Iron therapy for pre‐operative anaemia
Abstract
Background
Pre‐operative anaemia is common and occurs in up to 76% of patients. It is associated with increased peri‐operative allogeneic blood transfusions, longer hospital lengths of stay and increased morbidity and mortality. Iron deficiency is one of the most common causes of this anaemia. Oral iron therapy has traditionally been used to treat anaemia but newer, safer parenteral iron preparations have been shown to be more effective in other conditions such as inflammatory bowel disease, chronic heart failure and post‐partum haemorrhage. A limited number of studies look at iron therapy for the treatment of pre‐operative anaemia. The aim of this Cochrane review is to summarise the evidence for use of iron supplementation, both enteral and parenteral, for the management of pre‐operative anaemia.
Objectives
The objective of this review is to evaluate the effects of pre‐operative iron therapy (enteral or parenteral) in reducing the need for allogeneic blood transfusions in anaemic patients undergoing surgery.
Search methods
We ran the search on 25 March 2015. We searched the Cochrane Injuries Group's Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R), EMBASE Classic and EMBASE (Ovid), CINAHL Plus (EBSCO), PubMed, clinical trials registries, conference abstracts, and we screened reference lists.
Selection criteria
We included all randomised controlled trials (RCTs) which compared pre‐operative iron monotherapy to placebo, no treatment, standard of care or another form of iron therapy for anaemic adults undergoing surgery. Anaemia was defined by haemoglobin values less than 13 g/dL for males and 12 g/dL for non‐pregnant females.
Data collection and analysis
Data were collected by two authors on the proportion of patients who receive a blood transfusion, amount of blood transfused per patient (units) and haemoglobin measured as continuous variables at pre‐determined time‐points: pre‐treatment, pre‐operatively but post‐treatment, and post‐operatively. Statistical analysis was performed using the Cochrane statistical software, Review Manager 2014. Outcome data were summarised in tables and a forest plot.
Main results
Three prospective randomised controlled studies evaluated pre‐operative iron therapy to correct anaemia (two in colorectal and one in gynaecological surgery) and included 114 patients in total. One compared oral iron versus standard care (Lidder 2007); one intravenous iron versus control (Edwards 2009); and one study compared oral versus intravenous iron (Kim 2009). Both colorectal trials reported the primary outcome (proportion of patients who received allogeneic blood transfusions) and meta‐analysis showed a reduction in blood transfusions with the administration of iron therapy, but the reduction was not statistically significant (risk ratio (RR) 0.56, 95% confidence interval (CI) 0.27 to 1.18). All studies reported haemoglobin change but data for the anaemic patients were only available for two studies (Edwards 2009 and Kim 2009). Edwards 2009 showed no difference in haemoglobin at the end of treatment pre‐operatively. The intravenous versus oral iron study showed an increase in haemoglobin with intravenous iron at the end of treatment pre‐operatively (MD 1.90 g/dL, 95% CI 1.16 to 2.64; participants = 56), but the results are at high risk of bias because participants with less than 80% compliance with therapy were excluded from the analysis and compliance was lower in the oral iron group due to the side‐effects of treatment (Kim 2009).
None of the studies reported quality of life, short‐ or long‐term mortality or post‐operative morbidity.
Authors' conclusions
The use of iron therapy for pre‐operative anaemia does not show a statistically significant reduction in the proportion of patients who received an allogeneic blood transfusion compared to no iron therapy. However, the 38 patients in our analysis falls far short of the 819 patients our information size calculation recommended to detect a 30% reduction in blood transfusions. Intravenous iron may be more effective than oral iron at increasing haemoglobin. However, all these conclusions are drawn from only three small randomised controlled studies. Further well designed, adequately powered randomised controlled studies are required to determine the true effectiveness of iron therapy for pre‐operative anaemia.
PICOs
Plain language summary
Iron treatment for low red blood cell count prior to surgery
Review question: We reviewed the evidence for iron treatment prior to major surgery, for reducing the need for blood transfusions in patients around the time of surgery. We found three studies that looked at this question.
Background: Low red blood cell count (anaemia) is common prior to surgery. It can cause dizziness, shortness of breath and lack of energy as well as increase the risks of surgery and the need for a blood transfusion. Anaemia is commonly due to lack of iron, and iron treatment (with tablets or injections) has been proven effective in other conditions to treat anaemia. Limited research has looked at whether iron treatment works before surgery.
Search date: 25 March 2015
Study characteristics: We looked at all adult patients with anaemia undergoing surgery who received iron treatment prior to their surgery. We included three studies and a total of 114 participants.
Key results: Iron treatment did not reduce the number of blood transfusions or improve the red blood cell count compared to no treatment. There is currently insufficient evidence that iron treatment works before surgery. To date, too few studies involving too small a number of people have been undertaken and so it is not possible to obtain a reliable result as to the effects of this treatment.
