Versión cefálica externa para la presentación podálida a término
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single centre, parallel‐group randomised controlled trial. 2‐arm trial with individual randomisation. | |
| Participants | Setting: Mardan Medical Complex, Pakistan. July 2010 to 31st Dec 2011. Inclusion criteria: singleton fetus in a breech presentation confirmed by ultrasound, between 33 weeks and 35 weeks' gestation. N = 123 women. Exclusion criteria: women with contraindication to ECV; contraindications to early ECV or contraindications to labour or vaginal birth (e.g. fetal heart rate abnormalities, vaginal bleeding, rupture of membranes, placental abruption, fetal growth restriction, previous caesarean section, low amniotic fluid index, fetal weight > 4 kg) or woman unwilling to undergo ECV. | |
| Interventions | Early ECV: ECV carried out between 34 (238 days) and 35 weeks of gestation. No tocolytics were used. Women were monitored for 3 hours before and 1 hour after the procedure. Up to 2‐3 attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities. N = 63. Control: ECV carried out at or after 37 weeks. No tocolytics were used. Women were monitored for 3 hours before and 1 hour after the procedure. Up to 2‐3 attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities. N = 60. | |
| Outcomes | Reported number of attempts at ECV, reasons for discontinuing ECV, maternal and fetal complications, presentation at delivery, mode of delivery. | |
| Notes | It was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject". There was no further description of methods used. We contacted the author for more information but have not yet had a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that this was the same as methods used in published multicentre randomised controlled trials. |
| Allocation concealment (selection bias) | Unclear risk | Not described. "Patients were randomly divided into two groups". |
| Blinding of participants and personnel (performance bias) | High risk | No blinding mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | All women appeared to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published report. We requested a copy of the protocol but this was not made available to us. |
| Other bias | Unclear risk | It was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject". There was no further description of methods used. We contacted the author for more information but have not yet had a response. |
| Methods | An international multicentre randomised controlled trial with randomisation stratified by parity using a centralised telephone randomisation system. Breech verified within 4 days of randomisation, and confirmed prior to ECV attempt. | |
| Participants | All nulliparous women with any breech presentation and multiparous women with a frank breech presentation were eligible for the trial if they had a live singleton fetus and a gestational age of between 34 weeks, 0 days and 36 weeks 0 days. Women were excluded if they had a parity > 4, if they planned to move to a non‐trial centre, or if there was any contraindication to labour or vaginal birth (such as placenta previa, or previous classical caesarean section), to ECV (such as fetal heart rate abnormalities, abruptio placenta, fetal anomalies, uterine anomalies, oligohydramnios, rupture of membranes, over distended uterus) or to early ECV (such as fetus engaged in the pelvis, an increased risk of preterm labour, increased risk of abruptio placenta). | |
| Interventions | ECV was begun between 34 weeks 0 days and 36 weeks 0 days in the early group (n = 117); and between 37 weeks 0 days and 38 weeks 0 days in the delayed group (n = 116). Tocolysis recommended either routinely or selectively in both groups; analgesia permitted. | |
