Versión cefálica externa para la presentación podálida a término
Resumen
Antecedentes
La versión cefálica externa (VCE) del feto de nalgas a término (después de las 37 semanas) ha mostrado ser efectiva para reducir el número de presentaciones de nalgas y cesáreas, pero las tasas de éxito son relativamente bajas. Esta revisión examina los estudios que inician la VCE antes del término (antes de las 37 semanas de gestación).
Objetivos
Evaluar la efectividad de una política de inicio de la VCE antes del término (antes de las 37 semanas de gestación) para la presentación de nalgas sobre la presentación fetal al parto, el método del parto y la tasa de parto prematuro, morbilidad perinatal, mortinatalidad o mortalidad neonatal.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (31 de marzo de 2015) y en las listas de referencias de los estudios recuperados.
Criterios de selección
Ensayos controlados aleatorizados (ECA) de VCE intentada antes del término (37 semanas de gestación) o iniciada antes del término, comparada con un grupo control de pacientes (con presentación de nalgas) en las que no se intentó la VCE o la VCE se intentó a término. Se consideraron elegibles para inclusión los ensayos aleatorizados grupales, pero no se identificaron. No fueron elegibles para inclusión los ensayos clínicos cuasialeatorizados ni los estudios que utilizaron un diseño cruzado.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, evaluaron los ensayos para inclusión y el riesgo de sesgo, extrajeron los datos y verificaron su exactitud. Los estudios se evaluaron con respecto al riesgo de sesgo y la calidad general de la evidencia para los resultados importantes se evaluó mediante los criterios GRADE.
Resultados principales
Se incluyen cinco estudios (2187 pacientes). No fue posible cegar la intervención y no está claro qué repercusión tendría la falta de cegamiento sobre los resultados informados. Para otros dominios del "Riesgo de sesgo", los estudios tuvieron riesgo bajo o incierto de sesgo.
Un estudio informó sobre la VCE que se realizó y completó antes de las 37 semanas de gestación, en comparación con ninguna VCE. No se encontraron diferencias en la tasa de presentación no cefálica al parto (riesgo relativo [RR] 1,04; intervalo de confianza [IC] del 95%: 0,64 a 1,69; participantes = 102). Un estudio informó sobre una política de VCE que se inició antes del término (33 semanas) y hasta las 40 semanas de gestación y que se pudo repetir hasta el parto, en comparación con ninguna VCE. Este estudio mostró una disminución en la tasa de presentación no cefálica al parto (RR 0,59; IC del 95%: 0,45 a 0,77; participantes = 179).
Tres estudios informaron sobre la VCE iniciada entre las 34 y 35 semanas de gestación, en comparación con el inicio entre las 37 y 38 semanas de gestación. Los resultados agrupados indicaron que la VCE temprana redujo el riesgo de presentación no cefálica al parto (RR 0,81; IC del 95%: 0,74 a 0,90; participantes = 1906; estudios = tres; I² = 0%; evidencia considerada de alta calidad), fracaso para lograr el parto cefálico vaginal (RR 0,90; IC del 95%: 0,83 a 0,97; participantes = 1888; estudios = tres; I² = 0%, evidencia considerada de alta calidad) y parto vaginal en presentación podálica (RR 0,44; IC del 95%: 0,25 a 0,78; participantes = 1888; estudios = tres; I² = 0%, evidencia considerada de alta calidad). La diferencia entre los grupos en el riesgo de cesárea no fue estadísticamente significativa (RR 0,92; IC del 95%: 0,85 a 1,00; participantes = 1888; estudios = tres; I² = 0%; evidencia considerada de alta calidad). Hubo evidencia de que el riesgo de trabajo de parto prematuro aumentó con la VCE temprana en comparación con la VCE después de las 37 semanas (6,6% en el grupo de VCE y 4,3% en los controles) (RR 1,51; IC del 95%: 1,03 a 2,21; participantes = 1888; estudios = tres; I² = 0%; evidencia considerada de alta calidad). No hubo diferencias claras entre los grupos en la puntuación de Apgar baja del lactante a los cinco minutos o la muerte perinatal (mortinatalidad más mortalidad neonatal hasta los siete días) (evidencia considerada de baja calidad para ambos resultados).
Conclusiones de los autores
En comparación con ningún intento de VCE, la VCE iniciada antes del término reduce la presentación no cefálica al parto. En comparación con la VCE a término, la VCE iniciada entre las 34 y 35 semanas puede tener algún efecto beneficioso en cuanto a la disminución de la tasa de presentación no cefálica y el riesgo de parto vaginal de nalgas. Sin embargo, la VCE temprana puede aumentar el riesgo de parto prematuro tardío, y es importante que cualquier estudios de investigación futuro informe sobre los resultados de morbilidad infantil. Los resultados de la revisión indican que se necesita una discusión cuidadosa con las pacientes acerca del momento del procedimiento de VCE para que puedan tomar decisiones informadas.
PICOs
Resumen en términos sencillos
Versión cefálica externa para la presentación podálida a término
Los fetos en los que las nalgas aparecen primero (en posición podálica) pueden tener más problemas durante el parto que los que nacen de cabeza (en posición cefálica) porque puede haber algún retraso en el parto de la cabeza y presión sobre el cordón umbilical cuando la cabeza pasa a través del canal de parto. Durante una versión cefálica externa (VCE), el feto que viene de nalgas es girado hacia abajo a la posición de cabeza presionando suavemente sobre el abdomen de la madre. Los estudios de investigación muestran que la VCE después de las 37 semanas reduce el número de fetos en posición de nalgas a término, así como el número de cesáreas.