Quality of evidence: The major limitation in study design for all trials was too few participants. More research in larger, well‐designed trials is needed before a definitive answer can be given regarding the effectiveness of iron therapy before surgery. Two of the three studies included in this review were at low risk of bias. This was despite a lack of participant blinding in both trials where oral iron was used, as the objective measurement of haemoglobin would unlikely be influenced by the participant or investigator. The results of one of the studies are at high risk of bias because patients who did not take all of their assigned treatment were not included in the analysis. Overall the quality of evidence is low according to the GRADE criteria. Including new research in the future will change the results.
Authors' conclusions
Summary of findings
| Iron therapy versus placebo or no iron therapy for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Iron therapy | |||||
| Proportion of patients who received a blood transfusion | 635 per 1000 | 356 per 1000 | RR 0.56 | 38 | ⊕⊕⊝⊝ | |
| Amount of blood transfused per patient (in units) | Data from two small studies could not be combined as they were skewed and reported as medians and ranges. One RCT in 18 people reported a difference in medians of 0 (interquartile range: 1) with iron therapy. Another RCT in 20 people reported a median difference of 1 unit with iron therapy (range 0 to 2). | ‐ | 38 | ⊕⊕⊝⊝ | It is not possible to combine the data because they are skewed. These are the raw data. | |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 11.9 g/dL (SD 2.6) | mean 11.2 g/d L(SD 1.95) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intervention groups was 0.7 g/dL lower | 18 | ⊕⊕⊝⊝ | Data from one study; the raw data are presented. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only two small randomised control trials and a subset of anaemic patients resulting in a very small number of participants. | ||||||
| Iron therapy: Intravenous versus oral administration for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron therapy | Intravenous iron therapy | |||||
| Proportion of patients who received a blood transfusion | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Amount of blood transfused per patient (in units) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 8.6 g/dL (SD 1.4) | mean 10.5 g/dL (SD 1.4) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intravenous group was | 56 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study excluded those with less than 80% compliance with therapy and compliance was lower in the oral administration group. | ||||||
Background
Description of the condition
Anaemia is defined as a total reduction in erythrocyte number, reduced amount of circulating haemoglobin, or decreased circulating red blood cell mass (Perkins 2006), resulting in a pathological state where the oxygen‐carrying capacity of blood is insufficient to meet physiological demand (Varat 1972). The World Health Organization (WHO) defines anaemia as a haemoglobin level of less than 120 g/L in non‐pregnant adult women, less than 110 g/L in pregnant adult women and less than 130 g/L in adult men. Anaemia is a common finding in pre‐operative patients, with a prevalence ranging from 5% to 76% depending on the age of the patient, the nature of the condition, and operation planned (Shander 2004). The most common form of anaemia is secondary to iron deficiency, which can occur secondary to excessive losses, such as chronic haemorrhage, or inadequate intake (Piednoir 2011).
Anaemia of sufficient severity can cause symptoms such as dizziness, shortness of breath, angina and lethargy. Anaemia in a pre‐operative setting is associated with an increased requirement for peri‐operative blood transfusion (Benoist 2001). In patients undergoing colorectal surgery, pre‐operative anaemia is an independent risk factor for post‐operative complications and a longer post‐operative hospital stay (Leichtle 2011). Other studies have shown that peri‐operative anaemia is associated with an increased risk of peri‐operative infection and mortality (Dunne 2002).
Description of the intervention
Oral iron supplementation and allogeneic blood transfusion are the current standard practice of treatment for pre‐operative anaemia. Pre‐operative oral iron supplementation has been proven to be effective in the treatment of anaemia and for reducing the need for blood transfusion in colorectal surgery (Lidder 2007; Quinn 2010). It is cheap, widely available and easily administered. However, oral iron is associated with a number of gastrointestinal side‐effects such as abdominal pain, constipation, diarrhoea and dyspepsia. Non‐compliance as a result of these side‐effects is a problem. Oral iron supplementation may also be insufficient to compensate for ongoing blood losses due to poor intestinal absorption.
Parenteral iron was first introduced in the early 20th century in the form of intramuscular and subcutaneous injections (Heath 1932). However, these early formulations caused severe toxic reactions leading to their disuse. Towards the latter half of the 20th century, high molecular weight iron dextran was introduced for both intravenous and intramuscular use. However, the use of high molecular weight iron dextran has been phased out and replaced with low molecular weight iron dextran and other newer formulations of intravenous iron such as iron sucrose, ferric gluconate, ferumoxytol, ferric carboxymaltose and iron isomaltoside. This was due to reports of anaphylactic‐type reactions with the use of high molecular weight iron dextran due to the instability of the molecule as well as the formation of anti‐dextran antibodies.
