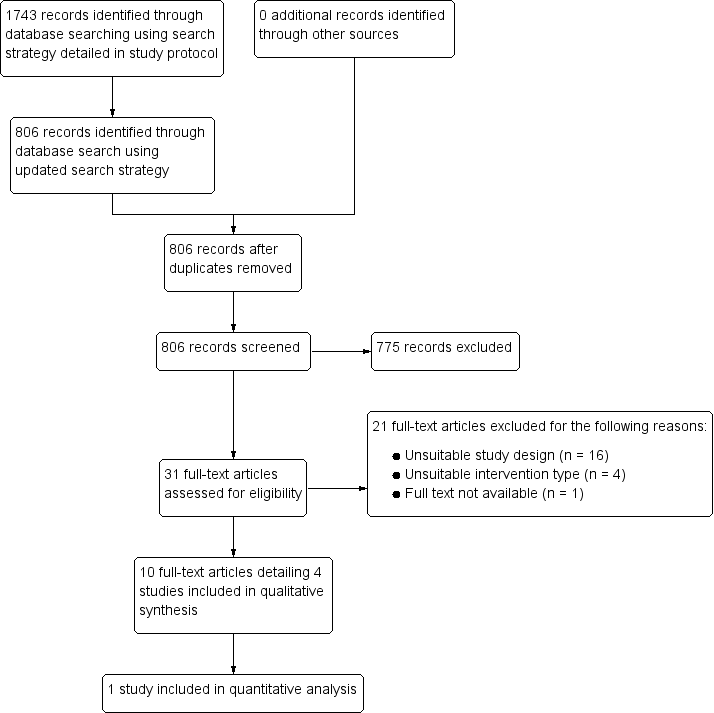
Rociamiento espacial con insecticida para la prevención de la transmisión del paludismo
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: controlled before‐and‐after (CBA) study | |
| Participants | Number of participants: 408:485 | |
| Interventions | Active ingredient and dosage: pyrethrin, 0.002 to 0.0025 lbs per acre Co‐interventions (type, access, compliance): not described | |
| Outcomes | Outcomes measured:
Length of follow‐up: 15th June to 15th September (both in 1973, pre‐spray, and 1974, during the intervention) | |
| Location Profile | Study location: villages in the coastal plain east of La Libertad, El Salvador. Plasmodium species: falciparum and vivax | |
| Vector Profile | Primary vector species: An albimanus Phenotypic resistance profile: moderately to highly resistant to dichlorodiphenyltrichloroethane (DDT), dieldrin, malathion, and propoxur Method of mosquito collection: mosquito densities were measured using New Jersey light‐traps 1 night a week, from sunset until sunrise. Densities were measures the night before a spray round | |
| Notes | CBA; not included in the meta‐analysis Funding source: unknown | |
| Methods | Study design: interrupted time series (ITS) (though other designs were also used within the study) to assess the impact of space spraying in an area on epidemiological, entomological and ecological outcomes. For some outcomes, the surrounding unsprayed area was reported as a comparison. The malaria incidence rate and adult mosquito density were the only outcomes meeting the study design criteria for this review. Adjustment for clustering: none | |
| Participants | Number of participants: 15,106 living in sprayed area (31,710 including unsprayed area) | |
| Interventions | Active ingredient and dosage: malathion 95%, 6 oz per acre for first cycle and 4.5 oz per acre for all subsequent cycles Co‐interventions (type, access, compliance): IRS with DDT | |
| Outcomes | Epidemiological outcomes measured:
Entomological:
Ecological (note‐ not following suitable study design for inclusion in review):
Length of follow‐up: 5 months (October 1972 – March 1973). Sprays were scheduled to begin when epidemic levels were reached (100 cases/month/10,000 population). | |
| Location Profile | Study location: Miragoane Valley, southern peninsula, Haiti. The area has a natural barrier of mountain ranges which were expected to limit immigration of mosquitoes from adjacent unsprayed areas Malaria endemicity: perennial and seasonal with persistent pattern of outbreaks, from October into January | |
| Vector Profile | Primary vector species: Anopheles albimanus Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): breed in marshes surrounding shallow lakes in the valley floor. Adults rest in dense sugar canes and banana groves Phenotypic resistance profile: susceptible to malathion (in preliminary tests: 92% mortality at 0.8% malathion, 100% mortality at 1.6% malathion. 0% mortality in controls) Method of mosquito collection: 9 updraft UV light‐traps at 3 collection sites, operating from 5.30pm to 5.30am Human‐baited biting collections measured over 1 hour at 2 locations in each of the 3 collection sites (1 near breeding sites and 1 near houses) | |
| Notes | Funding source: unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the intervention independent of other changes? | Unclear risk | There is a lack of detail of concurrent control measures such as IRS, and environmental factors such as rainfall; the impact of these is therefore hard to measure |
| Was the shape of the intervention effect pre‐specified? | Low risk | The point of the analysis is the point of the intervention |
| Was the intervention unlikely to affect data collection? | Low risk | The sources and methods of data collection were the same before and after the intervention |
