Tratamiento para la deglución para la disfagia en el accidente cerebrovascular agudo y subagudo
Resumen
Antecedentes
La disfagia (problemas para tragar), que es frecuente después de un accidente cerebrovascular, se asocia con un mayor riesgo de muerte o dependencia, la aparición de neumonía, una disminución de la calidad de vida y una estancia hospitalaria más prolongada. Los tratamientos proporcionados para mejorar la disfagia tienen como objetivo acelerar la recuperación de la función de deglución y reducir esos riesgos. Ésta es una actualización de una revisión publicada por primera vez en 1999 y actualizada en 2012.
Objetivos
Evaluar los efectos del tratamiento para la deglución sobre la muerte o la dependencia entre los supervivientes de un accidente cerebrovascular con disfagia en los seis meses siguientes a su aparición.
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos del Grupo Cochrane de Accidentes Cerebrales Vasculares (Cochrane Stroke Group) (26 de junio de 2018), en el Registro Cochrane Central de Ensayos Controlados (CENTRAL); 2018, número 6) en la Cochrane Library (búsqueda del 26 de junio de 2018), MEDLINE (26 de junio de 2018), Embase (26 de junio de 2018), el Cumulative Index to Nursing and Allied Health Literature (CINAHL) (26 de junio de 2018), Web of Science Core Collection (26 de junio de 2018), SpeechBITE (28 de junio de 2016), ClinicalTrials.Gov (26 de junio de 2018), y la World Health Organization International Clinical Trials Registry Platform (26 de junio de 2018). También se realizaron búsquedas en Google Scholar (7 de junio de 2018) y en las listas de referencias de los ensayos y artículos de revisión pertinentes.
Criterios de selección
Se intentó incluir los ensayos controlados aleatorizados (ECA) de intervenciones para pacientes con disfagia y accidente cerebrovascular reciente (en el transcurso de seis meses).
Obtención y análisis de los datos
Dos autores de la revisión aplicaron de forma independiente los criterios de inclusión, extrajeron los datos, evaluaron el riesgo de sesgo, utilizaron el enfoque GRADE para evaluar la calidad de la evidencia y resolvieron los desacuerdos mediante discusión con el tercer autor de la revisión (PB). Se utilizaron modelos de efectos aleatorios para calcular los odds ratios (OR), las diferencias de medias (DM) y las diferencias de medias estandarizadas (DME), y se proporcionaron los intervalos de confianza (IC) del 95% para cada uno.
El resultado primario fue el resultado funcional, definido como la muerte o la dependencia (o la muerte o la discapacidad), al final del ensayo. Los resultados secundarios fueron la mortalidad al final del ensayo, la duración de la estancia hospitalaria, la proporción de participantes con disfagia al final del ensayo, la capacidad de deglución, la puntuación de penetración aspiración o la neumonía, el tiempo de tránsito faríngeo, la institucionalización y la nutrición.
Resultados principales
Se añadieron 27 nuevos estudios (1777 participantes) a esta actualización para incluir un total de 41 ensayos (2660 participantes).
Se evaluó la eficacia del tratamiento para la deglución en general y en subgrupos por tipo de intervención: acupuntura (11 estudios), intervenciones conductuales (nueve estudios), tratamiento farmacológico (tres estudios), estimulación eléctrica neuromuscular (EENM; seis estudios), estimulación eléctrica faríngea (EEF; cuatro estudios), estimulación física (tres estudios), estimulación transcraneal con corriente directa (ETCD; dos estudios) y estimulación magnética transcraneal (EMT; nueve estudios).
El tratamiento para la deglución no tuvo efectos sobre el resultado primario (muerte o dependencia/discapacidad al final del ensayo) según los datos de un ensayo (dos conjuntos de datos) (OR 1,05; IC del 95%: 0,63 a 1,75; 306 participantes; dos estudios; I² = 0%; p = 0,86; evidencia de calidad moderada). El tratamiento para la deglución no tuvo efectos sobre la mortalidad al final del ensayo (OR 1,00; IC del 95%: 0,66 a 1,52; 766 participantes; 14 estudios; I² = 6%; p = 0,99; evidencia de calidad moderada). La tratamiento para la deglución probablemente redujo la duración de la estancia hospitalaria (DM ‐2,9; IC del 95%: ‐5,65 a ‐0,15; 577 participantes; ocho estudios; I² = 11%; p = 0,04; evidencia de calidad moderada). Los investigadores no encontraron evidencia de un efecto de subgrupo sobre la base de la prueba de las diferencias de subgrupo (p = 0,54). El tratamiento para la deglución puede haber reducido la proporción de participantes con disfagia al final del ensayo (OR 0,42; IC del 95%: 0,32 a 0,55; 1487 participantes; 23 estudios; I² = 0%; p = 0,00001; evidencia de calidad baja). Los resultados de los ensayos no muestran evidencia de un efecto de subgrupo sobre la base de la prueba de las diferencias de subgrupo (p = 0,91). El tratamiento para la deglución puede mejorar la capacidad de deglución (DME ‐0,66; IC del 95%: ‐1,01 a ‐0,32; 1173 participantes; 26 estudios; I² = 86%; p = 0,0002; evidencia de calidad muy baja). No se encontró evidencia de un efecto de subgrupo sobre la base de la prueba de las diferencias de subgrupo (p = 0,09). Se observó una heterogeneidad de moderada a significativa entre los ensayos de estas intervenciones. El tratamiento para la deglución no redujo la puntuación de penetración aspiración (es decir, no redujo la aspiración radiológica) (DME ‐0,37; IC del 95%: ‐0,74 a ‐0,00; 303 participantes; 11 estudios; I² = 46%; p = 0,05; evidencia de calidad baja). El tratamiento para la deglución puede reducir la incidencia de infección respiratoria o neumonía (OR 0,36; IC del 95%: 0,16 a 0,78; 618 participantes; nueve estudios; I² = 59%; p = 0,009; evidencia de calidad muy baja).
Conclusiones de los autores
Evidencia de calidad moderada y baja indica que el tratamiento para la deglución no tuvo un efecto significativo sobre los resultados de muerte o dependencia/discapacidad, mortalidad al final del ensayo o puntuación de penetración aspiración. Sin embargo, el tratamiento para la deglución puede haber reducido la duración de la estancia hospitalaria, la disfagia y las infección respiratoria, y puede haber mejorado la capacidad de deglución. No obstante, estos resultados se basan en evidencia de calidad variable, que involucra una variedad de intervenciones. Se necesitan más ensayos de alta calidad para comprobar si las intervenciones específicas son eficaces.
PICO
Resumen en términos sencillos
Tratamiento para la deglución para las dificultades para tragar en los supervivientes de accidente cerebrovascular que han tenido un accidente cerebrovascular reciente
Pregunta
Se deseaba evaluar la eficacia del tratamiento para la deglución en los supervivientes de un accidente cerebrovascular con disfagia (dificultad para tragar). Se buscaron tratamientos para la deglución en los sobrevivientes hasta seis meses después del accidente cerebrovascular.
Antecedentes
El accidente cerebrovascular a menudo provoca dificultades para tragar. Esto puede provocar asfixia, infecciones respiratorias, una disminución de la calidad de vida, una estancia hospitalaria más prolongada y un mayor riesgo de muerte o de ser dado de alta a un centro asistencial. El tratamiento para mejorar la deglución tiene como objetivo acelerar la recuperación de la función de deglución y reducir estos riesgos.
Características de los estudios
Ésta es una actualización de una revisión Cochrane publicada originalmente en 1999 y actualizada anteriormente en 2012. Ahora se han incluido 41 estudios (2660 participantes), y la evidencia está vigente hasta junio de 2018. El tratamiento para la deglución comprende varios tipos de tratamiento diferentes y se examinaron ocho de ellos: acupuntura (11 estudios), intervenciones conductuales (nueve estudios), tratamiento farmacológico (tres estudios), estimulación eléctrica neuromuscular (EENM; seis estudios), estimulación eléctrica faríngea (EEF; cuatro estudios), estimulación física (tres estudios), estimulación transcraneal con corriente directa (ETCD; dos estudios) y estimulación magnética transcraneal (EMT; nueve estudios).
Resultados clave
El tratamiento para la deglución no dio lugar a menos muerte o discapacidad entre los supervivientes de un accidente cerebrovascular, ni tampoco dio lugar a una deglución más segura después del tratamiento. Sin embargo, algunos tratamiento individuales para la deglución parecieron reducir la duración de la estancia hospitalaria, disminuir las posibilidades de contraer una infección respiratoria o una neumonía, o mejorar la capacidad de deglución y la recuperación de los problemas de deglución. Muchos de los tratamientos para la deglución implicaban diferentes métodos de administración, por lo que todavía no está claro qué enfoque es el más eficaz para cada tipo de tratamiento.
Calidad de la evidencia
La calidad de la evidencia generalmente fue muy baja, baja o moderada. Se necesitan estudios adicionales de alta calidad.