| Outcomes | Primary: presentation at delivery. | |
| Notes | n = 233. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification by parity, random block sizes. External randomisation service. |
| Allocation concealment (selection bias) | Low risk | External telephone randomisation service. |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Interim analysis was carried out by blinded assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Analysis by intention‐to‐treat. 233 women randomised, outcome data available for 132 women and babies. |
| Selective reporting (reporting bias) | Low risk | Protocol provided by the authors. Outcome reporting bias not apparent. |
| Other bias | Low risk | Other bias not apparent. |
| Methods | 2‐arm, unblinded, multicentre, parallel‐group randomised controlled trial. Stratification for parity and centre. Individual randomisation. | |
| Participants | Setting: 68 centres in 21 countries. Hospital setting, with clinicians who were experienced in ECV and birth facilities that were deemed to meet Canadian standards. 1543 women randomised. Inclusion criteria: women with singleton fetus in a breech presentation who had a recent screening ultrasound, between 33+0/7 weeks' and 35+6/7 weeks' gestation. Exclusion criteria: women with contraindications to ECV (e.g. fetal heart rate abnormalities, placental abruption, major life‐threatening fetal anomalies, uterine anomalies, hyper‐extended fetal head, rupture of fetal membranes, severe oligohydramnios or hydramnios); contraindications to early ECV (e.g. increased risk of preterm labour or placental abruption); or contraindications to labour or vaginal birth (e.g. placenta praevia, previous classical caesarean section); or if they had been prior participants in the trial; were at increased risk of unstable lie (such as grand multiparity); or if they planned to give birth by caesarean section even if the fetus turned to a cephalic position, or if they planned a vaginal birth if the fetus remained breech. | |
| Interventions | Early ECV: (n = 767) ECV carried out between 34+0/7 and 35+6/7 weeks of gestation, and within 7 days of randomisation. Fetal presentation was confirmed by ultrasound immediately before the ECV procedure. Fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus later reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman. Delayed ECV: (n = 774) ECV carried out at or after 37+0/7. Fetal presentation was confirmed by ultrasound immediately before the ECV procedure. Fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus later reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman. (Overall, tocolytics were used during all ECV attempts in 68% of cases.) | |
| Outcomes | Primary: rate of caesarean section. Secondary: rate of preterm birth (< 37 weeks), non‐cephalic presentation at birth, admission to NICU for more than 24 hours, serious neonatal morbidity or death, maternal morbidity or death, pain and maternal satisfaction. | |
| Notes | 1 of the review authors was an investigator on this trial. Data extraction and assessment of risk of bias were carried out by 2 independent review authors. This study was funded by Canadian institutes of Health Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with a computerised randomisation program, using computer‐generated random block sizes and 1:1 allocation. |
| Allocation concealment (selection bias) | Low risk | Central randomisation by telephone. |