| Was knowledge of the allocated interventions adequately prevented? | Unclear risk | Unlikely that the outcomes were assessed blindly. Incidence measurements depended on self‐reporting of fever symptoms that may be influenced by participant knowledge of the intervention, although parasitaemia was confirmed by blood smear. Mosquito density measurements were objective and unlikely to be influenced by this knowledge |
| Were incomplete outcome data adequately addressed? | Low risk | No missing outcome data likely to bias the results |
| Was the study free from selective outcome reporting? | Low risk | The study design states that vector densities were also recorded in unsprayed areas but these are not reported. This may show a reduction in densities for reasons other than spraying. However, as time series data were used for this outcome, we did not consider this selective reporting likely to cause a bias in the results reported in this review |
| Was the study free from other risks of bias? | Low risk | There is no evidence of other risk of biases |
| Methods | Study design: CBA study with 4 treatment arms:
Unit of allocation: village Incidence rate was monitored by passive (through the Ranau health office) and active case detection (through monthly mass blood surveys covering ˜70% of the population) Mosquitoes were monitored using bare leg catches. The infectious status of the female anophelines was determined by microscopic examination Adjustment for clustering: none | |
| Participants | Number of participants: 178 (intervention); 216 (control). A further 285 participants were included in the remaining arms of the study not relevant to this review | |
| Interventions | Active ingredient and dosage: alphacypermethrin, 2 g AI/104 x m2 Co‐interventions (type, access, compliance): IRS and the use of insecticide‐impregnated mosquito nets (lambdacyhalothrin and deltamethrin nets) | |
| Outcomes | Outcomes measured:
Length of follow‐up: 2 years. Spraying from November 1998 until December 1999, with further spraying monthly from March 2000 to August 2000. | |
| Location Profile | Study location: Ranau District, Sabah State, situated in the north of Borneo Island, Malaysia | |
| Vector Profile | Primary vector species: Anopheles balabacensis Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): Exophilic and exophagic behaviour (up to 27 times more bites outside than inside). Early biting from 18.00 Phenotypic resistance profile: susceptibility tests have shown the anopheline mosquitoes have resistance to 4% DDT and 0.75% permethrin. Female mosquitoes in Sabah have also been demonstrated to avoid walls treated with DDT for a period of 3 ‐ 4 months after an IRS treatment Method of mosquito collection: mosquitoes were caught outdoors using the bare leg catch technique from 18.00 to 24.00 hrs. Surveillance was conducted 2 days before spraying, (about 4 weeks after the previous spray), except for June to August 2000, which was conducted 2 weeks after the previous spray, to correlate the effectiveness of the spraying with the mosquito life cycle | |
| Notes | CBA; not included in the meta‐analysis Funding source: Malysian government and Valent Biosciences | |
| Methods | Study design: ITS (though other designs were also used within the study) to assess the impact on epidemiological and short‐term entomological outcomes, following spraying with a variety of equipment and formulations. For some outcomes, nearby unsprayed areas are reported as a comparison. In Pudupettai, however, the control group was contaminated as it received space spraying 3 times, and because there was only 1 cluster in this arm, the control group is not a valid comparison. The malaria incidence rate is the only outcome that meets the study design criteria for this review. Adjustment for clustering: none | |
| Participants | Number of participants: the population receiving the intervention is not reported. For the analysis, we have estimated population sizes using census data | |
