Aspirina para el tratamiento agudo de la cefalea tensional episódica en adultos
Information
- DOI:
- https://doi.org/10.1002/14651858.CD011888.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 13 January 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
All authors have participated in the writing of the protocol and review.
Sources of support
Internal sources
-
The Oxford Pain Research Trust, UK.
Institutional support
External sources
-
No sources of support supplied
Declarations of interest
SD: none known.
PW: none known.
RAM has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Acknowledgements
The Oxford Pain Research Trust provided institutional support for this protocol.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 13 | Aspirin for acute treatment of episodic tension‐type headache in adults | Review | Sheena Derry, Philip J Wiffen, R Andrew Moore | |
| 2015 Sep 25 | Aspirin for acute treatment of episodic tension‐type headache in adults | Protocol | Guy Stephens, Sheena Derry, R Andrew Moore | |
Differences between protocol and review
We have clarified our wording on IHS diagnostic criteria in the 'Description of the condition' section, and have included information on dosage of aspirin in the 'Description of the intervention' section.
In the protocol, we said that we would "look primarily for studies using aspirin alone, but also seek studies that used aspirin in combination with another active oral treatment". We have clarified this so that it now reads "We looked primarily for studies using aspirin alone, but also sought studies that compared a combination of aspirin and another active oral treatment with the non‐aspirin component alone".
We have changed the order in which secondary outcomes are reported. The new order is more logical, and is in line with the other reviews in this series. We have also added a post‐hoc analysis of Patient Global Evaluation of treatment as an exploratory analysis, in the absence of other data.
We have clarified our assessment of potential performance and assessment bias in the 'Methods' section. In this review, all outcomes were self‐assessed, so the same considerations apply to detection bias as performance bias.
In addition, we have modified Appendix 4 (GRADE) to align it more closely with the wording used in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and extended the description in the 'Methods' section of the GRADE assessment for exceptional circumstances to explain possible decisions.
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised until 2022 following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acetaminophen [therapeutic use];
- Administration, Oral;
- Analgesics [administration & dosage, adverse effects, *therapeutic use];
- Aspirin [administration & dosage, adverse effects, *therapeutic use];
- Codeine [therapeutic use];
- Dipyrone [therapeutic use];
- Mentha piperita;
- Pain Measurement;
- Plant Oils [therapeutic use];
- Randomized Controlled Trials as Topic;
- Tension‐Type Headache [*drug therapy];
- Time Factors;
- Treatment Outcome;
Medical Subject Headings Check Words
Adult; Aged; Humans; Middle Aged;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
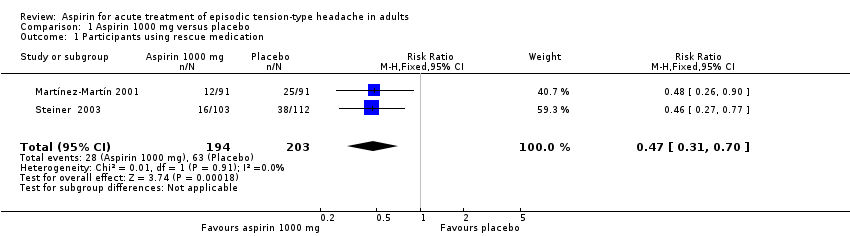
Comparison 1 Aspirin 1000 mg versus placebo, Outcome 1 Participants using rescue medication.
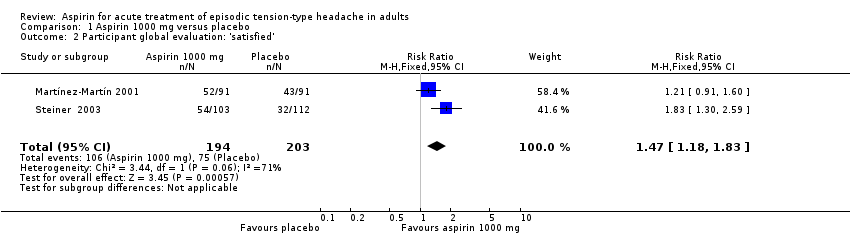
Comparison 1 Aspirin 1000 mg versus placebo, Outcome 2 Participant global evaluation: 'satisfied'.

Comparison 1 Aspirin 1000 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 2 Aspirin 500 mg or 650 mg versus placebo, Outcome 1 Participants with any adverse event.
| Aspirin 1000 mg compared with placebo for episodic tension‐type headache | ||||||
| Patient or population: adults with episodic tension‐type headache Settings: community Intervention: aspirin 1000 mg Comparison: placebo | ||||||
| Outcomes | Outcome with | Outcome with | RR NNT, NNTp or NNH (95% CI) | No of studies, participants, events | Quality of the evidence | Comments |
| Pain‐free at 2 hours | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free at other time points (1, 4, 24 hours) | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free or mild pain at 2 hours | 61/112 | 78/103 | Not calculated | 1 study, 215 participants, 139 events | Very low | Downgraded three levels due to sparse data: single study with 215 participants in comparison |
| Use of rescue medication | 310 in 1000 | 140 in 1000 | RR 0.47 (0.31 to 0.70) NNTp 6.0 (4.1 to 12) | 2 studies, 397 participants, 91 events | Low | Downgraded two levels due to sparse data: small number of studies, participants and events |
| Patient Global Evaluation at end of study | 370 in 1000 | 550 in 1000 | RR 1.5 (1.2 to 1.8) NNT 5.7 (3.7 to 12) | 2 studies, 397 participants, 181 events | Very low | Downgraded three levels due to sparse data: small number of studies, participants, differences in scales and timing for the outcome, and post‐hoc nature of the analysis |
| Any adverse event | 140 in 1000 | 160 in 1000 | RR 1.1 (0.75 to 1.5) NNH not calculated | 3 studies, 723 participants, 107 events | Low | Downgraded two levels due to sparse data: small number of studies and events |
| Specific adverse events | Inconsistently reported and too few events for analysis Included gastrointestinal upset or dyspepsia, nausea, dizziness, and somnolence | ‐ | 4 studies, 767 participants | Very low | Downgraded three levels due to sparse data: small number of studies, few events, inconsistent reporting | |
| Serious adverse events | No events reported | No events reported | ‐ | 4 studies, 767 participants, no events | Very low | Downgraded three levels due to sparse data: small number of studies and no events |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence (EPOC 2015). High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants using rescue medication Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| 2 Participant global evaluation: 'satisfied' Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.18, 1.83] |
| 3 Participants with any adverse event Show forest plot | 3 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.77, 2.02] |