Authors' conclusions
Summary of findings
| Swallowing therapy compared to placebo for dysphagia in acute and subacute stroke | ||||||
| Patient or population: dysphagia in acute and subacute stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with swallowing therapy | |||||
| Death or dependency at end of trial | Study population | OR 1.05 | 306 | ⊕⊕⊕⊝ | a | |
| 693 per 1000 | 703 per 1000 | |||||
| Case fatality at end of trial | Study population | OR 1.00 | 766 | ⊕⊕⊕⊝ | b | |
| 197 per 1000 | 197 per 1000 | |||||
| Length of inpatient stay (days) | Mean length of inpatient stay (days) ranged from 19 to 119 | MD 2.9 lower | ‐ | 577 | ⊕⊕⊕⊝ | c |
| Proportion of participants with dysphagia at end of trial | Study population | OR 0.42 | 1487 | ⊕⊕⊝⊝ | d | |
| 570 per 1000 | 357 per 1000 | |||||
| Swallowing ability | Mean swallowing ability was 0 | SMD 0.66 lower | ‐ | 1173 | ⊕⊝⊝⊝ | e |
| Penetration aspiration score | Mean penetration aspiration score was 0 | SMD 0.37 lower | ‐ | 303 | ⊕⊕⊝⊝ | f |
| Adverse event: chest infection or pneumonia | Study population | OR 0.34 | 676 | ⊕⊝⊝⊝ | g | |
| 343 per 1000 | 151 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by one level due to lack of precision (one study split into two trials). bDowngraded by one level for indirectness of the evidence (i.e. multiple different interventions). cDowngraded by one level due to indirectness of the evidence (i.e. multiple different interventions). Note also that two studies had unclear blinding. dDowngraded by two levels due to indirectness of the evidence and blinding ‐ a large number of studies did not clarify blinding status. eDowngraded by three levels due to indirectness of the evidence (i.e. multiple different interventions), considerable heterogeneity, and fair number of studies did not clarify blinding status. fDowngraded by two levels due to indirectness of the evidence (i.e. multiple different interventions) and moderate heterogeneity. gDowngraded by three levels due to indirectness of the evidence (i.e. multiple different interventions), substantial heterogeneity, and fair number of studies did not clarify blinding status. | ||||||
Background
Description of the condition
Dysphagia after stroke is common, affecting 27% to 64% of stroke survivors (Gordon 1987; Wolfe 1993; Odderson 1995; Smithard 1996; Mann 2000; Singh 2006a; Rofes 2013). Although dysphagia improves spontaneously in many people with stroke (by two weeks in about half), some will die and 15% of stroke survivors will still have swallowing problems at one month (Smithard 1993); many of these individuals require long‐term feeding with significant impairment of function, recovery, and quality of life (Barer 1989; Smithard 1997; Mann 1999; Perry 2004). Complications of dysphagia include aspiration leading to chest infection and pneumonia, malnutrition, inability to rehabilitate, increased risk of infection, prolonged length of stay in hospital, and increased risk of death (Smithard 1993; Odderson 1995; Finestone 1996; Smithard 1996; Sharma 2001; Martino 2005; Arnold 2016). Early identification and management of dysphagia have been shown to reduce pneumonia rates (Odderson 1995; Ramsey 2003; Hinchey 2005; Lakshminarayan 2010). Cohen 2016 recently reviewed this topic.
Description of the intervention
Speech and language therapists (SLTs) often administer interventions for treating dysphagia. These interventions involve behavioural approaches that may be compensatory or rehabilitative in nature. Compensatory approaches include modification of fluid and food consistencies, postural techniques such as adopting a chin tuck position, and swallow strategies such as a supraglottic swallow. Rehabilitative methods include swallowing exercises that focus on muscle strength; resistance or skill training, or both, such as tongue exercises, effortful swallow, and Mendelsohn’s manoeuvre (Mendelsohn 1987); and the Shaker exercise (Shaker 2002). Rehabilitative methods also include peripheral sensory stimulation, such as physical stimulation with tactile, thermal, or sour stimulation (Lazarra 1986; Logemann 1991; Logemann 1993; Rosenbek 1996; U1111‐1188‐0335); carbonation (Krival 2008); electrical stimulation (Power 2006); and air pulses (Theurer 2013). Researchers have also studied chemical and pharmacological agents, including capsaicin, black pepper oil, cabergoline, angiotensin‐converting enzyme (ACE) inhibitors, and nifedipine (Arai 2003; Ebihira 2004; Ebihira 2005).
Practitioners in China routinely use acupuncture techniques to treat dysphagia (Wong 2012).
Several other stimulation methods to promote recovery from dysphagia post stroke have emerged in recent years, in particular peripheral and central stimulation methods. Peripheral methods include pharyngeal electrical stimulation (PES), as reported in Scutt 2015, and neuromuscular surface electrical stimulation (NMES), as described in Chen 2016. Central stimulation methods, also known as non‐invasive brain stimulation, include transcranial magnetic stimulation (TMS) (Momosaki 2016; Pisegna 2016), as well as transcranial direct current stimulation (tDCS) (Momosaki 2016; Pisegna 2016).
How the intervention might work
The swallowing network is asymmetrically represented in both cerebral hemispheres, with one hemisphere showing dominance for swallowing (Hamdy 1998). Following unilateral stroke, TMS studies have demonstrated that recovery from dysphagia is associated with improved function of the non‐lesioned hemisphere (Hamdy 1998). The aim of most of the interventions described in this review is to accelerate this process of plasticity in acute and sub‐acute stroke patients with dysphagia. The exact process by which this is achieved is not fully understood, although it is thought that some interventions specifically aim to improve swallowing by enhancing sensory drive to the brain, causing increased activity in motor swallowing areas.
Why it is important to do this review
Dysphagia post stroke affects quality of life, carries increased risks of mortality and dependency (Smithard 1996; Arnold 2016), prolongs hospital stay (Smithard 1996; Smithard 1997; Arnold 2016), increases healthcare costs, and often leads to discharge from hospital to a care home (Smithard 1996; Arnold 2016). Despite all of this, the previous two versions of this review concluded in 1999 and 2012 that overall, current evidence for interventions was insufficient, and that no definitive treatments for dysphagia were available (Bath 1999; Geeganage 2012).
An updated version of this review is therefore needed to appraise current evidence regarding the effectiveness of interventions for dysphagia post stroke. This information will provide support for clinical practice; will inform stroke survivors, clinicians, and healthcare funders regarding which interventions are most effective; and may help guide policy and funding decisions. This review assesses the effectiveness of swallowing therapy for treatment of dysphagia in stroke survivors with acute or subacute stroke.
Objectives
To assess the effects of swallowing therapy on death or dependency among stroke survivors with dysphagia within six months of stroke onset.
Methods
Criteria for considering studies for this review
Types of studies
We identified randomised controlled trials (RCTs) of swallowing therapy for stroke survivors with acute or subacute stroke and dysphagia.
We excluded trials if they compared two or more active treatments (i.e. treatment was confounded), recruited participants after six months following stroke onset, involved a large proportion of participants with non‐stroke causes of dysphagia, or used a cross‐over design by which we could not just use data from the first treatment phase.
For this third version of the review, we removed most trials examining postural studies and all trials examining modified fluids because they lacked a true control group. We also excluded trials of free water protocols, oral hygiene, cough reflex testing, and swallow screening, as we do not consider these to be interventions for dysphagia per se. We also excluded trials involving the use of antibiotics.
Types of participants
Definitions
Acute or subacute stroke
Participants recruited with a clinical diagnosis of stroke within six months of onset.
Stroke type
Ischaemic or haemorrhagic.
Dysphagia
Diagnosed clinically (water swallow tests, modified diet or fluid assessments, swallowing test scores) by a clinician (typically a nurse or SLT), or by a videofluoroscopy swallow study (VFSS) or fibreoptic endoscopic evaluation of swallowing (FEES).
Types of interventions
-
Acupuncture versus no acupuncture or routine acupuncture or sham acupuncture
-
Behavioural interventions such as swallowing exercises, or positioning versus limited, usual, or no treatment
-
Drug intervention versus none or placebo
-
Neuromuscular electrical stimulation (NMES) versus none or sham stimulation
-
Pharyngeal electrical stimulation (PES) versus none or sham stimulation
-
Physical stimulation such as thermal or tactile versus limited, usual, or no treatment
-
Transcranial direct current stimulation (tDCS) versus none or sham stimulation
-
Transcranial magnetic stimulation (TMS) versus none or sham stimulation
We combined different interventions, collectively referred to as 'swallowing therapy', for the purpose of analysing their effects on the main outcomes. Given that the science of intervention development for dysphagia is at an early stage, it is reasonable to ask the question whether any intervention is better than no intervention, and to try to establish where the most positive effects are seen and for what topics more research is needed.
Types of outcome measures
We obtained information on the following outcome measures, as available, for each trial.
Primary outcomes
-
Functional outcome assessed as death or dependency (modified Rankin Scale: mRS > 2), or death or disability (Barthel Index: BI < 60), at the end of the trial
We chose functional outcome (i.e. death or dependency/disability) as the primary outcome because dysphagia is associated with increased risk of death or dependency in acute and subacute stroke. Whilst swallowing therapy aims to reduce dysphagia, we needed to assess whether evidences shows that people receiving swallowing therapy are less likely to die or remain dependent. We listed other important outcomes relevant to swallowing function as secondary outcomes.
