نقش داروهای کولینرژیک در درمان تاردیو دیسکینزی ناشی از آنتیسایکوتیکها
چکیده
پیشینه
تاردیو دیسکینزی (tardive dyskinesia; TD) یک عارضه جانبی و مضر داروهای آنتیسایکوتیک (نورولپتیک) مرسوم است. به نظر میرسد که TD جزیی از نقص کولینرژیک مرکزی است. داروهای کولینرژیک برای درمان TD استفاده شدهاند
اهداف
تعیین تاثیرات داروهای کولینرژیک (آروکولین (arecoline)، کولین (choline)، دیانول (deanol)، لسیتین (lecithin)، مکلوفنوکسات (meclofenoxate)، فیزوستیگمین (physostigmine)؛ RS 86، تاکرین (tacrine)، متوکسیتاکرین (metoxytacrine)، گالانتامین (galantamine)، ایپیداکرین (ipidacrine)، دونپزیل (donepezil)، ریواستیگمین (rivastigmine)، اپتاستیگمین (eptastigmine)، متریفونات (metrifonate)، زانوملین (xanomeline)، سویملین (cevimeline)) در درمان افراد مبتلا به TD ناشی از آنتیسایکوتیک در اسکیزوفرنی یا بیماریهای روانی مزمن دیگر.
روشهای جستوجو
یک جستوجوی الکترونیک در پایگاه ثبت کارآزماییهای مطالعه‐محور گروه اسکیزوفرنی در کاکرین (16 جولای 2015 و اپریل 2017) انجام دادیم. این پایگاه ثبت، مجموعهای از پژوهشهای گسترده از کارآزماییهای تصادفیسازی و کنترل شده در بسیاری از بانکهای اطلاعاتی الکترونیک، پایگاههای ثبت کارآزماییها، خلاصه مقالات کنفرانسها و پایاننامهها است. منابع تمام مطالعات شناسایی شده برای استنادهای کارآزماییها جستوجو شدند.
معیارهای انتخاب
ما گزارشهای شناسایی شده از طریق جستوجو را در این مرور گنجاندیم، به شرط اینکه کارآزماییهای کنترل شده شامل افراد مبتلا به TD ناشی از آنتیسایکوتیک و بیماریهای روانی مزمن بودند و شرکتکنندگان به طور تصادفی به گروه عامل کولینرژیک یا گروه دارونما (placebo) یا گروه عدم مداخله تخصیص داده شده بودند. دو نویسنده مرور بهطور مستقل از هم کیفیت روششناسی کارآزماییها را ارزیابی کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور دادهها را استخراج کرده و تا جایی که امکانپذیر بود خطر نسبی (RR) یا تفاوت میانگین (MD) را با 95% فواصل اطمینان (CI) آنها تخمین زدیم. ما دادهها را بر اساس قصد درمان (intention‐to‐treat) تجزیهوتحلیل کردیم، با این فرض که افرادی که مطالعه را زودهنگام ترک کردند بهبودی نداشتند. خطر سوگیری (bias) را ارزیابی کرده و جدول «خلاصه یافتهها» را با استفاده از سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ایجاد کردیم.
نتایج اصلی
14 مطالعه را که در آنها داروهای کولینرژیک با دارونما مقایسه شده و بین سالهای 1976 تا 2014 منتشر شده بودند، در این مرور گنجاندیم. تمام مطالعات شرکتکنندگان اندکی داشتند (پنج تا 60 فرد). سه مطالعه که داروهای کولینرژیک جدید آلزایمر را برای درمان TD بررسی کرده بودند، برای به روز کردن این مرور، جدید بودند. به طور کلی خطر سوگیری در مطالعات وارد شده نامشخص بود و دلیل آن نیز گزارشدهی ضعیف، عدم شفافسازی پنهانسازی تخصیص (allocation concealment)، عدم تصریح ایجاد توالی (generation of the sequence)، عدم کورسازی واضح مطالعات بود، ما درباره کامل بودن دادهها مطمئن نیستیم و غالبا به طور ضعیف و انتخابی گزارش شده بودند.
درباره تاثیرات داروهای قدیمی یا جدید کولینرژیک در مقایسه با دارونما از نظر پیامد «عدم بهبودی مهم از نظر بالینی در نشانههای TD» نامطمئن هستیم، کیفیت شواهد بسیار پائین بود (RR: 0.89؛ 95% CI؛ 0.65 تا 1.23؛ 27 نفر؛ 4 RCT). هشت کارآزمایی نشان دادند که داروهای کولینرژیک تفاوتی اندک یا عدم تفاوت در بدتر شدن نشانههای TD ایجاد میکنند (شواهد با کیفیت پائین؛ RR: 1.11؛ 95% CI؛ 0.55 تا 2.24؛ 147 نفر). باز هم به دلیل شواهد با کیفیت بسیار پائین، درباره تاثیرات داروهای کولینرژیک بر این موارد نامطمئن هستیم: وضعیت روانی (RR: 50.0؛ 95% CI؛ 0.10 تا 2.61؛ 77 نفر؛ 5 RCT)، حوادث جانبی (RR: 0.56؛ 95% CI؛ 0.15 تا 2.14؛ 106 نفر؛ 4 RCT) و ترک زودهنگام مطالعه (RR: 1.09؛ 95% CI؛ 0.56 تا 2.10؛ 288 نفر؛ 12 RCTs). در هیچ مطالعهای پیامدهای پذیرش درمان، اعتماد به نفس در جامعه، درگیر شدن در جامعه، درگیر شدن در شبکههای اجتماعی یا کیفیت زندگی شخصی بیمار گزارش نشده بود.
نتیجهگیریهای نویسندگان
TD یک مشکل اصلی بهداشت عمومی است. تاثیرات بالینی داروهای قدیمی کولینرژیک و عوامل جدید کولینرژیک که در حال حاضر برای درمان آلزایمر استفاده میشوند نامشخص است و به دلیل مطالعات کوچک و اندک، سوالات بسیاری بدون پاسخ مانده است. داروهای کولینرژیک باید علاقه محققین باشند و در حال حاضر جایگاه اندکی در کارهای بالینی مرسوم دارند. با این حال، با ارائه عوامل کولینرژیک جدید که اکنون برای درمان آلزایمر استفاده میشوند، حوزهای برای کارآزماییهای اطلاعاتی بیشتر فراهم آمده است. اگر قرار است این عوامل کولینرژیک جدید برای درمان افراد مبتلا به TD بررسی شوند، تاثیرات آنها باید در کارآزماییهای تصادفیسازی شده، بزرگ و با طراحی مناسب تعیین شود.
PICO
خلاصه به زبان ساده
داروهای کولینرژیک در درمان تاردیو دیسکینزی ناشی از آنتیسایکوتیکها
سوال مطالعه مروری
آیا داروهای کولینرژیک برای درمان عوارض جانبی ناخوشایند مانند تاردیو دیسکینزی (tardive dyskinesia) در افراد مبتلا به اسکیزوفرنی (schizophrenia) یا بیماران روانی مشابه دیگر و تحت درمان با داروهای آنتیسایکوتیک مفید هستند یا خیر.
پیشینه.
بیماران مبتلا به اسکیزوفرنی غالبا صداهایی میشنوند و چیزهایی میبینند (توهم) و باورهای عجیب و غریب دارند (هذیان). این نشانهها معمولا با داروهای آنتیسایکوتیک درمان میشوند. اما این داروها عوارض جانبی ناتوان کنندهای (debilitating) دارند. تاردیو دیسکینزی حرکات غیر‐ارادی است که باعث ایجاد تشنج، اسپاسم و شکلک در صورت، دهان، زبان و فک میشود. این بیماری به وسیله تجویز طولانی‐مدت یا دوز بالای آنتیسایکوتیکها ایجاد میشود، درمان این بیماری مشکل است و حتی میتواند درمانناپذیر باشد. به نظر میرسد که تاردیو دیسکینزی میتواند به دلیل نقص کولینرژیک باشد. داروهای کولینرژیک قدیمی مانند دیانول (deanol)، لسیتین (lecithin) و مکلوفنوکسات (meclofenoxate) برای درمان تاردیو دیسکینزی استفاده شدهاند. داروهای کولینرژیک جدید مانند دونپزیل (donepezil)، گالانتامین (galantamine) و ریواستیگمین (rivastigmine) که برای درمان آلزایمر استفاده میشوند ممکن است در درمان تاردیو دیسکینزی مجاز باشند.
ویژگیهای مطالعه
ما در پایگاه ثبت کارآزماییهای گروه اسکیزفرنی در کاکرین، کارآزماییهای مربوط را جستوجو کردیم (جولای 2015 و اپریل 2017). این مرور شامل 14 مطالعه بود که در آنها استفاده از داروهای کولینرژیک با دارونما (placebo) مقایسه شده بود. تمام مطالعات شرکتکنندگان اندکی داشتند (پنج تا 60 نفر) که به طور تصادفی تخصیص داده شده بودند، این شرکتکنندگان مبتلا به اسکیزفرنی یا بیماریهای روانی مزمن بوده و مبتلا به تاردیو دیسکینزی ناشی از آنتیسایکوتیک بودند.
