Danazol para el dolor pélvico asociado con endometriosis
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, placebo‐controlled open trial | |
| Participants | Country: Italy | |
| Interventions | Danazol 600 mg/day for 3 months versus no treatment | |
| Outcomes | Pelvic pain recurrence, pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, placebo‐controlled double blind trial | |
| Participants | Country: Finland | |
| Interventions | Treatments: MPA 100 mg x 1/day + placebo x 2/day; danazol 200 mg x 3/day | |
| Outcomes | AFS scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, placebo‐controlled double blind trial | |
| Participants | Country: Finland | |
| Interventions | Treatments: MPA 100 mg/day; danazol 200 mg 3 x/day | |
| Outcomes | AFS scores (peritoneal implants component) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, placebo‐controlled double blind trial | |
| Participants | Country: Finland | |
| Interventions | Treatments: MPA 100 mg/day; danazol 200 mg 3 x/day | |
| Outcomes | AFS scores (peritoneal implants component) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Country: Finland | |
| Interventions | Treatments: MPA 100 mg/day; danazol 200 mg 3 x/day | |
| Outcomes | Levels of hormonal parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Outcomes published relate to infertility only | |
| No outcomes of interest, unclear randomisation process. | |
| Did not include outcomes of interest to this review | |
| Treatment period was post opertative and included surgery | |
| Women had oviarian cysts and endometriosis was not confirmed | |
| Outcomes published relate to infertility only | |
| Outcomes published relate to infertility only |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 1 Total pain. | ||||
| 1.1 Three months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐4.95 [‐6.61, ‐3.29] |
| 1.2 Six months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐7.51, ‐3.89] |
| 1.3 Six months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐7.50 [‐9.38, ‐5.62] |
| 2 Pelvic pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 2 Pelvic pain. | ||||
| 2.1 Three months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐0.90, ‐0.40] |
| 2.2 Six months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.68, ‐1.12] |
| 2.3 Six months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.33, ‐0.77] |
| 3 Low back pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 3 Low back pain. | ||||
| 3.1 Six months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.25, ‐0.55] |
| 3.2 Six months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.55, ‐0.85] |
| 4 Defaecation pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 4 Defaecation pain. | ||||
| 4.1 6 months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.10, ‐0.44] |
| 4.2 6 months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.99, ‐0.37] |
| 5 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 5 Adverse events. | ||||
| 5.1 Oedema at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.8 [1.38, 118.32] |
| 5.2 Acne at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 25.14 [2.70, 234.17] |
| 5.3 Vaginal spotting at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.36, 9.05] |
| 5.4 Muscle cramps at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.2 [0.94, 353.55] |
| 6 AFS scores, total ‐ 12 months (six months after stopping treatment) Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.58, 0.78] |
| Analysis 1.6  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 6 AFS scores, total ‐ 12 months (six months after stopping treatment). | ||||
| 7 AFS scores, total ‐ change in Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐4.16, 0.36] |
| Analysis 1.7  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 7 AFS scores, total ‐ change in. | ||||
| 8 Total or partial resoultion of peritoneal endometriotic implants Show forest plot | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.83, 30.28] |
| Analysis 1.8  Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 8 Total or partial resoultion of peritoneal endometriotic implants. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 1 Total pain. | ||||
| 1.1 3 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐3.59, ‐0.81] |
| 1.2 6 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐4.2 [‐5.71, ‐2.69] |
| 1.3 6 months or more after treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.18, ‐0.42] |
| 2 Pelvic pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 2 Pelvic pain. | ||||
| 2.1 3 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.80, ‐0.24] |
| 2.2 6 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐1.38, ‐0.82] |
| 2.3 6 months after treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.75, ‐0.19] |
| 3 Moderate or severe pain 6 months or more after followup Show forest plot | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.20, 2.05] |
| Analysis 2.3  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 3 Moderate or severe pain 6 months or more after followup. | ||||
| 4 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 4 Adverse events. | ||||
| 4.1 vaginal spotting | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.75 [2.02, 173.94] |
| 4.2 acne | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.75 [2.02, 173.94] |
| 5 Satisfaction with treatment Show forest plot | 1 | 34 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.94 [2.61, 37.81] |
| Analysis 2.5  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 5 Satisfaction with treatment. | ||||
| 6 Weight gain Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [1.34, 4.66] |
| Analysis 2.6  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 6 Weight gain. | ||||
| 7 AFS scores, total ‐ 12 months (six months after stopping treatment) Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐5.27, ‐1.73] |
| Analysis 2.7  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 7 AFS scores, total ‐ 12 months (six months after stopping treatment). | ||||
| 8 AFS scores, total ‐ change in Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐3.02, 1.22] |
| Analysis 2.8  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 8 AFS scores, total ‐ change in. | ||||
| 9 AFS scores, peritoneal and ovarian ‐ 12 months (six months after stopping treatment) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐3.90, ‐0.30] |
| Analysis 2.9  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 9 AFS scores, peritoneal and ovarian ‐ 12 months (six months after stopping treatment). | ||||
| 10 Resolution of endometriotic implants at laparoscopy Show forest plot | 1 | 34 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.44, 6.74] |
| Analysis 2.10  Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 10 Resolution of endometriotic implants at laparoscopy. | ||||

Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 1 Total pain.

Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 2 Pelvic pain.
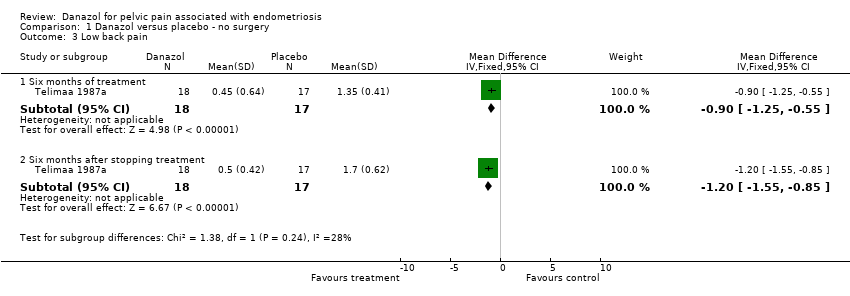
Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 3 Low back pain.

Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 4 Defaecation pain.
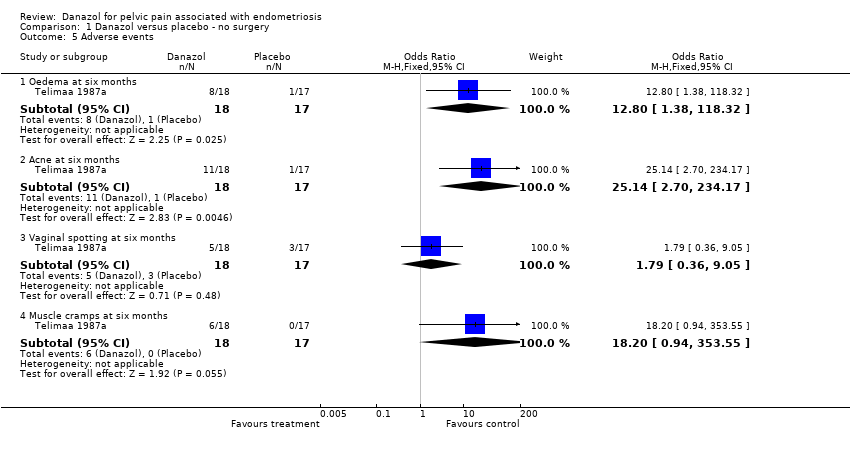
Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 5 Adverse events.

Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 6 AFS scores, total ‐ 12 months (six months after stopping treatment).
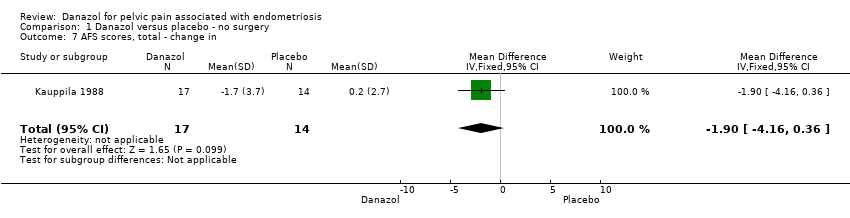
Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 7 AFS scores, total ‐ change in.
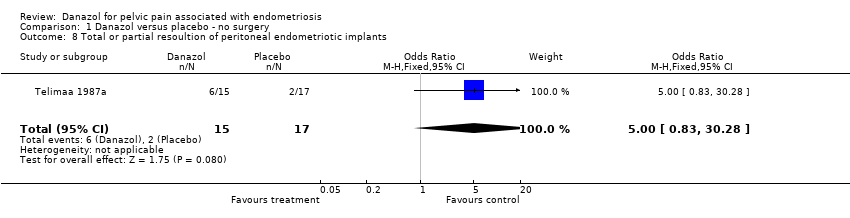
Comparison 1 Danazol versus placebo ‐ no surgery, Outcome 8 Total or partial resoultion of peritoneal endometriotic implants.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 1 Total pain.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 2 Pelvic pain.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 3 Moderate or severe pain 6 months or more after followup.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 4 Adverse events.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 5 Satisfaction with treatment.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 6 Weight gain.
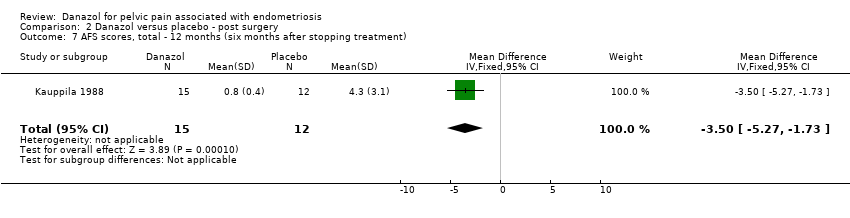
Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 7 AFS scores, total ‐ 12 months (six months after stopping treatment).
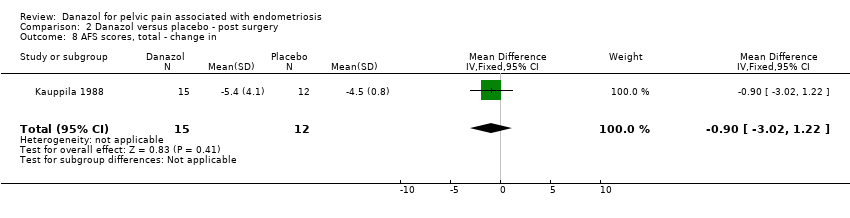
Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 8 AFS scores, total ‐ change in.