Secondary outcomes
-
Case fatality at the end of the trial
-
Length of inpatient stay
-
Proportion of patients with dysphagia at the end of the trial
-
Swallowing ability based on assessments of dysphagia impairment using the dysphagia severity rating scale (DSRS), the functional oral intake scale (FOIS), the dysphagia outcome and severity scale (DOSS), or water swallowing tests
-
Penetration Aspiration score determined by VFSS and FEES and quantified on a scale such as the Penetration Aspiration Scale (PAS)
-
Chest infection or pneumonia, determined clinically or radiologically
-
Swallow timings from VFSS measurements (e.g. pharyngeal transit time (PTT))
-
Nutritional measure based on blood albumin
-
Institutionalisation with discharge to a residential, care, or nursing home, or to an extended care facility
-
Neurological impairment within four weeks (e.g. using National Institutes of Health Stroke Scale (NIHSS) or Scandinavian Stroke Scale)
-
Quality of life (e.g. using Short Form‐36 (SF‐36) or EuroQoL (measure of health‐related quality of life))
Search methods for identification of studies
See the Cochrane Stroke Group search methods. We searched for trials in all languages and arranged translation of relevant articles published in languages other than English. We have listed publications requiring translation in the Characteristics of studies awaiting classification section.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched on 26 June 2018). In addition, we searched:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (Appendix 1) in the Cochrane Library (searched 26 June 2018);
-
MEDLINE Ovid (1946 to 26 June 2018) (Appendix 2);
-
Embase (1974 to 26 June 2018) (Appendix 3);
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO) (1982 to 26 June 2018) (Appendix 4);
-
Science Citation Index Expanded, Social Sciences Citation Index, Conference Proceedings Citation Index‐ Science (Web of Science Core Collection; 1900 to 26 June 2018) (Appendix 5); and
-
SpeechBITE (searched 28 June 2018) (Appendix 6).
In an effort to identify further published, unpublished, and ongoing trials, we searched:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 26 June 2018; Appendix 7);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 26 June 2018; Appendix 8); and
-
Google Scholar (searched 7 June 2018; Appendix 9).
Searching other resources
Additionally, we searched the reference lists of relevant trials and review articles and our own reference lists.
For a previous version of this review (Geeganage 2012), we contacted researchers and the UK Royal College of Speech and Language Therapists Special Interest Group for information on adult‐acquired dysphagia trials.
Data collection and analysis
Selection of studies
For this update, two review authors (HSL, LE) scanned the titles and abstracts of records identified through searches of electronic bibliographic databases and excluded obviously irrelevant articles. We independently reviewed the full text of remaining studies and selected relevant trials according to the listed inclusion criteria; we resolved disagreements through discussion with the third review author (PB).
Data extraction and management
For this update, two review authors (HSL, LE) extracted data using a predefined proforma, and entered the data into RevMan 5 (RevMan 2014); we resolved disagreements through discussion and consultation with the third review author (PB). We assessed information on randomisation, blinding, numbers of participants randomised, timing of treatment from stroke, types of dysphagia therapy, participant withdrawals and losses to follow‐up, and relevant outcomes (Types of outcome measures). We aggregated outcome data from dose escalation or dose comparison trials into one active treatment group.
Assessment of risk of bias in included studies
We assessed potential for bias using the 'Risk of bias' tool as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This assessment includes sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other issues.
Measures of treatment effect
We assessed weighted estimate of the typical treatment effect across trials using odds ratios (ORs) and 95% confidence intervals (CIs) for binary data, mean differences (MDs) and 95% CIs for continuous data, and standardised mean differences (SMDs) and 95% CIs for continuous data based on different scales. We performed analyses using RevMan 5 (RevMan 2014). We calculated OR using the Mantel‐Haenszel method, and MDs using the inverse variance method.
Unit of analysis issues
When outcome measures included different scores, we converted these to grades in the same direction of mild to severe and analysed them using MDs. When studies compared graduations of therapy (high‐medium‐low intensity), we divided the middle‐intensity group in two and analysed study data by comparing high intensity versus medium intensity, and medium intensity versus low intensity or no treatment. Similarly, if a trial compared high‐ versus low‐frequency stimulation or unilateral versus bilateral stimulation, we divided control group participants equally between treatment groups to prevent control participants from being counted more than once, and thereby artificially narrowing the CIs. We entered each set of data as a separate trial.
Dealing with missing data
If a trial publication did not provide relevant data or if data were missing but we felt it appropriate otherwise, we placed studies into Characteristics of studies awaiting classification.
Assessment of heterogeneity
We used the random‐effects model to assess heterogeneity by looking at forest plots to see how CIs overlapped (non‐overlapping studies are exhibiting statistical heterogeneity) along with the I² statistic (Higgins 2011). We defined thresholds for interpreting heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions, whereby 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We assessed selective outcome reporting as reported in the 'Risk of bias' table (Characteristics of included studies).
Data synthesis
We performed meta‐analysis using functionality within RevMan 5 (RevMan 2014): we used random‐effects models (Mantel‐Haenszel method) and presented data as number (%) or mean (standard deviation), with OR, MD, or SMD. We used random‐effects models because we expected that trials would be heterogeneous in design and delivery, including different types of participants and interventions.
Grade and 'Summary of findings' table
We assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for the following main outcomes of analysis.
-
Death or dependency/disability at the end of the trial.
-
Case fatality at the end of the trial.
-
Length of inpatient stay.
-
Proportion of participants with dysphagia at the end of the trial.
-
Swallowing ability.
-
Penetration aspiration score.
-
Adverse event: chest infection or pneumonia.
We have presented in summary of findings Table for the main comparison key findings of the review, including a summary of the quantity of data, the magnitude of effect size, and the overall quality of evidence.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on the eight different types of swallowing therapy to provide more specific information pertaining to the different interventions. We assessed for significant subgroup interactions by testing for subgroup differences for each main outcome.
Sensitivity analysis
We did not perform sensitivity analyses due to the small number of studies.
Results
Description of studies
We identified 27 new RCTs involving a total of 1777 acute or subacute stroke survivors with dysphagia.
Results of the search
We have presented the PRISMA study flow diagram in Figure 1. In total, we identified 2902 references, removed 860 duplicates, and screened 2042 records. We excluded 1874 records, leaving a total of 168 records. After full‐text review, we excluded 41 studies. We added these newly excluded studies to the existing list of 39 excluded studies, for a total of 80 (Excluded studies). We added 22 studies into the ongoing studies section (Ongoing studies). We also added 78 new studies to the eight existing studies awaiting classification, yielding a total of 86 (Studies awaiting classification); these studies have been completed and are awaiting publication or are awaiting translation, or we are seeking full‐text articles. External assessment of this review led to a request to further update the searches; an updated search revealed further potentially relevant studies, and we have added these to the Studies awaiting classification section; we will assess these when we prepare the next update of this review. Finally, we added 27 new studies to the existing 14 studies, yielding a total of 41 included studies (47 data sets) (Included studies). This resulted in the addition of 1777 participants to the existing 883, for a total of 2660 participants.
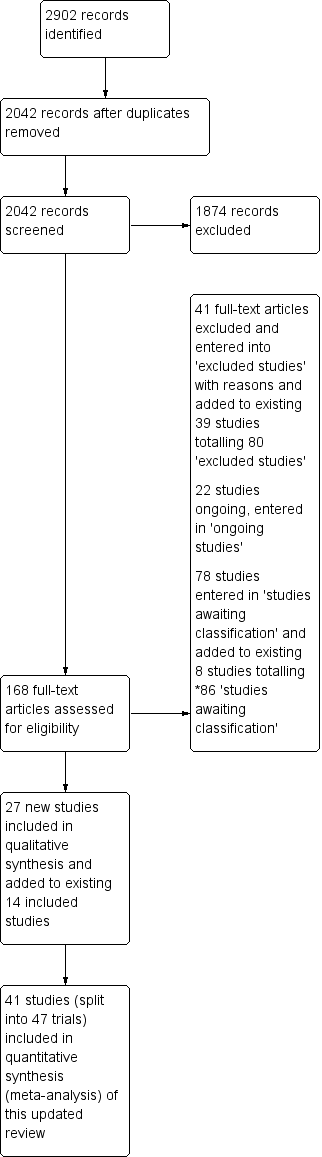
Study Flow Diagram, *86 studies awaiting classification.
Included studies
We included 41 trials in this updated review (mean participant age 67.8 years). These trials looked at various forms of swallowing therapy after stroke.
When outcome measures included different scores, we converted these to grades in the same direction of mild to severe and analysed them using mean differences (MDs). Two studies compared graduations of therapy (high‐medium‐low intensity) (Yuan 2003i; Yuan 2003ii; Carnaby 2006i; Carnaby 2006ii;); here, we divided the middle‐intensity group in two and analysed the study data by comparing high intensity versus medium intensity, and medium intensity versus low intensity or no treatment. Similarly, one trial of TMS compared high‐ versus low‐frequency stimulation or unilateral versus bilateral stimulation (Kim 2012i; Kim 2012ii; Du 2016i; Du 2016ii; Park 2016 (a) i; Park 2016 (a) ii); here, we divided control group participants equally between treatment groups to prevent control participants from being counted more than once and thereby artificially narrowing the confidence intervals (CIs). We entered each set of data as a separate trial; hence, although the total number of included studies was 41, the total number of data sets entered for analysis was 47.
Acupuncture
Eleven studies tested acupuncture in 998 participants (Liu 2000; Han 2004; Liu 2004; Wei 2005; Jia 2006a; Bai 2007i; Bai 2007ii; Huang 2010; Chan 2012; Chen 2016a; Xia 2016a).
Behavioural interventions
Nine studies investigated behavioural interventions in 632 participants (Yuan 2003i; Yuan 2003ii; Song 2004; Carnaby 2006i; Carnaby 2006ii; Kang 2012; Zheng 2014; Heo 2015; Park 2016b). Behavioural interventions consisted of swallowing exercises, environmental modifications such as upright positioning for feeding, safe swallowing advice, dietary modifications, kinesio‐taping, and expiratory muscle strength training.
