Lamotrigina complementaria para la epilepsia parcial resistente a los fármacos
Resumen
Antecedentes
Esta es una versión actualizada de la revisión Cochrane publicada en The Cochrane Library 2010, Número 1.
La epilepsia es un trastorno neurológico frecuente que afecta a casi el 0,5% al 1% de la población. En casi el 30% de estos pacientes, la epilepsia es refractaria a los fármacos disponibles actualmente. El tratamiento farmacológico aún es la primera opción para controlar la epilepsia. La lamotrigina es uno de los fármacos antiepilépticos nuevos y es el tema de esta revisión. La lamotrigina en combinación con otros fármacos antiepilépticos (complementaria) puede reducir las convulsiones, pero con algunos efectos adversos. El objetivo de esta revisión sistemática fue examinar la evidencia actual de la eficacia y la tolerabilidad de la lamotrigina cuando se utiliza como tratamiento complementario para los pacientes con epilepsia parcial refractaria.
Objetivos
Determinar los efectos de la lamotrigina sobre (1) las convulsiones, (2) el perfil de efectos adversos y (3) la cognición y la calidad de vida, en comparación con los controles placebo, cuando se administra como tratamiento complementario para los pacientes con epilepsia parcial refractaria.
Métodos de búsqueda
Para la versión anterior de la revisión, los autores realizaron búsquedas en el Registro Especializado de Ensayos Controlados del Grupo Cochrane de Epilepsia (Cochrane Epilepsy Group) (enero 2010), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, The Cochrane Library 2010,Número 1), MEDLINE (1950 a enero 2010) y en las listas de referencias de los artículos.
Para esta actualización se realizaron búsquedas en el Registro Especializado del Grupo Cochrane de Epilepsia (Cochrane Epilepsy Group) (28 de mayo 2015), CENTRAL (The Cochrane Library 2015, Número 4), MEDLINE (Ovid, 1946 a mayo 2015) y en las listas de referencias de los artículos. También se estableció contacto con los fabricantes de lamotrigina (GlaxoSmithKline). No se impusieron restricciones de idioma.
Criterios de selección
Ensayos aleatorizados controlados con placebo de pacientes con epilepsia parcial resistente a los fármacos de cualquier edad, en los que se utilizó un método adecuado de ocultación de la aleatorización. Los estudios fueron doble o simple ciego, o no se cegaron. En los estudios cruzados (cross‐over), el primer período de tratamiento se analizó como un ensayo paralelo. Los participantes elegibles fueron adultos o niños con epilepsia parcial resistente a los fármacos.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron los ensayos para su inclusión y extrajeron los datos. Los resultados investigados incluyeron una reducción del 50% o más en la frecuencia de las convulsiones, el retiro del tratamiento, los efectos adversos, los efectos sobre la cognición y la calidad de vida. Los análisis primarios fueron por intención de tratar. Se realizaron análisis de sensibilidad en los mejores y peores casos para tener en cuenta los datos de resultados faltantes. Se estimaron los riesgos relativos (RR) agrupados con los intervalos de confianza (IC) del 95% para los resultados primarios de la frecuencia de las convulsiones y el retiro del tratamiento. Para los efectos adversos se calcularon los RR y los IC del 99%.
Resultados principales
No se identificaron estudios nuevos para esta actualización, por lo que los resultados no han cambiado.
En la versión anterior de la revisión, los autores encontraron cinco estudios paralelos del fármaco complementario y ocho estudios cruzados en adultos o niños con epilepsia focal refractaria, y un estudio paralelo del fármaco complementario con un diseño enriquecido según la respuesta en lactantes. En total, estos 14 estudios incluyeron 1958 participantes (38 lactantes, 199 niños y 1721 adultos). Las fases iniciales variaron entre cuatro y 12 semanas; y las fases de tratamiento entre ocho y 36 semanas. En general, 11 estudios (n = 1243 participantes) se calificaron como bajo riesgo de sesgo, y tres (n = 715 participantes) tuvieron un riesgo de sesgo incierto debido a la falta de información sobre el diseño del estudio. Se informó de un cegamiento efectivo en tres estudios (n = 504 participantes). El riesgo relativo (RR) general para una reducción del 50% o más de la frecuencia de las convulsiones fue 1,80 (IC del 95%: 1,45 a 2,23; 12 ECA) para 12 estudios (n = 1322 participantes, adultos y niños), lo que indica que la lamotrigina fue significativamente más efectiva que placebo para reducir la frecuencia de las convulsiones. El RR general de retirada del tratamiento (por cualquier motivo) fue 1,11 (IC del 95%: 0,90 a 1,36; 14 ECA) para catorce estudios (n = 1958 participantes). Los efectos adversos asociados significativamente a la lamotrigina fueron: ataxia, mareos, diplopía y náuseas. Los RR de estos efectos adversos fueron los siguientes: ataxia 3,34 (IC del 99%: 2,01 a 5,55; 12 ECA; n = 1524); mareo 2,00 (IC del 99%: 1,51 a 2,64;13 ECA; n = 1767); diplopía 3,79 (IC del 99%: 2,15 a 6,68; tres ECA; n = 943); náuseas 1,81 (IC del 99%: 1,22 a 2,68; 12 ECA; n = 1486). Los datos limitados disponibles impidieron establecer conclusiones sobre los efectos en la cognición y la calidad de vida. No hubo heterogeneidad importante entre los estudios para los resultados. En general, la evidencia se evaluó como de calidad alta a moderada debido a los datos incompletos de algunos resultados.
Conclusiones de los autores
La lamotrigina como tratamiento complementario de las convulsiones parciales parece ser efectiva para reducir la frecuencia de las convulsiones, y parece ser bastante bien tolerada. Sin embargo, los ensayos fueron de duración relativamente corta y no aportaron evidencia a largo plazo. Se necesitan ensayos adicionales para evaluar los efectos a largo plazo de la lamotrigina, y compararla con otros fármacos complementarios.
Debido a que no se encontraron estudios nuevos, las conclusiones no han cambiado.
PICOs
Resumen en términos sencillos
Agregado de lamotrigina para la epilepsia parcial resistente a los fármacos
Antecedentes
La epilepsia es un trastorno en el que descargas eléctricas inesperadas del cerebro causan convulsiones. Aproximadamente un tercio de los pacientes con epilepsia se mantienen con convulsiones a pesar del tratamiento con los fármacos antiepilépticos (FAE) (más antiguos) que se utilizan actualmente. Además, los FAE más antiguos tienen muchos efectos adversos.
Por lo tanto, el desarrollo de nuevos tratamientos efectivos para el tratamiento de las convulsiones refractarias reviste una importancia considerable. Como resultado, se ha desarrollado una variedad de nuevos FAE como tratamientos "complementarios". La lamotrigina es uno de ellos.
