Reduction in saturated fat intake for cardiovascular disease
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT | |
| Participants | People with non‐melanoma skin cancer (USA) Ethnicity: white 100% (excluded from study if of Asian, Black, Hispanic or American Indian ancestry) Statins use allowed: Unclear % taking statins: Not reported | |
| Interventions | Reduced fat vs usual diet Control aims: no dietary advice Control methods: no dietary change, 4‐month intervals clinic examination by dermatologist Intervention methods: 8 x weekly classes plus monthly follow‐up sessions, with behavioural techniques being taught following individual approach (not clear if in a group or individual). 4‐month intervals clinic examination by dermatologist Intervention delivered face‐to‐face by a dietitian Total fat intake, %E ("during study" months 4 ‐ 24): cont 37.8 (SD 4.1), int 20.7 (SD 5.5) (mean difference ‐17.10, 95% CI ‐18.88 to ‐15.32) significant reduction Saturated fat intake, %E ("during study", months 4 ‐ 24): cont 12.8 (SD 2.0), int 6.6 (SD 1.8), (mean difference ‐6.20, 95% CI ‐6.90 to ‐5.50) significant reduction PUFA intake, %E ("during study", months 4 ‐ 24): cont 7.8 (SD 1.4), int 4.5 (SD 1.3), (mean difference ‐3.30, 95% CI ‐3.79 to ‐2.81) significant reduction PUFA n‐3 intake: not reported PUFA n‐6 intake: Linoleic acid, Control 16.9 (SD 5.6) g, Int 8.5 (SD 3.3) g MUFA intake, %E ("during study", months 4 ‐ 24): cont 14.4 (SD 1.7), int 7.6 (SD 2.2), (mean difference ‐6.80, 95% CI ‐7.52 to ‐6.08) significant reduction CHO intake, %E ("during study", months 4 ‐ 24): cont 44.6 (SD 6.9), int 60.3 (SD 6.3), (mean difference 15.70, 95% CI 13.29 to 18.11) significant increase Protein intake, %E ("during study", months 4 ‐ 24): cont 15.7 (SD 2.4), int 17.7 (SD 2.2), (mean difference 2.00, 95% CI 1.16 to 2.84) significant increase Trans fat intake: not reported Replacement for saturated fat: CHO and protein (by dietary aims and achievements) Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: incidence of actinic keratosis and non‐melanoma skin cancer Secondary outcomes: cancer deaths (none) Tertiary outcomes: none (weight data provided, but no variance info) | |
| Notes | Study duration 24 months. Study aim was to achieve low‐fat diet, but the study achieved a statistically significant reduction in saturated fat intake in the low‐fat group compared to control. SFA reduction achieved. Total serum cholesterol: not reported At 2 years control ‐1.5 kg n = 50?, intervention ‐1 kg n = 51? Trial dates: Study dates not reported (but still recruiting at first publication in 1994) Funding: National Cancer Institute Declarations of Interest of primary researchers: none stated, all authors work for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "list of randomly generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | High risk | Physician blinding: adequate |
| Incomplete outcome data (attrition bias) | Low risk | For mortality. Unclear for other outcomes |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists asked for data |
| Free of systematic difference in care? | High risk | Minor, all have 4‐monthly clinic visits, the intervention group had 8 behavioural technique classes that the control group did not have |
| Stated aim to reduce SFA | High risk | Aim to reduce SFA not stated |
| Achieved SFA reduction | Low risk | Statistically significant SFA reduction achieved |
| Achieved TC reduction | Unclear risk | Not reported |
| Other bias | Low risk | None noted |
| Methods | Factorial RCT | |
| Participants | Men recovering from an MI (UK) Ethnicity: not stated Statins use allowed? Unclear, but there do not appear to have been any medication‐based exclusion criteria and included participants were taking anti‐hypertensives, anti‐anginals, anti‐coagulants, anti‐platelet, digoxin and "other cardiac drugs". % taking statins: Not reported, but only 5.4% were taking "other cardiac drugs" which may have included statins | |
| Interventions | Reduced and modified fat vs usual diet Control aims: no dietary advice on fat, weight reducing advice if BMI > 30 Note: This was a factorial trial, and so some in each group were randomised to increased fatty fish and/or increased cereal fibre. Control methods: dietitians provided 'sensible eating' advice without specific information on fats Intervention methods: dietitians provided the participants and their wives with initial individual advice and a diet information sheet; participants were revisited for further advice, recipes, encouragement at 1, 3, 6, 9, 12, 15, 18 and 21 months Intervention delivered individually face‐to‐face by a dietitian Total fat intake, %E (through study): cont 35 (SD 6), int 31 (SD 7) (mean difference ‐4.00, 95% CI ‐4.57 to ‐3.43) significant reduction Saturated fat intake, %E (through study): cont 15 (SD3), int 11 (SD3), (mean difference ‐4.00, 95% CI ‐4.26 to ‐3.74) significant reduction PUFA intake (through study)⁑: cont 7 (SD unclear), int 9 (SD unclear), (mean difference 2.00, 95% CI 1.57 to 2.43 assuming SDs of 5) significant increase PUFA n‐3 intake: EPA, Control 0.6 (SD 0.7) g/wk, Int 2.4 (SD 1.4) g/wk PUFA n‐6 intake: not reported MUFA intake (through study)⁑: cont 13 (SD unclear), int 11 (SD unclear) (mean difference ‐2.00, 95% CI ‐2.43 to ‐1.57 assuming SDs of 5) significant reduction CHO intake (through study): cont 44 (SD 6),int 46 (SD 7) (mean difference 2.00, 95% CI 1.43 to 2.57) significant increase Protein intake (through study): cont 17 (SD 4), int 18 (SD 4) (mean difference 1.00, 95% CI 0.65 to 1.35) significant increase Trans fat intake: not reported Replacement for saturated fat: PUFA and CHO (by dietary aims), PUFA, CHO and protein (by dietary achievements) Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: mortality, reinfarction Secondary outcomes: cancer deaths, total MI, non‐fatal MI, CHD mortality, CHD events (total MI) Tertiary outcomes: total and HDL cholesterol | |
| Notes | Study duration 24 months Study aim was to achieve low fat diet with raised P/S ratio and saturated fat intake in the intervention group was significantly lower than in the control group. SFA reduction aimed and achieved. Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.26 (95% CI ‐0.36 to ‐0.16), statistically significant reduction ⁑Estimated by subtraction (assuming total fat = SFA + PUFA + MUFA) or using the ratio (assuming P/S = PUFA/SFA) Trial dates: Study dates not reported (published in 1989) Funding: Welsh Scheme for the Development of Health and Social Research, Welsh Heart Research Foundation, Flora Project, Health Promotion Research Trust. (Seven Seas Health Care and Duncan Flockhart provided the MaxEPA capsules and Norgene provided 'Fybranta' tablets ‐ but these were not used in the comparison discussed in this systematic review) Declarations of Interest of primary researchers: none stated, all authors work for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomised using sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Unclear if envelopes were opaque |
| Blinding (performance bias and detection bias) | High risk | Physician blinding: yes |
| Incomplete outcome data (attrition bias) | Low risk | GPs contacted for information on mortality and morbidity when participants did not attend |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as we asked all trialists for data |
| Free of systematic difference in care? | High risk | Different levels of advice appear to have been provided. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | Low risk | Statistically significant TC fall |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Adults with newly‐diagnosed diabetes (The Netherlands) Control: 51 randomised, unclear how many analysed (all analysed re deaths) Mean years in trial: unclear (max duration 6 years) Baseline total fat intake: int cont Baseline saturated fat intake: int cont Ethnicity: not stated Statins use allowed? Unclear % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat vs usual diet Control aims: SFA 35%E, CHO 50%E, protein 15%E Control methods: unclear, surveyed by dietitian Intervention methods: unclear, surveyed by dietitian Intervention appears to be delivered by dietitian but no clear details on format or frequency. Total fat intake: not reported Saturated fat intake: not reported (mean difference unclear) PUFA intake: not reported PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake: not reported CHO intake: not reported Protein intake: not reported Trans fat intake: not reported Replacement for saturated fat: mainly PUFA (based on dietary aims) Style: diet advice? Setting: community | |
| Outcomes | Stated trial outcomes: progression of diabetic retinopathy Secondary outcomes: total cholesterol, TGs (data read off graph), CHD mortality (fatal MI), CHD events (MI, angina) | |
| Notes | Study duration 6 years. Study aim was for control group to take 35%E as saturated fat, and the intervention group 40%E from fat, of which 33% was from linoleic acid (so saturated fat < 27%E), but saturated fat intake during trial not reported SFA reduction aimed (unclear whether achieved). Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.47(95% CI ‐0.76 to ‐0.18), statistically significant reduction Trial dates: Study recruitment 1973 to (unclear) Funding: Dutch Heart Foundation Declarations of Interest of primary researchers: none stated, all authors work for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants matched in pairs then randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | High risk | Neither participants nor physicians appear blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, deaths, cancer and CV events are drop‐outs, trialists asked for data ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as we asked all trialists for data |
| Free of systematic difference in care? | Unclear risk | Level and type of intervention unclear. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Unclear risk | SFA intake not reported |
| Achieved TC reduction | Low risk | Statistically significant TC fall |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | People with impaired glucose intolerance or high normal blood glucose (New Zealand) Ethnicity: European 67% int, 77% control, Maori 11% int, 7% control, Pacific islander 20% int, 13% control, Other 3% int, 4% control (outcomes not provided by ethnicity) Statins use allowed? Unclear % taking statins: Not reported | |
| Interventions | Reduced fat vs usual diet Control aims: usual diet Control methods: usual intake plus general advice on healthy eating consistent with the New Zealand guidelines and standard dietary information for people with nutrition‐related problems upon entering the trial Intervention methods: monthly small group meetings to follow a 1‐year structured programme aimed at reducing fat in the diet, includes education, personal goal setting, self monitoring Total fat intake, %E (at 1 year): int 26.1 (SD 7.7), cont 33.6 (SD 7.8) (mean difference ‐7.50, 95% CI ‐10.37 to ‐4.63) significant reduction Intervention delivered in small face‐to‐face groups but unclear by whom Saturated fat intake, %E (at 1 year): cont 13.4 (SD 4.7), int 10.0 (SD 4.2) (mean difference ‐3.40, 95% CI ‐5.05 to ‐1.75) significant reduction PUFA intake, %E (at 1 year): cont 4.8 (SD 1.6), int 4.0 (SD 1.4) (mean difference ‐0.80, 95% CI ‐1.36 to ‐0.24) significant reduction PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (at 1 year): cont 11.8 (SD 3.1), int 8.9 (SD 2.8) (mean difference ‐2.90, 95% CI ‐3.99 to ‐1.81) significant reduction CHO intake, %E (at 1 year): cont 45.8 (SD 10.9), int 54.2 (SD 10.5) (mean difference 8.40, 95% CI 4.44 to 12.36) significant increase Protein intake, %E (at 1 year): cont 16.6 (SD 3.9), int 18.4 (SD 3.5), (mean difference 1.80, 95% CI 0.43 to 3.17) significant increase Trans fat intake: not reported Replacement for saturated fat: carbohydrate and protein (based on dietary achievements) Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: lipids, glucose, blood pressure Secondary outcomes: total MI, stroke, cancer diagnoses, cancer deaths, CHD events (MI or angina) Tertiary outcomes: weight, total, LDL and HDL cholesterol, TGs, BP | |
| Notes | Study duration over 4 years Study aim was to reduce total fat (not saturated fat), but saturated fat intake in the intervention group was significantly lower than in the control group. SFA reduction achieved. Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.05 (95% CI ‐0.46 to 0.36), NO statistically significant reduction and smaller than 0.20 Trial dates: Recruitment 1988 to 1990 Funding: National Heart Foundation of New Zealand, Aukland Medical Research Foundation, Lotteries Medical Board and the Health Research Council of New Zealand. Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unmarked opaque envelopes were opened by the person recruiting, unable to alter allocation later |
| Allocation concealment (selection bias) | Low risk | Unmarked opaque envelopes were opened by the person recruiting, unable to alter allocation later |
| Blinding (performance bias and detection bias) | High risk | Participants were not blinded, outcome assessors were |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, deaths, cancer and CV events are drop‐outs, trialists were asked for data ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as we asked all trialists for data |
| Free of systematic difference in care? | High risk | See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | High risk | Aim to reduce SFA not stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | High risk | TC fall small (0.05 mmol/L only) and not statistically significant |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Middle‐aged siblings of people with early CHD, with at least 1 CVD risk factor (USA) Ethnicity: African‐American 18% int, 25% control (remainder of group ethnicity not described, and outcomes not presented by ethnicity) Statins use allowed? Unclear (raised LDL cholesterol was a condition of entry, so use of statins probably minimal) % taking statins: Not reported | |
| Interventions | Reduced fat intake vs usual diet Control aim: usual care Intervention aim: total fat 40 g/d or less Control methods: usual physician care with risk factor management at 0, 1 and 2 years Intervention methods: Individualised counselling by trained nurse, appointments 6 ‐ 8 weekly for 2 years Intervention delivered individually, face‐to‐face by a trained nurse. Total fat intake, %E (at 2 years): int 34.1 (SD unclear), cont 38.0 (SD unclear) (mean difference ‐3.90, 95% CI ‐6.46 to ‐1.34 assuming SDs of 10) significant reduction Saturated fat intake, %E (at 2 years): int 11.5 (SD unclear), cont 14.4 (SD unclear) (mean difference ‐2.90, 95% CI ‐4.18 to ‐1.62 assuming SDs of 5) significant reduction PUFA intake: not reported PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake: not reported CHO intake: not reported Protein intake: not reported Trans fat intake: not reported Replacement for saturated fat: unclear Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: dietary intake Secondary outcomes: cancer diagnoses (no events), cancer deaths (none), stroke, total and non‐fatal MI, CHD mortality (none), CHD events (MI or angina) Tertiary outcomes: BMI, HDL and LDL cholesterol, TG | |
