Intensity of continuous renal replacement therapy for acute kidney injury
Abstract
Background
Acute kidney injury (AKI) is a common condition among patients in intensive care units (ICU), and is associated with substantial morbidity and mortality. Continuous renal replacement therapy (CRRT) is a blood purification technique used to treat the most severe forms of AKI but its effectiveness remains unclear.
Objectives
To assess the effects of different intensities (intensive and less intensive) of CRRT on mortality and recovery of kidney function in critically ill AKI patients.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 9 February 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov. We also searched LILACS to 9 February 2016.
Selection criteria
We included all randomised controlled trials (RCTs). We included all patients with AKI in ICU regardless of age, comparing intensive (usually a prescribed dose ≥35 mL/kg/h) versus less intensive CRRT (usually a prescribed dose < 35 mL/kg/h). For safety and cost outcomes we planned to include cohort studies and non‐RCTs.
Data collection and analysis
Data were extracted independently by two authors. The random‐effects model was used and results were reported as risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CI).
Main results
We included six studies enrolling 3185 participants. Studies were assessed as being at low or unclear risk of bias. There was no significant difference between intensive versus less intensive CRRT on mortality risk at day 30 (5 studies, 2402 participants: RR 0.88, 95% CI 0.71 to 1.08; I2 = 75%; low quality of evidence) or after 30 days post randomisation (5 studies, 2759 participants: RR 0.92, 95% CI 0.80 to 1.06; I2 = 65%; low quality of evidence). There were no significant differences between intensive versus less intensive CRRT in the numbers of patients who were free of RRT after CRRT discontinuation (5 studies, 2402 participants: RR 1.12, 95% CI 0.91 to 1.37; I2 = 71%; low quality of evidence) or among survivors at day 30 (5 studies, 1415 participants: RR 1.03, 95% CI 0.96 to 1.11; I2 = 69%; low quality of evidence) and day 90 (3 studies, 988 participants: RR 0.98, IC 95% 0.94 to 1.01, I2 = 0%; moderatequality of evidence). There were no significant differences between intensive and less intensive CRRT on the number of days in hospital (2 studies, 1665 participants): MD ‐0.23 days, 95% CI ‐3.35 to 2.89; I2 = 8%; low quality of evidence) and the number of days in ICU (2 studies, 1665 participants: MD ‐0.58 days, 95% CI ‐3.73 to 2.56, I2 = 19%; low quality of evidence). Intensive CRRT increased the risk of hypophosphataemia (1 study, 1441 participants: RR 1.21, 95% CI 1.11 to 1.31; high quality evidence) compared to less intensive CRRT. There was no significant differences between intensive and less intensive CRRT on numbers of patients who experienced adverse events (3 studies, 1753 participants: RR 1.08, 95% CI 0.73 to 1.61; I2 = 16%; moderate quality of evidence). In the subgroups analysis by severity of illness and by aetiology of AKI, intensive CRRT would seem to reduce the risk mortality (2 studies, 531 participants: RR 0.73, 95% CI 0.61 to 0.88; I2 = 0%; high quality of evidence) only in the subgroup of patients with post‐surgical AKI.
Authors' conclusions
Based on the current low quality of evidence identified, more intensive CRRT did not demonstrate beneficial effects on mortality or recovery of kidney function in critically ill patients with AKI. There was an increased risk of hypophosphataemia with more intense CRRT. Intensive CRRT reduced the risk of mortality in patients with post‐surgical AKI.
PICOs
Plain language summary
Intensity of continuous renal replacement therapy for acute kidney injury
What is the issue?
Acute kidney injury (AKI) is very common among patients admitted to intensive care units (ICU), it is associated with a high death rated and characterised by the rapid loss of the kidney function. Patients with AKI show increased levels of serum uraemic toxins (creatinine and urea), serum potassium and metabolic acids, accumulation of water and in the most cases a reduction in urine output. In this population these chemicals and fluid overload are related to increased rates of death. Theoretically, effective removal of toxins and excess water from the bloodstream might improve patient outcomes (such as mortality rate and recovery of kidney function).
Continuous renal replacement therapy (CRRT) is a blood purification technique that enables removal of excess water and toxins. CRRT involves blood being diverted from the patient via a catheter (a hollow, flexible tube placed into a vein) through a filtering system which continuously and steadily removes excess water and toxins; purified blood is then returned to the patient via the catheter. Higher intensity CRRT improves the removal of toxins and excess water. The aim of this review was to investigate the effect of different intensities of CRRT (intensive or less intensive) on death, recovery of kidney function, and adverse events in people with AKI who are critically ill.
What did we do?
We searched the literature up until February 2016 and identified six studies enrolling 3185 patients with AKI that were evaluated in this review.
What did we find?
Six randomised studies enrolling 3185 participants were included in our review. Compared to less intensive CRRT, intensive CRRT did not reduce the risk of death, improve the recovery of kidney function, or reduce the risk of adverse events (such as bleeding) in patients with AKI. Intensive CRRT was associated with an increased risk of low blood phosphate levels.
