Benzodiacepinas para la discinesia tardía inducida por antipsicóticos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000205.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 20 enero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
HB: update of review, 2015 and 2017 searches: study selection, data extraction and assimilation, summary of findings, report writing.
PSB: update of review 2006: searching, data extraction, data assimilation, final report writing.
KSW: data extraction and assimilation for the first three versions of the review.
Sources of support
Internal sources
-
Enhance Reviews Ltd, UK.
Logistics support for Hanna Bergman for the 2016 update.
External sources
-
Cochrane Schizophrenia Group, Leeds, UK.
-
NIHR HTA Project Grant, reference number: 14/27/02, UK.
Salary support for Hanna Bergman.
Support for patient involvement consultation.
Support for traceable data database.
Declarations of interest
HB worked for Enhance Reviews Ltd. during preparation of this review and was paid for her contribution to this review. Enhance Reviews Ltd. was a private company that performs systematic reviews of literature. HB works for Cochrane Response, an evidence consultancy linked to Cochrane that take commissions from healthcare guideline developers and policy makers.
PSB: none known.
KSW is the Deputy Editor‐in‐Chief for Cochrane and Cochrane Innovations. When the National Institute for Health Research Health Technology Assessment (NIHR HTA) programme grant relevant to this review update was awarded, KSW was the Managing Director of Enhance Reviews Ltd.
Acknowledgements
John McGrath (Queensland, Australia) was author for this review from 1996 to 2002. He supported the first version and it would not exist today without his painstaking attention to detail. During the period of John's contribution, he was a member of the following advisory boards: Janssen‐Cilag Australia, Eli Lilly Australia, and Lundbeck Australia. In addition, John had been a co‐investigator on studies of antipsychotic medications produced by the following companies: Astra, Janssen‐Cilag, Eli Lilly, Zeneca (ICI), Sandoz, and Pfizer. The same companies had provided travel and accommodation expenses for John to attend relevant investigator meetings and scientific symposia. No funds have been paid directly to John. Payments related to participation in drug trials and board attendances have been paid to a Government‐audited trust account to support schizophrenia research.
Paul Walker (previously known as Paul Umbrich) was the author of the review update in 2002. In the update, he searched the citations, extracted and assimilated data, and wrote the final report.
The authors wish to thank Clive Adams, Tessa Grant, and Gill Rizzello for their support. Thanks also to Winson Wong, Sai Zhao, and Jun Xia for helping to translate Chinese articles; to Ben Gray for writing the plain language summary; and to Farhad Sokraneh for carrying out the trial search. We are also grateful to Dawn‐Marie Walker, Ruth Sayers, Megan Lees, and Vanessa Pinfold from McPin Foundation for organising and holding the public and patient involvement consultation with TD service users that contributed to selecting outcomes for the 'Summary of findings' tables and to guide future research. Finally, we wish to thank Rosie Asher and Antonio Grande for screening literature and helping with data extraction for the 2016 update, and Nicholas Henschke and Loukia Spineli for assisting with updating the report.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jan 20 | Benzodiazepines for antipsychotic‐induced tardive dyskinesia | Review | Hanna Bergman, Paranthaman S Bhoopathi, Karla Soares‐Weiser | |
| 2006 Jul 19 | Benzodiazepines for neuroleptic‐induced tardive dyskinesia | Review | Paranthaman Sethupathi Bhoopathi, Karla Soares‐Weiser | |
| 2003 Apr 22 | Benzodiazepines for neuroleptic‐induced tardive dyskinesia | Review | Paul Walker, K VS Soares, Karla Soares‐Weiser | |
Differences between protocol and review
The protocol as published with this review has evolved over time. The revisions of protocol are in line with the development of Review Manager and in keeping with Cochrane guidance. We think the revisions have greatly improved and enhanced this review. We do not think, however, that it has materially affected our conduct of the review or interpretation of the results.
There was a substantial update to the protocol in the 2017 search update with main changes being:
-
change of the title from 'Benzodiazepines for neuroleptic‐induced tardive dyskinesia;'
-
broaden the inclusion criteria by adding the comparison: 'Benzodiazepines compared with any other intervention for the treatment of tardive dyskinesia;'
-
updated list of outcomes following consultation with consumers; and
-
addition of the 'Summary of findings' tables.
The previous methods are reproduced in Appendix 1.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti‐Anxiety Agents [*therapeutic use];
- Antipsychotic Agents [adverse effects];
- Benzodiazepines [*therapeutic use];
- Clonazepam [therapeutic use];
- Dyskinesia, Drug‐Induced [*drug therapy, etiology];
- GABA Modulators [*therapeutic use];
- Phenobarbital [therapeutic use];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO

Message from one of the participants of the public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
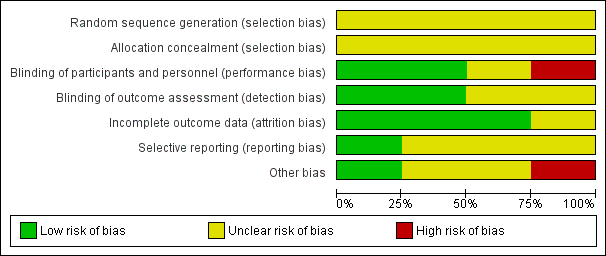
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.

Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale).

Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 2 TD symptoms: not any improvement.
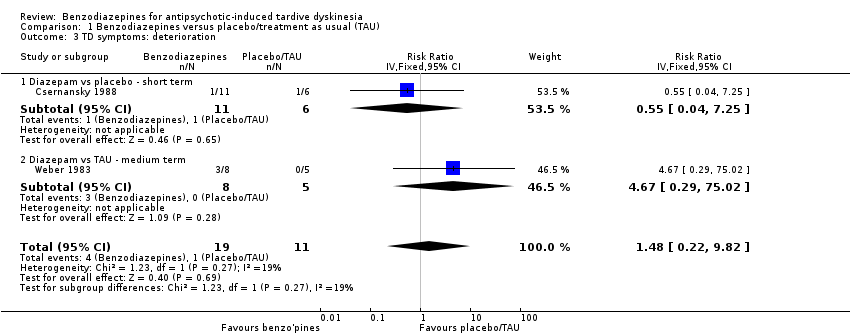
Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 3 TD symptoms: deterioration.
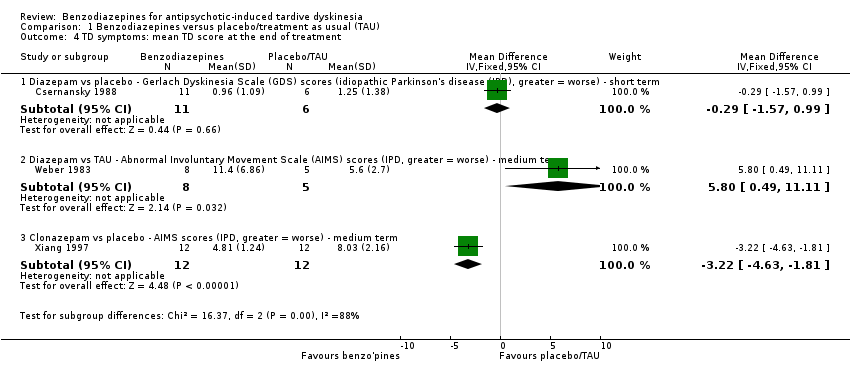
Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 4 TD symptoms: mean TD score at the end of treatment.
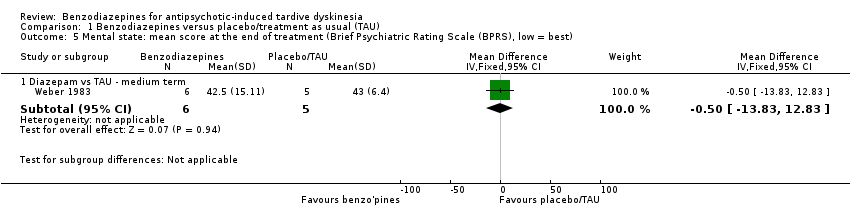
Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 5 Mental state: mean score at the end of treatment (Brief Psychiatric Rating Scale (BPRS), low = best).

Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 6 Leaving the study early.

Comparison 2 Benzodiazepines vs other compounds, Outcome 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) ‐ short term.
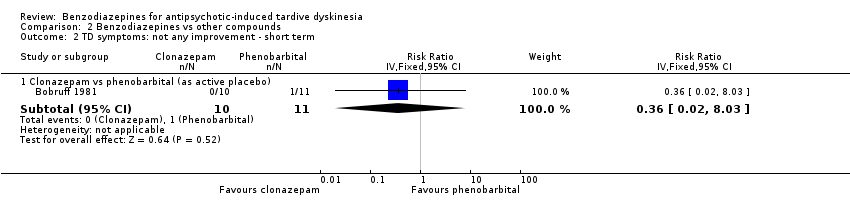
Comparison 2 Benzodiazepines vs other compounds, Outcome 2 TD symptoms: not any improvement ‐ short term.
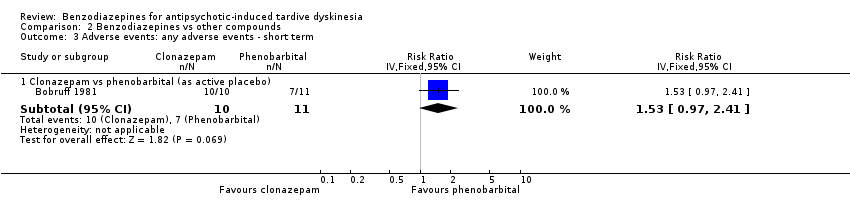
Comparison 2 Benzodiazepines vs other compounds, Outcome 3 Adverse events: any adverse events ‐ short term.

Comparison 2 Benzodiazepines vs other compounds, Outcome 4 Leaving the study early ‐ short term.
| Study tag | Participants | Comparison | Review |
| Antipsychotic‐induced akathisia | Clonazepam vs placebo | ||
| Benztropine vs propranolol | Anticholinergics for neuroleptic‐induced acute akathisia; Central action beta‐blockers versus placebo for neuroleptic‐induced acute akathisia. | ||
| Benzodiazepines vs artane (trihexyphenidyl hydrochloride). | Benzodiazepines for neuroleptic‐induced acute akathisia; Anticholinergics for neuroleptic‐induced acute akathisia. | ||
| Antipsychotic‐induced tardive dyskinesia | Naltrexone vs placebo | Miscellaneous treatments for neuroleptic‐induced tardive dyskinesia. | |
| Naltrexone + clonazepam vs clonazepam + placebo |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: serious mental illness treated by antipsychotic drugs for a protracted period. Tardive dyskinesia.a |
| Interventions | 1. Clonazepam 6‐12 mg oral daily dose. |
| Outcomes | Tardive dyskinesia: any clinically important improvement in tardive dyskinesia, any improvement, deterioration.b |
| aThis could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. bPrimary outcome. The same applies to the measure of primary outcome as for diagnosis. Not everyone may need to have operational criteria applied if clinical impression is proved to be accurate. | |
| n: number of participants. | |
| Benzodiazepines compared with placebo for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with benzodiazepines | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 1.12 | 32 | ⊕⊝⊝⊝ | ‐ | |
| 545 per 1000 | 611 per 1000 | |||||
| Tardive dyskinesia: deterioration in symptoms | Study population | RR 1.48 | 30 | ⊕⊝⊝⊝ | ‐ | |
| 91 per 1000 | 135 per 1000 | |||||
| Adverse effect: any adverse event | None of the included studies reported on these outcomes. | |||||
| Adverse effect: no clinically significant extrapyramidal adverse effects | ||||||
| Acceptability of the treatment (measured by participants leaving the study early) | Study population | RR 2.73 | 56 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: none of the studies adequately described randomisation procedure or allocation concealment, one study did not blind participants and personnel, and one study was a post hoc subgroup analysis of participants with tardive dyskinesia. 2Downgraded two levels for imprecision: small sample size, and 95% CI of effect estimate includes both appreciable benefit and appreciable harm for benzodiazepines. | ||||||
| Benzodiazepines compared with phenobarbital (as active placebo) for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with phenobarbital (active placebo) | Risk with benzodiazepines | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.44 | 21 | ⊕⊝⊝⊝ | ‐ | |
| 909 per 1000 | 400 per 1000 | |||||
| Tardive dyskinesia: deterioration in symptoms ‐ not measured | The included study did not report on this outcome. | |||||
| Adverse events: any | Study population | RR 1.53 | 21 | ⊕⊝⊝⊝ | ‐ | |
| 636 per 1000 | 974 per 1000 | |||||
| Adverse effect: extrapyramidal symptoms ‐ not reported | The included study did not report on this outcome. | |||||