Objetivos de la revisión
El objetivo de esta revisión fue determinar los efectos de la lamotrigina sobre las convulsiones, los efectos adversos, la cognición (capacidad de aprender y comprender) y la calidad de vida en comparación con los controles placebo, cuando se utiliza como tratamiento complementario para los pacientes con epilepsia parcial que no responden a los FAE existentes.
Para esta actualización no se identificaron estudios nuevos para agregar, por lo que las conclusiones nos e han modificado. La revisión incluyó 14 ensayos controlados aleatorizados con 1958 participantes.
Resultados
La lamotrigina, utilizada en combinación con otros FAE en pacientes con epilepsia parcial resistente a los fármacos puede disminuir aún más la frecuencia de las convulsiones. Sin embargo, el agregado de lamotrigina al tratamiento habitual se asocia con mayor frecuencia con un aumento de los efectos adversos como inestabilidad (ataxia), mareos, visión doble (diplopía) y náuseas.
Calidad de la evidencia
Los ensayos se evaluaron con respecto al riesgo de sesgo. En general se consideró que la calidad de la evidencia fue alta.
Conclusiones
Se necesitan más estudios de investigación de calidad alta para evaluar completamente la eficacia y la tolerabilidad de la lamotrigina y compararla con otros FAE más recientes.
La evidencia está actualizada hasta el 28 de mayo 2015.
Authors' conclusions
Summary of findings
| Lamotrigine versus placebo for drug‐resistant partial epilepsy | ||||||
| Patient or population: participants with drug‐resistant partial epilepsy Settings: outpatient setting Intervention: Lamotrigine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lamotrigine | |||||
| 50% or greater reduction in seizure frequency ‐ ITT analysis | 157 per 1000 | 283 per 1000 | RR 1.80 (95% CI 1.45 to 2.23) | 1322 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Treatment withdrawal | 159 per 1000 | 176 per 1000 | RR 1.11 (95% CI 0.90 to 1.36) | 1805 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Ataxia | 45 per 1000 | 150 per 1000 | RR 3.34 (99% CI 2.01 to 5.55) | 1524 | ⊕⊕⊕⊝ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Dizziness | 128 per 1000 | 256 per 1000 | RR 2.00 (99% CI 1.51 to 2.64) | 1767 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Fatigue | 113 per 1000 | 93 per 1000 | RR 0.82 (99% CI 0.55 to 1.22) | 1551 (12 studies) | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Nausea | 83 per 1000 | 150 per 1000 | RR 1.81 (99% CI 1.22 to 2.68) | 1486 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes4. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One or two studies do not contribute to the analysis, but no other bias. 2 All studies contributed to the analysis. 3 Wide confidence intervals. 4Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment). | ||||||
Background
This review is an update of a previously published review in The Cochrane Library 2010, Issue 1.
Description of the condition
Epilepsy is characterised by recurrent and unprovoked seizures, constituting a transient sign and symptom of abnormal, excessive electrical activity in the cerebral cortex (Fisher 2005). Epilepsy is one the most common serious neurological conditions worldwide, with significant psychosocial and physical morbidity. Its management requires expertise and good pharmacological knowledge of the available options (Lyer 2014). The condition affects approximately 50 million people worldwide. The total annual cost in Europe is approximately 15.5 billion Euros (Mula 2013). Between 2% and 3% of the population will be given a diagnosis of epilepsy at some time in their lives (Hauser 1993), the majority of whom will become seizure free. However, up to 30% will continue to have seizures, refractory to treatment with adequate doses of antiepileptic drugs (AEDs), which are often given in combination (Cockerell 1995). There is no internationally accepted definition of "drug‐resistant". For the purposes of this review, people will be considered to be drug‐resistant if they have failed to respond to a minimum of two AEDs, given as monotherapy. The majority of drug‐resistant people have partial onset (also called focal or localization related) seizures. In other words, seizures start in one part of the brain and during the course of the seizure, the abnormal electrical activity remains localised or spreads to other parts of the brain. Partial seizures can be divided into three types: simple partial; complex partial, and secondarily generalized tonic‐clonic seizures (Commission 1989).
Description of the intervention
Although more than a dozen new antiepileptic drugs (AEDs) have entered the market since 1993, up to 30% of patients remain refractory to current treatments. Thus, a concerted effort continues to identify and develop new therapies that will help these patients (Barker‐Haliski 2014). Pharmacological treatment remains the first choice for controlling epilepsy (Loscher 2002), although recent decades have seen advances in vagal stimulation (Panebianco 2015), and surgery (West 2015). Given that our standard drugs (e.g. carbamazepine, phenytoin, valproate) do not leave all people seizure free and are not without adverse effects, over the past 15 to 20 years, there has been renewed interest in the development of newer AEDs. One such drug is lamotrigine (LTG).
Lamotrigine is a novel AED, widely used in the treatment of epilepsy as adjunctive treatment for partial, secondarily generalised, and tonic‐clonic seizures in patients with refractory epilepsy, and bipolar disorder (Yamamoto 2012).
How the intervention might work
Lamotrigine was approved by the Food and Drug Administration in the USA in 1994 for use in partial‐onset seizures. It was ultimately approved for monotherapy in 1998. Lamotrigine is effective against a broad spectrum of seizure types and has a favourable metabolic profile. It has gained widespread use in the USA as both an immediate and an extended‐release agent (Moore 2012). Lamictal (GlaxoSmithKline) is considered the reference drug (Girolineto 2012). In vitro pharmacological studies suggest that the main mechanism of action of lamotrigine is to inhibit voltage‐sensitive sodium channels, thereby stabilizing neuronal membranes and consequently modulating presynaptic transmitter release of excitatory aminoacids, e.g. glutamate and aspartate (Leach 1995). Lamotrigine has been demonstrated to be effective as both an antiepileptic drug and a mood stabiliser (Vajda 2013).
Why it is important to do this review
In this review, we summarise evidence from randomised controlled trials where the efficacy and tolerability of lamotrigine for people with drug‐resistant partial epilepsy have been investigated, in order to aid clinical decision‐making when considering LTG as add‐on treatment within this population.
Antiepileptic drugs may impair people's cognitive abilities, and in this review, we include outcomes that assess cognitive effects. In addition, we have chosen to include quality‐of life outcomes, to assess the global impact of this drug on people's well‐being.
Objectives
To determine the effects of lamotrigine on (1) seizures, (2) adverse effect profile, and (3) cognition and quality of life, compared to controls, when used as an add‐on treatment for people with refractory partial epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
We included trials that met all the following criteria:
-
Randomised controlled trials, in which an adequate method of concealment of randomisation was used (e.g. allocation of sequentially sealed packages of medication, sealed opaque envelopes, telephone randomisation);
-
Double‐, single‐blind trials and unblinded trials;
-
Placebo‐controlled trials;
-
Parallel group and cross‐over studies. For cross‐over studies, the first treatment period was treated as a parallel trial, for the purposes of analysis of efficacy and safety data (i.e. only data from the first treatment period was used).