| Interventions | A variety of formulations and spraying machines were used in the course of the study to measure each one’s impact on entomological outcomes. Active ingredient and dosage: malathion, 325 – 375 mL per hectare Vanapuram (except Sanathur Dam): Active ingredient and dosage: malathion, 150 mL per hectare Melpallipattu (and Sanathur Dam): Active ingredient and dosage: malathion, 263 ‐ 300 mL per hectare Time of spraying: 2000 hr – 2200 hr, and 0500 hr – 0700hr. Co‐interventions (type, access, compliance): residual spraying with malathion conducted 3 times each year (April ‐ May, July ‐ August and September ‐ October) | |
| Outcomes | Outcomes measured:
Length of follow‐up: 4 years (January 1981 – December 1984). Spraying operations were conducted in
| |
| Location Profile | Study location: 3 sites along the Thenpennai riverine tract in Tamil Nadu state, India. These are Pudupettai (then South Arcot district; now Viluppuram district), Vanapuram, and Melpallipattu (then North Arcot district, now Tiruvannamalai district). Due to the unique epidemiological challenges of malaria transmission in Sathanur Dam village in Vanapuram, the results from Sathanur Dam are reported separately to Vanapuram, providing 4 distinct study sites Plasmodium species: 73.1% vivax, 21.5% falciparum, 5.4% mixed | |
| Vector Profile | Primary vector species: An culicifacies Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): Shows some exophilic behaviour Phenotypic resistance profile: resistant to DDT. Susceptible to malathion Method of mosquito collection: indoor/outdoor timed resting catches (days 1, 2, 3 and 11 after each spray), and indoor/outdoor all‐night human baited collections (day 3 after spraying) | |
| Notes | Funding source: unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the intervention independent of other changes? | Low risk | The concurrent IRS rounds were conducted in the same way as previous years and so would not explain a change in trend. The possibility that the decline was caused by lower than average rainfall in 1982 is unlikely, as villages sprayed in 1981 saw a decline in the same year, and the downward trend continued despite normal to heavy rainfall in 1983 and 1984 |
| Was the shape of the intervention effect pre‐specified? | Low risk | The point of analysis is the point of the intervention |
| Was the intervention unlikely to affect data collection? | Low risk | The methods of data collection were the same before and after the intervention |
| Was knowledge of the allocated interventions adequately prevented? | Unclear risk | Unlikely that the outcomes were assessed blindly. Incidence measurements depended on self‐reporting of fever symptoms that may be influenced by participant knowledge of the intervention, although parasitaemia was confirmed by blood smear |
| Were incomplete outcome data adequately addressed? | Low risk | No missing outcome data likely to bias the results |
| Was the study free from selective outcome reporting? | High risk | The report states that 24 villages in Vanapuram were sprayed but a time series of the number of cases and slide positivity rate is only presented for 4 of these villages |
| Was the study free from other risks of bias? | Unclear risk | A variety of spray equipment and formulations are used and is unclear at which times and locations each has been used |
Abbreviations: AI: active ingredient; CBA: controlled before‐and‐after; DDT: dichlorodiphenyltrichloroethane; ITS: interrupted time series; N/A: not applicable; N/S: not stated.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Did not meet inclusion criteria for study design. The report documents a control campaign using multiple vector control activities undertaken simultaneously including indoor spraying, larviciding, drainage and destruction of potential breeding sites, as well as outdoor spraying. The campaign did not compare an intervention area with an untreated control group or provide a time series with data points prior to the intervention. | |
