Lamotrigina complementaria para la epilepsia parcial resistente a los fármacos
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Double‐blind, placebo‐controlled, randomised, parallel‐group study. Three arms: 1 placebo, 1 lamotrigine, and 1 pregabalin. Baseline period = 6 weeks; double‐blind treatment period = 17 weeks, which included initial 5 weeks dosage titration for lamotrigine and 6 weeks maintenance at 300 mg/day and additional treatment period of 6 weeks with dose escalation to 400 mg/day for those with continuing seizures. Double‐blind treatment period was followed by an open‐label study or a 2‐week taper phase. | |
| Participants |
Exclusion Criteria:
| |
| Interventions | Group I (n = 141): received placebo. Group II (n = 141): received lamotrigine 300 mg/day after dose titration over 5 weeks, and if seizures occurred during 6‐week maintenance, further dose escalation to 400 mg/day from week 12 to 17. Group III (n = 152): received pregabalin. | |
| Outcomes | (1) Seizure frequency. (2) Adverse events, including changes in physical and neurologic examinations, 12‐lead electrocardiograms (ECGs), and clinical laboratory tests (hematology, blood chemistry, pregnancy, and urinalysis). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | The details were not mentioned in the publication. |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided regarding blinding of participants, study personnel, and outcome assessors. Regarding the medications, blinding was maintained by administering the same numbers of capsules per day per group. |
| Incomplete outcome data (attrition bias) | Low risk | 35 withdrew from placebo group and 40 from lamotrigine group. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable to check a priori outcomes, but appears all expected and pre‐specified outcomes are reported. |
| Other bias | Unclear risk | Responder rates were mentioned as percentages and actual numbers were not given. Author has been contacted regarding actual number of responders in each group. This study was sponsored by Pfizer Inc. |
| Methods | Randomized, double‐blind, cross‐over study. Two treatment arms: 1 placebo, and 1 lamotrigine. Baseline period = 8 weeks.Treatment I and II = 12 weeks each. Washout = 6 weeks, including taper period. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine, or placebo. Median daily dose of lamotrigine was 200 mg. Participants on valproate received lower doses. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated sequentially‐numbered sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Low risk | Participants and parents were blinded. An unblinded investigator with knowledge of the medication and plasma concentrations instructed the blinded investigators about dispensing the trial medications. Identical tablets and packaging used. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. No participant withdrew from the study during the first treatment phase. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check to priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomized, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. | |
| Participants |
| |
| Interventions | Lamotrigine or placebo was added to the patients' existing AEDs. The dose of lamotrigine varied from 75 to 400 mg. Participants on valproate received lower doses. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Partcipants were allocated sequentially‐numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided regarding blinding of participants, study personnel, and outcome assessors. All treatments (tablets) and packaging were identical. Prepacked coded medication was dispensed by pharmacy. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 10 participants withdrew from the study; 8 randomised to lamotrigine and 2 to placebo. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomized, double blind, parallel group, multi‐centre study. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. Median dose ranged from 2.7 to 12.9 mg/kg/day depending upon concurrent use of other AEDs. Participants on valproate received lower doses. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised with a blocked randomisation scheme to treatment with add‐on lamotrigine or matched placebo in bottles labelled with pre‐generated participant numbers. |