نتایج کلیدی
ما دریافتیم که تاثیرات داروهای کولینرژیک قدیمی و جدید نامشخص هستند و دلیل آن هم مطالعات کوچک و اندک موجود بود، در نتیجه شواهد زیادی وجود ندارد و سوالات بدون پاسخ زیادی باقی ماندهاند.
کیفیت شواهد.
شواهد موجود ضعیف، محدود و در مقیاس اندک هستند. بنابراین توصیه به استفاده از این داروها برای درمان تاردیو دیسکینزی بر اساس یافتههای ما امکانپذیر نیست. بررسی کامل تاثیرات مثبت داروهای کولینرژیک در افراد مبتلا به تاردیو دیسکینزی نیازمند مطالعات طولانی‐مدت، بزرگ و با طراحی مناسب است، خصوصا درباره داروهای کولینرژیک جدیدی که در حال حاضر برای درمان بیماری آلزایمر استفاده میشود.
بن گری (Ben Gray) ‐ محقق ارشد بنیاد McPin
Authors' conclusions
Summary of findings
| CHOLINERGIC DRUGS versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: people with various psychiatric disorders (mainly schizophrenia) and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | CHOLINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 to 12 weeks | 1000 per 1000 | 890 per 1000 | RR 0.89 | 27 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin) found a significant difference between cholinergic drug and placebo. |
| Tardive dyskinesia: Deterioration follow‐up: 9 days to 12 weeks | 116 per 1000 | 129 per 1000 | RR 1.11 | 147 | ⊕⊕⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Mental state: Deterioration follow‐up: 11 days to 12 weeks | 56 per 1000 | 28 per 1000 | RR 0.50 | 77 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, lecithin) found a significant difference between cholinergic drug and placebo. |
| Adverse effects: Any adverse events follow‐up: 9 days to 8 weeks | 98 per 1000 | 55 per 1000 | RR 0.56 | 106 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Acceptability of treatment: Leaving the study early follow‐up: 9 days to 12 weeks | 90 per 1000 | 98 per 1000 | RR 1.09 | 288 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, galantamine, meclofenoxate, lecithin) found a significant difference between cholinergic drug and placebo. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: for many studies it was unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
Background
Description of the condition
Since the 1950s antipsychotic (neuroleptic) medication has been used extensively to treat people with chronic mental illnesses such as schizophrenia. These drugs can effectively control symptoms such as abnormal perceptions (hallucinations), disordered thinking and fixed false beliefs (delusions). In addition, maintenance therapy with antipsychotics is associated with a reduced risk of relapse (Schooler 1993). However, antipsychotic medication has also been associated with a wide range of adverse effects, including movement disorders. The appearance of these disorders can be extremely disfiguring, compounds stigma, and is associated with poor compliance to antipsychotic treatment (Barnes 1993; Tarsy 2011).
Tardive dyskinesia (TD) is one such movement disorder and is characterised by abnormal, repetitive and involuntary movements. The clinical features include: tongue protrusion, side‐to‐side or rotatory movement of the jaw, lip smacking, puckering and pursing, and rapid eye blinking (Casey 1999). In some people rapid movements of the arms, legs, and trunk may also occur. TD is a chronic condition of insidious onset, the severity of which fluctuates spontaneously (APA 1992). Although the most frequent cause of TD is the use of antipsychotic medication, it is striking that dose reduction can lead to a temporary exacerbation in symptoms. Conversely, increasing the dose is often associated with a temporary remission (Cavallaro 1993; Smith 1980). Studies on the natural history of TD have reported widely variable remission rates (1% to 62%) depending on the patient's age, psychiatric diagnosis, course of the psychiatric disorder, and duration of therapy (Bergen 1989; Fernandez 2001; Glazer 1990).
The exact mechanisms of the pathophysiology of TD are unknown. Antipsychotic drugs block certain chemical receptor sites in the brain ‐ one of these is specific for dopamine (Casey 1995). One hypothesis explaining the cause of antipsychotic‐induced TD is that long‐term blockade of the dopamine receptors may lead to an imbalance between the activity of dopamine cells and others that employ choline (Alphs 1983; Casey 1995). This theory implies that there is an over activity of dopaminergic transmission in relation to cholinergic transmission in the striatum. Another hypothesis suggests that the chronic use of antipsychotics may also cause an abnormal production of highly active atoms and chemical groups (cytotoxic free radicals), which may damage specific cells in the brain. This, in turn, could contribute to the pathophysiology of TD (Andreassen 2000; Andreassen 2001; Cadet 1989). The latter theory is supported by the persistent nature of the syndrome, once established.
TD occurs in more than 20% of people that use first‐generation antipsychotic drugs continually for longer than three months (Kane 1982; Glazer 2000; Tarsy 2011). Every year 4% to 5% of adults and 25% to 30% of elderly persons who continually use these drugs begin to show signs of TD (APA 1992; Correll 2004). Advancing age is a risk factor for both TD's prevalence and severity, with those who are < 60 years of age being three times more likely to spontaneously remit (Jeste 2000; Smith 1980).
When the second‐generation antipsychotic drugs were introduced in the 1990’s many hoped that they would not cause TD (Miller 2007; Rosenheck 2007). Although the risk of developing TD with second‐generation antipsychotic drugs does seem to be reduced, TD risks have not been eliminated (Miller 2007; Tarsy 2011). There is even some evidence to indicate that rates of TD do not differ at all between first‐ and second‐generation antipsychotic drugs (Leucht 2009; Rosenheck 2007; Woods 2010). The large, definitive, US randomised trial of antipsychotic treatments for schizophrenia (CATIE), with a four‐year period of follow‐up, obtained an incidence rate of TD of around 17% (Miller 2008). Due to widespread use of second‐generation antipsychotic drugs, increased off‐label use, and an ageing population, the frequency of TD is likely to be higher than thought (Cloud 2014; Maher 2012), and increasing. The problem will be considerably greater for people in countries where use of newer drugs is less prevalent (Ballesteros 2000; Martins 2011).
Description of the intervention
Medications affecting the cholinergic pathway have been tested for the treatment of TD due to the hypothesis that TD is correlated with damage in cholinergic cells in striatal subregions. This damage might be caused by changes in dopaminergic transmission, as a result of prolonged antipsychotic treatment (Grimm 2001). A variety of cholinergic medications have been used in TD, starting from the 1970's. The cholinergic compounds included in this review have various functions in relation to acetylcholine, the main neurotransmitter and neuromodulator in the cholinergic pathway.
How the intervention might work
This review looked at compounds such as physostigmine, choline, and lecithin (containing phosphatidyl choline), which were some of the early cholinergic medications used for TD, as they were thought to be of potential help in treating TD by enhancing acetylcholine synthesis (Wurtman 1978). It was thought that high levels of choline in the blood (which, for instance, phosphatidyl choline converts into after digestion) would enhance acetylcholine synthesis in neurons by making the precursor more available. Other treatments included, such as deanol and meclofenoxate, have been tested due to their cholinomimetic actions (Casey 1975; Izumi 1986). Deanol, a synthetic substance, is also thought to be a precursor of acetylcholine, but this is unproven, and deanol might be an acetylcholine agonist or even a suppressor of acetylcholine synthesis. During the last 20 years many new central nervous system (CNS)‐active cholinergic compounds, predominantly acetylcholinesterase‐inhibitors such as galantamine (Loy 2006), rivastigmine (Birks 2015) and donepezil (Birks 2006b), have emerged for the treatment of Alzheimer's disease and dementia. These compounds were also included in this review, due to their potential for treating TD symptoms (Caroff 2001).
Why it is important to do this review
Several atypical antipsychotic drugs have been produced in the last decades that claim to cause less or no TD (Lieberman 1996). These claims may or may not be true, and certainly evidence does point to the fact that thoughtful use of older generation drugs is not associated with any more problems of TD than with newer treatments (Chouinard 2008). However, in a global context, it is likely that the less expensive and more familiar drugs ‐ such as chlorpromazine or haloperidol ‐ will continue to be the mainstay of treatment of people with schizophrenia (WHO Essential List 2010). Use of drugs such as these is associated with the emergence of TD and, therefore, this condition will remain a problem for years to come.
TD can result in considerable social and physical disability (Barnes 1993) and symptoms are often irreversible (Bergen 1989; Fernandez 2001; Gerlach 1988; Glazer 1990). Additionally, TD is frequently associated with lower quality of life (Ascher‐Svanum 2008) and a greater mortality rate (Chong 2009). Given the high incidence and prevalence of TD among people taking antipsychotic medication, the need for prevention or treatment is clear. Unfortunately, there has been sparse evidence to guide clinicians (NICE 2014; Taylor 2009). Although many treatments have been tested, no one intervention has been shown clearly to be effective. Cessation or reduction of the dose of antipsychotic medication would be the ideal management for TD. In clinical practice this is not always possible, not least because in many individuals such a reduction would lead to relapse. This review focuses on whether the addition of different types of cholinergic medications to those already receiving antipsychotic medication is likely to help TD.
