Aspirina para el tratamiento agudo de la cefalea tensional episódica en adultos
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | R, DB, PC, parallel groups Single episode treated with one of the study interventions when PI ≥ moderate | |
| Participants | Episodic TTH (IHS criteria) for ≥ 1 year, 2 to 10 episodes/month Excluded: unable to differentiate migraine or migraine frequency > 1/month N = 559 (487 for efficacy) M 183, F 304 Mean age 37 years (range 18 to 66) Baseline pain ≥ moderate | |
| Interventions | Aspirin 1000 mg, n = 223 Paracetamol 300 mg + codeine 30 mg, n = 233 Placebo, n = 103 Rescue medication: usual product at usual dose (no time limit specified) | |
| Outcomes | PI: 4‐point categorical scale PR: 5‐point categorical scale over 4 h SPID over 4 h TOTPAR over 4 h PID over 4 h Use of rescue medication AEs | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Appears to use DD method with aspirin and placebo caplets and paracetamol and placebo capsules |
| Blinding of outcome assessment (detection bias) | Low risk | Appears to use DD method with aspirin and placebo caplets and paracetamol and placebo capsules |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawals mainly due to non‐compliance with study protocol, and not equal between groups. Does not affect safety results, but imputation method not mentioned for efficacy results ‐ did not contribute to meta‐analysis |
| Size | Unclear risk | 103 participants in placebo treatment group |
| Methods | R, DB, AC and PC, cross‐over study 4 episodes treated, 1 with each study medication | |
| Participants | Tension headache (IHS) Age range 18 to 65 years N = 44 (completers, included in analysis) | |
| Interventions | Aspirin 1000 mg Peppermint oil (oleum menthae piperitae) solution Ll 170, 10 g Placebo Application of solution on forehead and temples, repeated 15 and 30 minutes after start of treatment | |
| Outcomes | PI: 5‐point scale (0 to 4) AEs | |
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced randomisation using Latin squares |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Capsules of identical appearance, placebo solution masked by adding traces of peppermint oil |
| Blinding of outcome assessment (detection bias) | Low risk | Capsules of identical appearance, placebo solution masked by adding traces of peppermint oil |
| Incomplete outcome data (attrition bias) | High risk | Completer analysis |
| Size | High risk | < 50 participants per treatment group |
| Methods | R, DB, AC and PC, parallel groups Single dose of one intervention to treat each of 2 attacks | |
| Participants | Episodic TTH (IHS criteria), 2 to 15 episodes/month, previous PR with a non‐opioid analgesic Age range 18 to 65 years Excluded: allergy or hypersensitivity or contraindication to study drugs; pregnant/breastfeeding; history alcohol or drug misuse; headache correlated with hormone contraception N = 360 (326 treated both attacks) M 89, F 271 Baseline pain ≥ moderate (mean 5.7/10) | |
| Interventions | Aspirin 1000 mg, n = 91 Metamizole 500 mg, n = 82 Metamizole 1000 mg, n = 92 Placebo, n = 91 Rescue medication after 2 h if required | |
| Outcomes | PI: 4‐point categorical scale over 4 h PR: 5‐point categorical scale over 4 h TOTPAR4 PGE: 4‐point categorical scale at 4 h Use of rescue medication AEs | |
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "using the double‐dummy technique" |
| Blinding of outcome assessment (detection bias) | Low risk | "using the double‐dummy technique" |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of imputation, unclear how data from two attacks was combined |
| Size | Unclear risk | 50 to 199 participants per treatment arm (82 to 92) |
| Methods | R, DB, PC, parallel‐group trial Single episode treated with one dose of study medication when pain was ≥ moderate intensity | |
| Participants | Headache (tension and tension‐vascular) clinically moderately severe, impairing efficiency, previously responsive to OTC medication: divided into tension headache and tension‐vascular headache Excluded: significant medical history; history other headache type; headache severe enough to render bed bound; intolerance to study medications N = 307 completed study (269 evaluated) M 53, F 216 (evaluated participants) Mean age 32 years Baseline pain moderately severe | |
