Ecografía para la confirmación de la colocación de la sonda gástrica
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Study characteristics | |||
| Patient sampling | Consecutive enrolment, no information available regarding participant exclusion. | ||
| Patient characteristics and setting | No information available among participant characteristics and setting. | ||
| Index tests | Ultrasound (with and without 60 mL of air injected) by trained nurses. Blinded to whoosh test. | ||
| Target condition and reference standard(s) | No detailed information available. | ||
| Flow and timing | Participants were first tested with ultrasound (with and without 60 mL of air injected) as compared with whoosh test performed by other nurses. No information available regarding timing of reference standard or dropout of participants. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | No detailed information available regarding random or consecutive sampling. Inclusion criteria: people benefiting from the prehospital insertion of an NG tube after tracheal intubation. Exclusion criteria: aged < 18 years; supported during an interhospital transportation; presenting a suspected fracture of the bones of the skull base; and who absorbed detergents, oil or foam products. | ||
| Patient characteristics and setting | Aged ≥ 18 years, intubated in prehospital setting by EMS team which included a physician. Excluded people with suspicion of cranial base fracture and who had a history of caustic agent ingestion. | ||
| Index tests | Titan ultrasound machine in all ambulances. Physicians on board, who were e‐FAST trained, received 1‐day training for ultrasound verification of NG tube placement. Standardized method included a left subcostal view with the ultrasound probe while NG tube inserted by EMS staff. If not initially seen on ultrasound, 50 mL of air injected through NG tube. X‐ray control carried out at hospital and compared with prehospital results. | ||
| Target condition and reference standard(s) | Chest X‐ray on arrival at the hospital. | ||
| Flow and timing | No exclusions described. Ultrasound test performed. If not initially seen on ultrasound, 50 mL of air was injected through the NG tube. X‐ray control carried out at hospital and compared with the prehospital results. | ||
| Comparative | |||
| Notes | 2 review authors (HT and YT) assessed based on the extracted data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Unclear | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Yes | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | No | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Yes | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single‐centre observational study performed between November 2012 and May 2013 in mobile emergency and resuscitation service. Inclusion criteria: aged ≥ 18 years receiving prehospital care and requiring GT insertion. Exclusion criteria: aged ≤ 18 years, pregnant women, contraindication at GT insertion, interhospital transfers and absence of X‐ray control. After verification of correct GT placement by the auscultation method or whoosh test (instillation of air in the tube with sounds heard simultaneously through a stethoscope placed over the stomach region) combined with the aspirate method (visual inspection of aspirate contents), ultrasound test was performed. | ||
| Patient characteristics and setting | Prior test: auscultation method or whoosh test (instillation of air in tube with sounds heard simultaneously through stethoscope placed over stomach region) combined with aspirate method (visual inspection of aspirate contents). Presentation: in prehospital setting, emergency physician checked GT placement using ultrasonography during GT insertion by nurse or just after epigastric auscultation and aspirate method was realized. Intended use of index test: to determine whether or not the GT could be viewed in the oesophagus, stomach, or both. | ||