| Did not meet inclusion criteria for study design. The report is not a trial, rather a history of the medical services of the Indian Armed Forces in the Second World War, including malaria control alongside other medical services such as nutrition and disease prevention. The two main methods described for controlling malaria were residual spraying with dichlorodiphenyltrichloroethane (DDT) and drug prophylaxis, adding to previous methods of larviciding and larval habitat modification. | |
| Did not meet inclusion criteria for intervention type. The study compared a village receiving ULV application with technical‐grade malathion with a village receiving no intervention. However, applications of malathion were both indoor and outdoor, and therefore the intervention is not suitable for inclusion in the review. The study also reported no epidemiological outcomes, only entomological indices (adult landing rates, resting densities, and ovitrap recordings). | |
| Did not meet inclusion criteria for intervention type. The report documents the response to an epidemic of malaria in Venezuela in 2002. The country reported the highest recorded incidence of malaria in its history with 51,264 cases, surpassing the previous high of 5893 in 1990. The primary intervention used was preventive treatment by mass drug administration using ‘cloroquinine' and ‘primaquinine'. This intervention was supplemented with space spraying. There was no control group for the intervention. | |
| Did not meet inclusion criteria for study design. The paper documents the response to an outbreak of malaria in São Paulo State of Brazil in 1984. It is not a trial and has no control group. Space spraying is conducted using DDT. The report documents the treatment of the cases. | |
| Did not meet inclusion criteria for intervention type. The intervention tested in the study was a residual insecticide dispenser which was placed within study houses, and dispensed dichlorvos insecticide. One study village was compared against one control village where houses did not receiver dichlorvos dispensers. Blood samples were drawn from all children up to 10 years old for smear and thick blood film examinations, once before and seven times after dichlorvos treatment. | |
| Did not meet inclusion criteria for study design. The report is not a trial, rather a history of the control methods employed to prevent malaria and other tropical diseases in the Second World War, South Pacific Campaign (1942 ‐ 1945). A number of interventions are described that were employed simultaneously, including careful choice of camp sites, habitat modification, larviciding from the ground (paris‐green dust, oil and later DDT solution or dust) or from aircraft (DDT solution), space spraying with pyrethrum aerosols and residual DDT preparations, impregnating bed nets, screening, semi‐permanent and permanent control work and suppressive medication. | |
| Did not meet inclusion criteria for study design. The report documents the incidence of malaria and density of the primary vector species in a coastal region of El Salvador, over a period in which several interventions were implemented in the study area. These included aerial application of the larvicide Abate, two cycles of mass drug distribution with amodiaquine, and one application cycle of the residual insecticide propoxur, applied to the exterior walls of each house in the area. The report is not a clinical trial and no control group was examined | |
| Did not meet inclusion criteria for study design. The report documents the slide positivity for malaria and density of the vector species in Farukkhabad district, India, over a period in which several interventions were implemented in the study area. These included indoor residual spraying (IRS) with DDT, space spraying with 5% or 6% malathion, larviciding with Baytex, and mass drug administration with chloroquine, primaquine, and metakelfin. The report is not a clinical trial and no control group was examined. | |