There has been major progress and development of newer formulations of intravenous iron. Earlier high molecular weight iron dextran was linked to fatal anaphylactic‐type reactions (Chertow 2004). Iron sucrose, a safer formulation not associated with anaphylactic‐type reactions, had to be given in small maximum dosages of 200 mg for each infusion, thus requiring several small‐dose infusions to achieve the calculated iron deficit. Newer agents such as ferric carboxymaltose and iron isomaltoside have since been developed which allow total dose infusion and have much higher maximum approved doses and have not been associated with anaphylactic‐type reactions (Auerbach 2010).
The use of intravenous iron has been investigated mainly for the treatment of anaemia in the setting of inflammatory bowel disease and chronic kidney disease. Early studies have shown that intravenous iron is effective in treating anaemia in inflammatory bowel disease, with a quicker result than oral iron and fewer side‐effects, an important factor in determining compliance (Kulnigg 2008). The use of intravenous iron in anaemic patients with chronic heart failure has been shown to significantly improve symptoms and quality of life (Okonko 2008; Anker 2009). In women with post‐partum iron deficiency anaemia, intravenous iron has been shown to be safe and at least as effective as oral iron but with fewer gastrointestinal side‐effects (Breymann 2008; Seid 2008). Kim 2009 showed that intravenous iron was more effective than oral iron in the treatment of pre‐operative anaemia in women with menorrhagia. The use of intravenous iron in patients with chronic kidney disease has been studied and shown to be more effective than oral iron and has fewer side‐effects (Qunibi 2011).
However, there have been a limited number of studies looking at the use of intravenous iron in a pre‐operative setting. The use of intravenous iron for pre‐operative anaemia has mainly been studied in orthopaedic surgery. Some of these studies have shown a reduced transfusion rate and infection rate with the use of intravenous iron (Cuenca 2004; Garcia‐Erce 2005). An observational study in patients undergoing major surgery (colorectal cancer resections, hysterectomies and lower limb arthroplasties) saw an average increase in haemoglobin level of 2 g/dL within a three‐ to five‐week period (Munoz 2009).
How the intervention might work
The bone marrow requires an internal iron turnover of 20 to 30 mg/day for erythropoiesis. The body absorbs 1 to 2 mg/day of dietary iron, despite the normal diet containing 15 to 20 mg of iron. Ferrous sulphate is one of the most commonly used oral iron supplements and a 200 mg tablet contains 65 mg iron. Oral iron is absorbed most readily on an empty stomach; however, this also increases the risk of gastrointestinal side‐effects. Therefore, iron supplements are often taken with food to minimise the side‐effects, although this may decrease the absorption by 40% to 66% (Swain 1996). Certain drugs such as antacids, proton pump inhibitors and tetracyclines reduce iron absorption. Oral iron is absorbed mainly in the duodenum where it is reduced into a ferrous state by the duodenal enterocytes and exported via the iron exporter, ferroportin, into the circulation bound to transferrin (Munoz 2009).
Current intravenous iron preparations consist of iron‐carbohydrate complexes. Following intravenous injection, the iron‐carbohydrate complex is taken up and phagocytosed by the reticulo‐endothelial system and the remaining iron core is exported out of the cell and transported for erythropoiesis and storage (Munoz 2009).
The use of intravenous iron bypasses the problems of poor absorption that arise with oral iron supplements. Intravenous iron is also better tolerated with far fewer gastrointestinal side‐effects than oral iron (Qunibi 2011). Newer formulations of intravenous iron such as ferric carboxymaltose can be given in large doses (up to 1000 mg) and studies have shown that intravenous iron results in a more rapid rise in haemoglobin when compared with oral iron supplementation (Qunibi 2011).
Why it is important to do this review
Pre‐operative anaemia is a predictor of peri‐operative allogeneic blood transfusion (Shander 2004). Despite screening of blood products, allogeneic blood transfusion carries risks such as viral transmission, immunomodulation, allergic reactions and allo‐immunisation (Vamvakas 2009). Blood is also an expensive and finite resource.
Studies have also associated pre‐operative anaemia to increased post‐operative morbidity and mortality and increased length of hospital stay (Carson 1996; Dunne 2002; Spahn 2010).
Intravenous iron is a method of management of anaemia that is fairly new in the role of managing pre‐operative anaemia. It can be given as a large, single‐dose regimen with fewer gastrointestinal side‐effects than the oral iron tablets.
The aim of this Cochrane review is to summarise the evidence for use of iron supplementation, both enteral and parenteral, for the management of pre‐operative anaemia. The evidence from this review will establish if there is justification for a large randomised controlled trial to investigate the use of intravenous iron in pre‐operative patients with colorectal cancer.
Objectives
The objective of this review is to evaluate the effects of pre‐operative iron therapy (enteral or parenteral) in reducing the need for allogeneic blood transfusions in anaemic patients undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) which compared pre‐operative iron monotherapy to placebo, no treatment, standard of care or another form of iron therapy.
Pre‐operative iron monotherapy is defined as any iron therapy started after the decision for surgery was made, and initiated before the day of surgery.
Types of participants
Anaemic adults over the age of 18 years undergoing surgery. Anaemia is defined by haemoglobin values less than 13 g/dL for males and 12 g/dL for non‐pregnant females (as per the WHO standard guidelines). We accepted different criteria for anaemia provided that there was a clear definition of anaemia by the study investigators.
We included trials that did not specify anaemic participants if there was stratification of results to an anaemic subgroup. Pregnant women were not eligible for inclusion in this review.
Types of interventions
We included trials that began the administration of iron between the day of decision for surgery and the day before surgery. We included trials with any dose, duration and formulation (enteral or parenteral) of iron.
The types of interventions were:
-
oral iron;
-
parenteral (including intravenous) iron.
We compared between an intervention and placebo/no treatment/standard of care (as per each trial protocol) or between two interventions. Where the effect of iron was combined with another co‐intervention, we excluded the trial.
Types of outcome measures
Primary outcomes
-
Proportion of patients who receive a blood transfusion
Secondary outcomes
-
Amount of blood transfused per patient (units)
-
Post‐operative mortality in the short term (within 30 days) and long term (greater than one year)
-
Post‐operative morbidity (including infection rates and adverse events)
-
Any validated measure of quality of life
-
Measurement value of the following haematologic parameters: haemoglobin, haematocrit, ferritin level and reticulocyte count
-
Measured as continuous variables at pre‐determined time‐points: pre‐treatment with iron/placebo; pre‐operatively but post‐treatment with iron/placebo; post‐operatively
-
Information size calculation for the primary outcome
Assuming 20% of patients in the control group require a blood transfusion, and a treatment effect of 30% (i.e. 14% require transfusion following iron therapy), 819 people need to be randomised to receive either iron therapy or control in order to obtain a reliable estimate of the treatment effect (alpha = 0.05, beta = 0.1) (Keeler 2015).
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group's Trials Search Co‐ordinator searched the following databases:
-
Cochrane Injuries Group Specialised Register (25/03/2015);
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library) (issue 3 of 12, 2015);
-
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 25/03/2015);
-
EMBASE Classic and EMBASE (Ovid SP) (1947 to 25/03/2015);
-
PubMed (25/03/2015);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 25/03/2015);
-
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 25/03/2015);
-
ClinicalTrials.gov (https://clinicaltrials.gov/) (25/03/2015);
-
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/) (25/03/2015).
All search strategies are listed in Appendix 1. We adapted the MEDLINE search strategy as necessary for each of the other databases: the added study filter is a modified version of the Ovid MEDLINE Cochrane Highly Sensitive Search Strategy for identifying randomised trials; to the EMBASE search strategy we added the study design terms as used by the UK Cochrane Centre (Lefebvre 2011).
Data collection and analysis
The Cochrane Injuries Group's Trials Search Co‐ordinator ran the searches and collated the search results before passing them on to two authors (ON and BK) for screening.
Selection of studies
Two authors (ON and BK) examined the citations independently and applied pre‐agreed selection criteria to identify all potentially eligible studies. Disagreements were resolved through consensus with five authors (ON, BK, AM, MB and AA). We describe the characteristics of excluded studies and reasons for their exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
One author (ON) extracted data relevant to each study using a standardised data extraction form and presented information in the 'Characteristics of included studies' table. Another author (BK) double‐checked the data. We resolved disagreements by discussion between the two authors and involved additional authors (AA, MB) when necessary. When information was unclear, we contacted the study investigators for further details.
Assessment of risk of bias in included studies
Two authors (ON and BK) independently assessed each study report for risk of bias by making judgements on the following questions according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Was the allocation sequence adequately generated (to check for possible selection bias)?
-
Was the allocation sequence adequately concealed (to check for possible selection bias)?
-
Was the study blinded with reference to participants, personnel and outcome assessors (to check for possible performance bias)?
-
Was there a suggestion of incomplete outcome data (to check for possible attrition bias through withdrawals, dropouts and protocol deviations)?
-
Was there an intent‐to‐treat analysis?
-
Was there any suggestion of selective outcome reporting?
-
Were there any other sources of bias?
-
What was the overall risk of bias?
We assessed the magnitude and direction of bias based upon our assessment of each study. If we considered bias likely to impact on findings, we explored the effect of the bias by undertaking sensitivity analyses.
Measures of treatment effect
We carried out statistical analysis using the Cochrane statistical software, Review Manager 2014.
For dichotomous data, we present results as summary risk ratios (RRs) with 95% confidence intervals (CIs).
We calculated mean differences (MDs) or standardised mean differences (SMDs) with 95% CIs between the study groups.
Unit of analysis issues
The unit of analysis is the participant.
Dealing with missing data
For included studies, we noted the levels of attrition in the 'Risk of bias' table. We carried out analyses on an intention‐to‐treat basis as far as possible.
Assessment of heterogeneity
We assessed included trials for heterogeneity by visually examining the forest plots for the estimated treatment effects. We used the I² statistic to assess statistical heterogeneity. We regarded heterogeneity as moderate when I² was greater than 30%.
Data synthesis
We present outcome data in tables and a forest plot. We created a 'Summary of findings' table according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Measures taken at final follow‐up were compared between treatment groups.
We used the fixed‐effect model in the meta‐analysis. The amount of blood transfused per patient was measured in units (where one unit contains approximately 250 millilitres of blood).
In the 'Summary of findings' table, we report the primary and secondary outcome measures.
Results
Description of studies
Results of the search
The search was performed on 25 March 2015 and retrieved 894 records. From these we identified six potential studies that used iron therapy as an intervention without the concomitant administration of another therapy (Crosby 1994; Andrews 1997; Lidder 2007; Edwards 2009; Kim 2009; Garrido‐Martin 2012), see Figure 1.

Study flow diagram.
Included studies
We found three prospective randomised controlled studies that evaluated the use of pre‐operative iron therapy to correct anaemia. Two studies were in colorectal surgery (Lidder 2007; Edwards 2009) and one in gynaecological surgery (Kim 2009). Three studies detailed the use of iron therapy prior to surgery for pre‐operative anaemia and included a total of 114 participants. One study compared oral iron versus no iron therapy (Lidder 2007), one study compared intravenous iron versus placebo (Edwards 2009) and one study compared intravenous iron versus oral iron (Kim 2009).
Lidder 2007 conducted an open label prospective randomised controlled trial looking at pre‐operative patients undergoing surgery for colorectal cancer. They identified 45 adult patients (28 male). Patients were randomised to receive oral ferrous sulphate (200 mg TDS for 2 weeks) or no iron therapy. 20 patients were anaemic at recruitment (6 in oral iron group, 14 in no iron group). They measured recruitment and pre‐operative haemoglobin, ferritin and reticulocyte count, and operative blood transfusions. Only the blood transfusion data are reported separately for the 20 patients with anaemia. This was a pilot study and no power calculation was conducted.
Edwards 2009 conducted a prospective, blinded, placebo‐controlled randomised trial involving patients undergoing surgery for colorectal cancer. Sixty patients (39 male) were randomised to receive 600 mg IV iron sucrose in 250 ml 0.9 percent saline or placebo a minimum of two weeks before surgery. Eighteen patients were anaemic at recruitment (nine patients in each arm). The main outcome measures were change in haemoglobin from recruitment to pre‐operatively and operative transfusion rates. Study was powered to detect a difference in the haemoglobin change between recruitment and surgery of 0.5 g/dL.
Kim 2009 conducted a phase IV open label prospective randomised controlled trial looking at pre‐operative patients undergoing gynaecological surgery for menorrhagia. They included 76 adult female patients with haemoglobin less than 90 g/L and randomised them to receive either intravenous iron sucrose (dose based on calculated total iron deficit) or oral iron (80 mg/day iron protein succinylate) in the three weeks preceding surgery. The study was powered to detect a haemoglobin change of 1 g/dL and evaluated change in haemoglobin from recruitment in the study to pre‐operatively.
Excluded studies
Three studies were excluded. One study excluded anaemic patients (Garrido‐Martin 2012), while a second had no subgroup analysis of anaemic patients (Andrews 1997). The third study only randomised non‐anaemic participants and gave all anaemic participants iron with no control arm for the group (Crosby 1994).
Risk of bias in included studies
A summary of the review authors' risk of bias judgements can be found in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Three studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies clearly report allocation using a computer‐generated randomisation sequence and suitable allocation concealment (Edwards 2009; Kim 2009). One study does not clearly report the methods of randomisation and the risk of bias is unclear (Lidder 2007).
Blinding
One study was placebo controlled (Edwards 2009). However, the nature of the intervention prevents blinding when comparing intravenous to oral iron and reportedly oral iron versus placebo due to effects of iron on stool colour (Edwards 2009; Kim 2009).
This absence of blinding is less likely to create bias in the objective outcome measures such as change in haemoglobin and ferritin. It would potentially influence subjective measures like quality of life questionnaires but none of the three studies report this outcome measure. Blood transfusion, unless administered under a strict transfusion protocol, could potentially be influenced by lack of blinding in these studies. One study reports clinicians directly treating participants were blind to the intervention received (Edwards 2009).
Incomplete outcome data
One study excluded participants with a compliance of less than 80% from the analysis instead of analysis on an intention‐to‐treat basis (Kim 2009). This is important, especially when considering oral iron therapy where compliance could be a major factor in the efficacy of the treatment.
Selective reporting
As reported in incomplete outcome data, one study selectively reported data only from participants in whom compliance was greater than 80%, hence data do not represent all randomised patients on an intention‐to‐treat basis (Kim 2009). The effect of this is likely to skew the results in favour of intravenous iron therapy.
Other potential sources of bias
We identified no other source of bias.
Effects of interventions
See: Summary of findings for the main comparison Iron therapy versus placebo or no iron therapy for pre‐operative anaemia; Summary of findings 2 Iron therapy: Intravenous versus oral administration for pre‐operative anaemia
Comparison 1: Iron therapy compared to placebo or standard care
Primary outcome: Proportion of patients who received a blood transfusion
Two studies reported the proportion of patients who received allogeneic blood transfusions (Lidder 2007; Edwards 2009). There was a reduction in the proportion of patients who received a blood transfusion, but the reduction was not statistically significant (RR 0.56, 95% CI 0.27 to 1.18; participants = 38; I² = 0%; Analysis 1.1).
Secondary outcomes:
-
Amount of blood transfused per patient (in units)
Two studies involving 38 people reported the number of units of blood transfused in each treatment group. It was not possible to combine the data because they are skewed. The raw data are given in the table below.
| Study | Iron group | Control |
| median 0 units (IQR 1 unit) (9 people) (unspecified number of total units transfused) | median 2 units (IQR 3 units) (9 people) (unspecified number of total units transfused) | |
| median 1 units (range 0 to 2 units) (6 people) (6 units transfused in total) | median 2.5 units (range 0 to 11 units) (14 people) (39 units transfused in total) |
-
Post‐operative mortality in the short term (within 30 days) and long term (greater than one year)
Neither of the studies reported mortality.
-
Post operative morbidity (including infections and adverse events)
Neither study reported post‐operative morbidity.
-
Any validated measure of quality of life
None of the studies reported quality of life.
-
Measurement value of the following haematologic parameters: haemoglobin, haematocrit, ferritin level and reticulocyte count. Pre‐specified measurement time points were: pre‐treatment, pre‐operatively post‐treatment, and post‐operatively post‐treatment.
Haemoglobin level
The Lidder 2007 study authors collected data on this outcome at two time points, pre‐treatment and pre‐operatively post‐treatment, but the data are not reported separately for the 20 anaemic patients.
In the Edwards 2009 study, pre‐treatment, there was no difference in haemoglobin levels between the control and intervention groups ((MD −0.10 g/dL (95% CI −1.75 to 1.55), 18 people, Analysis 1.2).
The Edwards 2009 study reported haemoglobin levels for the 18 anaemic patients at the end of treatment, pre‐operatively. There was no difference in haemoglobin level between the control and intervention groups (MD −0.70 g/dL (95% CI −2.82 to 1.42), 18 people, Analysis 1.3).
Post‐operatively in the Edwards 2009 study, haemoglobin levels were slightly higher among people in the iron therapy group (MD −1.10 g/dL (95% CI −2.07 to −0.13), 18 people, Analysis 1.4). However, such a small difference in haemoglobin level is not clinically relevant and may be a chance finding as the data are based on such a small group of people. More research will clarify the true effect of treatment.
Haematocrit level
The Edwards 2009 study collected data on haematocrit levels pre‐treatment, and the end of treatment pre‐operatively, and after treatment post‐operatively, but no standard deviation values are reported and so it is not possible to analyse the data.
Ferritin
The Lidder 2007 study authors collected data on this outcome at two time points, pre‐treatment and pre‐operatively post‐treatment, but the data are not reported separately for the 20 anaemic patients.
The Edwards 2009 study collected data on ferritin levels pre‐treatment, and the end of treatment pre‐operatively, and after treatment post‐operatively, but no standard deviation values are reported and so it is not possible to analyse the data.
Reticulocyte count
The Lidder 2007 study authors collected data on this outcome at two time points, pre‐treatment and pre‐operatively post‐treatment, but the data are not reported separately for the 20 anaemic patients.
Comparison 2: Intravenous compared with oral iron therapy
One study compared intravenous iron therapy with oral iron therapy (Kim 2009). There were 39 people randomised to the intravenous group, and 37 people randomised to the oral iron therapy group. The investigators only took measurements from people who received at least 80% of the treatment they were intended to receive. Data are reported on 30/39 people (77%) randomised to IV therapy, and 26/37 people (70%) randomised to oral therapy. The results are at risk of bias because 1) there was lower compliance with oral iron therapy treatment, and 2) the data available for analysis do not describe the iron levels of people who took only some or none of the treatment they were assigned.
Primary outcome: Proportion of patients who received a blood transfusion
The Kim 2009 study did not measure this outcome.
Secondary outcomes:
-
Amount of blood transfused per patient (in units)
The Kim 2009 study did not measure this outcome.
-
Post‐operative mortality in the short term (within 30 days) and long term (greater than one year)
The Kim 2009 study did not measure this outcome.
-
Post‐operative morbidity (including infections and adverse events)
The Kim 2009 study reported "no severe adverse events were observed in the two groups, and only some tolerable adverse events were observed in each group. Two cases of myalgia and one case of injection pain developed in the intravenous iron group and one event of nausea and one event of dyspepsia was observed in the oral iron group" (p.39).
-
Any validated measure of quality of life
The Kim 2009 study did not measure this outcome.
-
Measurement value of the following haematologic parameters: haemoglobin, haematocrit, ferritin level and reticulocyte count. Pre‐specified measurement time points were: pre‐treatment, pre‐operatively post‐treatment, and post‐operatively post‐treatment.
Haemoglobin level
In the Kim 2009 study, pre‐treatment, there was no difference in haemoglobin levels between the intravenous and oral iron therapy groups (MD −0.30 g/dL (95% CI −0.90 to 0.30), 56 participants, Analysis 2.1).
The Kim 2009 study measured haemoglobin levels pre‐operatively, post‐treatment. Haemoglobin levels were slightly higher in the intravenous iron therapy group, compared to the oral iron therapy group (MD 1.90 g/dL (95% CI 1.16 to 2.64), 56 participants, Analysis 2.2). However, this result is at risk of bias and should be interpreted with caution because compliance with the intervention varied between treatment groups. Compliance was lower in the oral iron therapy group, and blood was analysed only from those who had taken more than 80% of their assigned treatment. People who did not receive or take more than 80% of their assigned treatment did not provide measurements for analysis and are not included in the results.
Haematocrit level
The Kim 2009 study did not measure this outcome.
Ferritin
The Kim 2009 study reported ferritin levels pre‐treatment, and there was no difference between the control and intervention groups (MD 75.80 µg/L (95% CI −46.57 to 198.17), Analysis 2.3).
The Kim 2009 study reported ferritin levels post‐operatively post‐treatment, and there was no difference between the control and intervention groups (MD 221.70 µg/L (95% CI −30.92 to 474.32), Analysis 2.4).
Reticulocyte count
The Kim 2009 study did not measure this outcome.
Discussion
Summary of main results
Three prospective randomised controlled studies evaluated pre‐operative iron therapy to correct anaemia, two in colorectal surgery (Lidder 2007, Edwards 2009), and one in gynaecological surgery (Kim 2009), and included 114 patients in total. The two colorectal trials reported the primary outcome (proportion of patients who received allogeneic blood transfusions) for 38 patients and meta‐analysis showed a reduction in blood transfusion with the administration of iron therapy which was not statistically significant (RR 0.56, 95% CI 0.27 to 1.18; participants = 38).
For the secondary outcomes, all studies reported haemoglobin change but data for the anaemic patients was only available for two studies (Edwards 2009; Kim 2009). Edwards 2009 showed no difference in haemoglobin at the end of treatment pre‐operatively with intravenous iron compared to placebo. The intravenous versus oral iron study showed an increase in haemoglobin with intravenous iron at the end of treatment pre‐operatively (MD 1.90 g/dL, 95% CI 1.16 to 2.64; participants = 56) (Kim 2009). However, the study authors likely bias their results with the exclusion of an important group of patients who had less than 80% compliance with treatment and therefore this finding should be interpreted with caution.
The amount of blood transfused was reported by Lidder 2007 and Edwards 2009 and showed a reduction with iron therapy. However, the skewed nature of data prevented analysis of these results. Other haematological parameters were reported but no data were available for the subgroup of anaemic patients thereby preventing analysis. Other secondary outcomes including quality of life, short‐ or long‐term mortality, and post‐operative morbidity were not reported by any of the studies.
Overall completeness and applicability of evidence
Evidence regarding iron therapy for pre‐operative anaemia is limited with currently only three randomised controlled studies, all with small sample sizes. Furthermore, the 38 patients available for analysis of the primary outcome does not meet the 819 patients recommended by the information size calculation, preventing us from reaching a reliable conclusion regarding the effects of iron therapy pre‐operatively.
These studies are also limited in their generalisability with only two surgical specialities represented. No assessment of quality of life, morbidity and mortality also limit the application of these data which fail to measure patient‐centred outcomes. These studies currently allow no conclusions to be drawn when comparing oral versus intravenous iron therapy either.
Quality of the evidence
Kim 2009, while the largest and better designed of the three trials, was still a small trial and made important omissions in not recording blood transfusions and quality of life outcomes. This study also excluded data in the final analysis from participants whose compliance was less than 80%, acknowledging that compliance is a major factor in the efficacy of oral iron therapy, but therefore not reflecting the reality that many patients do not adhere to oral iron due to side‐effects.
Both Edwards 2009 and Lidder 2007 did not exclude non‐anaemic patients or assess for iron deficiency. While they include a subgroup analysis of anaemic patients these studies have not been powered to show a difference in the group of patients that require iron to correct their anaemia, namely those with iron deficiency anaemia. As a result these two already small studies have even fewer patients with which to determine the true effect of iron therapy.