| Index tests | Probe placed transversely on the anterior neck just superior to the suprasternal notch midline at level of thyroid gland and focused on visible part of oesophagus, with longitudinal and transversal viewing, then probe placed in subxiphoid area and oriented towards left upper abdominal quadrant to visualize stomach, with transverse and longitudinal viewing. Antrum imaged in a transversal plane in epigastric area using left lobe of liver as an internal landmark, angling transducer towards left subcostal area imaged the gastric body. Ultrasound examination considered positive when GT was visualized, appearing as an hyperechogenic circle posterior to the thyroid tissue adjacent to trachea, and as a hyperechogenic line in stomach. When GT was seen in oesophagus and not in stomach, 50 mL of air injected through GT, if ultrasonography showed dynamic fogging in stomach, GT considered in stomach. | ||
| Target condition and reference standard(s) | X‐ray on arrival at hospital. Details of interpretation: unclear. | ||
| Flow and timing | No participants excluded. After verification of correct GT placement by auscultation method or whoosh test (instillation of air in tube with sounds heard simultaneously through stethoscope placed over stomach region) combined with the aspirate method (visual inspection of aspirate contents), ultrasound test was performed. If not initially seen on ultrasound, 50 mL of air injected through NG tube. X‐ray control carried out at hospital and compared with prehospital results. Unclear time interval. No information comparing index test and reference standard. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | No | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Yes | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study in people undergoing GT insertion in prehospital setting conducted in 2 French towns (Marseille and Grasse) over 1‐year period from May 2010 to May 2011. Inclusion criteria: aged ≥ 18 years, prehospital settings and requiring GT insertion. Exclusion criteria: aged < 18 years, pregnant, interhospital transfers and absence of X‐ray control. After insertion and securing of GT by auscultation or whoosh test, emergency physician verified correct placement of GT by ultrasound. | ||
| Patient characteristics and setting | Prior test: auscultation method or whoosh test (instillation of air in tube with sounds heard simultaneously through stethoscope placed over stomach region) combined with aspirate method (visual inspection of aspirate contents). Presentation: after insertion and securing of GT, emergency physician verified correct placement of GT by ultrasound. Intended use of index and setting: confirming accurate GT placement. Setting: prehospital. | ||
| Index tests | After insertion and securing of GT, emergency physician verified correct placement of GT by ultrasound. Technique standardized; probe placed in the subxiphoid area then oriented towards left upper abdominal quadrant to visualize stomach, with transverse viewing, antrum imaged in a transversal plane in epigastric area using left lobe of liver as internal landmark, gastric body imaged by angling transducer towards left subcostal area. Ultrasound examination considered positive when GT appeared as a hyperechogenic line in stomach. Videorecorded showing GT tip; 2 radiologists reviewed each video to confirm results. | ||
| Target condition and reference standard(s) | Final confirmation of GT placement was X‐ray on arrival at hospital. | ||
| Flow and timing | No participants excluded. Time interval and interventions between index tests and reference standard not described. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | No | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Yes | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Inclusion criteria: mechanically ventilated participants monitored in ICU between February and July 2014 who received ultrasound‐guided NG tube placement. Exclusion criteria: history of neck surgery (e.g. tracheotomy), anatomic deformity, nasal fracture or severe coagulopathy; aged < 16 years. Tube removed if coughing and dyspnoea occurred during placement. | ||
| Patient characteristics and setting | Prior test: none. Presentation: 56 mechanically ventilated participants monitored in ICU between February and July 2014 who received ultrasound‐guided NG tube placement. Intended use of index test: 'real‐time' imaging of passage of NG tube through oesophagus. Setting: ICU. | ||