| Did not meet inclusion criteria for study design. The report documents the success of a control programme in Basrah Liwa, Iraq, as it replaced DDT for use in IRS with malathion in some areas, where high resistance to DDT was detected alongside susceptibility to malathion. During the study, high amounts of flooding contributed to higher than usual transmission of the disease. To combat this, a range of measures were introduced, including space spraying with diazinon, intensified larviciding measures, aerial spraying with DDVP, and mass drug administration. The report is not a clinical trial and no control group was examined. | |
| Did not meet inclusion criteria for study design. The study evaluated the impact of space spraying on lab‐reared caged mosquitoes only, which were placed at different sites in a region sprayed with malathion. The report is not a clinical trial and no control villages were studied, although control caged mosquitoes were monitored, in cages placed outside of spraying areas. | |
| Did not meet inclusion criteria for study design. The report documents the incidence of malaria and density of the primary vector species in a military camp in South Korea, over a period in which several interventions were implemented in the study area. These included personal protection such as topical repellents, permethrin‐treated clothing and mosquito nets, window screens, indoor spraying with permethrin, and ULV space spraying with piperanyl butoxide. The report is not a clinical trial and no control group was examined. | |
| Did not meet inclusion criteria for study design. The impact of ULV using malathion, delivered using vehicle‐mounted Leco machines (where villages were accessible by road) and hand‐held Fontan sprayers (where villages were not accessible by road) on mosquito density was evaluated in the Solomon Islands. No control group was monitored. Two outcomes were monitored: the man‐biting rate, and mosquito sensitivity to spraying (using caged mosquitoes caught in the previous nights' man‐biting collections). Man‐biting rates were recorded before, during, and several days after spraying. Without three time points prior to the intervention implementation, the study did not meet the criteria for an ITS study. | |
| Did not meet inclusion criteria for study design. The report is not a trial, but summarizes the actions of the Bombay State malaria organization set up in 1942 and its impact on malaria transmission and vector populations. The paper describes the range of interventions that have been used including drug administration, mosquito larvicides, habitat modification and space spraying, before the introduction of IRS with DDT. | |
| Did not meet inclusion criteria for intervention type. The report is an investigation into the impact on sprayers' (i.e. those who have carried out IRS in a trial in Haiti) urinary metabolites and blood cholinesterase levels. | |
| Full text was not available. We consider the study unlikely to be included, as the abstract appears to describe IRS (though published before the term was commonly used) rather than space spraying. |
Abbreviations: DDT: dichlorodiphenyltrichloroethane; IRS: indoor residual spraying.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of malaria (step rate ratio: indicating the impact of space spraying at the first pre‐intervention time point) Show forest plot | 1 | Rate Ratio (Random, 95% CI) | 1.00 [0.51, 1.92] | |
| Analysis 1.1  Comparison 1 Space spraying versus no space spraying, Outcome 1 Incidence of malaria (step rate ratio: indicating the impact of space spraying at the first pre‐intervention time point). | ||||
| 2 Incidence of malaria (slope rate ratio: indicating the proportion of cases reduced per post‐intervention time point) Show forest plot | 1 | Rate Ratio (Random, 95% CI) | 0.85 [0.79, 0.91] | |
| Analysis 1.2 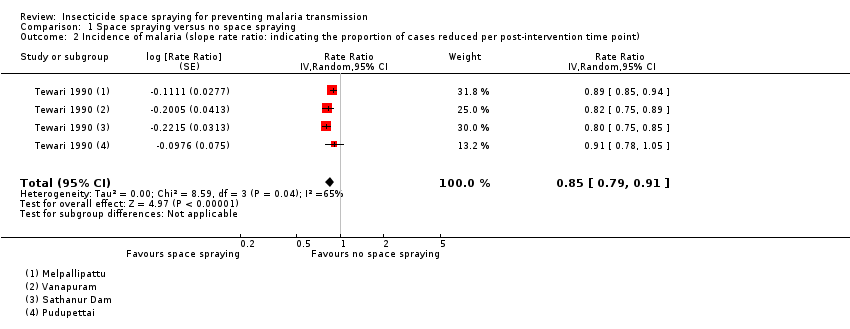 Comparison 1 Space spraying versus no space spraying, Outcome 2 Incidence of malaria (slope rate ratio: indicating the proportion of cases reduced per post‐intervention time point). | ||||