Esta revisión incluyó cinco estudios controlados aleatorizados con 2187 mujeres; los estudios tuvieron riesgo de sesgo bajo o incierto, aunque no fue posible "cegar" a las pacientes y al personal a esta intervención. Los resultados mostraron que si la VCE se realiza alrededor de la mitad del tercer trimestre (32 a 34 semanas), aumentan las probabilidades de que el feto esté con la cabeza hacia abajo al término. Tres ensayos que incluyeron 1888 pacientes encontraron que al iniciar la VCE entre las semanas 34 y 36, en comparación con el inicio de la VCE después de las 37 semanas (a término), hubo una disminución del 19% en la tasa de presentación no cefálica al parto, una reducción del 10% en el riesgo de no lograr un parto vaginal cefálico y una reducción considerable en la probabilidad de un parto vaginal de nalgas; sin embargo, la VCE temprana puede aumentar significativamente las probabilidades de un parto prematuro tardío. Por lo tanto, la calidad de la evidencia para estos resultados se consideró baja. La evidencia sobre las posibles ventajas y desventajas de la versión cefálica externa (VCE) temprana (antes de las 37 semanas) requerirá de una discusión cuidadosa con las pacientes sobre el momento del procedimiento de la VCE para que puedan tomar decisiones informadas.
Conclusiones de los autores
Summary of findings
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 | 1906 | ⊕⊕⊕⊕ | ||
| 523 per 1000 | 424 per 1000 | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 | 1888 | ⊕⊕⊕⊕ | ||
| 600 per 1000 | 540 per 1000 | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 | |||||
| Caesarean section | Study population | RR 0.92 | 1888 | ⊕⊕⊕⊕ | ||
| 565 per 1000 | 519 per 1000 | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 | |||||
| Vaginal breech birth | Study population | RR 0.44 | 1888 | ⊕⊕⊕⊕ | ||
| 35 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 | 1759 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 | 1887 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 | 1888 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide 95% CI crossing the line of no effect and low event rate. | ||||||
Antecedentes
Descripción de la afección
Al final del embarazo, la mayoría de los fetos de embarazos únicos se colocan con la cabeza hacia abajo listos para el parto; los fetos que están en presentación podálica (las nalgas primero) pueden tener un mayor riesgo durante el parto vaginal, ya que puede haber retraso en el parto de la cabeza y compresión del cordón umbilical a medida que la cabeza pasa a través de la pelvis ósea. Aproximadamente entre el 3% y el 4% de todas las embarazadas que llegan a término tendrán un feto en presentación podálica y el parto en podálica se asocia con un mayor riesgo para el neonato, independientemente de la forma del parto (Schutte 1985).
La presentación podálica puede ser causada por una anomalía fetal o materna subyacente, o puede ser un suceso aparentemente al azar, o estar relacionada con una variante benigna en otros aspectos, como la ubicación de la placenta en un cuerno uterino. En estos últimos casos, la presentación podálica hace que un feto y una madre saludables estén en una situación de mayor riesgo de parto vaginal complicado o de operación cesárea. No es sorprendente que, a través de los años, la posibilidad de manipular al feto para cambiar la presentación podálica a cefálica haya despertado el interés de los obstetras.
Descripción de la intervención
Durante una versión cefálica externa (VCE), el feto que viene de nalgas es girado hacia abajo a la posición de cabeza presionando suavemente sobre el abdomen de la madre. La versión cefálica externa (VCE) antes del término se introdujo en la práctica obstétrica habitual debido a su evidente efectividad inmediata, así como por los resultados tranquilizadores de varios ensayos no aleatorizados, y a pesar de los resultados negativos del único ensayo con asignación al azar publicado antes de 1980 (Brosset 1956). La popularidad de la VCE antes del término comenzó a declinar después de mediados de los años 70, debido en parte a trabajos que informaron un aumento significativo de la mortalidad perinatal asociada con el procedimiento (Bradley‐Watson 1975) y a la consideración creciente de la cesárea como una opción más segura que la VCE para el parto en presentación podálica.
De qué manera podría funcionar la intervención
Para el feto único en presentación podálica, se ha demostrado que la cesárea es más segura que el parto vaginal (Hofmeyr 2003). Aunque muchas pacientes preferirían un parto vaginal (Gamble 2000; Geary 1997; Hildingsson 2002; Turnbull 1999), la mayoría optaría por la cesárea si hubiera una indicación médica, lo que daría lugar a que la mayoría de los fetos en presentación podálica nacieran por cesárea. Los riesgos asociados con la cesárea son bajos, pero la cesárea no está exenta de riesgos para la madre, y en los países desarrollados aún es el factor principal que contribuye a la incidencia de mortalidad y morbilidad maternas después del parto (Liu 2007; Minkoff 2003). Las estimaciones de la incidencia de mortalidad asociada con la cesárea electiva casi se triplicaron en comparación con el parto vaginal (Cooper 2002; Hall 1999), y se ha demostrado que la morbilidad materna grave se ha quintuplicado (Liu 2007). Entre los fetos en presentación podálica, una revisión Cochrane de cesárea programada versus parto vaginal programado para el embarazo con presentación podálica a término informó que, aunque el 45% de las embarazadas del grupo de parto programado dieron a luz por cesárea, la cesárea programada se asoció con un aumento de la morbilidad materna (cociente de riesgos 1,29; intervalo de confianza del 95%: 1,03 a 1,61) (Hofmeyr 2003). Además del aumento de la morbilidad inmediata después de la cesárea, también se pueden producir adherencias intraabdominales, lo que provoca infertilidad posterior (LaSala 1987). La presencia de la cicatriz uterina hace que los embarazos futuros tengan un mayor riesgo de complicaciones como embarazo ectópico, placenta previa, acreta y abruptio placentae (desprendimiento de la placenta), así como de rotura uterina (Dashe 2002; Gilliam 2002; Lydon‐Rochelle 2001; Minkoff 2003). Otro factor desalentador de la cesárea es que el procedimiento requiere de la experiencia de un obstetra u otro médico con formación quirúrgica y limita la función de los profesionales sanitarios para la atención obstétrica de bajo riesgo, como las parteras y los médicos de familia
Una revisión de las estrategias para reducir las tasas de cesárea identificó la versión cefálica externa (VCE) como la única intervención clínica con evidencia de Nivel 1 demostrada para reducir las tasas de cesárea primaria en general (Walker 2002). La VCE realizada a término ha mostrado ser efectiva para disminuir de forma moderada la tasa de presentación no cefálica al parto y evitar la cesárea (Hofmeyr 2015).