| Blinding of participants and personnel (performance bias) | High risk | “The nature of the intervention did not lend itself to blinding of either participants or clinicians.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | Some efforts were made to avoid detection bias. Blinding of initial assessment and recording of outcomes is not described. An independent Data Safety and Monitoring Board “reviewed all stillbirths and neonatal deaths, blinded to allocation group, for the existence of any anomaly considered incompatible with life and to make a determination regarding exclusion of any women from the analysis of perinatal/neonatal outcomes”. An interim analysis of results was also carried out by the independent Data Safety and Monitoring Board, blinded to group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | 2 women, 1 in each group, asked to be removed from the study. 8 women were lost to follow‐up (2 assigned to early ECV, 6 to delayed ECV). This left 1533 women (99.4%), so although the losses to follow‐up were unequal the numbers were small in the context of the whole study. A small amount of missing data (2 early ECV, 1 delayed ECV) accounted for in table 5. An intention‐to‐treat analysis was conducted. Perinatal and neonatal deaths were excluded from the analyses of measures of neonatal morbidity. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes appear to have been reported, including those showing no differences between groups. Multiple reports available for this study including trial registration. |
| Other bias | Low risk | Baseline characteristics were similar in the 2 groups. No other bias apparent. |
| Methods | Allocation at 32 weeks' gestation by randomised sealed envelopes, stratified by parity. Breech verified by ultrasound. | |
| Participants | Singleton breech presentation before term (from 32 weeks). Exclusion criteria: contraindication to external version. | |
| Interventions | External cephalic version attempt without tocolysis (n = 50) compared with no ECV attempt (n = 52). ECV was attempted by an assistant in training. If failed, a further attempt was made by an obstetrician 1 week later. | |
| Outcomes | Non‐cephalic births; caesarean section; 1 minute Apgar score < 7; Umbilical vein pH < 7.2; neurological deficit in newborn; perinatal mortality. The perinatal death was due to placental abruption. | |
| Notes | Groningen, The Netherlands. The authors ascribe the low success rate to the gentleness with which external cephalic version was attempted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocations were concealed in sealed envelopes. It was not clear whether all envelopes were used in sequential order and that all were accounted for. |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not possible to blind the intervention. It was not clear whether outcomes were recorded by blinded assessors. |
| Incomplete outcome data (attrition bias) | Low risk | 102 women were randomised. All appear to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from original paper, translated notes and correspondence. It was not clear whether all outcomes were fully reported. |
| Other bias | Unclear risk | The description of study methods was very brief. Other bias was not apparent. |
| Methods | Random allocation of women using sealed envelopes, stratified by parity. | |
| Participants | Healthy white Dutch women with uncomplicated pregnancy of 33‐40 weeks' gestation and a live singleton breech fetus attending antenatal clinic of Ikazia Hospital, Rotterdam, The Netherlands. | |
| Interventions | Repeated ECV performed between 33 and 40 weeks' gestation with no tocolysis, analgesia or anaesthesia compared to no ECV. | |
| Outcomes | Presentation at delivery; mode of delivery; neonatal outcome. | |
| Notes | n = 180. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Randomisation stratified by parity. |