| Index tests | Image of empty oesophagus obtained, then inserted NG tube 10‐14 Fr in thickness from appropriate nostril by adjusting nasal passage. Subsequently, NG tube gently advanced, and passage visualized with ultrasound. Oesophagus primarily viewed in transverse plain then attempt made to obtain a longitudinal view. Ultrasound performed to obtain sonographic image of oesophagus before removing guidewire of NG tube. | ||
| Target condition and reference standard(s) | After ultrasound‐guided tube insertion, gastric placement of the NG tube tip confirmed with abdominal X‐ray. All reference standard results interpreted by a single person. Used prespecified criteria of correct position, i.e. NG tube tip below the diaphragm; should follow straight course down midline of chest to a point below diaphragm. | ||
| Flow and timing | No participants excluded. Unclear time interval. No test performed between index tests and reference standard. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | No | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Yes | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Were the criteria of reference standard for target condition prespecified? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective study performed between May and September 2011 in a local emergency centre. Included participants with low consciousness in whom correct placement of NG tube was ultimately verified by chest X‐ray. Inclusion criteria: aged > 18 years, undergoing NG tube insertions for reasons including drug overdose, suspicion of gastric bleeding, endotracheal intubation and others. 10 participants excluded because they did not receive X‐ray confirmation. | ||
| Patient characteristics and setting | Prior test: auscultation, pH testing and ultrasound performed in random order. Presentation: participants with low consciousness in whom correct placement of NG tube was ultimately verified by chest X‐ray. Intended use of index test: to verify gastric intubation. Setting: EMS. | ||
| Index tests | Ultrasound examinations included a transversal scan performed prior to tube insertion from either right or left side of the participant's neck to verify that oesophagus was located behind respiratory tract. Attempted visualization of NG tube in separate scans of fundus and antrum of stomach. Used linear probe for study of neck and convex probe for stomach. If visualization not possible, 40 mL of normal saline and 10 mL of air were injected through NG tube and if ultrasonography showed dynamic fogging in stomach, gastric placement of tube was verified. | ||
| Target condition and reference standard(s) | Chest X‐rays interpreted by emergency medicine specialist who did not perform ultrasound examinations. | ||
| Flow and timing | 10 participants excluded because they did not receive X‐ray confirmation; no other participants excluded from analysis. Auscultation, pH testing and ultrasound performed in random order. If ultrasound visualization not possible, 40 mL of normal saline and 10 mL of air were injected through NG tube and if ultrasonography showed dynamic fogging in stomach, gastric placement of tube was verified. After these tests, an emergency medicine specialist who did not perform ultrasound examinations interpreted chest X‐rays. Unclear time interval. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Inclusion criteria: people in ICU, endotracheal intubation and ventilation or independent breathing. Exclusion criteria: percutaneous endoscopic gastrostomy tube. How were participants' coughs managed: not reported. | ||
| Patient characteristics and setting | Adults aged 16‐84 years. In 50/60 procedures, participants were endotracheally intubated and ventilated. In 10/60 procedures, participants were breathing spontaneously, none had a tracheostomy. Prior test: not reported. Presentation: people in ICU, endotracheal intubation and ventilation or independent breathing. Intended use of index test: to replace X‐ray for verification of GTs. Setting: ICU. | ||
| Index tests | Correct placement of tip of tube in stomach ascertained by ultrasound by detecting a 50 mL air jet applied with a syringe via the GT. Ultrasound performed by 10 experienced examiners/practitioners. Reference standard test (radiological control) done after index test (ultrasound). Results of ultrasound and other control methods compared to radiological control of tube. | ||
| Target condition and reference standard(s) | X‐ray of the lower thorax or upper abdomen. Incorrect localization of tube defined as localization of tube in oesophagus or lungs. | ||
| Flow and timing | In 60 GT insertions (with 50 participants on artificial ventilation) performed on a medical ICU, correct placement of tube was controlled by auscultation, pH measurement of aspirate and ultrasound. In ultrasound, correct placement of tip of tube in stomach ascertained by detecting a 50 mL air jet applied with a syringe via GT. Results of ultrasound and other control methods compared to radiological control of tube. Period between placement of GT and radiological control was within 24 hours. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | No | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | No | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Prospective study in a 5‐bed ICU performed between May and September 2005. | ||
| Patient characteristics and setting | Included 16 participants, 9 men and 7 women, mean (± SD) age 66.3 ± 7.1 years, mean (± SD) APACHE II score 21 ± 5.2. All participants intubated and mechanically ventilated. Prior test: not reported. Presentation: people in ICU over 5‐month period. Intended use of index test: to replace radiology for verification of GTs. Setting: ICU. | ||
| Index tests | Ultrasound confirmation of NG tube position by identifying air bubbles after injecting a 10 mL mixture of 5% dextrose and air and by standard X‐ray. No detailed information available regarding interpretation of results. | ||
| Target condition and reference standard(s) | NG tube position also confirmed by X‐ray. No detailed information available. | ||
| Flow and timing | Median (± SD) procedure time 14.93 ± 1.71 minutes for ultrasound and 84 ± 30.64 minutes for X‐ray (P < 0.001). No detailed information regarding timing, time gap, withdrawals and any intervention between index test and reference standard available. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | No detailed information available. | ||
| Patient characteristics and setting | No detailed information available. | ||
| Index tests | Anterolateral neck scanned in high frequency to visualize GT tube's characteristic echogenic surface with posterior anechoic shadow in oesophagus. Then, right diaphragm location identified by low‐frequency imaging. Comparisons of ultrasound findings made to chest X‐ray findings. Data collected by a single internal medicine resident. | ||
| Target condition and reference standard(s) | No detailed information available. Data collected by a single internal medicine resident. | ||
| Flow and timing | No detailed information regarding timing, time gap, withdrawals and any intervention between index test and reference standard. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | Unclear | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Unclear | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Were the criteria of reference standard for target condition prespecified? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Unclear | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Consecutive participants during a 2‐month period who received a weighted tip NG tube (12CH, Cair, France) for enteral feeding between 8:30 a.m. and 8:00 p.m. Exclusion criteria: NG tubes inserted during other periods, when only 1 physician was on duty. When participants coughed too much, tube removed and reinserted (author's reply). Examiner did not reinsert the tube when there was no auscultation (author's reply). | ||
| Patient characteristics and setting | 35 weighted tip NG tubes inserted in 33 participants (18 men, 15 women; mean (± SD) age 62.2 ± 19.8 years; mean (± SD) Simplified Acute Physiology Score II score 48 ± 20.7; mean (± SD) body mass index 24.8 ± 5.8). 26 (79%) participants mechanically ventilated at the time tube insertion and 19 (73%) sedated. Main diagnoses on ICU admission were acute aggravation of chronic respiratory failure (n = 8), community‐acquired pneumonia (n = 4), pulmonary pneumocystosis (n = 1), acute respiratory distress syndrome (n = 3), septic shock (n = 6), myasthenia gravis (n = 1), stroke (n = 3) and other disorders (n = 5). Prior test: none described. Presentation: daytime weighted NG tube insertion. Intended use of index: ensuring correct tube placement. Setting: ICU. Did not differentiate gastric or enteric tube (i.e. possibly passing through pyloric ring to the duodenum). | ||
| Index tests | Duodenum examined in middle epigastric area; if duodenum or NG tip (or both) not visualized, probe oriented towards left upper abdominal quadrant to visualize gastric area. If NG tip still not visible, 5 mL normal saline mixed with 5 mL air injected into tube to visualize the hyperechogenic 'fog' exiting tip. NG tube tip considered correctly located when surrounded by hydric and echogenic moving formations (related to peristalsis). Did not specifically record cases of pneumothorax or intrabronchial NG tube location or precise gastric/duodenal location of tip. Examiner did not use prespecified criteria of ultrasound (author's reply). | ||
| Target condition and reference standard(s) | As soon as tube was correctly inserted, radiology department performed confirmatory X‐ray. Blinded design of study required NG tube tip verification by 2 physicians, 1 to interpret X‐rays and 1 to perform ultrasound examination. Each physician was unaware of the other's findings. Examiner did not use prespecified criteria of X‐ray (author's reply). | ||
| Flow and timing | No detailed information regarding timing, withdrawals and any intervention between index test and reference standard. Time gap between index test and reference standard not recorded (author's response). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| How were participants' coughs managed? Did the study avoid reinsertion of the tube when participants coughed too much? | No | ||
| How were auscultation findings (e.g. bubbling sounds) dealt with? Did the study avoid reinsertion of the tube when auscultation was not found? | Yes | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom the GT was difficult to place? | Yes | ||
| Did the study avoid inappropriate exclusions? Did the study avoid excluding participants for whom tubes were difficult to visualize? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were the criteria of reference standard for target condition prespecified? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Was there an appropriate interval between the index test and reference standard? We set an arbitrary 4 hours for this review | Yes | ||
| Were all participants included in the analysis? Consider withdrawals and withdrawals who were likely to impact on study results. Also consider the exclusion of 'difficult' participants | Yes | ||
| Low | |||
APACHE II: Acute Physiology and Chronic Health Evaluation II; eFAST: extended Focused Assessment with Sonography for Trauma; EMS: emergency medical service; GT: gastric tube; ICU: intensive care unit; n: number of participants; NG: nasogastric; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Editorial. | |
| Nasoenteric tubes inserted into the postpyloric area. | |
| Transpyloric tube. | |
| Not a diagnostic test accuracy study. Compared method for placing feeding tubes. | |
| Case report. | |
| Letter. | |
| pH‐specific paper used as reference standard. | |
| Case series. | |
| Nasal‐jejunal tube. |
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Ultrasound Show forest plot | 10 | 550 |
| Test 1 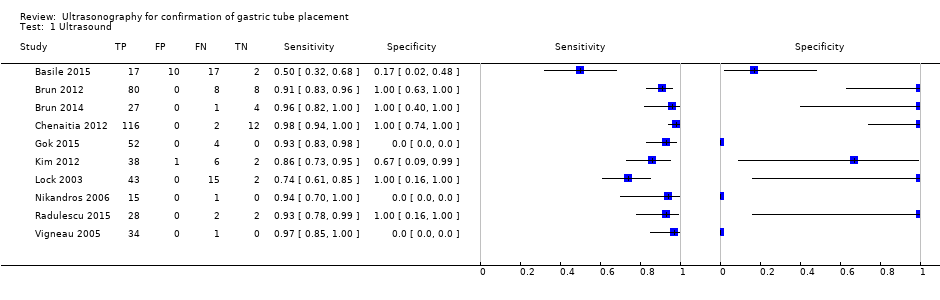 Ultrasound. | ||

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
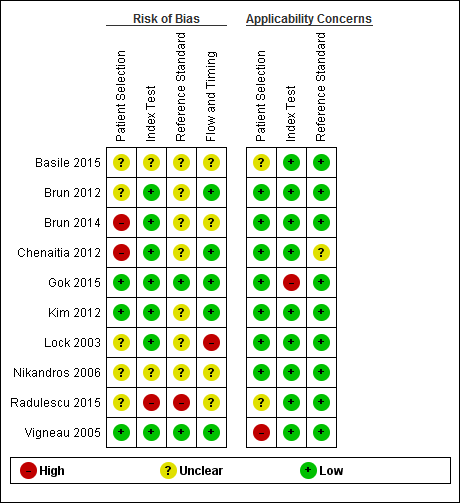
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Forest plot of diagnostic accuracy of ultrasound in different ways. Four studies reported the diagnostic accuracy of ultrasound (Brun 2012; Chenaitia 2012; Gok 2015; Radulescu 2015), while the others reported the diagnostic accuracy of ultrasound combined with other methods. Gok 2015 reported the diagnostic accuracy of ultrasound during tube insertion (ultrasound‐guide insertion). We found three visualization methods (echo window) of ultrasound: neck (Gok 2015), epigastric (Brun 2012; Chenaitia 2012; Kim 2012; Lock 2003; Vigneau 2005), and a combination (Brun 2014; Radulescu 2015). Studies used air injection during ultrasound (Basile 2015; Brun 2014), saline injection (Vigneau 2005), both air and saline injection (Kim 2012), and dextrose and air injection (Nikandros 2006). Two studies did not report the echo window (Basile 2015; Nikandros 2006).
| Accuracy of ultrasound for confirmation of gastric tube placement | |||||||||
| Population | Adults in any settings (prehospital, ICU, EMS or unclear) | ||||||||
| Index test | Ultrasound (any methods) | ||||||||
| Reference standard | X‐ray | ||||||||
| Studies | Cross‐sectional study or unclear study designa | ||||||||
| Study ID | TPb | FPb | FNb | TNb | Participants | Sensitivity | Specificity | Methodc | Echo windowc |
| 17 | 10 | 17 | 2 | 46 | 0.50 (0.32 to 0.68) | 0.17 (0.02 to 0.48) | Ultrasound + air injection after insertion | NR | |
| Brun 2012a,d | 80 | 0 | 8 | 8 | 96 | 0.91 (0.83 to 0.96) | 1.00 (0.63 to 1.00) | Ultrasound after insertion | Epigastric |
| 27 | 0 | 1 | 4 | 32 | 0.96 (0.82 to 1.00) | 1.00 (0.40 to 1.00) | Ultrasound + air injection after insertion | Neck + epigastric | |
| 116 | 0 | 2 | 12 | 130 | 0.98 (0.94 to 1.00) | 1.00 (0.74 to 1.00) | Ultrasound after insertion | Epigastric | |
| 52 | 0 | 4 | 0 | 56 | 0.93 (0.83 to 0.98) | Not estimable | Ultrasound during insertion | Neck | |
| 38 | 1 | 6 | 2 | 47 | 0.86 (0.73 to 0.95) | 0.67 (0.09 to 0.99) | Ultrasound + saline and air injection | Neck + epigastric | |
| 43 | 0 | 15 | 2 | 55 (60 measurements)e | 0.74 (0.61 to 0.85) | 1.00 (0.16 to 1.00) | Ultrasound + air injection after insertion | Epigastric | |
| 15 | 0 | 1 | 0 | 16 | 0.94 (0.70 to 1.00) | Not estimable | Ultrasound + dextrose and air injection after insertion | NR | |
| 28 | 0 | 2 | 2 | 32 | 0.93 (0.78 to 0.99) | 1.00 (0.16 to 1.00) | Ultrasound after insertion | Neck + epigastric | |
| 34 | 0 | 1 | 0 | 35 | 0.97 (0.85 to 1.00) | Not estimable | Ultrasound + saline injection after insertion | Epigastric | |
| CI: confidence interval; EMS: emergency medical service; ICU: intensive care unit: FN: false negative; FP: false positive; NR: not reported; TN: true negative; TP: true positive. | |||||||||
| Accuracy of ultrasound for confirmation of gastric tube placement for drainage in settings where X‐ray facilities are not readily available | |||||||||
| Population | Adults underwent gastric tube insertion for drainage in settings where X‐ray facilities are not readily available (prehospital or EMS) | ||||||||
| Index test | Ultrasound (any methods) | ||||||||
| Reference standard | X‐ray | ||||||||
| Studies | Cross‐sectional study or unclear study designa | ||||||||
| Study ID | TPb | FPb | FNb | TNb | Participants | Sensitivity | Specificity | Methodc | Echo windowc |
| Brun 2012c,d | 80 | 0 | 8 | 8 | 96 | 0.91 (0.83 to 0.96) | 1.00 (0.63 to 1.00) | Ultrasound after insertion | Epigastric |
| 27 | 0 | 1 | 4 | 32 | 0.96 (0.82 to 1.00) | 1.00 (0.40 to 1.00) | Ultrasound+ air injection after insertion | Neck + epigastric | |
| 116 | 0 | 2 | 12 | 130 | 0.98 (0.94 to 1.00) | 1.00 (0.74 to 1.00) | Ultrasound after insertion | Epigastric | |
| 38 | 1 | 6 | 2 | 47 | 0.86 (0.73 to 0.95) | 0.67 (0.09 to 0.99) | Ultrasound + saline and air injection | Neck + epigastric | |
| CI: confidence interval; EMS: emergency medical service; FN: false negative; FP: false positive; TN: true negative; TP: true positive. | |||||||||
| Study ID | Male:female | Age | BMI | Children | Non‐sedated | Sedated | Intubated | Diameter | Setting |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 56:24 | 52 ± 23 | NR | 0 | 0 | 96 | 96 | 14 or 16 | Prehospital | |
| 18:14 | 57 ± 17 | NR | 0 | 22 | 10 | 32 | 14 or 16 | Prehospital | |
| 77:53 | 55.7 ± 19.8 | NR | 0 | 0 | 130 | 130 | 14‐18 | Prehospital | |
| 32:24 | 48.4 ± 28.9 | 27.1 ± 6.4 | 0 | 0 | 56 | 56 | 10‐14 | ICU | |
| 28:19 | 57.6 ± 17.2 | NR | 0 | 0 | 47 | 27 | 16 | EMS | |
| NR | 59.2 ± 16.2 | NR | NR | NR | NR | 50 | 14 or 16 | ICU | |
| 9:7 | 66.3 ± 7.1 | NR | NR | 0 | 16 | 16 | NR | ICU | |
| NR | N/R | NR | NR | NR | NR | NR | NR | NR | |
| 18:16 | 62.2 ± 19.8 | 24.8 ± 5.8 | 0 | 14 | 19 | 26 | 12 | ICU | |
| BMI: body mass index; EMS: emergency medical service; ICU: intensive care unit; NR: not reported; SD: standard deviation. a Reports from the same research group. | |||||||||
| Test | No. of studies | No. of participants |
| 1 Ultrasound Show forest plot | 10 | 550 |