| Acceptability of the treatment (measured by participants leaving the study early) | Study population | Not estimable | 21 | ⊕⊝⊝⊝ | No events were reported; no one left the study early. | |
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not measured | The included study did not report on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure, allocation concealment, or blinding. 2Downgraded two levels for imprecision: only one study with a very small sample size. | ||||||
| Interventions | Reference |
| Anticholinergic medication | Soares‐Weiser 1997; Soares‐Weiser 2000; 2016 update to be published. |
| Benzodiazepines | This review. |
| Calcium channel blockers | Essali 2011; 2016 update to be published. |
| Cholinergic medication | Tammenmaa 2002; 2016 update to be published. |
| Gamma‐aminobutyric acid agonists | Alabed 2011; 2016 update to be published. |
| Miscellaneous treatments | Soares‐Weiser 2003; 2016 update to be published. |
| Neuroleptic reduction or cessation (or both) and neuroleptics | Soares‐Weiser 2006; 2016 update to be published. |
| Non‐neuroleptic catecholaminergic drugs | El‐Sayeh 2006; 2016 update to be published. |
| Vitamin E | Soares‐Weiser 2011; 2016 update to be published. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) Show forest plot | 2 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.60, 2.09] |
| 1.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.23] |
| 1.2 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.71, 3.16] |
| 2 TD symptoms: not any improvement Show forest plot | 2 | 32 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [0.33, 6.74] |
| 2.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.04, 7.25] |
| 2.2 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 2.5 [0.39, 16.05] |
| 3 TD symptoms: deterioration Show forest plot | 2 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.48 [0.22, 9.82] |
| 3.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.04, 7.25] |
| 3.2 Diazepam vs TAU ‐ medium term | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 4.67 [0.29, 75.02] |
| 4 TD symptoms: mean TD score at the end of treatment Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Diazepam vs placebo ‐ Gerlach Dyskinesia Scale (GDS) scores (idiopathic Parkinson's disease (IPD), greater = worse) ‐ short term | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.57, 0.99] |
| 4.2 Diazepam vs TAU ‐ Abnormal Involuntary Movement Scale (AIMS) scores (IPD, greater = worse) ‐ medium term | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [0.49, 11.11] |
| 4.3 Clonazepam vs placebo ‐ AIMS scores (IPD, greater = worse) ‐ medium term | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.22 [‐4.63, ‐1.81] |
| 5 Mental state: mean score at the end of treatment (Brief Psychiatric Rating Scale (BPRS), low = best) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Diazepam vs TAU ‐ medium term | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐13.83, 12.83] |
| 6 Leaving the study early Show forest plot | 3 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.15, 48.04] |
| 6.1 Clonazepam vs placebo ‐ medium term | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.15, 48.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 0.44 [0.20, 0.96] |
| 2 TD symptoms: not any improvement ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.02, 8.03] |
| 3 Adverse events: any adverse events ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.97, 2.41] |
| 4 Leaving the study early ‐ short term Show forest plot | 1 | Risk Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Difference (IV, Fixed, 95% CI) | 0.0 [‐0.17, 0.17] |