Se ha planteado la hipótesis de que, en comparación con la espera hasta el término, comenzar el procedimiento de VCE un poco más temprano en el embarazo, antes de que el feto se fije en la pelvis y mientras haya niveles máximos de líquido amniótico presente, puede disminuir de manera adicional la tasa de presentación no cefálica al parto y favorecer el parto vaginal en cefálica (Hutton 2011b).
Por qué es importante realizar esta revisión
Antes de mediados de los años 70, la versión cefálica externa (VCE) se intentaba habitualmente antes del término, debido a la creencia de que el procedimiento pocas veces sería exitoso a término. Estudios posteriores mostraron que, con la administración de tocolisis, la VCE se podía realizar en una cantidad importante de embarazadas con presentación podálica a término (Cluver 2015). La VCE a término difiere en varios aspectos fundamentales de la VCE realizada antes del término. Estas diferencias incluyen el hecho de que el feto está maduro y se puede extraer más fácilmente en caso de complicaciones, y que la versión espontánea sin intentar la VCE, o la reversión después de una VCE exitosa, son menos frecuentes a término. Una revisión Cochrane de la VCE a término (comenzada a las 37 semanas) informó un aumento de la probabilidad de que el feto esté en cefálica en el momento del parto, así como una reducción de las cesáreas (Hofmeyr 2015). Por lo tanto, la VCE se ha recomendado para todas las embarazadas con un feto en presentación podálica a término, cuando no hay contraindicaciones. Sin embargo, el procedimiento a menudo no es exitoso, particularmente en los contextos de América del Norte y Europa, (Hofmeyr 2015; Hutton 1999), y un estudio que comparó los resultados cuando la VCE se inició antes (34 a 35 semanas de gestación) con la VCE a término (después de 37 semanas de gestación), informó una disminución clínicamente importante en la proporción de pacientes con presentación no cefálica al parto (Hutton 2011b).
Los lectores deben consultar las revisiones previas del tema (Hofmeyr 1989, Hofmeyr 1991, Hofmeyr 1992, Hofmeyr 1993). Ver también las revisiones Cochrane relacionadas: "Versión cefálica externa para la presentación podálica a término" (Hofmeyr 2015); "Intervenciones para ayudar a rotar al feto a término de presentación podálica a cefálica mediante versión cefálica externa" (Cluver 2015); y "Versión cefálica mediante tratamiento postural para la presentación podálica" (Hofmeyr 2012).
Objetivos
Evaluar la efectividad de una política de inicio de la versión cefálica externa (VCE) antes del término para la presentación podálica sobre la presentación fetal al parto y el método del parto, así como la tasa de parto prematuro, la morbilidad perinatal, los mortinatos o la mortalidad neonatal, con el uso de la mejor evidencia disponible.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos clínicos aleatorizados que compararan los efectos de la versión cefálica externa (VCE) antes del término o iniciada antes del término con un grupo control (ningún intento de VCE o VCE a término). Se consideraron elegibles para inclusión los ensayos aleatorizados grupales, pero no se identificaron. No fueron elegibles para inclusión los ensayos clínicos cuasialeatorizados ni los estudios que utilizaron un diseño cruzado. Se planificó incluir los estudios informados como resumen, siempre que proporcionaran información suficiente que permitiera evaluar el riesgo de sesgo.
Tipos de participantes
Embarazadas con un feto único vivo en presentación podálica antes del término.
Tipos de intervenciones
Intento de versión cefálica externa antes del término (37 semanas de gestación) o iniciado antes del término, en comparación con ningún intento de VCE o VCE a término. Las comparaciones se dividen en las tres categorías siguientes.
-
VCE antes del término comparada con ninguna VCE.
-
Una política de iniciar la VCE antes del término, pero continuar si es necesario hasta el término, en comparación con ninguna VCE.
-
Una política de iniciar la VCE antes del término comparada con una política de iniciar la VCE después de las 37 semanas.
Los estudios que reclutaron pacientes antes y después del término serían elegibles para inclusión en la comparación uno, siempre que los resultados se informaran por separado para las embarazadas en el grupo antes del término.
Tipos de medida de resultado
Los resultados se incluyeron si se determinó que eran clínicamente significativos, los datos estuvieron disponibles para el análisis según la asignación original, independientemente de las violaciones del protocolo, y si los datos estuvieron disponibles en un formato adecuado para el análisis. Como parte de la evaluación del riesgo de sesgo, se evaluó si se habían tomado medidas razonables para minimizar el sesgo de observador, y se confirmó que los datos faltantes no fueron suficientes para influir materialmente en las conclusiones.
Resultados primarios
-
Tasa de presentación no cefálica al nacer
-
No se ha logrado el parto vaginal en cefálica (cesárea más parto vaginal en presentación podálica)
-
Método del parto (cesárea, parto vaginal en presentación podálica, parto vaginal en presentación cefálica)
Resultados secundarios
-
Parto prematuro
-
Resultados perinatales, que incluyen morbilidad grave (definida por el autor del ensayo), mortinato, mortalidad neonatal y mortalidad perinatal
-
Puntuación de Apgar del neonato < 7 a los cinco minutos
Métodos de búsqueda para la identificación de los estudios
Búsquedas electrónicas
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 March 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional searching carried out in the previous version of this review (Hutton 2006), see: Appendix 1.]