This review is one in a series of Cochrane reviews (see Table 1) evaluating treatments for antipsychotic‐induced TD, and is an update of a Cochrane review first published in 1997 (McGrath 1997), and previously updated in 2002 (Tammenmaa 2002).
Objectives
The primary objective of this review is to determine the effects of cholinergic drugs (arecoline, choline, deanol, lecithin, meclofenoxate, physostigmine, RS 86, tacrine, metoxytacrine, galantamine, ipidacrine, donepezil, rivastigmine, eptastigmine, metrifonate, xanomeline, cevimeline) for treating antipsychotic‐induced TD in people with schizophrenia or other chronic mental illness.
The secondary objectives are:
1. to examine whether duration of treatment has an effect on treatment response;
2. to examine whether there is difference in treatment effect for the various cholinergic compounds;
3. to examine whether treatment response differs in people with schizophrenia who are older (above 65 years) and for whom the prevalence of spontaneous dyskinesias is estimated to be higher. (This secondary objective was added in response to a comment from peer review in the first substantial up‐date of the review).
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. Where a trial was described as 'double‐blind' but it implied that the study was randomised and the demographic details of each group were similar, it was included and we conducted a sensitivity analysis to evaluate the effect of the presence or absence of these data. We excluded quasi‐randomised studies, such as those allocated by using alternate days of the week.
Types of participants
People with schizophrenia or any other serious mental illness, diagnosed by any criteria, irrespective of gender, age or nationality who:
1. required the use of antipsychotics for more than three months;
2. developed TD during antipsychotic treatment (diagnosed by any criteria at baseline of the trial and at least one other occasion);
3. for whom the dose of antipsychotic medication had been stable for one month or more before the trial and during the trial (the same applies for those free of antipsychotics).
Types of interventions
1. The cholinergic drugs
Arecoline, choline, deanol, lecithin, meclofenoxate, physostigmine, RS 86.
In the first substantial update of the review the following cholinergic compounds were assessed to be relevant and added to the scope of the review: 7‐methoxytacrine, cevimeline, donepezil, eptastigmine, galantamine, ipidacrine, metrifonate, rivastigmine, tacrine, xanomeline, .
2. Control condition
Placebo or no intervention.
For the 2017 update a post hoc decision was made to also include studies evaluating the above mentioned cholinergic drugs compared to any other intervention for the treatment of TD.
Types of outcome measures
When appropriate, the we grouped outcomes into time periods ‐ short term (less than six weeks), medium term (between six weeks and six months) and long term (over six months).
Primary outcomes
1. Tardive dyskinesia
No clinically important improvement in the symptoms of individuals, defined as more than 50% improvement on any TD scale ‐ any time period.
2. Adverse effects
No clinically significant extrapyramidal adverse effects ‐ any time period.
Secondary outcomes
1. Tardive dyskinesia
1.1 Any improvement in the symptoms of individuals on any TD scale, as opposed to no improvement.
1.2 Deterioration in the symptoms of individuals, defined as any deleterious change on any TD scale.
1.3 Average change in severity of TD during the trial period.
1.4 Average difference in severity of TD at the end of the trial.
2. Global outcome measures (this category of outcome measures was added in the first substantial update of the review).
2.1 The number of people per treatment group who died for any reason.
2.2 Treatment group mean and standard deviation of endpoint score on any scale of quality of life.
2.3 Treatment group mean and standard deviation of endpoint score on any scale of level of functioning.
3. General mental state changes
3.1 Deterioration in general psychiatric symptoms (such as delusions and hallucinations) defined as any deleterious change on any scale.
3.2 Average difference in severity of psychiatric symptoms at the end of the trial.
4. Acceptability of the treatment
4.1 Acceptability of the intervention to the participant group as measured by numbers of people dropping out during the trial.
5. Adverse effects
5.1 Use of any anti‐parkinsonism drugs.
5.2 Average score/change in extrapyramidal adverse effects.
5.3 Acute dystonia.
6. Other adverse effects, general and specific
7. Hospital and service utilisation outcomes
7.1 Hospital admission.
7.2 Average change in days in hospital.
7.3 Improvement in hospital status (for example: change from formal to informal admission status, use of seclusion, level of observation).
8. Economic outcomes
8.1 Average change in total cost of medical and mental health care.
8.2 Total indirect and direct costs.
9. Social confidence, social inclusion, social networks, or personalised quality of life measures
9.1. No significant change in social confidence, social inclusion, social networks, or personalised quality of life measures.
9.2 Average score/change in social confidence, social inclusion, social networks, or personalised quality of life measures.
10. Behaviour
10.1 Clinically significant agitation.
10.2 Use of adjunctive medication for sedation.
10.3 Aggression to self or others.
11. Cognitive state
11.1 No clinically important change.
11.2 No change, general and specific.
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADEpro to export data from this review to create a 'Summary of findings' table. This table provides outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of the available data on all outcomes we rated as important to patient care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
1. Tardive dyskinesia
1.1 Improved to a clinically important extent
1.2 Deteriorated
2. Mental state
2.1 Deteriorated
3. Adverse effect
3.1 Any adverse event
3.2 Adverse effects: no clinically significant extrapyramidal adverse effects
4. Acceptability of treatment
4.1 Leaving the study early
5. Social confidence, social inclusion, social networks, or personalised quality of life measures*
5.1 No significant change in social confidence, social inclusion, social networks, or personalised quality of life measures for either recipients of care or caregivers.
* Outcome designated important to patients. We wished to add perspectives from people’s personal experience with TD to the research agenda. A consultation with service users was planned where a previously published version of a review in the Cochrane TD series (Soares‐Weiser 2011; see Table 1) and a lay overview of that review gave the foundation for the discussions. The session was planned to provide time to reflect on current research on TD and consider gaps in knowledge. The report is published in the Health Technology Assessment (HTA) report for the UK National Institute of Health Research (Bergman 2017). We have added one figure showing one service user's expression of frustration concerning this neglected area of research (Figure 1). Informed by the results of the consultation, for this review, we updated outcomes for the 'Summary of findings' table.

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
Search methods for identification of studies
Electronic searches
The 2017 review update was carried out in parallel with updating eight other TD reviews, see Table 1 for details. The search for trials covered all nine TD reviews.
1. Cochrane Schizophrenia Group’s Study‐Based Register of Trials
We searched the register on 16 July, 2015 and 26 April 2017 using the following string:
*Tardive Dyskinesia* in Healthcare Condition Field of Study
In such a study‐based register, searching the major concept retrieves all the synonym keywords and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
2. Details of previous electronic searches
See Appendix 1.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
Where necessary, we contacted the first author of each included study for information regarding unpublished trials. We noted the outcome of this contact in the included studies table.
Data collection and analysis
Selection of studies
For the 2017 update, review author RA and Antonio Grande (AG) (see Acknowledgements) inspected all abstracts of studies identified as above and identified potentially relevant reports. We resolved disagreements by discussion, or where there was still doubt, we acquired the full‐text article for further inspection. We acquired the full‐text articles of all relevant reports/abstracts meeting the initial criteria for reassessment and carefully inspected for a final decision on inclusion (see Criteria for considering studies for this review). RA and AG were not blinded to the names of the authors, institutions or journal of publication. Where difficulties or disputes arose, we asked review author HB for assistance and had it not been possible to decide, or if adequate information was not available to make a decision, we planned to add these studies to those awaiting assessment and contact the authors of the papers for clarification.
Data extraction and management
1. Extraction
For the 2017 update, review authors RA and HB independently extracted data from all included studies. Again, we discussed any disagreement and documented decisions. With remaining problems KSW helped clarify issues and we documented these final decisions. We extracted data presented only in graphs and figures whenever possible, but included only if two review authors independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multicentre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
For the 2017 update we extracted data to simple forms. Extracted data are available here with a link to the original source PDF for each item.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we noted in Description of studies if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we preferred to use mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant data before inclusion.
We entered data from studies of at least 200 participants in the analysis, because skewed data pose less of a problem in large studies. We also entered all relevant change data as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not.
For endpoint data from studies < 200 participants:
(a) when a scale started from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than 1, it strongly suggests a skew and we excluded these data. If this ratio was higher than 1 but below 2, there is suggestion of skew. We entered these data and tested whether its inclusion or exclusion changed the results substantially. Finally, if the ratio was larger than 2, we included these data, because skew is less likely (Altman 1996; Higgins 2011).