Authors' conclusions
Summary of findings
| Intensive versus less intensive CRRT for AKI | ||||||
| Patient or population: patients with AKI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Less intensive CRRT | Intensive CRRT | |||||
| Mortality at day 30 | Study population | RR 0.88 | 2402 (5) | ⊕⊕⊝⊝ | ||
| 430 per 1000 | 420 per 1000 | |||||
| Moderate | ||||||
| Mortality after 30 days post‐randomisation | Study population | RR 0.92 | 2759 (5) | ⊕⊕⊝⊝ | ||
| 514 per 1000 | 483 per 1000 | |||||
| Moderate | ||||||
| 593 per 1000 | 557 per 1000 | |||||
| Patients free of RRT after discontinuing CRRT | Study population | RR 1.12 | 2402 (5) | ⊕⊕⊝⊝ | ||
| 483 per 1000 | 541 per 1000 | |||||
| Moderate | ||||||
| 390 per 1000 | 437 per 1000 | |||||
| Patients free of RRT after discontinuing CRRT | Study population | RR 0.98 | 988 (3) | ⊕⊕⊕⊝ | ||
| 923 per 1000 | 904 per 1000 | |||||
| Moderate | ||||||
| 800 per 1000 | 784 per 1000 | |||||
| Adverse events: hypophosphataemia | Study population | RR 1.21 | 1441 (1) | ⊕⊕⊕⊕ | ||
| 540 per 1000 | 654 per 1000 | |||||
| Moderate | ||||||
| 540 per 1000 | 653 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ inconsistency: due to substantial heterogeneity (I2 values ranged from 73% to 78%) ² imprecision: due to wide CI which crossed the threshold for clinically meaningful effects ³ Indirectness: critically ill patients with AKI in CRRT have high short‐term mortality risk; mortality is a competing end point for kidney recovery at day 90 | ||||||
| Intensive versus less intensive CRRT for AKI: subgroups | ||||||
| Patient or population: patients with AKI who need CRRT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard dose | High dose | |||||
| Mortality: patients with sepsis | Study population | RR 0.94 | 966 (5) | ⊕⊕⊝⊝ | ||
| 524 per 1000 | 492 per 1000 | |||||
| Moderate | ||||||
| 618 per 1000 | 581 per 1000 | |||||
| Mortality: patients without sepsis | Study population | RR 0.89 | 1216 (4) | ⊕⊕⊝⊝ | ||
| 465 per 1000 | 414 per 1000 | |||||
| Moderate | ||||||
| 564 per 1000 | 502 per 1000 | |||||
| Mortality: patients with AKI related to cardiac or general surgery | Study population | RR 0.73 | 531 (2) | ⊕⊕⊕⊕ | ||
| 505 per 1000 | 368 per 1000 | |||||
| Moderate | ||||||
| 459 per 1000 | 335 per 1000 | |||||
| Mortality: patients with AKI not related to surgery | Study population | RR 0.94 | 1871 (3) | ⊕⊕⊝⊝ | ||
| 414 per 1000 | 389 per 1000 | |||||
| Moderate | ||||||
| 550 per 1000 | 517 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ inconsistency: due to substantial heterogeneity (I2 values ranged from 73% to 78%) ² imprecision: due to wide CI which crossed the threshold for clinically meaningful effects | ||||||
Background
Description of the condition
Acute kidney injury (AKI) is a complex clinical entity characterised by an abrupt decline in kidney function (Mehta 2007). AKI incidence among adults admitted to intensive care units (ICU) range from 5% to 20% (Hoste 2006; Joannidis 2005); in children the incidence is 10% (Schneider 2010). Despite its potential to be reversed, AKI is associated with high rates of morbidity and mortality (Bagshaw 2007). AKI‐related mortality substantially increases among people with multi‐organ failure, sepsis or who are receiving renal replacement therapy (RRT) (Metnitz 2002; Sutherland 2010). More than 70% of people with AKI need renal support therapies. Despite advances in clinical care, people with AKI are at high risk of mortality and morbidity, and require significant healthcare resources (Sutherland 2010; Uchino 2005).
Description of the intervention
Continuous renal replacement therapy (CRRT) is an extracorporeal blood purification therapy, intended to support impaired kidney function. CRRT slowly removes fluid over prolonged periods (Foland 2004; Gibney 2008; Goldstein 2001; Mehta 1999), removes higher molecular weight solutes efficiently (Brunnet 1999; Clark 1999; Liao 2003; Ronco 2002; Sieberth 1995), and confers beneficial haemodynamic stability effects. CRRT modalities are defined by their main solute clearance mechanism. These are convection (continuous venovenous haemofiltration (CVVH)), diffusion (continuous venovenous haemodialysis (CVVHD)), or a combination of both convection and diffusion (continuous venovenous haemodiafiltration, CVVHDF) (Palevsky 2002). Several interventions have been used over the past three decades with the aim of improving poor prognoses of people with AKI. A significant factor that may impact on CRRT outcomes is intensity of treatment (timing of CRRT for AKI is being investigated in another Cochrane review, Fayad 2013a).
CRRT intensity is generally related to the quantity of solute removal required to improve outcomes in people with AKI. CRRT intensity can be analysed based either on solute removal from the blood, or appearance of solutes in effluent fluid. Some published studies have used effluent flow rates, expressed as total effluent volume/weight and unit of time (mL/kg/h), as a dose surrogate (RENAL Study 2006; Ronco 2000a), while accounting for effects of pre‐dilution and modality differences (Claure‐Del Granado 2011). Elsewhere, authors have considered that dialysis doses delivered as total effluent volume/clearance of solutes such as urea, creatinine is a better method to measure dose (Lyndon 2012). Equivalent renal urea clearance also provides a good estimate of delivered dialysis dose in CRRT (Claure‐Del Granado 2012) which can be converted to effluent rate and expressed as mL/kg/h (Marshall 2006).
Few studies have assessed other dimensions of dose such as electrolyte and acid‐base homeostasis (Bellomo 2013; Bihorac 2005; Morimatsu 2003; Uchino 2001) and fluid balance/fluid overload (Bouchard 2009; Davenport 2010; Sutherland 2010) using effluent volume as the dose measure.
How the intervention might work
A hypothesis that high intensity of RRT may improve survival has emerged from animal and human studies. These findings include indirect evidence from patients with ESKD (Lowrie 1981; Parker 1994).
Intensity based on a urea kinetics model was evaluated in animal studies by Grootendorst 1992, and in severe ill patients (sepsis, sepsis‐shock) who received high dose (60 to 80 mL/kg/h) reported improvement in haemodynamic state with possible benefits in clinical outcomes (Honore 2000). A retrospective study found that dose correlated with survival in patients with intermediate scores of illness (Paganini 1996). Although prospective dose studies demonstrated association of improved survival or kidney recovery with high dose dialysis (Phu 2002; Ronco 2000a; Saudan 2006), these advantages were not universally observed (ATN Study 2005; Negash 2011; RENAL Study 2006; Vesconi 2009; Van Wert 2010).
Few studies have researched other components of dose that play important roles in clinical results. These include fluid balance and fluid overload associated with increased mortality risk (Bouchard 2009; Goldstein 2001), adequate homeostasis of electrolytes (sodium, potassium and hydrogen ions) related to cardiovascular stability, and the maintenance of kidney blood flow (Uchino 2001).
Why it is important to do this review
Studies assessing CRRT intensity (intensive versus less intensive) have either not reported investigation of all variables inherent in therapy for people with AKI or report inconsistent results. We investigated the relationship between different intensities of CRRT and clinical outcomes for people with AKI. Review evidence could have direct relevance to decisions about optimal intensity of CRRT to improve survival in critically ill patients with AKI.
Objectives
To assess the effects of different intensities (intensive and less intensive) of CRRT on mortality and recovery of kidney function in critically ill AKI patients.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at CRRT modalities for people with AKI in ICU settings were eligible for inclusion. For outcomes such us safety and costs, non‐RCTs and cohort studies were also to be included if sufficiently high quality, sampling was clearly described, patients characterised, proportions of patients experiencing any adverse events or who dropped out because of adverse events was adequately reported, co‐interventions were described, and at least 80% of patients included were analysed after treatment.
Types of participants
Inclusion criteria
We included all patients with AKI in ICU being treated with CRRT regardless of age or gender. We assigned AKI definitions cited by the included studies.
Exclusion criteria
We excluded patients who received dialysis treatment before admission to ICU, patients admitted for drug overdose (doses exceeding therapeutic requirements), or with acute poisoning (all toxins).
Types of interventions
We compared intensive (usually a prescribed dose ≥ 35 mL/kg/h) versus less intensive CRRT (usually a prescribed dose < 35 mL/kg/h). These categories of intensities were defined as published in the original publications. We included all CRRT modalities (CVVH, CVVHD and CVVHDF).