Búsqueda de otros recursos
We manually searched the reference lists of all retrieved articles and contacted expert in this research field.
We did not apply any language or date restrictions.
Obtención y análisis de los datos
For methods used in the previous version of this review, see Hutton 2006.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search (this section of the review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group).
Selección de los estudios
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Extracción y manejo de los datos
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses.
For this update the quality of the evidence has been assessed using the GRADE approach (Schunemann 2009) for the comparison ECV commenced before term versus ECV at term. This comparison was considered to be the most clinically relevant, as external cephalic version at term has been demonstrated to be effective in reducing the chance of non‐cephalic presentation at birth and caesarean section and should be regarded as the standard of care (Hofmeyr 2015). Comparisons of early ECV with no ECV are now mainly of historical interest. The quality of the evidence was assessed for the following outcomes.
-
Rate of non‐cephalic presentation at birth.
-
Vaginal cephalic birth not achieved (caesarean section plus vaginal breech delivery).
-
Caesarean birth.
-
Breech vaginal birth.
-
Preterm birth.
-
Perinatal mortality (stillbirth plus neonatal death up to seven days).
-
Apgar score less than seven at five minutes.
GRADE profiler (GRADEpro 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Medidas del efecto del tratamiento
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Cuestiones relativas a la unidad de análisis
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials if they were otherwise eligible. In this version of the review no such trials were identified. If cluster trials are eligible for future updates we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials have not been included.
Studies with multiple treatment arms
In this version of the review we have not included any trials with more than two treatment arms; if such trials are included in updates we will use the methods described in the Handbook to analyse findings.
Manejo de los datos faltantes
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Evaluación de la heterogeneidad
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Evaluación de los sesgos de notificación
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Síntesis de los datos
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. If we use random‐effects analyses in updates, the random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Análisis de subgrupos e investigación de la heterogeneidad
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses. We planned subgroup analysis for:
-
nulliparous versus multiparous women (as nulliparity is a well established to be associated with decreased likelihood of success of ECV);
-
type of breech (frank breech, where the fetus has hips flexed and legs extended making the ECV more difficult versus non‐frank);
-
use of tocolytics versus no tocolytics, (as tocolytics have been shown to increase the likelihood of success in ECV at term, and variation in use may explain heterogeneity between trials);
-
gestational age at randomisation ( 33 weeks 0 days to 34 weeks 6 days; and 35 weeks 0 days to 36 weeks 6 days).
We planned subgroup analysis for primary outcomes only. In this version of the review the study samples however were insufficient to make this analysis meaningful. We will carry out planned subgroup analysis if more data become available in future updates. We will assess subgroup differences by interaction tests available in RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Análisis de sensibilidad
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this would make any difference to the overall result. In this version of the review too few studies were included to allow these additional analyses.
Results
Description of studies
Results of the search
In the previous published version of this review, three studies were included (Hutton 2003; Mensink 1980; Van Veelen 1989). In this update, two further studies were identified and were assessed as eligible for inclusion (Akhtar 2013; Hutton 2011). SeeCharacteristics of included studies.
In this version of the review, altogether, five studies were excluded (Brosset 1956; Dafallah 2004; El‐Muzaini 2008; Kasule 1985; Rust 2005) and one is still ongoing (Belizan 1989).Two controlled trials which had been included in an earlier version of this review were subsequently excluded for concerns relating to methodological soundness (Brosset 1956; Kasule 1985). Neither of these trials used random assignment to treatment groups. The Brosset 1956 study states that "cases were divided into two groups" while in the Kasule 1985 trial women were "allocated to a version or non‐version group depending on the day they attended antenatal clinic".
Included studies
Comparison one: ECV before term (from 32 weeks with one repeat attempt) compared with no ECV
Mensink 1980 included women in early third trimester (as early as 32 weeks' gestation) in a randomised controlled trial undertaken in Gronigen, The Netherlands. Allocation was undertaken using randomised sealed envelopes, stratified by parity. Breech was verified by ultrasound. Women with a singleton breech presentation before term (from 32 weeks) were included. Women with any contraindication to external version were excluded. External cephalic version (ECV) was attempted without tocolysis by an assistant in training (n = 50) compared with no ECV attempt (n = 52). If the ECV failed, a further attempt was made by an obstetrician one week later. Outcomes included: non‐cephalic births; caesarean section; one minute Apgar score less than seven; umbilical vein pH less than 7.2; neurological deficit in newborn; and perinatal mortality; neonatal morbidity at the time of delivery was reported, but this was not defined.
Comparison two: ECV commencing before term (33 to 40 weeks with repeated attempts) compared with no ECV
Van Veelen 1989 enrolled 180 healthy white Dutch women with uncomplicated pregnancy at 33 to 40 weeks' gestation and a live singleton breech fetus attending antenatal clinic of Ikazia Hospital, Rotterdam, The Netherlands. Random allocation of women used sealed envelopes, and was stratified by parity. Repeated ECV was performed between 33 and 40 weeks' gestation up to four times with no tocolysis, analgesia or anaesthesia compared with no ECV. The outcomes included: presentation at delivery; mode of delivery; neonatal outcome including perinatal death.
Comparison three: ECV commencing before term compared with ECV commencing after term (37 weeks' gestation)
In this update three studies are now included in this comparison (Akhtar 2013; Hutton 2003; Hutton 2011).