(b) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), (Kay 1986)), which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
Where relevant, to facilitate comparison between trials, we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we converted continuous outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this can be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for cholinergic medication. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not un‐improved'), we presented data where the left of the line indicates an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review authors RA and HB independently assessed risk of bias within the included studies by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, we made the final rating by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. If non‐concurrence occurred, we reported this.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios as odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference SMD). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If any of the included trials had randomised participants by clusters, and where clustering had not been accounted for in primary studies, we would have presented such data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, we will seek to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported it will be assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary we simply added and combined within the two‐by‐two table. If data were continuous, we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not use data where the additional treatment arms were not relevant.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table by down‐rating quality. We also planned to downgrade quality within the 'Summary of findings' table should loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). We assumed all those leaving the study early had no improvement. We undertook a sensitivity analysis to test how prone the primary outcomes were to change by comparing data only from people who completed the study to that point to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
We reported and used data where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either 'P' value or 't' value available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, we preferred to use the more sophisticated approaches. (e.g. MMRM or multiple‐imputation) and only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item "incomplete outcome data" of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise and discussed them in the text if they arose.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise and discussed them in the text if they arose.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, can be interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 Cochrane Handbook for Systematic Reviews of InterventionsHiggins 2011). We explored and discussed in the text potential reasons for substantial levels of heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In future versions of this review, if funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Duration of treatment
We anticipated a subgroup analysis to examine whether any improvement occurred with short periods of intervention (less than six weeks) and, if this did occur, whether this effect was maintained at longer periods of follow‐up.
1.2 Cholinergic compound
As different cholinergic drug compounds may have differential effects on antipsychotic‐induced TD, we performed a subgroup analysis to compare the effects of different cholinergic drugs. We proposed to undertake comparisons only for primary outcomes to minimise the risk of multiple comparisons.
1.3 Older participants
We also wanted to examine whether the treatment response differs in people with schizophrenia who are older (above 65 years) and for whom the prevalence of spontaneous dyskinesias is estimated to be higher. We had hoped to present data for this subgroup for the primary outcomes.
2. Investigation of heterogeneity
We reported inconsistency if it was high. First, we investigated whether data were entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, we would not pool such data but discuss relevant issues. We know of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we simply discussed these. We did not undertake sensitivity analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
If trials were described in some way as to imply randomisation we undertook a sensitivity analyses for the primary outcomes. We included these studies in the analyses and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we used relevant data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported and discussed these results, but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with completer data only. We undertook a sensitivity analysis to test how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported and discussed these results, but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that we judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, we included data from these trials in the analysis
4. Imputed values
Had we included cluster trials, we would have undertaken a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect.
If we found substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed and random effects
We synthesised data using a fixed‐effect model, however, we also synthesised data for the primary outcome using a random‐effects model to evaluate whether this altered the significance of the results.
Results
Description of studies
Please see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The 2015 and 2017 update searches were part of an update of nine Cochrane reviews, see Table 1. The 2015 search retrieved 704 references for 344 studies, see Figure 2 for study flow diagram. After having excluded irrelevant references at title and abstract screening, we screened full texts of 72 references (63 studies). Three of these reports were new included studies to this review (Caroff 2007; Jahanian 2014; Ogunmefun 2009), added to the 11 already included studies. One of these studies (Caroff 2007) was an ongoing study in the previously published version of this review. We were able to exclude four studies that were awaiting assessment in the previously published version of this review (Gelenberg 1989; Joe 1985; Marsalek 1994; Perez Cruet 1981), and we excluded one more study for this update (Bartels 1981), added to the 44 already excluded studies from the previously published version of this review. No studies await assessment, and as far as we are aware there are no ongoing studies that would be relevant to this review.
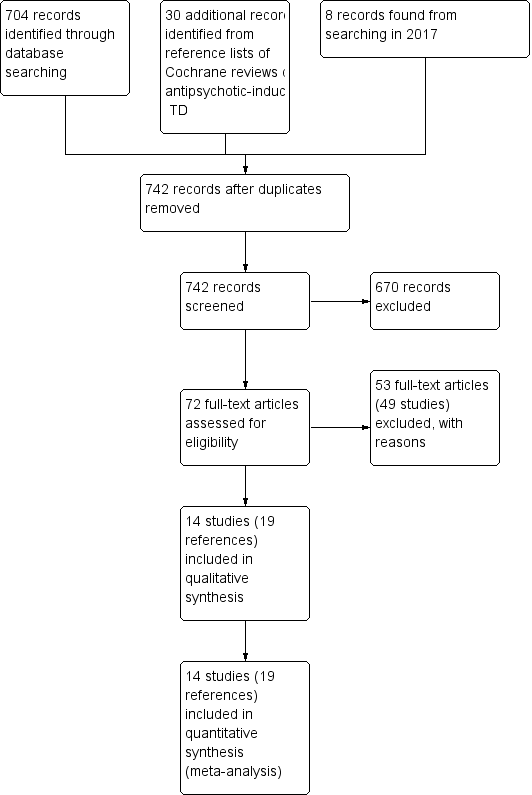
Study flow diagram for 2015 and 2017 searches.
The 2017 search found eight records (five studies). The editorial base of the Cochrane Schizophrenia Group screened these records and no new studies were relevant to this review. They could be relevant to the other reviews in this series of TD reviews (see Table 1), and were put into awaiting assessment of the miscellaneous treatments review (Soares‐Weiser 2003).
Included studies
Overall, the review now includes 14 studies with 364 participants published between 1976 and 2014. Three included studies are new to this update (Caroff 2007; Jahanian 2014; Ogunmefun 2009).
1. Methods
All studies were stated to be randomised and double‐blinded. For further details, please see sections below on Allocation (selection bias) and Blinding (performance bias and detection bias).
2. Design
All included studies presented a parallel longitudinal design. Eight of the 14 studies used a cross‐over design with two periods (Caroff 2007; Gelenberg 1990; Jackson 1978; Jackson 1979; Kocher 1980; Lucius 1976; Ogunmefun 2009; Tarsy 1977). We had considered this as likely when embarking on the review and have used only the data from before the first cross‐over for the reasons outlined above (Unit of analysis issues).
3. Duration
TD is often a chronic condition and symptoms tend to fluctuate and show considerable variability across time. Only four studies, however, all cross‐over trials, had a duration longer than six weeks (Caroff 2007 (12 weeks first treatment phase, 30 weeks total duration); Gelenberg 1990 (eight weeks first treatment phase, 18 to 20 weeks total duration); Jackson 1978 (12 weeks first treatment phase, 32 weeks total duration); Tarsy 1977 (eight weeks first treatment phase, 16 weeks total duration)). All the other included studies were of short duration (nine days to six weeks).
4. Participants
Participants now total 364 people. Both sexes were recruited and the age range was wide, though most people were men in their 50s. Diagnoses included various chronic psychiatric disorders, but mainly schizophrenia. All had antipsychotic‐induced TD diagnosed using Schooler and Kane’s research diagnostic criteria, or by a clinical psychiatrist. The number of participants ranged from five to 60 (median 21).
5. Setting
Most trials were conducted in hospital. Only two studies specifically recruited outpatients (Gelenberg 1990; Tarsy 1977). The studies themselves were from around the world, with eight conducted in the USA (Beckham 1981; Caroff 2007; Gelenberg 1990; Jackson 1978; Jackson 1979; Ogunmefun 2009; Price 1982; Tarsy 1977), and one each in Japan (Yagi 1990), Canada (de Montigny 1979), Australia (George 1981), Iran (Jahanian 2014), Switzerland (Kocher 1980) and Germany (Lucius 1976).
6. Interventions
6.1 Cholinergic drugs
6.1.1 Deanol
Six studies (de Montigny 1979; George 1981; Jackson 1978; Kocher 1980; Lucius 1976; Tarsy 1977) used deanol in doses ranging from 600 mg/day to 2000 mg/day.
6.1.2 Lecithin
Four studies (Beckham 1981; Gelenberg 1990; Jackson 1979; Price 1982) used lecithin in doses ranging from 50 g/day to 60 g/day which contained 20 g/day to 35g/day phosphatidyl choline (a theoretical precursor of acetylcholine).
6.1.3 Meclofenoxate hydrochloride
Yagi 1990 applied meclofenoxate hydrochloride in a dose of 900 mg/day.
6.1.4 Galantamine
Caroff 2007 used galantamine in doses increasing to 12 mg twice per day over 12 weeks.
6.1.5 Rivastigmine
Jahanian 2014 used rivastigmine in a dose of 1.5 mg twice per day.
6.1.6 Donepezil
Ogunmefun 2009 used donepezil in doses ranging from 5 mg to 10 mg per day.
6.2 Comparison group
In all studies placebo was used as a comparison group, with no further details given. In one study a comparison between two doses of deanol was also made (George 1981). None of the included studies compared cholinergic drugs with another active intervention.
Participants remained on schizophrenia treatment antipsychotic medication during the trials.
7. Outcomes
7.1 General
Some outcomes were presented in graphs, with inexact P values of differences, or a statement of significant or non‐significant difference. This made it impossible to acquire raw data for synthesis. Some continuous outcomes could not be extracted due to missing number of participants or missing means, standard deviations, or standard errors. We have shown details of the scales that provided usable data below. We have provided reasons for exclusions of data under 'Outcomes' in the Characteristics of included studies table.