Types of outcome measures
Primary outcomes
Death
-
Death from any cause at days 7, 15, 30, 60, and 90
-
Death or non‐recovery at 90 days.
Recovery of kidney function
-
Numbers of patients free of RRT after discontinuing CRRT
-
Numbers of patients free of RRT after discontinuing CRRT at days 30, 60, and 90.
Secondary outcomes
Metabolic balance
-
Numbers of patients who normalised serum electrolytes (potassium, sodium) concentration during CRRT
-
Numbers of patients who normalised serum bicarbonate and base‐excess concentration during CRRT
-
Numbers of patients who normalised serum urea and creatinine concentration during CRRT.
Fluid balance
-
Numbers of patients who achieved adequate fluid balance during CRRT.
Adverse events
-
Numbers of patients who dropped out because of adverse events (technique or patient‐dependent factors)
-
Numbers of patients experiencing any adverse events
-
Numbers of patients with intervention‐related complications (e.g. disequilibrium, hypokalaemia, hypophosphataemia, hypocalcaemia, bleeding, hypotension)
-
Numbers of patients with catheter‐related complications (early and late).
We looked for differences in overall dropout rates and any adverse effects by type (mild or severe). We defined adverse events severity where medical therapeutic interventions were implied in reporting. Withdrawals due to protocol violation or loss to follow‐up were not included in counts of adverse events.
Length of stay
-
Days in hospital
-
Days in ICU.
Cost
We planned to assess costs of CRRT modalities including:
-
Type and number of dialyser filters
-
Use/no use of anticoagulation
-
Types of anticoagulation and anticoagulants
-
Use of replacement fluid
-
Numbers of days on CRRT.
All costs were to be reported in international monetary units.
-
Cost per day of CRRT (expressed in international monetary units)
-
Length of hospital stay with CRRT
-
Length of ICU stay with CRRT.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 9 February 2016 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
-
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference, proceedings and currents awareness alert, available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
LILACS (Latin American and Caribbean Health Sciences) (from March 1980 to February 2016)
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies with potential relevance to the review. Titles and abstracts were screened independently by two authors who discarded studies that were not applicable; however studies and reviews that could include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary, the full text of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. We resolved any discrepancy by discussion.
Assessment of risk of bias in included studies
The following items were independently assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For normally distributed outcomes, we calculated summary estimates of treatment effects using the inverse variance method. For dichotomous outcomes (mortality, kidney recovery and adverse events) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (length of stay, cost) the mean difference (MD) was used or the standardised mean difference (SMD) if different scales were used.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g., last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
If possible, funnel plots were to be used to assess the potential existence of small study bias (Higgins 2011).
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (such as intervention, participant and study quality). Heterogeneity among participants could relate to age, gender, fluid overload (< 10% and > 10% body weight relative to baseline), Intensive CRRT for AKI in homogenous subpopulations such as cardiac surgery or sepsis patients, effects of intensive continuous therapy on severity of illness ‐ high, intermediate and low. We used appropriate scores of illness severity, such as Pediatric Risk of Mortality (PRISM), Pediatric Index of Mortality (PIM), Acute Physiology and Chronic Health Evaluation (Apache), Sequential Organ Failure Assessment (SOFA), and Cleveland Clinic ICU Acute Renal Failure (CCF).
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect size.
-
Repeating the analysis excluding unpublished studies
-
Repeating the analysis taking account of risk of bias
-
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
-
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). Two summary of findings tables were created. summary of findings Table for the main comparison summarizes the main findings for the comparison "Intensive versus less intensive RRT for acute kidney injury". We presented the following outcomes.
-
Mortality until day 30 post‐randomisation
-
Mortality after 30 days post‐randomisation
-
Kidney function recovery: number of patients free of RRT after discontinuing CRRT
-
Kidney function recovery: number of patients free of RRT after discontinuing CRRT until day 90, among survivals
-
Adverse events: number of patients with hypophosphataemia
summary of findings Table 2 summarizes the main mortality findings for the subgroups of patients with AKI with and without sepsis, and related or not to surgery.
Results
Description of studies
See. Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We identified 185 records from electronic databases (MEDLINE, EMBASE, CENTRAL, Cochrane Kidney and Transplant's Specialised Register, LILACS) to 9 February 2016. We screened these titles and abstracts excluded 117 records. We assessed the full text of 68 potentially eligible records (21 studies). Six studies (52 records) were included in our review (ATN Study 2005; Bouman 2002; RENAL Study 2006; Ronco 2000a; Saudan 2006; Tolwani 2008). One study has recently been completed but no results have been published (NCT01560650). Fourteen studies (15 records) were excluded (Figure 1). There was no disagreement among authors regarding inclusion of studies.

Study flow diagram
Included studies
Six included studies (ATN Study 2005; Bouman 2002; RENAL Study 2006; Ronco 2000a; Saudan 2006; Tolwani 2008) enrolled a total of 3185 participants.
Study participants were all admitted to ICU. The mean age ranged from 51 and 68 years, and the proportion of male ranged from 55% to 71%. Sepsis was the primary cause of AKI in four studies (ATN Study 2005; RENAL Study 2006; Saudan 2006; Tolwani 2008) and surgery or cardio‐surgery the main cause in the other two (Bouman 2002; Ronco 2000a)
All studies were reported between 2000 and 2008. Three were single‐centre studies (Ronco 2000a; Saudan 2006; Tolwani 2008) and three were multicentre (ATN Study 2005; Bouman 2002; RENAL Study 2006).
Four studies used one CRRT modality exclusively; RENAL Study 2006 and Tolwani 2008 used CVVHDF, and Bouman 2002 and Ronco 2000a used CVVH. Saudan 2006 used CVVH and CVVHDF and ATN Study 2005 used intermittent haemodialysis (IHD) and CVVHDF or sustained low‐efficiency haemodialysis (SLED), depending on the haemodynamic stability of the participant. Replacement fluid was administered either pre filter (ATN Study 2005; Saudan 2006; Tolwani 2008) or post filter (Bouman 2002; Ronco 2000a; RENAL Study 2006) when either CVVH or CVVHDF were used.
Five studies assessed the effects of two intensities of continuous therapy (ATN Study 2005; Bouman 2002; RENAL Study 2006; Saudan 2006; Tolwani 2008), whereas one assessed the effects of three CRRT intensities (standard, intermediate and high) (Ronco 2000a). For the purpose of the analysis, we combined the intermediate with high‐dose arm of this study to create one high intensity arm. In Bouman 2002, two arms received the same less intensive CRRT dose but differed only in the timing of CRRT initiation. We combined these two treatment arms to create one less intensive arm. ATN Study 2005 randomly assigned critically ill patients with AKI to high‐intensity or low‐intensity RRT. Within treatment groups, patients were allocated to intermittent (IHD) or prolonged (SLED) and continuous RRT according to cardiovascular SOFA score. Continuous RRT was provided to 69.7% of patients as their initial therapy. However, unstable patients assigned to CRRT had a variable number of switches in treatment modality: none (≈36.1%) or 1 (≈24%) and ≥ 2 modalities (≈10%) (Palevsky 2009). For the purpose of the analysis, we included all patients initially allocated to continuous CRRT independently of the switches in treatment modality (intention‐to‐treat analysis), who survived to day 60.