Drug therapy
Three studies assessed several different drugs in 148 participants (Perez 1997; Lee 2015; Warusevitane 2015). Drug interventions included nifedipine in 17 participants (Perez 1997), lisinopril in 71 participants (Lee 2015), and metoclopramide in 60 participants (Warusevitane 2015).
Neuromuscular electrical stimulation (NMES)
Six studies tested NMES in 312 participants (Lim 2009; Xia 2011; Park 2012; Lee 2014; Li 2014; Terre 2015). Researchers most often compared NMES versus traditional dysphagia therapy. One study combined NMES and effortful swallow (Park 2012).
Pharyngeal electrical stimulation (PES)
Four studies involving 214 participants assessed PES (Jayasekeran 2010a; Jayasekeran 2010b; STEPS 2016; Vasant 2016).
Physical stimulation (thermal, tactile)
Three studies enrolled 155 participants. Types of stimulation included tactile stimulation (Bath 1997), electrical stimulation (Power 2006), and Tongyan spray (Feng 2012).
Transcranial direct current stimulation (tDCS)
Two studies assessed tDCS in 34 participants (Kumar 2011; Shigematsu 2013).
Transcranial magnetic stimulation (TMS)
Nine studies involving 167 participants investigated TMS (Khedr 2009; Khedr 2010; Kim 2012i; Kim 2012ii; Park 2013; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii).
Excluded studies
We excluded 80 studies from this updated review, most commonly because investigators compared two active treatments (confounded) or because the trials were not RCTs. We excluded 10 studies as reported outcomes were not relevant to this review. We excluded 11 studies because of lack of outcome data; some of these might be relevant to this review should outcome data become available (Characteristics of excluded studies).
Risk of bias in included studies
Key sources of bias follow; we have summarised risk of bias in Figure 2.
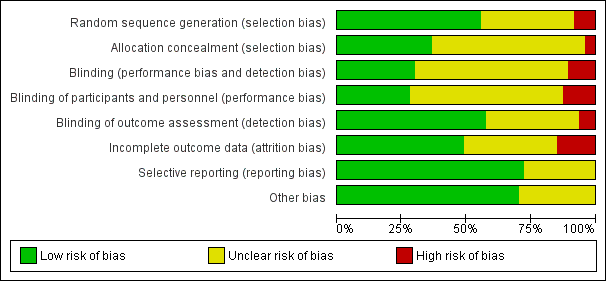
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Random sequence generation
-
Randomisation by computer occurred in 15 studies (low risk of bias) (Bath 1997; Perez 1997; Carnaby 2006i; Carnaby 2006ii; Jayasekeran 2010a; Jayasekeran 2010b; Park 2012; Park 2013; Lee 2014; Li 2014; Lee 2015; Terre 2015; Chen 2016a; STEPS 2016; Vasant 2016).
-
Randomisation via random number tables occurred in 10 studies (low risk of bias) (Song 2004; Bai 2007i; Bai 2007ii; Chan 2012; Feng 2012; Shigematsu 2013; Warusevitane 2015; Du 2016i; Du 2016ii; Xia 2016a).
-
Simple randomisation occurred in four studies (low risk of bias) (Han 2004; Kumar 2011; Heo 2015; Park 2016b).
-
Method of randomisation was unclear in 16 studies (unclear risk of bias) (Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Wei 2005; Power 2006; Khedr 2009; Huang 2010; Khedr 2010; Xia 2011; Kang 2012; Kim 2012i; Kim 2012ii; Zheng 2014; Park 2016a (i); Park 2016a (ii)).
-
Two studies used non‐randomised methods (high risk of bias) (Jia 2006a; Lim 2009).
Allocation concealment
-
Researchers ensured allocation concealment in 17 studies (low risk of bias) (Han 2004; Carnaby 2006i; Carnaby 2006ii; Khedr 2009; Chan 2012; Feng 2012; Park 2012; Park 2013; Shigematsu 2013; Li 2014; Lee 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016b; Vasant 2016).
-
Allocation concealment was unclear in 28 studies (unclear risk of bias) (Bath 1997; Perez 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Song 2004; Wei 2005; Power 2006; Bai 2007i; Bai 2007ii; Huang 2010; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Kumar 2011; Xia 2011; Kang 2012; Kim 2012i; Kim 2012ii; Lee 2014; Zheng 2014; Heo 2015; Terre 2015; Park 2016a (i); Park 2016a (ii); STEPS 2016; Xia 2016a).
-
Two studies did not ensure allocation concealment (high risk of bias) (Jia 2006a; Lim 2009).
Baseline prognostic factors matching between intervention and control groups
-
Baseline factors were similar in 34 studies (low risk of bias) (Perez 1997; Song 2004; Carnaby 2006i; Carnaby 2006ii; Bai 2007i; Bai 2007ii; Khedr 2009; Jayasekeran 2010b; Khedr 2010; Xia 2011; Chan 2012; Feng 2012; Kang 2012; Kim 2012i; Kim 2012ii; Park 2012; Park 2013; Shigematsu 2013; Lee 2014; Li 2014; Zheng 2014; Heo 2015; Lee 2015; Terre 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Park 2016b; STEPS 2016; Vasant 2016; Xia 2016a).
-
Baseline factor matching was unclear in 13 studies (unclear risk of bias) (Bath 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Han 2004; Liu 2004; Wei 2005; Jia 2006a; Power 2006; Lim 2009; Huang 2010; Jayasekeran 2010a; Kumar 2011).
Blinding
Performance bias
-
Both participants and investigators were blinded in three studies (low risk of bias) (Perez 1997; Kumar 2011; Warusevitane 2015).
-
Participants were blinded in nine studies (low risk of bias) (Khedr 2009; Chan 2012; Park 2012; Park 2013; Terre 2015; Du 2016i; Du 2016ii; STEPS 2016; Vasant 2016).
-
Both participants and investigators were unblinded in five studies (high risk of bias) (Carnaby 2006i; Carnaby 2006ii; Chen 2016a; Park 2016a (i); Park 2016a (ii)).
-
Blinding of participants and investigators was uncertain in 14 studies (unclear risk of bias) (Bath 1997; Han 2004; Bai 2007i; Bai 2007ii; Lim 2009; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Xia 2011; Shigematsu 2013; Li 2014; Lee 2015; Park 2016b; Xia 2016a).
Detection bias
-
Outcomes were blinded in 28 studies (low risk of bias) (Perez 1997; Han 2004; Wei 2005; Carnaby 2006i; Carnaby 2006ii; Khedr 2009; Lim 2009; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Xia 2011; Chan 2012; Park 2012; Park 2013; Shigematsu 2013; Li 2014; Lee 2015; Terre 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Park 2016b; STEPS 2016; Vasant 2016; Xia 2016a).
-
Outcomes were not blinded in three studies (high risk of bias) (Bath 1997; Bai 2007i; Bai 2007ii).
Overall, 16 studies did not report on any blinding procedures (i.e. for participants, investigators, or outcome assessors) (unclear risk of bias) (Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Song 2004; Wei 2005; Jia 2006a; Power 2006; Huang 2010; Feng 2012; Kang 2012; Kim 2012i; Kim 2012ii; Lee 2014; Zheng 2014; Heo 2015).
Incomplete outcome data
-
Ten studies reported no loss of participants during follow‐up (low risk of bias) (Han 2004; Jayasekeran 2010a; Chan 2012; Kang 2012; Kim 2012i; Kim 2012ii; Park 2013; Shigematsu 2013; Lee 2014; Warusevitane 2015).
-
Twelve studies reported loss of participants during follow‐up, but we judged them to be at low risk of bias (Perez 1997; Carnaby 2006i; Carnaby 2006ii; Khedr 2009; Khedr 2010; Feng 2012; Park 2012; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Vasant 2016).
-
We judged seven studies to be at high risk of bias due to incomplete outcome data (Lim 2009; Jayasekeran 2010b; Li 2014; Lee 2015; Chen 2016a; Park 2016b; STEPS 2016).
-
Loss of participants during follow‐up was unclear in 18 studies (unclear risk of bias) (Bath 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Song 2004; Wei 2005; Jia 2006a; Power 2006; Bai 2007i; Bai 2007ii; Huang 2010; Kumar 2011; Xia 2011; Zheng 2014; Heo 2015; Terre 2015; Xia 2016a).
-
Data were not available for quality of life.
Selective reporting
-
We judged 34 studies to be at low risk of reporting bias (Perez 1997; Carnaby 2006i; Carnaby 2006ii; Power 2006; Khedr 2009; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Kumar 2011; Xia 2011; Chan 2012; Feng 2012; Kang 2012; Kim 2012i; Kim 2012ii; Park 2012; Park 2013; Shigematsu 2013; Lee 2014; Li 2014; Zheng 2014; Heo 2015; Lee 2015; Terre 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Park 2016b; STEPS 2016; Vasant 2016; Xia 2016a).
-
In the remaining 13 studies, it was unclear if reported data were complete (unclear risk of bias) (Bath 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Han 2004; Liu 2004; Song 2004; Wei 2005; Jia 2006a; Bai 2007i; Bai 2007ii; Lim 2009; Huang 2010).
Other potential sources of bias
We assessed seven studies based on translations of the original text (Yuan 2003i; Yuan 2003ii; Song 2004; Wei 2005; Bai 2007i; Bai 2007ii; Huang 2010). Native Chinese speakers performed translations from Chinese to English.
We aggregated outcome data from dose escalation or comparison trials to form one active treatment group in one trial (Jayasekeran 2010b).
