Versión cefálica externa para la presentación podálida a término
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000084.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 29 julio 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
GJ Hofmeyr prepared the initial review of this topic. E Hutton has revised and will maintain the review. T Dowswell was involved in updating the review.
Sources of support
Internal sources
-
(GJH) Effective Care Research Unit, University of the Witwatersrand/Fort Hare, Eastern Cape Department of Health, South Africa.
-
(TD) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
E Hutton is an author of two of the included trials (Hutton 2003; Hutton 2011) but was not involved in data extraction or assessment of risk of bias for these trials.
Justus Hofmeyr: I have received royalties for three chapters that I have authored in Up‐to‐date (http://www.uptodate.com/contents/search).
-
http://www.uptodate.com/contents/external‐cephalic‐version
-
http://www.uptodate.com/contents/overview‐of‐breech‐presentation
-
http://www.uptodate.com/contents/delivery‐of‐the‐fetus‐in‐breech‐presentation
Therese Dowswell: I am employed by the University of Liverpool on an NIHR Cochrane Programme grant to work on a range of Cochrane Reviews. The Funders have no influence on the content or conclusions of the reviews that I work on.
Acknowledgements
T Dowswell is supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jul 29 | External cephalic version for breech presentation before term | Review | Eileen K Hutton, G Justus Hofmeyr, Therese Dowswell | |
| 2006 Jan 25 | External cephalic version for breech presentation before term | Review | Eileen K Hutton, G Justus Hofmeyr | |
| 1996 Apr 22 | External cephalic version for breech presentation before term | Review | G Justus Hofmeyr | |
Differences between protocol and review
The protocol for this review was modified in April 2005 to include comparisons of external cephalic version (ECV) commenced before term but continued if necessary up to term.
In this updated version of the review two additional primary outcomes have been added. The purpose of ECV is to avoid a caesarean birth or a breech vaginal birth; we have therefore added a new composite outcome: cephalic vaginal birth not achieved (caesarean section + breech vaginal birth). The individual outcomes are presented separately; vaginal breech birth is now reported, caesarean section was one of the original outcomes. In this version we have also added an additional secondary outcome: Infant Apgar score < seven at five minutes. Infant morbidity was not consistently reported and low infant Apgar score provides some indication of infant wellbeing at birth. We have now reported more information re neonatal outcome. We also report a non‐prespecified outcome: maternal pain scores.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 3 Caesarean section.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 4 Vaginal breech birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 5 Apgar score < 7 at 1 minute.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 6 Perinatal mortality.
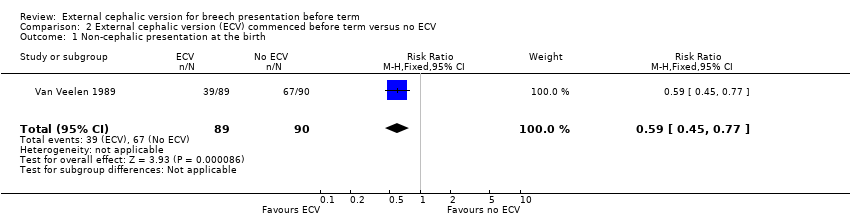
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.
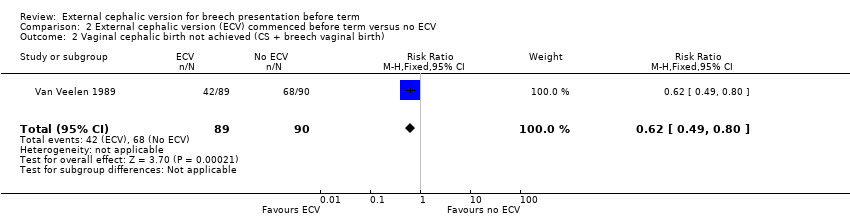
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 3 Caesarean section.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 4 Vaginal breech birth.
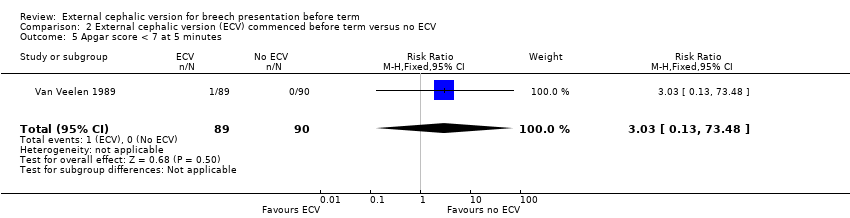
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 6 Stillbirth and neonatal mortality < 7 days.