| Allocation concealment (selection bias) | Unclear risk | "by means of drawing a sealed envelope." Othe information not provided. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was no mention of whether or not outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 180 women were randomised. 1 was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published report. |
| Other bias | Unclear risk | Little information on study methods was provided. Other bias was not apparent. |
ECV: external cephalic version
NICU: neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Method to group allocation is described as "dividing the cases up into two groups". It is highly unlikely that randomisation was used and there is risk of enrolment bias. This study is of historical interest in that it is an early example of a controlled trial. | |
| The methods of randomisation were not described for this study. Participants recruited included women between 36 to 38 weeks' gestation, some of the women were ≥ 37 weeks' gestation; separate data were not reported for women before term. | |
| It was not clear that this was a randomised controlled trial and participants recruited to the study were women with singleton pregnancies with breech presentation at term (≥ 37 weeks). | |
| Method to group allocation is described as being dependant on the day that women attended at antenatal clinic (on Monday and Wednesday ECV was performed, whereas on Tuesday and Thursday it was not), and there is significant risk of enrolment bias. It is also unclear in this study how the breech pregnancies were confirmed. In addition, 25% of the study population were grand‐multiparous women who are at increased risk of unstable lie, and are at an increased risk of encountering complications. | |
| In this trial women were recruited from 36 up to 386/7 weeks. Most included women were at term and separate data were not reported for women less than 37 weeks' gestation. |
ECV: external cephalic version
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Early external cephalic version in antenatal care. A randomized trial. |
| Methods | |
| Participants | Women with breech presentation at 31 weeks' pregnancy. |
| Interventions | ECV for breech presentation versus no ECV. |
| Outcomes | Caesarean section; length of postpartum stay. |
| Starting date | June 1989. |
| Contact information | Belizan JM. Centro Rosarino de Estudios Perinatales, Bv OroNo 500, 2000 Rosario, Argentina. |
| Notes | It is not clear whether or not this trial was completed. |
ECV: external cephalic version
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| Analysis 1.1  Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth. | ||||
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| Analysis 1.2  Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth). | ||||
| 3 Caesarean section Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.57, 5.84] |
| Analysis 1.3  Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 3 Caesarean section. | ||||
| 4 Vaginal breech birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.52] |
| Analysis 1.4  Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 4 Vaginal breech birth. | ||||
| 5 Apgar score < 7 at 1 minute Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.59] |
| Analysis 1.5  Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 5 Apgar score < 7 at 1 minute. | ||||
| 6 Perinatal mortality Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| Analysis 1.6 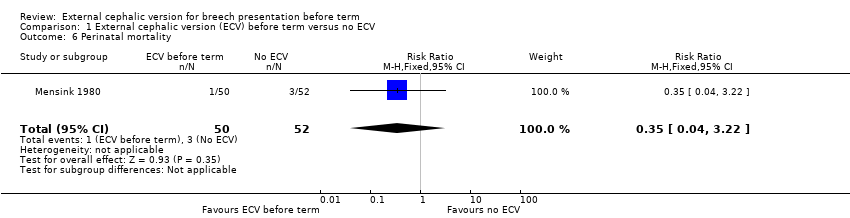 Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 6 Perinatal mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| Analysis 2.1  Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth. | ||||
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| Analysis 2.2 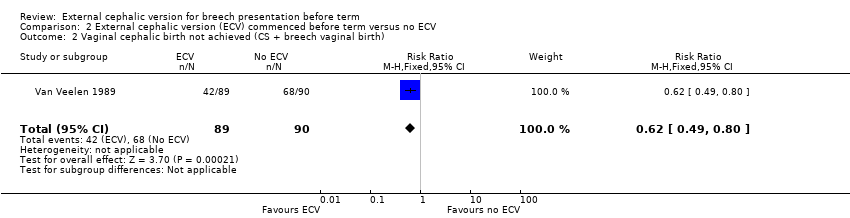 Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth). | ||||
| 3 Caesarean section Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.43] |
| Analysis 2.3 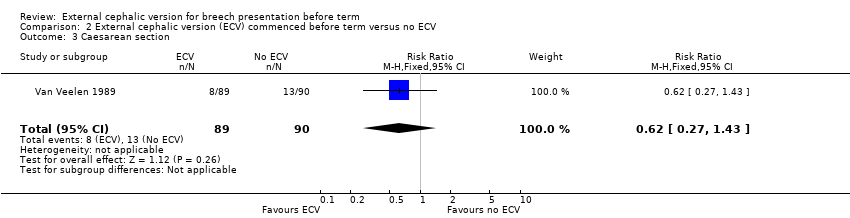 Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 3 Caesarean section. | ||||
| 4 Vaginal breech birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.85] |
| Analysis 2.4  Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 4 Vaginal breech birth. | ||||
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.48] |
| Analysis 2.5  Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 5 Apgar score < 7 at 5 minutes. | ||||
| 6 Stillbirth and neonatal mortality < 7 days Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| Analysis 2.6 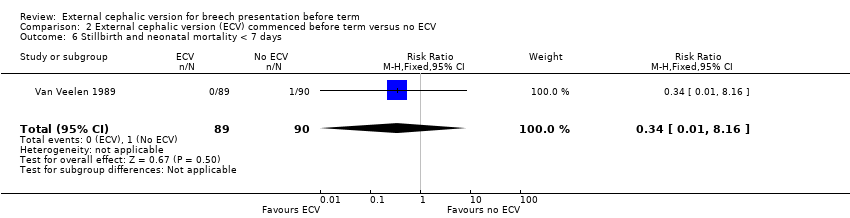 Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 6 Stillbirth and neonatal mortality < 7 days. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| Analysis 3.1  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 1 Non‐cephalic presentation at the birth. | ||||
| 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth) Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| Analysis 3.2  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth). | ||||
| 3 Caesarean section Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| Analysis 3.3 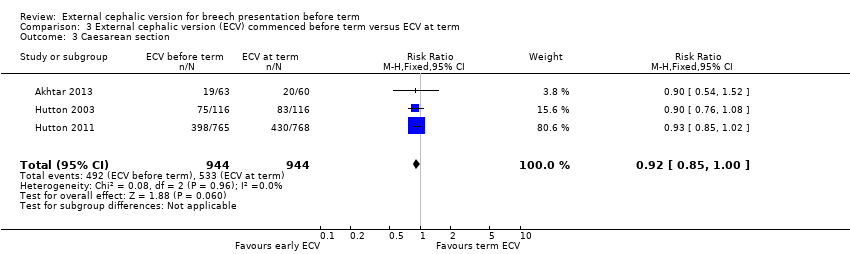 Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 3 Caesarean section. | ||||
| 4 Vaginal breech birth Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.78] |
| Analysis 3.4  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 4 Vaginal breech birth. | ||||
| 5 Apgar score < 7 at 5 minutes Show forest plot | 2 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.44] |
| Analysis 3.5  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 5 Apgar score < 7 at 5 minutes. | ||||
| 6 Stillbirth or neonatal mortality < 7 days Show forest plot | 3 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.34] |
| Analysis 3.6  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 6 Stillbirth or neonatal mortality < 7 days. | ||||
| 7 Preterm birth < 37 weeks Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.21] |
| Analysis 3.7 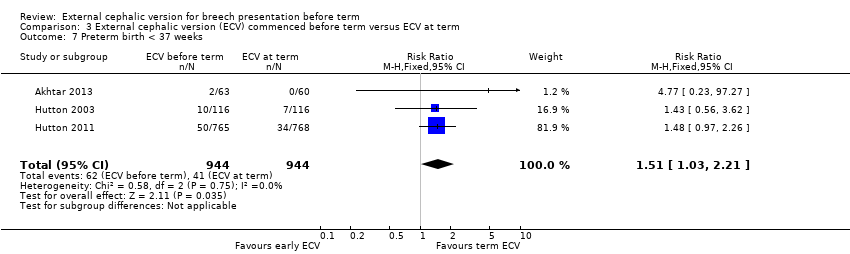 Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 7 Preterm birth < 37 weeks. | ||||
| 8 One or more serious fetal complications following randomisation Show forest plot | 2 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| Analysis 3.8  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 8 One or more serious fetal complications following randomisation. | ||||
| 9 NICU stay 4 days or longer Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.63] |
| Analysis 3.9  Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 9 NICU stay 4 days or longer. | ||||
| 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain) Show forest plot | 1 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |
| Analysis 3.10 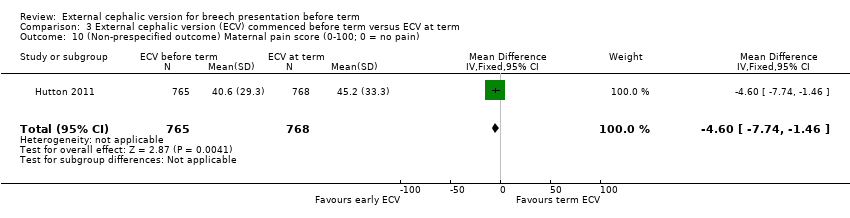 Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain). | ||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 3 Caesarean section.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 4 Vaginal breech birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 5 Apgar score < 7 at 1 minute.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 6 Perinatal mortality.
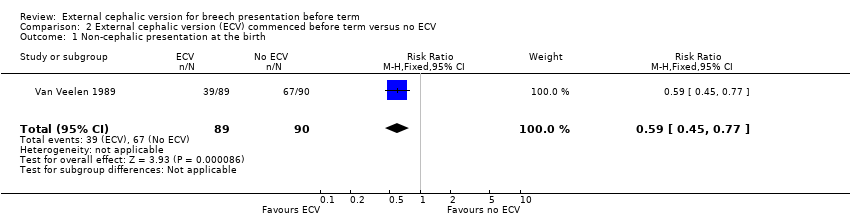
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.
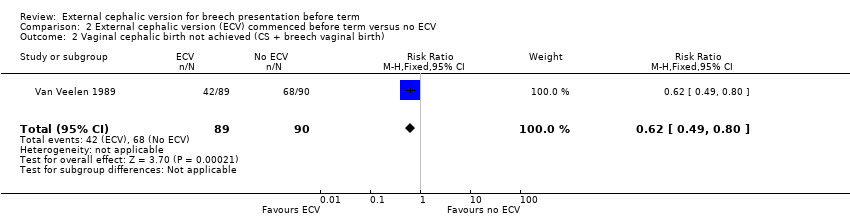
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 3 Caesarean section.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 4 Vaginal breech birth.
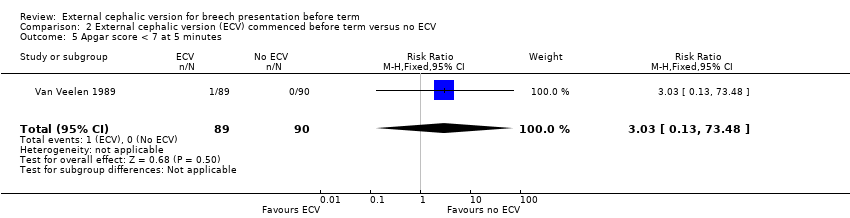
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 6 Stillbirth and neonatal mortality < 7 days.