Types of participants
Individuals of any age with partial epilepsy (i.e. experiencing simple partial, complex partial, or secondarily generalized tonic‐clonic seizures) who had failed to respond to at least one AED (drug‐resistant epilepsy).
Types of interventions
-
The treatment group received lamotrigine in addition to conventional antiepileptic drug (AED) treatment.
-
The control group received conventional AED treatment plus a matched placebo, or 'no treatment' control.
Types of outcome measures
Primary outcome
Greater than 50% reduction in seizure frequency
The primary outcome was the proportion of participants with a 50% or greater reduction in seizure frequency during the treatment period, compared to the pre‐randomisation baseline frequency. We chose this outcome as it is commonly reported in this type of study and can be calculated for studies that do not report this outcome, provided that baseline seizure data were recorded.
Secondary outcomes
Treatment withdrawal
The proportion of participants who had treatment withdrawn during the course of the treatment period was chosen as a measure of global effectiveness. Treatment may be withdrawn due to adverse effects, lack of efficacy, or a combination, and this is an outcome to which the individual makes a direct contribution. However, in studies of relatively short duration, such as studies that would be included in this review, adverse effects were likely to be the main reason for treatment withdrawal.
Adverse effects
(a) The proportion of participants experiencing any of the following adverse effects, which we considered to be the most common and important adverse effects of AEDs:
-
ataxia;
-
dizziness;
-
fatigue;
-
nausea;
-
somnolence;
-
diplopia;
-
headache.
(b) The proportion of participants who experienced the five most common adverse effects in a study, if different from those stated above.
Cognitive effects
The difference between intervention and control group(s) means for cognitive assessments used in the individual studies.
Quality of life
The difference between intervention and control group(s) means for quality of life (QOL) assessments used in the individual studies.
Search methods for identification of studies
Electronic searches
For the previous version of the review, the authors searched the following databases for relevant studies (Ramaratnam 2001):
-
The Cochrane Epilepsy Group Specialized Register (January 2010).
-
The Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2010).
-
MEDLINE (1950 to January 2010).
For this update, we searched the following databases for relevant studies:
-
The Cochrane Epilepsy Group Specialized Register (28 May 2015), using the strategy outlined in Appendix 1.
-
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2015, Issue 4), using the strategy outlined in Appendix 2.
-
MEDLINE (Ovid, 1946 to 28 May 2015), using the strategy outlined in Appendix 3.
We imposed no language restrictions. We tailored searches to individual databases, and adapted from those used in the previous review.
Searching other resources
For the original review and this update, we checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials, and authors and manufacturers of lamotrigine (GlaxoSmithKline) for additional information.
Data collection and analysis
Selection of studies
For this update, two review authors (SR and MP) independently assessed trials for inclusion. Any disagreements were resolved by discussion with a third author (AM). Two review authors (SR and MP) independently extracted data and assessed the risk of bias for included trials; again, disagreements were resolved by mutual discussion.
Data extraction and management
We extracted the following data for each trial, using a data extraction form:
(1) Methods and trial design:
(a) Method of randomisation.
(b) Method of allocation concealment.
(c) Method of blinding.
(d) Whether any participants had been excluded from reported analyses.
(e) Duration of baseline period.
(f) Duration of treatment period.
(g) Dose(s) of LTG tested.
(h) Information on Sponsorship and Funding.
(2) Participant and demographic information:
(a) Total number of participants allocated to each treatment group.
(b) Age and sex.
(c) Number with partial and generalized epilepsy.
(d) Seizure types.
(e) Seizure frequency during the baseline period.
(f) Number of background drugs.
Outcomes
We recorded the number of participants who experienced each outcome (seeTypes of outcome measures) per randomised group, and contacted authors of trials for any missing information.
Assessment of risk of bias in included studies
Two review authors (SR and MP) independently assessed the risk of bias for each trial, using the Cochrane 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We discussed and resolved disagreements. We completed a 'Risk of bias' table for each included study in RevMan (RevMan 2014). We rated all included studies as having a low, high or unclear risk of bias on six domains applicable to randomised controlled trials: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting, and other sources of bias. We created 'Summary of findings' tables, using GRADEpro and the GRADE approach for assessing the quality of evidence (GRADEpro 2014)
Measures of treatment effect
We analysed the primary outcome of seizure reduction as a binary outcome and presented it as a risk ratio. We also analysed secondary outcomes, including treatment withdrawal and adverse effects as binary outcomes and presented risk ratio. We had also planned to present cognitive effects and quality of life as continuous outcomes via mean differences if the same measurement scales were used or via standardised mean differences if different measurement scales were used to measure the same outcome. However, due to the limited amount of data available for these outcomes, we have presented these outcomes in a narrative discussion
Unit of analysis issues
We included eight cross‐over studies in the review. We analysed data from these studies from the first treatment period only; we analysed parallel and cross‐over design studies in separate subgroups.
Dealing with missing data
We sought missing data by contacting the study authors. We carried out intention‐to‐treat (ITT), best case and worst case analysis on the primary outcome to account for any missing data (see Data synthesis). We presented all analyses in the main report.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials (e.g. age, seizure type, duration of epilepsy, number of antiepileptic drugs taken at time of randomisation), and trial factors (e.g. randomisation concealment, blinding, losses to follow up). We examined statistical heterogeneity using a Chi² test and I² statistic. When we found no significant heterogeneity (P < 0.10), we used a fixed‐effect model. Had we found heterogeneity (> 50%), we had planned to use a random‐effects model for the analysis.
Assessment of reporting biases
We requested protocols for all included studies to enable a comparison of outcomes of interest. If outcome reporting bias was suspected for any included study, we had planned to further investigate using the ORBIT matrix system (Kirkham 2010). We had planned an examination of asymmetry funnel plots to establish publication bias, but such an assessment was not possible due to the small number of studies included in the review.
Data synthesis
We used a fixed‐effect model meta‐analysis to synthesise the data. We measured the effect of each comparison on our preset primary and secondary outcomes, if data were available. Comparisons we expected to carry out included:
-
usual treatment plus lamotrigine versus usual treatment plus placebo
-
usual treatment plus lamotrigine versus no treatment
-
usual treatment plus lamotrigine versus usual treatment
Our preferred estimator for all binary outcomes was the Mantel‐Haenzsel Risk Ratio (RR). For the outcomes 50% or greater reduction in seizure frequency and treatment withdrawal, we used 95% confidence intervals (Cls). For individual adverse effects we used 99% Cls to make an allowance for multiple testing.
Our analyses included all participants in the treatment groups to which they had been allocated following randomisation.