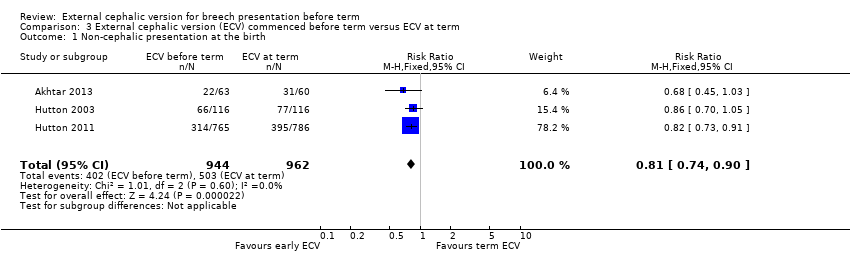
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth).

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 3 Caesarean section.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 4 Vaginal breech birth.
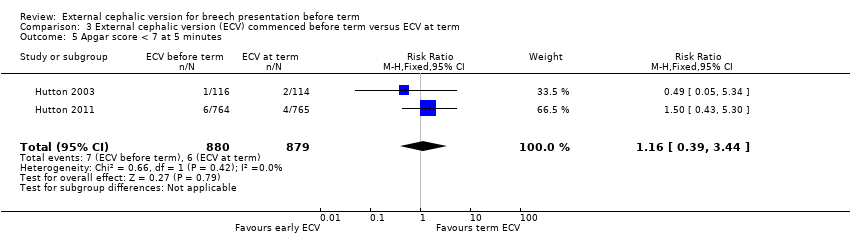
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 6 Stillbirth or neonatal mortality < 7 days.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 7 Preterm birth < 37 weeks.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 8 One or more serious fetal complications following randomisation.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 9 NICU stay 4 days or longer.
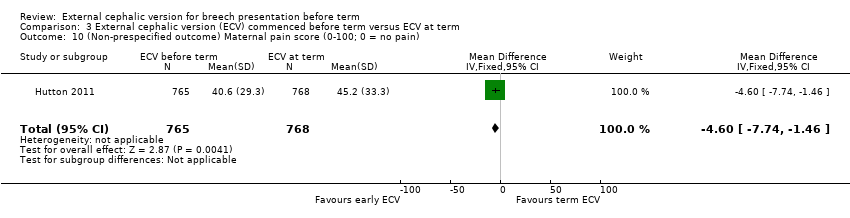
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain).
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 | 1906 | ⊕⊕⊕⊕ | ||
| 523 per 1000 | 424 per 1000 | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 | 1888 | ⊕⊕⊕⊕ | ||
| 600 per 1000 | 540 per 1000 | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 | |||||
| Caesarean section | Study population | RR 0.92 | 1888 | ⊕⊕⊕⊕ | ||
| 565 per 1000 | 519 per 1000 | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 | |||||
| Vaginal breech birth | Study population | RR 0.44 | 1888 | ⊕⊕⊕⊕ | ||
| 35 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 | 1759 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 | 1887 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 | 1888 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide 95% CI crossing the line of no effect and low event rate. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| 3 Caesarean section Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.57, 5.84] |
| 4 Vaginal breech birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.52] |
| 5 Apgar score < 7 at 1 minute Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.59] |
| 6 Perinatal mortality Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| 3 Caesarean section Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.43] |
| 4 Vaginal breech birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.85] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.48] |
| 6 Stillbirth and neonatal mortality < 7 days Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth) Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3 Caesarean section Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 4 Vaginal breech birth Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.78] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 2 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.44] |
| 6 Stillbirth or neonatal mortality < 7 days Show forest plot | 3 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.34] |
| 7 Preterm birth < 37 weeks Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.21] |
| 8 One or more serious fetal complications following randomisation Show forest plot | 2 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| 9 NICU stay 4 days or longer Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.63] |
| 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain) Show forest plot | 1 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |

