Interventions for dysphagia and nutritional support in acute and subacute stroke
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Computerised randomisation Double blind Baseline prognostic factors balanced between treatment groups | |
| Participants | 1 centre in Italy 48 patients Mean age 72 years | |
| Interventions | Rx: calorie‐protein supplementation (n = 24) C: routine care (n = 24) For 21 days | |
| Outcomes | Anthropometric and nutritional variables Cognitive function | |
| Notes | Exclusions: aphasic patients, chronic renal failure, diabetes on hypoglycaemic therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list derived through random generator procedure using SAS software |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation Randomisation list identified the blinded treatments as A or B |
| Blinding (performance bias and detection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded to the supplementation |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Random numbers table Outcomes not blinded | |
| Participants | 1 centre in China Baseline characteristics similar No cross‐overs or drop‐outs identified | |
| Interventions | A1: shallow needling (control) (n = 35) = low intensity | |
| Outcomes | Watian drinking test grade | |
| Notes | Exclusions: needle phobia, infection risk, dementia, inability to co‐operate with treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | (High versus medium dataset) | |
| Participants | As data set 1 | |
| Interventions | A1: shallow needling (control) | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Methods | Computerised randomisation by minimisation Unblinded outcome assessment Analysis by ITT Cross‐overs: 3 NGT to PEG, 0 PEG to NGT Balancing of baseline prognostic factors between treatment groups unclear | |
| Participants | 1 centre in UK 19 patients: 8 male Mean age 77 (SD 11) years 13 ischaemic stroke, 6 haemorrhagic stroke 100% CT Enrolment within 2 weeks of stroke onset | |
| Interventions | Factorial trial: PEG versus NGT; intensive versus conservative swallowing therapy PEG:NGT: up to 3 NGTs Intensive swallowing therapy: as for conservative, plus voluntary control (tongue‐holding), sensory stimulation (tactile, oromotor exercises, swallow practice) Conservative swallowing therapy: review, advice regarding feeding route, postural/dietary modification, safe swallowing methods | |
| Outcomes | Primary outcomes: resumption of safe feeding at 12 weeks, weight loss < 5% at 6 weeks, discharge by 6 weeks Secondary outcomes: impairment, disability, handicap, quality of life, tube failures, chest infection, oropharyngeal delay time (by videofluoroscopy) at 4 weeks | |
| Notes | Exclusions: oro‐gastrointestinal disease, concurrent severe illness, coagulopathy, pre‐morbid dependency, severe dementia, psychiatric illness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by minimisation |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded outcome assessment |
| Methods | Computer‐based randomisation Allocation sequence was concealed from researchers and participants Outcome measurements and the intervention were not blinded to group allocation Analysis by ITT Baseline prognostic factors balanced between treatment groups | |
| Participants | 4 centres in UK 104 patients with acute stroke (stroke onset to randomisation median 4 days; IQR 3 to 6 days) Mean age 80 years | |
| Interventions | Rx: NGT + nasal loop (n = 51) C: NGT + conventional adhesive dressing (n = 53) | |
| Outcomes | Primary outcome: proportion of prescribed feed and fluids delivered via NGT over 2 weeks after randomisation Secondary outcome measures at 2 weeks: mean volume of feed and fluids delivered, proportion of participants not receiving any NGT feed, supplementary parenteral fluids, number of NGT insertions, number of chest X‐rays to check NGT position, change in weight, treatment failure, adverse events, and tolerability Secondary outcome measures at 3 months: mortality, length of hospital stay, PEG use, residential status, and Barthel Index | |
| Notes | Exclusions: contraindications to NGT feeding, NGT had been established for more than 7 days elsewhere | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated pseudo‐random list using random permutated blocks of randomly varying size and stratified by site and stroke severity |
| Allocation concealment (selection bias) | Low risk | Recruits were consecutively randomised, and the allocation sequence was concealed from researchers and participants until the end of the trial once all analyses were complete |
| Blinding (performance bias and detection bias) | High risk | Outcome measurements and the intervention were not blinded to group allocation, owing to the nature of the intervention and concurrent data collection |
| Blinding of participants and personnel (performance bias) | High risk | As above Data were analysed independently by the study statistician, who was blinded to group allocation |
| Blinding of outcome assessment (detection bias) | High risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computerised randomisation Blinded outcome assessments by SLT ITT Baseline prognostic factors balanced between treatment groups | |
| Participants | 1 centre in Australia 306 patients, baseline characteristics similar Enrolment within 2 weeks of stroke onset: mean/median 2 days, range 0 to 12 days Clinical and videofluoroscopic evidence of dysphagia | |
| Interventions | Rx 1: standardised high‐intensity swallowing therapy (n = 102) | |
| Outcomes | Outcomes: time to return to normal diet; aspiration pneumonia; dysphagia (PHAD score < 85) | |
| Notes | Trial completed and published 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation was based on a computer‐generated random numbers list generated with the SPSS statistical package |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was held in the trial office, remote from the study environment; assignment to 1 of 3 treatment options by giving a telephone |
| Blinding (performance bias and detection bias) | High risk | All people involved in the study were unaware of the treatment allocation, apart from the patients and the study speech pathologist who treated the patients |
| Blinding of participants and personnel (performance bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome was assessed by an independent speech pathologist, who was unaware of the treatment allocation, every month for 6 months after randomisation |
| Incomplete outcome data (attrition bias) | Low risk | 3 patients were lost to follow‐up before the 6‐month analysis |
| Selective reporting (reporting bias) | Low risk | |
| Methods | (High vs. medium data set) | |
| Participants | As data set 1 | |
| Interventions | High = 102 (high intensity) | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation was based on a computer‐generated random numbers list generated with the SPSS statistical package |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was held in the trial office, remote from the study environment; assignment to 1 of 3 treatment options by giving a telephone |
| Blinding (performance bias and detection bias) | High risk | All people involved in the study were unaware of the treatment allocation, apart from the patients and the study speech pathologist who treated the patients |
| Blinding of participants and personnel (performance bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome was assessed by an independent speech pathologist, who was unaware of the treatment allocation, every month for 6 months after randomisation |
| Incomplete outcome data (attrition bias) | Low risk | 3 patients were lost to follow‐up before the 6‐month analysis |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computerised randomisation by minimisation Blinded outcome assessment by post or telephone Cross‐overs: 3 normal diet to supplement, 48 supplement to normal diet, 79 did not receive allocated supplements Baseline prognostic factors balanced between treatment groups | |
| Participants | 125 centres in 15 countries 4023 non‐dysphagic patients: 2149 male Mean age 71 (SD 13) years Stroke 99% Enrolment within 30 days of stroke onset | |
| Interventions | Rx: protein (22.5 g per day) energy (540 kcal) supplements + normal hospital diet (n = 2011) | |
| Outcomes | Primary outcomes: dead or dependent (mRS 3 to 6); death at 6 months | |
| Notes | Exclusions: dysphagia, SAH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated minimisation algorithm |
| Allocation concealment (selection bias) | Low risk | The randomisation systems were housed on a secure server with access permitted, via a password, only to those members of the co‐ordinating team who had been fully trained how to use the systems Participating centres were issued with codes in order for them to access the randomisation services |
| Blinding (performance bias and detection bias) | High risk | FOOD was an open trial, with both the randomising person and the patient being aware of the treatment allocation The only blinded assessment was the 6‐month follow‐up |
| Blinding of participants and personnel (performance bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | 11 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computerised randomisation by minimisation (age, country, predicted poor outcome) Blinded outcome assessment by post or telephone Cross‐overs: 58 avoid to early group, 60 did not receive early tube Baseline prognostic factors balanced between treatment groups | |
| Participants | 83 centres in 15 countries 859 dysphagic patients: 394 male Mean age 76 (SD 11) years Stroke 99.5% Enrolment within 7 days of stroke onset | |
| Interventions | Rx: early (within 7 days) enteral feeding (n = 429) | |
| Outcomes | Primary outcomes: dead or dependent (mRS 4 to 6); death at 6 months | |
| Notes | Exclusions: SAH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated minimisation algorithm |
| Allocation concealment (selection bias) | Low risk | The randomisation systems were housed on a secure server with access permitted, via a password, only to those members of the coordinating team who had been fully trained how to use the systems Participating centres were issued with codes in order for them to access the randomisation services |
| Blinding (performance bias and detection bias) | High risk | FOOD was an open trial, with both the randomising person and the patient being aware of the treatment allocation The only blinded assessment was the 6‐month follow‐up |
| Blinding of participants and personnel (performance bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | 1 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computerised randomisation by minimisation Blinded outcome assessment by post or telephone Cross‐overs: 13 in NGT group received early PEG, 23 allocated to PEG received NGT Baseline prognostic factors balanced between treatment groups | |
| Participants | 47 centres in 11 countries 321 dysphagic patients: 144 male Mean age 76 (SD 10) years Stroke 100% Enrolment within 30 days of stroke onset | |
| Interventions | Rx: PEG feeding (within 3 days of enrolment) (n = 162) | |
| Outcomes | Primary outcomes: dead or dependent (mRS 4 to 6); death at 6 months | |
| Notes | Exclusions: SAH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated minimisation algorithm |
| Allocation concealment (selection bias) | Low risk | The randomisation systems were housed on a secure server with access permitted, via a password, only to those members of the coordinating team who had been fully trained how to use the systems Participating centres were issued with codes in order for them to access the randomisation services |
| Blinding (performance bias and detection bias) | High risk | FOOD was an open trial, with both the randomising person and the patient being aware of the treatment allocation The only blinded assessment was the 6‐month follow‐up |
| Blinding of participants and personnel (performance bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation: block randomisation by telephone, concealment unclear Blinding of nutritional outcome measurement only Analysis by ITT unclear Cross‐overs unclear Baseline factors balanced | |
| Participants | 1 centre in UK 42 non‐dysphagic patients with impaired nutritional status (defined as MAC and TSF ≤ 1 SD below the mean expected) Mean age 78 years in intervention and 80 years in control group All ischaemic stroke Enrolment within 1 week of stroke onset | |
| Interventions | Rx: daily enteral sip feeding and usual hospital food, treatment for 4 weeks (n = 21) C: usual hospital food (n = 21) | |
| Outcomes | Primary outcomes: energy intake and nutritional status (weight, TSF, MAC, albumin, transferrin, and iron) | |
| Notes | Exclusions: dysphagia, normal nutritional status, haemorrhagic stroke, active gastrointestinal disease, renal or liver failure, heart failure, sepsis, or malignancy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by telephone |
| Allocation concealment (selection bias) | Unclear risk | Concealment unclear |