Hutton 2003 is an international multicentre randomised controlled trial (n = 233). All nulliparous women with any breech presentation and multiparous women with a frank breech presentation were eligible for the trial if they had a live singleton fetus and a gestational age of between 34 weeks, 0 days and 36 weeks 0 days. Women were excluded if they had a parity greater than four, if they planned to move to a non‐trial centre, or if there was any contraindication to labour or vaginal birth (such as placenta previa, or previous classical caesarean section), to ECV (such as fetal heart rate abnormalities, abruptio placenta, fetal anomalies, uterine anomalies, oligohydramnios, rupture of membranes, over distended uterus) or to early ECV (such as fetus engaged in the pelvis, an increased risk of preterm labour, increased risk of abruptio placenta). ECV was begun between 34 weeks 0 days and 36 weeks 0 days in the early group (n = 117); and between 37 weeks 0 days and 38 weeks 0 days in the delayed group (n = 116). Tocolysis was recommended to be used either routinely or selectively in both groups; analgesia was permitted. The primary outcome was presentation at delivery; other outcomes included: caesarean section; serious fetal complication; preterm birth less than 37 weeks; women's views about ECV. The study was funded by Canadian Institutes of Health Research.
The Hutton 2011 study included 68 centres in 21 countries with ECV carried out by clinicians who were experienced in the procedure and with birth facilities that were deemed to meet Canadian standards. One‐thousand, five‐hundred and forty‐three women were randomised. The study recruited women with a singleton fetus in a breech presentation, between gestation ages of 33 weeks 0 days and 35 weeks six days. Women with contraindications to ECV (e.g. fetal heart rate abnormalities, placental abruption, major life‐threatening fetal anomalies, uterine anomalies, hyper‐extended fetal head, rupture of fetal membranes, severe oligohydramnios or hydramnios); contraindications to early ECV (e.g. increased risk of preterm labour or placental abruption); or contraindications to labour or vaginal birth (e.g. placenta praevia, previous classical caesarean section); or if they had been prior participants in the trial; were at increased risk of unstable lie (such as grand multiparity); or if they planned to give birth by caesarean section even if the fetus turned to a cephalic position, or if they planned a vaginal birth if the fetus remained breech were excluded. In the early ECV group (n = 767), ECV carried out between 34 weeks 0 days and 35 weeks six days gestation, and within seven days of randomisation. In the delayed ECV group (n = 774) ECV carried out at or after 37 weeks' gestation. In both groups fetal presentation was confirmed by ultrasound, fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman.
The study by Akhtar 2013 is a single‐centre, parallel‐group randomised controlled trial carried out in a hospital in Pakistan. The study included women with a singleton fetus with breech presentation between 33 and 35 weeks' gestation (n = 123 women). Women with contraindications to ECV, contraindications to early ECV or contraindications to labour or vaginal birth (e.g. fetal heart rate abnormalities, vaginal bleeding, rupture of membranes, placental abruption, fetal growth restriction, previous CS, low amniotic fluid index, fetal weight greater than 4 kg) or women unwilling to undergo ECV were excluded. In the early ECV group, ECV was carried out between 34 (238 days) and 35 weeks of gestation (n = 63). In the delayed ECV group ECV was carried out at or after 37 weeks. No tocolytics were used in either group and women were monitored for three hours before and one hour after the procedure. Up to two‐three attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities (n = 60).
Excluded studies
Five studies were excluded. Two studies were excluded for methodological reasons; it was not clear in the study by Brosset 1956 that allocation to groups was random and in the Kasule 1985 study allocation was by day of the week. Both of these studies are at high risk of selection bias. The remaining studies (Dafallah 2004; El‐Muzaini 2008; Rust 2005) were excluded because the intervention group mainly included women recruited at term and separate results were not available for women with preterm pregnancies. See Characteristics of excluded studies.
Ongoing studies
We have limited information on the study by Belizan 1989; it is not clear whether this study was completed, more information is set out in Characteristics of ongoing studies.
Risk of bias in included studies
See table Characteristics of included studies.
Allocation
Hutton 2003 and Hutton 2011 used a centralised telephone randomisation service and these studies were assessed as low risk of bias for sequence generation and allocation concealment. The remaining studies did not fully describe the methods used for generating the randomisation sequence. Mensink 1980 and Van Veelen 1989 used randomised, sealed envelopes to conceal allocation (it was not clear whether or not envelopes were opaque and sequentially numbered). Akhtar 2013 reported using the same methods as those used in the Hutton 2003 and Hutton 2011 trials but no further information was provided. All studies were stratified for parity at randomisation.
Blinding
Blinding women and care providers is not feasible for the intervention under study. It was not clear whether there was any attempt to achieve observer blinding in the collection of the outcome data in any of the studies. Although lack of blinding would not be likely to effect outcomes such as presentation at delivery, it is not clear whether lack of blinding had an impact on some of the other outcomes reported such as caesarean section.
Incomplete outcome data
All included studies were assessed to be at low risk of bias for this domain. There were no losses to follow‐up in Akhtar 2013, Mensink 1980 or Van Veelen 1989. Hutton 2003 reported one loss to follow‐up in the early ECV group following randomisation but prior to any ECV procedure being done. Hutton 2011 included more than 99% of women randomised in the analysis. All used an intention‐to‐treat approach to analyses.
Selective reporting
In the two multicentre trials (Hutton 2003; Hutton 2011) study protocols were available and there did not appear to have been any outcome reporting bias. In the remaining studies assessment of bias was from published reports and it was not clear whether all outcome data were reported.
Other potential sources of bias
In the Akhtar 2013 trial it was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject" (Hutton 2003; Hutton 2011). There was no further description of methods used. We contacted the author for more information but have not yet had a response (September 2014).