For the efficacy outcome (50% or greater reduction in seizure frequency) we undertook three analyses:
(a) Primary (intention‐to‐treat) analysis
Participants not completing follow‐up or with inadequate seizure data were assumed to be non‐responders. To test the effect of this assumption, we undertook the following sensitivity analyses. Analysis by ITT was done where this was reported by the included studies.
(b) Worst case analysis
Participants not completing follow‐up or with inadequate seizure data were assumed to be non‐responders in the lamotrigine group, and responders in the placebo group.
(c) Best case analysis
Participants not completing follow‐up or with inadequate seizure data were assumed to be responders in the lamotrigine group, and non‐responders in the placebo group.
Subgroup analysis and investigation of heterogeneity
We considered adults and children and doses in our subgroup analyses. We performed a subgroup analysis for adverse events.
Sensitivity analysis
We had intended to carry out sensitivity analyses if peculiarities were found between study quality, characteristics of participants, interventions and outcomes.
Results
Description of studies
Results of the search
The latest search (carried out 28 May 2015) identified 141 records from the databases outlined above. We screened 96 records after duplicates were removed for inclusion in the review. We excluded 82 at this point, and requested 14 full‐text articles to assess for eligibility. We contacted authors of these trials for more information, providing their contact details were available. Following this, we excluded all 14 studies (please see Figure 1 and Characteristics of excluded studies for reason of exclusion). Thus, no new studies were included in this review.

Study flow diagram for update.
Included studies
We did not find any new studies for this update.
In the previous version of this review, the authors included 14 randomised controlled trials that investigated the use of add‐on lamotrigine compared to placebo, in 1958 participants with uncontrolled partial seizures (38 infants, 199 children, and 1721 adults). Trial characteristics are summarised below. For further information on each trial, please see Characteristics of included studies.
The 14 studies included five parallel group studies (Baulac 2010; Duchowny 1999; Matsuo 1993; Naritoku 2007; Schachter 1995); one parallel group study in infants with a responder‐enriched design, in which all patients received adjunctive lamotrigine during an open‐label phase and those who had a 40% or greater reduction in the frequency of partial seizures during the last four weeks were randomly assigned to double‐blind treatment for up to eight weeks with continued lamotrigine or placebo (Piña‐Garza 2008); and eight cross‐over studies (Binnie 1989; Boas 1996; Jawad 1989; Loiseau 1990; Messenheimer 1994; Schapel 1993; Schmidt 1993; Smith 1993). All the studies but two recruited adults; Duchowny 1999 recruited only children, and Piña‐Garza 2008 enrolled only infants aged one to 24 months of age. One trial used extended‐release formulation of lamotrigine (Naritoku 2007), while the others used immediate‐release formulations. In general, the individuals included in these studies had at least three to four partial seizures a month, despite therapy with a stable AED regime consisting of two or three AEDs, which were appropriate for the type of epilepsy, and were given in adequate doses.
Almost all studies excluded people with: intellectual disabilities, progressive neurological disease, major psychiatric problems, associated pseudo seizures, newly‐diagnosed epilepsy, status epilepticus in the 24 weeks preceding the trial, associated systemic diseases, abnormal laboratory investigations not explained by enzyme induction by AEDs, a history of non‐compliance, failure to keep reliable records of seizures or adverse effects, irregular clinic visits, recent use of any other investigational AED, abuse of alcohol or other prescription or non‐prescription drugs; people receiving chronic medication, especially antipsychotic drugs, women who were pregnant or at risk of pregnancy, lactating women. For the cross‐over studies, participants were not randomised to a single dose, but took a range of doses, depending on their clinical response and the concurrent administration of other AEDs. Use of valproate was not permitted in three studies (Matsuo 1993; Messenheimer 1994; Schachter 1995), two excluded people on valproate monotherapy (Schapel 1993; Smith 1993), while others used lower dosages of lamotrigine for people on valproate. One parallel study tested doses of 300 mg and 500 mg of lamotrigine per day (Matsuo 1993), whereas the others tested a range of doses between 75 mg and 600 mg per day (median between 200 mg and 400 mg/day). The length of the treatment period varied from eight to 24 weeks.
All studies were published as full articles, except Schmidt 1993, which was only published as an abstract. All studies, except Baulac 2010 (which was sponsored by Pfizer Inc), were sponsored by GlaxoSmithKline, manufacturers of lamotrigine, as part of their pre‐licensing programme.
Excluded studies
In this update, we excluded 14 studies for the following reasons: four studies were not randomised trials; five studies did not study an eligible population; study results were not available for three studies; one study did not include LTG as part of the add‐on therapy; and one study was not a placebo‐controlled trial. The details of these studies are given in the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias in each included study. Each study was allocated an overall rating for risk of bias: low, high, or unclear. See below for specific domain ratings.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
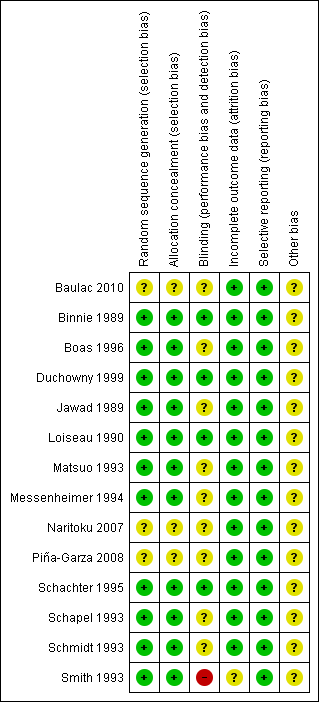
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated the method by which allocation was randomised as having a low risk of bias in eleven trials (n = 1243 participants), because they used a computer‐generated randomisation schedule or random number tables (Binnie 1989; Boas 1996; Duchowny 1999; Jawad 1989; Loiseau 1990; Matsuo 1993; Messenheimer 1994; Schachter 1995; Schapel 1993; Schmidt 1993; Smith 1993). The investigators did not provide clear methods In three trials (n = 715 participants), which were rated as unclear (Baulac 2010; Naritoku 2007; Piña‐Garza 2008). For sequence generation, we rated the same 11 studies as having a low risk of bias because they dispensed sequentially numbered packages to each participant, and random permuted blocks were used to generate the allocation sequence. We rated three studies (n = 715 participants) as unclear due to a lack of details on the methods used (Baulac 2010; Naritoku 2007; Piña‐Garza 2008).
Blinding
We rated three studies (n = 504 participants) as having a low risk of bias for this particular domain because participants, parents and investigators were blinded (Binnie 1989; Jawad 1989; Schachter 1995). We judged blinding of participants as unclear in ten papers (n = 1373 participants) because no details of the method of blinding were provided (Baulac 2010; Boas 1996; Duchowny 1999; Loiseau 1990; Matsuo 1993; Messenheimer 1994; Naritoku 2007; Piña‐Garza 2008; Schapel 1993; Schmidt 1993). One study (n = 81 participants) was rated as high risk of bias because patients and investigators were able to identify the lamotrigine treatment (Smith 1993).