| Blinding (performance bias and detection bias) | Low risk | Treatment assignments were unknown to all study‐site personnel, patients and sponsors. Lamotrigine and matching placebo were provided as berry‐flavoured, chewable, dispersible caplets or tablets in strengths of 5, 25, and 100 mg. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 2 enrolled participants withdrew before randomisation. 14 participants allocated to lamotrigine and 18 participants allocated to placebo withdrew during treatment phase. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. Five phases: baseline period = 8 weeks; Treatment I = 12 weeks; Washout I = 6 weeks; treatment II = 12 weeks; Washout II = 6 weeks. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. The median daily dose of lamotrigine was 250 mg. Participants on valproate received lower doses. Unblinded investigator wrote prescriptions based on plasma concentration. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Partcipants were allocated by sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided regarding blinding of participants, study personnel, and outcome assessors. Identical tablets and packaging used. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. One participant who was allocated to lamotrigine withdrew from the study (the reason for exclusion was reported) and none withdrew from the placebo group. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. The median daily lamotrigine dose was 300 mg. Participants on valproate received lower doses. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Low risk | Neurologists, participants and parents were blinded. Investigators were blinded. All treatments (tablets) and packaging were identical. Pre‐packed coded medication dispensed by pharmacy. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 2 participants withdrew from the study; 1 receiving lamotrigine and 1 receiving placebo. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, parallel group, multi‐centre study. Pre‐randomisation baseline = 12 weeks. Treatment phase = 24 weeks. Taper and follow‐up = 3 weeks. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine 300 mg or lamotrigine 500 mg or placebo. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Randomisation concealment: allocated sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided regarding blinding of participants, study personnel, and outcome assessors. Identical tablets and packaging used. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 25 participants withdrew from the study; 7 receiving lamotrigine 300 mg, 12 receiving lamotrigine 500 mg and 6 receiving placebo. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable to check a priori outcomes, but appears all expected and pre‐specified outcomes were reported. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. Total study duration was 43 weeks. Pre‐randomisation baseline = 8 weeks. Treatment A = 14 weeks (including 2 weeks blinded tapering). Follow‐up period = 3 weeks. Treatment B = 14 weeks (including 2 weeks blinded tapering). Washout = 4 weeks. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. Median lamotrigine dose 400 mg/day. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Unclear risk | Investigators were blinded. No more information provided regarding blinding of neurologists, participants, and parents. All treatments (tablets) and packaging were identical. Pre‐packed coded medication dispensed by pharmacy. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 6 participants withdrew from the study; 2 receiving lamotrigine and 4 receiving placebo. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Double‐blind, randomised, parallel‐group, placebo‐controlled multi‐centre global study. Screening phase of up to 2 weeks during which eligibility was determined; an 8‐week baseline phase serving to exclude from randomisation patients who did not meet the minimum seizure frequency criterion; a 7‐week, double‐blind escalation phase during which lamotrigine XR (Extended Release) was introduced and titrated to its target dose; and a 12‐week, double‐blind maintenance phase during which dosage of study medication and concomitant AED were maintained. | |
| Participants |
Exclusion criteria included: presence of primary generalized seizures, status epilepticus during or within 24 weeks before the start of the baseline phase, chronic treatment with three or more AEDs, current or previous use of lamotrigine, current use of felbamate or adherence to a ketogenic diet, and pregnancy or planned pregnancy during the study or within 3 weeks after the last dose of study medication. | |
| Interventions | Treatment group received lamotrigine XR (Extended Release); other group received identical placebo. Dosage of lamotrigine XR was escalated gradually up to 200 mg/day in those receiving valproate, 300 mg/day in those receiving valproate and an enzyme inducing AED, and up to 500 mg/day in those receiving enzyme inducing AEDs without valproate. | |
| Outcomes | (1) Seizure frequency. (2) Adverse events (3) Withdrawals from study. US subjects had following additional assessments: Profile of Mood States (POMS), Center for Epidemiologic Studies‐Depression Scale (CES‐D), research version of the Neurological Disorders Depression Inventory‐Epilepsy (NDDI‐E), Quality of Life in Epilepsy‐31‐P (QOLIE‐31‐P), Liverpool Adverse Experience Profile (AEP), Seizure Severity Questionnaire (SSQ), and Epworth Sleepiness Scale (ESS). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported in the publication. |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | 24 subjects withdrew from treatment group and 16 from placebo group. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | There was no protocol available to check a priori outcomes,but appears all expected and pre‐specified outcomes were reported. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, multi‐centre placebo‐controlled trial. Responder‐enriched design in which all patients received adjunctive lamotrigine during an open‐label phase (wherein dose was escalated to achieve optimal response); those who had a 40% or greater reduction in the frequency of partial seizures during the last 4 weeks of the optimisation period were randomly assigned to double‐blind treatment for up to 8 weeks with continued lamotrigine or placebo. | |
| Participants |
Exclusion Criteria: subjects with progressive myoclonic epilepsy; progressive neurologic disease, seizures unrelated to epilepsy or resulting from drug withdrawal; use of felbamate, adrenocorticotropic hormone, previous use of lamotrigine, two AEDs as maintenance treatment, presence of hepatic dysfunction, having a functioning vagus nerve stimulator; or being on a ketogenic diet. | |
| Interventions | Intervention group was continued on lamotrigine. Control group subjects had their lamotrigine dose tapered and changed to placebo. The maximum maintenance dose was 5.1 mg/kg/day for those on non–enzyme‐inducing AEDs or valproate and 15.6 mg/kg/day for those on enzyme‐inducing AEDs. | |
| Outcomes | (1) Percentage of patients who had treatment failures during the double‐blind phase. (2) Cumulative percentage of patients who met escape criteria as a function of days on double‐blind study medication. Subjects were withdrawn from study if they met one of the following criteria:
| |
| Notes | The protocol was amended midway through the study to randomly assign all patients with at least 40% reduction in seizure frequency, instead of planned inclusion of subjects with 40% to 80% reduction in seizure frequency. 43 subjects who had more than 80% reduction in seizure frequency before the protocol amendment were not included in the double‐blind study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported in the publication. |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | 11 patients (8 in the lamotrigine group and 3 in placebo group) completed the double‐blind phase, 25 (9 in the lamotrigine group and 16 in placebo group) met escape criteria, and 2 (both in the lamotrigine group) prematurely withdrew because of protocol violations. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported. Protocol was not available. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, parallel‐group study. Pre‐randomisation baseline = 4 weeks. Treatment phase = 24 weeks. Taper and follow‐up = 3 weeks. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. | |
| Outcomes | (1) Withdrawals from treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Low risk | Neurologists, participants and parents were blinded. Investigators were blinded. Identical tablets and packaging used. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 73 participants withdrew from the study; 53 receiving lamotrigine and 20 receiving placebo. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section of paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. Median daily dose of lamotrigine was 300 mg. Participants receiving valproate received lower doses. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | Banks 1991 is linked to this study and investigated cognitive functions (concentration and attention; general cerebral efficiency; mnestic function). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided regarding blinding of participants, study personnel, and outcome assessors. All treatments and packaging were identical. Pre‐packed coded medication dispensed by pharmacy. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. None withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable, but appears all expected and pre‐specified outcomes were reported. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. Pre‐randomisation baseline not known. Treatment I and II = 12 weeks each, including 2‐week tapering period. Washout = 2 weeks . | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. Dosage varied from 50 mg to 450 mg (median dose was 300 mg). | |
| Outcomes | (1) 50% responder rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided regarding blinding of participants and parents. Unblinded investigator wrote prescriptions based on plasma concentration. Identical tablets and packaging were used. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from analysis. 1 participant receiving lamotrigine and 9 receiving placebo withdrew from the study. The reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable, but appears all expected and pre‐specified outcomes were reported. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
| Methods | Randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. Pre‐randomisation baseline = 4 weeks. Treatment I and II = 18 weeks each. Washout = 6 weeks. | |
| Participants |
| |
| Interventions | Add‐on lamotrigine or placebo. Lamotrigine dose up to 400 mg/day. Median daily dose was 300 mg. Participants on valproate received lower doses. | |
| Outcomes | (1) 50% responder rates. | |
| Notes | HRQL model was completed by 40 to 54 of 81 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Partecipants were allocated sequentially numbered, sealed packages containing either lamotrigine or placebo. |
| Blinding (performance bias and detection bias) | High risk | Patients (46/73) and investigators (52/73) were able to identify lamotrigine treatment. Identical tablets and packaging were used. Prepacked coded medication dispensed by pharmacy. |
| Incomplete outcome data (attrition bias) | Unclear risk | No participants were excluded from analysis. 9 people withdrew from the study; 6 receiving lamotrigine and 3 receiving placebo. The reasons for exclusion were reported. Patients who discontinued prematurely did not complete the HRQOL measure at the time of discontinuation, the exclusion of treatment failures may introduce a bias in favour of lamotrigine. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable, but appears all expected and pre‐specified outcomes were reported. |
| Other bias | Unclear risk | This study was sponsored by GlaxoSmithKline, the manufactures of LTG. |
AED: antiepileptic drug
LTG: lamotrigine
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Ineligible population: subjects included had primary generalized epilepsy and not partial seizures | |
| Inelegible population: subjects included in the study had uncontrolled partial epilepsy and generalised tonic‐clonic seizures. | |
| Inelegible population: subjects included in the study had uncontrolled partial epilepsy and generalised tonic‐clonic seizures. | |
| Published as conference abstract: details of the methods and results are not available. | |
| Published as conference abstract: details of the methods and results are not available. | |
| Not randomised controlled trial. | |
| No randomised controlled trial. | |
| Comparative study among therapeutic equivalents of lamotrigine. Not placebo controlled. | |
| Published as conference abstract: details of the methods and results are not available. | |
| Inelegible population: subjects included in the study had epilepsy, migraine, pain, psychiatric disorders. | |
| Subjects included in the study had all epileptic types. Lacosamide as add‐on for epilepsy and in comparison with lamotrigine and topiramate. | |
| Inelegible population: subjects included in the study had all epileptic types. | |
| Published as conference abstract: details of the methods and results are not available. | |
| Comparative study of lamotrigine and zonisamide. Not placebo controlled | |
| Study of institutionalised people with severe epilepsy. Subjects included in the study had all epileptic types. | |
| No LTG add‐on. | |
| Details of the results are not available. | |
| Published as conference abstract: details of the methods and results are not available. | |
| Not randomised controlled trial. | |
| Published as conference abstract: details of the methods and results are not available. | |
| Not randomised controlled trial. |
AED: antiepileptic drug
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intention‐to‐treat analysis Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 1 Intention‐to‐treat analysis. | ||||
| 1.1 Cross‐over | 8 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.44, 4.61] |
| 1.2 Parallel group lamotrigine ‐ 300 mg | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.57, 2.67] |
| 1.3 Parallel group lamotrigine ‐ 500 mg | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.08, 4.20] |
| 1.4 Parallel group (children) | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.59, 4.38] |
| 1.5 Parallel Group ‐Adults‐Lamotrigine ER | 1 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.16, 2.50] |
| 1.6 Parallel Group 300 to 600mg | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.75] |
| 1.7 Any dose lamotrigine, adults or children | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.45, 2.23] |
| 2 Worst case scenario Show forest plot | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| Analysis 1.2 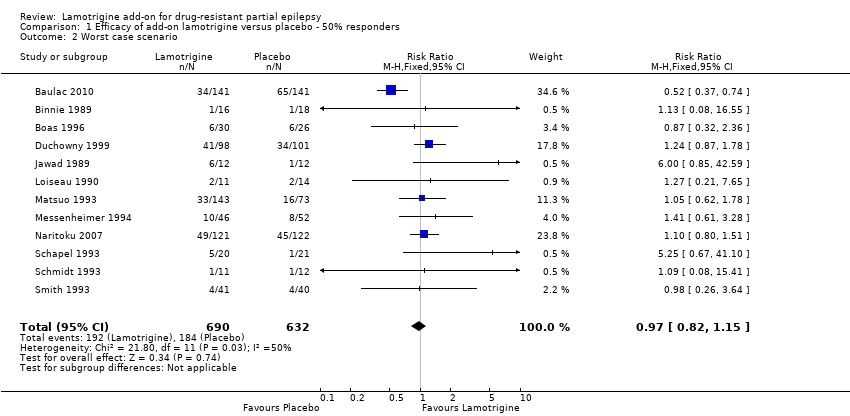 Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 2 Worst case scenario. | ||||
| 3 Best case scenario Show forest plot | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [2.36, 3.50] |
| Analysis 1.3  Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 3 Best case scenario. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawal from treatment Show forest plot | 14 | 1805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.90, 1.36] |
| Analysis 2.1  Comparison 2 Treatment withdrawal (global outcome), Outcome 1 Withdrawal from treatment. | ||||
| 1.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.50] |
| 1.2 Parallel studies in children | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.42, 1.52] |
| 1.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.89, 3.72] |
| 1.4 Parallel Studies in Infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ataxia Show forest plot | 12 | 1524 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.34 [2.01, 5.55] |
| Analysis 3.1  Comparison 3 Adverse effects, Outcome 1 Ataxia. | ||||
| 1.1 Parallel studies ‐ adults | 3 | 943 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.40 [1.67, 6.90] |
| 1.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.15 [0.72, 36.64] |
| 1.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.98 [1.38, 6.41] |
| 2 Dizziness Show forest plot | 13 | 1767 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.00 [1.51, 2.64] |
| Analysis 3.2 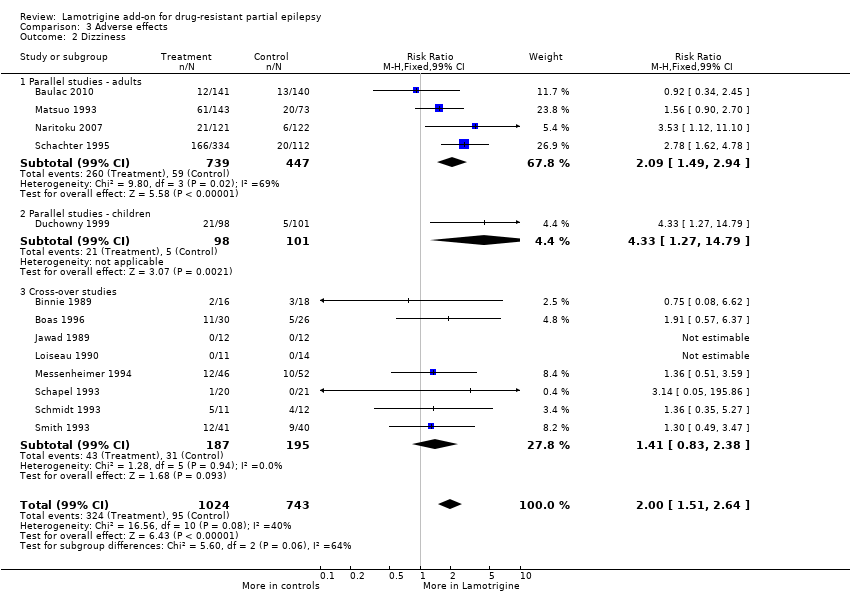 Comparison 3 Adverse effects, Outcome 2 Dizziness. | ||||
| 2.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.09 [1.49, 2.94] |
| 2.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.33 [1.27, 14.79] |
| 2.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.83, 2.38] |
| 3 Fatigue Show forest plot | 12 | 1551 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.82 [0.55, 1.22] |
| Analysis 3.3 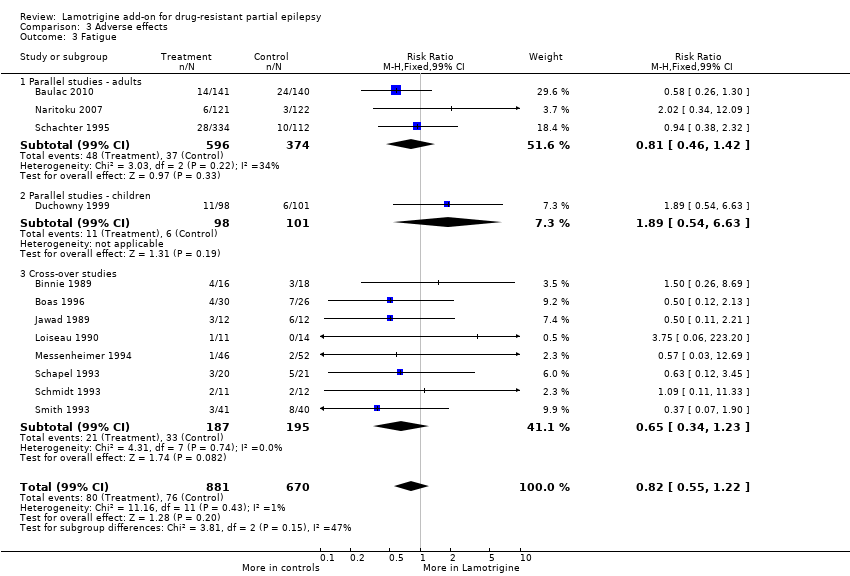 Comparison 3 Adverse effects, Outcome 3 Fatigue. | ||||
| 3.1 Parallel studies ‐ adults | 3 | 970 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.46, 1.42] |
| 3.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.89 [0.54, 6.63] |
| 3.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.65 [0.34, 1.23] |
| 4 Nausea Show forest plot | 12 | 1486 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.81 [1.22, 2.68] |
| Analysis 3.4  Comparison 3 Adverse effects, Outcome 4 Nausea. | ||||
| 4.1 Parallel Studies ‐ adults | 3 | 905 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.68 [1.02, 2.78] |
| 4.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.67 [0.81, 39.69] |
| 4.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.67 [0.85, 3.29] |
| 5 Somnolence Show forest plot | 13 | 1767 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.96, 2.00] |
| Analysis 3.5 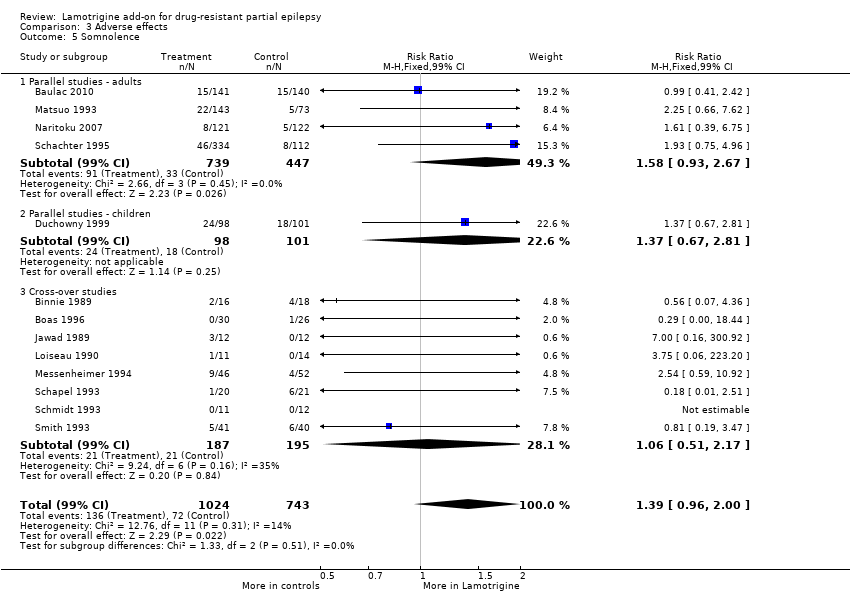 Comparison 3 Adverse effects, Outcome 5 Somnolence. | ||||
| 5.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.58 [0.93, 2.67] |
| 5.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.37 [0.67, 2.81] |
| 5.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.06 [0.51, 2.17] |
| 6 Diplopia Show forest plot | 3 | 943 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.79 [2.15, 6.68] |
| Analysis 3.6 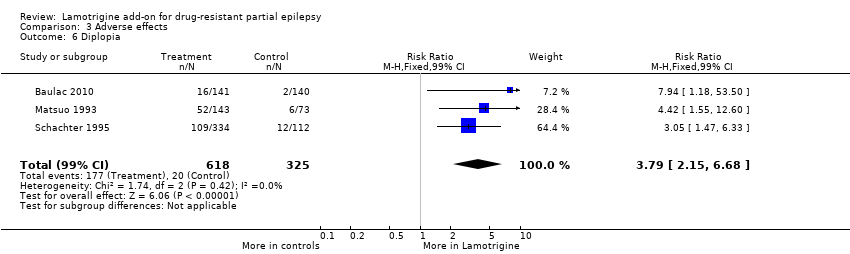 Comparison 3 Adverse effects, Outcome 6 Diplopia. | ||||
| 7 Headache Show forest plot | 5 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.13 [0.87, 1.45] |
| Analysis 3.7  Comparison 3 Adverse effects, Outcome 7 Headache. | ||||

Study flow diagram for update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
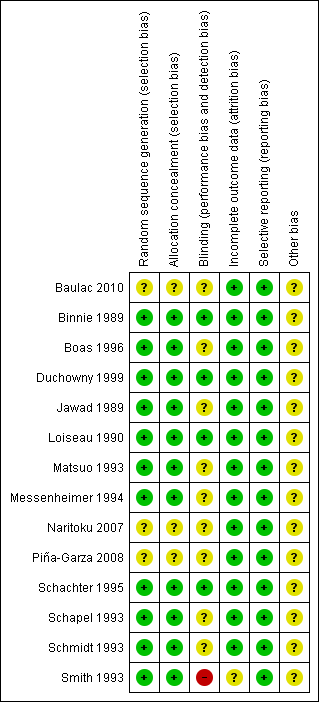
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 1 Intention‐to‐treat analysis.

Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 2 Worst case scenario.

Comparison 1 Efficacy of add‐on lamotrigine versus placebo ‐ 50% responders, Outcome 3 Best case scenario.

Comparison 2 Treatment withdrawal (global outcome), Outcome 1 Withdrawal from treatment.

Comparison 3 Adverse effects, Outcome 1 Ataxia.

Comparison 3 Adverse effects, Outcome 2 Dizziness.

Comparison 3 Adverse effects, Outcome 3 Fatigue.

Comparison 3 Adverse effects, Outcome 4 Nausea.

Comparison 3 Adverse effects, Outcome 5 Somnolence.

Comparison 3 Adverse effects, Outcome 6 Diplopia.

Comparison 3 Adverse effects, Outcome 7 Headache.
| Lamotrigine versus placebo for drug‐resistant partial epilepsy | ||||||
| Patient or population: participants with drug‐resistant partial epilepsy Settings: outpatient setting Intervention: Lamotrigine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lamotrigine | |||||
| 50% or greater reduction in seizure frequency ‐ ITT analysis | 157 per 1000 | 283 per 1000 | RR 1.80 (95% CI 1.45 to 2.23) | 1322 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Treatment withdrawal | 159 per 1000 | 176 per 1000 | RR 1.11 (95% CI 0.90 to 1.36) | 1805 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Ataxia | 45 per 1000 | 150 per 1000 | RR 3.34 (99% CI 2.01 to 5.55) | 1524 | ⊕⊕⊕⊝ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Dizziness | 128 per 1000 | 256 per 1000 | RR 2.00 (99% CI 1.51 to 2.64) | 1767 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Fatigue | 113 per 1000 | 93 per 1000 | RR 0.82 (99% CI 0.55 to 1.22) | 1551 (12 studies) | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| Nausea | 83 per 1000 | 150 per 1000 | RR 1.81 (99% CI 1.22 to 2.68) | 1486 | ⊕⊕⊕⊕ | RR >1 indicates outcome is more likely in Lamotrigine group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes4. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One or two studies do not contribute to the analysis, but no other bias. 2 All studies contributed to the analysis. 3 Wide confidence intervals. 4Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment). | ||||||
| Outcome | Study | Number tested | Lamotrigine mean | Placebo mean |
| Stroop time | 41 | 93.98 | 98.39 | |
| Stroop error | 44 | 2.18 | 2.41 | |
| Stroop colour word (Total score) | 10 | 32.4+/‐10.9 | 35.6+/‐9.42 | |
| Number cancellation: AC | 44 | 51.36 | 49.7 | |
| Number cancellation: AE | 43 | 3.6 | 3.04 | |
| Number cancellation: BC | 42 | 48.21 | 48.54 | |
| Number cancellation: C | 42 | 38.19 | 39.29 | |
| Critical flicker fusion | 40 | 30.44 | 30.37 | |
| Choice reaction time | 40 | 0.675 | 0.669 | |
| Digit symbol (Scaled score) | 10 | 5 +/‐2.45 | 6.6 +/‐ 2.71 | |
| Rey complex figure recall percentile | 10 | 22+/‐17.51 | 30.5+/‐27.33 | |
| Trail making part B percentile | 10 | 26+/‐30.35 | 30.5+/‐32.09 |
| Outcome | Number tested | Lamotrigine ‐ Mean | Placebo ‐ Mean | Clinical relevance |
| PSYCHOLOGICAL: | ||||
| Depression | 54 | 4.24 | 4.26 | No significant difference |
| Happiness | 51 | 3.8 | 1.96 | Higher scores in LTG group; P = 0.003 |
| Mood | 50 | 24.36 | 26.8 | No significant difference |
| Self‐esteem | 50 | 30.06 | 29.16 | No significant difference |
| Mastery | 50 | 20.02 | 18.78 | Higher scores in LTG group; P = 0.003 |
| Anxiety | 54 | 6.87 | 6.83 | No significant difference |
| PHYSICAL (Nottingham Health Profile): | ||||
| Energy | 53 | 0.68 | 0.68 | No significant difference |
| Pain | 53 | 0.6 | 0.69 | No significant difference |
| Emotional reaction | 53 | 1.96 | 1.96 | No significant difference |
| Sleep | 53 | 0.89 | 0.76 | No significant difference |
| Social isolation | 53 | 0.92 | 0.94 | No significant difference |
| Physical mobility | 53 | 0.96 | 0.91 | No significant difference |
| SEIZURE SEVERITY SCALE: | ||||
| Percept | 53 | 25.19 | 25.47 | No significant difference |
| Ictal | 53 | 19.47 | 20.53 | Less severe seizures in LTG group; P = 0.017 |
| Caregivers | 53 | 20.35 | 21.80 | Less severe seizures in LTG group; P = 0.035 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intention‐to‐treat analysis Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Cross‐over | 8 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.44, 4.61] |
| 1.2 Parallel group lamotrigine ‐ 300 mg | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.57, 2.67] |
| 1.3 Parallel group lamotrigine ‐ 500 mg | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.08, 4.20] |
| 1.4 Parallel group (children) | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.59, 4.38] |
| 1.5 Parallel Group ‐Adults‐Lamotrigine ER | 1 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.16, 2.50] |
| 1.6 Parallel Group 300 to 600mg | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.75] |
| 1.7 Any dose lamotrigine, adults or children | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.45, 2.23] |
| 2 Worst case scenario Show forest plot | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 3 Best case scenario Show forest plot | 12 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [2.36, 3.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawal from treatment Show forest plot | 14 | 1805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.90, 1.36] |
| 1.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.50] |
| 1.2 Parallel studies in children | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.42, 1.52] |
| 1.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.89, 3.72] |
| 1.4 Parallel Studies in Infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ataxia Show forest plot | 12 | 1524 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.34 [2.01, 5.55] |
| 1.1 Parallel studies ‐ adults | 3 | 943 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.40 [1.67, 6.90] |
| 1.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.15 [0.72, 36.64] |
| 1.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.98 [1.38, 6.41] |
| 2 Dizziness Show forest plot | 13 | 1767 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.00 [1.51, 2.64] |
| 2.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.09 [1.49, 2.94] |
| 2.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.33 [1.27, 14.79] |
| 2.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.83, 2.38] |
| 3 Fatigue Show forest plot | 12 | 1551 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.82 [0.55, 1.22] |
| 3.1 Parallel studies ‐ adults | 3 | 970 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.46, 1.42] |
| 3.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.89 [0.54, 6.63] |
| 3.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.65 [0.34, 1.23] |
| 4 Nausea Show forest plot | 12 | 1486 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.81 [1.22, 2.68] |
| 4.1 Parallel Studies ‐ adults | 3 | 905 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.68 [1.02, 2.78] |
| 4.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.67 [0.81, 39.69] |
| 4.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.67 [0.85, 3.29] |
| 5 Somnolence Show forest plot | 13 | 1767 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.96, 2.00] |
| 5.1 Parallel studies ‐ adults | 4 | 1186 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.58 [0.93, 2.67] |
| 5.2 Parallel studies ‐ children | 1 | 199 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.37 [0.67, 2.81] |
| 5.3 Cross‐over studies | 8 | 382 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.06 [0.51, 2.17] |
| 6 Diplopia Show forest plot | 3 | 943 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.79 [2.15, 6.68] |
| 7 Headache Show forest plot | 5 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.13 [0.87, 1.45] |