Five studies prescribed dose according to patients’ weight at the time of admission (ATN Study 2005; RENAL Study 2006; Ronco 2000a; Saudan 2006; Tolwani 2008), and only one prescribed dose per unit of time (Bouman 2002).
Overall, the prescribed dose of CRRT in the less intensive arm of included studies ranged between 20 to 25 mL/kg/h and in intensive arm ranged between 35 to 48 mL/kg/h.
For full details see Characteristics of included studies.
Excluded studies
Fourteen studies were excluded. Cole 2002 and Vesconi 2009 compared continuous dialysis therapy versus no haemofiltration or other RRT (intermittent haemodialysis). Boussekey 2008, IVOIRE Study 2013, Sanchez 2010b and Zhang 2012 and two ongoing studies (NCT01191905; NCT01251081) compared different intensity‐arms treatment. Ghani 2006 and Payen 2009 did not provide relevant outcomes for this review. Two studies were not RCTs (Brause 2003; Zha 2012). In HEROICS Study 2015, 36% of control‐arm patients did not receive CRRT. Jiang 2005 had different inclusion criteria in relation to our review. Only six patients (16%) with severe pancreatitis have AKI and were treated with CRRT (Characteristics of excluded studies).
Risk of bias in included studies
Included studies were generally at low or unclear risk of bias for all domains (See Figure 2; Figure 3).
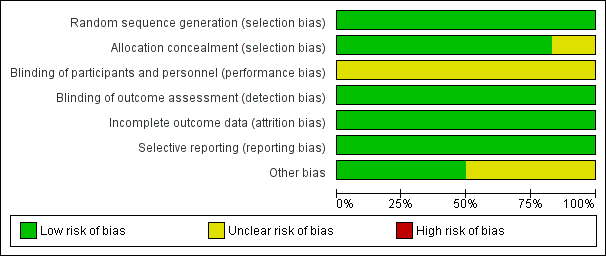
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Bouman 2002 and Ronco 2000a did not provide detailed information about random sequence generation and allocation concealment processes. Authors were contacted and we were informed that the random sequence generation was appropriate (computer‐generated) and sealed opaque envelopes were used for the allocation process. All studies were assessed as being at low risk of selection bias due to appropriate random sequence generation and five were allocation concealment processes. The allocation process was considered unclear in Saudan 2006 due to disease severity imbalance observed between the study arms.
Blinding
All included studies were assessed at low risk of detection bias (outcome measurement was unlikely to be influenced by lack of blinding), and unclear risk of performance bias (insufficient information to enable judgment).
Incomplete outcome data
The risk of attrition bias was low in all included studies. Intention‐to‐treat analysis was performed in all studies.
Selective reporting
Published records included all expected outcomes and were considered at low risk of bias.
Other potential sources of bias
Three studies provided complete information on grant support for authors and studies (ATN Study 2005; RENAL Study 2006; Tolwani 2008). This information was unclear in the remaining three studies.
Effects of interventions
See: Summary of findings for the main comparison Intensive versus less intensive continuous renal replacement therapy (CRRT) for acute kidney injury (AKI); Summary of findings 2 Intensive versus less intensive continuous renal replacement therapy (CRRT) for acute kidney injury (AKI): subgroups
The effects of intensive CRRT versus less intensive CRRT for main results and the quality of the evidence are summarised in summary of findings Table for the main comparison
Mortality
All six studies assessed the effect of different intensities of CRRT (intensive versus less intensive treatment) on mortality. These studies varied in mortality reporting timing: at 90 days (RENAL Study 2006; Saudan 2006), 60 days (ATN Study 2005), 28 days after randomisation or at ICU discharge (Bouman 2002; Tolwani 2008), and 15 days after cessation of CRRT (Ronco 2000a).
There was no significant difference between intensive versus less intensive CRRT on mortality risk at day 30 (Analysis 1.1.1 (5 studies, 2402 participants): RR 0.88, 95% CI 0.71 to 1.08; I2 = 75%) or after 30 days post randomisation (Analysis 1.1.2 (5 studies, 2759 participants): RR 0.92, 95% CI 0.80 to 1.06; I2 = 65%). There was substantial heterogeneity among studies. We downgraded the quality of evidence from high to low due to this inconsistency and imprecision (summary of findings Table for the main comparison).
Subgroup analysis and investigation of heterogeneity
There was evidence of significant heterogeneity in the magnitude of the effect among the included studies that measured mortality at different times of randomisation (at day 30 and after 30 days). To assess heterogeneity among participants we planned to perform the following pre‐specified subgroup analyses: by age, gender, fluid overload (≤ 10% and > 10% in body weight relative to baseline), according to aetiology of AKI and severity of illness. Only data for aetiology of AKI and severity of illness were available.
The effect of the severity of illness at baseline was assessed using two subgroups: patients with and without sepsis and patients with high and low SOFA cardiovascular scores (≥ 3 and < 3). The was no significant differences in mortality between intensive versus less intensive CRRT in patients with sepsis (Analysis 1.2.1 (5 studies, 966 participants): RR 0.94; 95% CI 0.69 to 1.27; I2 = 72%) or without sepsis (Analysis 1.2.2 (4 studies, 1216 participants): RR 0.89, 95% CI 0.69 to 1.15; I2 = 73%), or in patients with SOFA scores < 3 (Analysis 1.2.3; 1 study, 404 participants: RR 0.91, 95% CI 0.71 to 1.18) or SOFA score ≥ 3 (Analysis 1.2.4; 1 study, 1056 participants: RR 1.04, 95% CI 0.92 to 1.18).
The effect of AKI aetiology was considered using two subgroups: patients with AKI secondary to surgical causes and patients with AKI related to non‐surgical causes. Compared to less intensive CRRT, Intensive CRRT reduced the risk of death in patients with post‐surgical AKI (Analysis 1.2.5 (2 studies, 531 participants): RR 0.73, 95% CI 0.61 to 0.88; I2 = 0%), but not in patients with AKI related to non‐surgical causes (Analysis 1.2.6 (3 studies, 1871 participants): RR 0.94, 95% CI 0.73 to 1.20; I2 = 76%).
The heterogeneity observed in subgroup analyses could be explained by AKI aetiology (test for subgroup differences: Chi2 = 9.56; P = 0.09; I2 = 47.7%). When the post‐surgery AKI group was removed from the analysis, I2 = 0% (test for subgroup differences: Chi2 = 1.96, P = 0.74).
We downgraded the quality of evidence from high to low due to inconsistency and imprecision on the following subgroup analyses: patients with and without sepsis, patients with high and low SOFA cardiovascular score and patients with non‐surgical AKI. High quality of evidence was found for the subgroup of patients with AKI related to surgery (summary of findings Table 2).