7.2 Scales used to measure TD symptoms
7.2.1 Abnormal Involuntary Movement Scale (AIMS)
The AIMS (Guy 1976a) is a 12‐item scale consisting of a standardised examination followed by questions rating the orofacial, extremity and trunk movements, as well as three global measurements. Each of these 10 items can be scored from zero (none) to four (severe). Two additional items assess the dental status. The AIMS ranges from zero to 40, with higher scores indicating greater severity.
7.2.2 Tardive Dyskinesia Rating Scale (TDRS)
The TDRS (Simpson 1979) is a 34‐item scale consisting of measurement of the movements around the orofacial region, neck, trunk and extremities. Each of these items can be scored from zero (absent) to five (severe). This scale ranges from 10 to 102, with higher scores indicating greater severity.
7.3 Scales used to measure global state
7.3.1 Clinical Global Impression
The CGI is a three‐item scale commonly used in studies on schizophrenia to enable clinicians to quantify severity of illness and overall clinical improvement (Guy 1976b). The items are: severity of illness; global improvement and efficacy index. A seven‐point scoring system is usually employed with low scores indicating decreased severity and/or greater recovery.
7.4 Mental state
Many trials recorded changes in general mental state, and many different ways were employed to rate these changes. No scores, however, were reported in sufficient detail (number of people, mean and SD) to be used in analysis. Only the outcome of 'deterioration of mental state' could be found and used.
7.5 Adverse effects
Possible worsening of acute extrapyramidal symptoms due to cholinergic medication was assessed in some trials, however, trialists did not report scores in detail. All trials assessed general adverse effects, but, again, we often found it impossible to extract useful data (see Characteristics of included studies table); in many cases it was reported that no events occurred. Eight trials assessed specific adverse events, such as gastric adverse events.
Excluded studies
There are 49 excluded studies (53 references). Thirty‐eight of these studies were not randomised and thus excluded. One RCT was excluded because participants were not on a stable dose of antipsychotics before the trial (Simpson 1977). One RCT provided no usable data (Marsalek 1997), and nor did another nine cross‐over RCTs that did not report outcome data for the first phase before crossing over to the next treatment (Chien 1978; Domino 1985; Gelenberg 1989; Joe 1985; Jus 1978; Lieberman 1988; Nasrallah 1986; Penovich 1978; Perez Cruet 1981). We contacted the authors of eight of these studies but received no more details on outcome data. We could not identify up‐to‐date contact details for authors of two of these studies published 35 to 20 years ago (Chien 1978; Marsalek 1997); they were also excluded as we assume it very unlikely to receive data so many years later.
Studies awaiting assessment
There are no studies awaiting assessment.
Ongoing studies
We know of no ongoing studies.
Risk of bias in included studies
Please refer to Figure 3 and Figure 4 for graphical overviews of the risk of bias in the included studies.
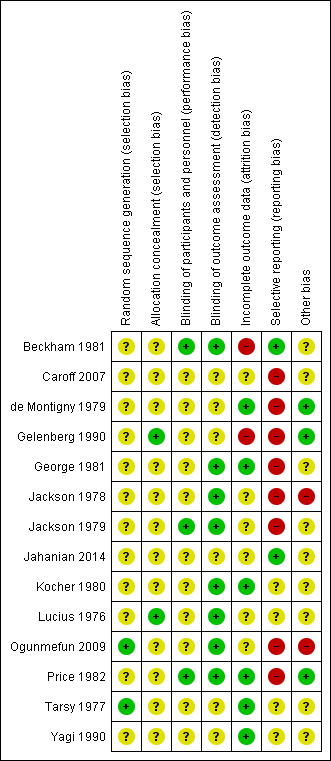
'Risk of bias, summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Ogunmefun 2009 and Tarsy 1977 reported using random number tables, though did not describe allocation concealment. Gelenberg 1990 and Lucius 1976 explicitly described an external allocation procedure. All other studies were not explicit about how the randomisation sequence was generated or how allocation was achieved other than using the word "randomized".
Blinding
Although all studies were conducted on a double‐blind basis, only three (Beckham 1981; Jackson 1979; Price 1982) explicitly described how this was undertaken and no study tested the blindness of raters, clinicians and trial participants. Eight trials (Beckham 1981; George 1981; Jackson 1978; Jackson 1979; Kocher 1980; Lucius 1976; Ogunmefun 2009; Price 1982) adequately described blinding of outcome assessors.
Incomplete outcome data
Two studies (Beckham 1981; Gelenberg 1990) had a drop‐out rate of greater than 30%. These studies were rated as having a high risk of attrition bias. In all cases, however, we tried to ensure that every person randomised was analysed.
Selective reporting
The majority of data in this review originates from published reports. Expected outcomes (impact on TD symptoms) were reported for most of the trials. Only Beckham 1981 and Jahanian 2014 were considered to have a low risk of reporting bias as they reported all outcomes that were stated in their published protocols (Beckham 1981 was a thesis which contained all detailed methods applied). All other studies did not fully report data for the stated outcome measures.
Other potential sources of bias
All studies had small or very small sample sizes (five to 60 participants per study). Eight of the 13 studies used a cross‐over design, and in most studies it was unclear whether other sources of bias may exist.
Effects of interventions
1. Comparison 1. Cholinergic drugs versus placebo
1.1 TD symptoms
We had chosen 'any improvement in TD symptoms of more than 50% on any TD scale ‐ any time period' as a primary outcome. Although the data we found in trials did not fit this exactly, we feel that the outcome 'not improved to a clinically important extent' fits best with what we had hoped to find.
1.1.1 Not improved to a clinically important extent
There was no significant difference for cholinergic drugs over placebo on clinically important improvement after two to 12 weeks treatment (very low‐quality evidence, 4 trials, 27 people, risk ratio (RR) 0.89, 95% confidence interval (CI) 0.65 to 1.23, I2 = 0%, Analysis 1.1).
1.1.2 Not any improvement
For the outcome of 'not any improvement in TD symptoms', we found no difference between cholinergic drugs and placebo after nine days to 12 weeks treatment (low‐quality evidence, 9 trials, 180 people, RR 0.87, 95% CI 0.71 to 1.07, I2=1%, Analysis 1.2). One study also provided self‐reported incidence of not any improvement in TD symptoms and found no difference between cholinergic drugs and placebo after nine to 11 days treatment (1 trial, 30 people, RR 0.92, 95% CI 0.62 to 1.36, Analysis 1.3).
1.1.3 Average endpoint scores
TD symptoms were also measured on the continuous AIMS and TDRS scales (see Description of studies above). No difference was found between cholinergic drugs and placebo on either average AIMS endpoint scores after two to 12 weeks treatment (low‐quality evidence, 7 studies, 171 people, mean difference (MD) ‐0.12, 95% CI ‐0.44 to 0.21, I2 = 44%, Analysis 1.4) or average TDRS endpoint scores after eight weeks treatment (5 participants, 1 study, not estimable, Analysis 1.5).
1.1.4 Deterioration of symptoms
There was also no difference between cholinergic drugs and placebo with regard to deterioration of TD symptoms after nine days to 12 weeks treatment (low‐quality evidence, 8 trials, 147 people, RR 1.11, 95% CI 0.55 to 2.24, I2=0%, Analysis 1.6). One study also provided self‐reported incidence of deterioration in TD symptoms and found no difference between cholinergic drugs and placebo after nine to 11 days treatment (1 trial, 30 people, RR 3.00, 95% CI 0.13 to 68.26, Analysis 1.7).
1.2 Global outcome measures
1.2.1 Death for any reason
No deaths were reported in any of the studies where this information could be extracted (11 trials, 278 people, Analysis 1.8). For cross‐over trials we counted only the first period before the cross‐over. However, in one cross‐over study (Tarsy 1977) one person died suddenly at home due to acute aspiration in the second cross‐over period, his placebo period. By this time he had completed eight weeks of deanol treatment and four weeks of placebo treatment.
1.2.2 Global outcomes
One study assessed global usefulness of meclofenoxate on the categorical Global Usefulness Rating. This study showed no difference between meclofenoxate and placebo after eight weeks treatment (60 people, RR 0.89, 95% CI 0.59 to 1.32, Analysis 1.9).
1.2.3 Global state
Another study assessed global state with the CGI and showed no difference between lecithin and placebo after 11 days treatment (31 people, RR ‐0.43, 95% CI ‐1.36 to 0.50, Analysis 1.10).
1.3 Mental state
1.3.1 Deterioration
There was no significant difference in deterioration of mental state between cholinergic drugs and placebo after 11 days to 12 weeks treatment (very low‐quality evidence, RR 0.50, 95% CI 0.10 to 2.61; 77 participants, 5 studies; I2 = 0%, Analysis 1.11).