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 9 AFS scores, peritoneal and ovarian ‐ 12 months (six months after stopping treatment).

Comparison 2 Danazol versus placebo ‐ post surgery, Outcome 10 Resolution of endometriotic implants at laparoscopy.
| Study ID | Concealed allocation | Method of randomisat | Losses to followup | Post random exclus | Intention to treat | Blinding |
| Bianci 1999 | Not stated | Computer generated list | None | None | yes | Open study |
| Kaupilla 1988 | Not stated | Not stated | None | None | yes | Double blind |
| Telimaa 1987a | Not stated | Not stated | None | 9 ‐ 4 in the MPA group and 2 in the danazol group and 3 in the placebo group (5 for pregnancies) | no | Double blind |
| Telimaa 1987b | Not stated | Not stated | None | 9 ‐ 3 in the MPA group, 2 in the danazol group and 4 in the placebo group, 8 for pregnancies | no | Double blind |
| Telimaa 1990 | Not stated | Not stated | None | None | yes | Unclear |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Three months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐4.95 [‐6.61, ‐3.29] |
| 1.2 Six months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐7.51, ‐3.89] |
| 1.3 Six months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐7.50 [‐9.38, ‐5.62] |
| 2 Pelvic pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Three months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐0.90, ‐0.40] |
| 2.2 Six months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.68, ‐1.12] |
| 2.3 Six months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.33, ‐0.77] |
| 3 Low back pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Six months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.25, ‐0.55] |
| 3.2 Six months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.55, ‐0.85] |
| 4 Defaecation pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months of treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.10, ‐0.44] |
| 4.2 6 months after stopping treatment | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.99, ‐0.37] |
| 5 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oedema at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.8 [1.38, 118.32] |
| 5.2 Acne at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 25.14 [2.70, 234.17] |
| 5.3 Vaginal spotting at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.36, 9.05] |
| 5.4 Muscle cramps at six months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.2 [0.94, 353.55] |
| 6 AFS scores, total ‐ 12 months (six months after stopping treatment) Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.58, 0.78] |
| 7 AFS scores, total ‐ change in Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐4.16, 0.36] |
| 8 Total or partial resoultion of peritoneal endometriotic implants Show forest plot | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.83, 30.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐3.59, ‐0.81] |
| 1.2 6 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐4.2 [‐5.71, ‐2.69] |
| 1.3 6 months or more after treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.18, ‐0.42] |
| 2 Pelvic pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.80, ‐0.24] |
| 2.2 6 months of treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐1.38, ‐0.82] |
| 2.3 6 months after treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.75, ‐0.19] |
| 3 Moderate or severe pain 6 months or more after followup Show forest plot | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.20, 2.05] |
| 4 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 vaginal spotting | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.75 [2.02, 173.94] |
| 4.2 acne | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.75 [2.02, 173.94] |
| 5 Satisfaction with treatment Show forest plot | 1 | 34 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.94 [2.61, 37.81] |
| 6 Weight gain Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [1.34, 4.66] |
| 7 AFS scores, total ‐ 12 months (six months after stopping treatment) Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐5.27, ‐1.73] |
| 8 AFS scores, total ‐ change in Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐3.02, 1.22] |
| 9 AFS scores, peritoneal and ovarian ‐ 12 months (six months after stopping treatment) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐3.90, ‐0.30] |
| 10 Resolution of endometriotic implants at laparoscopy Show forest plot | 1 | 34 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.44, 6.74] |