Sensitivity analysis
The sensitivity analysis was performed excluding studies by risk of bias and large studies. When the analysis was developed taking risk of bias into account we observed that Saudan 2006 contributed to heterogeneity, and when excluded, heterogeneity was not significant (P = 0.63; I2 = 0%). The reason for exclusion was significant imbalance in the severity of illness observed between treatment arms. The effect of high intensity on mortality changed, but the direction of effects remained constant. We found no changes in heterogeneity when the study with larger sample size was excluded.
Data on death or non‐recovery at 90 days was not available.
Recovery of kidney function
Five studies reported information on recovery of kidney function (in all patients and among survivors). Studies varied in reporting of kidney recovery timing: 90 days after randomisation (RENAL Study 2006; Saudan 2006); 28 days (RENAL Study 2006; Saudan 2006; Tolwani 2008); at hospital discharge (Bouman 2002;Tolwani 2008); or 15 days after cessation of CRRT (Ronco 2000a).
Overall, there was no significant difference between intensive versus less intensive CRRT in the numbers of patients who were free of RRT after CRRT discontinuation (Analysis 1.3.1 (5 studies, 2402 participants): RR 1.12, 95% CI 0.91 to 1.37; I2 = 71%). There was substantial heterogeneity among studies. We downgraded the quality of evidence from high to low due to inconsistence and imprecision.
Similarly there was no significant difference between intensive versus less intensive CRRT on recovery of kidney function among survivors who discontinued CRRT at day 30 (Analysis 1.3.2 (5 studies, 1415 participants): RR 1.03, 95% CI 0.96 to 1.11; I2 = 69%) or at day 90 (Analysis 1.3.3 (3 studies, 988 participants): RR 0.98, IC 95% 0.94 to 1.01, I2 = 0%). We downgraded the quality of evidence from high to low due to inconsistency and indirectness and rated as moderate quality of evidence, due to indirectness, respectively (summary of findings Table for the main comparison)
Subgroup analysis and investigation of heterogeneity
There was evidence of significant heterogeneity in the magnitude of the effect among the included studies that measured recovery of kidney function at different times in relation to randomisation. To assess heterogeneity among participants, we performed prespecified subgroup analyses by age, gender, fluid overload (≤ 10% and > 10% in body weight in relation to baseline), according to AKI aetiology and severity of illness. Only data for AKI aetiology were available. The effect of AKI aetiology was assessed using subgroups: patients with AKI predominantly related to surgical causes and patients with AKI related to non‐surgical causes. Compared to less intensive CRRT, intensive CRRT increased recovery of kidney function in patients with post‐surgical AKI (Analysis 1.4.1 (2 studies, 531 participants): RR 1.27, 95% CI 1.05 to 1.53, P = 0.01, I2= 0%), but there were no difference on recovery of kidney function in patients with AKI related to non‐surgical causes (Analysis 1.4.2 (3 studies, 1870 participants): RR 1.12, 95% CI 0.73 to 1.71, P = 0.61, I2= 82%). There was no heterogeneity between groups (test for subgroup differences: Chi2 = 0.27, P = 0.60, I2 = 0%).
Sensitivity analysis
There was evidence of heterogeneity in recovery of kidney function up to 30 days after discontinuation of CRRT (I2 = 69%). We performed sensitivity analyses to explore the above listed factors on the effect size. Few data were available. Sensitivity analysis was performed excluding studies with high risk of bias and those with large sample sizes. When the analysis was developed taking risk of bias into account, we observed that Ronco 2000a contributed to heterogeneity. When excluded, heterogeneity was not significant (P = 0.63; I2 = 0%). Ronco 2000a included a non‐validated outcome (kidney recovery 15 days after cessation of CRRT) and enrolled patients with high incidence of post‐surgical AKI, which may have contributed to a better prognosis on recovery of kidney function. The effect of intensive CRRT remained constant and the direction of effects did not change.
Length of stay
RENAL Study 2006 and Tolwani 2008 compared the effects of intensity of CRRT on length of stay. There were no significant differences between intensive and less intensive CRRT on the number of days in hospital (Analysis 1.5.1 (2 studies, 1665 participants): MD ‐0.23 days, 95% CI ‐3.35 to 2.89; I2 = 8%) and the number of days in ICU (Analysis 1.5.2 (2 studies, 1665 participants): MD ‐0.58 days, 95% CI ‐3.73 to 2.56, I2 = 19%). We downgraded the quality of evidence from high to low due to substantial imprecision and risk of bias.
Metabolic control
There was no significant difference between intensive and less intensive CRRT on the numbers of patients who normalised metabolic acidosis (Analysis 1.6.1 (1 study, 115 participants): RR 1.05, 95% CI 0.73 to 1.51). We rated this as moderate quality evidence due to imprecision.
Adverse events
The effects of intensity of CRRT on adverse events were reported in three studies (Bouman 2002; RENAL Study 2006; Saudan 2006). Intensive CRRT increased the risk of hypophosphataemia (Analysis 1.7.2 (1 study, 1441 participants): RR 1.21, 95% CI 1.11 to 1.31) compared to less intensive CRRT. We rated this as high quality evidence.
There were no significant differences between intensive and less intensive CRRT on the numbers of patients who experienced adverse events (Analysis 1.7.1 (3 studies, 1753 participants): RR 1.08, 95% CI 0.73 to 1.61; I2 = 16%), hypokalaemia (Analysis 1.7.3 (1 study, 1455 participants): RR 0.96, 95% CI 0.80 to 1.15), arrhythmia (Analysis 1.7.4 (1 study, 1463 participants): RR 0.92, 95% CI 0.80 to 1.06), and bleeding (Analysis 1.7.5 (3 studies, 1775 participants): RR 0.78, 95% CI 0.27 to 2.24; I2 = 0%). We downgraded the quality of evidence from high to moderate due to imprecision and for the last outcome we rate as low quality evidence due to substantial imprecision (summary of findings Table for the main comparison).
Fluid balance and costs of CRRT were not reported in any of the included studies.
Evaluation of publication bias
We constructed a funnel plot to investigate potential publication bias. Meta‐analysis of mortality at day 30 was analysed. We found reasonable symmetry indicating a low risk of publication bias (Figure 4).

Funnel plot of comparison: 1 High Intensity versus less intensive CRRT, outcome: 1.1 Mortality
Discussion
Summary of main results
Our systematic review and subsequent meta‐analysis examines the effect of different intensities of CRRT on mortality, kidney recovery function and adverse events among 3185 critically ill patients with AKI. Most of the included studies were assessed at low or unclear risk of bias for all domains. Within the intensity ranges assessed, more intensive CRRT did not demonstrate beneficial effects on mortality at different time points post randomisation (day 30 or after 30 days) and kidney recovery function (in all patients and among survivals at 30 and 90 days before randomisation) compared with less intensive therapy. The overall estimated effects on these outcomes are not statistically significant; the confidence intervals are sufficiently wide to include clinically benefits and harm (imprecision), with high level of heterogeneity (inconsistency). Treatment with more intensive CRRT increased the risk of hypophosphataemia, although it did not increase other adverse events when compared to less intensive CRRT. All results (except hypophosphataemia) were imprecise because the confidence intervals were wide which crossed the threshold for clinically meaningful effects.
An important limitation of this systematic review was the substantial heterogeneity found in the main results as mortality at day 30 or after 30 days (I2 = 75% and I2 = 65% respectively) and recovery of kidney function in all patients and among survivals at 30 (I2 = 71% and I2 = 69% respectively). However, there was no heterogeneity identified for recovery of kidney function at 90 days (I2 = 0%). We explored this heterogeneity by two prespecified clinical subgroup analyses; severity of acute illness (patients with and without sepsis and high and low SOFA cardiovascular scores) and by aetiology of AKI. We found that in patients with surgery‐acquired AKI, intensive CRRT reduced mortality risk at day 30 compared to those patients with non‐surgically‐acquired AKI. Therefore, AKI aetiology was identified as a source of heterogeneity in the size of effect among included studies.
More intensive CRRT had uncertain effects on length of stay, number of days in ICU and number of days in hospital. These results should be interpreted with caution owing to the fact that only two small studies reported these data. Some studies have reported days in hospital and days in ICU but, in patients with a high short‐term mortality risk, the interpretation of such results may be misleading given the mortality is a competing end point for length of stay.
Overall completeness and applicability of evidence
See Description of studies; Characteristics of included studies
Six randomised studies (ATN Study 2005; Bouman 2002;RENAL Study 2006; Ronco 2000a; Saudan 2006; Tolwani 2008) evaluated the effect of different intensities of CRRT on survival and recovery of kidney function in critically ill patients with AKI. Two studies (Ronco 2000a; Saudan 2006) favoured more intensive therapy. In contrast, four other studies (ATN Study 2005; Bouman 2002;RENAL Study 2006; Tolwani 2008) have not demonstrated beneficial effects with an increased intensity of therapy on clinical outcomes. These results are consistent with those reported in our review.
Disparity and the heterogeneity found in these results among the studies probably may explained by several factors such as differences in methodological quality of studies, patients characteristics, delivered dialysis dose, and timing of CRRT initiation.
In a study of 425 patients, Ronco 2000a reported a decrease in mortality at day 15 from 59% to 43% with an increased in the intensity from 20 mL/kg/h to 35 or 45 mL/kg/h. We observed some limitations in this study: an absence of detailed description of randomisation and allocation concealment process (limiting the internal validity); a low incidence of patients with AKI‐related to sepsis (15%); and a non‐validated short‐term outcome (limiting the external validity). In Saudan 2006 (206 patients with AKI) there was a 26% reduction in all‐cause of mortality at day 90 (from 62% to 36%) with an increase in the intensity of CRRT from 25 mL/kg/h to 45 mL/kg/h. This study has an important imbalance in the severity of illness observed between CVVH treatment arms (limiting the internal validity). Additionally, both studies were unblinded, single‐centre studies (limiting the internal and external validity respectively). In contrast, three studies evaluating intensity in continuous therapy, and one study in combined modalities (Intermittent and continuous), did not demonstrate any effect of increased intensity of therapy on survival. Bouman 2002 conducted a small study evaluating both intensity and timing of initiation of CVVH in 106 critical patients with AKI. There were no differences on the survival for either intensities or initiation time. It is interesting to note that the actual delivered therapy in the high‐intensity arm was much less than the prescribed intensity. Furthermore, survival was greater than expected (survival at 28 days 69% to 75% in all groups) probably related to a low incidence of patients with AKI‐related to sepsis (limiting the external validity). The study was underpowered due to the small sample size. Similarly, Tolwani 2008 evaluated 200 patients with AKI. They found no difference on survival with intensive continuous therapy. This was an unblinded, single‐centre study (limiting the internal and external validity respectively). Finally, two large multicentre studies were conducted. In ATN Study 2005, 1124 critically ill patients with AKI were randomised to high‐intensity or low‐intensity. Within treatment groups, patients were allocated to CVVHDF or SLED and IHD according to cardiovascular SOFA score. In RENAL Study 2006, 1508 patients were randomly assigned to two intensities of CVVHDF (intensive or less intensive). All patients received CRRT as their first mode of RRT; only a small proportion of patients received IHD (7%). Both studies reported no beneficial effect on mortality and recovery of kidney function associated with a more intensive RRT.
There were also differences in the prescribed and delivered doses of CRRT. In ATN Study 2005, the dose delivered was 89% of that prescribed for higher‐intensity treatment, Tolwani 2008 reported a value of 83%, and in RENAL Study 2006 the delivered dose was 84%. For the lower‐intensity treatment, the doses delivered were 95% in ATN Study 2005, 85% in Tolwani 2008, and 88% in RENAL Study 2006. In all other studies (Bouman 2002; Ronco 2000a; Saudan 2006) delivered doses were less than 85% of the prescribed doses. The difference between the prescribed and the delivered dose highlights the risk of overestimating the effective delivery of therapy and the need to improve operational measures in CRRT.
Although the analysis included data obtained from a comprehensive and rigorous search, we identified gaps in several areas. The majority of participants of the included studies were adults, limiting the applicability of our finding to children. In general, the incidence of AKI secondary to sepsis is very high in ICU (50% to 60%); however, in two studies it was observed that the majority of patients had post‐surgical AKI, and relatively few had sepsis or pre‐existing chronic kidney disease, limiting the applicability of our results to general ICU population. Three included studies were single‐centre studies, limiting the external validity of the results.
While the urea kinetics remains widely used to measure intensity of RRT in AKI‐patients, this approach provides an incomplete assessment of dose of RRT, especially in the critically ill patients with AKI.
An important challenge when examining the evidence of dialysis intensity in patients with AKI is to determine the exact number of patients who received IHD or CRRT in those studies using a combination of both strategies (ATN Study 2005), as well as distinguishing which patients remained dialysis‐depend after ICU discharge or received transitory IHD without regard to the original assigned treatment. In view of this, we contacted the authors for more information. The long‐term kidney outcomes after hospital discharge among survivors of AKI remain poorly characterised. The studies did not report data on mortality and kidney function recovery in patients with pre‐existing chronic kidney disease and with low or intermediate scores of severity of illness. The results on length of stay (days in hospital and in the ICU) and recovery of kidney function should be interpreted with caution, especially when the mortality risk is taken into account.
We are aware that an important aspect to consider in term of efficacy is the timing in which CRRT are indicated. Currently, we are trying to answer this question with a systematic review specified (Fayad 2013a).
We included only RCTs with the purpose of reducing bias.
Quality of the evidence
We conducted this review according to the process described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Our review was based on evidence from six RCTs (3185 participants) that compared different intensities of CRRT in critically ill patients with AKI. The quality of evidence for our main outcomes was drawn from studies assessed at low risk of bias for random sequence generation and allocation concealment processes, incomplete outcome data, intention to treat analysis, selective outcomes reporting, performance bias and other sources of bias; and unclear risk for detection bias.
Data comparing the effect of higher intensity versus lower intensity CRRT on mortality at day 30 or after were obtained from six well‐conducted RCTs respectively, but we downgraded the quality of evidence to low, mainly due to inconsistencies (I2 values of 65% and 75%) and imprecision (CIs included a range of plausible values with clinically important benefits but also included harms). Similarly, we downgraded the quality of evidence to low for recovery of kidney function in all patients and among survivors at day 30 due to inconsistencies (I2 values of 71% and 69%) and rated as moderate data obtained for recovery of kidney function among survivors at day 90 by indirectness (the recovery of kidney function in this high risk group is affected when the risk of death is taken into account).
Data used to assess the impact of intensive versus less intensive CRRT on adverse events were obtained from three well‐conducted RCTs, providing treatment effects with clinically important harms; however, we downgraded the quality of evidence to moderate due to imprecision (CIs included both clinically important benefits and harm). One study provided data on hypophosphataemia. We rated this as high quality evidence.
Potential biases in the review process
While this review was conducted according to rigorous methods developed by the Cochrane Collaboration, some bias may be present in the review process. We searched for all relevant studies using sensitive and validated strategies in major medical databases and grey literature sources. However, it is possible that some studies (such as unpublished data and studies with negative or no effects) were not identified. An analysis for evidence to assess the risk of publication bias was not possible for all outcomes due to the small number of studies available in each meta‐analysis (Figure 4).
It was difficult to identify the number of patients who received IHD in the included study using a combination of both therapies (intermittent and continuous), as well also in studies evaluating CRRT intensity, in which patients remained dialysis‐dependent after ICU or hospital discharge, many were likely to have transitioned to IHD regardless of the original study‐assigned dose of CRRT.
Several subgroup analyses were planned to explore potential sources of heterogeneity in our review, however a lack of data prevented us from doing these analyses.
Agreements and disagreements with other studies or reviews
Our systematic review in keeping with previous meta‐analysis on intensity in CRRT (Negash 2011) or in mixed modality, combining intermittent, sustained and continuous dialysis (Jun 2010; Van Wert 2010), has not found beneficial effects of more intensive RRT with respect to mortality and kidney recovery function in critically ill patients with AKI compared to less intensive therapy.
There has been increased interest in recovery of kidney function. Indeed, lack of recovery of kidney function implies the need for long‐term dialysis associated with low quality of life. Our review has not demonstrated benefits on recovery of kidney function with intensive therapy. These findings are consistent with four individual RCTs (ATN Study 2005; Bouman 2002; RENAL Study 2006; Tolwani 2008) and do not agree with those reported of two previous RCTs (Ronco 2000a; Saudan 2006). It is important to note that relevant differences on recovery of kidney function between ATN Study 2005 and RENAL Study 2006 were observed (45.2% versus 13.3% of survivors depend on RRT at day 28 respectively). These differences may be due to several factors including different populations, prevalence of intermittent dialysis, pre‐existing chronic kidney disease and timing of RRT initiation. A review by Palevsky 2005 on factors affecting kidney recovery following AKI did not recommend either intensities with regard to recovery of kidney function when the mortality risk is taken into account (given that mortality is a competing end point for recovery of kidney function).
The hypothesis that in critically ill patients, especially those with sepsis or systemic inflammatory responses, could benefit from an intensive CRRT was proposed by several researchers. It is interesting to note that we did not find benefit from higher intensity CRRT in this subgroup of patients in our review. These results were consistent with previous meta‐analysis (Jun 2010; Van Wert 2010). Additionally, previous reviews explored the effect of high volume haemofiltration (HVHF) specifically in critically ill patients with severe sepsis or septic shock in an ICU setting (Borthwick 2013; Clark 2014; Lehner 2014). These reviews applied different thresholds for HVHF: Borthwick 2013 defined HVHF as > 35 mL/kg/h, while more recent reviews define HVHF as >50 mL/kg/h (Clark 2014) and HVHF and pulse high volume haemofiltration (PHVHF) as 85 mL/Kg/h (Lehner 2014). These reviews included studies we excluded from our review due to the very‐high intensity applied (Boussekey 2008; IVOIRE Study 2013; Sanchez 2010b; Zhang 2012) or no requirement of AKI for enrolment (Jiang 2005) These reviews found insufficient evidence of a therapeutic benefit for routine use of HVHF for septic AKI.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Funnel plot of comparison: 1 High Intensity versus less intensive CRRT, outcome: 1.1 Mortality
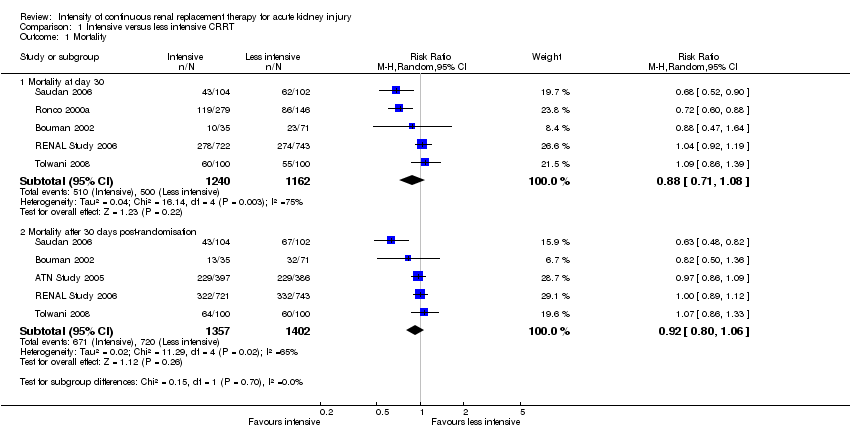
Comparison 1 Intensive versus less intensive CRRT, Outcome 1 Mortality.

Comparison 1 Intensive versus less intensive CRRT, Outcome 2 Mortality in prespecified groups.

Comparison 1 Intensive versus less intensive CRRT, Outcome 3 Recovery of kidney function.

Comparison 1 Intensive versus less intensive CRRT, Outcome 4 Kidney function recovery in prespecified subgroup.
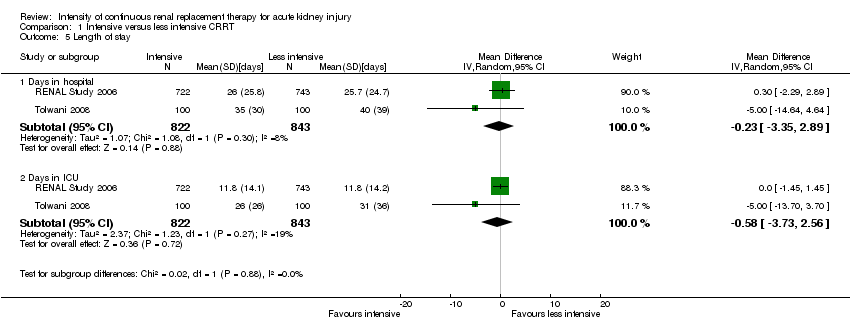
Comparison 1 Intensive versus less intensive CRRT, Outcome 5 Length of stay.