| Blinding (performance bias and detection bias) | High risk | Single blind Blinding of nutritional outcome measurement only |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and nurses were aware of the group to which they were allocated |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | 4 lost to follow‐up immediately after randomisation owing to early discharge as a result of complete recovery |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computerised randomisation Outcomes assessed unblinded Analysis by ITT No cross‐overs, exclusions post‐randomisation, or losses to follow‐up Baseline prognostic factors balanced between treatment groups | |
| Participants | 1 centre in USA 20 patients with documented aspiration of thin fluids only: 14 male, 6 female Mean age 76.8 years Stroke types unclear Enrolment within 3 weeks of stroke onset: mean 12.8 days, range 4 to 19 days | |
| Interventions | Rx: thickened fluids and free water (n = 10) C: thickened fluids only (n = 10) Treatment until aspiration resolved (7 to 64 days) | |
| Outcomes | Outcomes: development of pneumonia, dehydration, and satisfaction Time to resolution of aspiration to thin fluids | |
| Notes | Exclusions: aspiration to thickened fluids Follow‐up: 30 days beyond resolution of aspiration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Concealment unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes assessed unblinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computer‐generated random numbers by research pharmacist, placebo‐controlled double blind. Outcomes unblinded | |
| Participants | 3 centres in the UK 100% stroke 58 with dysphagia, baseline characteristics similar | |
| Interventions | Rx: selective decontamination of digestive tract with antibacterial oral gel for 3 weeks (n = 25) | |
| Outcomes | Pneumonia rates | |
| Notes | Exclusions: on antibiotics, steroids, or previous stroke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes unblinded |
| Incomplete outcome data (attrition bias) | High risk | Of the 203 patients, 20 died during their hospitalisation, 19 withdrew and full follow‐up was obtained for the remaining 164 |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computer‐based randomisation Blinding unknown Baseline prognostic factors were balanced between treatment groups | |
| Participants | 1 centre in Norway 170 patients < 3 days of acute stroke 5 excluded after randomisation, 41 lost to follow‐up (22 died, 19 refused to participate in follow‐up) Mean age 79 years | |
| Interventions | Rx: individualised nutritional treatment (n = 58) C: routine care (n = 66) | |
| Outcomes | Primary: percentage of patients with weight loss > 5% Secondary: quality of life, hand grip strength, length of hospital stay | |
| Notes | Exclusions: stroke diagnosis unclear, critically ill, severe dementia, could not be weighed, planned discharge < 24 hours after the first visit by trial assessor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence of treatment allocation was prepared from a computer‐generated randomisation list by a person not involved in patient assessments |
| Allocation concealment (selection bias) | Low risk | Patients randomised to individualised, nutritional treatment or to routine care in blocks of 20 patients using sequentially numbered, non‐transparent envelopes containing the treatment allocation information |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding unknown |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding unknown |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding unknown |
| Incomplete outcome data (attrition bias) | High risk | 41 lost to follow‐up (22 died, 19 refused to participate in follow‐up) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Computer block randomisation, no cross‐overs Unblinded outcome measures | |
| Participants | 1 centre in Malaysia | |
| Interventions | Rx: PEG (n = 10) | |
| Outcomes | Case fatality, nutritional measures (TSF, MAC, albumin) | |
| Notes | Exclusions: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer block randomisation |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded outcome measures |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unknown Blinding unknown | |
| Participants | 1 centre in China 97 patients with post‐stroke dysphagia | |
| Interventions | Group 1: electric stimulation (n = 35) Group 2: rehabilitation training group (n = 30) Group 3: acupuncture (n = 32) | |
| Outcomes | Swallowing function | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unknown |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding unknown |
| Blinding of participants and personnel (performance bias) | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | Computerised randomisation by minimisation Blinded outcome measures Balancing of prognostic baseline factors between treatment groups unclear | |
| Participants | 2 centres in UK 28 patients with acute anterior circulation cerebral infarct or haemorrhage (< 3 weeks) Mean age 75 years | |
| Interventions | Rx: bedside pharyngeal electrical stimulation C: sham stimulation | |
| Outcomes | Airway aspiration at 2 weeks' post intervention | |
| Notes | Exclusion: dementia, pacemaker or implantable cardiac defibrillator, severe receptive aphasia, unstable cardiopulmonary status, distorted oropharyngeal anatomy (e.g. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by minimisation |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded outcome measures |
| Incomplete outcome data (attrition bias) | High risk | 3 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unclear: patients were assigned randomly to receive real or sham repetitive transcranial magnetic stimulation using closed envelopes Blinded outcome assessment Allocation sequence was concealed from participants Baseline prognostic factors were balanced between treatment groups | |
| Participants | 1 centre in Egypt 26 patients between the 5th and 10th days post stroke (monohemispheric) Mean age 56 years | |
| Interventions | Rx: repetitive transcranial magnetic stimulation of the affected motor cortex (n = 14) C: sham stimulation (n = 12) | |
| Outcomes | Primary outcome: score on the dysphagia rating scale Secondary outcomes: motor power of hand grip, Barthel Index, measures of oesophageal motor evoked potentials from both hemispheres before and 1 month after sessions | |
| Notes | Exclusion: head injury or neurological disease other than stroke, unstable cardiac dysrhythmia, fever, infection, hyperglycaemia, and prior administration of tranquilliser | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was concealed from participants |
| Blinding (performance bias and detection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) | Low risk | Patients were informed of which group they had been allocated at the end of the last assessment |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | All patients apart from 1 in the sham treatment group who died completed the trial and follow‐up periods |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomisation using simple randomisation Double blind Analysis by ITT unclear Balancing of prognostic baseline factors between treatment groups unclear | |
| Participants | 1 centre in USA 14 patients with subacute (24 to 168 hours) unilateral hemispheric infarction Mean age 75 years | |
| Interventions | Rx: anodal transcranial direct current stimulation C: sham stimulation For 5 consecutive days | |
| Outcomes | Swallowing impairment using dysphagia outcome and severity scale | |
| Notes | Exclusions: patients with difficulty following instructions because of obtundation or cognitive impairment, pre‐existing swallowing problems, other contraindications to transcranial direct current stimulation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation using simple randomisation |
| Allocation concealment (selection bias) | Unclear risk | As above |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unclear: participants were divided into 2 groups according to the order of enrolment Blinding of outcomes unclear Analysis by ITT unclear Balancing of prognostic baseline factors between treatment groups unclear | |
| Participants | 1 centre in Korea 22 patients with CT or MRI confirmed stroke < 6 months from onset Mean age 64 years | |
| Interventions | Rx: neuromuscular electrical stimulation + thermal‐tactile stimulation (n = 13) C: thermal‐tactile stimulation (n = 9) | |
| Outcomes | Outcomes: swallow function, scoring system, penetration‐aspiration scale and pharyngeal transit time | |
| Notes | Exclusions: inability to receive the treatment for 1 hour, neurological disease other than stroke, combined behavioural disorder that interfered with administration of therapy, current illness or upper gastrointestinal disease, inability to give informed consent because of cognitive impairment or receptive aphasia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear. Participants were divided into 2 groups according to the order of enrolment |
| Allocation concealment (selection bias) | Unclear risk | As above |
| Blinding (performance bias and detection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details available |
| Incomplete outcome data (attrition bias) | High risk | 36 enrolled to the study. Only 28 patients completed the study (16 in the experimental group and 12 in the control group) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unclear Blinding of outcomes unclear Analysis by ITT unclear Balancing of prognostic baseline factors between treatment groups unclear | |
| Participants | 1 centre in China 84 patients with bulbar palsy and CT/MRI documented stroke: male 54, female 30 Age 50 to 78 years Infarct 56, haemorrhage 28 Enrolment within 2 months of stroke onset | |
| Interventions | Rx: acupuncture ‐ Tiantu (CV 22), Lieque (LU 7), Zhaohai (KI 6) ‐ once daily for 10 days (n = 54) C: (n = 30) | |
| Outcomes | Outcome: bulbar function (phonation, swallowing, cough reflex) Timing unclear | |
| Notes | Exclusions: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unclear: patients were randomly allocated using closed envelopes Outcome assessments unblinded Analysis by ITT No cross‐overs, exclusions post‐randomisation, or losses to follow‐up Balancing of baseline prognostic factors for treatment groups unclear | |
| Participants | 2 centres in UK 30 participants: 11 male Mean age 77 years Stroke types not given; CT performed in 25 patients Enrolment 14 (± 3) days post‐admission All patients were unconscious at admission with a dense hemiplegia Dysphagia assessed by absence of normal gag reflex or inability to swallow 50 mL of sterile water without choking | |
| Interventions | PEG tube (12 French gauge Fresenius or 24 French gauge Wilson Cook) inserted using percutaneous approach with pull‐through. Antibiotic (cefuroxime 750 mg iv) given prophylactically; sedation with 5 to 10 mg diazepam (n = 16) NGT (Flocare 500); all patients got standard enteral feed (Nutrison); feed delivered via Flowcare 500 at 50 mL/hour for first 24 hours increased to 100 mL/hour; patients fed in a semi‐recumbent position for 6 weeks (n = 14) | |
| Outcomes | Case fatality at 6 weeks Amount of feed administered Change in nutritional status (MAC, serum albumin, TSF, weight change) Treatment failure Length of hospital stay Number of times tube inserted | |
| Notes | Exclusions: previous history of gastrointestinal disease, unfit for endoscopy or iv sedation Follow‐up: 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated using closed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessments unblinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Methods | Method of randomisation: using a specific list Double blind 20 patients lost to follow‐up Baseline prognostic factors were balanced between treatment groups | |
| Participants | 72 patients, < 60 days from ictus | |
| Interventions | Rx: Nutristroke diet + antioxidants (n = 16) C: Nutristroke diet + placebo (n = 18) For 12 months Mean age 65 years | |
| Outcomes | Anthropometric measures, neurological/functional status | |
| Notes | Exclusions: > 60 days from ictus, haemorrhagic lesions, other chronic disabling pathologies, inability or refusal to give consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using a specific list |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Low risk | No patient, research assistant, investigator or any other medical or nursing staff could distinguish the placebo from the supplements during the study |
| Blinding of participants and personnel (performance bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | High risk | 20 drop‐outs (27.2%) with 4 deaths (3 males, 1 female) form cardiovascular events |
| Selective reporting (reporting bias) | Low risk | |
| Methods | ‐ | |
| Participants | ‐ | |
| Interventions | Rx: Nutristroke diet + n3 fatty acid (n = 20) Mean age 61 years | |
| Outcomes | ‐ | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using a specific list |
| Blinding (performance bias and detection bias) | Low risk | No patient, research assistant, investigator, or any other medical or nursing staff could distinguish the placebo from the supplements during the study |
| Blinding of participants and personnel (performance bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | High risk | 20 drop‐outs (27.2%) with 4 deaths (3 males, 1 female) from cardiovascular events |
| Selective reporting (reporting bias) | Low risk | |
| Methods | ‐ | |
| Participants | ‐ | |
| Interventions | Rx: Nutristroke diet + antioxidants + n3 fatty acid (n = 18) Mean age 66 years | |
| Outcomes | ‐ | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using a specific list |
| Blinding (performance bias and detection bias) | Low risk | No patient, research assistant, investigator, or any other medical or nursing staff could distinguish the placebo from the supplements during the study |
| Blinding of participants and personnel (performance bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | High risk | 20 drop‐outs (27.2%) with 4 deaths (3 males, 1 female) from cardiovascular events |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unclear Outcome assessment blinding unclear Cross‐overs not given Baseline prognostic factors balanced between treatment groups | |
| Participants | 6 centres in UK 63 dysphagic patients: gender ratio unclear Mean age 75 (SD 8) years Enrolled at 5 to 7 days post stroke | |
| Interventions | Rx: PEG within 10 days of stroke (n = 32) | |
| Outcomes | Primary outcome: unclear | |
| Notes | Exclusions: none given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Methods | Computerised randomisation Triple‐blind trial; outcomes assessed by blinded therapist Analysis by ITT No cross‐overs or losses to follow‐up 1 participant withdrawn with heart failure (nifedipine group) Baseline prognostic factors balanced between treatment groups | |
| Participants | 1 centre in UK 17 patients; 8 male Mean age 77 (SD 7) years All first ischaemic stroke 100% CT Enrolment 2 weeks after stroke | |
| Interventions | Rx: nifedipine (LA 30 mg orally daily, Bayer UK) (n = 8) Pl: matching tablet; treatment for 4 weeks (n = 9) | |
| Outcomes | Primary outcome: clinical improvement in swallowing Other outcomes: incidence of silent aspiration, pharyngeal transit time and response duration, swallowing delay (all assessed by videofluoroscopy), death | |
| Notes | Exclusions: unable to sit, high clinical risk of aspiration, receptive dysphasia, cognitive impairment, pre‐stroke dysphagia, existing neurological or psychiatric disease, current treatment with calcium channel blockers or aminophylline Follow‐up: 4 weeks. 1 patient withdrawn with heart failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Blinding (performance bias and detection bias) | Low risk | Triple‐blind trial |
| Blinding of participants and personnel (performance bias) | Low risk | Triple‐blind trial |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessed by blinded therapist |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant withdrawn with heart failure (nifedipine group) No cross‐overs |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation unclear CT scans were analysed by a neuroradiologist who was blinded to the patients clinical presentation and videofluoroscopic swallowing status Baseline data unclear | |
| Participants | 1 centre in UK 16 patients | |
| Interventions | Rx: actual electrical stimulation following threshold setting exercise | |
| Outcomes | Changes on videofluoroscopy 60 minutes post intervention | |
| Notes | Exclusions: prior dysphagia, intercurrent illness, other neurological disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation: sealed opaque envelope block randomisation of 10 patients Double blind Baseline prognostic factors were balanced between treatment groups | |
| Participants | 1 centre in US < 4 weeks of stroke Mean age 74 years | |
| Interventions | Rx: intensive nutritional supplementation (n = 51) C: routine nutritional supplementation (n = 51) | |
| Outcomes | Primary: change in total score on the FIM Secondary: FIM motor and cognitive subscores, length of stay, 2‐minute and 6‐minute timed walk tests measured at admission and on discharge and discharge disposition | |
| Notes | Exclusions: prior history of alcohol abuse, renal and liver disease, malabsorption, medically unstable or demented, terminally ill, participating any other therapeutic trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Identical sealed opaque envelope containing block randomisation of 10 patients |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Blinding of participants and personnel (performance bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | 2 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Method of randomisation: random numbers table Allocation method and concealment unclear | |
| Participants | 1 centre in China 53 patients; 46 male All dysphagia identified by water swallow test Baseline characteristics reported as similar | |
| Interventions | Rx: nurse‐led swallowing exercises, oral stimulation and oral care (n = 29) C (n = 24) | |
| Outcomes | Primary and secondary outcomes not defined Resolution of dysphagia by water swallow test and dietary ability, pneumonia rates | |
| Notes | Exclusions and whether ITT not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | Method of randomisation unclear Outcomes blinded | |
| Participants | 1 centre in China | |
| Interventions | Rx: Shuiti acupoint injection with stellate ganglion block for 40 days of treatment (n = 32) | |
| Outcomes | Resolution of dysphagia: water swallow test score | |
| Notes | Exclusions: needle phobia, organ failure, head and neck tumours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | Method of randomisation unclear Blinding unclear (Medium‐ versus low‐intensity data set) | |
| Participants | 1 centre in China | |
| Interventions | R1: enteral nutrition agent with thickener and swallowing therapy (high data set = 18) | |
| Outcomes | Length of stay, pneumonia rates, nutritional measures, resolution of dysphagia (Swallow test grade) | |
| Notes | Exclusions: terminal illness, organ failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | (High versus medium data set) | |
| Participants | As data set 1 | |
| Interventions | High intensity (n = 18) | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
BMI: body mass index
C: control group
CT: computer tomography
FIM: Functional Independence Measure
ITT: intention‐to‐treat analysis
IQR: interquartile range
iv: intravenous
MAC: mid‐upper arm circumference
MD: mean difference
MRI: magnetic resonance imaging
mRS: modified Rankin Score
NGT: nasogastric tube
OR: odds ratio
PEG: percutaneous endoscopic gastrostomy
PHAD: Paramatta Hospital's Assessment for Dysphagia score
Pl: placebo group
Rx: treatment group
SAH: subarachnoid haemorrhage
SD: standard deviation
SLT: speech and language therapist (speech pathologist)
SSS: Scandinavian Stroke Scale
TSF: triceps skinfold
VF: videofluoroscopy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT assessing transcutaneous electrical stimulation versus control 12 patients with chronic stroke and episodes of choking while eating or drinking Outcome: latency time in swallowing reflex Excluded: no outcome data | |
| RCT assessing PEG insertion methods and antibiotic prophylaxis in dysphagic patients, oropharyngeal carcinoma (n = 56, 56%), neurogenic (n = 32, 32%), other (n = 12, 12%) | |
| CCT assessing ACE inhibitors in dysphagic and non‐dysphagic stroke patients | |
| Non‐RCT comparing imidapril with losartan 53 patients with hypertension, symptomless dysphagia, and history of stroke Outcome: serum substance P level Excluded: (1) non‐RCT; (2) comparing 2 active treatments; (3) no outcome data | |
| RCT | |
| Study comparing effect of neck posture on swallowing latency in stroke patients and controls | |
| RCT comparing PEG and NGT feeding in 90 dysphagic patients with neurological problems (n = 42, 47%), ear nose and throat disease (n = 39, 43%) or post‐surgery (n = 9, 10%) | |
| Cluster RCT assessing effect of oral supplements (400 kcal per day) on pressure ulcers | |
| RCT assessing hydration routes in 17 dysphagic stroke patients | |
| RCT assessing neuromuscular electrical stimulation versus traditional swallowing therapy in 25 stroke patients with dysphagia Outcomes: videoradiographic swallowing evaluation, nutritional status, oral motor function test, and a visual analogue scale (VAS) for self‐evaluation of complaints Excluded: (1) no outcome data | |
| RCT assessing hydration routes in 34 elderly acute stroke patients with either impaired consciousness or dysphagia No difference in serum osmolality; subcutaneous hydration cheaper | |
| RCT assessing effectiveness of electric stimulation versus traditional dysphagia therapy in patients with acute stroke (< 6 weeks) Outcomes: The American Speech Language Hearing Association National outcome measurement system swallowing level Excluded: no outcome data | |
| RCT assessing tongue acupuncture + ice massage + general medical treatment (n = 50) versus general medical treatment (n = 46) in acute dysphagic stroke patients Outcome: dysphagia recovery assessed using videofluoroscopy Excluded: (1)unable to obtain data | |
| RCT assessing electroacupuncture + rehabilitation (n = 34) versus rehabilitation alone (n = 34) in dysphagia patients with pseudobulbar palsy including stroke Treated for 10 days Outcome: dysphagia recovery after stroke Excluded: no outcome data | |
| RCT assessing feed fibre content on diarrhoea severity and frequency | |
| RCT assessing timing of feeding: 3 hours (n = 21) versus 24 hours (n = 20) ‐ following insertion of PEG tube in 41 dysphagic patients (stroke n = 17) requiring PEG | |
| Case control study assessing acupuncture at Lianquan (Ren 23) and Chize (Lu 5) in 150 patients with stroke causing pseudo bulbar palsy | |
| Quasi‐RCT (alternate assignment) comparing 2 nasogastric tubes in 41 dysphagic patients on a ventilator (n = 28, 68%) or with stroke or head injury (n = 13, 32%) | |
| Observational study to determine the effect of sour and cold food in the pharyngeal transit times of 30 patients with stroke Outcome: pharyngeal transit time using a videofluoroscopy swallow test Excluded: (1) non1RCT | |
| Unpublished study comparing high versus low glucose in NGT feeding in 70 dysphagic stroke patients No further information on trial design, protocol, patients, interventions, outcomes | |
| RCT comparing early NGT feeding with a standard formula containing hydrolyzed casein versus a formula containing hydrolyzed whey protein in 31 acute (< 48 hours) ischaemic stroke patients Outcome: changes in the serum levels of glutathione peroxidase, C‐reactive protein, and interleukin 6 Excluded: (1) treatment is confounded, i.e. 2 active groups and no control | |
| RCT comparing 3 active interventions in 115 dysphagic stroke patients taught compensatory swallowing techniques | |
| RCT comparing 3 enteral diets differing only in lipid composition in 36 dysphagic patients with head injury (n = 15), stroke (n = 13) or other neurological problems (n = 8) | |
| Crossover RCT comparing liquid and spoon‐thick (pudding‐like) feeds in 61 inpatients diagnosed with stroke Outcome: aspiration using nasoendoscopy Excluded: (1) compared 2 active treatments; (2) no relevant outcome data | |
| RCT comparing PEG tube size in 52 dysphagic patients (83% stroke) | |
| RCT assessing dose response relationship of capsaicin (1E‐9‐1E‐6 mol/L) on swallowing reflex in 20 patients with stroke or vascular dementia | |
| RCT | |
| RCT | |
| RCT | |
| RCT including 50 patients with dysphagia following stroke Evaluation of gastrointestinal tolerance of a new thickening powder versus current thickening powder Outcome: GI symptoms (measurements: stool frequency and consistency, GI symptoms and food and fluid intake) Excluded: 2 active treatment groups with no control group (confounded) | |
| RCT including 16 acute stroke (< 4 days from ictus) patients with dysphagia Transcranial magnetic stimulation versus none Outcome: pharyngeal electromyographic responses Excluded: (1) no outcome data | |
| CCT comparing 3 active interventions in 112 patients with aspiration | |
| Quasi‐RCT (alternate assignment) comparing electrical stimulation with thermal‐tactile stimulation in 110 dysphagic stroke patients | |
| Non‐RCT transcutaneous electrical stimulation applied submentally to 11 patients with recent oropharyngeal dysphagia (> 8 weeks) induced by a hemispheric (n = 7) or brainstem (n = 4) stroke, with pharyngeal residue and/or laryngeal aspiration diagnosed by videofluoroscopy Outcome: dysphagia handicap index questionnaire, videofluoroscopy, and cortical mapping of pharyngeal muscles Excluded: (1) no control group | |
| A case‐controlled study of early rehabilitation treatment (5 days/week for 2 weeks) in acute dysphagic stroke patients Excluded: (1) non‐RCT | |
| RCT assessing 2 antibiotic regimes with control in 347 patients with cancer or neurological disorders | |
| RCT assessing methods for preparing thickened fluids in 46 dysphagic stroke patients | |
| Non‐RCT assessing the use of PEG for enteral nutrition in patients admitted for stroke Control: patients with other diseases Excluded: (1) non‐RCT | |
| RCT assessing amino acid regimes versus control in 69 patients with SAH requiring post‐operative parenteral nutrition | |
| Study assessing feed protein content in 39 dysphagic tube‐fed stroke patients | |
| RCT comparing the direct method using a 24 Fr bumper‐button‐type device with the pull method for percutaneous endoscopic gastrostomy in 140 patients with stroke and other CNS disorders Outcome: rate of peristomal infections Excluded: (1) time since stroke onset to randomisation not provided for stroke patients | |
| Study of 96 consecutive patients within 24 hours of acute stroke Before and after study of swallowing exercises delivered by trained nurse Excluded: (1) not RCT | |
| Pharyngeal electrical stimulation Excluded: (1) healthy volunteers; (2) not RCT | |
| Retrospective case‐matched controlled study in 193 stroke patients with a PEG tube and matched 193 controls Outcome: length of rehabilitation hospital stay, improvement in FIM scores, FIM efficiency score, need for transfer back to acute care hospital, diagnosis for which transfer was required, final discharge destination, and survival status Excluded: (1) non‐RCT | |
| CCT assessing Banxia Houpo Tang in 32 patients with previous ischaemic stroke and pneumonia | |
| RCT comparing 2 different commercial enteral formulas in 12 acute (≤ 3 months) stroke patients Outcome: nutritional biomarkers and an oxidative stress biomarker, malondialdehyde (MDA), quality of life Excluded: (1) comparison between 2 active treatments, no control group | |
| Case control study Excluded: (1) not RCT | |
| Case control group | |
| Non‐RCT assessing the effects of swallowing with oropharyngeal sensory stimulation in nasogastric tube insertion in 32 stroke patients Outcome: oro‐pharyngeal swallowing function score Excluded: (1) non‐RCT | |
| RCT comparing 2 food thickeners on swallowing function in 51 patients with stroke Outcome: changes of videofluoroscopic swallowing study clinical score Excluded: (1) no time since stroke onset; (2) comparing 2 treatments, no control group; (3) no outcome data | |
| Randomised crossover trial assessing levodopa in 27 patients with basal ganglia infarction and 20 healthy volunteers | |
| Non‐randomised crossover study assessing fluid consistency (thin, thick, ultra‐thick) and delivery method (teaspoon, cup) in 190 dysphagic patients | |
| Crossover trial comparing liquid meal versus saline on gastro‐oesophageal reflux in 15 PEG gastrojejunal tube‐fed stroke patients (9 with, 6 without oesophagitis) | |
| RCT assessing either traditional swallowing therapy or the Shaker exercise in patients with prolonged oropharyngeal dysphagia and aspiration Outcomes: occurrence of aspiration (preswallow, intraswallow, postswallow) at the 6‐week follow‐up period, occurrence of residue in the oral cavity, valleculae, or pyriform sinuses and the Performance Status Scale for Diet Excluded: (1) head and neck cancer and stroke; (2) no outcome data | |
| RCT assessing liquid diets (thickener, gelatinised water) in 16 dysphagic patients with stroke | |
| Implanted neuroprosthesis (neuro control implantable receiver‐stimulator) to stimulate the laryngeal nerve versus sensory training in dysphagic patients including stroke > 6 months post onset Excluded: (1) no control group, 2 active groups compared; (2) no outcome data | |
| Observational Excluded: (1) proportion of stroke unclear; (2) chronic dysphagic patients; (3) not RCT | |
| Crossover trial assessing thickening agents in 8 dysphagic patients (stroke n = 6) and 13 volunteers | |
| RCT comparing pre‐thickened, standarised consistency fluids for 6 weeks versus fluids thickened at the bedside using modified maize starch for 6 weeks in 11 dysphagic patients in residential care Outcomes: Barthel Index, Mini Mental State Examination Excluded: (1) no control group, 2 active interventions | |
| RCT comparing traditional swallowing therapy versus Shaker Exercise in 6 stroke and 5 cancer patients Outcome: deglutitive thyrohyoid shortening before and after completion of assigned therapy regimen Excluded: (1) no time of onset for stroke patients; (2) no separate results for stroke (3) no outcome data | |
| RCT comparing transcranial magnetic stimulation versus sham stimulation 12 stoke patients with dysphagia Outcome: pharyngeal electromyographic responses Excluded: (1) no outcome data | |
| RCT comparing transcranial magnetic stimulation versus pharyngeal electrical stimulation versus paired associative stimulation versus sham stimulation in 14 dysphagic stroke patients Outcome: videofluoroscopic swallowing assessments Excluded: (1) no outcome data | |
| RCT comparing amantadine (100 mg daily) versus control in 185 ischaemic stroke patients | |
| RCT comparing 5 mg imidapril or placebo in randomised, double‐blind, crossover design. Patients were normotensive patients had at least one episode of aspiration and healthy volunteers Outcome: swallowing reflex Excluded: (1) no outcome data | |
| Non‐RCT comparing several techniques designed to improve the ability to swallow in stroke patients with chronic dysphagia with healthy volunteers Outcome: swallowing safety Excluded: (1) non‐RCT | |
| RCT assessing intramuscular stimulation device implanted in the neck versus vibrotactile stimulation of the throat in 20 patients with dysphagia secondary to stroke or chronic neurological disease Outcome: swallowing safety for 10 mL of thin liquid and 5 mL of pudding with and without stimulation Excluded: (1) comparing 2 active treatments no control (confounded) | |
| RCT comparing nicergoline (15 mg tds) versus control in 50 ischaemic stroke patients Outcome: substance P level Excluded: (1) no outcome data | |
| Retrospective case control study assessing timing of feeding (< 72 hours versus > 72 hours of admission) in 52 dysphagic stroke patients | |
| Non‐RCT assessing effects of changes in bolus consistency involving 60 stroke patients and 20 healthy non‐neurologically impaired patients Outcomes: stage transition duration and laryngeal closure duration Excluded: (1) non‐RCT; (2) no outcome data | |
| RCT assessing PEG tube size in 56 dysphagic patients (51% stroke) | |
| RCT comparing PEG with NGT feeding in 40 dysphagic patients | |
| Single case study assessing oral (palatal) electrical stimulation in 4 stroke patients with chronic dysphagia | |
| RCT | |
| Non‐RCT measuring initiation of laryngeal closure and laryngeal closure duration in 3 groups of patients: (1) 10 stroke patients who aspirated before and during the swallow, (2) 10 stroke patients who did not aspirate, and (3) 10 normal control patients Outcome: initiation of laryngeal closure and laryngeal closure duration Excluded: (1) non‐RCT | |
| RCT Group 1: neuromuscular electrical stimulation (n = 12) Group 2: rehabilitation swallowing therapy (n = 11) All stroke Excluded: (1) counfounded, i.e. comparison of 2 active treatments | |
| RCT assessing disease specific enteral formula versus standard formula in 105 patients with type 2 diabetes mellitus and neurological dysphagia Outcome: total insulin requirements, fasting glucose, afternoon blood glucose, HbA1C and safety criteria Excluded: (1) time since stroke onset to randomisation unclear | |
| Non‐randomised crossover study assessing fluid consistency (thin, thick) and volume (5 ml, 10 ml, cup) in 21 dysphagic stroke patients | |
| RCT assessing thickened fluids versus postural and/or swallowing strategies in 50 patients with post‐stroke dysphagia: a further group of patients who were not dysphagic for liquids and who were given normal fluids compared with the RCT Outcome: development of chest infection and dehydration Excluded: (1) no control group, 2 interventional groups were compared in the RCT | |
| Before and after intervention study | |
| Crossover trial assessing oral energy load (glucose or xylose) in 9 patients with stroke and 8 matched control participants | |
| Randomised crossover trial assessing thermal stimulation in 7 male dysphagic patients with multiple previous strokes | |
| Randomised crossover trial assessing thermal stimulation in 23 dysphagic patients with multiple previous strokes | |
| Dose comparison RCT of thermal stimulation (150, 300, 450, 600 trials per week) in 45 dysphagic stroke patients recruited within 12 weeks | |
| Nasogastric tube versus percutaneous radiologic gastrostomy in critically ill patients admitted to intensive care unit and requiring gastric tubing Excluded: (1) no control, 2 active treatments; (2) no data for stroke patients | |
| RCT comparing enteral formulae ‐ rich in monounsaturated fatty acid versus rich in carbohydrates ‐ in 15 diabetic dysphagic stroke patients | |
| Multi fibre enriched formula for 2 weeks versus fibre‐free formula in dysphagic patients on long‐term enteral nutrition Outcome: faecal short‐chain fatty acids and microbiota Excluded: (1) no control group, confounded trial | |
| Randomised trial | |
| Case control study assessing ACE inhibitors in stroke patients | |
| RCT comparing head‐raising exercise with sham exercise in 27 dysphagic patients | |
| RCT comparing NGT and NJT feeding in patients with dysphagia following stroke Excluded: (1) unable to obtain data | |
| Crossover trial comparing pureed and moulded peaches in 15 dysphagic patients (stroke n = 10) and 15 normal volunteers | |
| RCT comparing enteral with parenteral nutrition in 49 tube fed patients post‐neurosurgery | |
| RCT assessing swallowing therapy (biofeedback) in 9 patients with dysphagia secondary to stroke or head injury Excluded: (1) dysphagia of mixed aetiology; (2) outcome measures (tongue and lip motor force) not relevant to this review | |
| RCT amino acid compositions in 30 patients 12 hours post‐surgery for SAH | |
| 3 meals per day (regular menu and portions) versus 5 meals of same energy content in elderly residents of an extended care facility suffering from dysphagia Outcome: effect on energy intake Excluded: (1) confounded, no control group, no data for stroke patients | |
| RCT assessing swallowing function using cilostazol versus placebo in 48 patients with dysphagia secondary to stroke Outcom: swallowing function Excluded: (1) onset of stroke to randomisation 1 to 6 months; (2) crossover study no access to data on the first phase | |
| 21 patients | |
| RCT assessing PEG tube composition in 106 patients with mixed indications for tube feeding | |
| Group 1: motor control programme (n = 30) | |
| Non‐RCT assessing repetitive transcranial magnetic stimulation in patients with post stroke dysphagia Outcomes: dysphagia handicap index and videofluoroscopy Excluded: (1) non‐RCT; (2) no control group | |
| Non‐RCT assessing submental sensitive transcutaneous electrical stimulation in 13 patients with neurogenic oropharyngeal dysphagia Outcomes: swallowing function using a standardised videofluoroscopic barium swallow Excluded: (1) non‐RCT; (2) no control group | |
| RCT assessing fluid consistency in 24 dysphagic acute stroke patients | |
| Non‐randomised assessment of speech and language therapy referrals for assessment of speech and swallowing in elderly patients, 40% of whom had a stroke | |
| Non‐RCT of acupuncture and sublingual blood letting Excluded: (1) timing unclear; (2) no control group (confounding); (3) not RCT | |
| Randomisation unclear, timing unclear | |
| Crossover trial assessing fibre supplementation in dysphagic stroke patients | |
| RCT comparing different depth of Chonggu (EX‐HN 27) by electroacupuncture in patients of dysphagia after stroke Chonggu (EX‐HN 27) deep insertion group (n = 99) Chonggu (EX‐HN 27) shallow insertion group (n = 94) Traditional acupuncture group (n = 90) Outcomes: Kubota's Water Drinking Test Scale, standard swallowing function scale and TCM Scale of Dysphagia After Stroke Excluded: (1) no outcome data | |
| High protein enteral nutrition formula versus standard enteral nutrition formula for 14 days in patients with severe stroke Outcome: survival, hypoalbumenia Excluded: (1) no control group confounded trial; (2) unable to obtain data |
ACE: angiotensin converting enzyme
CCT: controlled clinical trial
CXR: chest x‐ray
FIM: Functional Independence Measure
GI: gastrointestinal
NGT: nasogastric tube
NJT: nasojejunal tube
PEG: percutaneous endoscopic gastrostomy
RCT: randomised controlled trial
SAH: subarachnoid haemorrhage
TCM: traditional Chinese medicine
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | Patients with dysphagia owing to neurological diseases |
| Interventions | PEG using transnasal endoscopy or transoral endoscope |
| Outcomes | Safety, pain, stress |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic patients |
| Interventions | NGT versus control |
| Outcomes | Swallowing function |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic stroke patients |
| Interventions | Shallow versus deep versus deep multi‐needling |
| Outcomes | Swallowing function |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Elderly critically ill inpatients at risk of pressure ulcer development |
| Interventions | Nutritional supplements versus control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT comparing of 2 medication delivery systems |
| Participants | Dysphagic patients |
| Interventions | Rapitab orally disintegrating pill versus conventional pill |
| Outcomes | Swallow effort, airway compromise and patient preference |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Acute dysphagic stroke patients |
| Interventions | Early intervention to improve swallowing including altering shape of food, posture, nasal feeding, throat swab training, and electroacupuncture versus control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Ischaemic stroke patients with pseudobulbar palsy |
| Interventions | Early throat muscle training versus control |
| Outcomes | Effects on vertebral and basilar artery blood flow |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Elderly patients (mostly dysphagic stroke patients) |
| Interventions | Intermittent versus continuous NGT feeding |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic nursing home residents |
| Interventions | 200 g (8 oz) versus 100 g (4 oz) servings of thickened drinks |
| Outcomes | Effect on food consumption and hydration potential |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Long‐term geriatric inpatients (including stroke) |
| Interventions | Comparison of 3 dietary supplements |
| Outcomes | Effect on dietary intake, anthropometric variables and biochemical analyses. |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Frail elderly nursing home patients (including stroke) |
| Interventions | Reformed foods and thickened beverages versus traditional food |
| Outcomes | Effect on dietary intake and weight |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with pseudobulbar dysphagia |
| Interventions | Pureed diet with thin liquids versus soft mechanical diet with thickened liquid |
| Outcomes | Incidence of aspiration pneumonia |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Acute stroke patients with dysphagia and dysarthria |
| Interventions | Scalp and neck acupuncture + electroacupuncture versus control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with dysphagia |
| Interventions | PEG placement by single physician using endoscope holder versus PEG placement by 2 physicians using conventional pull method |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Chronic dysphagic stroke patients |
| Interventions | Repetitive transcranial magnetic stimulation versus sham stimulation over the unaffected pharyngeal motor cortex |
| Outcomes | Measurements of cortico‐pharyngeal excitability |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Mechanically ventilated stroke and head injury patients |
| Interventions | Percutaneous gastrostomy versus NGT |
| Outcomes | Ventilator‐associated pneumonia |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Stroke patients with dysphagia |
| Interventions | Structured swallowing training programme versus no training |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Stroke patients with pseudobulbar paralysis |
| Interventions | Scalp acupuncture + sublingual needling versus scalp acupuncture + control needling |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with stroke |
| Interventions | Continuous versus intermittent nasogastric feeding |
| Outcomes | Gastrointestinal haemorrhage |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic patients |
| Interventions | Peg placement with or without an over tube |
| Outcomes | Peristomal infection |
| Notes | In the process of retrieving full‐text article |
| Methods | Randomised cross‐over study |
| Participants | Dysphagic stroke patients |
| Interventions | Clear fluid (10 mL tap water) versus thickened fluid (10 mL tap water with a scoop of Nutilis thickener) |
| Outcomes | Aspiration and oxygen saturation |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic stroke patients |
| Interventions | Manual + electro‐acupuncture (6 to 8 treatments 2 to 3 times per week for 3 weeks) versus control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with severe cerebral infarction |
| Interventions | Modified enteral nutrition versus traditional nutrition |
| Outcomes | Nutritional status and gut function |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Tube‐fed type II diabetic patients with neurological dysphagia (primarily stroke) |
| Interventions | Comparison of 2 enteral feeding formulae (low carbohydrates + high monounsaturated fatty acids (Diben) versus standard formula) |
| Outcomes | Glycaemic control |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Post stroke patients with dysphagia |
| Interventions | Vital stimulation (and electrotherapy intervention) versus traditional treatment |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Acute dysphagic stroke patients |
| Interventions | Pharyngeal electrical stimulation versus no treatment |
| Outcomes | Aspiration scores at 2 weeks |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Hemiplegic stroke patients |
| Interventions | Carnitine versus placebo |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients referred for placement of feeding gastrostomy (majority neurological) |
| Interventions | Operative gastrostomy versus PEG |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with dysphagia after stroke |
| Interventions | Acupuncture at Lianquan, Yamen and Tian Zhu acupoints versus VitalStim therapy |
| Outcomes | Swallowing function |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Acute stroke patients needing gastrointestinal tube feeding |
| Interventions | Tube feeding by immunonutrition‐oriented or protein‐oriented food |
| Outcomes | Short‐term clinical outcomes |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic patients (including stroke) |
| Interventions | Extra‐corporeal PEG versus pull method PEG |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Acute haemorrhage stroke patients |
| Interventions | Continuous parenteral nutrition for 7 days versus glucose control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with post‐stroke dysphagia |
| Interventions | Early rehabilitation + acupuncture versus control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Post stroke dysphagic patients |
| Interventions | FES 40 minutes/day versus FES 40 minutes twice daily |
| Outcomes | Swallowing function |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic stroke patients with poor elevation of the larynx |
| Interventions | Comparison of 2 methods of larynx elevation (15 minutes, 5 x day for 4 weeks) |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Acute stroke patients with dysphagia within 72 hours of admission |
| Interventions | NGT feeding versus nasal feeding of liquid diet |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Dysphagic stroke patients 15 to 40 days post stroke |
| Interventions | Head acupuncture versus body acupuncture versus control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
| Methods | RCT |
| Participants | Patients with severe stroke |
| Interventions | High protein enteral nutrition formula versus standard enteral nutrition formula |
| Outcomes | Survival and risk of hypoalbuminaemia |
| Notes | In the process of retrieving full‐text article |
FES: functional electrical stimulation
NGT: nasogastric tube
PEG: percutaneous endoscopic gastrostomy
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Adjunctive Neuromuscular electrical Stimulation for the Rehabilitation of Swallowing "ANSRS" |
| Methods | RCT, double blind (participant, investigator, outcomes assessor) |
| Participants | 53 stroke patients with dysphagia |
| Interventions | Arm 1: usual care, Arm 2: sham neuromuscular electrical stimulation, Arm 3: neuromuscular electrical stimulation |
| Outcomes | Clinical response at 3 weeks' and 3 months' post treatment |
| Starting date | 2008 |
| Contact information | Giselle Carnaby‐Mann, University of Florida |
| Notes | Funding: University of Florida, National Center for Medical Rehabilitation Research |
| Trial name or title | Effect of transcutaneous electrical stimulation on post‐stroke dysphagic patients "EETI‐01" |
| Methods | RCT, safety and efficacy study |
| Participants | Post‐stroke dysphagic patients |
| Interventions | Sensory stimulation versus motor stimulation |
| Outcomes | Not provided |
| Starting date | 2011 |
| Contact information | Pere Clavé, MD, [email protected] 937417700 |
| Notes | Funding: Hospital de Mataró Lead, CIBEREHD |
| Trial name or title | A randomised controlled trial of pharyngeal electrical stimulation in the treatment of dysphagia after brain injury |
| Methods | RCT Phase II |
| Participants | Hospitalised stroke patients within 6 weeks of their stroke |
| Interventions | Pharyngeal electrical stimulation versus control |
| Outcomes | Swallow function |
| Starting date | 2003 |
| Contact information | Prof Shaheen Hamdy, Hope Hospital, Clinical Sciences Building, Department of GI Sciences, Hope Hospital, Stott Lane, Salford, Greater Manchester, M6 8HD, UK |
| Notes | Funding: NIHR Research for Patient Benefit |
| Trial name or title | Clinical evaluation of dysphagia therapeutic apparatus on cerebrovascular disease |
| Methods | RCT |
| Participants | Stroke patients 2 to 60 days from onset |
| Interventions | Dysphagia therapeutic apparatus on acupoints versus regular dysphagia rehabilitation versus both |
| Outcomes | Dysphagia therapeutic apparatus versus control |
| Starting date | 2009 |
| Contact information | Chengqi He, No. 37, Guoxue Alley, Wuhou District, Chengdu, Sichuan, China |
| Notes | Funding: State Plan for High‐Tech Research and Development |
| Trial name or title | Respiratory muscle training in stroke. Evaluation of respiratory muscle strengthening to reduce chest infections in stroke patients with swallowing problems |
| Methods | RCT Phase II |
| Participants | 60 ischaemic stroke patients with dysphagia aged between 50 to 80 years |
| Interventions | Expiratory muscle, inspiratory muscle or sham training |
| Outcomes | Aspiration, cough, chest infections, respiratory muscle strength |
| Starting date | 2011 |
| Contact information | Prof Lalit Kalra, King's College Hospital NHS Trust, King's College Hospital NHS Trust, Bessemer Road, London, SE5 9PJ, UK |
| Notes | Funding: NIHR ‐ Central commissioning facility |
| Trial name or title | Comparison of intravenous and subcutaneous bolus infusion in post‐stroke hydration |
| Methods | ‐ |
| Participants | Patients: 150 |
| Interventions | Intravenous versus subcutaneous hydration |
| Outcomes | ‐ |
| Starting date | 2000 |
| Contact information | Prof M Lye, Department of Geriatrics, 3rd Floor Duncan Building, Daulby Street, Liverpool, L69 3GB UK |
| Notes | Funding: NHS Executive North West (£16,500) |
| Trial name or title | Effect of electrical stimulation in post‐stroke patients with dysphagia |
| Methods | RCT, open but assessors are blinded |
| Participants | Post‐stroke patients with dysphagia |
| Interventions | Electrical stimulation versus control |
| Outcomes | Videofluorography, Fujishima's grade, motion analysis |
| Starting date | 2010 |
| Contact information | Shuji Matsumoto, Department of Rehabilitation and Physical Medicine, Kagoshima Universit, y3930‐7 Takachiho, Makizono‐cho, Kirishima City, Japan |
| Notes | Funding: self funding |
| Trial name or title | Identifying and treating arousal related deficits in neglect and dysphagia |
| Methods | Randomised, double‐blind (participant, investigator), cross‐over assignment |
| Participants | Stroke patients with neglect, dysphagia |
| Interventions | Modafinil 200 mg once daily versus placebo for 3 days |
| Outcomes | Predicting response to modafinil among participants with neglect, dysphagia |
| Starting date | 2010 |
| Contact information | Gary McCullough, [email protected] |
| Notes | Funding: University of Arkansas, Eunice Kennedy Shriver National Institute of Child Health and Human Development |
| Trial name or title | Exercise for swallowing problems after stroke |
| Methods | Randomised, open label |
| Participants | 200 post‐stroke patients |
| Interventions | Group 1: lingual press (high‐intensity, oral, non‐swallowing) Group 2: effortful swallowing (high‐intensity swallowing) Group 3: natural swallowing (high‐frequency, low‐intensity swallowing) Group 4: non‐oral sham (control) exercise |
| Outcomes | Composite score of Penetration/Aspiration Scale and Residue Scale with no worsening of either at baseline, week 4, and week 8 |
| Starting date | 2011 |
| Contact information | Jacqueline Hind, MS [email protected] |
| Notes | Funding: Department of Veterans Affairs Lead, University of Wisconsin, Madison |
| Trial name or title | SQACU01 ‐ a randomised trial of acupuncture as adjuvant therapy for dysphagia due to recent stroke |
| Methods | |
| Participants | Acute stroke < 1 week |
| Interventions | Acupuncture versus sham acupuncture for 16 sessions |
| Outcomes | Tube feeding, pneumonia, mortality, each at 6 months |
| Starting date | 2001 |
| Contact information | Dr D Heng, Clinical Trials & Epidemiology Research Unit, Ministry of Health, Block A, Unit 02‐02, 226 Outram Road, Singapore 169039 |
| Notes | Funding: ? |
| Trial name or title | Tongue Pressure Profile Training for dysphagia post stroke "TPPT" |
| Methods | Randomised, single blind (outcomes assessor) |
| Participants | 60 patients with thin liquid flow‐control difficulties secondary to stroke or acquired brain injury |
| Interventions | Compare 2 different tongue‐pressure resistance training protocols Tongue‐pressure profile training versus tongue‐pressure strength‐and‐accuracy training |
| Outcomes | Primary: change in penetration‐aspiration scale Secondary: change in bolus control for thin liquids on videofluoroscopy versus baseline |
| Starting date | 2011 |
| Contact information | Catriona M Steele, Toronto Rehabilitation Institute, Canada |
| Notes | Funding: Toronto Rehabilitation Institute |
| Trial name or title | Swallowing Treatment using Electrical Pharyngeal Stimulation (STEPS) study |
| Methods | Randomised, single blind, outcome blind |
| Participants | 140 patients with post‐stroke dysphagia < 6 weeks |
| Interventions | Pharyngeal electrical stimulation: active versus sham |
| Outcomes | Primary: change in penetration‐aspiration scale at 2 weeks from baseline; secondary: Toronto Bedside Swallowing Screening Test, Dysphagia Severity Rating Scale, National Institute of Health Stroke Scale, modified Rankin Scale |
| Starting date | March 2012 |
| Contact information | Philip M Bath, University of Nottingham, 0115 823 1765 |
| Notes | Funding: Phagenesis Ltd |
| Trial name or title | Randomised controlled open label trial to evaluate tolerance and safety of a new pre‐thickened energy dense sip feed in patients in need of oral nutritional support |
| Methods | RCT, open label |
| Participants | Dysphagic patients requiring oral nutritional support |
| Interventions | Pre‐thickened sip feed versus a standard sip feed thickened with a commercially available thickening powder |
| Outcomes | Stool frequency, incidence and intensity of gastrointestinal symptoms, safety parameters in blood |
| Starting date | 2009 |
| Contact information | Dr A Vriesema, Numico Research BV, PO Box 7005, 20 Bosrand Road, Wageningen, 6700 CA, The Netherlands |
| Notes | Funding: Danone Research BV |
| Trial name or title | Cortical neuromodulation in post stroke dysphagia |
| Methods | RCT, double blind (participant, investigator), efficacy study |
| Participants | 20 patients with post‐stroke dysphagia |
| Interventions | Sub‐motor threshold stimulation of mylohyoid muscles versus control |
| Outcomes | Videofluoroscopy before and after (once a day for 5 consecutive days) |
| Starting date | 2007 |
| Contact information | Eric Verin, Rouen University, France |
| Notes | Funding: University Hospital, Rouen |
| Trial name or title | Randomised controlled study on the acupuncture for dysphagia in convalescence phase of apoplexy |
| Methods | RCT |
| Participants | Patients with dysphagia in the convalescence phase of stroke (2 and 6 months) |
| Interventions | Combination of body acupuncture, scalp acupuncture and electroacupuncture versus routine rehabilitation training |
| Outcomes | Safety and tolerability of the acupuncture |
| Starting date | 2007 |
| Contact information | Yue Xie, 9‐312, No. 32 Fuxing Road, Haidian District, Beijing, China |
| Notes | Funding: Beijing Public Health Bureau |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Swallowing therapy, Outcome 1 Case fatality at end of trial. | ||||
| 1.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 1.2 Drug therapy | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.06, 21.87] |
| 1.3 Pharyngeal electrical stimulation | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 4.31 [0.19, 98.51] |
| 1.4 Physical stimulation (thermal, tactile) | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.92] |
| 1.5 Transcranial magnetic stimulation | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 7.12] |
| 2 Death or dependency at end of trial Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Swallowing therapy, Outcome 2 Death or dependency at end of trial. | ||||
| 2.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.75] |
| 3 Institutionalisation Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Swallowing therapy, Outcome 3 Institutionalisation. | ||||
| 3.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.39, 1.48] |
| 4 Length of stay (days) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Swallowing therapy, Outcome 4 Length of stay (days). | ||||
| 4.1 Behavioural interventions | 4 | 370 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.68, 0.28] |
| 5 Chest infection or pneumonia Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Swallowing therapy, Outcome 5 Chest infection or pneumonia. | ||||
| 5.1 Behavioural interventions | 5 | 423 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 5.2 Drug therapy | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.67] |
| 5.3 Pharyngeal electrical stimulation | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.09] |
| 6 Dysphagia at end of trial Show forest plot | 13 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Swallowing therapy, Outcome 6 Dysphagia at end of trial. | ||||
| 6.1 Acupuncture | 4 | 256 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.13, 0.46] |
| 6.2 Behavioural interventions | 5 | 423 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.88] |
| 6.3 Drug therapy | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.07, 3.35] |
| 6.4 Neuromuscular electrical stimulation | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.50] |
| 6.5 Physical stimulation (thermal, tactile) | 1 | 7 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 11.34] |
| 6.6 Transcranial direct current stimulation | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 8.39] |
| 7 Pharyngeal transit time (seconds) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Swallowing therapy, Outcome 7 Pharyngeal transit time (seconds). | ||||
| 7.1 Drug therapy | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.91, 0.49] |
| 7.2 Pharyngeal electrical stimulation | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.51, 0.20] |
| 7.3 Physical stimulation (thermal, tactile) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] |
| 8 Swallow score Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Swallowing therapy, Outcome 8 Swallow score. | ||||
| 8.1 Acupuncture | 3 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.53, 0.72] |
| 8.2 Neuromuscular electrical stimulation versus behavioural interventions | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Physical stimulation (thermal, tactile) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐2.58, 5.38] |
| 8.4 Transcranial direct current stimulation | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.50, 2.50] |
| 9 Nutritional (albumin) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Swallowing therapy, Outcome 9 Nutritional (albumin). | ||||
| 9.1 Behavioural interventions | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.77, 5.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Route of feeding, Outcome 1 Case fatality at end of trial. | ||||
| 1.1 PEG versus NGT | 5 | 455 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.42, 1.56] |
| 1.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.27, 1.33] |
| 2 Death or dependency at end of trial Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Route of feeding, Outcome 2 Death or dependency at end of trial. | ||||
| 2.1 PEG versus NGT | 3 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.12, 5.55] |
| 2.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.18, 1.57] |
| 3 Institutionalisation Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Route of feeding, Outcome 3 Institutionalisation. | ||||
| 3.1 PEG versus NGT | 2 | 364 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.15, 2.57] |
| 3.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.78, 3.81] |
| 4 Length of stay in hospital (days) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Route of feeding, Outcome 4 Length of stay in hospital (days). | ||||
| 4.1 PEG versus NGT | 2 | 384 | Mean Difference (IV, Random, 95% CI) | 14.32 [‐12.04, 40.68] |
| 4.2 NGT with loop versus NGT | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 7.0 [‐8.48, 22.48] |
| 5 Pressure sores Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Route of feeding, Outcome 5 Pressure sores. | ||||
| 5.1 PEG versus NGT | 1 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 3.1 [0.98, 9.83] |
| 5.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.28, 3.84] |
| 6 Chest infection or pneumonia Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Route of feeding, Outcome 6 Chest infection or pneumonia. | ||||
| 6.1 PEG versus NGT | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.86] |
| 6.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.39, 1.84] |
| 7 Dysphagia at end of trial Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Route of feeding, Outcome 7 Dysphagia at end of trial. | ||||
| 7.1 PEG versus NGT | 2 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.05, 11.77] |
| 8 Treatment failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Route of feeding, Outcome 8 Treatment failure. | ||||
| 8.1 PEG versus NGT | 3 | 72 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.51] |
| 8.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.64, 4.34] |
| 9 Gastrointestinal bleeding Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Route of feeding, Outcome 9 Gastrointestinal bleeding. | ||||
| 9.1 PEG versus NGT | 1 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.69] |
| 9.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.43, 6.17] |
| 10 Feed delivery (%) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Route of feeding, Outcome 10 Feed delivery (%). | ||||
| 10.1 PEG versus NGT | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 22.0 [16.15, 27.85] |
| 10.2 NGT with loop versus NGT | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 18.0 [6.66, 29.34] |
| 11 Weight at end of trial (last value carried forward) (kg) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.11  Comparison 2 Route of feeding, Outcome 11 Weight at end of trial (last value carried forward) (kg). | ||||
| 11.1 PEG versus NGT | 2 | 34 | Mean Difference (IV, Random, 95% CI) | 4.08 [‐4.32, 12.48] |
| 12 Mid‐arm circumference (last value carried forward) (cm) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.12  Comparison 2 Route of feeding, Outcome 12 Mid‐arm circumference (last value carried forward) (cm). | ||||
| 12.1 PEG versus NGT | 3 | 58 | Mean Difference (IV, Random, 95% CI) | 2.29 [‐0.30, 4.89] |
| 13 Albumin (last value carried forward) (g/L) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2 Route of feeding, Outcome 13 Albumin (last value carried forward) (g/L). | ||||
| 13.1 PEG versus NGT | 3 | 63 | Mean Difference (IV, Random, 95% CI) | 4.92 [0.19, 9.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Timing of feeding, Outcome 1 Case fatality at end of trial. | ||||
| 1.1 Early versus late feeding | 1 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.04] |
| 2 Death or disabled at end of trial Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Timing of feeding, Outcome 2 Death or disabled at end of trial. | ||||
| 2.1 Early versus late feeding | 1 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.31] |
| 3 Institutionalisation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Timing of feeding, Outcome 3 Institutionalisation. | ||||
| 3.1 Early versus late feeding | 1 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of dysphagia (days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Fluid supplementation, Outcome 1 Time to resolution of dysphagia (days). | ||||
| 1.1 Free thin fluids | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐8.10 [‐20.84, 4.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Nutritional supplementation, Outcome 1 Case fatality at end of trial. | ||||
| 1.1 Sip feeding | 7 | 4343 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.28, 1.21] |
| 2 Death or dependency at end of trial Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Nutritional supplementation, Outcome 2 Death or dependency at end of trial. | ||||
| 2.1 Sip feeding | 1 | 4023 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.20] |
| 3 Institutionalisation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Nutritional supplementation, Outcome 3 Institutionalisation. | ||||
| 3.1 Sip feed | 1 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.07] |
| 4 Length of stay in hospital (days) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Nutritional supplementation, Outcome 4 Length of stay in hospital (days). | ||||
| 4.1 Sip feeding | 2 | 4114 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐0.81, 3.60] |
| 5 Pressure sores Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Nutritional supplementation, Outcome 5 Pressure sores. | ||||
| 5.1 Sip feeding | 2 | 4125 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.96] |
| 6 Energy intake (kcal/day) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.6  Comparison 5 Nutritional supplementation, Outcome 6 Energy intake (kcal/day). | ||||
| 6.1 Sip feeding | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 430.18 [141.61, 718.75] |
| 7 Protein intake (g/day) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.7  Comparison 5 Nutritional supplementation, Outcome 7 Protein intake (g/day). | ||||
| 7.1 Sip feeding | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 17.28 [1.99, 32.56] |
| 8 Albumin (last value carried forward) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.8  Comparison 5 Nutritional supplementation, Outcome 8 Albumin (last value carried forward). | ||||
| 8.1 Sip feeding | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.65, 1.24] |

Study flow diagram.* Further 38 studies are awaiting assessment.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

Comparison 1 Swallowing therapy, Outcome 1 Case fatality at end of trial.

Comparison 1 Swallowing therapy, Outcome 2 Death or dependency at end of trial.

Comparison 1 Swallowing therapy, Outcome 3 Institutionalisation.

Comparison 1 Swallowing therapy, Outcome 4 Length of stay (days).

Comparison 1 Swallowing therapy, Outcome 5 Chest infection or pneumonia.
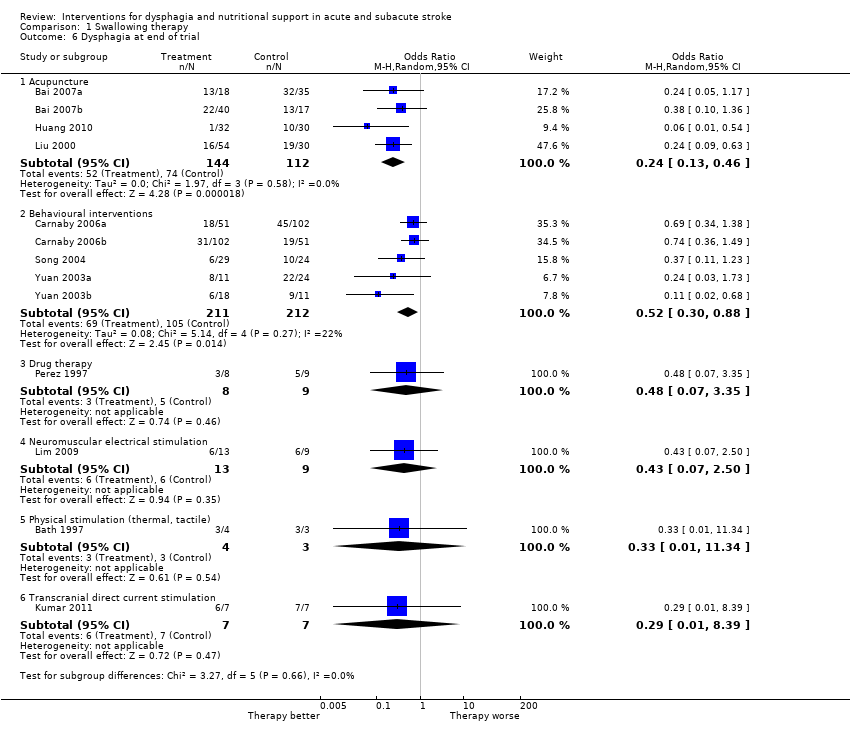
Comparison 1 Swallowing therapy, Outcome 6 Dysphagia at end of trial.

Comparison 1 Swallowing therapy, Outcome 7 Pharyngeal transit time (seconds).
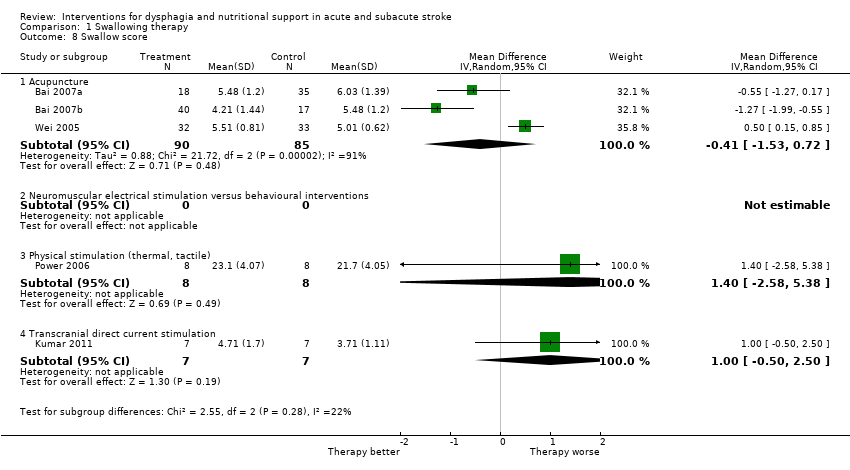
Comparison 1 Swallowing therapy, Outcome 8 Swallow score.
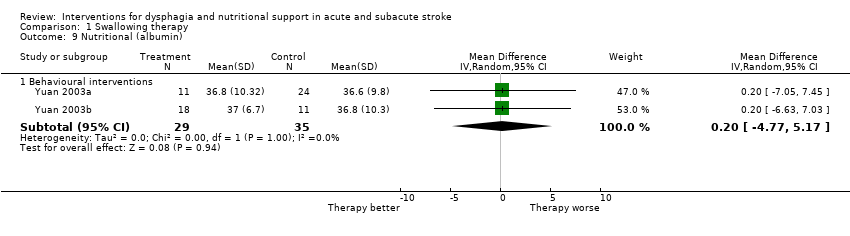
Comparison 1 Swallowing therapy, Outcome 9 Nutritional (albumin).
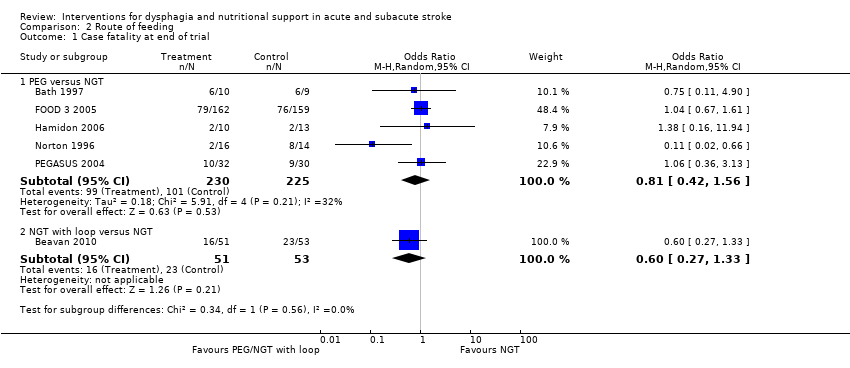
Comparison 2 Route of feeding, Outcome 1 Case fatality at end of trial.

Comparison 2 Route of feeding, Outcome 2 Death or dependency at end of trial.

Comparison 2 Route of feeding, Outcome 3 Institutionalisation.
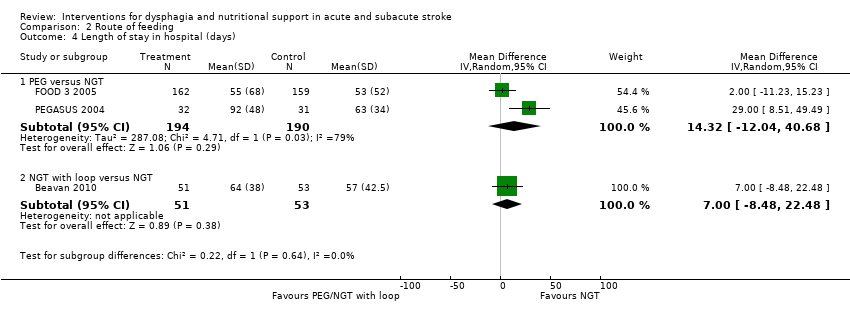
Comparison 2 Route of feeding, Outcome 4 Length of stay in hospital (days).

Comparison 2 Route of feeding, Outcome 5 Pressure sores.
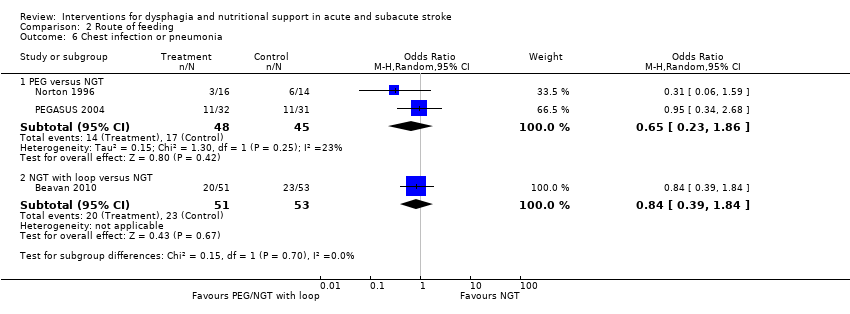
Comparison 2 Route of feeding, Outcome 6 Chest infection or pneumonia.

Comparison 2 Route of feeding, Outcome 7 Dysphagia at end of trial.

Comparison 2 Route of feeding, Outcome 8 Treatment failure.

Comparison 2 Route of feeding, Outcome 9 Gastrointestinal bleeding.

Comparison 2 Route of feeding, Outcome 10 Feed delivery (%).
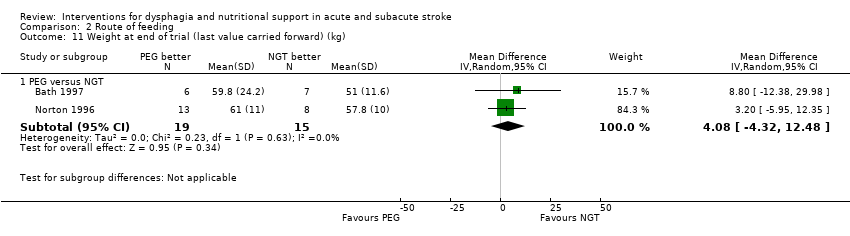
Comparison 2 Route of feeding, Outcome 11 Weight at end of trial (last value carried forward) (kg).

Comparison 2 Route of feeding, Outcome 12 Mid‐arm circumference (last value carried forward) (cm).

Comparison 2 Route of feeding, Outcome 13 Albumin (last value carried forward) (g/L).

Comparison 3 Timing of feeding, Outcome 1 Case fatality at end of trial.
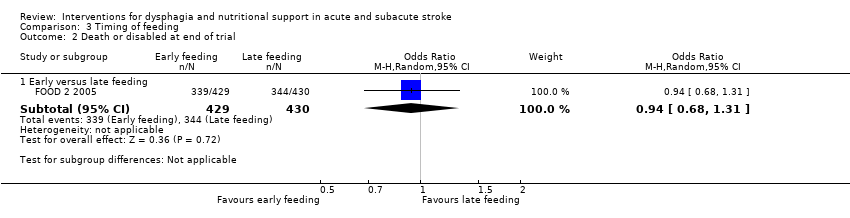
Comparison 3 Timing of feeding, Outcome 2 Death or disabled at end of trial.

Comparison 3 Timing of feeding, Outcome 3 Institutionalisation.

Comparison 4 Fluid supplementation, Outcome 1 Time to resolution of dysphagia (days).

Comparison 5 Nutritional supplementation, Outcome 1 Case fatality at end of trial.
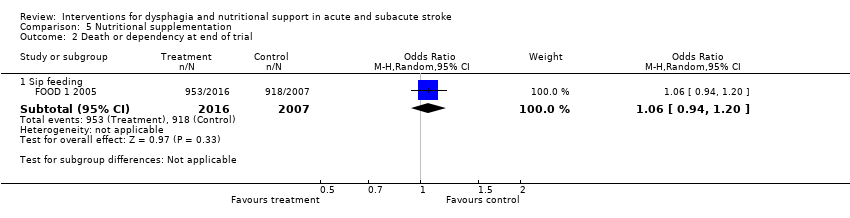
Comparison 5 Nutritional supplementation, Outcome 2 Death or dependency at end of trial.

Comparison 5 Nutritional supplementation, Outcome 3 Institutionalisation.

Comparison 5 Nutritional supplementation, Outcome 4 Length of stay in hospital (days).

Comparison 5 Nutritional supplementation, Outcome 5 Pressure sores.

Comparison 5 Nutritional supplementation, Outcome 6 Energy intake (kcal/day).
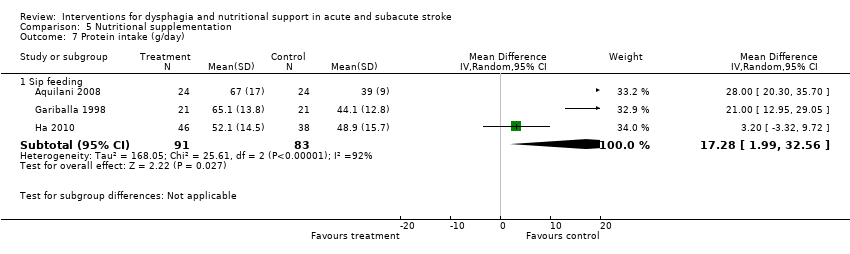
Comparison 5 Nutritional supplementation, Outcome 7 Protein intake (g/day).

Comparison 5 Nutritional supplementation, Outcome 8 Albumin (last value carried forward).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 1.2 Drug therapy | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.06, 21.87] |
| 1.3 Pharyngeal electrical stimulation | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 4.31 [0.19, 98.51] |
| 1.4 Physical stimulation (thermal, tactile) | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.92] |
| 1.5 Transcranial magnetic stimulation | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 7.12] |
| 2 Death or dependency at end of trial Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.75] |
| 3 Institutionalisation Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.39, 1.48] |
| 4 Length of stay (days) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Behavioural interventions | 4 | 370 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.68, 0.28] |
| 5 Chest infection or pneumonia Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Behavioural interventions | 5 | 423 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 5.2 Drug therapy | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.67] |
| 5.3 Pharyngeal electrical stimulation | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.09] |
| 6 Dysphagia at end of trial Show forest plot | 13 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Acupuncture | 4 | 256 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.13, 0.46] |
| 6.2 Behavioural interventions | 5 | 423 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.88] |
| 6.3 Drug therapy | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.07, 3.35] |
| 6.4 Neuromuscular electrical stimulation | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.50] |
| 6.5 Physical stimulation (thermal, tactile) | 1 | 7 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 11.34] |
| 6.6 Transcranial direct current stimulation | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 8.39] |
| 7 Pharyngeal transit time (seconds) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Drug therapy | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.91, 0.49] |
| 7.2 Pharyngeal electrical stimulation | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.51, 0.20] |
| 7.3 Physical stimulation (thermal, tactile) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] |
| 8 Swallow score Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Acupuncture | 3 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.53, 0.72] |
| 8.2 Neuromuscular electrical stimulation versus behavioural interventions | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Physical stimulation (thermal, tactile) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐2.58, 5.38] |
| 8.4 Transcranial direct current stimulation | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.50, 2.50] |
| 9 Nutritional (albumin) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Behavioural interventions | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.77, 5.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 PEG versus NGT | 5 | 455 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.42, 1.56] |
| 1.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.27, 1.33] |
| 2 Death or dependency at end of trial Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 PEG versus NGT | 3 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.12, 5.55] |
| 2.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.18, 1.57] |
| 3 Institutionalisation Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 PEG versus NGT | 2 | 364 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.15, 2.57] |
| 3.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.78, 3.81] |
| 4 Length of stay in hospital (days) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 PEG versus NGT | 2 | 384 | Mean Difference (IV, Random, 95% CI) | 14.32 [‐12.04, 40.68] |
| 4.2 NGT with loop versus NGT | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 7.0 [‐8.48, 22.48] |
| 5 Pressure sores Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 PEG versus NGT | 1 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 3.1 [0.98, 9.83] |
| 5.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.28, 3.84] |
| 6 Chest infection or pneumonia Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 PEG versus NGT | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.86] |
| 6.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.39, 1.84] |
| 7 Dysphagia at end of trial Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 PEG versus NGT | 2 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.05, 11.77] |
| 8 Treatment failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 PEG versus NGT | 3 | 72 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.51] |
| 8.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.64, 4.34] |
| 9 Gastrointestinal bleeding Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 PEG versus NGT | 1 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.69] |
| 9.2 NGT with loop versus NGT | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.43, 6.17] |
| 10 Feed delivery (%) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 PEG versus NGT | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 22.0 [16.15, 27.85] |
| 10.2 NGT with loop versus NGT | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 18.0 [6.66, 29.34] |
| 11 Weight at end of trial (last value carried forward) (kg) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 PEG versus NGT | 2 | 34 | Mean Difference (IV, Random, 95% CI) | 4.08 [‐4.32, 12.48] |
| 12 Mid‐arm circumference (last value carried forward) (cm) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 PEG versus NGT | 3 | 58 | Mean Difference (IV, Random, 95% CI) | 2.29 [‐0.30, 4.89] |
| 13 Albumin (last value carried forward) (g/L) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 PEG versus NGT | 3 | 63 | Mean Difference (IV, Random, 95% CI) | 4.92 [0.19, 9.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Early versus late feeding | 1 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.04] |
| 2 Death or disabled at end of trial Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early versus late feeding | 1 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.31] |
| 3 Institutionalisation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Early versus late feeding | 1 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of dysphagia (days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Free thin fluids | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐8.10 [‐20.84, 4.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at end of trial Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Sip feeding | 7 | 4343 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.28, 1.21] |
| 2 Death or dependency at end of trial Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Sip feeding | 1 | 4023 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.20] |
| 3 Institutionalisation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Sip feed | 1 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.07] |
| 4 Length of stay in hospital (days) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Sip feeding | 2 | 4114 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐0.81, 3.60] |
| 5 Pressure sores Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Sip feeding | 2 | 4125 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.96] |
| 6 Energy intake (kcal/day) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Sip feeding | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 430.18 [141.61, 718.75] |
| 7 Protein intake (g/day) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Sip feeding | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 17.28 [1.99, 32.56] |
| 8 Albumin (last value carried forward) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Sip feeding | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.65, 1.24] |