| Notes | Study duration 2 years Study aim was to reduce total fat based on ATPII dietary guidelines, and preliminary work established that this intervention reduced saturated fat and dietary cholesterol, and saturated fat intake was significantly lower than in the control group SFA reduction aimed and achieved Total serum cholesterol not reported, but LDL was, difference between intervention and control, mmol/L: ‐0.29 (95% CI ‐0.54 to ‐0.04), statistically significant reduction Trial dates: Study recruitment 1991 to 1994 Funding: National Institute of Nursing Research, General Clinical Research Center of the National Institutes of Health Declarations of Interest of primary researchers: none stated, all authors work for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned via computerised schema after all eligible siblings from a family had been screened |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | High risk | Participants and trialists clear about their allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, deaths, cancer and CV events are drop‐outs, trialists were asked for data ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists asked for data |
| Free of systematic difference in care? | High risk | Differences in frequency of follow up, but unclear what differences in care occurred between the physician and nurse‐led care. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | Low risk | Statistically significant LDL fall (though TC not reported) |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Free‐living men who have survived a first MI (UK) Ethnicity: not stated Statins use allowed? Unclear (anti‐coagulants allowed, but few other medications appear to have been used) % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat vs usual diet Control aims: usual diet Control methods: usual diet plus reducing diet (reduced CHO) for weight management for overweight men Intervention methods: instructed to follow a dietary regimen removing saturated fat from the diet plus daily dose of 85 g soya oil; half of it had to be taken unheated. Reduced CHO diet for weight management in overweight men Intervention appears to be delivered and supervised by trial dietitian but unclear how often. Total fat intake, %E (at 3.5 years): int 46 (SD unclear), cont 43 (SD unclear) (mean difference 3.00, 95% CI 0.91 to 5.09 assuming SDs of 10) significant increase Saturated fat intake: not reported (mean difference unclear) PUFA intake: not reported PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake: not reported CHO intake: not reported Protein intake: not reported Trans fat intake: not reported Replacement for saturated fat: mainly PUFA (based on dietary goals) Style: diet advice & supplement (soy oil) Setting: community | |
| Outcomes | Stated trial outcomes: MI or sudden death Secondary outcomes: total and non‐fatal MI, stroke, cancer deaths, CHD mortality, CHD events (CHD mortality or non‐fatal MI) Tertiary outcomes: none (data for weight, total cholesterol and BP, but no variance info) | |
| Notes | Study duration over 6 years Study aim for intervention "saturated fats were replaced by polyunsaturated fats", but saturated fat intakes during trial were not reported. SFA reduction aimed Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.64 (95% CI unclear), reduction > 0.20 For all, data at 4 years, control n = 89, intervention n = 88 Weight change: control ‐3 kg, intervention 0 kg Total cholesterol change: control ‐0.47 mmol/L, intervention ‐1.11 mmol/L Systolic BP change: control 0 mmHg, intervention +2 mmHg Diastolic BP change: control +3 mmHg, intervention ‐1 mmHg Trial dates: Study recruitment 1960 to 1965, analysed 1967 Funding: Medical Research Council Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using random numbers, by blocks within hospitals" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | High risk | Physician blinding: adequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Data collection was thorough, but some participants dropped out and contact was lost, so some events may have been missed |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Unlikely as control group continued diet as usual, intervention group were likely to have had additional contact. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Unclear risk | SFA intake not reported |
| Achieved TC reduction | Low risk | Although statistical significance was not reported or calculable, TC in the intervention group was 0.64 mmol/L lower than in the control group, a large fall (and almost certainly statistically significant) |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Men with previous MI (Norway) Ethnicity: ethnicity not mentioned Statins use allowed? Unclear (medications not mentioned as exclusion criteria, most appeared to be on anti‐coagulant medications, statins not mentioned) % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat diet vs control Control aims: no dietary advice but direct questions answered, supplement = 1 vitamin tablet daily Control methods: usual diet Intervention methods: continuous instruction and supervision by dietitian, including home visits, letters and phone calls Total fat intake: unclear (note ‐ intake of total fat, carbohydrate, protein and sugar was assessed in 17 "especially conscientious and positive" as well as intelligent dieters, but this is not reported here as unlikely to be representative, and lacking control group data) Saturated fat intake: unclear (mean difference unclear) PUFA intake: unclear PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake: unclear CHO intake: unclear Protein intake: unclear Trans fat intake: unclear Replacement for saturated fat: PUFA (based on dietary goals) Style: diet advice and supplement (food) Setting: community | |
| Outcomes | Stated trial outcomes: coronary heart disease morbidity and mortality Secondary outcomes: non‐fatal and total MI, stroke, CHD mortality (fatal MI and sudden death), CHD events (MI, angina and sudden death) Tertiary outcomes: weight, total cholesterol, systolic and diastolic BP (but no variance information is provided) | |
| Notes | Study duration over 4 years Study aim was to reduce serum cholesterol by a diet "low in saturated fats and in cholesterol, and rich in highly unsaturated fats", saturated fat intakes during study were not reported SFA reduction aimed (reduction unclear as not measured except in a highly compliant subgroup) Total serum cholesterol, difference between intervention and control, mmol/L: ‐1.07 (95% CI unclear), reduction > 0.20 Weight change from baseline was ‐0.5 kg in the control group (n ˜ 155), ‐2.5 kg in the intervention group (n ˜ 160) to 51 months Total cholesterol change from baseline was ‐0.46 mmol/L in the control group and ‐1.53 mmol/L in the intervention group at 51 months Systolic BP at baseline was 153.8 mmHg in control and 159.0 in intervention, and mean sBP through trial was 154.3 mmHg in control and 158.2 mmHg in the intervention group Diastolic BP at baseline was 93.5 mmHg in control and 97.1 mmHg in intervention, through trial mean dBP was 95.5 mmHg in control and 98.6 mmHg in intervention participants Trial dates: Recruitment 1956 to 1958 Funding: Det Norske Råd for Hjerte‐ og karsyk‐dommer, A/S Freia Chokoladefabriks Arbeidsfond for Ernærings‐forskning, JL Tiedemanns Tobaksfabrik Joh H Andresens medisinske fond, plus A/S Farmacöytisk Industri provided a multivitamin free of charge, DE‐NO‐FA and Lillleborg Fabriker provided soy bean oil at reduced prices, the Research Laboratory of the Norwegian Canning Industry, Stavanger Preserving Co and Kommendal Packing Comp provided Norwegian sardines in cod liver oil free to those in the intervention group. Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers used", by Prof Knut Westlund |
| Allocation concealment (selection bias) | Low risk | Randomisation appears to have occurred before medical examination within the study |
| Blinding (performance bias and detection bias) | High risk | Participants were aware of their allocation as was the main trialist. Outcomes were categorised by a diagnostic board, but their blinded status was unclear |
| Incomplete outcome data (attrition bias) | Low risk | The participants who could not be directly followed up for the 5 years were followed until death or study end through personal interviews, or contact with their physicians or relatives |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Dietetic input level very different, although medical care appeared similar. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Unclear risk | SFA intake not reported |
| Achieved TC reduction | Low risk | Although statistical significance was not reported or calculable, TC in the intervention group was 1.07 mmol/L lower than in the control group, a large fall (and almost certainly statistically significant) |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Newly‐diagnosed non‐insulin‐dependent diabetics (UK) Ethnicity: not stated Statins use allowed? Unclear % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Reduced and modified dietary fat vs average diet Control aims: total fat 40%E, PUFA 12%E, protein 20%E, CHO 40%E (reducing simple sugars), 1500 kcal/day Control methods: dietary advice from diabetes dietitian Intervention methods: dietary advice from diabetes dietitian Total fat intake, %E (at 7 ‐ 9 years)§: int 32 (SD unclear), cont 41 (SD unclear) (mean difference ‐9.00, 95% CI ‐11.48 to ‐6.52 assuming SDs of 10) significant reduction Saturated fat intake, %E (at 7 ‐ 9 years)§: int 10.7 (SD unclear), cont 20.4 (SD unclear) (mean difference ‐9.70, 95% CI ‐10.94 to ‐8.46 assuming SD of 5) significant reduction PUFA intake, %E (at 7 ‐ 9 years)§: int 11.8 (SD unclear), cont 2.1 (SD unclear) (mean difference 9.70, 95% CI 8.46 to 10.94 assuming SDs of 5) significant increase PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (at 7 ‐ 9 years)§: int 9.5 (SD unclear), cont 18.6 (SD unclear) (mean difference ‐9.10, 95% CI ‐10.34 to 7.86 assuming SDs of 5) significant reduction Carbohydrate intake: not reported Protein intake: not reported Trans fat intake: not reported Replacement for saturated fat: PUFA and CHO (based on dietary goals and achievements) Style: diet advice Setting: community (outpatients clinic) | |
| Outcomes | Stated trial outcomes: retinopathy Secondary outcomes: none Tertiary outcomes: BMI, total cholesterol | |
| Notes | Study duration over 9 years Study aim was to reduce total fat and increase PUFAs (so reducing saturates), and saturated fat intake in the intervention group was significantly lower than in the control group SFA reduction achieved. Total serum cholesterol, difference between intervention and control, mmol/L: 0.07 (95% CI ‐0.34 to 0.48), NO statistically significant reduction and smaller than 0.20 §validity of these data is questionable as it represents only 3 intervention and 3 control participants. Source: Lopez‐Espinoza 1984 Trial dates: Recruitment 1973 to 1976 Funding: Oxford Diabetes Trust, British Diabetic Association, International Sugar Research Foundation Inc Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number sequence, provided and allotted by a separate agency" (Prof Richard Peto) |
| Allocation concealment (selection bias) | Low risk | "random number sequence, provided and allotted by a separate agency" (Prof Richard Peto) |
| Blinding (performance bias and detection bias) | High risk | Participants not blinded, physicians unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, deaths, cancer and CV events are drop‐outs ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | Low risk | Dietetic advice for both groups. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | High risk | Aim to reduce SFA not stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | High risk | No statistically significant TC fall, and difference only 0.07 mmol/L |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Men (?) with angina or following MI (UK) Intervention ‐ corn: randomised 26, analysed 13 Ethnicity: not stated Statins use allowed? Unclear (anti‐coagulants not allowed, but all participants received conventional treatments at the discretion of their physicians) % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat vs usual diet Control aims: usual diet Intervention aims ‐ corn: restrict dietary fat, plus 80 g/day corn oil provided Control methods: usual physician care plus follow‐up clinic monthly, then every 2 months, no dietary fat advice or oil provided Intervention methods: usual physician care plus follow‐up clinic monthly, then every 2 months, dietary fat advice plus oil provided Unclear how the advice was delivered or by whom Total fat intake, %E (at 18 months): corn 50.5 (SD unclear), cont 32.6 (SD unclear) (mean difference 17.90, 95% CI 10.77 to 25.03 assuming SDs of 10) significant increase Saturated fat intake: unclear (mean difference unclear) PUFA intake: unclear PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake: unclear CHO intake, %E (at 18 months): corn 36.5 (SD unclear), cont 51.5 (|SD unclear) (mean difference ‐15.00, 95% CI ‐29.27 to ‐0.73 assuming SDs of 20) significant reduction Protein intake, %E (at 18 months): corn 11.0 (SD unclear), cont 13.2 (SD unclear) (mean difference ‐2.20, 95% CI ‐5.77 to 1.37 assuming SDs of 5) no significant difference Trans fat intake: unclear Replacement for saturated fat: mainly PUFA (based on aims and achievements) Style: diet advice and supplement (oil) Setting: community | |
| Outcomes | Stated trial outcomes: cardiac events Secondary outcomes: stroke (none), non‐fatal and total MI, CHD mortality (fatal MI and sudden death), CHD events (all MI and sudden death) Tertiary outcomes: total cholesterol | |
| Notes | Study duration 2 years Study aim was to reduce total fat (by restricting fatty meat, sausages, pastry, ice cream, cheese, cake, milk, eggs and butter) and prescribe vegetable oil (so reducing saturates), but saturated fat intakes during intervention were not reported SFA reduction aimed (but unclear whether achieved as SFA intake not reported) Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.58 (95% CI ‐1.42 to 0.26), NO statistically significant reduction but > 0.20 Trial dates: unclear, published in 1965 Funding: probably unfunded (they thank the Paddington General Hospital for clinic facilities, and St Mary's and Paddington General Hospital physicians for referral of patients, but no funding acknowledged) Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Unclear if envelopes were opaque |