Comparison 1 Intensive versus less intensive CRRT, Outcome 6 Metabolic control.
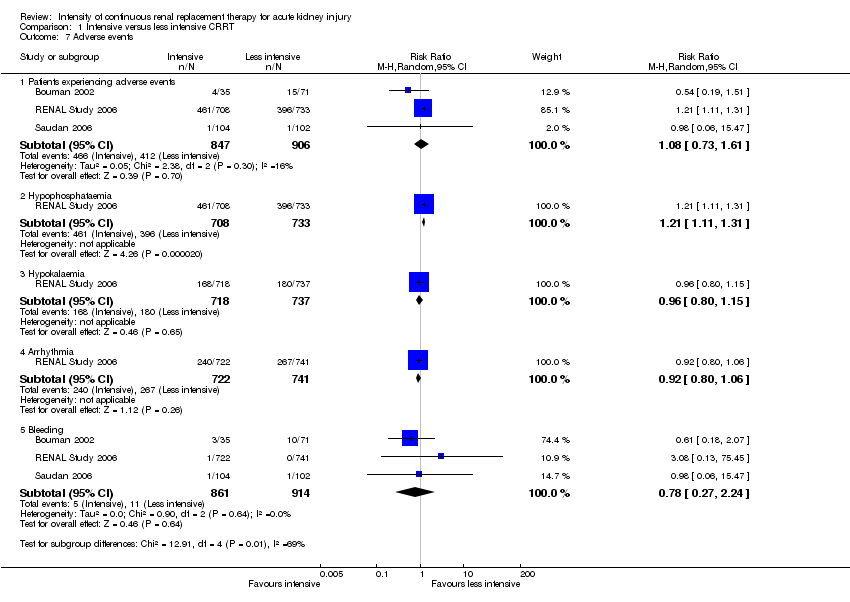
Comparison 1 Intensive versus less intensive CRRT, Outcome 7 Adverse events.
| Intensive versus less intensive CRRT for AKI | ||||||
| Patient or population: patients with AKI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Less intensive CRRT | Intensive CRRT | |||||
| Mortality at day 30 | Study population | RR 0.88 | 2402 (5) | ⊕⊕⊝⊝ | ||
| 430 per 1000 | 420 per 1000 | |||||
| Moderate | ||||||
| Mortality after 30 days post‐randomisation | Study population | RR 0.92 | 2759 (5) | ⊕⊕⊝⊝ | ||
| 514 per 1000 | 483 per 1000 | |||||
| Moderate | ||||||
| 593 per 1000 | 557 per 1000 | |||||
| Patients free of RRT after discontinuing CRRT | Study population | RR 1.12 | 2402 (5) | ⊕⊕⊝⊝ | ||
| 483 per 1000 | 541 per 1000 | |||||
| Moderate | ||||||
| 390 per 1000 | 437 per 1000 | |||||
| Patients free of RRT after discontinuing CRRT | Study population | RR 0.98 | 988 (3) | ⊕⊕⊕⊝ | ||
| 923 per 1000 | 904 per 1000 | |||||
| Moderate | ||||||
| 800 per 1000 | 784 per 1000 | |||||
| Adverse events: hypophosphataemia | Study population | RR 1.21 | 1441 (1) | ⊕⊕⊕⊕ | ||
| 540 per 1000 | 654 per 1000 | |||||
| Moderate | ||||||
| 540 per 1000 | 653 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ inconsistency: due to substantial heterogeneity (I2 values ranged from 73% to 78%) ² imprecision: due to wide CI which crossed the threshold for clinically meaningful effects ³ Indirectness: critically ill patients with AKI in CRRT have high short‐term mortality risk; mortality is a competing end point for kidney recovery at day 90 | ||||||
| Intensive versus less intensive CRRT for AKI: subgroups | ||||||
| Patient or population: patients with AKI who need CRRT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard dose | High dose | |||||
| Mortality: patients with sepsis | Study population | RR 0.94 | 966 (5) | ⊕⊕⊝⊝ | ||
| 524 per 1000 | 492 per 1000 | |||||
| Moderate | ||||||
| 618 per 1000 | 581 per 1000 | |||||
| Mortality: patients without sepsis | Study population | RR 0.89 | 1216 (4) | ⊕⊕⊝⊝ | ||
| 465 per 1000 | 414 per 1000 | |||||
| Moderate | ||||||
| 564 per 1000 | 502 per 1000 | |||||
| Mortality: patients with AKI related to cardiac or general surgery | Study population | RR 0.73 | 531 (2) | ⊕⊕⊕⊕ | ||
| 505 per 1000 | 368 per 1000 | |||||
| Moderate | ||||||
| 459 per 1000 | 335 per 1000 | |||||
| Mortality: patients with AKI not related to surgery | Study population | RR 0.94 | 1871 (3) | ⊕⊕⊝⊝ | ||
| 414 per 1000 | 389 per 1000 | |||||
| Moderate | ||||||
| 550 per 1000 | 517 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ inconsistency: due to substantial heterogeneity (I2 values ranged from 73% to 78%) ² imprecision: due to wide CI which crossed the threshold for clinically meaningful effects | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mortality at day 30 | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.08] |
| 1.2 Mortality after 30 days post‐randomisation | 5 | 2759 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 2 Mortality in prespecified groups Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Patients with sepsis | 5 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 2.2 Patients without sepsis | 4 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 2.3 Patients with SOFA cardiovascular score < 3 | 1 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.18] |
| 2.4 Patients with SOFA cardiovascular ≥ 3 | 1 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 2.5 Patients with AKI related to surgical causes | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.88] |
| 2.6 Patients with AKI unrelated to surgical causes | 3 | 1871 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 3 Recovery of kidney function Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Free of RRT after discontinuing CRRT | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.37] |
| 3.2 Free of RRT after discontinuing CRRT at day 30 | 5 | 1416 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 3.3 Free of RRT after discontinuing CRRT at day 90 | 3 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.01] |
| 4 Kidney function recovery in prespecified subgroup Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Patients with AKI related to surgical causes | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.05, 1.53] |
| 4.2 Patients with AKI related to non‐surgical causes | 3 | 1870 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.71] |
| 5 Length of stay Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Days in hospital | 2 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐3.35, 2.89] |
| 5.2 Days in ICU | 2 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐3.73, 2.56] |
| 6 Metabolic control Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Normalised metabolic acidosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Patients experiencing adverse events | 3 | 1753 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.61] |
| 7.2 Hypophosphataemia | 1 | 1441 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.11, 1.31] |
| 7.3 Hypokalaemia | 1 | 1455 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.15] |
| 7.4 Arrhythmia | 1 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 7.5 Bleeding | 3 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.24] |