1.4 Adverse effects
1.4.1 Any adverse effects
Although four studies (106 participants) reported on any adverse events, only one study reported that any events occurred, 3/31 in the meclofenoxate group compared with 5/29 in the placebo group after eight weeks treatment (very low‐quality evidence, RR 0.56, 95% CI 0.15 to 2.14; 60 participants; Analysis 1.12). The three studies reporting on donepezil or lecithin reported no events.
1.4.2 Various specific adverse effects
Out of eight studies (130 participants) that reported on various specific adverse events, only two studies reported that specific adverse events occurred. For deanol, one study reported that 4/10 participants had gastric adverse events versus 0/10 participants on placebo after three weeks treatment (RR 9.00, 95% CI 0.55 to 147.95; 5 studies, 61 participants; Analysis 1.13), and two studies reported that 8/32 participants had sedation, peripheral cholinergic effects, and undesirable body odour events, versus 0/22 participants on placebo after three to four weeks treatment (RR 6.83, 95% CI 0.99 to 47.25; 6 studies, 94 participants; I2 = 0%; Analysis 1.13). The four other studies reporting on deanol reported that no specific adverse events occurred, and two studies evaluating lecithin reported that no specific adverse events occurred (see Analysis 1.13).
1.5 Leaving the study early
Using cholinergic drugs did not significantly increase the risk of a person leaving the study early after nine days to 12 weeks treatment (very low‐quality evidence, 12 studies, 288 people, RR 1.09, 95% CI 0.56 to 2.10, I2 = 47%, Analysis 1.14).
We did not identify any studies that reported on hospital and service utilisation outcomes, economic outcomes, social confidence, social inclusion, social networks, personalised quality of life, behaviour, or cognitive state.
1.6 Subgroup analysis
1.6.1 Duration of treatment
We stratified analyses by duration. Any effects that cholinergic drugs may have had did not clearly change in relation to duration of treatment.
1.6.2 Cholinergic compound
We stratified analyses by cholinergic compound. Any effects that cholinergic drugs may have did not clearly change in relation to type of compound.
1.6.3 Older participants
It was not possible to evaluate whether participants aged > 65 years responded differently to younger participants, since no trial reported data for different age groups that could be extracted for separate analyses.
1.7 Heterogeneity
Data were mostly homogeneous. We detected statistical heterogeneity as described in Assessment of heterogeneity for two of the outcomes.
-
TD: average endpoint score on the AIMS (I2 = 44%, P = 0.10): seven studies reported on this outcome, but there is no clear outlier.
-
Three studies reported an effect estimate favouring cholinergic drug over placebo, but none of the studies reported statistically significant differences between groups (see Analysis 1.4): lecithin versus placebo after two weeks treatment (short term) (MD ‐1.07, 95% CI ‐2.21 to 0.07; 6 participants, 1 RCT), lecithin versus placebo after eight weeks treatment (medium term) (MD ‐0.10, 95% CI ‐1.04 to 0.84; 14 participants, 1 RCT), and meclofenoxate versus placebo after eight weeks treatment (medium term) (MD ‐0.19, 95% CI ‐0.58 to 0.20; 60 participants, 1 RCT).
-
Four studies reported an effect estimate favouring placebo over cholinergic drug, but again, none of the studies reported statistically significant differences between groups (see Analysis 1.4): rivastigmine versus placebo after eight weeks treatment (short term) (MD 2.20, 95% CI ‐1.16 to 5.56; 40 participants, 1 RCT), deanol versus placebo after 12 weeks treatment (medium term) (MD 1.42, 95% CI ‐0.29 to 3.13; 6 participants, 1 RCT), galantamine versus placebo after 12 weeks treatment (medium term) (MD 1.50, 95% CI ‐0.44 to 3.44; 35 participants, 1 RCT), donepezil versus placebo after four to six weeks treatment (short term) (MD 1.10, 95% CI ‐4.22 to 6.42; 10 participants, 1 RCT).
-
-
Acceptability of treatment: leaving the study early (I2 = 47%, P = 0.13): 12 studies reported on this outcome, but only four reported that any events occurred (= that any participants left the study early), and none of these found a statistically significant difference between groups. When removing from the analysis a study that from visual inspection of the graph appears to be an outlier, the I2 statistic goes down to 0% (analysis not shown). This study reported an effect estimate in the direction favouring placebo over galantamine after 12 weeks treatment (RR 3.00, 95% CI 0.96 to 9.39; 38 participants, 1 RCT, Analysis 1.14); the other studies reported effect estimates in the direction favouring lecithin or deanol over placebo after 11 days to eight weeks treatment (see Analysis 1.14).
1.8 Sensitivity analyses
1.8.1 Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. As all studies were stated to be randomised we have not undertaken this sensitivity analysis.
1.8.2 Assumptions for lost binary data
We would have undertaken a sensitivity analysis assessing where assumptions had to be made regarding people lost to follow‐up for the primary outcome (see Dealing with missing data). We intended to compare the findings when we used our assumption compared with completer data only. No assumptions had to be made, studies that reported data for the primary outcome reported for all randomised participants.
1.8.3 Risk of bias
When excluding three trials from the primary outcome that we judged to be at high risk of bias across one or more of the domains, there was no substantial alteration to the direction of effect or the precision of the effect estimates (RR 1.00, 95% CI 0.43 to 2.34; 1 study, 5 people, analysis not shown).
1.8.4 Imputed values
We would have undertaken a sensitivity analysis to assess the effects of including data from cluster‐randomised trials where we used imputed values for ICC in calculating the design effect. No cluster‐randomised trials were included.
1.8.5 Fixed and random effects
We also synthesised data for the primary outcome using a random‐effects model. This did not alter the significance of the results (RR 0.94, 95% CI 0.71 to 1.25; 4 studies, 27 people, analysis not shown).
2. Comparison 2. Cholinergic drugs versus other cholinergic drugs
Only one study (George 1981) was identified that reported on this comparison. When comparing a higher dose of deanol (2000 mg/day) to a lower dose (1000 mg/day) there was a slight improvement in TD scores for the group with the higher dose (1 trial, 22 people, RR 0.50, 95% CI 0.25 to 0.98, Analysis 2.1). No events were reported between the different doses for death or leaving the study early (Analysis 2.2; Analysis 2.3).
We did not identify any studies that reported on adverse events, mental state, hospital and service utilisation outcomes, economic outcomes, social confidence, social inclusion, social networks, personalised quality of life, behaviour, or cognitive state.
Discussion
Summary of main results
1. The search
This 2017 update has identified additional data from three studies (Caroff 2007; Jahanian 2014; Ogunmefun 2009) of new CNS‐active cholinergic agents mainly used for the treatment of Alzheimer's disease (donezepil, galantamine, and rivastigmine). It is probable, however, that the effect of these modern cholinergic agents for TD has not been comprehensively investigated within randomised trials. This update did not identify any new trials of the old cholinergic drugs (deanol, lecithin, meclofenoxate); trials of these drugs were published from 1976 to 1990.
2. Few data
Only a little over 350 people have been included in this review. It is possible that real, and important, effects have not been highlighted because of the necessarily wide CIs of the findings. Many outcomes were not measured at all (see Overall completeness and applicability of evidence), including one of our pre‐stated outcome measures. We may have been overambitious in hoping for some of these outcomes in TD trials, but simple reporting of quality of life still does not seem too demanding and does remain of interest.
3. Comparison 1. Cholinergic drugs versus placebo
3.1 TD symptoms
We are uncertain about the evidence on no clinically important improvement in TD symptoms comparing cholinergic drugs and placebo as the evidence was of very low quality (RR 0.89, 95% CI 0.65 to 1.23, 4 trials, 27 people). There may be little or no difference in deterioration of TD symptoms between cholinergic drugs and placebo, evidence was of low quality (RR 1.11, 95% CI 0.55 to 2.24, 8 trials, 147 people).
3.2 Mental state
We are uncertain about the evidence on deterioration of mental state comparing cholinergic drugs and placebo as the evidence was of very low quality (RR 0.50, 95% CI 0.10 to 2.61, 77 people, 5 trials).
3.3 Adverse events
We are uncertain about the evidence on adverse events comparing cholinergic drugs and placebo as the evidence was of very low quality (RR 0.56, 95% CI 0.15 to 2.14, 106 people, 4 trials).
3.4 Acceptability of treatment
It is always unclear what leaving the study early means. It could be to do with the participant not accepting treatment for a series of reasons, or of participants finding the trial intolerable. It also could be a function of a trial design in which willing participants are still asked to leave because of some degree of protocol violation. In any event, four studies reported that 14/53 people left the cholinergics group compared with a not significantly different 12/50 people in the placebo group. Eight studies reported that no adverse events occurred.
3.5 Social confidence, social inclusion, social networks, or personalised quality of life
This group of outcomes was selected as being of importance to patients for the 2017 review update following a service user consultation. None of the included studies reported on this outcome.
See summary of findings Table for the main comparison for a summary of the evidence.