Effects of interventions
Summary of findings for main outcomes of swallowing therapy in general
We entered the important outcomes in this review into summary of findings Table for the main comparison, and we reported outcomes for 'swallowing therapy' versus 'no swallowing therapy'. This means that overall, for each outcome (e.g. length of inpatient stay), we combined several different interventions to test for efficacy. In this way, we have provided information on the effectiveness of swallowing therapy as a whole for each outcome. We assessed three additional outcomes (pharyngeal transit time, institutionalisation, and nutrition) but did not include them in summary of findings Table for the main comparison (a maximum of seven outcomes are allowed); therefore, we did not assess the quality of studies for these outcomes using the GRADE approach, and we have not reported their outcomes in the main findings.
We also undertook subgroup analysis for each different type of intervention.
The number of outcomes reported varied considerably across studies.
-
Primary outcome of death or dependency/disability at end of trial in one trial (split into two data sets).
-
Case fatality at end of trial in 14 trials.
-
Length of inpatient stay in eight trials.
-
Proportion of patients with dysphagia at end of trial in 23 trials.
-
Swallowing ability in 26 trials.
-
Penetration aspiration score (PAS) in 11 trials.
-
Chest infections or pneumonia in nine trials.
-
Swallow timing in six trials.
-
Nutrition in three trials.
-
Institutionalisation in three trials.
Primary outcome
Functional outcome: death or dependency or death or disability at end of trial
Swallowing therapy had no effect on death or dependency, or death or disability, at end of trial (odds ratio (OR) 1.05, 95% confidence interval (CI) 0.63 to 1.75; 306 participants; 2 studies; I² = 0%; P = 0.86: moderate‐quality evidence; Analysis 1.1). One trial (two data sets) of behavioural interventions reported on this outcome.
Secondary outcomes
Case fatality at end of trial
Swallowing therapy had no effect on case fatality at end of trial (OR 1.00, 95% CI 0.66 to 1.52; 766 participants; 14 studies; I² = 6%; P = 0.99: moderate‐quality evidence; Analysis 1.2). Trials of behavioural interventions, drug therapy, pharyngeal electrical stimulation, physical stimulation, and transcranial magnetic stimulation reported on this outcome.
Length of inpatient stay
Swallowing therapy probably reduced length of inpatient stay (mean difference (MD) ‐2.90, 95% CI ‐5.65 to ‐0.15; 577 participants; 8 studies; I² = 11%; P = 0.04: moderate‐quality evidence; Analysis 1.3). Trials of behavioural interventions and PES reported on this outcome. Subgroup analysis showed that the interventions did not differ (Analysis 1.3).
Proportion of participants with dysphagia at end of trial
Swallowing therapy probably reduced the proportion of participants with dysphagia at end of trial (OR 0.42, 95% CI 0.32 to 0.55; 1487 participants; 23 studies; I² = 0%; P = 0.00001: low‐quality evidence; Analysis 1.4). Trials of acupuncture, behavioural interventions, drug therapy, NMES, PES, physical stimulation, and tDCS reported on this outcome. Subgroup analysis showed that acupuncture (OR 0.31, 95% CI 0.20 to 0.49; 676 participants; 8 studies; I² = 0%; P < 0.00001) and behavioural interventions (OR 0.45, 95% CI 0.28 to 0.74; 511 participants; 6 studies; I² = 28%; P = 0.001) each reduced dysphagia but did not differ from each other (P = 0.91; Analysis 1.4).
Swallowing ability
Swallowing therapy probably improved swallowing ability (standardised mean difference (SMD) ‐0.66, 95% CI ‐1.01 to ‐0.32; 1173 participants; 26 studies; I² = 86%; P = 0.0002: very low‐quality evidence; Analysis 1.5). Trials of acupuncture, behavioural interventions, drug therapy, NMES, PES, physical stimulation, tCDS, and TMS reported on this outcome. Subgroup analysis showed that behavioural interventions (SMD ‐0.56, 95% CI ‐1.07 to ‐0.05; 121 participants; 3 studies; I² = 47%; P = 0.03) and TMS (SMD ‐1.29, 95% CI ‐2.37 to ‐0.21; 141 participants; 8 studies; I² = 85%; P = 0.02) each improved swallowing ability but did not differ from each other (P = 0.09; Analysis 1.5). Review authors noted moderate to substantial heterogeneity between trials (Analysis 1.5).
Penetration aspiration score
Swallowing therapy did not significantly reduce aspiration assessed as penetration aspiration score (SMD ‐0.37, 95% CI ‐0.74 to ‐0.00; 303 participants; 11 studies; I² = 46%; P = 0.05: low‐quality evidence; Analysis 1.6). Trials of behavioural interventions, NMES, PES, and TMS reported on this outcome. However, given that results show no overall benefit, we have not commented on subgroup analysis (Analysis 1.6).
Chest infection or pneumonia
Swallowing therapy probably reduced the incidence of chest infection or pneumonia (OR 0.36, 95% CI 0.16 to 0.78; 618 participants; 9 studies; I² = 59%; P = 0.009: very low‐quality evidence; Analysis 1.7). Trials of behavioural interventions, drug therapy, NMES, and PES reported on this outcome. Subgroup analysis showed that drug therapy (OR 0.06, 95% CI 0.01 to 0.21; 60 participants; 1 study; I² not applicable; P < 0.0001) significantly reduced the incidence of chest infection or pneumonia at end of trial ‐ a result that differed significantly from other interventions (P = 0.008; Analysis 1.7).
Pharyngeal transit time (PTT)
Swallowing therapy may have reduced PTT (MD ‐0.23, 95% CI ‐0.32 to ‐0.15; 187 participants; 6 studies; I² = 29%; P < 0.00001; Analysis 1.8). Trials of drug therapy, NMES, PES, and physical stimulation reported on this outcome. Subgroup analysis showed that NMES (MD ‐0.23, 95% CI ‐0.39 to ‐0.08; 126 participants; 3 studies; I² = 63%; P = 0.003; Analysis 1.8) and physical stimulation in one small study (MD ‐0.19; 95% CI ‐0.34 to ‐0.04; 16 participants; 1 study; I² not applicable; P = 0.01) each reduced PTT but did not differ from each other, i.e. these findings are likely due to chance and not‐significant. (P = 0.98; Analysis 1.8).
Institutionalisation
Swallowing therapy did not reduce the incidence of institutionalisation (OR 0.75, 95% CI 0.47 to 1.19; 447 participants; 3 studies; I² = 0%; P= 0.22; Analysis 1.9). Trials of behavioural interventions and pharyngeal electrical stimulation reported on this outcome.
Nutrition (albumin)
Swallowing therapy did not reduce nutrition (MD 0.37, 95% CI ‐1.5 to 2.24; 169 participants; 3 studies; I² = 0%; P = 0.70; Analysis 1.10). Trials of behavioural interventions and pharyngeal electrical stimulation reported on this outcome.
Detailed subgroup analysis: summary of findings per type of intervention
Not all interventions addressed all outcomes. We have reported available data.
Acupuncture
Acupuncture resulted in significant results (i.e. < 1.0) for reducing the proportion of participants with dysphagia at end of trial. However, these findings may be due to chance, given that testing for subgroup differences did not yield significant results. Acupuncture did not reduce swallowing ability. Data on the effects of acupuncture on other outcomes were not available.
-
Proportion of participants with dysphagia at end of trial (OR 0.31, 95% CI 0.20 to 0.49; 676 participants; 8 studies; I² = 0%; P < 0.00001; Analysis 1.4).
-
Swallowing ability (SMD ‐0.55, 95% CI ‐1.20 to 0.11; 496 participants; 6 studies; I² = 91%; P = 0.10). We noted significant heterogeneity (Analysis 1.5).
Behavioural interventions
Behavioural interventions produced significant results (i.e. < 1.0) for improving swallowing ability and reducing the proportion of participants with dysphagia at the end of the trial. However, both of these findings may be due to chance, given that testing for subgroup differences for each outcome did not yield significant results. Although behavioural interventions also reduced penetration aspiration score (i.e. < 1.0), results show no overall benefit for this outcome and this finding is likely due to chance. Behavioural interventions did not reduce length of inpatient stay, chest infection or pneumonia, case fatality at end of trial, functional outcome, institutionalisation, or nutrition. Behavioural interventions addressed more outcomes when compared with most interventions.
-
Swallowing ability (SMD ‐0.56, 95% CI ‐1.07 to ‐0.05; 121 participants; 3 studies; I² = 47%; P = 0.03; Analysis 1.5).
-
Proportion of participants with dysphagia at end of trial (OR 0.45, 95% CI 0.28 to 0.74; 511 participants; 6 studies; I² = 28%; P = 0.001; Analysis 1.4).
-
Penetration aspiration score (SMD ‐0.88, 95% CI ‐1.68 to ‐0.08; 27 participants; 1 study; I² not applicable; P = 0.03; Analysis 1.6).
-
Length of inpatient stay (MD ‐2.70, 95% CI ‐5.68 to 0.28; 370 participants; 4 studies; I² = 19%; P = 0.08; Analysis 1.3).
-
Chest infection or pneumonia (OR 0.56, 95% CI 0.31 to 1.00; 473 participants; 6 studies; I² = 21%; P = 0.05; Analysis 1.7).
-
Case fatality at end of trial (OR 0.83, 95% CI 0.46 to 1.51; 306 participants; 2 studies; I² = 0%; P = 0.54; Analysis 1.2).