| Blinding (performance bias and detection bias) | High risk | Physician blinding: inadequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Some lost to follow‐up by 2 years, so some events may have been missed |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | Low risk | All received conventional treatments at the discretion of the physicians, all attended a special follow‐up clinic. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Unclear risk | SFA intake not reported |
| Achieved TC reduction | High risk | Although the TC in the intervention group was 0.58 mmol/L lower than in the control group, this was not statistically significant in this small study |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Men (?) with angina or following MI (UK) Mean years in trial: control 1.7, olive 1.5 Ethnicity: Not stated Statins use allowed? Unclear (anti‐coagulants not allowed, but all participants received conventional treatments at the discretion of their physicians) % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat vs usual diet Control aims: usual diet Control methods: usual physician care plus follow‐up clinic monthly, then every 2 months, no dietary fat advice or oil provided Intervention methods: usual physician care plus follow‐up clinic monthly, then every 2 months, dietary fat advice plus oil provided Unclear how the advice was delivered or by whom Total fat intake, %E (at 18 months): olive 46.2 (SD unclear), cont 32.6 (SD unclear) (mean difference 13.60, 95% CI 6.30 to 20.90 assuming SDs of 10) significant increase Saturated fat intake: unclear (mean difference unclear) PUFA intake: unclear PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake: unclear CHO intake, %E (at 18 months): olive 42.2 (SD unclear), cont 51.5 (SD unclear) (mean difference ‐9.30, 95% CI ‐23.91 to 5.31 assuming SDs of 20) no significant difference Protein intake, %E (at 18 months): olive 9.6 (SD unclear), cont 13.2 (SD unclear) (mean difference ‐3.60, 95% CI ‐7.25 to 0.05 assuming SDs of 5) no significant difference Trans fat intake: unclear Replacement for saturated fat: mainly MUFA (based on dietary aims) Style: diet advice and supplement (oil) Setting: community | |
| Outcomes | Stated trial outcomes: cardiac events Secondary outcomes: stroke (none), non‐fatal and total MI, CHD mortality (fatal MI and sudden death), CHD events (all MI and sudden death) Tertiary outcomes: total cholesterol | |
| Notes | Study duration 2 years Study aim was to reduce total fat (by restricting fatty meat, sausages, pastry, ice cream, cheese, cake, milk, eggs and butter) and prescribe vegetable oil (so reducing saturates), but saturated fat intakes during intervention were not reported SFA reduction aimed (but unclear whether achieved as SFA intake not reported) Total serum cholesterol, difference between intervention and control, mmol/L: 0.30 (95% CI ‐0.93 to 1.53), NO statistically significant reduction, mean total cholesterol rose Trial dates: unclear, published in 1965 Funding: probably unfunded (they thank the Paddington General Hospital for clinic facilities, and St Mary's and Paddington General Hospital physicians for referral of patients, but no funding acknowledged) Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Methods | RCT | |
| Participants | Women with a high risk of breast cancer (USA) Ethnicity: White 89%, African‐American 9%, Hispanic 2% Statins use allowed? No (those on lipid‐lowering medications were excluded) % taking statins: 0% | |
| Interventions | Reduced fat vs usual diet Control aims: usual diet Control methods: continued usual diet Intervention methods: Bi‐weekly individual dietetic appointments over 3 months followed by monthly individual or group appointments, including education, goal setting, evaluation, feedback and self monitoring Intervention delivered face‐to‐face by a dietitian Total fat intake, %E (at 12 months)§: int 17.6 (SD 5.8), cont 33.8 (SD 7.4) (mean difference ‐16.20, 95% CI ‐18.34 to ‐14.06) significant reduction Saturated fat intake, %E (at 12 months)§: int 6.0 (SD 3.0), cont 12.1 (SD 5.2) (mean difference ‐6.10, 95% CI ‐7.47 to ‐4.73) significant reduction PUFA intake, %E (at 12 months)§: int 3.8 (SD 1.7), cont 7.3 (SD 4.1) (mean difference ‐3.50, 95% CI ‐4.51 to ‐2.49) significant reduction PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (at 12 months)§: int 6.1 (SD 3.0), cont 12.8 (SD 6.3) (mean difference ‐6.70, 95% CI ‐8.29 to ‐5.11) significant reduction CHO intake: not reported Protein intake: not reported Trans fat intake: not reported Replacement for saturated fat: unclear, either carbohydrate or protein (based on aims and achievements) Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: intervention feasibility Secondary outcomes: cancer diagnosis (8 diagnoses, but not clear in which arms) Tertiary outcomes: weight, total, LDL and HDL cholesterol, TGs | |
| Notes | Study duration 2 years Study aim was to reduce total fat to 15%E (saturated fat not mentioned), but saturated fat intake in the intervention group was significantly lower than in the control group SFA reduction achieved Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.34 (95% CI ‐0.64 to ‐0.04), statistically significant reduction Trial dates: Recruitment 1987 to 1989 Funding: Marilyn J Smith Fund, Harper‐Grace Hospitals, the Wesley Foundation, National Cancer Institute, Karmanos Cancer Institute Core Grant, the United Foundation of Detroit Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions except PN Kim who was affiliated with Wesley Health Strategies (now Health Strategies, which offers a "full‐service health and fitness centre with an educated fitness staff and spacious workout areas", see healthstrategiesfitness.com/) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age and randomised (block size 2) |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | High risk | Participants knew their allocation, unclear whether physicians did |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, deaths, cancer and CV events are drop‐outs ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Very different contact time with dietitian, but medical appointments same in both groups. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | High risk | Aim to reduce SFA not stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | Low risk | Statistically significant TC fall |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Men with angina referred for angiography (UK) Ethnicity: not stated Statins use allowed? No (1 arm of the trial, not described here, prescribed cholestyramine) % taking statins: 0% | |
| Interventions | Reduced and modified fat diet vs usual diet Control aims: no diet intervention but advised to lose weight if BMI > 25 Control methods: usual care but no formal dietetic counselling. They were counselled against smoking if appropriate and advised about daily exercise level Intervention methods: Usual care plus dietetic individual assessment of diet and advice. Further dietetic counselling and food stuffs were given to participants who did not achieve or maintain certain levels of serum cholesterol reduction Initial intervention was delivered individually face‐to‐face by a dietitian and follow‐up by a clinician Total fat intake, %E (through study): int 27 (SD 7), cont 37 (SD 5) (mean difference ‐10.00, 95% CI ‐13.35 to ‐6.65) significant reduction Saturated fat intake, %E (through study): int 9 (SD 3), cont 16 (SD 4) (mean difference ‐7.00, 95% CI ‐8.97 to ‐5.03) significant reduction PUFA intake, %E (through study)§: int 7 (SD 2), cont 5 (SD 2) (mean difference 2.00, 95% CI 0.89 to 3.11) significant increase PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (through study)§: int 10 (SD 4), cont 17 (SD 5) (mean difference ‐7.00, 95% CI ‐9.52 to ‐4.48) significant reduction CHO intake, %E (through study)§: int 49 (SD 7), cont 41 (SD 7) (mean difference 8.00, 95% CI 4.12 to 11.88) significant increase Protein intake, %E (through study)§: int 19 (SD 4), cont 18 (SD 2) (mean difference 1.00, 95% CI ‐0.73 to 2.73) no significant effect Trans fat intake: not reported Replacement for saturated fat: CHO and PUFA (based on aims and achievements) Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: angiography Secondary outcomes: total, HDL, LDL cholesterol, TGs, total/HDL and LDL/HDL ratios, 2‐hour post‐load glucose (weight and BP "remained similar" but were not reported, Lp(a) reported but as geometric means) | |
| Notes | Study duration 3 years Study aim was to reduce saturated fats (to 8 ‐ 10%E), and saturated fat intake in the intervention group was significantly reduced SFA reduction aimed and achieved Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.76 (95% CI ‐1.19 to ‐0.33), statistically significant reduction Trial dates: Study dates not reported (published in 1992) Funding: Unilever plc, the Chemical Pathology Fund of St Thomas' Hospital, and Bristol‐Meyers Ltd Declarations of Interest of primary researchers: none stated, all authors work for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "blinded random cards issued centrally by statistician advisor" |
| Allocation concealment (selection bias) | Low risk | "blinded random cards issued centrally by statistician advisor" |
| Blinding (performance bias and detection bias) | High risk | Physician blinding: unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, deaths, cancer and CV events are drop‐outs ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Usual care in both groups, dietetic counselling only in the intervention group. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | Low risk | Statistically significant TC fall |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Men with previous MI (Australia) Ethnicity: not stated Statins use allowed? Unclear (use of medication did not appear to be an exclusion criteria) % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat diet vs usual diet Control aims: reduction in energy if overweight, no other specific dietary advice, allowed to use PUFA margarine instead of butter Control methods: no specific dietary instruction (except re weight) Intervention methods: advised and tutored individually, diet assessed 3 times in 1st year and twice annually thereafter Intervention was delivered face‐to‐face individually but unclear by whom Total fat intake, %E ("during follow up"): int 38.3 (SD 5.9), cont 38.1 (SD 5.4) (mean difference 0.20, 95% CI ‐0.88 to 1.28) no significant difference Saturated fat intake, %E ("during follow up"): int 9.8 (SD 2.6), cont 13.5 (SD 3.2) (mean difference ‐3.70, 95% CI ‐4.25 to ‐3.15) significant reduction PUFA intake, %E ("during follow up"): int 15.1 (SD 4.3), cont 8.9 (SD 3.5) (mean difference 6.20, 95% CI 5.45 to 6.95) significant increase PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E ("during follow up"): int 11.5 (SD 2.1), cont 13.8 (SD 2.5) (mean difference ‐2.30, 95% CI ‐2.74 to ‐1.86) significant reduction CHO intake, %E ("during follow up"): int 40.9 (SD 7.3), cont 40.3 (SD 7.3) (mean difference 0.60, 95% CI ‐0.79 to 1.99) no significant difference Protein intake, %E ("during follow up"): int 15.2 (SD 2.8), cont 15.7 (SD 3.4) (mean difference ‐0.50, 95% CI ‐1.09 to 0.09) no significant difference Trans fat intake: not reported Primary replacement for saturated fat: mainly PUFA (based on dietary aims and achievements) Style: diet advice Setting: community | |
| Outcomes | Stated trial outcomes: cardiovascular mortality and morbidity Secondary outcomes: CHD deaths (exact events included not stated) Tertiary outcomes: total cholesterol, TG, BMI, sBP, dBP | |
| Notes | Study duration 7 years Study aim was saturated fat 10%E, and saturated fat intake in the intervention group was less than 80% of that in the control (73%) SFA reduction aimed and achieved Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.30 (95% CI ‐0.51 to ‐0.09), statistically significant reduction Trial dates: Recruitment 1966 to [unclear] and followed for 2 to 7 years Funding: Life Insurance Medical Research Fund of Australia and New Zealand Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | High risk | Physician blinding: adequate |
| Incomplete outcome data (attrition bias) | Low risk | Survival analysis used |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Advice and follow‐up in intervention group, not in control. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | Low risk | Statistically significant TC fall |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Men living at the Veterans Administration Center (USA) Ethnicity: White 90%, African‐American 7%, Asian 1%, Mexican 1%, other 1% Statins use allowed? Unclear (only 4 participants were taking nicotinic acid, 17 diuretics, 56 digitalis, none on heparin) % taking statins: Not reported (probably none as too early, pre‐1980) | |
| Interventions | Modified fat vs usual diet Control aims: provided, total fat 40%E Control methods: whole diet provided Intervention methods: whole diet provided Total fat intake, %E (during trial): int 38.9 (SD unclear), cont 40 (SD unclear) (mean difference ‐1.10, 95% CI ‐2.45 to 0.25 assuming SDs of 10) no significant difference Saturated fat intake, %E (during trial): int 8.3 (SD unclear), cont 18.5 (SD unclear) (mean difference ‐10.20, 95% CI ‐10.87 to ‐9.53 assuming SDs of 5) significant reduction PUFA intake, %E (during trial)§: int 16.0 (SD ?), cont 4.9 (SD 0.10) (mean difference 11.10, 95% CI 10.62 to 11.58 assuming missing SD was 5) significant increase PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (during trial)⁑: not reported, approx int 14.0, cont 17.2 (mean difference ‐3.20, 95% CI ‐3.87 to ‐2.53) significant reduction CHO intake, %E (during trial)⁑: not reported, approx int 45.9, cont 44.8 (mean difference 1.10, 95% CI ‐1.60 to 3.80 assuming SDs of 20) no significant difference Protein intake, %E (during trial)§: int 15.2 (SD ?), cont 15.2 (SD ?) (mean difference 0.00, 95% CI ‐0.67 to 0.67 assuming SDs of 5) no significant difference Trans fat intake: not reported Replacement for saturated fat: mainly PUFA (based on dietary aims and achievements) Style: diet provided Setting: residential institution | |
| Outcomes | Stated trial outcomes: mortality, heart disease Secondary outcomes: cancer deaths, cancer diagnoses, stroke, non‐fatal MI, total MI, CHD deaths (fatal MI and sudden death due to CHD), CHD events (any MI or sudden death due to CHD) Tertiary outcomes: none (some data on total cholesterol, but no variance info) | |
| Notes | Study duration over 8 years Study aim was to replace 66% of saturated fat by unsaturated fats, and saturated fat intake in the intervention group was significantly lower than in control SFA reduction aimed and achieved Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.37 (95% CI ‐0.77 to 0.03), NO statistically significant reduction but reduction > 0.20 ⁑Estimated by subtraction (assuming total fat = SFA + PUFA + MUFA or energy intake = energy from fat + CHO + protein) Trial dates: Recruitment 1959 to 1967 Funding: Veterans Administration, Aruthur Dodd Fuller Foundation, National Heart Institute, Los Angeles County Heart Association, plus gifts of foods from Mazola corn oil and Mazola margarine, the National Soybean Processors Association, Pitman‐Moore Company (Emdee margarine) and Hi‐Saff Imitation Ice‐cream from Frozen Desserts Company. Edgmar Farms donated milk refrigeration equipment. Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers used" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) | Low risk | physician blinding: adequate |
| Incomplete outcome data (attrition bias) | Low risk | All followed up via Veterans Admin system |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | Low risk | All ate centre food as usual. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | High risk | No statistically significant TC fall, though fall was > 0.20 mmol/L |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Post‐menopausal women aged 50 ‐ 79 with CVD at baseline (USA) Ethnicity (women both with and without CVD at baseline): white 82%, black 11%, Asian or pacific islander 2%, unknown 1%, American Indian or Alaskan native < 1%. No statistically significant effects of the intervention on CHD events was seen for any ethnic subgroup Statins use allowed? Yes % taking statins: 12% of women recruited were on lipid‐lowering medication (these were a mixture of participants with and without CVD at baseline) | |
| Interventions | Reduced fat vs usual diet Control: diet‐related education materials Control methods: given copy of 'Dietary Guidelines for Americans' Intervention methods: 18 group sessions with trained and certified nutritionists in the 1st year, quarterly maintenance sessions thereafter, focusing on diet and behaviour modification Intervention delivered face‐to‐face in a group by nutritionists Intake data all relate to the full WHI cohort (not divided by whether participants have CVD at baseline or not): Total fat intake, %E (at 6 years): int 28.8 (SD 8.4), cont 37.0 (SD 7.3) (mean difference ‐8.20, 95% CI ‐8.34 to ‐8.06) significant reduction Saturated fat intake, %E (at 6 years): int 9.5 (SD3.2), cont 12.4 (SD3.1) (mean difference ‐2.90, 95% CI ‐2.96 to ‐2.84 for full WHI population) significant reduction PUFA intake, %E (at 6 years)§: int 6.3 (SD?), cont 7.6 (SD?) (mean difference ‐1.30, 95% CI ‐1.72 to ‐0.88 assuming missing SDs were 5) significant reduction PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (at 6 years)§: int 11.1 (SD?), cont 14.3 (SD?) (mean difference ‐3.20, 95% CI ‐3.62 to ‐2.78 assuming unclear SDs were 5) significant reduction CHO intake, %E (at 6 years)§: int 53.9 (SD?), cont 46.3 (SD?) (mean difference 7.60, 95% CI 5.91 to 9.29 assuming SDs of 20) significant increase Protein intake, %E (at 6 years)§: int 17.7 (SD?), cont 17.0 (SD?) (mean difference 0.70, 95% CI 0.28 to 1.12 assuming SDs of 5) significant increase Trans fat intake, %E (at 6 years)§: int 1.8 (SD?), cont 2.4 (SD?) (mean difference unclear, no SDs assumed) Replacement for saturated fat: mainly CHO, some protein (based on dietary achievement) Style: dietary advice Setting: community | |
| Outcomes | Stated trial outcomes: breast cancer, mortality, other cancers, cardiovascular events, diabetes Data available on total mortality? yes Secondary outcomes: cancer deaths*, cancer diagnoses*, stroke, non‐fatal MI Tertiary outcomes: weight, BMI, total, LDL and HDL cholesterol, TGs, systolic and diastolic BP * these are only available for the whole cohort, not split between low and high CVD risk groups | |
| Notes | Study duration over 8 years Study aim was to reduce total fat to 20%E, reduce saturated fat to 7%E and increase fruit and vegetable intake (Patterson 2003), and saturated fat intake in the intervention group was significantly lower than in control SFA reduction aimed and achieved. Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.09 (95% CI ‐0.15 to ‐0.02), statistically significant reduction §Amongst the 881 intervention and 1373 control participants with blood samples at baseline, with or without CVD at baseline (Howard 2010). Trial dates: Recruitment was between 1993 and 1998 Funding: National Heart, Lung and Blood Institute of the National Institutes of Health Declarations of Interest of primary researchers: Declarations vary from paper to paper, but this is a typical one from Beresford 2006 "Dr Black has received research grants from Pfizer and AstraZeneca, was on the speakers bureaus for Pfizer, Novartis, Sanofi‐Aventis, Bristol‐Meyers Squibb, Searle, Pharmacia, and Boehringer and served as a consultant of on an advisory board for Myogen, Merck Sharp and Dohme, Novartis, Mylan‐Bertek, Pfizer, Bristol‐Meyers Squibb, and Sanofi‐Aventis. Dr Howard has served on the advisory boards of Merck, Schering Plough, and the Egg Nutrition Council, has received research support from Merck and Pfizer, and has consulted for General Millls. Dr Assaf is an employee of Pfizer. No other disclosures were reported." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm |
| Allocation concealment (selection bias) | Low risk | Computer algorithm |
| Blinding (performance bias and detection bias) | High risk | Participants aware of allocation |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Intervention participants received 18 group sessions with behavioural modification plus quarterly maintenance sessions thereafter, control groups received a leaflet. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | Low risk | Aim to reduce SFA stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | Low risk | Statistically significant TC fall |
| Other bias | Low risk | None noted |
| Methods | RCT | |
| Participants | Post‐menopausal women aged 50 ‐ 79 without CVD at baseline (USA) Ethnicity (women both with and without CVD at baseline): white 82%, black 11%, Asian or pacific islander 2%, unknown 1%, American Indian or Alaskan native < 1%. No statistically significant effects of the intervention on CHD events was seen for any ethnic subgroup Statins use allowed? Yes % taking statins: 12% of women recruited were on lipid‐lowering medication (these were a mixture of participants with and without CVD at baseline) | |
| Interventions | Reduced fat vs usual diet Control: diet‐related education materials Control methods: given copy of 'Dietary Guidelines for Americans' Intervention methods: 18 group sessions with trained and certified nutritionists in the 1st year, quarterly maintenance sessions thereafter, focusing on diet and behaviour modification Intervention delivered face‐to‐face in a group by nutritionists Intake data all relate to the full WHI cohort (not divided by whether participants have CVD at baseline or not): Total fat intake, %E (at 6 years): int 28.8 (SD 8.4), cont 37.0 (SD 7.3) (mean difference ‐8.20, 95% CI ‐8.34 to ‐8.06) significant reduction Saturated fat intake, %E (at 6 years): int 9.5 (SD3.2), cont 12.4 (SD3.1) (mean difference ‐2.90, 95% CI ‐2.96 to ‐2.84 for full WHI population) significant reduction PUFA intake, %E (at 6 years)§: int 6.3 (SD?), cont 7.6 (SD?) (mean difference ‐1.30, 95% CI ‐1.72 to ‐0.88 assuming missing SDs were 5) significant reduction PUFA n‐3 intake: not reported PUFA n‐6 intake: not reported MUFA intake, %E (at 6 years)§: int 11.1 (SD?), cont 14.3 (SD?) (mean difference ‐3.20, 95% CI ‐3.62 to ‐2.78 assuming unclear SDs were 5) significant reduction CHO intake, %E (at 6 years)§: int 53.9 (SD?), cont 46.3 (SD?) (mean difference 7.60, 95% CI 5.91 to 9.29 assuming SDs of 20) significant increase Protein intake, %E (at 6 years)§: int 17.7 (SD?), cont 17.0 (SD?) (mean difference 0.70, 95% CI 0.28 to 1.12 assuming SDs of 5) significant increase Trans fat intake, %E (at 6 years)§: int 1.8 (SD?), cont 2.4 (SD?) (mean difference unclear, no SDs assumed) Replacement for saturated fat: mainly carbohydrate, some protein (based on dietary achievement) Style: dietary advice Setting: community | |
| Outcomes | Stated trial outcomes: breast cancer, mortality, other cancers, cardiovascular events, diabetes Data available on total mortality? yes* Secondary outcomes: cancer deaths*, cancer diagnoses*, stroke, non‐fatal MI, diabetes diagnosis* Tertiary outcomes: weight, BMI, total, LDL and HDL cholesterol, TGs, systolic and diastolic BP (Lp(a) and HOMA reported as geometric means) * these are only available for the whole cohort, not split between low and high CVD risk groups | |
| Notes | Study duration over 8 years Study aim was to reduce total fat to 20%E, reduce saturated fat to 7%E and increase fruit and vegetable intake (Patterson 2003), and saturated fat intake in the intervention group was significantly lower than in control SFA reduction aimed and achieved Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.09 (95% CI ‐0.15 to ‐0.02), statistically significant reduction §Amongst the 881 intervention and 1373 control participants with blood samples at baseline, with or without CVD at baseline (Howard 2010). Trial dates: Recruitment was between 1993 and 1998 Funding: National Heart, Lung and Blood Institute of the National Institutes of Health Declarations of Interest of primary researchers: Declarations vary from paper to paper, but this is a typical one from Beresford 2006 "Dr Black has received research grants from Pfizer and AstraZeneca, was on the speakers bureaus for Pfizer, Novartis, Sanofi‐Aventis, Bristol‐Meyers Squibb, Searle, Pharmacia, and Boehringer and served as a consultant of on an advisory board for Myogen, Merck Sharp and Dohme, Novartis, Mylan‐Bertek, Pfizer, Bristol‐Meyers Squibb, and Sanofi‐Aventis. Dr Howard has served on the advisory boards of Merck, Schering Plough, and the Egg Nutrition Council, has received research support from Merck and Pfizer, and has consulted for General Millls. Dr Assaf is an employee of Pfizer. No other disclosures were reported." | |
| Methods | RCT | |
| Participants | Women with localised resected breast cancer (USA) Control: 1462 randomised, 1462 analysed Intervention: 975 randomised, 975 analysed Mean years in trial: overall 5.0 Ethnicity: 85% white, 5% black, 4% Hispanic, 5% Asian or pacific islander, <1% American Indian or unknown (no outcome data based on ethnicity) Statins use allowed? Not stated (statins not mentioned in inclusion or exclusion criteria within trial protocol) % taking statins: Not reported | |
| Interventions | Reduced fat intake vs usual diet Control aims: minimal nutritional counselling focused on nutritional adequacy Control methods: 1 baseline dietetic session plus 3‐monthly sessions Intervention methods: 8 bi‐weekly individual dietetic sessions plus 3‐monthly contact and optional monthly group sessions, incorporating individual fat gram goals, social cognitive theory, self monitoring, goal setting, modelling, social support and relapse prevention and management Intervention was delivered face‐to‐face individually by trained dietitian Total fat intake, %E (at 1 year): int 20.3 (SD 8.1), cont 29.2 (SD 7.4) (mean difference ‐8.90, 95% CI ‐9.53 to ‐8.27) Total fat %E (at 5 years): int 23.2 (SD 8.4) n = 380, cont 31.2 (SD 8.9) n = 648 (mean difference ‐8.00, 95% CI ‐9.09 to ‐6.91) significant reduction Saturated fat intake*, %E (at 1 year): int 6.4 (SD 0.14 [4.4]), cont 9.8 (SD 0.15 [5.7]) (mean difference ‐3.40, 95% CI ‐3.80 to ‐3.00 assuming reported SDs were actually SEs) significant reduction PUFA intake*, %E (at 1 year): int 4.5 (SD 0.09 (2.8)), cont 6.4 (SD 0.10 (3.8)) (mean difference ‐1.90, 95% CI ‐2.16 to ‐1.64) significant reduction PUFA n‐3 intake: not reported by study arm PUFA n‐6 intake: not reported by study arm MUFA intake*, %E (at 1 year): int 7.6 (SD 0.14 (4.4)), cont 11.5 (SD 0.16 (6.1)) (mean difference ‐3.90, 95% CI ‐4.32 to ‐3.48) significant reduction CHO intake, %E (at 6 months): int 60.8 (SD 19.6), cont 50.5 (SD 14.8) (mean difference 10.30, 95% CI 8.85 to 11.75) significant increase Protein intake, %E (at 6 months): int 19.1 (SD 5.2), cont 17.6 (SD 4.1) (mean difference 1.50, 95% CI 1.11 to 1.89) significant increase Trans fat intake: not reported Replacement for saturated fat: CHO and protein (based on dietary achievement) Style: dietary advice Setting: community | |
| Outcomes | Stated trial outcomes: dietary fat intake, total cholesterol, weight and waist Data available on total mortality? yes Secondary outcomes: cancer diagnoses Tertiary outcomes: weight, BMI, total cholesterol | |
| Notes | Study duration 5 years Study aim was to reduce total fat to 15 ‐ 20%E, but saturated fat intake in the intervention group was significantly lower than in control SFA reduction achieved Total serum cholesterol, difference between intervention and control, mmol/L: ‐0.14 (95% CI ‐0.34 to 0.05), NO statistically significant reduction and reduction < 0.20 *SDs appear incorrect, probably SEs? Trial dates: Recruitment 1994 to 2001 Funding: National Cancer Institute, Breast Cancer Research Foundation, American Institute for Cancer Research Declarations of Interest of primary researchers: none stated, all authors worked for academic or health institutions except that Njeri Karanja worked for Kaiser Permanente Center for Health Research, Bette Caan for Kaiser Permanente Medical Group, and Barbara L Winters for Campbell's Soup Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random stratified permuted block design, carried out at the statistical co‐ordinating centre of WINS |
| Allocation concealment (selection bias) | Low risk | Random stratified permuted block design, carried out at the statistical co‐ordinating centre of WINS |
| Blinding (performance bias and detection bias) | High risk | Participants not blinded, not relevant for assessment of mortality by researchers |
| Incomplete outcome data (attrition bias) | Low risk | All assessed. |
| Selective reporting (reporting bias) | Low risk | Not relevant for primary and secondary outcomes as all trialists were asked for data |
| Free of systematic difference in care? | High risk | Differences in attention ‐ more time for those in intervention group. See Control and Intervention Methods in Interventions section of the Table of Characteristics of Included Studies |
| Stated aim to reduce SFA | High risk | Aim to reduce SFA not stated |
| Achieved SFA reduction | Low risk | SFA reduction achieved |
| Achieved TC reduction | High risk | No statistically significant TC fall |
| Other bias | Low risk | None noted |
%E = percent of total energy intake
ATPII ‐ Adult treatment panel II
CABG = coronary artery bypass graft
CHD = coronary heart disease
CHO = carbohydrates
chol = cholesterol
CI = confidence interval
CVD = cardiovascular disease
dBP = diastolic blood pressure
DVT = deep vein thrombosis
HOMA = homeostatic model assessment
Lp(a) = lipoprotein
MI = myocardial infarction
MUFA = monounsaturated fats
P/S = polyunsaturated/saturated fat ratio
PCTA = percutaneous transluminal coronary angioplasty
PUFA = polyunsaturated fats
PVD = peripheral vascular disease
RCT = randomised controlled trial
sBP = systolic blood pressure
SD = standard deviation
SE = standard error
SFA = saturated fats
TC = total cholesterol
TG = triglyceride
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Follow‐up less than 24 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Follow‐up less than 24 months | |
| Multifactorial intervention | |
| Study aim was to assess effects of a low‐fat diet and methods state that the "nature of the fat consumed was not altered". Saturated fat content of diet was not reported. | |
| Weight reduction encouraged in the conventional diet, but not in the vegan diet arm | |
| No appropriate control group (and not low fat vs modified fat) | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Complex paper in Italian, unclear whether cardiovascular events occurred, contact with authors not established | |
| Intervention and randomised follow‐up less than 6 months | |
| Study aim was to reduce total fat intake to 15%E with no specific intervention on saturated fat. Saturated fat in intervention group was more than 80% of that in the control group. | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Multifactorial intervention | |
| Trial, unclear if randomised, contact could not be established with trialists | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Follow‐up less than 24 months | |
| Unclear whether any relevant events occurred, not able to contact trialists | |
| No appropriate control group (and not low fat vs modified fat) | |
| Follow‐up less than 24 months | |
| Intervention is not dietary fat modification or low fat diet | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Unable to establish contact with authors to provide data on numbers of deaths and CV events | |
| Follow‐up less than 24 months | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Intervention is not dietary fat modification or low fat diet | |
| Follow‐up less than 24 months | |
| Intervention is not dietary fat modification or low‐fat diet | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Neither mortality nor cardiovascular morbidity data available as study data have been lost | |
| Multifactorial intervention | |
| Duration 1 year only | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention is not dietary fat modification or low fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Duration 1 year only | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Unable to establish contact with authors to establish whether relevant events occurred | |
| Intervention aim was for a "mediterranean diet" with total fat 27 ‐ 30%E, protein 15 ‐ 18%E, CHO 50 ‐ 55%E, no specific aim to reduce saturated fat (though polyunsaturated margarine given to intervention group), and intervention group saturated fat was more than 80% of that in the control. | |
| No appropriate control group (and not low fat vs modified fat) | |
| Follow‐up less than 24 months | |
| Multifactorial intervention | |
| Study authors confirmed that no deaths or cardiovascular events occurred during the study. | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Not randomised (cluster‐randomised, but < 6 clusters) | |
| Intervention and randomised follow‐up less than 6 months | |
| Authors confirmed that differences between intervention and control groups included smoking and physical activity, as well as dietary changes | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention is not dietary fat modification or low fat diet | |
| Weight reduction in 1 arm but not the other | |
| Follow‐up less than 24 months | |
| Participants were newly diagnosed with cancer | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Aim was to reduce total fat or reduce carbohydrate, but no saturated fat aims were stated, and effects of the diets on saturated fat intakes were unclear. | |
| Intervention is not dietary fat modification or low fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| The study aimed for weight loss in 1 arm and not in the comparison arm | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Not randomised, Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Unclear whether randomised, unable to contact authors to discuss | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| Follow‐up less than 24 months | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Unable to establish contact with authors to gain access to data on health outcomes (none reported in paper) | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low‐fat diet | |
| No appropriate control group (and not low fat vs modified fat) | |
| Both intervention groups aimed to lose weight, while the control group did not. | |
| Intervention and randomised follow‐up less than 6 months | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Intervention aim was to reduce total fat and increase dietary fibre (saturated fat not mentioned), and no saturated fat intakes during trial reported | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| Weight reduction for some low‐fat diet participants (those with BMI > 25) but not in Mediterranean group | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| Diet plus stress management vs no intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| No appropriate control group (and not low fat vs modified fat) ‐ the high CHO diet is neither 'usual' or 'low fat' to compare with the modified fat diet | |
| Although the study proceeded for over 4 years participants (patients) came and went and mean follow‐up was only 1 year | |
| No appropriate control group (and not low fat vs modified fat) | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Follow‐up less than 24 months | |
| Diet and breast self examination vs no intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| Intervention and randomised follow‐up less than 6 months | |
| Follow‐up less than 24 months | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Multifactorial intervention | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| The study aimed for weight loss in 1 arm and not in the other arm | |
| Multifactorial intervention | |
| Follow‐up less than 24 months | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention aim was to reduce total fat and increase dietary fibre, fruit and vegetables (saturated fat not mentioned), and no saturated fat intakes during trial reported. | |
| All study arms (low or high total fat) prescribed low saturated fat intake (8%E), no usual fat comparator | |
| Total fat goals in the low‐fat arm were unclear and authors confirmed that aims were non‐specific (if aims < 30%E this study would be included) | |
| Follow‐up less than 24 months | |
| The study aimed for weight loss in 1 arm and not in the comparison arm | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Not randomised | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Follow‐up less than 24 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Follow‐up less than 24 months | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| The study aimed for weight loss in 1 arm and not in the comparison arm | |
| Intervention and randomised follow‐up less than 6 months | |
| The study aimed for weight loss in 1 arm and not in the comparison arm | |
| Intervention and randomised follow‐up less than 6 months | |
| Multifactorial intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Diet plus breast self examination vs no intervention | |
| No appropriate control group (and not low fat vs modified fat) | |
| Follow‐up less than 24 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Follow‐up less than 24 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| All study arms prescribed low saturated fat intake, no usual fat comparator | |
| Multifactorial intervention | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention and randomised follow‐up less than 6 months | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Multifactorial intervention | |
| Longest duration only 12 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months | |
| Unable to establish contact with authors to assess whether any relevant events occurred | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low fat diet | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| No appropriate control group (and not low fat vs modified fat) | |
| Intervention is not dietary fat modification or low fat diet | |
| Intervention and randomised follow‐up less than 6 months | |
| Study aimed to reduce total fat, but saturated fat goals were not mentioned, and saturated fat intake in the intervention group was more than 80% of that in the control (81%) | |
| Multifactorial intervention | |
| Neither mortality nor cardiovascular morbidity data available as such data were not collected in the study | |
| Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least 1 author) | |
| Intervention and randomised follow‐up less than 6 months | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Intervention is not dietary fat modification or low‐fat diet | |
| No appropriate control group (and not low fat vs modified fat) | |
| No appropriate control group (and not low fat vs modified fat) | |
| Stated aim was to reduce total fat by 50%, no saturated fat aims | |
| Lifestyle intervention includes exercise and weight as well as diet | |
| Intervention is not dietary fat modification or low‐fat diet | |
| Multifactorial intervention including smoking, weight, exercise and alcohol components | |
| Multifactorial intervention | |
| Intervention and randomised follow‐up less than 6 months |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 12 | 55858 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| Analysis 1.1 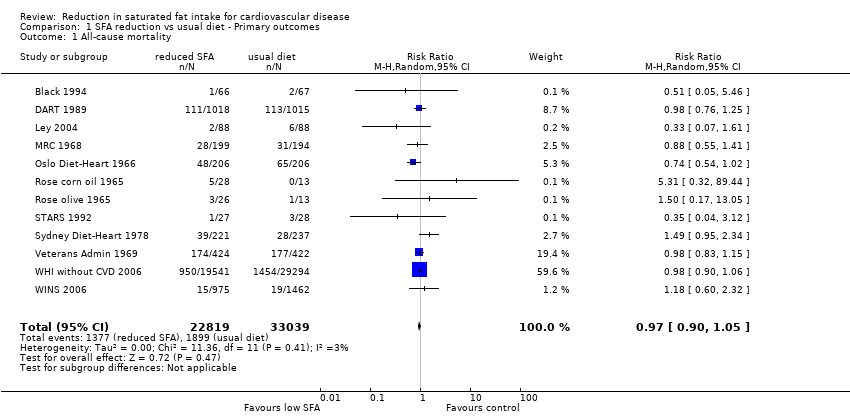 Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 1 All‐cause mortality. | ||||
| 2 Cardiovascular mortality Show forest plot | 12 | 53421 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.12] |
| Analysis 1.2  Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 2 Cardiovascular mortality. | ||||
| 3 Combined cardiovascular events Show forest plot | 13 | 53300 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| Analysis 1.3 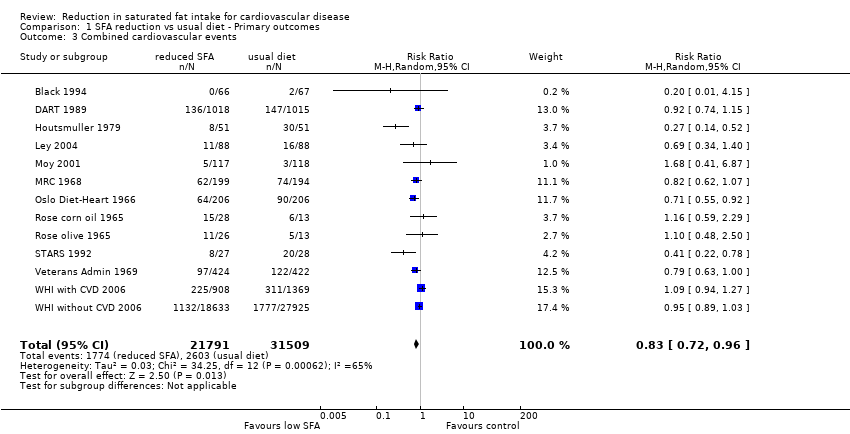 Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 3 Combined cardiovascular events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarctions Show forest plot | 11 | 53167 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| Analysis 2.1  Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 1 Myocardial infarctions. | ||||
| 2 Non‐fatal MI Show forest plot | 9 | 52834 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.13] |
| Analysis 2.2 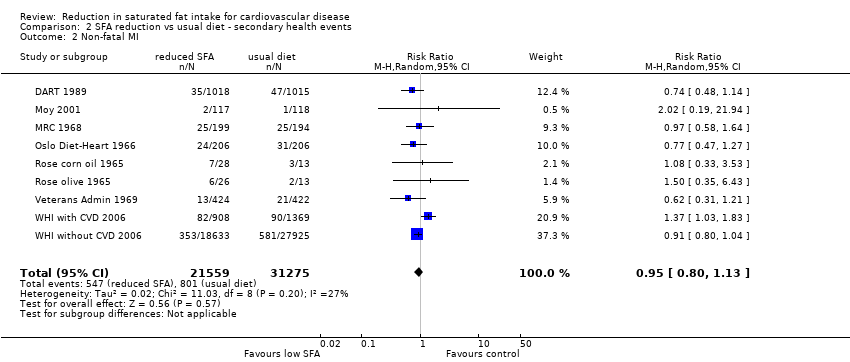 Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 2 Non‐fatal MI. | ||||
| 3 Stroke Show forest plot | 8 | 50952 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| Analysis 2.3  Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 3 Stroke. | ||||
| 4 CHD mortality Show forest plot | 10 | 53159 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.15] |
| Analysis 2.4 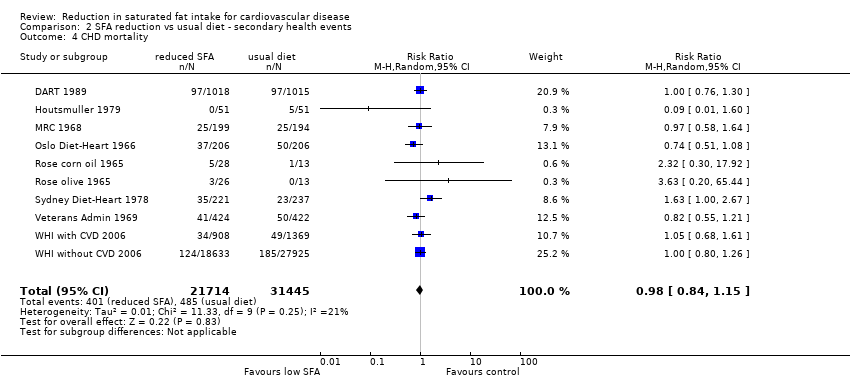 Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 4 CHD mortality. | ||||
| 5 CHD events Show forest plot | 12 | 53199 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.74, 1.03] |
| Analysis 2.5  Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 5 CHD events. | ||||
| 6 Diabetes diagnoses Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6 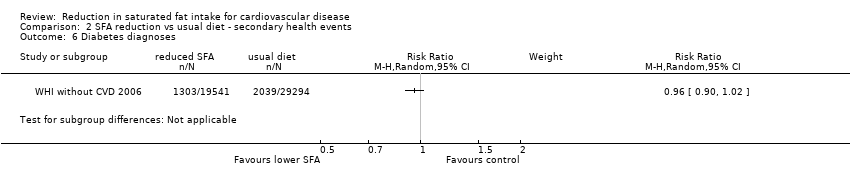 Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 6 Diabetes diagnoses. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol, mmol/L Show forest plot | 14 | 7115 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.36, ‐0.13] |
| Analysis 3.1  Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 1 Total cholesterol, mmol/L. | ||||
| 2 LDL cholesterol, mmol/L Show forest plot | 5 | 3291 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| Analysis 3.2 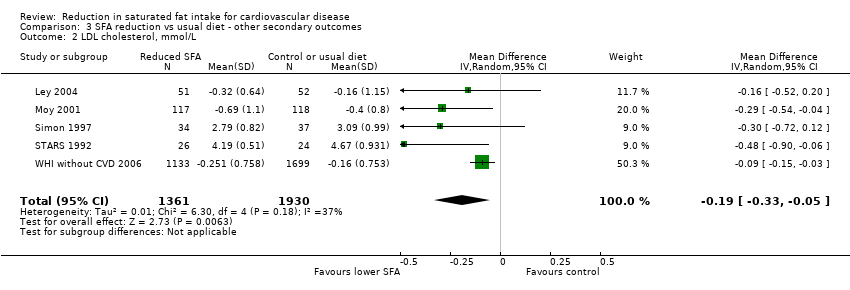 Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 2 LDL cholesterol, mmol/L. | ||||
| 3 HDL cholesterol, mmol/L Show forest plot | 6 | 5147 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| Analysis 3.3 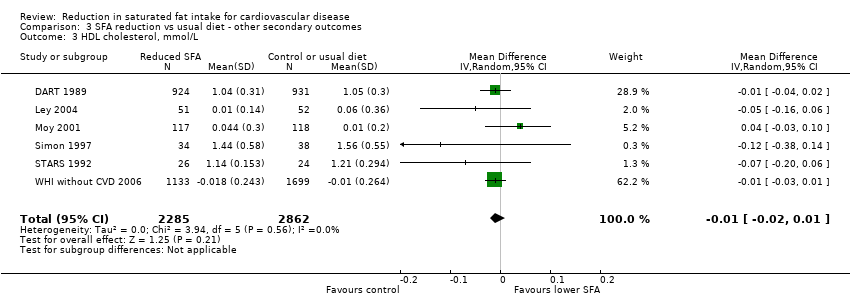 Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 3 HDL cholesterol, mmol/L. | ||||
| 4 Triglycerides, mmol/L Show forest plot | 7 | 3845 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.21, 0.04] |
| Analysis 3.4  Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 4 Triglycerides, mmol/L. | ||||
| 5 total cholesterol /HDL ratio Show forest plot | 3 | 2985 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| Analysis 3.5  Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 5 total cholesterol /HDL ratio. | ||||
| 6 LDL /HDL ratio Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 6 LDL /HDL ratio. | ||||
| 7 Lp(a), mmol/L Show forest plot | 2 | 2882 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| Analysis 3.7  Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 7 Lp(a), mmol/L. | ||||
| 8 Insulin sensitivity Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.8 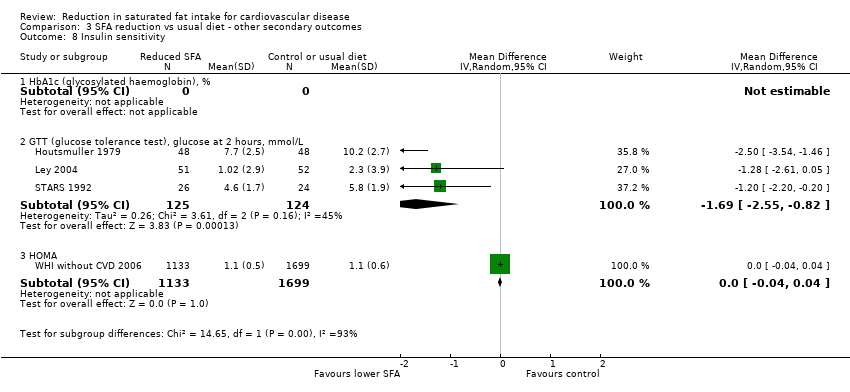 Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 8 Insulin sensitivity. | ||||
| 8.1 HbA1c (glycosylated haemoglobin), % | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 GTT (glucose tolerance test), glucose at 2 hours, mmol/L | 3 | 249 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.55, ‐0.82] |
| 8.3 HOMA | 1 | 2832 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Please note that while Rose 1965 (Rose corn oil 1965; Rose olive 1965) and WHI 2006 (WHI with CVD 2006; WHI without CVD 2006) each appear twice in this summary, they are each a single trial. Rose 1965 was a 3‐arm trial and we have used the two intervention arms separately in the review, while WHI 2006 provided some data separately for people with or without CVD at baseline.

Forest plot of comparison: 1 SFA reduction vs usual diet ‐ health events, outcome: 1.1 All‐cause mortality.
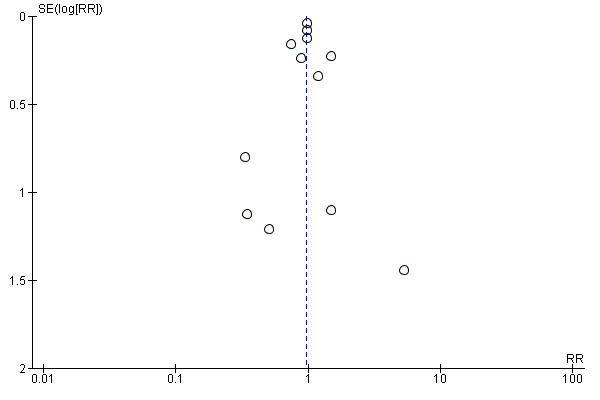
Funnel plot of comparison: fat modification or reduction vs usual diet ‐ total mortality.
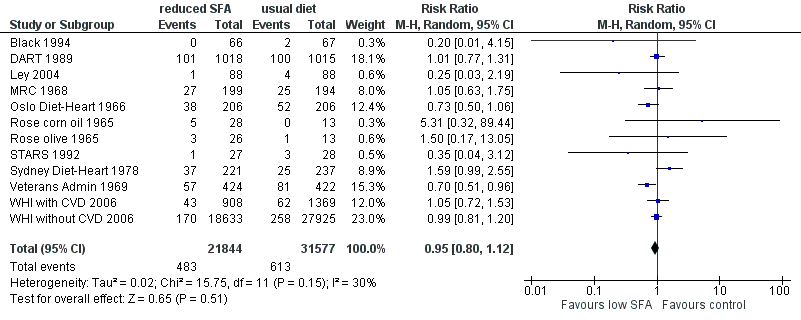
Forest plot of comparison: 1 SFA reduction vs usual diet ‐ Primary outcomes, outcome: 1.2 Cardiovascular mortality.

Forest plot of comparison: 1 SFA reduction vs usual diet ‐ Primary outcomes, outcome: 1.3 Combined cardiovascular events.

Funnel plot of comparison: fat modification or reduction vs usual diet ‐ combined cardiovascular events.

Exploring saturated fat cut‐offs.

Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 1 All‐cause mortality.

Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 2 Cardiovascular mortality.

Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 3 Combined cardiovascular events.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 1 Myocardial infarctions.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 2 Non‐fatal MI.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 3 Stroke.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 4 CHD mortality.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 5 CHD events.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 6 Diabetes diagnoses.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 1 Total cholesterol, mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 2 LDL cholesterol, mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 3 HDL cholesterol, mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 4 Triglycerides, mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 5 total cholesterol /HDL ratio.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 6 LDL /HDL ratio.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 7 Lp(a), mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 8 Insulin sensitivity.
| Low saturated fat compared with usual saturated fat for CVD risk | |||||
| Patient or population: people at any baseline risk of CVD Intervention: reduction of saturated fat intake Comparison: usual saturated fat intake | |||||
| Outcomes | Relative effect | Absolute effects (per 10,000) | No of Participants | Quality of the evidence | Comments |
| All‐cause mortality follow‐up mean duration 56 months1 | RR 0.97 (0.90 to 1.05) | 17 fewer (from 57 fewer to 29 more) | 55,858 | ⊕⊕⊕⊝ | Critical importance |
| Cardiovascular mortality follow‐up mean duration 53 months1 | RR 0.95 (0.80 to 1.12) | 10 fewer | 53,421 | ⊕⊕⊕⊝ | Critical importance |
| Combined cardiovascular events follow‐up mean duration 52 months1 | RR 0.83 (0.72 to 0.96) | 138 fewer (from 33 fewer to 228 fewer) | 53,300 | ⊕⊕⊕⊝ | Critical importance |
| Myocardial infarctions follow‐up mean duration 55 months | RR 0.90 (0.80 to 1.01) | 32 fewer (from 63 fewer to 3 more) | 53,167 | ⊕⊕⊕⊝ | Critical importance |
| Non‐fatal MI follow‐up mean duration 55 months1 | RR 0.95 (0.80 to 1.13) | 13 fewer (from 51 fewer to 33 more) | 52,834 | ⊕⊕⊕⊝ | Critical importance |
| Stroke follow‐up mean duration 59 months1 | RR 1.00 (0.89 to 1.12) | 0 fewer (from 25 fewer to 25 more) | 50,952 | ⊕⊕⊕⊝ | Critical importance |
| CHD mortality follow‐up mean duration 65 months1 | RR 0.98 (0.84 to 1.15) | 3 fewer (from 25 fewer to 23 more) | 53,159 | ⊕⊕⊕⊝ | Critical importance |
| CHD events follow‐up mean duration 59 months1 | RR 0.87 (0.74 to 1.03) | 80 fewer (from 160 fewer to 19 more) | 53,199 | ⊕⊕⊝⊝ | Critical importance |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Minimum study duration was 24 months 2These large RCTs of relatively long duration were well randomised but fewer than half had good allocation concealment (the rest were unclear). Blinding was only well‐conducted in 1 RCT, however blinding is very difficult in trials of dietary fat intake. Incomplete outcome data were variable, and most included studies had systematic differences in care (i.e. intervention group had more time or attention than the control group). We noted no other biases. We downgraded each study once for a combination of these issues around validity and issues around precision. The level of compliance with interventions involving long‐term behaviour change, such as those used in these studies, can vary widely. This is likely to attenuate the pooled effect and bias it towards the null. 3No important heterogeneity; I² ≤ 30% 4These RCTs directly assessed the effect of lower vs usual saturated fat intake on health outcomes of interest. Participants included men and women with and without CVD at baseline (also some participants with CVD risk factors like diabetes, or at risk of cancers). 5The 95% CI crosses 1.0 and does not exclude important benefit or harm. 6The funnel plot did not suggest any small‐study (publication) bias. 7Potentially important heterogeneity was identified; I² = 65%. However, the heterogeneity was partly explained by the degree of saturated fat reduction, and the degree of cholesterol lowering achieved (in subgrouping and in meta‐regression). For this reason we did not downgrade the study for inconsistency. 8The 95% CI does not cross 1.0 or a threshold of important harm. 9Too few studies to reliably assess small‐study bias (< 10 RCTs) 10Important heterogeneity; I² = 66%. | |||||
| Reference | Population | CVD risk category | Is intervention delivered to Individual or group? | intervention given by? | Face‐to‐face or other? | Number of visits | Is intervention advice only or other intervention? |
| People with non‐melanoma skin cancer | Low | Unclear | Dietitian | Face‐to‐face | 8 x weekly classes then monthly follow‐up sessions | Advice (behaviour techniques learning) | |
| Men recovering from a MI | High | Individual | Dietitian | Face‐to‐face | 9 | Advice (diet advice, recipes and encouragement) | |
| Adults with newly‐diagnosed diabetes | Moderate | Unclear | Dietitian | Unclear | Unclear | Advice? | |
| People with impaired glucose intolerance or high normal blood glucose | Moderate | Small group | Unclear | Face‐to‐face | Monthly meetings | Advice (education, personal goal setting, self‐monitoring) | |
| Middle‐aged siblings of people with early CHD, with at least 1 CVD risk factor | Moderate | Individual | Trained nurse | Face‐to‐face | 6 ‐ 8 weekly for 2 years | Advice (individualised counselling sessions) | |
| Free‐living men who have survived a 1st MI | High | Individual | Dietitian | Face‐to‐face | Unclear | Advice and supplement (soy oil) | |
| Men with previous MI | High | Individual | Dietitian | Face‐to‐face and other | Unclear | Advice and supplement (food) | |
| Newly‐diagnosed non‐insulin‐dependent diabetics | Moderate | Individual | Diabetes dietitian | Face‐to‐face | After 1 month then at 3‐month intervals | Advice | |
| Men (?) with angina or following MI | High | Unclear | Unclear | Unclear | Follow‐up clinic monthly, then every 2 months | Advice and supplement (oil) | |
| Men (?) with angina or following MI | High | Unclear | Unclear | Unclear | Follow‐up clinic monthly, then every 2 months | Advice and supplement (oil) | |
| Women with a high risk of breast cancer | Low | Individual followed by individual or group | Dietitian | Face‐to‐face | Bi‐weekly over 3 months followed by monthly | Advice (individualised eating plan and counselling sessions) | |
| Men with angina referred for angiography | High | individual | Dietitian | Face‐to‐face | Clinic visits at 3‐months intervals | Advice | |
| Men with angina referred for angiography | High | Individual | Unclear | Face‐to‐face | 3 times in 1st year and twice annually thereafter | Advice | |
| Men living at the Veterans Administration Center | Low | Individual | Unclear (whole diet provided) | N/A | N/A | Diet provided | |
| Post‐menopausal women aged 50 ‐ 79 with CVD at baseline | High | Group | Nutritionists | Face‐to‐face | 18 sessions/ 1st yr and quarterly maintenance sessions after | Advice | |
| Post‐menopausal women aged 50 ‐ 79 without CVD at baseline | Low | Group | Nutritionists | Face‐to‐face | 18 sessions/ 1st yr and quarterly maintenance sessions after | Advice | |
| Women with localised resected breast cancer | Low | Individual followed by group | Dietitian | Face‐to‐face | 8 bi‐weekly sessions, then 3‐monthly contact and optional monthly sessions | Advice | |
| N/A: not applicable | |||||||
| Participants | All‐cause mortality | CV mortality | CVD events | MI | Non‐fatal MI | Stroke | CHD mortality | CHD events | Diabetes Diagnoses | |
| 133 | 133 | 133 | 133 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2033 | 2033 | 2033 | 2033 | 2033 | 2033 | 0 | 2033 | 2033 | 0 | |
| 102 | 0 | 0 | 102 | 102 | 0 | 0 | 102 | 102 | 0 | |
| 176 | 176 | 176 | 176 | 176 | 0 | 176 | 0 | 176 | 0 | |
| 267 | 0 | 0 | 235 | 235 | 235 | 235 | 0 | 267 | 0 | |
| 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 0 | |
| 412 | 412 | 412 | 412 | 412 | 412 | 412 | 412 | 412 | 0 | |
| 249 (data not provided by arm) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 41 | 41 | 41 | 41 | 41 | 41 | 0 | 41 | 41 | 0 | |
| 39 | 39 | 39 | 39 | 39 | 39 | 0 | 39 | 39 | 0 | |
| 194 (data not provided by arm) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 60 | 55 | 55 | 55 | 55 | 0 | 55 | 0 | 55 | 0 | |
| 458 | 458 | 458 | 0 | 0 | 0 | 0 | 458 | 0 | 0 | |
| 846 | 846 | 846 | 846 | 846 | 846 | 846 | 846 | 846 | 0 | |
| 2277 | 0 | 2277 | 2277 | 0 | 2277 | 2277 | 2277 | 2277 | 0 | |
| 48835 | 48835 | 46558 | 46558 | 48835 | 46558 | 46558 | 46558 | 46558 | 48835 | |
| 2437 | 2437 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total Participants | 58509 | 55858 | 53421 | 53300 | 53167 | 52834 | 50952 | 53159 | 53204 | 48835 |
| Percent of participants for this outcome | 100% | 95% | 91% | 91% | 91% | 90% | 87% | 91% | 91% | 83% |
| These numbers are the numbers of participants in each study who were available for assessment of outcomes within meta‐analysis (not necessarily the number of participants randomised within the trial). | ||||||||||
| Participants | Total cholesterol | LDL cholesterol | HDL cholesterol | Triglycerides | TG/HDL ratio | Total cholesterol/HDL ratio | LDL/HDL ratio | LP (a) | Insulin sensitivity | |
| 133 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2033 | 1855 | 0 | 1855 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 102 | 96 | 0 | 0 | 96 | 0 | 0 | 0 | 0 | 96 | |
| 176 | 103 | 103 | 103 | 103 | 0 | 103 | 0 | 0 | 103 | |
| 267 | 0 | 235 | 235 | 235 | 0 | 0 | 0 | 0 | 0 | |
| 393 | 177 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 412 | 329 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 249 | 58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 41 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 39 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 194 | 72 | 71 | 72 | 71 | 0 | 0 | 0 | 0 | 0 | |
| 55 | 50 | 50 | 50 | 50 | 0 | 50 | 50 | 50 | 50 | |
| 458 | 458 | 0 | 0 | 458 | 0 | 0 | 0 | 0 | 0 | |
| 846 | 843 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2277 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 48835 | 2832 | 2832 | 2832 | 2832 | 0 | 2832 | 0 | 2832 | 2832 | |
| 2437 | 196 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total Participants | 58952 | 7115 | 3291 | 5147 | 3845 | 0 | 2985 | 50 | 2882 | 3081 |
| Percent of participants for this outcome | 100% | 12% | 6% | 9% | 7% | 0% | 5% | 0.1% | 5% | 5% |
| These numbers are the numbers of participants in each study who were available for assessment of outcomes within meta‐analysis (not necessarily the number of participants randomised within the trial). | ||||||||||
| Analysis | RR (95% CI) of all‐cause mortality | I² | No. of events | No. of participants | |
| Main analysis | 0.97 (0.90 to 1.05) | 3% | 3276 | > 55,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.97 (0.89 to 1.06) | 11% | 3231 | > 53,000 |
| SFA significantly reduced | 0.99 (0.92 to 1.06) | 0% | 3095 | > 54,000 | |
| TC significantly reduced | 0.96 (0.83 to 1.11) | 33% | 2871 | > 52,000 | |
| Minus WHI | 0.96 (0.84 to 1.11) | 12% | 872 | > 7000 | |
| Mantel‐Haenszel fixed‐effect | 0.98 (0.91 to 1.04) | 3% | 3276 | > 55,000 | |
| Peto fixed‐effect | 0.97 (0.90 to 1.05) | 16% | 3276 | > 55,000 | |
| SFA: saturated fatty acids | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of all‐cause mortality | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.79 | PUFA replacement | 0.96 (0.82 to 1.13) | 26% | 824 | > 4000 |
| MUFA replacement | 3.00 (0.33 to 26.99) | N/A | 4 | 52 | |
| CHO replacement | 0.98 (0.91 to 1.05) | 0% | 2677 | > 53,000 | |
| Protein replacement | 0.98 (0.91 to 1.06) | 0% | 2673 | > 53,000 | |
| Subgroup by duration, P = 0.60 | Up to 24 months | 0.99 (0.78 to 1.26) | 0% | 236 | > 2000 |
| > 24 to 48 months | 0.96 (0.83 to 1.12) | 0% | 414 | > 1000 | |
| > 48 months | 1.00 (0.79 to 1.27) | 55% | 2618 | > 52,000 | |