See Figure 1 and Figure 2 for a summary of findings for risk of bias.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Comparison one: ECV attempt before term (with one repeat attempt) compared with no ECV: one trial involving 102 women (Mensink 1980)
Primary outcomes
The rate of non‐cephalic presentation at birth in the ECV group was 40% and in the no ECV group was 39% (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.64 to 1.69) Analysis 1.1. (The trial authors ascribe the low success rate to the gentleness with which ECV was attempted.) There was no clear difference between groups for failure to achieve vaginal cephalic birth (caesarean section plus breech vaginal birth) (RR 1.04, 95% CI 0.67 to 1.62) Analysis 1.2. (The rate of caesarean section was 14% in the ECV group and 8% in the no ECV group (RR 1.82, 95% CI 0.57 to 5.84) Analysis 1.3. The number of women undergoing vaginal breech delivery was comparable in the two groups (RR 0.87, 95% CI 0.49 to 1.52) Analysis 1.4.
Secondary outcomes
There was no clear evidence of differences between groups for other outcomes.
-
The rate of one minute Apgar scores less than seven (RR 0.62, 95% CI 0.25 to 1.59) Analysis 1.5.
-
The rate of stillbirth or neonatal mortality less than seven days (RR 0.35, 95% CI 0.04 to 3.22) Analysis 1.6.
Rates of premature delivery were similar in the two groups (two premature deliveries in the intervention group and three in the control group). "Perinatal morbidity" at the time of delivery was also reported although this was not defined (with one event in the intervention group and three in the control group).
Comparison two: ECV commencing before term compared with no ECV (repeated attempts): one trial involving 179 women (Van Veelen 1989)
Primary outcomes
The ECV group had 44% non‐cephalic presentation at birth compared to 74% in the no ECV group (RR 0.59, 95% CI 0.45 to 0.77); this difference between groups was statistically significant Analysis 2.1 . Women in the ECV group were at reduced risk of failing to achieve cephalic vaginal birth (RR 0.62, 95% CI 0.49 to 0.80) Analysis 2.2. The rate of caesarean section delivery was 11% in the ECV group compared to 14% in the no ECV group (RR 0.62, 95% CI 0.27 to 1.43) Analysis 2.3. The frequency of vaginal breech delivery was reduced in the ECV group (RR 0.63, 95% CI 0.46 to 0.85) Analysis 2.4.
Secondary outcomes
There was insufficient information on other outcomes.
-
The rate of five minute Apgar scores less than seven (one event in the intervention group) Analysis 2.5.
-
The rate of stillbirth or neonatal mortality less than seven days (one event in the control group) Analysis 2.6.
The authors reported no "major complications" in either group.
Comparison three: ECV commencing before term compared with ECV commencing after term (37 weeks' gestation): three trials involving 1906 women (Akhtar 2013; Hutton 2003; Hutton 2011)
Primary outcomes
The rate of non‐cephalic presentation at birth was lower when ECV was started before term (RR 0.81, 95% CI 0.74 to 0.90; participants = 1906; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.1. Women who had early ECV were at slightly less risk of failing to achieve a cephalic vaginal birth (RR 0.90, 95% CI 0.83 to 0.97; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.2. The rate of caesarean section was reduced when ECV was started before 37 weeks' gestation although the difference between groups did not reach statistic significance (RR 0.92, 95% CI 0.85 to 1.00; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.3. Women who were randomised to early ECV were at a considerably reduced risk of having a vaginal breech birth; the difference between groups for this outcome was statistically significant (RR 0.44, 95% CI 0.25 to 0.78; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.4.
Secondary outcomes
The rate of preterm birth less than 37 weeks was increased in the early ECV group (RR 1.51, 95% CI 1.03 to 2.21; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.7.
There was no strong evidence of differences between groups identified for:
-
the rate of five minute Apgar scores less than seven (RR 1.16, 95% CI 0.39 to 3.44; participants = 1759; studies = two; I2 = 0%, evidence graded low quality due to imprecision) Analysis 3.5;
-
the rate of stillbirth or neonatal mortality less than seven days (RR 0.23, 95% CI 0.04 to 1.34; participants = 1887; studies = three; I2 = 0%, evidence graded low quality due to imprecision) Analysis 3.6;
-
The studies by Hutton 2003 and Hutton 2011 reported several outcomes relating to neonatal outcome but these were not mutually exclusive and so a single composite outcome was reported: one or more serious fetal complication (RR 0.87, 95% CI 0.42 to 1.79; participants = 1761; studies = two; I2 = 0%) Analysis 3.8.
-
There no clear difference between groups for NICU stay for four days or longer (RR 2.50, 95% CI 0.49 to 12.63; participants = 232; studies = 1) Analysis 3.9.
Non‐prespecified outcome
One study reported maternal pain scores following the ECV attempt; pain scores were lower when ECV was commenced before term (mean difference (MD) ‐4.60, 95% CI ‐7.74 to ‐1.46; participants = 1533).
Discusión
Resumen de los resultados principales
Existe evidencia convincente para apoyar la versión cefálica externa (VCE) que se inicia al término, es decir, después de las 37 semanas de gestación. Una revisión Cochrane sobre la VCE concluyó que es una maniobra útil para disminuir la tasa de presentación no cefálica y de cesárea cuando se inicia después de las 37 semanas de gestación (Hofmeyr 2015), y las principales sociedades obstétricas recomiendan ofrecer la VCE a las embarazadas con bajo riesgo y con un embarazo en presentación podálica. De los estudios de VCE a término, los realizados en centros europeos o norteamericanos informan una tasa relativamente baja de éxito con la VCE y una tasa notablemente mayor de presentación no cefálica al nacer, en comparación con los ensayos africanos. Es posible que haya diferencias en las características de las poblaciones. En un estudio de cohortes, Hofmeyr 1986 informó tasas más altas de éxito con el procedimiento de VCE en un grupo de pacientes africanas, en comparación con las pacientes caucásicas.