-
Functional outcome (OR 1.05, 95% CI 0.63 to 1.75; 306 participants; 2 studies; I² = 0%; P = 0.86; Analysis 1.1).
-
Institutionalisation (OR 0.76, 95% CI 0.39 to 1.48; 306 participants; 2 studies; I² = 12%; P = 0.42; Analysis 1.9).
-
Nutrition (albumin) (MD 0.20, 95% CI ‐4.77 to 5.17; 64 participants; 2 studies; I² = 0%; P = 0.94; Analysis 1.10).
Drug therapy
Drug therapy was probably effective for reducing chest infection or pneumonia in one study ‐ a result that differed from those of other interventions. Drug therapy did not improve swallowing ability, nor did it reduce case fatality, proportion of participants with dysphagia at end of trial, or pharyngeal transit time. Data on effects of drug therapy on other outcomes were not available.
-
Chest infection or pneumonia (OR 0.06, 95% CI 0.01 to 0.21; 60 participants; 1 study; I² not applicable; P < 0.0001; Analysis 1.7).
-
Swallowing ability (SMD ‐0.46, 95% CI ‐0.93 to 0.01; 71 participants; 1 study; I² not applicable; P = 0.06; Analysis 1.5).
-
Case fatality (OR 1.40, 95% CI 0.31 to 6.28; 148 participants; 3 studies; I² = 70%; P = 0.66; Analysis 1.2).
-
Proportion of participants with dysphagia at end of trial (OR 0.48, 95% CI 0.07 to 3.35; 17 participants; 1 study; I² not applicable; P = 0.46; Analysis 1.4).
-
Pharyngeal transit time (MD ‐0.21, 95% CI ‐0.91 to 0.49; 17 participants; 1 study; I² not applicable; P = 0.56; Analysis 1.8).
Neuromuscular electrical stimulation (NMES)
NMES was probably effective for reducing pharyngeal transit time (i.e. < 1.0). NMES did not reduce the proportion of participants with dysphagia at end of trial or penetration aspiration score, and did not improve swallowing ability.
-
Pharyngeal transit time (MD ‐0.23, 95% CI ‐0.39 to ‐0.08; 126 participants; 3 studies; I² = 63%; P = 0.003; Analysis 1.8).
-
Proportion of participants with dysphagia at end of trial (OR 0.51, 95% CI 0.18 to 1.49; 76 participants; 2 studies; I² = 7%; P = 0.22; Analysis 1.4).
-
Penetration aspiration score (SMD 0.57, 95% CI ‐0.38 to 1.52; 18 participants; 1 study; I² not applicable; P = 0.24; Analysis 1.6).
-
Swallowing ability (SMD ‐1.34, 95% CI ‐3.39 to 0.71; 100 participants; 2 studies; I² = 93%; P = 0.20; Analysis 1.5).
Pharyngeal electrical stimulation (PES)
PES studies addressed many outcomes but did not show an effect for case fatality, length of inpatient stay, proportion of participants with dysphagia at end of trial, swallowing ability, penetration aspiration score, chest infection or pneumonia, pharyngeal transit time, institutionalisation, or nutrition.
-
Case fatality (OR 0.92, 95% CI 0.38 to 2.26; 215 participants; 4 studies; I² = 0%; P = 0.86; Analysis 1.2).
-
Length of inpatient stay (MD ‐6.05, 95% CI ‐16.40 to 4.31; 207 participants; 4 studies; I² = 27%; P = 0.25; Analysis 1.3).
-
Proportion of participants with dysphagia at end of trial (OR 0.55, 95% CI 0.15 to 2.11; 66 participants; 3 studies; I² = 0%; P = 0.39; Analysis 1.4).
-
Swallowing ability (SMD 0.06, 95% CI ‐0.22 to 0.34; 194 participants; 3 studies; I² = 0%; P = 0.69; Analysis 1.5).
-
Penetration aspiration score (SMD ‐0.17, 95% CI ‐0.53 to 0.19; 177 participants; 4 studies; I² = 12%; P = 0.35; Analysis 1.6).
-
Chest infection (OR 0.43, 95% CI 0.06 to 3.09; 28 participants; 1 study; I² not applicable; P = 0.40; Analysis 1.7).
-
Pharyngeal transit time (MD ‐0.15, 95% CI ‐0.67 to 0.37; 28 participants; 1 study; I² not applicable; P = 0.56; Analysis 1.8).
-
Institutionalisation (OR 0.73, 95% CI 0.36 to 1.48; 141 participants; 1 study; I² not applicable; P = 0.38; Analysis 1.9).
-
Nutrition (MD 0.40; 95% CI‐1.62 to 2.42; 105 participants; 1 study; I² not applicable; P = 0.70; Analysis 1.10).
Physical stimulation (thermal, tactile)
Physical stimulation reduced pharyngeal transit time in one small study (i.e. < 1.0). However, these findings may be due to chance, given that testing for subgroup differences did not yield significant findings.
Physical stimulation had no effect on case fatality at end of trial nor on proportion of participants with dysphagia at end of trial and did not improve swallowing ability.
-
Pharyngeal transit time (MD ‐0.19, 95% CI ‐0.34 to ‐0.04; 16 participants; 1 study; I² not applicable; P = 0.01; Analysis 1.8).
-
Case fatality at end of trial (OR 1.05, 95% CI 0.16 to 6.92; 19 participants; 1 study; I² not applicable; P = 0.96; Analysis 1.2).
-
Proportion of participants with dysphagia at end of trial (OR 0.65, 95% CI 0.07 to 5.85; 127 participants; 2 studies; I² = 0%; P = 0.70; Analysis 1.4).
-
Swallowing ability (SMD ‐0.30, 95% CI ‐1.29 to 0.68; 16 participants; 1 study; I² not applicable; P = 0.55; Analysis 1.5).
Transcranial direct current stimulation (tDCS)
tDCS did not alter the proportion of participants with dysphagia at end of trial and did not improve swallowing ability. Data on other outcomes were not available.
-
Proportion of participants with dysphagia at end of trial (OR 0.29, 95% CI 0.01 to 8.39; 14 participants; 1 study; I² not applicable; P = 0.47; Analysis 1.4).
-
Swallowing ability (SMD ‐0.33, 95% CI ‐2.22 to 1.56; 34 participants; 2 studies; I² = 85%; P = 0.73; Analysis 1.5).
Transcranial magnetic stimulation (TMS)
TMS improved swallowing ability at end of trial (i.e. < 1.0), although this finding may be due to chance, given that testing for subgroup differences did not yield significant results. We also noted considerable heterogeneity. TMS did not alter case fatality at end of trial nor penetration aspiration score. Data on other outcomes were not available.
-
Swallowing ability (SMD ‐1.29, 95% CI ‐2.37 to ‐0.21; 141 participants; 8 studies = 8; I² = 85%; P = 0.02; Analysis 1.5).
-
Case fatality at end of trial (OR 0.28, 95% CI 0.03 to 2.93; 78 participants; 4 studies; I² = 0%; P = 0.29; Analysis 1.2).
-
Penetration aspiration score (SMD ‐0.53, 95% CI ‐1.22 to 0.16; 81 participants; 5 studies; I² = 51%; P = 0.13; Analysis 1.6).
In summary, acupuncture, behavioural interventions, and TMS appeared to be individually effective for reducing some outcomes. However, as results of testing for subgroup differences were not significant, none of these interventions are convincingly different from the summary result. Drug therapy was the only intervention that was significantly less than 1.0, and findings were significantly different for testing of subgroup differences, although this result was based on very low‐quality evidence.
Discussion
Summary of main results
We included 41 studies in this updated review of swallowing therapy in people with stroke. We identified 22 additional studies that are ongoing (Characteristics of ongoing studies), along with 86 studies that are awaiting classification (Characteristics of studies awaiting classification).
Researchers assessed eight types of stimulatory techniques ‐ acupuncture, behavioural therapy, drug therapy, neuromuscular electrical stimulation (NMES), pharyngeal electrical stimulation (PES), physical stimulation, transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation (TMS). Swallowing therapy had no effect on functional outcomes (death or dependency, or death or disability), although only one trial reported this outcome (two data sets). Swallowing therapy also had no effect on case fatality at end of trial, nor on penetration aspiration score. However, swallowing therapy probably reduced length of inpatient stay, the proportion of participants with dysphagia at end of trial, and the incidence of chest infection or pneumonia (with one study reporting significant effects for drug therapy). Swallowing therapy also probably improved swallowing ability. In the absence of significant effects on the primary outcome, statistically significant findings in secondary and explanatory outcomes are hypothesis‐generating and might reflect chance, for example, due to multiple‐comparison testing. Hence, further trials are needed to test these observations.
Overall completeness and applicability of evidence
Results of this review are incomplete at this time because of the significant number of ongoing studies and those awaiting classification identified by review authors. Nevertheless, the addition of new studies to this version of the review has tightened confidence intervals, although the overall conclusion that dysphagia treatment does not alter functional outcome has not changed.
Quality of the evidence
The quality of evidence ranged from very low and low through moderate to high, as presented in summary of findings Table for the main comparison. The most common reasons for reduced quality of evidence were lack of blinding, moderate to considerable heterogeneity between trials, and lack of precision (i.e. inclusion of multiple different interventions).
Potential biases in the review process
Results of the present analysis are subject to several caveats. First, we combined different interventions together for analysis, to assess whether trial results show any effect of swallowing therapy as a whole as opposed to no intervention or usual care. This means that decisions on which specific types of interventions are effective cannot be made upon analysis of these data. Future reviews will focus on assessing effects of specific interventions on main outcomes. Second, we excluded 80 studies from the analysis. One common reason for exclusion is that studies compared two active treatments without including a control or placebo group. We also excluded trials due to lack of uniformity in usage of outcome measures and lack of data on clinical outcomes, such as dependency, mortality, institutionalisation, and chest infection or pneumonia. Further, included trials used various swallowing assessment techniques, cortical excitability techniques, and videofluoroscopic measurements. So, trialists are encouraged to design future trials that include a control or placebo group, and to incorporate standard outcome measures. Third, a further 86 studies are awaiting assessment, subject to the availability of full‐text articles; such omission of multiple studies will inevitably bias review results. Fourth, with regard to acupuncture, data from three studies may have been confounded due to use of 'routine' acupuncture or a different type of acupuncture as control, variation in delivery of therapy, and risk of language bias, in that some of the acupuncture literature is available in full only in Chinese language journals. Similarly, we included data from an NMES study (Park 2012), which considered sensory stimulation as a control; therefore we cannot be certain that this trial is not confounded. Last, the present analysis included only studies up to six months from stroke onset, and the effects of later treatments for post‐stroke dysphagia remain unclear.
It is important to note that many trials are ongoing and should add substantially to the existing data once complete.
Agreements and disagreements with other studies or reviews
This is the largest, most inclusive, and most up‐to‐date review on this topic. It combines all current interventions for dysphagia in the acute and subacute phases of stroke. A number of separate systematic reviews exploring individual interventions for stroke survivors have been published, including some examining acupuncture in stroke (Xie 2008; Long 2012; Wong 2012), behavioural interventions in neurogenic dysphagia (Ashford 2009), TMS in stroke and acquired brain injury (Yang 2015; Liao 2016; Momosaki 2016; Pisegna 2016), tDCS in stroke and acquired brain injury (Yang 2015; Momosaki 2016; Pisegna 2016), NMES in stroke and neurological impairment (Chen 2016; Ding 2016), and PES in stroke (Scutt 2015). However, these reviews have examined the efficacy of individual interventions, whereas the current review has examined the efficacy of swallowing therapy overall; hence direct comparisons are difficult to make.

Study Flow Diagram, *86 studies awaiting classification.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
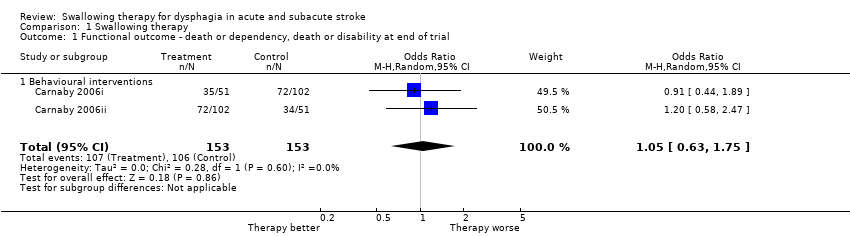
Comparison 1 Swallowing therapy, Outcome 1 Functional outcome ‐ death or dependency, death or disability at end of trial.
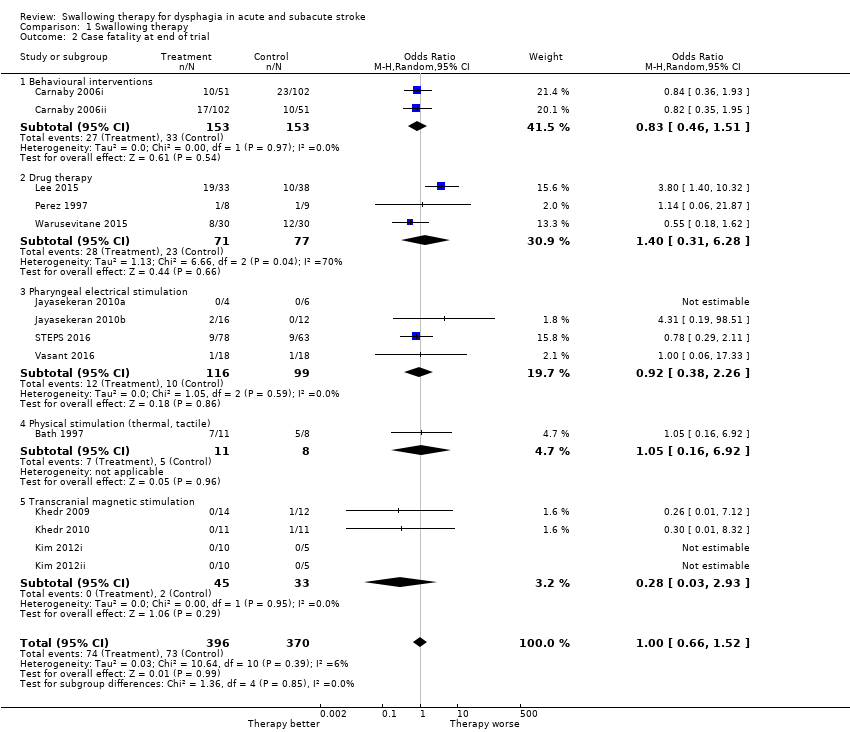
Comparison 1 Swallowing therapy, Outcome 2 Case fatality at end of trial.
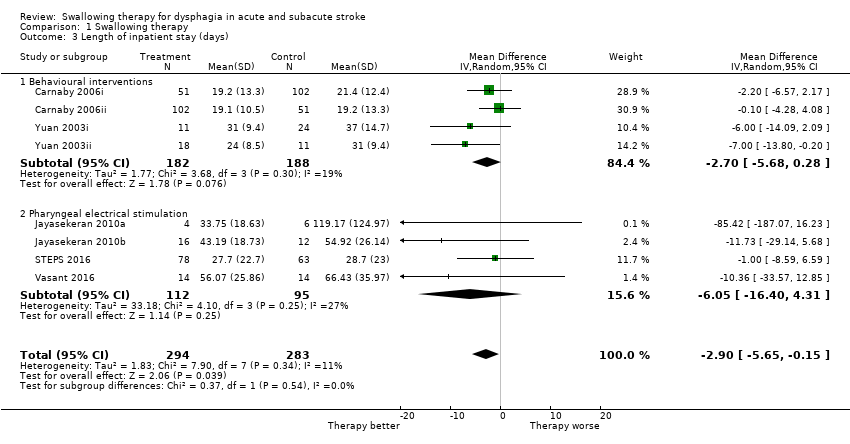
Comparison 1 Swallowing therapy, Outcome 3 Length of inpatient stay (days).

Comparison 1 Swallowing therapy, Outcome 4 Proportion of participants with dysphagia at end of trial.
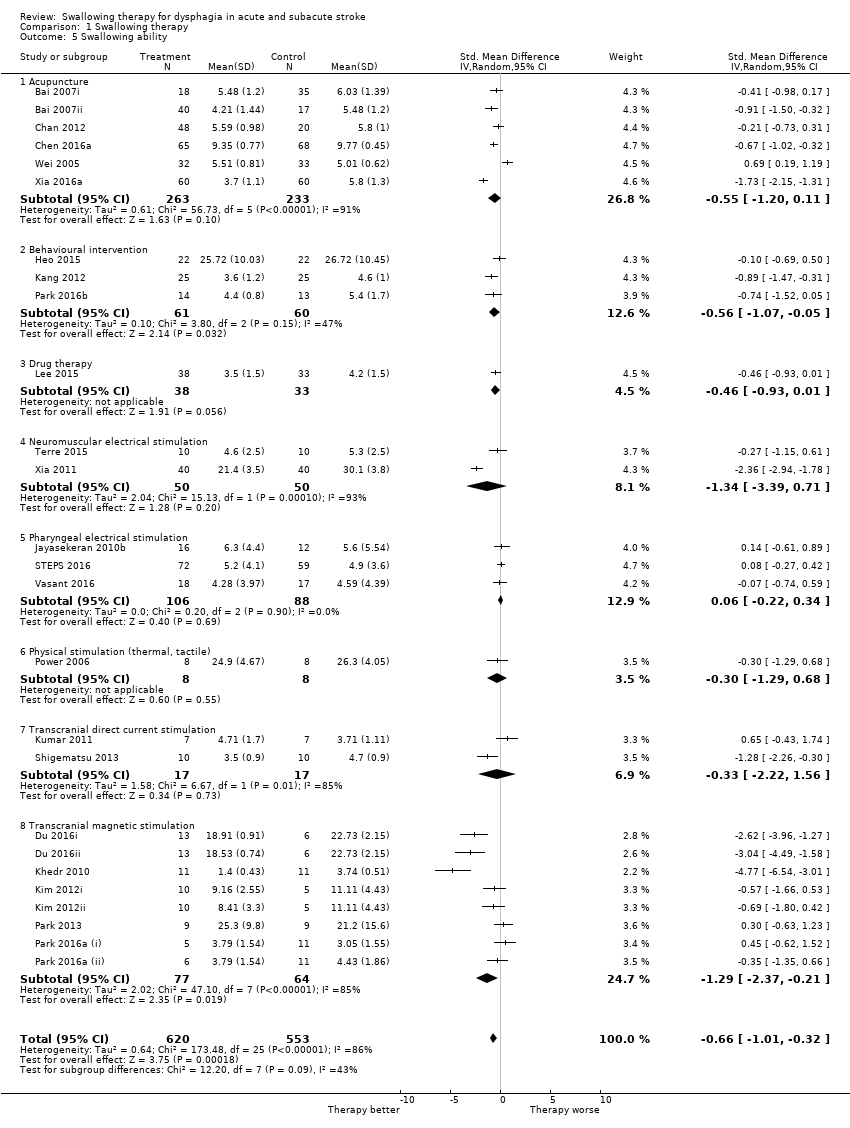
Comparison 1 Swallowing therapy, Outcome 5 Swallowing ability.