| Unclear duration | 0.33 (0.07 to 1.61) | N/A | 8 | > 100 | |
| Subgroup by baseline SFA, P = 0.48 | Up to 12%E SFA | 1.18 (0.60 to 2.32) | N/A | 34 | > 2400 |
| > 12 to 15%E SFA | 1.01 (0.86 to 1.19) | 26% | 2706 | > 51,000 | |
| > 15 to 18%E SFA | 0.35 (0.04 to 3.12) | N/A | 4 | 55 | |
| > 18%E SFA | 0.98 (0.83 to 1.15) | N/A | 351 | 846 | |
| Subgroup by SFA change, P = 0.31 | Up to 4%E SFA difference | 1.02 (0.88 to 1.19) | 26% | 2737 | > 53,000 |
| > 4 to 8%E SFA difference | 0.41 (0.08 to 2.07) | 0% | 7 | > 100 | |
| > 8%E SFA difference | 0.98 (0.83 to 1.15) | N/A | 351 | > 800 | |
| Subgroup by sex, P = 0.40 | Men | 0.96 (0.83 to 1.11) | 13% | 830 | > 4000 |
| Women | 0.98 (0.91 to 1.06) | 0% | 2438 | > 51,000 | |
| Mixed, men and women | 0.33 (0.07 to 1.61) | N/A | 8 | 176 | |
| Subgroup by CVD risk, P = 0.40 | Low CVD risk | 0.98 (0.91 to 1.05) | 0% | 2792 | > 52,000 |
| Moderate CVD risk | 0.33 (0.07 to 1.61) | N/A | 8 | 176 | |
| Existing CVD | 0.97 (0.90 to 1.05) | 33% | 476 | > 3000 | |
| Subgroup by serum TC reduction, P = 0.85 | TC reduced by ≥ 0.2 mmol/L | 0.96 (0.81 to 1.14) | 32% | 823 | > 4000 |
| TC reduced by < 0.2 mmol/L | 0.98 (0.91 to 1.06) | 0% | 2450 | > 51,000 | |
| Unclear TC change | 0.51 (0.05 to 5.46) | N/A | 3 | > 100 | |
| Subgroup by decade of publication, P = 0.28 | 1960s | 0.92 (0.80 to 1.07) | 2% | 532 | > 1700 |
| 1970s | 1.49 (0.95 to 2.34) | N/A | 67 | 458 | |
| 1980s | 0.98 (0.76 to 1.25) | N/A | 224 | 2033 | |
| 1990s | 0.41 (0.08 to 2.07) | 0% | 7 | 188 | |
| 2000s | 0.98 (0.83 to 1.15) | 5% | 2446 | > 51,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CVD mortality | I² | No. of events | No. of participants | |
| Main analysis | 0.95 (0.80 to 1.12) | 30% | 1096 | >53000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.96 (0.81 to 1.13) | 32% | 1089 | > 53,000 |
| SFA significantly reduced | 0.96 (0.79 to 1.18) | 42% | 945 | > 52,000 | |
| TC significantly reduced | 1.00 (0.86 to 1.16) | 19% | 942 | > 52,000 | |
| Minus WHI | 0.92 (0.72 to 1.18) | 40% | 563 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.95 (0.85 to 1.07) | 30% | 1096 | > 53,000 | |
| Peto fixed‐effect | 0.95 (0.84 to 1.08) | 41% | 1096 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CVD mortality | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.79 | PUFA replacement | 0.95 (0.73 to 1.25) | 55% | 553 | > 4000 |
| MUFA replacement | 3.00 (0.33 to 26.99) | N/A | 4 | 52 | |
| CHO replacement | 0.99 (0.86 to 1.14) | 0% | 745 | > 51,000 | |
| Protein replacement | 0.99 (0.86 to 1.14) | 0% | 741 | > 51,000 | |
| Subgroup by duration, P = 0.33 | Up to 24 months | 1.26 (0.54 to 2.94) | 26% | 213 | > 2000 |
| > 24 to 48 months | 0.79 (0.57 to 1.08) | 14% | 194 | > 1000 | |
| > 48 months | 1.01 (0.79 to 1.30) | 54% | 685 | > 49,000 | |
| Unclear duration | 0.25 (0.03 to 2.19) | N/A | 5 | > 100 | |
| Subgroup by baseline SFA, P = 0.13 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.04 (0.88 to 1.24) | 19% | 803 | > 51,000 | |
| > 15 to 18%E SFA | 0.35 (0.04 to 3.12) | N/A | 4 | 55 | |
| > 18%E SFA | 0.70 (0.51 to 0.96) | N/A | 138 | 846 | |
| Subgroup by SFA change, P = 0.08 | Up to 4%E SFA difference | 1.05 (0.89 to 1.24) | 21% | 801 | >51000 |
| > 4 to 8%E SFA difference | 0.29 (0.05 to 1.70) | 0% | 6 | > 100 | |
| > 8%E SFA difference | 0.70 (0.51 to 0.96) | N/A | 152 | > 900 | |
| Subgroup by sex, P = 0.45 | Men | 0.96 (0.73 to 1.25) | 48% | 559 | > 4000 |
| Women | 1.00 (0.84 to 1.19) | 0% | 533 | > 48,000 | |
| Mixed, men and women | 0.25 (0.03 to 2.19) | NA | 5 | 176 | |
| Subgroup by CVD risk, p=0.26 | Low CVD risk | 0.84 (0.60 to 1.16) | 54% | 568 | > 47,000 |
| Moderate CVD risk | 0.25 (0.03 to 2.19) | NA | 5 | 176 | |
| Existing CVD | 1.04 (0.83 to 1.31) | 33% | 524 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.57 | TC reduced by ≥ 0.2 mmol/L | 0.95 (0.73 to 1.25) | 55% | 553 | > 4000 |
| TC reduced by < 0.2 mmol/L | 1.00 (0.84 to 1.18) | 0% | 542 | > 49,000 | |
| Unclear TC change | 0.20 (0.01 to 4.15) | N/A | 2 | > 100 | |
| Subgroup by decade of publication, P = 0.04 | 1960s | 0.78 (0.63 to 0.97) | 2% | 289 | > 1700 |
| 1970s | 1.59 (0.99 to 2.55) | N/A | 62 | 458 | |
| 1980s | 1.01 (0.77 to 1.31) | N/A | 201 | > 2000 | |
| 1990s | 0.29 (0.05 to 1.70) | 0% | 6 | 188 | |
| 2000s | 0.99 (0.83 to 1.18) | 0% | 538 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CVD events | I² | No. of events | No. of participants | |
| Main analysis | 0.83 (0.72 to 0.96) | 65% | 4377 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.84 (0.72 to 0.97) | 69% | 4354 | > 52,000 |
| SFA significantly reduced | 0.91 (0.79 to 1.04) | 53% | 4012 | > 52,000 | |
| TC significantly reduced | 0.81 (0.68 to 0.98) | 77% | 4092 | > 52,000 | |
| Minus WHI | 0.75 (0.61 to 0.91) | 51% | 932 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.93 (0.88 to 0.98) | 65% | 4377 | > 53,000 | |
| Peto fixed‐effect | 0.92 (0.86 to 0.98) | 72% | 4377 | > 53,000 | |
| SFA: saturated fatty ac5d | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CVD events | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.14 | PUFA replacement | 0.73 (0.58 to 0.92) | 69% | 884 | > 3000 |
| MUFA replacement | 1.00 (0.53 to 1.89) | NA | 22 | 52 | |
| CHO replacement | 0.93 (0.79 to 1.08) | 57% | 3785 | > 51,000 | |
| Protein replacement | 0.98 (0.90 to 1.06) | 15% | 3757 | > 51,000 | |
| Subgroup by duration, P = 0.15 | Up to 24 months | 0.96 (0.78 to 1.16) | 0% | 330 | > 2000 |
| > 24 to 48 months | 0.73 (0.56 to 0.95) | 50% | 383 | > 1000 | |
| > 48 months | 0.93 (0.79 to 1.11) | 75% | 3599 | > 49,000 | |
| Unclear duration | 0.43 (0.17 to 1.08) | NA | 65 | > 200 | |
| Subgroup by baseline SFA, P = 0.13 | Up to 12%E SFA | NA | |||
| > 12 to 15%E SFA | 0.98 (0.91 to 1.05) | 6% | 3765 | > 51,000 | |
| > 15 to 18%E SFA | 0.41 (0.22 to 0.78) | NA | 28 | 55 | |
| > 18%E SFA | 0.79 (0.63 to 1.00) | NA | 219 | 846 | |
| Subgroup by SFA change, P = 0.005 | Up to 4%E SFA difference | 0.98 (0.91 to 1.05) | 6% | 3763 | > 51,000 |
| > 4 to 8%E SFA difference | 0.40 (0.22 to 0.74) | 0% | 30 | > 100 | |
| > 8%E SFA difference | 0.79 (0.63 to 1.00) | NA | 219 | > 800 | |
| Subgroup by sex, P = 0.05 | Men | 0.80 (0.69 to 0.93) | 24% | 859 | > 3000 |
| Women | 1.00 (0.88 to 1.14) | 60% | 3445 | > 48,000 | |
| Mixed, men and women | 0.59 (0.23 to 1.49) | 71% | 73 | > 500 | |
| Subgroup by CVD risk, P = 0.67 | Low CVD risk | 0.89 (0.75 to 1.06) | 40% | 3130 | > 47,000 |
| Moderate CVD risk | 0.59 (0.23 to 1.49) | 71% | 73 | > 500 | |
| Existing CVD | 0.86 (0.71 to 1.05) | 63% | 1174 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.03 | TC reduced by ≥ 0.2 mmol/L | 0.74 (0.59 to 0.92) | 63% | 887 | > 4000 |
| TC reduced by < 0.2 mmol/L | 0.99 (0.90 to 1.08) | 15% | 3488 | > 49,00 | |
| Unclear TC change | 0.20 (0.01 to 4.15) | NA | 2 | > 100 | |
| Subgroup by decade of publication, P , 0.0001 | 1960s | 0.79 (0.69 to 0.91) | 0% | 546 | > 1700 |
| 1970s | 0.27 (0.14 to 0.52) | NA | 38 | 102 | |
| 1980s | 0.92 (0.74 to 1.15) | NA | 283 | > 2000 | |
| 1990s | 0.40 (0.22 to 0.74) | 0% | 30 | 188 | |
| 2000s | 0.99 (0.89 to 1.11) | 25% | 3480 | > 49,000 | |
| CHO: carbohydrate | |||||
| Regression factor | No. of studies | Constant | Coefficient (95% CI) | P value | Proportion of between study variation explained |
| Change in SFA as %E | 8 | 0.01 | 0.05 (‐0.03 to 0.13) | 0.16 | 89% |
| Change in SFA as % of control | 8 | 0.26 | 0.01 (‐0.01 to 0.03) | 0.14 | 89% |
| Baseline SFA as %E | 8 | 0.68 | ‐0.06 (‐0.15 to 0.04) | 0.19 | 81% |
| Change in TC, mmol/L | 12 | 0.03 | 0.69 (0.05 to 1.33) | 0.04 | 99% |
| Change in PUFA as %E | 5 | ‐0.01 | ‐0.02 (‐0.08 to 0.03) | 0.25 | 100% |
| Change in MUFA as %E | 5 | ‐0.26 | ‐0.03 (‐0.14 to 0.09) | 0.50 | ‐87% |
| Change in CHO as %E | 7 | ‐0.11 | ‐0.00 (‐0.05 to 0.05) | 0.92 | ‐273% |
| Change in total fat intake as %E | 9 | ‐0.17 | ‐0.01 (‐0.03 to 0.01) | 0.28 | 100% |
| Gender* | 13 | ‐0.17 | ‐0.14 (‐0.63 to 0.35) | 0.55 | ‐13% |
| Study duration | 13 | ‐0.47 | 0.00 (‐0.01 to 0.02) | 0.76 | ‐24.8 |
| CVD risk at baseline** | 13 | ‐0.44 | 0.03 (‐0.48 to 0.55) | 0.89 | ‐39% |
| *0 = women, 1 = mixed, 2 = men | |||||
| Analysis | RR (95% CI) of any MI | I² | No. of events | No. of participants | |
| Main analysis | 0.90 (0.80 to 1.01) | 10% | 1714 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.89 (0.78 to 1.02) | 17% | 1707 | > 53,000 |
| SFA significantly reduced | 0.94 (0.85 to 1.04) | 0% | 1520 | > 52,000 | |
| TC significantly reduced | 0.89 (0.76 to 1.05) | 26% | 1561 | > 52,000 | |
| Minus WHI | 0.85 (0.73 to 0.98) | 1% | 608 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.92 (0.84 to 1.01) | 10% | 1714 | > 53,000 | |
| Peto fixed‐effect | 0.92 (0.83 to 1.01) | 31% | 1714 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of any MI | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.48 | PUFA replacement | 0.83 (0.67 to 1.02) | 29% | 591 | > 3000 |
| MUFA replacement | 1.40 (0.51 to 3.85) | N/A | 12 | 52 | |
| CHO replacement | 0.96 (0.86 to 1.06) | 0% | 1392 | > 51,000 | |
| Protein replacement | 0.96 (0.86 to 1.07) | 0% | 1389 | > 51,000 | |
| Subgroup by duration, P = 0.78 | Up to 24 months | 0.95 (0.77 to 1.17) | 0% | 300 | > 2000 |
| > 24 to 48 months | 0.83 (0.64 to 1.06) | 0% | 207 | > 1000 | |
| > 48 months | 0.81 (0.54 to 1.24) | 78% | 1194 | > 49,000 | |
| Unclear duration | 0.41 (0.02 to 7.73) | 71% | 13 | > 200 | |
| Subgroup by baseline SFA, P = 0.50 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 0.96 (0.87 to 1.07) | 0% | 1392 | > 51,000 | |
| > 15 to 18%E SFA | 0.52 (0.05 to 5.39) | N/A | 3 | 55 | |
| > 18%E SFA | 0.76 (0.55 to 1.05) | N/A | 125 | 846 | |
| Subgroup by SFA change, P = 0.50 | Up to 4%E SFA difference | 0.96 (0.87 to 1.07) | 0% | 1392 | > 51,000 |
| > 4 to 8%E SFA difference | 0.52 (0.05 to 5.39) | N/A | 3 | 55 | |
| > 8%E SFA difference | 0.76 (0.55 to 1.05) | N/A | 125 | > 800 | |
| Subgroup by sex, P = 0.35 | Men | 0.85 (0.73 to 0.98) | 0% | 592 | > 3000 |
| Women | 0.97 (0.86 to 1.09) | N/A | 1106 | > 48,000 | |
| Mixed, men and women | 0.75 (0.13 to 4.47) | 51% | 16 | > 500 | |
| Subgroup by CVD risk, P = 0.96 | Low CVD risk | 0.90 (0.72 to 1.13) | 49% | 1231 | > 49,000 |
| Moderate CVD risk | 0.75 (0.13 to 4.47) | 51% | 16 | > 500 | |
| Existing CVD | 0.87 (0.74 to 1.03) | 0% | 467 | > 2000 | |
| Subgroup by serum TC reduction, P = 0.12 | TC reduced by ≥ 0.2 mmol/L | 0.83 (0.70 to 0.98) | 9% | 592 | > 4000 |
| TC reduced by < 0.2 mmol/L | 0.98 (0.87 to 1.10) | 0% | 1122 | > 49,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P = 0.23 | 1960s | 0.80 (0.64, 1.00) | 10% | 313 | 1731 |
| 1970s | 0.08(0.0, 1.33) | N/A | 6 | 102 | |
| 1980s | 0.91 (0.73, 1.14) | N/A | 276 | 2033 | |
| 1990s | 0.52 (0.05, 5.39) | N/A | 3 | 55 | |
| 2000s | 0.98 (0.87, 1.10) | 0% | 1116 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of non‐fatal MI | I² | No. of events | No. of participants | |
| Main analysis | 0.95 (0.80 to 1.13) | 27% | 1348 | > 52,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.95 (0.80 to 1.13) | 27% | 1348 | > 52,000 |
| SFA significantly reduced | 0.91 (0.72 to 1.25) | 60% | 1225 | > 51,000 | |
| TC significantly reduced | 0.97 (0.79 to 1.19) | 45% | 1296 | > 51,000 | |
| Minus WHI | 0.81 (0.64 to 1.04) | 0% | 242 | > 3000 | |
| Mantel‐Haenszel fixed‐effect | 0.94 (0.85 to 1.05) | 27% | 1348 | > 52,000 | |
| Peto fixed‐effect | 0.94 (0.84 to 1.05) | 27% | 1348 | > 52,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of non‐fatal MI | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.62 | PUFA replacement | 0.80 (0.63 to 1.03) | 0% | 233 | > 3000 |
| MUFA replacement | 1.20 (0.42 to 3.45) | N/A | 11 | 52 | |
| CHO replacement | 0.99 (0.73 to 1.35) | 75% | 1188 | > 50,000 | |
| Protein replacement | 0.99 (0.73 to 1.35) | 75% | 1188 | > 50,000 | |
| Subgroup by duration, P = 0.64 | Up to 24 months | 0.83 (0.57 to 1.22) | 0% | 103 | > 2000 |
| > 24 to 48 months | 0.82 (0.53 to 1.27) | 10% | 84 | > 1000 | |