Incomplete outcome data
We rated all the included studies, except one (Smith 1993) (n = 1877 participants), as having a low risk of bias for this domain as minimal missing data were reported; either intention‐to‐treat analysis was employed, or there were no concerns of missing data having an effect on the overall outcome estimate. Smith 1993 was rated as unclear risk of bias because participants who discontinued prematurely did not complete the HRQOL measure at the time of discontinuation.
Selective reporting
We requested the protocols for all included studies to compare a priori methods and outcomes to the published report, but none of the protocols for the included studies were available. We rated all included studies as low risk of bias for this domain as there was no suspicion of selective outcome reporting bias. All expected outcomes were reported in each of the publications.
Other potential sources of bias
All these studies, except one (Baulac 2010, which was sponsored by Pfizer Inc.), were sponsored by GlaxoSmithKline, the manufacturers of the LTG, and therefore, we rated all but one study as having an unclear risk of bias for this domain.
Effects of interventions
See: Summary of findings for the main comparison
For the cross‐over trials, we analysed data from the first treatment phase for the efficacy, treatment, withdrawal, and adverse effects. These data were unpublished and obtained from Glaxo Wellcome, the sponsors of all but one study (Baulac 2010).
Primary Outcome
Greater than 50% reduction in seizure frequency
A Chi² test for responses to lamotrigine indicated no significant heterogeneity between trials (Chi² = 11.02; df = 11; P = 0.44; I² = 0%), so a fixed‐effect model was used to measure efficacy.
For twelve studies (n = 1322 participants, adults and children), the RR was 1.80; 95% CI 1.45 to 2.23 for any dose of lamotrigine added to regular anti‐epileptic drug therapy versus placebo. The RR from eight cross‐over studies (N = 382 participants) was 2.58; 95% CI 1.44 to 4.61 (Analysis 1.1).The RR for the best case and worst case scenarios were RR 2.88 (95% CI 2.36 to 3.50) and RR 0.97 (95% CI 0.82 to 1.15) respectively (Analysis 1.2, Analysis 1.3).
The RR for a daily dose of 300 mg of lamotrigine was 1.23; 95% CI 0.57 to 2.67; the RR was 2.13; 95% CI 1.08 to 4.20 for lamotrigine 500 mg per day, compared to placebo (Matsuo 1993). For children, the RR was 2.64; 95% CI 1.59 to 4.38 (Duchowny 1999).
We could not calculate responder rates for Schachter 1995 because baseline seizure counts were not obtained, or for the infants in Piña‐Garza 2008, where the primary end point was exit due to treatment failure,
Secondary Outcomes
Treatment withdrawal
Fourteen studies (n = 1958 participants) were included in this analysis. The overall RR for treatment withdrawal for any reason was (RR 1.11; 95% CI 0.90 to 1.36); thus there was insufficient evidence to conclude that participants were more likely to discontinue lamotrigine than placebo (Analysis 2.1).
We obtained the following data: 186 participants withdrew from treatment and 77 participants withdrew from control groups in parallel studies in adults (Baulac 2010, Matsuo 1993, Naritoku 2007, Schachter 1995); 14 participants withdrew from treatment and 18 from control groups in a parallel study in children (Duchowny 1999); 19 participants withdrew from treatment and 10 participants withdrew from control groups in cross‐over studies in adults (Binnie 1989, Boas 1996, Jawad 1989, Loiseau 1990, Messenheimer 1994, Schapel 1993, Schmidt 1993, Smith 1993); 11 participants withdrew from treatment and 16 withdrew from control groups in a parallel study in infants (Piña‐Garza 2008).
Insufficient data were available to undertake the planned dose‐response subgroup analyses.
Adverse effects
In addition to reports of ataxia, dizziness, fatigue, and nausea, some studies reported somnolence, diplopia and headache among the five most common adverse effects and are included in the analysis. Ataxia, dizziness, diplopia, and nausea were significantly more likely with lamotrigine. The RR for individual adverse effects were:
-
ataxia ‐ RR 3.34; (99% CI 2.01 to 5.55) (12 studies, 1524 participants, Analysis 3.1);
-
dizziness ‐ RR 2.00 (99% CI 1.51 to 2.64) (13 studies, 1767 participants, Analysis 3.2);
-
fatigue ‐ RR 0.82 (99% CI 0.55 to 1.22) (12 studies, 1551 participants, Analysis 3.3);
-
nausea ‐ RR 1.81(99% CI 1.22 to 2.68) (12 studies, 1486 participants, Analysis 3.4);
-
somnolence ‐ RR 1.39 (99% CI 0.96 to 2.00) (13 studies, 1767 participants, Analysis 3.5);
-
diplopia ‐ RR 3.79 (99% CI 2.15 to 6.68) (3 studies, 943 participants, Analysis 3.6); and
-
headache ‐ RR 1.13 (99% CI 0.87 to 1.45) (5 studies, 1385 participants, Analysis 3.7).
Cognitive effects and Quality of life
Two studies incorporated measures of cognitive functions (Banks 1991; Smith 1993; n = 54 participants). No significant differences were found on any of the tests used. However, participants receiving lamotrigine showed a marginal (not significant) reduction in general cerebral efficiency as assessed by the third segment of the Stroop colour word test‐a test of concentration and distractibility (Table 1).
| Outcome | Study | Number tested | Lamotrigine mean | Placebo mean |
| Stroop time | 41 | 93.98 | 98.39 | |
| Stroop error | 44 | 2.18 | 2.41 | |
| Stroop colour word (Total score) | 10 | 32.4+/‐10.9 | 35.6+/‐9.42 | |
| Number cancellation: AC | 44 | 51.36 | 49.7 | |
| Number cancellation: AE | 43 | 3.6 | 3.04 | |
| Number cancellation: BC | 42 | 48.21 | 48.54 | |
| Number cancellation: C | 42 | 38.19 | 39.29 | |
| Critical flicker fusion | 40 | 30.44 | 30.37 | |
| Choice reaction time | 40 | 0.675 | 0.669 | |
| Digit symbol (Scaled score) | 10 | 5 +/‐2.45 | 6.6 +/‐ 2.71 | |
| Rey complex figure recall percentile | 10 | 22+/‐17.51 | 30.5+/‐27.33 | |
| Trail making part B percentile | 10 | 26+/‐30.35 | 30.5+/‐32.09 |
We provided a narrative discussion for this outcome and more information in Table 1. Meta‐analysis of the two studies was not possible due to the differences in cognitive function measured in the two studies.
