) |
| Asthma exacerbation (adverse effects) caused by inactivated influenza vaccine, measured in the first 2 weeks following vaccination ‐ children (over 3 years of age) given inactivated influenza vaccine | 33 per 100 | 34 per 100 (29 to 38) | See comment | 712 | ⊕⊕⊕⊝ | Risks were calculated from paired proportions in a single cross‐over study (at low risk of bias) |
| Results from trials in adults3 | ||||||
| Protection from experiencing an asthma exacerbation of any cause over the influenza season ‐ adults given inactivated influenza vaccine | See comment | See comment | See comment | See comment | See comment | 2 parallel‐group studies in adults did not contribute to this outcome due to low levels of influenza infection in the season following vaccination |
| Protection from experiencing an influenza‐related asthma exacerbation over the influenza season ‐ adults given inactivated influenza vaccine | See comment | See comment | See comment | See comment | See comment | 2 parallel‐group studies in adults did not contribute to this outcome due to low levels of influenza infection in the season following vaccination |
| Asthma exacerbation (adverse effects) caused by inactivated influenza vaccine, measured in the first 2 weeks following vaccination ‐ adults given inactivated influenza vaccine | 25 per 100 | 27 per 100 (24 to 29) | See comment | 1526 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences (from paired proportions in 2 cross‐over studies) |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the reported risk difference of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Single study on children with 95% CI that included no difference between vaccination and placebo. 2 95% CI from the pooled results of two studies excluded the pre‐specified threshold of a 6% increase in the number of participants with an asthma exacerbation following influenza vaccination. 3One trial at low risk of bias and one trial at unclear risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐related asthma exacerbations Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Number of participants with influenza‐related exacerbations | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Number of patients with any asthma exacerbation | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration of influenza‐related asthma exacerbation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Severity of influenza‐related asthma exacerbation (symptom score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Difference in symptom score during influenza positive weeks Show forest plot | 1 | Mean difference (Fixed, 95% CI) | Totals not selected | |
| 5 Proportion of patients with minimum important difference in total symptom score (influenza‐positive weeks) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 FEV1 (% predicted) during influenza positive weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Asthma exacerbation within 2 weeks Show forest plot | 2 | 2238 | Risk Difference (Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 1.1 Adults | 2 | 1526 | Risk Difference (Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 1.2 Children | 1 | 712 | Risk Difference (Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2 Asthma exacerbation within 3 days Show forest plot | 2 | 2212 | Risk Difference (Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 3 Asthma exacerbation within 2 weeks (subgrouped by previous vaccination status) Show forest plot | 2 | 2206 | Risk Difference (Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 3.1 First‐time vaccinees | 2 | 474 | Risk Difference (Random, 95% CI) | 0.04 [‐0.03, 0.12] |
| 3.2 Repeat vaccinees | 2 | 1732 | Risk Difference (Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 4 Hospital admission (0 to 14 days post vaccination) Show forest plot | 1 | Risk Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Number of symptom‐free days in 2 weeks after vaccination Show forest plot | 1 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 6 ≥ 1 day off school or work Show forest plot | 2 | 2648 | Risk Difference (Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7 Medical consultation (0 to 14 days after immunisation) Show forest plot | 3 | 2894 | Risk Difference (Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 8 Patients at least 15% fall in FEV1 within 5 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 First dose of vaccination | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Second dose of vaccination | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Fall in mean peak flow (% baseline) days 2 to 4 Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 New or increased oral corticosteroid use (0 to 14 days after immunisation) Show forest plot | 2 | 2209 | Risk Difference (Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 11 Increased nebuliser usage within 3 days Show forest plot | 1 | Risk Difference (Fixed, 95% CI) | Totals not selected | |
| 12 Increased use of rescue medication following vaccination (days 1 to 3) Show forest plot | 4 | 2810 | Risk Difference (Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 13 Change in airways responsiveness Show forest plot | Other data | No numeric data | ||
| 14 Change in asthma symptoms in the week following vaccination Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital admission for asthma exacerbation Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Asthma exacerbations in the month after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Asthma exacerbations in the week following vaccination Show forest plot | Other data | No numeric data | ||
| 4 Mean FEV1 at 2 to 5 days post vaccination (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with significant fall in FEV1 (over 12% to 15% or 50 mL) on day 2 to 4 Show forest plot | 2 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.12, 0.15] |
| 6 Fall in mean FEV1 (L) (day 2 to 4) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of puffs of beta2‐agonist per day (in month following vaccination) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Morning peak flow of greater than 30% below baseline at least once in the 4 weeks after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in incidence of asthma exacerbation over total study period Show forest plot | 1 | % Rate difference (Fixed, 95% CI) | Totals not selected | |
| 2 Hospitalisations due to respiratory illness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Days off school or work (incidence rates) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 4 Unscheduled healthcare visits (incidence rates) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | Totals not selected | |
| 5 Children with serious adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subjects reporting wheeze in the first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Subjects reporting runny nose or nasal congestion in the first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Subjects reporting bronchospasm as an adverse event in first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Subjects reporting rhinitis as an adverse event in the first 15 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