4. Cholinergic drugs versus other cholinergic drugs
One study (George 1981) included study arms investigating two doses of deanol, 1 g and 2 g per dose, and found that twice as many participants experienced any improvement in TD symptoms with the higher dose (RR 0.50, 95% CI 0.25 to 0.98, 22 people). Very few participants were randomised, so the evidence is uncertain. Mental state, adverse events, or social confidence, social inclusion, social networks, or personalised quality of life were not reported. No events were reported for leaving the study early.
Overall completeness and applicability of evidence
1. Completeness
The most important finding of this review is that a systematic search of the literature still results in a review that is considerably underpowered to really investigate the clinical efficacy and safety of cholinergic agents in TD. All data are inconclusive. We were unable to detect any effect, good or bad of these drugs for TD. With the advent of new CNS‐active cholinergic agents for treatment of Alzheimer's disease and dementia (e.g., Birks 2006a; Birks 2006b; Birks 2015; Li 2015; Loy 2006; Maidment 2006; Rolinski 2012), the theoretical base for this review is strengthened. However, the actual central cholinergic transmission ‐enhancing effect of old cholinergic drugs, such as lecithin and especially deanol, remains unclear. Even if any of these old compounds had an effect on cholinergic transmission, the impact would probably be limited. Modern cholinergic drugs use a different mode of action and are worthy of investigation, although they are associated with gastrointestinal adverse events (Birks 2006a; Birks 2006b; Birks 2015; Li 2015; Loy 2006; Maidment 2006; Rolinski 2012). However, to date, these newer drugs have not been fully investigated in RCTs in the treatment of TD.
2. Applicability
All but two trials were hospital‐based but were nevertheless on people who would be recognisable in everyday care. Trials were set in high‐income countries in Asia, Australia, Europe and North America. Cholinergic drugs are readily accessible and most outcomes understandable in terms of clinical practice. Should cholinergic drugs have had important effects the findings may well have been applicable.
Quality of the evidence
Overall, the certainty of the evidence in this review is low to very low. This means that we have very little confidence in the effect estimates, and the true effects are likely to be substantially different from the estimates of the effect. The following are the main reasons for our low confidence in the evidence.
-
Poor study methodology and reporting of methods and data (see Figure 3 and Figure 4) resulting in downgrading evidence for risk of bias. Allocation concealment was not described, generation of the sequence was not explicit, studies were not clearly blinded, we are unsure if data are incomplete, and data were often poorly or selectively reported.
-
Very small sample sizes resulting in downgrading evidence for imprecision. The largest trial in this review randomised only 24 people. A trial of this size is unable to detect subtle, yet important differences due to cholinergic drugs with any confidence. In order to detect a 20% difference between groups, probably about 150 people are needed in each arm of the study (alpha 0.05, beta 0.8).
-
Wide CIs (often due to low event rates) that included appreciable benefit or harm for the intervention as well as no effect, resulting in downgrading evidence for imprecision.
Please see summary of findings Table for the main comparison for full details.
Potential biases in the review process
1. Missing studies
Every effort was made to identify relevant trials. However, these studies are all small and it is likely that we have failed to identify other studies of limited power. It is likely that such studies would also not be in favour of the cholinergics group. If they had been so, it is more likely that they would have been published in accessible literature. We do not, however, think it likely that we have failed to identify large relevant studies.
2. Introducing bias
We have tried to be balanced in our appraisal of the evidence but could have inadvertently introduced bias. We welcome comments or criticisms. New methods and innovations now make it possible to report data where, in the past, we could not report data at all or had to report data in a different way. We believe that 'Summary of findings' tables are a valuable innovation – but problematic to those not ‘blind’ to the outcome data. It is possible to ‘cherry pick’ significant findings for presentation in this table. We have tried to decrease the chance of doing this by asking a new review author (HB) to select outcomes relevant for this table before becoming familiar with the data.
Agreements and disagreements with other studies or reviews
The only other relevant quantitative reviews we know of is the previous Cochrane review (Tammenmaa 2002) and another systematic review that included the same RCTs as the Cochrane review (Tammenmaa 2004). These reviews found no evidence to support administration of the old cholinergic agents lecithin, deanol, and meclofenoxate to patients with TD, and concluded that further investigation of the clinical effects of novel cholinergic agents in TD is warranted. This update identified three new studies to include (as discussed in Results of the search), but does not substantially change these findings or conclusions.

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
Study flow diagram for 2015 and 2017 searches.

'Risk of bias, summary: review authors' judgements about each risk of bias item for each included study.
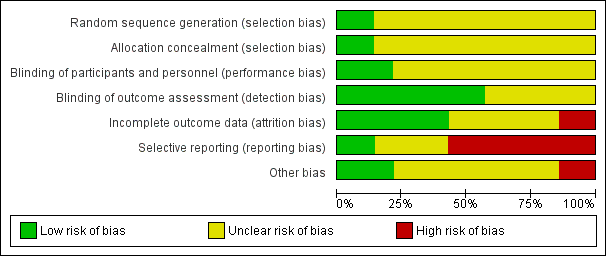
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
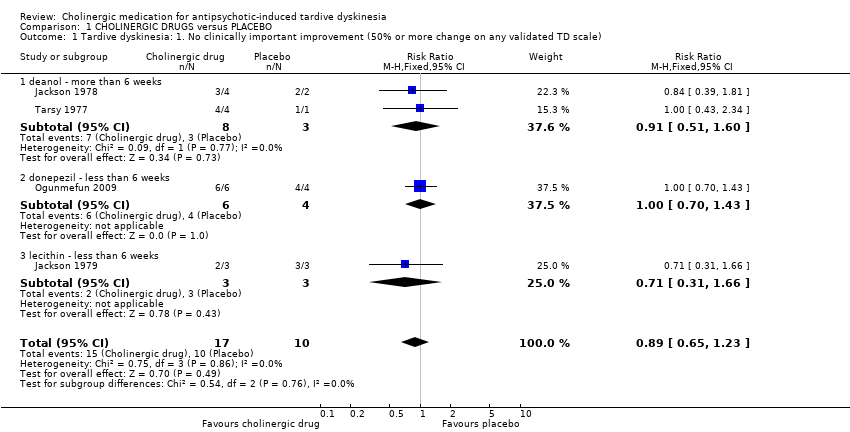
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater).
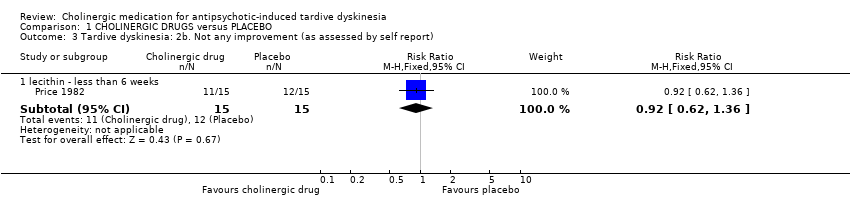
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better).
| Study | Intervention | Mean | SD | N | Comments |
| deanol ‐ more than 6 weeks | |||||
| Tarsy 1977 | Deanol | 10 | 5.48 | 4 | |
| Tarsy 1977 | Placebo | 10 | 0 | 1 | The confidence interval of mean difference was not estimable because the placebo group only had one participant. |
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater).
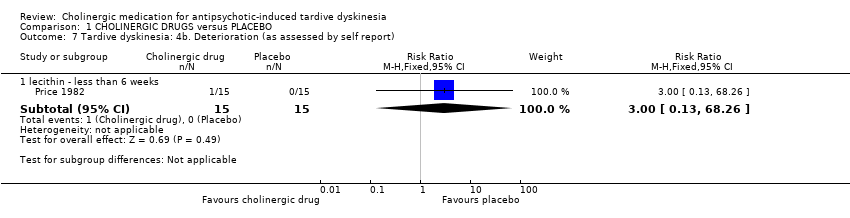
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 8 Global outcome: Death for any reason.
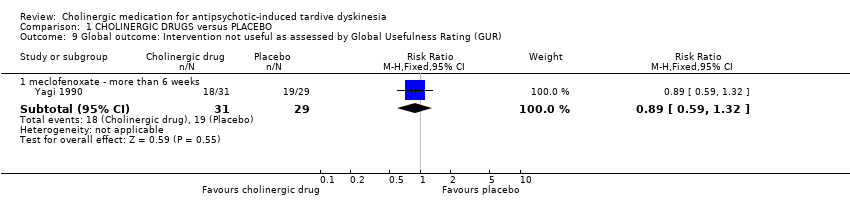
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR).
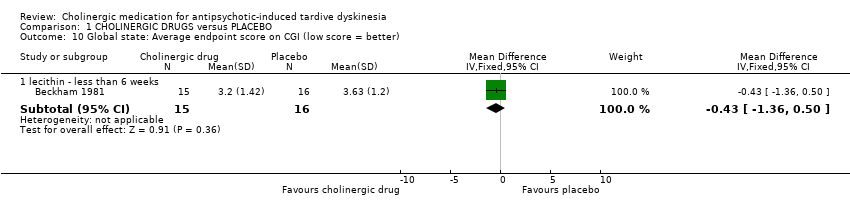
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 10 Global state: Average endpoint score on CGI (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 11 Mental state: Deterioration.
