Vaccines for preventing influenza in people with asthma
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomisation: no details. | |
| Participants | Location:Houston, Texas. | |
| Interventions | Vaccine Type: Intranasal bivalent (H3N2+H1N1) influenza A vaccine. 0.25 ml per nostril. | |
| Outcomes | Early: Lung function tests on days 0, 3‐4, and 7; performed in the mornings (no bronchodilators taken before testing). The authors regarded a reduction in FEV1 of 13% (or greater) from baseline to be clinically significant. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: by hospital number | |
| Participants | Location: Denver, Colorado. Residential Asthma Care Centre. | |
| Interventions | Vaccination Type: Bivalent (A/Port Chalmers/1/73 and B/Hong Kong/5/72) vaccine containing killed influenza virus. 0.25 ml or 0.5 ml given. | |
| Outcomes | Early: Change in peak flow and mean number of nebulised treatments given. | |
| Notes | First arm of crossover trial included. Data expressed as Mean difference in % change in predicted Peak Flow, and Nebuliser usage, between vaccinated and non‐vaccinated groups. SD calculated from published SEM. CAUTION: No baseline comparability of the two groups is reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Methods | Randomisation took place by the manufacturer when packing vaccine and placebo, from a computer generated list. | |
| Participants | Location: Rotterdam, Netherlands community based study. | |
| Interventions | Vaccination type: inactivated influenza vaccine intramuscular injection. The vaccine composition for 1999‐2000 was a combination of A/Sydney/5/97 H3N2‐like, A/Beijing/262/95‐like and B/Beijing/184/93‐like strains and for 2000‐2001 A/Moscow/10/99 H3N2‐like, A/New Caledonia/20/99 H1N1‐like and B/Beijing/184/93‐like strains as advised by the World Health Organisation Placebo group: The placebo consisted of a buffered phosphate solution with the same pH value and similar appearance as the inactivated influenza vaccine. | |
| Outcomes | Primary outcome: Influenza‐related asthma exacerbations (number, duration and severity). | |
| Notes | Power calculations suggested 600 patients needed to be enrolled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Cross‐over design. | |
| Participants | Location: 19 centres in the USA | |
| Interventions | Vaccination type: Heat‐killed trivalent split‐virus influenza type A and B vaccine (Fluzone, Aventis‐Pasteur). | |
| Outcomes | Primary outcome: Exacerbation of asthma within 14 days of vaccination. | |
| Notes | Bubble sizes were noted to be larger in the placebo syringes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomisation: Stratified by four morbidity categories | |
| Participants | Location: Netherlands | |
| Interventions | Vaccination type: purified split vaccine H1N1, H3N2, B45/90, B1/87 given intramuscularly. | |
| Outcomes | Early: adverse reactions (recalled by the patients after 4 weeks). | |
| Notes | No serologically confirmed influenza was seen in either the immunised or the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomisation: Stratified by baseline FEV1 (no details of allocation concealment) | |
| Participants | Location: Wurzburg, Germany | |
| Interventions | Vaccination types: | |
| Outcomes | Lung function measurements in Clinic, (two weeks before and after treatment). Home measurement of peak flow (best of three, twice daily) and symptoms recorded by patients (including breathing difficulty). | |
| Notes | No lung function measurements documented, only "no significant change in lung function following either vaccination or placebo" (even in the patients on systemic steroids). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: no details | |
| Participants | Location: Istanbul, Turkey | |
| Interventions | Vaccination type: Inactivated influenza vaccine given subcutaneously | |
| Outcomes | PC20 for methacholine challenge before vaccine and after 24 hours. | |
| Notes | PC20 (SD) in the placebo group was 7.02 (9.3) before challenge and 7.3 (3.6) after 24 hours. In the vaccine group PC20 was 9.5(10.6) before vaccine and 9.8(9.3) afterwards. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: no details | |
| Participants | Location: Minami‐Fukuoka chest hospital, Japan. In‐patients on asthma ward. | |
| Interventions | Vaccination Type: intranasal cold‐adapted recombinant trivalent influenza vaccine (H1N1, H3N2, B). Dose 0.3 ml by nasal spray. | |
| Outcomes | Early: asthma attacks | |
| Notes | Serology at the start was NOT comparable with 17/19 in the vaccinated group having a starting titre over 1:64 whereas only 8/25 in the non‐vaccinated group had a starting titre over 1:64 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: sealed envelopes, computer‐generated randomisation code provided by vaccine manufacturer. | |
| Participants | Location: nine respiratory centres and two asthma clinics in the United Kingdom. | |
| Interventions | Crossover design with two intramuscular injections given two weeks apart in random order. | |
| Outcomes | Outcome measures: primary clinical outcome was an asthma exacerbation within 72 hours of injection (defined as 20% fall in Peak Flow compared to lowest of the three days before vaccination). Also measured were change in mean PEF, inhaled Beta‐agonist use (72 hours before and after injection), antibiotic and oral steroid use for 7 days after injection, unscheduled medical attendance and hospital admission for 7 days after each injection. Symptom scores were also analysed for 72 hours before and after injection of vaccine or placebo. | |
| Notes | Peak flow was examined using percentage change for individuals of the worst test for 3 days before and after injection and also using the mean test result over the same periods. On all occasions only the best of three blows was used for the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomisation: Stratified by lung function results | |
| Participants | Location: Germany | |
| Interventions | Vaccination type: Whole virus, Split virus and Subunit vaccines. (A/Texas, A/USSR, B/Hong Kong). Patients were revaccinated at 6 weeks. | |
| Outcomes | Pulmonary function measured for 7 days before vaccination and compared with 3 days after vaccination. | |
| Notes | No placebo group and results stated as "no significant change in Lung function for individual or for the combined vaccines." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: computer generated random numbers | |
| Participants | Location: Two paediatric allergy practices in Seattle (Washington) and one in Stockton. | |
| Interventions | Vaccination type: Intranasal influenza virus trivalent, types A and B, live, cold‐adapted (CAIV‐T). | |
| Outcomes | The primary outcome index was the percent change in percent predicted FEV1 before and after vaccination. Peak flows, clinical asthma symptom scores and nighttime awakening scores were measured daily from 7 days pre‐ to 28 days postvaccination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: no details. | |
| Participants | Location: Newcastle, UK | |
| Interventions | Parallel design double blind. | |
| Outcomes | Spirometry (FEV1) and airways responsiveness (PD 20 methacholine). Both were measured twice at an interval of two weeks before vaccination and compared with measurements at 48 and 96 hours post‐vaccination. | |
| Notes | Data presented without standard deviations. The study was powered to detect a halving of the geometric mean PD 20. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomisation: no details | |
| Participants | Location: Ankara, Turkey | |
| Interventions | Cross‐over design, single blind. | |
| Outcomes | Asthma symptoms, morning and evening PEF, bronchodilator use all for one week following vaccination. Spirometry with methacholine challenge at baseline and 2 weeks after vaccination. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Randomisation: stratified into three age groups (15‐29, 30‐49, 50 or more) Patients selected themselves by choosing a folded peice of paper marked A or B inside. | |
| Participants | Location: 9 centres in Finland, asthmatic patients living in the community. | |
| Interventions | Vaccination Type: split influenza vaccine (H3N2, B) with subviron component (H1N1) 0.5 ml intramuscular injection. | |
| Outcomes | Early: daily PEF readings, symptom score, daily medication for first week. | |
| Notes | The incidence of influenza was very low in Finland in the follow‐up period. Sub‐group analysis was performed on the early outcomes to investigate the change in peak flow in different asthma types. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomisation: no details | |
| Participants | Location: Minami‐Fukuoka chest hospital, Japan. In‐patients on asthma ward. | |
| Interventions | Vaccination Type: intranasal cold‐adapted recombinant trivalent influenza vaccine (H1N1, H3N2, B). Dose 0.3 ml both nostrils by nasal spray. | |
| Outcomes | Early: "Asthma attacks", school absence. | |
| Notes | Baseline serology was similar in vaccinated and placebo groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Non‐randomised before and after study | |
| Mixed population of patients with rhinitis and asthma with no separate data for asthmatics | |
| No randomisation. No separate asthma data, mixed group of allergic patients. | |
| Not clearly stated as being randomised and no response from authors. | |
| Not randomised. | |
| Not stated as randomised and no response from authors. | |
| Not randomised | |
| No asthma outcomes measured. | |
| No randomisation of vaccination in asthmatics (no control intervention). | |
| No separate data on asthmatic patients (study of children in seven chronic disease categories). | |
| No randomisation of vaccination (comparison of influenza vaccination in asthmatics without asthma symptoms or with acute asthma). | |
| No asthma outcomes measured. | |
| Self‐selected treatment group (no randomisation). | |
| Not randomised. | |
| No randomisation of asthmatic patients. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Patients with an exacerbation of asthma Show forest plot | 2 | 4412 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] | ||||||||||||
| Analysis 1.1  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 1 Patients with an exacerbation of asthma. | ||||||||||||||||
| 1.1 First‐time vaccinees | 2 | 948 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] | ||||||||||||
| 1.2 Repeat vaccinees | 2 | 3464 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] | ||||||||||||
| 2 Patients with a fall in PEF of over 30% Show forest plot | 2 | 4252 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] | ||||||||||||
| Analysis 1.2  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 2 Patients with a fall in PEF of over 30%. | ||||||||||||||||
| 2.1 First‐time vaccinees | 1 | 194 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.11] | ||||||||||||
| 2.2 Repeat vacinees | 1 | 328 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] | ||||||||||||
| 2.3 Vaccination status unspecified | 1 | 3730 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] | ||||||||||||
| 3 Fall in mean Peak Flow (% baseline) days 2‐4 Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 1.3  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 3 Fall in mean Peak Flow (% baseline) days 2‐4. | ||||||||||||||||
| 4 Change in airways responsiveness Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 1.4
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 4 Change in airways responsiveness. | ||||||||||||||||
| 5 Increased nebuliser usage (days 1‐3) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 1.5  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 5 Increased nebuliser usage (days 1‐3). | ||||||||||||||||
| 6 Increased use of bronchodilators following vaccination (days 1‐3) Show forest plot | 3 | 4228 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] | ||||||||||||
| Analysis 1.6  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 6 Increased use of bronchodilators following vaccination (days 1‐3). | ||||||||||||||||
| 7 Hospital admission (0‐14 days post‐immunisation) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 1.7  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 7 Hospital admission (0‐14 days post‐immunisation). | ||||||||||||||||
| 8 Medical consultation (0‐14 days after immunisation) Show forest plot | 2 | 4396 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] | ||||||||||||
| Analysis 1.8  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 8 Medical consultation (0‐14 days after immunisation). | ||||||||||||||||
| 9 New or increased oral steroid use (0‐14 days after immunisation) Show forest plot | 2 | 4419 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] | ||||||||||||
| Analysis 1.9  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 9 New or increased oral steroid use (0‐14 days after immunisation). | ||||||||||||||||
| 10 One or more day off school or work Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 1.10  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 10 One or more day off school or work. | ||||||||||||||||
| 11 Number of symptom free days in fortnight after vaccination Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 1.11  Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 11 Number of symptom free days in fortnight after vaccination. | ||||||||||||||||
| 12 Change in asthma symptoms in the week following vaccination. Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 1.12
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 12 Change in asthma symptoms in the week following vaccination.. | ||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza related asthma exacerbations Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Split virus or Surface Antigen vaccine v. Placebo (Late Outcomes), Outcome 1 Influenza related asthma exacerbations. | ||||
| 1.1 Number of participants with influenza related exacerbations | 1 | 696 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 1.2 Number of patients with any asthma exacerbation | 1 | 696 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.00] |
| 2 Duration of influenza related asthma exacerbation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Split virus or Surface Antigen vaccine v. Placebo (Late Outcomes), Outcome 2 Duration of influenza related asthma exacerbation (days). | ||||
| 3 Severity of influenza related asthma exacerbation (symptom score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Split virus or Surface Antigen vaccine v. Placebo (Late Outcomes), Outcome 3 Severity of influenza related asthma exacerbation (symptom score). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||
| 1 Hospital admission for asthma exacerbation Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.1  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 1 Hospital admission for asthma exacerbation. | ||||||||||||
| 2 Asthma exacerbations in the month after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.2  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 2 Asthma exacerbations in the month after vaccination. | ||||||||||||
| 3 Asthma exacerbations in the week following vaccination Show forest plot | Other data | No numeric data | ||||||||||
| Analysis 3.3
Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 3 Asthma exacerbations in the week following vaccination. | ||||||||||||
| 4 Mean FEV1 at 2‐5 days post vaccination (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.4  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 4 Mean FEV1 at 2‐5 days post vaccination (% predicted). | ||||||||||||
| 5 Numer of patients with significant fall in FEV1 (over 12%‐15% or 50mls) on day 2‐4 Show forest plot | 2 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.12, 0.15] | ||||||||
| Analysis 3.5  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 5 Numer of patients with significant fall in FEV1 (over 12%‐15% or 50mls) on day 2‐4. | ||||||||||||
| 6 Fall in mean FEV1 in litres (day 2‐4) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.6  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 6 Fall in mean FEV1 in litres (day 2‐4). | ||||||||||||
| 7 Number of puffs of beta‐2 agonist per day (in month following vaccination) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.7  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 7 Number of puffs of beta‐2 agonist per day (in month following vaccination). | ||||||||||||
| 8 Morning Peak Flow of >30% below baseline at least once in the 4 weeks after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||
| Analysis 3.8  Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 8 Morning Peak Flow of >30% below baseline at least once in the 4 weeks after vaccination. | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All outcomes | Other data | No numeric data |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||
| 1 Home Peak Flow measurements before and after vaccination Show forest plot | Other data | No numeric data | ||||||||
| Analysis 5.1
Comparison 5 Immunisation with Whole virus v. Split virus v. Subunit vaccine (Early outcomes), Outcome 1 Home Peak Flow measurements before and after vaccination. | ||||||||||
| 2 Lung function measurements Show forest plot | Other data | No numeric data | ||||||||
| Analysis 5.2
Comparison 5 Immunisation with Whole virus v. Split virus v. Subunit vaccine (Early outcomes), Outcome 2 Lung function measurements. | ||||||||||

Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 1 Patients with an exacerbation of asthma.

Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 2 Patients with a fall in PEF of over 30%.
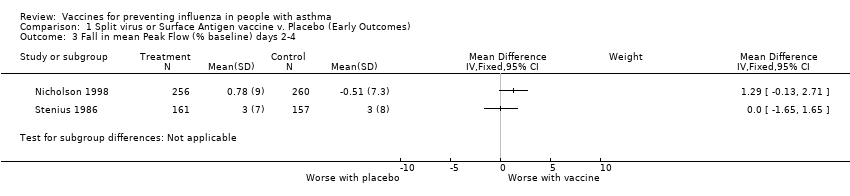
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 3 Fall in mean Peak Flow (% baseline) days 2‐4.
| Study | |
| Kut 1999 | No significant change in PC20 following either placebo or vaccine. |
| Reid 1998 | No significant difference found in placebo group (n=5) or vaccination group (n=17) in either mean PD20 or mean FEV1 (tested by analysis of variance ANOVA). No individual patient in either group showed a change of PD20 of more than two‐fold. |
| Sener 1999 | No significant difference between placebo and vaccine in PD20 at 2 weeks. Vaccine 2.96(SD 3.2) and placebo 2.76 (SD 2.91) |
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 4 Change in airways responsiveness.

Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 5 Increased nebuliser usage (days 1‐3).

Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 6 Increased use of bronchodilators following vaccination (days 1‐3).
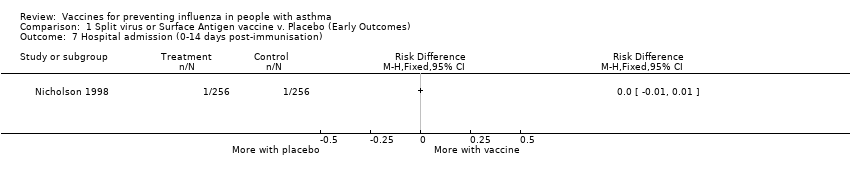
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 7 Hospital admission (0‐14 days post‐immunisation).
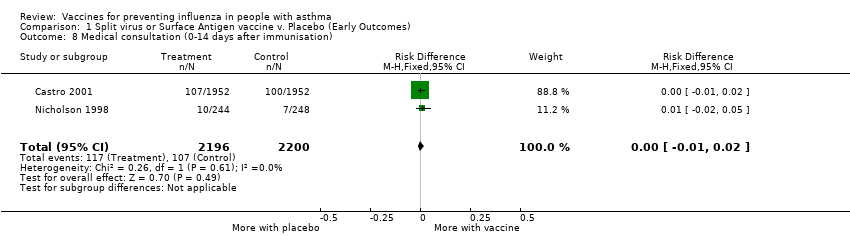
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 8 Medical consultation (0‐14 days after immunisation).

Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 9 New or increased oral steroid use (0‐14 days after immunisation).
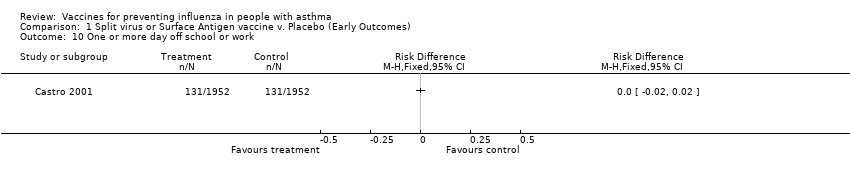
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 10 One or more day off school or work.

Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 11 Number of symptom free days in fortnight after vaccination.
| Study | |
| Govaert 1992 | No adverse reactions on asthma symptoms reported from any of the 14 asthmatics immunised with split‐virus vaccine or the 11 astmatics given placebo. (Communication from author) |
| Hahn 1980 | No significant deterioration in home Peak Flow measurement in the split vaccine (25 patients), subunit vaccine (25 patients) or placebo group (16 patients) in the two weeks following vaccination. No numerical data given. |
| Sener 1999 | No significant difference in symptom scores in the week after vaccine. Placebo mean score 4.66 (SD 7.3), vaccine mean score 4.92 (SD 7.56) |
| Stenius 1986 | Similar in the vaccine and placebo groups. No numerical data provided. |
Comparison 1 Split virus or Surface Antigen vaccine v. Placebo (Early Outcomes), Outcome 12 Change in asthma symptoms in the week following vaccination..

Comparison 2 Split virus or Surface Antigen vaccine v. Placebo (Late Outcomes), Outcome 1 Influenza related asthma exacerbations.

Comparison 2 Split virus or Surface Antigen vaccine v. Placebo (Late Outcomes), Outcome 2 Duration of influenza related asthma exacerbation (days).

Comparison 2 Split virus or Surface Antigen vaccine v. Placebo (Late Outcomes), Outcome 3 Severity of influenza related asthma exacerbation (symptom score).

Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 1 Hospital admission for asthma exacerbation.

Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 2 Asthma exacerbations in the month after vaccination.
| Study | |
| Miyazaki 1993 | No asthma attacks were apparent following vaccination. Evaluation was made difficult by an Adenovirus outbreak during the study period. No defintion of asthma attack provided by the authors. |
| Tanaka 1993 | No asthma attacks were observed following vaccination (20 patients given CR vaccine and 25 given placebo). No defintion of asthma attack provided by the authors. |
Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 3 Asthma exacerbations in the week following vaccination.

Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 4 Mean FEV1 at 2‐5 days post vaccination (% predicted).

Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 5 Numer of patients with significant fall in FEV1 (over 12%‐15% or 50mls) on day 2‐4.

Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 6 Fall in mean FEV1 in litres (day 2‐4).
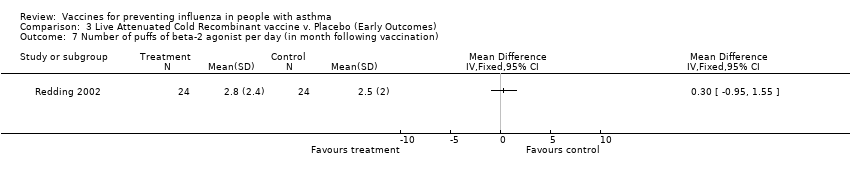
Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 7 Number of puffs of beta‐2 agonist per day (in month following vaccination).

Comparison 3 Live Attenuated Cold Recombinant vaccine v. Placebo (Early Outcomes), Outcome 8 Morning Peak Flow of >30% below baseline at least once in the 4 weeks after vaccination.
| Study | |
| Ortwein 1987 | No significant differences found in home Peak Flow measurements in the three days following vaccination in any of the vaccine groups individually or together. No numerical data provided in the paper. |
Comparison 5 Immunisation with Whole virus v. Split virus v. Subunit vaccine (Early outcomes), Outcome 1 Home Peak Flow measurements before and after vaccination.
| Study | |
| Ortwein 1987 | No deterioration in Lung Function measured in the laboratory in the 3 days following immunisation (either no change or small improvements seen.) No numerical data provided. |
Comparison 5 Immunisation with Whole virus v. Split virus v. Subunit vaccine (Early outcomes), Outcome 2 Lung function measurements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with an exacerbation of asthma Show forest plot | 2 | 4412 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 1.1 First‐time vaccinees | 2 | 948 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 1.2 Repeat vaccinees | 2 | 3464 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 2 Patients with a fall in PEF of over 30% Show forest plot | 2 | 4252 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] |
| 2.1 First‐time vaccinees | 1 | 194 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 2.2 Repeat vacinees | 1 | 328 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 2.3 Vaccination status unspecified | 1 | 3730 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 3 Fall in mean Peak Flow (% baseline) days 2‐4 Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Change in airways responsiveness Show forest plot | Other data | No numeric data | ||
| 5 Increased nebuliser usage (days 1‐3) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Increased use of bronchodilators following vaccination (days 1‐3) Show forest plot | 3 | 4228 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7 Hospital admission (0‐14 days post‐immunisation) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Medical consultation (0‐14 days after immunisation) Show forest plot | 2 | 4396 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 9 New or increased oral steroid use (0‐14 days after immunisation) Show forest plot | 2 | 4419 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 10 One or more day off school or work Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Number of symptom free days in fortnight after vaccination Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Change in asthma symptoms in the week following vaccination. Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza related asthma exacerbations Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Number of participants with influenza related exacerbations | 1 | 696 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 1.2 Number of patients with any asthma exacerbation | 1 | 696 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.00] |
| 2 Duration of influenza related asthma exacerbation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Severity of influenza related asthma exacerbation (symptom score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital admission for asthma exacerbation Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Asthma exacerbations in the month after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Asthma exacerbations in the week following vaccination Show forest plot | Other data | No numeric data | ||
| 4 Mean FEV1 at 2‐5 days post vaccination (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Numer of patients with significant fall in FEV1 (over 12%‐15% or 50mls) on day 2‐4 Show forest plot | 2 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.12, 0.15] |
| 6 Fall in mean FEV1 in litres (day 2‐4) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of puffs of beta‐2 agonist per day (in month following vaccination) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Morning Peak Flow of >30% below baseline at least once in the 4 weeks after vaccination Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All outcomes | Other data | No numeric data |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Home Peak Flow measurements before and after vaccination Show forest plot | Other data | No numeric data | ||
| 2 Lung function measurements Show forest plot | Other data | No numeric data | ||