Los estudios de VCE antes del término son menos claros. En el ensayo Mensink 1980, que comparó la VCE antes del término con ninguna VCE, el procedimiento se realizó en una etapa temprana del embarazo (32 semanas de gestación), cuando las tasas de versión espontánea aún son altas. A pesar de los hallazgos de este estudio inicial de VCE antes del término, que claramente no mostró diferencias entre el grupo de VCE y el grupo de ninguna VCE, los ensayos más recientes indican que puede haber efectos beneficiosos al comenzar la VCE antes del término, pero cerca de éste, particularmente en las poblaciones donde las tasas de éxito a término son bajas. El estudio Van Veelen 1989, que inició la VCE a las 33 semanas (pero hasta las 40 semanas, con una media de edad gestacional al momento de la VCE de 35 semanas), en comparación con ninguna VCE, mostró una disminución del 30% en la tasa de presentación no cefálica. Este ensayo mostró que las pacientes del grupo de VCE tuvieron un riesgo menor de no lograr un parto vaginal cefálico, aunque no se encontraron diferencias en la tasa de cesárea. Lo anterior se debe probablemente a la mayor proporción de embarazadas que planifican un parto vaginal en presentación podálica cuando el feto permaneció en podálica al llegar al término, ya que el estudio se realizó antes de la publicación de los resultados del Term Breech Trial (Hannah 2000), y es evidente la existencia de una política de parto vaginal en presentación podálica. El estudio fue muy pequeño para descartar de manera significativa diferencias en las puntuaciones de Apgar menores de 7 a los cinco minutos, en los mortinatos o en la mortalidad neonatal antes de los siete días. En el estudio Van Veelen 1989, el tiempo medio de inicio de la VCE fue de 35 semanas de gestación, y no está claro si el efecto beneficioso encontrado se puede atribuir al inicio del procedimiento más temprano en el embarazo, o a que algunos de los procedimientos no se iniciaron hasta después del término.
Tres ensayos compararon el inicio temprano de la VCE entre las 34 y 36 semanas de gestación con el inicio de la VCE entre las 37 y 38 semanas de gestación (Akhtar 2013; Hutton 2003; Hutton 2011). En comparación con las pacientes a las que se les realizó una VCE a término, las embarazadas asignadas al azar a VCE antes del término tuvieron una disminución del 19% en la tasa de presentación no cefálica al parto, una reducción del 10% en el riesgo de no lograr un parto vaginal cefálico, una disminución del 8% en la tasa de cesárea y una reducción considerable en el riesgo de someterse a un parto vaginal en presentación podálica. La calidad de la evidencia para todos estos resultados se consideró alta. Estos resultados son clínicamente importantes y, excepto el hallazgo relacionado con la cesárea, estas diferencias (a favor de la VCE temprana) fueron estadísticamente significativas. Sin embargo, las embarazadas asignadas al azar a la VCE temprana parecieron tener un riesgo mayor de parto prematuro tardío (el riesgo aumentó en el 51%), y aunque en general el número de pacientes que dieron a luz antes del término fue relativamente pequeño (6,6% en el grupo de VCE y 4,3% en los controles), el posible aumento de los partos prematuros tardíos se debe sopesar con los resultados positivos asociados con la VCE.
Calidad de la evidencia
Los ensayos incluidos en la revisión fueron de calidad metodológica mixta. Tres de los estudios no proporcionaron descripciones adecuadas de los métodos utilizados (Akhtar 2013; Mensink 1980; Van Veelen 1989). El cegamiento no fue posible en estos estudios y es difícil saber qué repercusión tuvo la falta de cegamiento sobre los resultados. Aunque los resultados medidos fueron objetivos y pueden no haber estado sujetos a sesgo de detección, es posible que la falta de cegamiento haya afectado el comportamiento de las embarazadas y los profesionales sanitarios, lo que puede haber tenido un efecto sobre la toma de decisiones clínicas y podría haber influido en resultados como la decisión de realizar o no una cesárea. En dos estudios los protocolos de los ensayos estuvieron disponibles; sin esta información es difícil evaluar el posible sesgo en el informe de los resultados.
Sesgos potenciales en el proceso de revisión
El proceso de revisión está sujeto a sesgos. Se intentó disminuir el sesgo en el proceso de revisión al hacer que dos autores de la revisión, de forma independiente, evaluaran el riesgo de sesgo y realizaran la extracción de los datos. Uno de los autores de la revisión (E Hutton) participó en dos de los ensayos incluidos (Hutton 2003; Hutton 2011); este autor no participó en la extracción de los datos ni en la evaluación del sesgo de estos ensayos.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 3 Caesarean section.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 4 Vaginal breech birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 5 Apgar score < 7 at 1 minute.
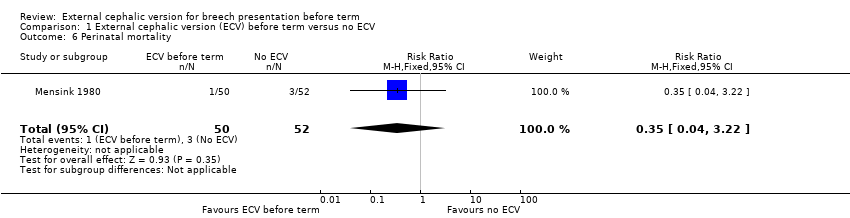
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 6 Perinatal mortality.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.