Agreements and disagreements with other studies or reviews
The three small randomised controlled trials presented here fail to support the conclusions of observational and case‐control studies that have demonstrated iron therapy reduces allogeneic blood transfusion and improves pre‐operative haemoglobin. These include studies from colorectal surgery (Okuyama 2005; Quinn 2010), orthopaedics (Cuenca 2004; Cuenca 2005), and gynaecological surgery (Breymann 2008).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Three studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 1 Proportion of patients who received a blood transfusion.

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 2 Haemoglobin levels pre‐treatment (g/dL).
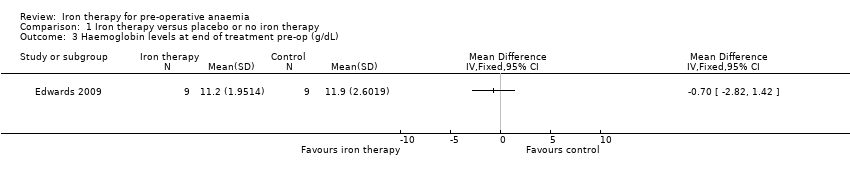
Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 3 Haemoglobin levels at end of treatment pre‐op (g/dL).
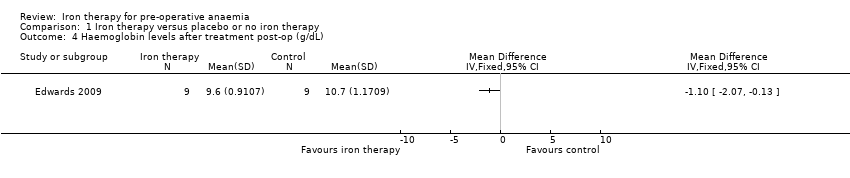
Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 4 Haemoglobin levels after treatment post‐op (g/dL).
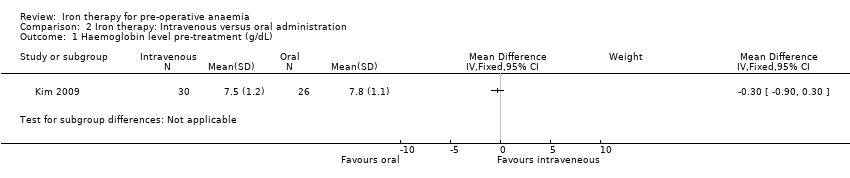
Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 1 Haemoglobin level pre‐treatment (g/dL).
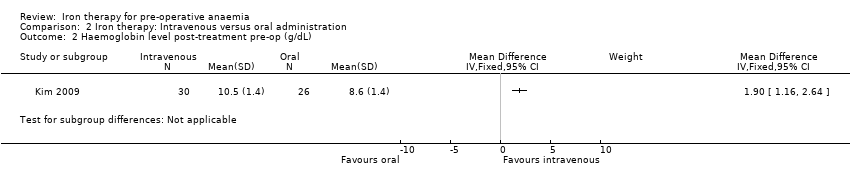
Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 2 Haemoglobin level post‐treatment pre‐op (g/dL).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 3 Ferritin level pre‐treatment (ɥg/L).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 4 Ferritin level post‐treatment (ɥg/L).
| Iron therapy versus placebo or no iron therapy for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Iron therapy | |||||
| Proportion of patients who received a blood transfusion | 635 per 1000 | 356 per 1000 | RR 0.56 | 38 | ⊕⊕⊝⊝ | |
| Amount of blood transfused per patient (in units) | Data from two small studies could not be combined as they were skewed and reported as medians and ranges. One RCT in 18 people reported a difference in medians of 0 (interquartile range: 1) with iron therapy. Another RCT in 20 people reported a median difference of 1 unit with iron therapy (range 0 to 2). | ‐ | 38 | ⊕⊕⊝⊝ | It is not possible to combine the data because they are skewed. These are the raw data. | |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 11.9 g/dL (SD 2.6) | mean 11.2 g/d L(SD 1.95) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intervention groups was 0.7 g/dL lower | 18 | ⊕⊕⊝⊝ | Data from one study; the raw data are presented. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only two small randomised control trials and a subset of anaemic patients resulting in a very small number of participants. | ||||||
| Iron therapy: Intravenous versus oral administration for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron therapy | Intravenous iron therapy | |||||
| Proportion of patients who received a blood transfusion | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Amount of blood transfused per patient (in units) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 8.6 g/dL (SD 1.4) | mean 10.5 g/dL (SD 1.4) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intravenous group was | 56 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study excluded those with less than 80% compliance with therapy and compliance was lower in the oral administration group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who received a blood transfusion Show forest plot | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 2 Haemoglobin levels pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Haemoglobin levels at end of treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Haemoglobin levels after treatment post‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Haemoglobin level pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Haemoglobin level post‐treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Ferritin level pre‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Ferritin level post‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