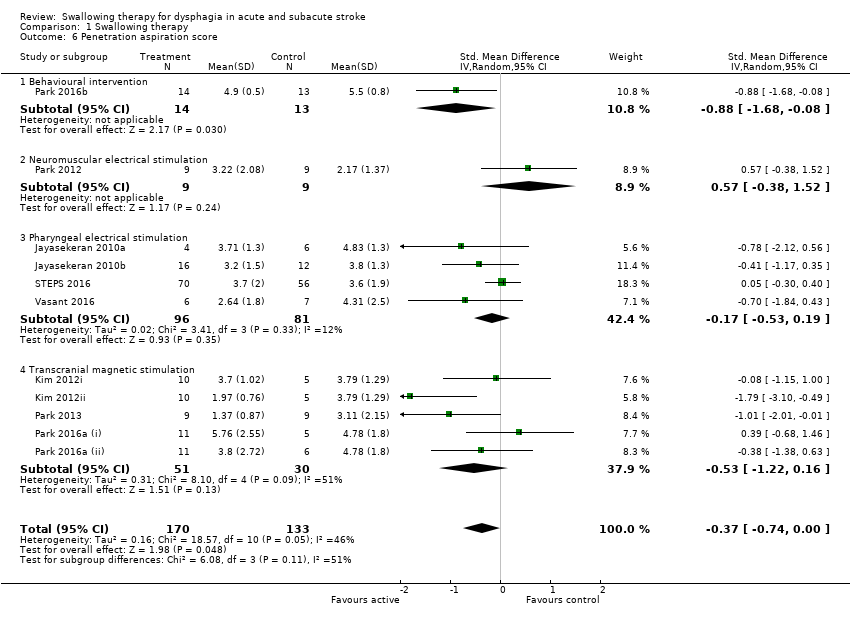
Comparison 1 Swallowing therapy, Outcome 6 Penetration aspiration score.

Comparison 1 Swallowing therapy, Outcome 7 Chest infection or pneumonia.

Comparison 1 Swallowing therapy, Outcome 8 Pharyngeal transit time (seconds).

Comparison 1 Swallowing therapy, Outcome 9 Institutionalisation.
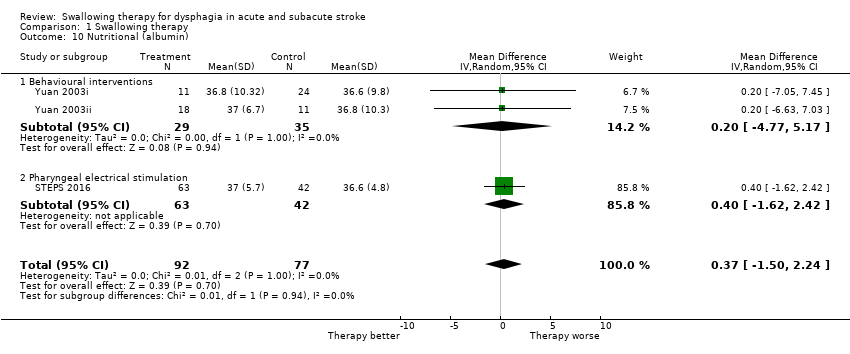
Comparison 1 Swallowing therapy, Outcome 10 Nutritional (albumin).
| Swallowing therapy compared to placebo for dysphagia in acute and subacute stroke | ||||||
| Patient or population: dysphagia in acute and subacute stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with swallowing therapy | |||||
| Death or dependency at end of trial | Study population | OR 1.05 | 306 | ⊕⊕⊕⊝ | a | |
| 693 per 1000 | 703 per 1000 | |||||
| Case fatality at end of trial | Study population | OR 1.00 | 766 | ⊕⊕⊕⊝ | b | |
| 197 per 1000 | 197 per 1000 | |||||
| Length of inpatient stay (days) | Mean length of inpatient stay (days) ranged from 19 to 119 | MD 2.9 lower | ‐ | 577 | ⊕⊕⊕⊝ | c |
| Proportion of participants with dysphagia at end of trial | Study population | OR 0.42 | 1487 | ⊕⊕⊝⊝ | d | |
| 570 per 1000 | 357 per 1000 | |||||
| Swallowing ability | Mean swallowing ability was 0 | SMD 0.66 lower | ‐ | 1173 | ⊕⊝⊝⊝ | e |
| Penetration aspiration score | Mean penetration aspiration score was 0 | SMD 0.37 lower | ‐ | 303 | ⊕⊕⊝⊝ | f |
| Adverse event: chest infection or pneumonia | Study population | OR 0.34 | 676 | ⊕⊝⊝⊝ | g | |
| 343 per 1000 | 151 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by one level due to lack of precision (one study split into two trials). bDowngraded by one level for indirectness of the evidence (i.e. multiple different interventions). cDowngraded by one level due to indirectness of the evidence (i.e. multiple different interventions). Note also that two studies had unclear blinding. dDowngraded by two levels due to indirectness of the evidence and blinding ‐ a large number of studies did not clarify blinding status. eDowngraded by three levels due to indirectness of the evidence (i.e. multiple different interventions), considerable heterogeneity, and fair number of studies did not clarify blinding status. fDowngraded by two levels due to indirectness of the evidence (i.e. multiple different interventions) and moderate heterogeneity. gDowngraded by three levels due to indirectness of the evidence (i.e. multiple different interventions), substantial heterogeneity, and fair number of studies did not clarify blinding status. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional outcome ‐ death or dependency, death or disability at end of trial Show forest plot | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.75] |
| 1.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.75] |
| 2 Case fatality at end of trial Show forest plot | 14 | 766 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.52] |
| 2.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 2.2 Drug therapy | 3 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.31, 6.28] |
| 2.3 Pharyngeal electrical stimulation | 4 | 215 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.26] |
| 2.4 Physical stimulation (thermal, tactile) | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.92] |
| 2.5 Transcranial magnetic stimulation | 4 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.93] |
| 3 Length of inpatient stay (days) Show forest plot | 8 | 577 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐5.65, ‐0.15] |
| 3.1 Behavioural interventions | 4 | 370 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.68, 0.28] |
| 3.2 Pharyngeal electrical stimulation | 4 | 207 | Mean Difference (IV, Random, 95% CI) | ‐6.05 [‐16.40, 4.31] |
| 4 Proportion of participants with dysphagia at end of trial Show forest plot | 23 | 1487 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.32, 0.55] |
| 4.1 Acupuncture | 8 | 676 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.49] |
| 4.2 Behavioural interventions | 6 | 511 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.28, 0.74] |
| 4.3 Drug therapy | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.07, 3.35] |
| 4.4 Neuromuscular electrical stimulation | 2 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.49] |
| 4.5 Pharyngeal electrical stimulation | 3 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 2.11] |
| 4.6 Physical stimulation (thermal, tactile) | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.07, 5.85] |
| 4.7 Transcranial direct current stimulation | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 8.39] |
| 5 Swallowing ability Show forest plot | 26 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.01, ‐0.32] |
| 5.1 Acupuncture | 6 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.20, 0.11] |
| 5.2 Behavioural intervention | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.07, ‐0.05] |
| 5.3 Drug therapy | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.93, 0.01] |
| 5.4 Neuromuscular electrical stimulation | 2 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐3.39, 0.71] |
| 5.5 Pharyngeal electrical stimulation | 3 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.34] |
| 5.6 Physical stimulation (thermal, tactile) | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.29, 0.68] |
| 5.7 Transcranial direct current stimulation | 2 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐2.22, 1.56] |
| 5.8 Transcranial magnetic stimulation | 8 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.37, ‐0.21] |
| 6 Penetration aspiration score Show forest plot | 11 | 303 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.74, ‐0.00] |
| 6.1 Behavioural intervention | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.68, ‐0.08] |
| 6.2 Neuromuscular electrical stimulation | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.38, 1.52] |
| 6.3 Pharyngeal electrical stimulation | 4 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.53, 0.19] |
| 6.4 Transcranial magnetic stimulation | 5 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.22, 0.16] |
| 7 Chest infection or pneumonia Show forest plot | 9 | 618 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.16, 0.78] |
| 7.1 Behavioural interventions | 6 | 473 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.00] |
| 7.2 Drug therapy | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.21] |
| 7.3 Neuromuscular electrical stimulation | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Pharyngeal electrical stimulation | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.09] |
| 8 Pharyngeal transit time (seconds) Show forest plot | 6 | 187 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.32, ‐0.15] |
| 8.1 Drug therapy | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.91, 0.49] |
| 8.2 Neuromuscular electrical stimulation | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.39, ‐0.08] |
| 8.3 Pharyngeal electrical stimulation | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.67, 0.37] |
| 8.4 Physical stimulation (thermal, tactile) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] |
| 9 Institutionalisation Show forest plot | 3 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.47, 1.19] |
| 9.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.39, 1.48] |
| 9.2 Pharyngeal electrical stimulation | 1 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.48] |
| 10 Nutritional (albumin) Show forest plot | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐1.50, 2.24] |
| 10.1 Behavioural interventions | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.77, 5.17] |
| 10.2 Pharyngeal electrical stimulation | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.62, 2.42] |