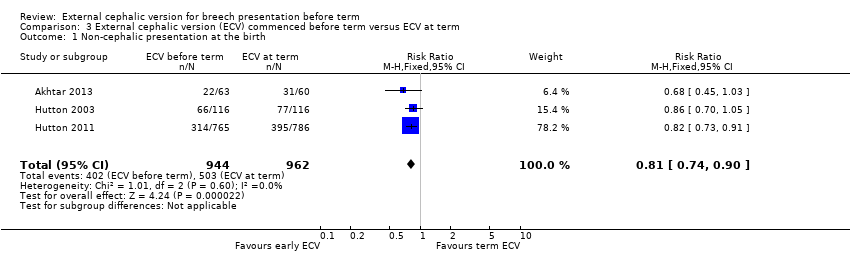
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth).

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 3 Caesarean section.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 4 Vaginal breech birth.
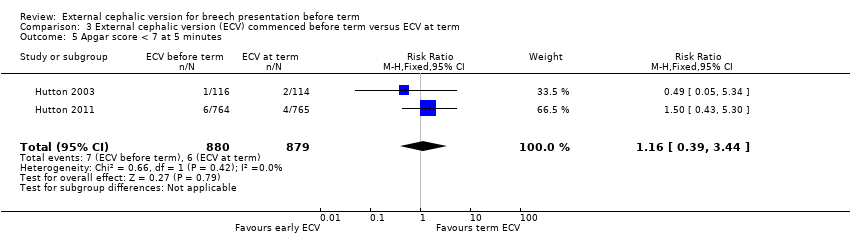
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 6 Stillbirth or neonatal mortality < 7 days.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 7 Preterm birth < 37 weeks.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 8 One or more serious fetal complications following randomisation.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 9 NICU stay 4 days or longer.
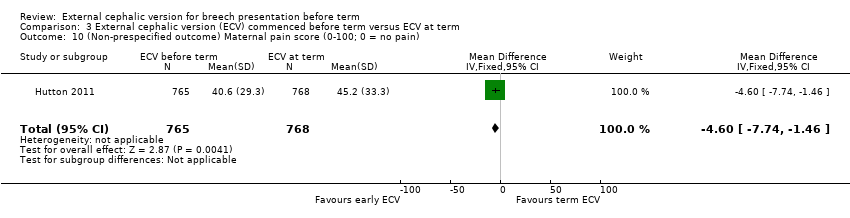
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain).
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 | 1906 | ⊕⊕⊕⊕ | ||
| 523 per 1000 | 424 per 1000 | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 | 1888 | ⊕⊕⊕⊕ | ||
| 600 per 1000 | 540 per 1000 | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 | |||||
| Caesarean section | Study population | RR 0.92 | 1888 | ⊕⊕⊕⊕ | ||
| 565 per 1000 | 519 per 1000 | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 | |||||
| Vaginal breech birth | Study population | RR 0.44 | 1888 | ⊕⊕⊕⊕ | ||
| 35 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 | 1759 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 | 1887 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 | 1888 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide 95% CI crossing the line of no effect and low event rate. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| 3 Caesarean section Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.57, 5.84] |
| 4 Vaginal breech birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.52] |
| 5 Apgar score < 7 at 1 minute Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.59] |
| 6 Perinatal mortality Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| 3 Caesarean section Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.43] |
| 4 Vaginal breech birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.85] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.48] |
| 6 Stillbirth and neonatal mortality < 7 days Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth) Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3 Caesarean section Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 4 Vaginal breech birth Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.78] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 2 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.44] |
| 6 Stillbirth or neonatal mortality < 7 days Show forest plot | 3 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.34] |
| 7 Preterm birth < 37 weeks Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.21] |
| 8 One or more serious fetal complications following randomisation Show forest plot | 2 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| 9 NICU stay 4 days or longer Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.63] |
| 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain) Show forest plot | 1 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |