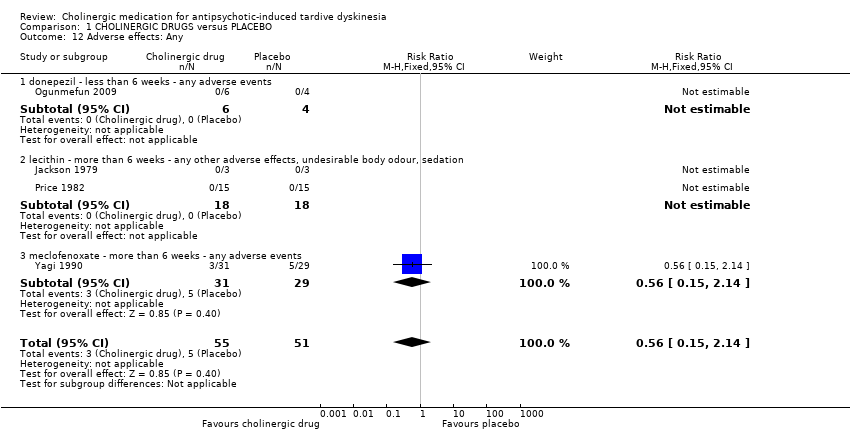
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 12 Adverse effects: Any.

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 13 Adverse effects: Various specific.
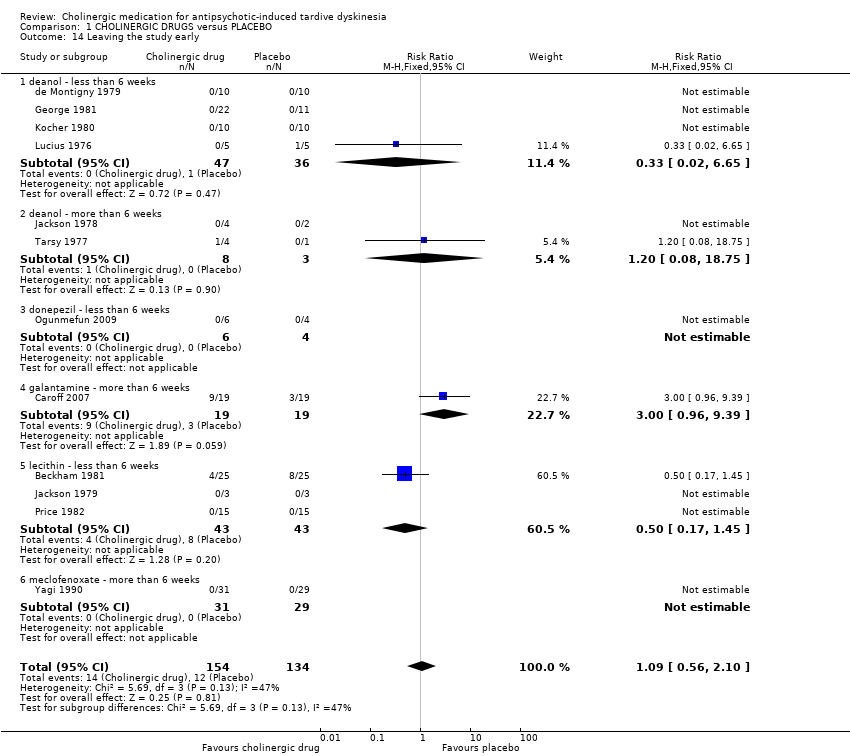
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 14 Leaving the study early.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 2 Global outcome: Death for any reason ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 3 Leaving the study early ‐ less than 6 weeks.
| Excluded study | Comparison | Treatment category | Relevant review | ||||
| #1 | #2 | ||||||
| Alpha‐methyl‐p‐tyrosine (AMPT) | versus choline chloride | Amino acid | Organic salt | ‐ | |||
| versus hydroxytryptophan | Amino acid (serotonin precursor) | ‐ | |||||
| versus valproic acid | Mood stabilisers | ‐ | |||||
| versus L‐DOPA | Amino acid | ‐ | |||||
| Benztropine versus bromocriptine | Anticholinergic | Dopamine agonist | ‐ | ||||
| Bromocriptine versus haloperidol | Dopamine agonist | Antipsychotic | ‐ | ||||
| Choline chloride | versus L‐DOPA | Organic salt | Amino acid | ‐ | |||
| versus hydroxytryptophan | ‐ | ||||||
| versus valproic | Anticonvulsant | ‐ | |||||
| Deanol | versus lithium carbonate | Antidepressant | Organic salt | ‐ | |||
| versus placebo | Placebo | ‐ | |||||
| versus sodium valproate | Anticonvulsant | ‐ | |||||
| versus oxpertine | Antipsychotic | ‐ | |||||
| Hydroxytryptophan versus L‐DOPA | Amino acid | Amino acid | ‐ | ||||
| L‐DOPA versus valproic acid | Anticonvulsant | ‐ | |||||
| Lithium carbonate versus placebo | Mood stabiliser | Placebo | ‐ | ||||
| Oxypertine versus sodium valproate | Antipsychotic | Anticonvulsant | ‐ | ||||
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | Specific cholinergic drug (N = 150) versus placebo (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| CHOLINERGIC DRUGS versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: people with various psychiatric disorders (mainly schizophrenia) and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | CHOLINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 to 12 weeks | 1000 per 1000 | 890 per 1000 | RR 0.89 | 27 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin) found a significant difference between cholinergic drug and placebo. |
| Tardive dyskinesia: Deterioration follow‐up: 9 days to 12 weeks | 116 per 1000 | 129 per 1000 | RR 1.11 | 147 | ⊕⊕⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Mental state: Deterioration follow‐up: 11 days to 12 weeks | 56 per 1000 | 28 per 1000 | RR 0.50 | 77 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, lecithin) found a significant difference between cholinergic drug and placebo. |
| Adverse effects: Any adverse events follow‐up: 9 days to 8 weeks | 98 per 1000 | 55 per 1000 | RR 0.56 | 106 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Acceptability of treatment: Leaving the study early follow‐up: 9 days to 12 weeks | 90 per 1000 | 98 per 1000 | RR 1.09 | 288 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, galantamine, meclofenoxate, lecithin) found a significant difference between cholinergic drug and placebo. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: for many studies it was unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | This review |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale) Show forest plot | 4 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] |
| 1.1 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.60] |
| 1.2 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| 1.3 lecithin ‐ less than 6 weeks | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.66] |
| 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) Show forest plot | 9 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| 2.1 deanol ‐ less than 6 weeks | 3 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 2.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.57] |
| 2.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.72, 2.44] |
| 2.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 2.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
| 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better) Show forest plot | 7 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.21] |
| 4.1 deanol ‐ more than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.29, 3.13] |
| 4.2 galantamine ‐ more than 6 weeks | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐0.44, 3.44] |
| 4.3 lecithin ‐ less than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐2.21, 0.07] |
| 4.4 lecithin ‐ more than 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.04, 0.84] |
| 4.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.58, 0.20] |
| 4.6 rivastigmine ‐ less than 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐1.16, 5.56] |
| 4.7 donepezil ‐ less than 6 weeks | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.22, 6.42] |
| 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better) Show forest plot | Other data | No numeric data | ||
| 5.1 deanol ‐ more than 6 weeks | Other data | No numeric data | ||
| 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater) Show forest plot | 8 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.24] |
| 6.1 deanol ‐ less than 6 weeks | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.48, 5.76] |
| 6.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.18] |
| 6.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.06, 7.85] |
| 6.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.31] |
| 6.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.18, 19.55] |
| 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 8 Global outcome: Death for any reason Show forest plot | 11 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.32] |
| 10 Global state: Average endpoint score on CGI (low score = better) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 lecithin ‐ less than 6 weeks | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.36, 0.50] |
| 11 Mental state: Deterioration Show forest plot | 5 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.61] |
| 11.1 deanol ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 11.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 11.3 lecithin ‐ less than 6 weeks | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 12 Adverse effects: Any Show forest plot | 4 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 12.1 donepezil ‐ less than 6 weeks ‐ any adverse events | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 lecithin ‐ more than 6 weeks ‐ any other adverse effects, undesirable body odour, sedation | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 meclofenoxate ‐ more than 6 weeks ‐ any adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 13 Adverse effects: Various specific Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 deanol ‐ less than 6 weeks ‐ gastric adverse effects | 5 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
| 13.2 deanol ‐ less than 6 weeks ‐ sedation, periferal cholinergic effects, undesirable body odour | 6 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.83 [0.99, 47.25] |
| 13.3 lecithin ‐ less than 6 weeks ‐ GI adverse effects | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Leaving the study early Show forest plot | 12 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.10] |
| 14.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 14.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 14.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.96, 9.39] |
| 14.5 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.45] |
| 14.6 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: Death for any reason ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Leaving the study early ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