| Interventions | Aspirin 650 mg, n = 90 Paracetamol 1000 mg, n = 87 Placebo, n = 92 No rescue medication allowed for 6 h | |
| Outcomes | PI: 3‐point scale over 6 h PR: 4‐point scale over 6 h AEs | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported Participants not randomised according to type of headache |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | DD method. "The tablets of each size were identical in appearance" |
| Blinding of outcome assessment (detection bias) | Low risk | DD method. "The tablets of each size were identical in appearance" |
| Incomplete outcome data (attrition bias) | Unclear risk | May be participants randomised who did not complete. Attrition > 10% due to protocol violations, no mention of discontinuations by headache type or of imputation |
| Size | Unclear risk | 50 to 199 participants per treatment arm (87 to 92) |
| Methods | R, DB, PC, parallel‐group study Single episode treated with one dose of study medication when pain was ≥ moderate intensity, 1 to 12 h after onset of headache | |
| Participants | Episodic tension headache (IHS), < 15/month Excluded: migraine; pregnant/breastfeeding; history gastrointestinal ulcer/haemorrhage; history alcohol or medication misuse N = 542 M:F ratio about 30:70 Mean age 40 years (SD 12; range 16 to 65 years) Mean baseline pain ≥ 57/100 (median ≥ 60/100) | |
| Interventions | Aspirin 500 mg, n = 111 Aspirin 1000 mg, n = 103 Paracetamol 500 mg, n = 105 Paracetamol 1000 mg, n = 111 Placebo, n = 112 Rescue medication allowed after 2 h | |
| Outcomes | PI: 100 mm VAS PR: 7‐point scale Functional ability: 4‐point scale, and time to return to normal function PGE: 5‐point scale AEs | |
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list" |
| Allocation concealment (selection bias) | Low risk | Assigned in numerical sequence |
| Blinding of participants and personnel (performance bias) | Low risk | DD method |
| Blinding of outcome assessment (detection bias) | Low risk | DD method |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis reported, with LOCF imputation |
| Size | Unclear risk | 50 to 199 participants per treatment arm (103 to 112) |
AC: active controlled; AE: adverse event; DB: double‐blind; DD: double‐dummy; F: female; h: hour; IHS: International Headache Society; ITT: intention to treat; LOCF: last observation carried forward; M: male; N: number of participants in study; n: number of participants in treatment arm; OTC: over‐the‐counter; PC: placebo controlled; PGE: Patient Global Evaluation; PI: pain intensity; PID: pain intensity difference; PR: pain relief; R: randomised; SD: standard deviation; SPID: summed pain intensity difference; TOTPAR: total pain relief; TTH: tension‐type headache; VAS: visual analogue scale; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Mean headache frequency > 15 days per month (chronic TTH) | |
| 84% of participants had migraine; no separate results for TTH | |
| Testing effect of adding tranquilliser to aspirin | |
| Mean headache frequency at upper limit (15/month) | |
| Study terminated, only 9 participants enrolled, no results posted | |
| Not stated to be randomised | |
| No diagnostic criteria reported, headache frequency unclear (> 1/month) so could include chronic TTH | |
| No diagnostic criteria reported, included participants with 'vascular headache' |
TTH: tension‐type headache.
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | R, DB (DD), AC and PC, parallel groups |
| Participants | Episodic TTH (diagnostic criteria provided, compatible with IHS), aged 18 to 65 years, men and women N = 1115 |
| Interventions | Aspirin 500 mg Aspirin 1000 mg Ibuprofen 200 mg Ibuprofen 400 mg Placebo Rescue medication: paracetamol |
| Outcomes | Meaningful relief at 2 h PR: categorical scale, to 4 h PGE at 24 h AEs |
| Notes | Completed, no results posted Request sent to Bayer for further information 30 August 2016; no response as of 15 September 2016 |
AC: active controlled; AE: adverse event; DB: double‐blind; DD: double‐dummy; h: hour; IHS: International Headache Society; PC: placebo controlled; PGE: Patient Global Evaluation; PR: pain relief; R: randomised; TTH: tension‐type headache.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants using rescue medication Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| Analysis 1.1 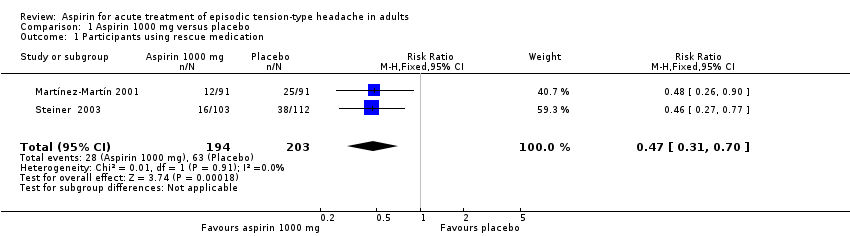 Comparison 1 Aspirin 1000 mg versus placebo, Outcome 1 Participants using rescue medication. | ||||
| 2 Participant global evaluation: 'satisfied' Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.18, 1.83] |
| Analysis 1.2 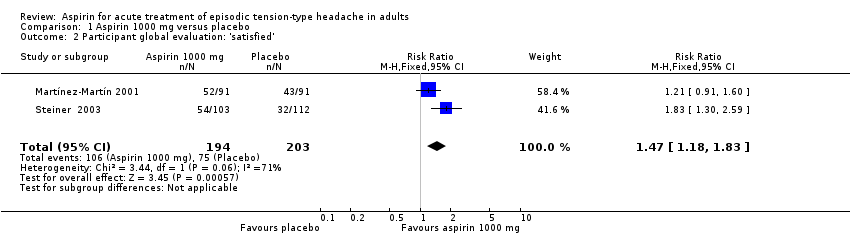 Comparison 1 Aspirin 1000 mg versus placebo, Outcome 2 Participant global evaluation: 'satisfied'. | ||||
| 3 Participants with any adverse event Show forest plot | 3 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.53] |
| Analysis 1.3  Comparison 1 Aspirin 1000 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.77, 2.02] |
| Analysis 2.1  Comparison 2 Aspirin 500 mg or 650 mg versus placebo, Outcome 1 Participants with any adverse event. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Aspirin 1000 mg versus placebo, Outcome 1 Participants using rescue medication.

Comparison 1 Aspirin 1000 mg versus placebo, Outcome 2 Participant global evaluation: 'satisfied'.

Comparison 1 Aspirin 1000 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 2 Aspirin 500 mg or 650 mg versus placebo, Outcome 1 Participants with any adverse event.
| Aspirin 1000 mg compared with placebo for episodic tension‐type headache | ||||||
| Patient or population: adults with episodic tension‐type headache Settings: community Intervention: aspirin 1000 mg Comparison: placebo | ||||||
| Outcomes | Outcome with | Outcome with | RR NNT, NNTp or NNH (95% CI) | No of studies, participants, events | Quality of the evidence | Comments |
| Pain‐free at 2 hours | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free at other time points (1, 4, 24 hours) | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free or mild pain at 2 hours | 61/112 | 78/103 | Not calculated | 1 study, 215 participants, 139 events | Very low | Downgraded three levels due to sparse data: single study with 215 participants in comparison |
| Use of rescue medication | 310 in 1000 | 140 in 1000 | RR 0.47 (0.31 to 0.70) NNTp 6.0 (4.1 to 12) | 2 studies, 397 participants, 91 events | Low | Downgraded two levels due to sparse data: small number of studies, participants and events |
| Patient Global Evaluation at end of study | 370 in 1000 | 550 in 1000 | RR 1.5 (1.2 to 1.8) NNT 5.7 (3.7 to 12) | 2 studies, 397 participants, 181 events | Very low | Downgraded three levels due to sparse data: small number of studies, participants, differences in scales and timing for the outcome, and post‐hoc nature of the analysis |
| Any adverse event | 140 in 1000 | 160 in 1000 | RR 1.1 (0.75 to 1.5) NNH not calculated | 3 studies, 723 participants, 107 events | Low | Downgraded two levels due to sparse data: small number of studies and events |
| Specific adverse events | Inconsistently reported and too few events for analysis Included gastrointestinal upset or dyspepsia, nausea, dizziness, and somnolence | ‐ | 4 studies, 767 participants | Very low | Downgraded three levels due to sparse data: small number of studies, few events, inconsistent reporting | |
| Serious adverse events | No events reported | No events reported | ‐ | 4 studies, 767 participants, no events | Very low | Downgraded three levels due to sparse data: small number of studies and no events |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence (EPOC 2015). High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants using rescue medication Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| 2 Participant global evaluation: 'satisfied' Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.18, 1.83] |
| 3 Participants with any adverse event Show forest plot | 3 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.77, 2.02] |