The results of the health‐related quality of life (HRQOL) assessments are given in Table 2. Smith 1993 (n = 54 participants), incorporated an HRQOL measure containing previously validated measures of physical, social and psychological functioning and a novel measure of seizure severity. There were no significant differences for the physical and social components of the HRQOL measure. Participants also reported significant improvements on the seizure severity scale when comparing lamotrigine versus placebo.
| Outcome | Number tested | Lamotrigine ‐ Mean | Placebo ‐ Mean | Clinical relevance |
| PSYCHOLOGICAL: | ||||
| Depression | 54 | 4.24 | 4.26 | No significant difference |
| Happiness | 51 | 3.8 | 1.96 | Higher scores in LTG group; P = 0.003 |
| Mood | 50 | 24.36 | 26.8 | No significant difference |
| Self‐esteem | 50 | 30.06 | 29.16 | No significant difference |
| Mastery | 50 | 20.02 | 18.78 | Higher scores in LTG group; P = 0.003 |
| Anxiety | 54 | 6.87 | 6.83 | No significant difference |
| PHYSICAL (Nottingham Health Profile): | ||||
| Energy | 53 | 0.68 | 0.68 | No significant difference |
| Pain | 53 | 0.6 | 0.69 | No significant difference |
| Emotional reaction | 53 | 1.96 | 1.96 | No significant difference |
| Sleep | 53 | 0.89 | 0.76 | No significant difference |
| Social isolation | 53 | 0.92 | 0.94 | No significant difference |
| Physical mobility | 53 | 0.96 | 0.91 | No significant difference |
| SEIZURE SEVERITY SCALE: | ||||
| Percept | 53 | 25.19 | 25.47 | No significant difference |
| Ictal | 53 | 19.47 | 20.53 | Less severe seizures in LTG group; P = 0.017 |
| Caregivers | 53 | 20.35 | 21.80 | Less severe seizures in LTG group; P = 0.035 |
We provided a narrative discussion for this outcome and more information in Table 2. Meta‐analysis was not possible as only a single study reported on this outcome.
Discussion
Summary of main results
Since publication of the previous version of this review, we found no new studies that met the selection criteria for this review.
The baseline phase in all but one trial ranged from 4 to 12 weeks, the treatment phase from 8 to 36 weeks (Schmidt 1993). Eleven of the fourteen included trials described adequate methods of concealment of randomisation, only four described adequate blinding. All but one trial was sponsored by the manufacturer of lamotrigine.
This meta‐analysis suggested that lamotrigine was more effective than placebo in reducing seizure frequency, when added to conventionally used antiepileptic drugs in people suffering from refractory partial epilepsy. We were unable to examine dose effects in planned subgroup analyses, but the results from Matsuo 1993 suggested increased efficacy with an increased dose. Only one study recruited children (Duchowny 1999), and one study recruited infants (Piña‐Garza 2008); we have no evidence from this review to indicate whether lamotrigine is more or less effective in infants and children than in adults. The use of 50% or more reduction in seizure frequency as a measure of efficacy could be criticized, given that seizure freedom would be a more relevant clinical measure. However, seizure freedom was rarely achieved in the studies involving people with refractory epilepsy.
For a drug to be an attractive option, it would need to have a favourable adverse effect profile, have little effect on cognition, and have positive effects on quality of life, in addition to reducing seizures. In our review, certain adverse effects (ataxia, dizziness, diplopia, and nausea) were significantly more likely to occur with lamotrigine. It was emphasized that researchers should routinely and regularly enquire about adverse effects, using a standardised check‐list thesaurus, and should not record only those volunteered by the patient. More participants had lamotrigine withdrawn than placebo, but this was not statistically significant.
The results of this review apply only to add‐on use of lamotrigine. Our results do not inform us how add‐on lamotrigine compares with other drugs when used as add‐ons, which is an extremely important issue for clinicians who are faced with an ever increasing number of antiepileptic drugs (AEDs) from which to choose, and who need to make an evidence‐based choice between the AEDs. Indirect comparisons can be made using results of other reviews, but such indirect comparisons require cautious interpretation.
Overall completeness and applicability of evidence
Only two studies evaluated the effects of add‐on lamotrigine therapy on cognition and quality of life. The results of these studies suggested that lamotrigine was probably not associated with any significant cognitive decline. Anyway, the limited data available and heterogenous measurement scales for quality of life outcome precluded us from drawing any conclusions about the effects on cognition and quality of life.
Caution is required when translating the results of clinical trials into everyday practice, since the individuals in trials are a highly selected population who may be better motivated, and are closely followed and monitored; participants who are uncooperative and non‐compliant, who are likely to have adverse effects and fewer benefits, are excluded. The results of this review cannot be extrapolated to people with generalized epilepsies, about whom there is a great paucity of data. The safety of lamotrigine during pregnancy and lactation cannot be ascertained from this review. The duration of the studies included in this review was insufficient to detect changes in cognition, social problems, or long‐term adverse effects. Trials that include a larger number of individuals, preferably who are using lamotrigine as monotherapy, and which are using reliable, validated measures and longer follow‐up are warranted. This review did not have the sensitivity to detect rare but serious adverse effects, such as psychosis, Steven Johnson's syndrome, or aplastic anaemia which may be seen with AEDs. Rare phenomena such as habituation and tolerance may not be evident in short‐term trials. The economic aspects of lamotrigine therapy also need to be examined.
Quality of the evidence
Overall, eleven studies were rated as having low risk of bias and three had an unclear risk of bias, due to lack of reported information around study design. Effective blinding of studies of lamotrigine was only reported in three studies. We rated all the included studies as low risk of bias for incomplete outcome data due to the intention‐to‐treat analyses undertaken by the study authors. The GRADE approach was used to rate the quality of evidence for each outcome and is presented in a 'Summary of findings' table (see summary of findings Table for the main comparison). For the main outcome of greater than 50% reduction in seizure frequency, the quality of evidence was rated as high (all studies contributed to the analysis). Tolerability outcomes (withdrawal and adverse effects) were judged as high to moderate quality due to the incomplete outcome data from some studies contributing to the analysis and a wide confidence intervals.
Potential biases in the review process
Although all protocols were requested, the time frame in which the majority of the studies were conducted made retrieval of all of these difficult. This could lead to potential bias through omitted information to which we did not have access. All studies but one were sponsored by GlaxoSmithKline, the manufacturers of lamotrigine and this could be a potential source of bias.

Study flow diagram for update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 1 Intention‐to‐treat analysis.
