Audífonos para la hipoacusia leve a moderada en adultos
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012023.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 25 September 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane ENT Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
MF conceived the review question, selected which studies to review, extracted data, assessed study quality, wrote the protocol and review, and co‐ordinated comments from the authors and reviewers.
PK extracted data, assessed study quality, conducted the data analyses, and contributed substantially to the writing of the protocol and review.
LY provided methodological advice on the protocol and the review.
MEJ provided statistical advice and conducted an independent verification of the data analyses.
FB joined as a co‐author for the full review, selected which studies to include and provided feedback on the review.
DH selected which studies to include, assessed study quality, and provided guidance and critical comment on the draft protocol and review.
Sources of support
Internal sources
-
National Institute for Health Research, UK.
MF, PK, MEJ and DH are funded by the National Institute for Health Research (NIHR) Biomedical Research Unit Programme, however the views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Melanie Ferguson: none known.
Pádraig T Kitterick: PK's employing organisation has received financial support from Cochlear Europe Ltd. and support in kind from Phonak UK Ltd. to conduct a multicentre clinical trial of cochlear implantation in single‐sided deafness co‐ordinated by PK. PK has accepted the hospitality of Cochlear Europe Ltd. to attend and speak at national and international scientific meetings.
Lee Yee Chong: none known.
Mark Edmondson‐Jones: none known.
Fiona Barker: none known
Derek Hoare: none known.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We acknowledge Helen Henshaw who helped to conceive the review question. We would also like to acknowledge Saoussen Ftouh (National Guideline Centre) for methodological support and advice. We thank the Cochrane ENT team for their invaluable help: Samantha Faulkner who designed the search strategy, Martin Burton for strategic advice and Jenny Bellorini for her support and substantial general advice. Finally, we thank the authors of included studies for their willingness to share information and respond to our queries.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 25 | Hearing aids for mild to moderate hearing loss in adults | Review | Melanie A Ferguson, Pádraig T Kitterick, Lee Yee Chong, Mark Edmondson‐Jones, Fiona Barker, Derek J Hoare | |
| 2015 Dec 22 | Hearing aids for mild to moderate hearing loss in adults | Protocol | Melanie A Ferguson, Pádraig T Kitterick, Mark Edmondson‐Jones, Derek J Hoare | |
Differences between protocol and review
The Types of participants section in the protocol was: "Adults (≥ 18 years old) with mild or moderate hearing loss, as defined by pure‐tone thresholds in the better‐hearing ear averaged across four frequencies (0.5 kHz, 1.0 kHz, 2.0 kHz and 4.0 kHz) of 26 to 40 dB HL (mild hearing loss) and 41 to 60 dB HL (moderate hearing loss). In the absence of confirmation that all participants in a study met these criteria (i.e. where participant‐level data were not reported or could not be obtained), we will include studies where the reported participant characteristics for the mean four‐frequency average as described above. If a mean frequency average is offered for a combination of frequencies other than 0.5 kHz, 1.0 kHz, 2.0kHz and 4.0 kHz, we will use studies where the reported value falls between 26 to 40 dB HL (mild hearing loss) or 41 to 60 dB HL (moderate hearing loss). If only qualitative descriptions of mild and moderate hearing loss are given with no supporting audiometric data, we will include such studies but will not include them in the meta‐analysis" (Ferguson 2015).
We revised the Types of participants section because there was concern about introducing a potential bias in the selection of studies such that those studies that were reported in less detail (e.g. group mean hearing threshold data or qualitative descriptions) were more likely to be included than those that either reported in more detail or provided us with participant‐level data. To minimise the risk of bias from the revision of the protocol, the revised definition for Types of participants and the final decisions about which studies met the inclusion criteria were subjected to an independent review by an external expert committee to ensure the decisions were appropriate for the review question.
The protocol stated that MF and FB would extract the data, but instead this was completed by MF and PK. The protocol did not state what would happen in the event of a disagreement on a risk of bias judgement between MF and PK. Where this occurred, DH was charged with making a final judgement. The protocol stated that MF would review the 'Summary of findings' table, but in addition LYC (who was not an author on the review protocol) also reviewed the 'Summary of findings' table.
The protocol stated in Measures of treatment effect that we would use the standardised mean difference at the trial endpoint. We instead used the mean difference for the hearing‐specific health‐related quality of life outcome at the trial endpoint as these data were collected using the same instrument (HHIE) across all studies that were included in the meta‐analysis.
Some of the pre‐specified subgroup and funnel plot analyses were not performed as the data were not available or were too limited.
We performed unplanned subgroup analyses for the hearing‐specific quality of life and listening ability outcomes due to the numerous participant and methodological differences between the Veterans Association studies and the other study included in the analyses (Humes 2017).
We made a minor adjustment to the wording of the review Objectives, changing 'effectiveness' to 'effects' to capture our interest in both positive and negative outcomes.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Aged, 80 and over; Humans;
PICOs

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Hearing aids versus no/placebo hearing aids, outcome: 1.1 Hearing‐specific health‐related quality of life. Assessed using Hearing Handicap Inventory for the Elderly (HHIE) in all studies.

Forest plot of comparison: 1 Hearing aids versus no/placebo hearing aids, outcome: 1.2 Health‐related quality of life.

Forest plot of comparison: 1 Hearing aids versus no/placebo hearing aids, outcome: 1.3 Listening ability.

Comparison 1 Hearing aids versus no/placebo hearing aids, Outcome 1 Hearing‐specific health‐related quality of life.
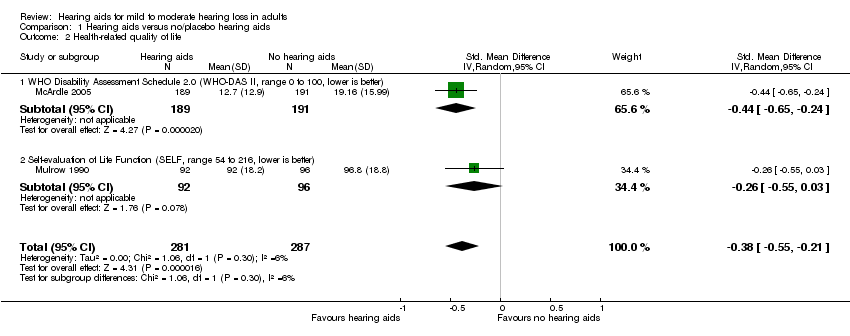
Comparison 1 Hearing aids versus no/placebo hearing aids, Outcome 2 Health‐related quality of life.

Comparison 1 Hearing aids versus no/placebo hearing aids, Outcome 3 Listening ability.
| Hearing aids versus no hearing aids for mild to moderate hearing loss in adults | |||||
| Patient or population: adults with mild to moderate hearing loss | |||||
| Outcomes No. of participants | Anticipated absolute effects (95% CI) | Quality | What happens* | ||
| Without hearing aids | With hearing aids | Difference | |||
| Hearing‐specific HRQoL assessed with: HHIE (range 0 to 100) Follow‐up: range 6 to 16 weeks No. of participants: 722 (3 RCTs) | The mean hearing‐specific HRQoL score was 39 | — | Mean 26 lower (42 to 11 lower) | ⊕⊕⊕⊝ | Lower score indicates better hearing‐specific HRQoL. The mean difference corresponds to a large effect size (SMD ‐1.38, 95% CI ‐2.02 to ‐0.75) favouring hearing aids. |
| Health‐related QoL assessed with: WHO‐DAS II (range 0 to 100) and the SELF (range 54 to 216) Follow‐up: range 2 months to 16 weeks No. of participants: 568 (2 RCTs) | — | — | SMD 0.38 lower (0.55 lower to 0.21 lower) | ⊕⊕⊕⊝ | Lower score indicates better HRQoL. The SMD corresponds to a small effect size favouring hearing aids, which is equivalent to a 6‐point decrease (9‐ to 3‐point decrease) on the 0 to 100 scale of the WHO‐DAS II5. |
| Listening ability assessed with: PHAP (range 0 to 1) and APHAB (range 0 to 100) Follow‐up: 6 weeks to 2 months No. of participants: 534 (2 RCTs) | — | — | SMD 1.88 lower (3.24 lower to 0.52 lower) | ⊕⊕⊕⊝ | Lower score indicates improved listening ability. The SMD corresponds to a large effect size favouring hearing aids, which is equivalent to a 29‐point decrease (50‐ to 8‐point decrease) on the 0 to 100 scale of the APHAB6. |
| Adverse effect ‐ pain No. of participants: 48 (1 RCT) | Adverse effects related to pain were measured in 1 study: none were reported. | ⊕⊝⊝⊝ VERY LOW7 | There was too little information to estimate the risk of pain. | ||
| Adverse effect ‐ noise‐induced hearing loss No. of participants: 48 (1 RCT) | Adverse effects related to noise‐induced hearing loss were measured in 1 study: none were reported. | ⊕⊝⊝⊝ VERY LOW7 | There was too little information to estimate the risk of noise‐induced hearing loss. | ||
| *The equivalent change in the intervention group (and its 95% confidence interval) is based on the standard deviation in the comparison group from a representative study (see footnotes for each outcome) and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Quality of evidence downgraded by one level because all studies have either a rating of unclear and/or high risk bias in at least one of these domains: selection bias, performance and/or detection bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hearing‐specific health‐related quality of life Show forest plot | 3 | 722 | Mean Difference (IV, Random, 95% CI) | ‐26.47 [‐42.16, ‐10.77] |
| 1.1 Subgroup A (community setting, male‐female balance, behind‐the‐ear hearing aids, placebo control) | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐10.54 [‐15.26, ‐5.82] |
| 1.2 Subgroup B (Veterans Association setting, mostly male, in‐the‐ear hearing aids, waiting list control) | 2 | 568 | Mean Difference (IV, Random, 95% CI) | ‐33.48 [‐36.72, ‐30.23] |
| 2 Health‐related quality of life Show forest plot | 2 | 568 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.55, ‐0.21] |
| 2.1 WHO Disability Assessment Schedule 2.0 (WHO‐DAS II, range 0 to 100, lower is better) | 1 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.65, ‐0.24] |
| 2.2 Self‐evaluation of Life Function (SELF, range 54 to 216, lower is better) | 1 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.55, 0.03] |
| 3 Listening ability Show forest plot | 2 | 534 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐3.24, ‐0.52] |
| 3.1 Profile of Hearing Aid Performance (PHAP, range 0 to 1, lower is better) | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.54, ‐0.81] |
| 3.2 Abbreviated Profile of Hearing Aid Benefit (APHAB, range 0 to 100, lower is better) | 1 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐2.84, ‐2.30] |