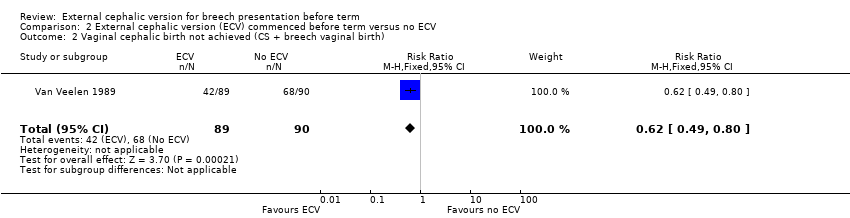
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).
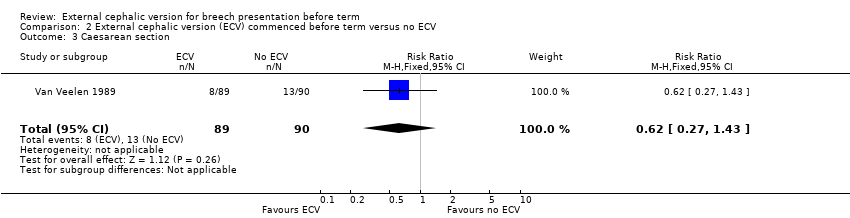
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 3 Caesarean section.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 4 Vaginal breech birth.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 5 Apgar score < 7 at 5 minutes.
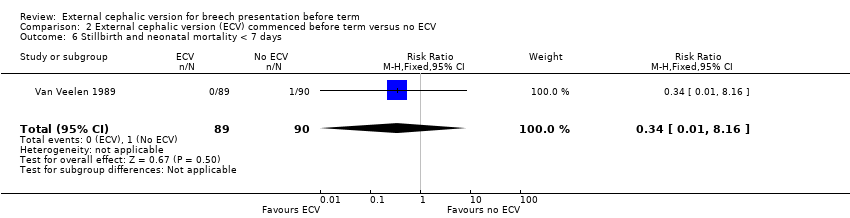
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 6 Stillbirth and neonatal mortality < 7 days.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth).
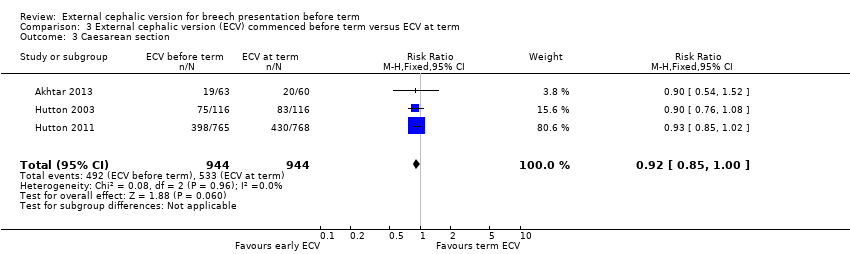
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 3 Caesarean section.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 4 Vaginal breech birth.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 6 Stillbirth or neonatal mortality < 7 days.
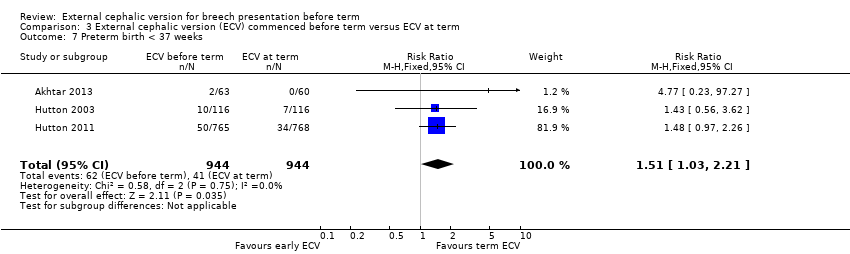
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 7 Preterm birth < 37 weeks.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 8 One or more serious fetal complications following randomisation.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 9 NICU stay 4 days or longer.
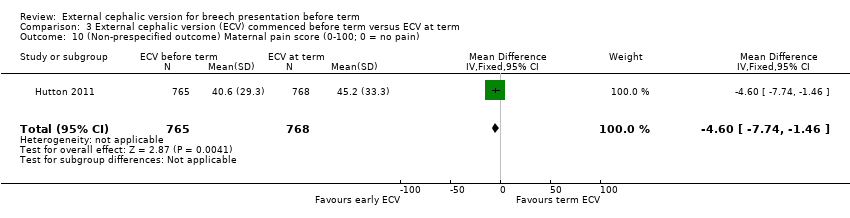
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain).
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 | 1906 | ⊕⊕⊕⊕ | ||
| 523 per 1000 | 424 per 1000 | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 | 1888 | ⊕⊕⊕⊕ | ||
| 600 per 1000 | 540 per 1000 | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 | |||||
| Caesarean section | Study population | RR 0.92 | 1888 | ⊕⊕⊕⊕ | ||
| 565 per 1000 | 519 per 1000 | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 | |||||
| Vaginal breech birth | Study population | RR 0.44 | 1888 | ⊕⊕⊕⊕ | ||
| 35 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 | 1759 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 | 1887 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 | 1888 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide 95% CI crossing the line of no effect and low event rate. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| 3 Caesarean section Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.57, 5.84] |
| 4 Vaginal breech birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.52] |
| 5 Apgar score < 7 at 1 minute Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.59] |
| 6 Perinatal mortality Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| 3 Caesarean section Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.43] |
| 4 Vaginal breech birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.85] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.48] |
| 6 Stillbirth and neonatal mortality < 7 days Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth) Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3 Caesarean section Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 4 Vaginal breech birth Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.78] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 2 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.44] |
| 6 Stillbirth or neonatal mortality < 7 days Show forest plot | 3 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.34] |
| 7 Preterm birth < 37 weeks Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.21] |
| 8 One or more serious fetal complications following randomisation Show forest plot | 2 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| 9 NICU stay 4 days or longer Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.63] |
| 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain) Show forest plot | 1 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |