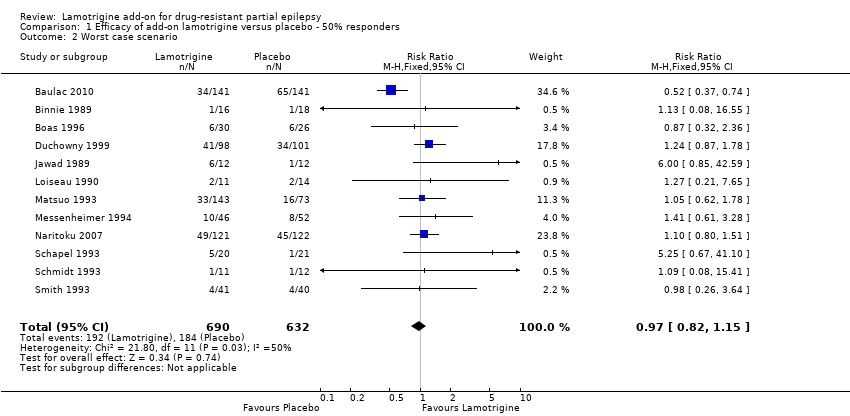
Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 2 Worst case scenario.

Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 3 Best case scenario.

Comparison 2 Treatment withdrawal (global outcome), Outcome 1 Withdrawal from treatment.

Comparison 3 Adverse effects, Outcome 1 Ataxia.
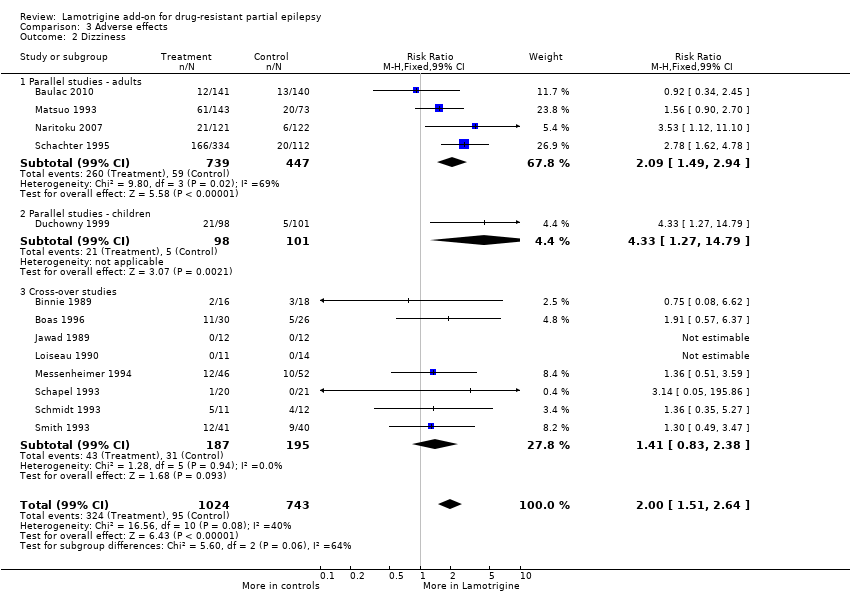
Comparison 3 Adverse effects, Outcome 2 Dizziness.
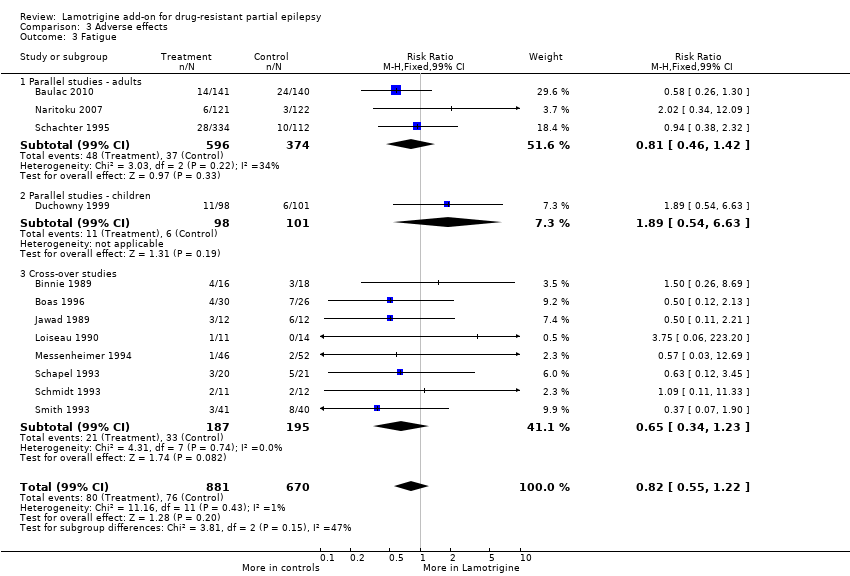
Comparison 3 Adverse effects, Outcome 3 Fatigue.

Comparison 3 Adverse effects, Outcome 4 Nausea.
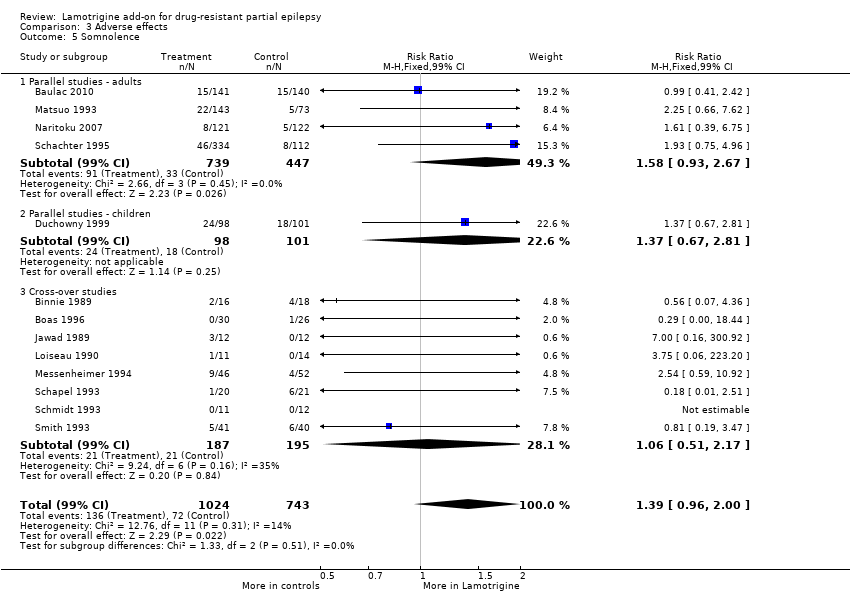
Comparison 3 Adverse effects, Outcome 5 Somnolence.
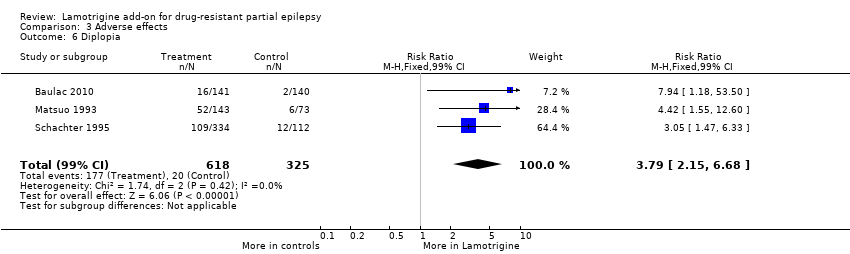
Comparison 3 Adverse effects, Outcome 6 Diplopia.

Comparison 3 Adverse effects, Outcome 7 Headache.
| Lamotrigine versus placebo for drug‐resistant partial epilepsy | ||||||
| Patient or population: participants with drug‐resistant partial epilepsy Settings: outpatient setting Intervention: Lamotrigine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lamotrigine | |||||
| 50% or greater reduction in seizure frequency ‐ ITT analysis | 157 per 1000 | 283 per 1000 | RR 1.80 (95% CI 1.45 to 2.23) | 1322 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Treatment withdrawal | 159 per 1000 | 176 per 1000 | RR 1.11 (95% CI 0.90 to 1.36) | 1805 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Ataxia | 45 per 1000 | 150 per 1000 | RR 3.34 (99% CI 2.01 to 5.55) | 1524 | ⊕⊕⊕⊝ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Dizziness | 128 per 1000 | 256 per 1000 | RR 2.00 (99% CI 1.51 to 2.64) | 1767 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Fatigue | 113 per 1000 | 93 per 1000 | RR 0.82 (99% CI 0.55 to 1.22) | 1551 (12 studies) | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Nausea | 83 per 1000 | 150 per 1000 | RR 1.81 (99% CI 1.22 to 2.68) | 1486 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes4. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One or two studies do not contribute to the analysis, but no other bias. 2 All studies contributed to the analysis. 3 Wide confidence intervals. 4Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment). | ||||||
| Outcome | Study | Number tested | Lamotrigine mean | Placebo mean |
| Stroop time | 41 | 93.98 | 98.39 | |
| Stroop error | 44 | 2.18 | 2.41 | |
| Stroop colour word (Total score) | 10 | 32.4+/‐10.9 | 35.6+/‐9.42 | |
| Number cancellation: AC | 44 | 51.36 | 49.7 | |
| Number cancellation: AE | 43 | 3.6 | 3.04 | |
| Number cancellation: BC | 42 | 48.21 | 48.54 | |
| Number cancellation: C | 42 | 38.19 | 39.29 | |
| Critical flicker fusion | 40 | 30.44 | 30.37 | |
| Choice reaction time | 40 | 0.675 | 0.669 | |
| Digit symbol (Scaled score) | 10 | 5 +/‐2.45 | 6.6 +/‐ 2.71 | |
| Rey complex figure recall percentile | 10 | 22+/‐17.51 | 30.5+/‐27.33 | |
| Trail making part B percentile | 10 | 26+/‐30.35 | 30.5+/‐32.09 |
| Outcome | Number tested | Lamotrigine ‐ Mean | Placebo ‐ Mean | Clinical relevance |
| PSYCHOLOGICAL: | ||||
| Depression | 54 | 4.24 | 4.26 | No significant difference |
| Happiness | 51 | 3.8 | 1.96 | Higher scores in LTG group; P = 0.003 |
| Mood | 50 | 24.36 | 26.8 | No significant difference |
| Self‐esteem | 50 | 30.06 | 29.16 | No significant difference |
| Mastery | 50 | 20.02 | 18.78 | Higher scores in LTG group; P = 0.003 |
| Anxiety | 54 | 6.87 | 6.83 | No significant difference |
| PHYSICAL (Nottingham Health Profile): | ||||
| Energy | 53 | 0.68 | 0.68 | No significant difference |
| Pain | 53 | 0.6 | 0.69 | No significant difference |
| Emotional reaction | 53 | 1.96 | 1.96 | No significant difference |
| Sleep | 53 | 0.89 | 0.76 | No significant difference |
| Social isolation | 53 | 0.92 | 0.94 | No significant difference |
| Physical mobility | 53 | 0.96 | 0.91 | No significant difference |
| SEIZURE SEVERITY SCALE: | ||||
| Percept | 53 | 25.19 | 25.47 | No significant difference |
| Ictal | 53 | 19.47 | 20.53 | Less severe seizures in LTG group; P = 0.017 |
| Caregivers | 53 | 20.35 | 21.80 | Less severe seizures in LTG group; P = 0.035 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intention‐to‐treat analysis Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Cross‐over | 8 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.44, 4.61] |
| 1.2 Parallel group lamotrigine ‐ 300 mg | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.57, 2.67] |
| 1.3 Parallel group lamotrigine ‐ 500 mg | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.08, 4.20] |
| 1.4 Parallel group (children) | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.59, 4.38] |
| 1.5 Parallel Group ‐Adults‐Lamotrigine ER | 1 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.16, 2.50] |
| 1.6 Parallel Group 300 to 600mg | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.75] |
| 1.7 Any dose lamotrigine, adults or children | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.45, 2.23] |
| 2 Worst case scenario Show forest plot | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 3 Best case scenario Show forest plot | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [2.36, 3.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawal from treatment Show forest plot | 14 | 1805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.90, 1.36] |
| 1.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.50] |
| 1.2 Parallel studies in children | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.42, 1.52] |
| 1.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.89, 3.72] |
| 1.4 Parallel Studies in Infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ataxia Show forest plot | 12 | 1524 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.34 [2.01, 5.55] |
| 1.1 Parallel studies ‐ adults | 3 | 943 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.40 [1.67, 6.90] |
| 1.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.15 [0.72, 36.64] |
| 1.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.98 [1.38, 6.41] |
| 2 Dizziness Show forest plot | 13 | 1767 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.00 [1.51, 2.64] |
| 2.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.09 [1.49, 2.94] |
| 2.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.33 [1.27, 14.79] |
| 2.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.83, 2.38] |
| 3 Fatigue Show forest plot | 12 | 1551 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.82 [0.55, 1.22] |
| 3.1 Parallel studies ‐ adults | 3 | 970 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.46, 1.42] |
| 3.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.89 [0.54, 6.63] |
| 3.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.65 [0.34, 1.23] |
| 4 Nausea Show forest plot | 12 | 1486 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.81 [1.22, 2.68] |
| 4.1 Parallel Studies ‐ adults | 3 | 905 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.68 [1.02, 2.78] |
| 4.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.67 [0.81, 39.69] |
| 4.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.67 [0.85, 3.29] |
| 5 Somnolence Show forest plot | 13 | 1767 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.96, 2.00] |
| 5.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.58 [0.93, 2.67] |
| 5.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.37 [0.67, 2.81] |
| 5.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.06 [0.51, 2.17] |
| 6 Diplopia Show forest plot | 3 | 943 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.79 [2.15, 6.68] |
| 7 Headache Show forest plot | 5 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.13 [0.87, 1.45] |