Space spraying with hand‐held equipment to control the mosquito population in Thailand

‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study. We did not assess the risk of bias for Hobbs 1976 or Seleena 2004, as the evidence of the effectiveness of space spraying in these studies has not been presented in this review or included in the analysis.

Incidence of clinical malaria per 1000 population in the Miragoane Valley of Haiti, March 1972 to February 1973. Incidence in the sprayed zone of the study site is shown in blue; the incidence in the surrounding untreated area is shown in red. The vertical red lines indicate the start and end of the space spraying intervention.

Number of cases of clinical malaria in Pudupettai, India, reported monthly between 1979 and 1982. The vertical red line indicates the start of the space spraying intervention.

Number of cases of clinical malaria in Vanapuram, India, reported monthly between 1980 and 1984. The vertical red line indicates the start of the space spraying intervention.

Number of cases of clinical malaria in Melpallipattu, India, reported monthly between 1980 and 1984. The vertical red line indicates the start of the space spraying intervention.

Number of cases of clinical malaria in Sathanur Dam, India, reported monthly between 1980 and 1984. The vertical red line indicates the start of the space spraying intervention.

Mosquito density measured in the sprayed region in Haiti using updraft UV light‐traps, March 1972 to February 1973. The initial implementation and end of the space spraying intervention are illustrated by vertical red lines.

Mosquito density measured as a human biting rate in the sprayed region in Haiti, March 1972 to February 1973. The initial implementation and end of the space spraying intervention are illustrated by vertical red lines.

Comparison 1 Space spraying versus no space spraying, Outcome 1 Incidence of malaria (step rate ratio: indicating the impact of space spraying at the first pre‐intervention time point).

Comparison 1 Space spraying versus no space spraying, Outcome 2 Incidence of malaria (slope rate ratio: indicating the proportion of cases reduced per post‐intervention time point).
| Space spraying compared to no space spraying for reducing malaria transmission | ||||||
| Patient or population: people of all ages | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with no space sprayinga | Risk following space sprayingb | |||||
| Malaria cases per month | 6 per 1000 | Instant effect: 6 per 1000 (3 to 12) Effect after 12 months follow‐up: 1 per 1000 (0 to 2 per 1000) | Step rate ratio: 1.00 Slope rate ratio: 0.85 | (1 observational study: 4 sites) | ⊕⊝⊝⊝ downgraded due to risk of bias, indirectness, and imprecision | We do not know if space spraying causes an immediate shift in the trend of malaria incidence over time or a change in the slope of the trend (that is, a proportional reduction in cases per month). |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe estimated the risk with no space spraying by calculating the mean monthly incidence of malaria across each of the study sites. We include only incidence data from complete years (January to December without intervention) in the calculation. | ||||||
| Compound and formulation | Concentration (g Al/ha) | |
| Cold fog | Thermal fog | |
| Deltamethrin ULV | 0.5 to 1.0 | 0.5 to 1.0 |
| Deltamethrin EW | 1.0 | — |
| Lambda‐cyhalothrin EC | 1.0 to 2.0 | 2.0 |
| Malathion EW and ULV | 112 to 600 | 112 to 600 |
| d‐d, trans‐cyphenothrin EC | 3.5 to 4.0 | 3.5 to 4.0 |
| Abbreviations: EC: emulsifiable concentrate; EW: emulsion, oil in water; ULV: ultra‐low volume liquid; AI: active ingredient | ||
| Study | Active ingredient/formulation/dose | Delivery method | Frequency and timing of application | Who implemented the intervention | Vector species |
| Haiti (Krogstad 1975) | Malathion 95%
| Aerial (Beech D‐18 aircraft) | Every 10 days | The Service National d'Eradication de la Malaria (SNEM), supported by USAID | An albimanus |
| India (Tewari 1990) | Malathion
See Characteristics of included studies for further details | Ground (hand‐held Fontan and Enfog sprayers, jeep‐mounted Tifa machines and handcart‐mounted Tiga machines | In Pudupettai, spraying was conducted weekly for 6 rounds, and subsequently applied in response to new cases or increases in vector density. Time of spraying: 8pm ‐ 10pm and 5am ‐ 7am | State National Malaria Elimination Programme (NMEP), with guidance from the Pondicherry Vector Control Research Centre | An culicifacies |
| El Salvador (Hobbs 1976) | 5% pyrethrin with 15% piperonyl butoxide
| Ground (truck‐mounted Leco sprayer) | Weekly. Time of spraying: 6pm ‐ 7pm | Central America Research Station (CARS) | An albimanus |
| Malaysia (Seleena 2004) | Alphacypermethrin
| Ground, with hand‐held sprayers | Monthly Time of spraying: not stated | Spray team of villagers, headed by a local public health inspector | (1o)Anopheles balabacensis (2o)An sundaicus, An flavirostris |
| Abbreviations: AI: active ingredient; ULV: ultra‐low volume. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of malaria (step rate ratio: indicating the impact of space spraying at the first pre‐intervention time point) Show forest plot | 1 | Rate Ratio (Random, 95% CI) | 1.00 [0.51, 1.92] | |
| 2 Incidence of malaria (slope rate ratio: indicating the proportion of cases reduced per post‐intervention time point) Show forest plot | 1 | Rate Ratio (Random, 95% CI) | 0.85 [0.79, 0.91] | |