| > 48 months | 1.01 (0.74 to 1.38) | 73% | 1161 | > 49,000 | |
| Unclear duration | |||||
| Subgroup by baseline SFA, P = 0.43 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.00 (0.75 to 1.35) | 64% | 1191 | > 51,000 | |
| > 15 to 18%E SFA | |||||
| > 18%E SFA | 0.62 (0.31 to 1.21) | N/A | 34 | 846 | |
| Subgroup by SFA change, P = 0.43 | Up to 4%E SFA difference | 1.00 (0.75 to 1.35) | 64% | 1191 | > 51,000 |
| > 4 to 8%E SFA difference | |||||
| > 8%E SFA difference | 0.62 (0.31 to 1.21) | N/A | 34 | > 800 | |
| Subgroup by sex, P = 0.35 | Men | 0.81 (0.63 to 1.03) | 0% | 239 | > 3000 |
| Women | 1.10 (0.73 to 1.64) | 85% | 1106 | > 48,000 | |
| Mixed, men and women | 2.02 (0.19 to 21.94) | N/A | 3 | > 200 | |
| Subgroup by CVD risk, P = 0.61 | Low CVD risk | 0.87 (0.68 to 1.12) | 19% | 968 | > 47,000 |
| Moderate CVD risk | 2.02 (0.19 to 21.94) | N/A | 3 | > 200 | |
| Existing CVD | 1.00 (0.76 to 1.31) | N/A | 377 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.14 | TC reduced by ≥ 0.2 mmol/L | 0.80 (0.62 to 1.03) | 0% | 234 | > 3000 |
| TC reduced by < 0.2 mmol/L | 1.11 (0.77 to 1.60) | 71% | 1114 | > 48,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P = 0.34 | 1960s | 0.84 (0.62, 1.13) | 0% | 157 | 1743 |
| 1970s | |||||
| 1980s | 0.74 (0.48, 1.14) | NA | 82 | 2033 | |
| 1990s | |||||
| 2000s | 1.11 (0.76, 1.61) | 71% | 1109 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of stroke | I² | No. of events | No. of participants | |
| Main analysis | 1.00 (0.89 to 1.12) | 0% | 1125 | > 50,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 1.00 (0.90 to 1.12) | 0% | 1119 | > 50,000 |
| SFA significantly reduced | 0.99 (0.88 to 1.12) | 2% | 1120 | > 50,000 | |
| TC significantly reduced | 1.02 (0.91 to 1.14) | 0% | 1084 | > 49,000 | |
| Minus WHI | 0.63 (0.35 to 1.14) | 0% | 49 | > 2000 | |
| Mantel‐Haenszel fixed‐effect | 0.99 (0.89 to 1.11) | 0% | 1125 | > 50,000 | |
| Peto fixed‐effect | 0.99 (0.88 to 1.13) | 18% | 1125 | > 50,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of stroke | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.69 | PUFA replacement | 0.68 (0.37 to 1.27) | 0% | 41 | > 1700 |
| MUFA replacement | |||||
| CHO replacement | 1.01 (0.90 to 1.13) | 0% | 1083 | > 49,000 | |
| Protein replacement | 1.01 (0.89 to 1.15) | 11% | 1082 | > 49000 | |
| Subgroup by duration, P = 0.17 | Up to 24 months | 1.01 (0.06 to 15.93) | N/A | 2 | > 200 |
| > 24 to 48 months | 0.57 (0.30 to 1.11) | 0% | 36 | > 900 | |
| > 48 months | 1.02 (0.91 to 1.14) | 0% | 1079 | > 49,000 | |
| Unclear duration | 0.20 (0.02 to 1.68) | N/A | 6 | 196 | |
| Subgroup by baseline SFA, P = 0.36 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.01 (0.90 to 1.13) | 0% | 1084 | > 49,000 | |
| > 15 to 18%E SFA | 0.35 (0.01 to 8.12) | N/A | 1 | 55 | |
| > 18%E SFA | 0.59 (0.30 to 1.15) | N/A | 35 | 846 | |
| Subgroup by SFA change, P = 0.36 | Up to 4%E SFA difference | 1.01 (0.90 to 1.13) | 0% | 1084 | > 49,000 |
| > 4 to 8%E SFA difference | 0.35 (0.01 to 8.12) | N/A | 1 | 55 | |
| > 8%E SFA difference | 0.59 (0.30 to 1.15) | N/A | 35 | > 800 | |
| Subgroup by sex, P = 0.35 | Men | 0.63 (0.33 to 1.18) | 0% | 39 | > 1300 |
| Women | 1.02 (0.91 to 1.14) | 0% | 1076 | > 48,000 | |
| Mixed, men and women | 0.37 (0.07 to 1.97) | 0% | 8 | > 400 | |
| Subgroup by CVD risk, P = 0.42 | Low CVD risk | 0.86 (0.52 to 1.42) | 59% | 597 | > 47,000 |
| Moderate CVD risk | 0.37 (0.07 to 1.97) | 0% | 8 | > 400 | |
| Existing CVD | 1.01 (0.86 to 1.18) | 0% | 518 | > 2000 | |
| Subgroup by serum TC reduction, P = 0.24 | TC reduced by ≥ 0.2 mmol/L | 0.70 (0.38 to 1.28) | 0% | 43 | > 1900 |
| TC reduced by < 0.2 mmol/L | 1.01 (0.89 to 1.15) | 11% | 1082 | > 49,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P=0.79 | 1960s | 0.92 (0.31, 2.69) | 23% | 40 | 1651 |
| 1970s | |||||
| 1980s | |||||
| 1990s | 0.35 (0.01, 8.12) | N/A | 1 | 55 | |
| 2000s | 1.01 (0.90, 1.13) | 0% | 1084 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CHD mortality | I² | No. of events | No. of participants | |
| Main analysis | 0.98 (0.84 to 1.15) | 21% | 886 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.98 (0.84 to 1.15) | 21% | 886 | > 53,000 |
| SFA significantly reduced | 1.02 (0.87 to 1.20) | 17% | 735 | > 52,000 | |
| TC significantly reduced | 1.00 (0.83 to 1.20) | 33% | 786 | > 52,000 | |
| Minus WHI | 0.97 (0.76 to 1.24) | 37% | 494 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.98 (0.86 to 1.12) | 21% | 886 | > 53,000 | |
| Peto fixed‐effect | 0.98 (0.85 to 1.13) | 39% | 886 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CHD mortality | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.80 | PUFA replacement | 0.98 (0.74 to 1.28) | 49% | 491 | > 4000 |
| MUFA replacement | 3.00 (0.33 to 26.99) | N/A | 4 | 52 | |
| CHO replacement | 1.01 (0.86 to 1.18) | 0% | 586 | > 50,000 | |
| Protein replacement | 1.01 (0.86 to 1.18) | 0% | 586 | > 50,000 | |
| Subgroup by duration, P = 0.33 | Up to 24 months | 1.02 (0.78 to 1.33) | 0% | 203 | > 2000 |
| > 24 to 48 months | 0.87 (0.64 to 1.19) | N/A | 141 | > 1000 | |
| > 48 months | 1.03 (0.79 to 1.34) | 52% | 537 | > 49,000 | |
| Unclear duration | 0.09 (0.01 to 1.60) | N/A | 5 | > 100 | |
| Subgroup by baseline SFA, P = 0.35 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.06 (0.90 to 1.25) | 11% | 644 | > 51,000 | |
| > 15 to 18%E SFA | |||||
| > 18%E SFA | 0.82 (0.55 to 1.21) | N/A | 91 | > 800 | |
| Subgroup by SFA change, P = 0.35 | Up to 4%E SFA difference | 1.06 (0.90 to 1.25) | 11% | 644 | > 51,000 |
| > 4 to 8%E SFA difference | |||||
| > 8%E SFA difference | 0.82 (0.55 to 1.21) | N/A | 91 | > 800 | |
| Subgroup by sex, P = 0.26 | Men | 0.98 (0.79 to 1.23) | 30% | 489 | > 4000 |
| Women | 1.01 (0.83 to 1.24) | 0% | 392 | > 48,000 | |
| Mixed, men and women | 0.09 (0.01 to 1.60) | N/A | 5 | > 100 | |
| Subgroup by CVD risk, P = 0.23 | Low CVD risk | 0.95 (0.78 to 1.16) | 0% | 400 | > 47,000 |
| Moderate CVD risk | 0.09 (0.01 to 1.60) | N/A | 5 | > 100 | |
| Existing CVD | 1.03 (0.83 to 1.27) | 22% | 481 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.73 | TC reduced by ≥ 0.2 mmol/L | 0.96 (0.75 to 1.24) | 42% | 491 | > 4000 |
| TC reduced by < 0.2 mmol/L | 1.02 (0.83 to 1.25) | 0% | 395 | > 48,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P = 0.62 | 1960s | 0.84 (0.66, 1.06) | 23% | 40 | 1651 |
| 1970s | 0.54 (0.03, 9.26) | 75% | 63 | 560 | |
| 1980s | 1.00 (0.76, 1.30) | N/A | 194 | 2033 | |
| 1990s | |||||
| 2000s | 1.01 (0.83, 1.24) | 0% | 392 | > 48,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CHD events | I² | No. of events | No. of participants | |
| Main analysis | 0.87 (0.74 to 1.03) | 66% | 3307 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.87 (0.74 to 1.03) | 66% | 3307 | > 53,000 |
| SFA significantly reduced | 1.95 (0.82 to 1.10) | 49% | 2988 | > 52,000 | |
| TC significantly reduced | 0.85 (0.70 to 1.03) | 75% | 3034 | > 52,000 | |
| Minus WHI | 0.80 (0.61 to 1.03) | 59% | 758 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.93 (0.87 to 0.99) | 66% | 3307 | > 53,000 | |
| Peto fixed‐effect | 0.92 (0.86 to 0.99) | 72% | 3307 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CHD events | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.28 | PUFA replacement | 0.76 (0.57 to 1.00) | 71% | 737 | > 3000 |
| MUFA replacement | 1.50 (0.62 to 3.61) | N/A | 15 | 52 | |
| CHO replacement | 0.98 (0.83 to 1.14) | 55% | 2846 | > 51,000 | |
| Protein replacement | 0.99 (0.88 to 1.12) | 41% | 2833 | > 51,000 | |
| Subgroup by duration, P = 0.72 | Up to 24 months | 1.01 (0.76 to 1.35) | 5% | 307 | > 2000 |
| > 24 to 48 months | 0.79 (0.55 to 1.14) | 50% | 251 | > 1000 | |
| > 48 months | 0.93 (0.76 to 1.14) | 79% | 2703 | > 49,000 | |
| Unclear duration | 0.60 (0.10 to 3.58) | 81% | 46 | > 200 | |
| Subgroup by baseline SFA, P = 0.09 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 0.99 (0.88 to 1.12) | 34% | 2837 | > 51,000 | |
| > 15 to 18%E SFA | 0.30 (0.09 to 0.98) | N/A | 13 | 60 | |
| > 18%E SFA | 0.77 (0.56 to 1.04) | N/A | 138 | > 800 | |
| Subgroup by SFA change, P = 0.09 | Up to 4%E SFA difference | 0.99 (0.88 to 1.12) | 34% | 2837 | > 51,000 |
| >4 to 8%E SFA difference | 0.30 (0.09 to 0.98) | N/A | 13 | 60 | |
| >8%E SFA difference | 0.77 (0.56 to 1.04) | N/A | 138 | > 800 | |
| Subgroup by sex, P = 0.39 | Men | 0.84 (0.70 to 1.02) | 35% | 708 | > 3000 |
| Women | 1.02 (0.84 to 1.23) | 77% | 2549 | > 48,000 | |
| Mixed, men and women | 0.88 (0.18 to 4.36) | 76% | 50 | > 500 | |
| Subgroup by CVD risk, P = 0.95 | Low CVD risk | 0.90 (0.76 to 1.05) | 33% | 2236 | > 47,000 |
| Moderate CVD risk | 0.88 (0.18 to 4.36) | 76% | 50 | > 500 | |
| Existing CVD | 0.94 (0.75 to 1.17) | 61% | 1021 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.06 | TC reduced by ≥ 0.2 mmol/L | 0.75 (0.58 to 0.99) | 65% | 738 | > 4000 |
| TC reduced by < 0.2 mmol/L | 1.03 (0.87 to 1.21) | 44% | 2569 | > 49,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P < 0.001 | 1960s | 0.84 (0.68, 1.05) | 30% | 419 | 1731 |
| 1970s | 0.27 (0.14, 0.52) | N/A | 38 | 102 | |
| 1980s | 0.91 (0.73, 1.14) | N/A | 276 | 2033 | |
| 1990s | 0.33 (0.10, 1.09) | N/A | 13 | 57 | |
| 2000s | 1.03 (0.86, 1.23) | 48% | 2561 | > 49,000 | |
| CHO: carbohydrate | |||||
| Outcome | Effect (95% CI) | I² | No. of studies (participants) | Differential effect by replacement? | Summary |
| TC, mmol/L | ‐0.24 (‐0.36 to ‐0.13) | 60% | 13 (7115) | No, P = 0.20 | TC reduced |
| LDL, mmol/L | ‐0.19 (‐0.33 to ‐0.05) | 37% | 5 (3291) | No, P = 0.16 | LDL reduced |
| HDL, mmol/L | ‐0.01 (‐0.02 to 0.01) | 0% | 7 (5183) | No, P = 0.99 | No effect |
| TG, mmol/L | ‐0.08 (‐0.21 to 0.04) | 51% | 7 (3845) | No, P = 0.12 | No effect |
| TG/HDL ratio | N/A | N/A | N/A | N/A | No data |
| TC/HDL ratio | ‐0.10 (‐0.33 to 0.13) | 24% | 3 (2985) | No, P = 0.45 | No effect |
| LDL/HDL ratio | ‐0.36 (‐0.92 to 0.20) | N/A | 1 (50) | N/A | Unclear |
| Lipoprotein (a), mmol/L | 0.00 (‐0.00 to 0.00) | 0% | 2 (2882) | No, P = 1.00 | No effect |
| Diabetes diagnosis | RR 0.96 (0.90 to 1.02) | N/A | 1 (> 48,000; 3342 diagnoses) | No, P = 1.00 | No effect |
| HbA1c, % | N/A | N/A | N/A | N/A | No data |
| Glucose 2 hrs post GTT, mmol/L | ‐1.69 (‐2.55 to ‐0.82) | 45% | 3 (249) | N/A | Reduced |
| HOMA | 0.00 (‐0.04 to 0.04) | NA | 1 (2832) | N/A | No effect |
| GTT: glucose tolerance test | |||||
| Outcome | Effect (95% CI) | I² | No. of studies (participants) | Differential effect by replacement? | Summary |
| Cancer diagnoses | RR 0.94 (0.83 to 1.07) | 33% | 4 (> 52,000; 5476 diagnoses) | No, P = 0.33 | No effect |
| Cancer deaths | RR 1.00 (0.61 to 1.64) | 49% | 5 (> 52,000; 2472 deaths) | No, P = 0.94 | No effect |
| Body weight, kg | MD ‐1.97 (‐3.67 to ‐0.27) | 72% | 6 (4541) | No, P = 1.00 | Weight loss |
| BMI, kg/m² | MD ‐0.50 (‐0.82 to ‐0.19) | 55% | 6 (5553) | No, P = 0.41 | BMI reduced |
| Systolic BP, mmHg | MD ‐0.19 (‐1.36 to 0.97) | 0% | 5 (3812) | No, P = 0.97 | No effect |
| Diastolic BP, mmHg | MD ‐0.36 (‐1.03 to 0.32) | 0% | 5 (3812) | No, P = 1.00 | No effect |
| BMI: body mass index | |||||
| Cut‐ off | RR of all‐cause mortality | RR of CVD mortality | RR of CVD events | RR of MI | RR of non‐fatal MI | RR of stroke | RR of CHD mortality | RR of CHD events |
| 7%E | 1.11 (0.58 to 2.12) | 0.20 (0.01 to 4.15) | 0.20 (0.01 to 4.15) | N/A | N/A | N/A | N/A | N/A |
| 8%E | 1.11 (0.58 to 2.12) | 0.20 (0.01 to 4.15) | 0.20 (0.01 to 4.15) | N/A | N/A | N/A | N/A | N/A |
| 9%E | 0.99 (0.84 to 1.15) | 0.69 (0.51 to 0.94) | 0.79 (0.62 to 0.99) | 0.76 (0.55 to 1.05) | 0.62 (0.31 to 1.21) | 0.59 (0.30 to 1.15) | 0.82 (0.55 to 1.21) | 0.77 (0.56 to 1.04) |
| 10%E | 0.99 (0.90 to 1.09) | 0.97 (0.74 to 1.26) | 0.89 (0.74 to 1.07) | 0.93 (0.80 to 1.08) | 0.99 (0.69 to 1.41) | 1.00 (0.89 to 1.12) | 1.05 (0.83 to 1.32) | 0.93 (0.75 to 1.14) |
| 11%E | 1.00 (0.88 to 1.12) | 0.95 (0.73 to 1.24) | 0.88 (0.74 to 1.05) | 0.94 (0.84 to 1.06) | 0.99 (0.69 to 1.41) | 0.98 (0.83 to 1.14) | 1.02 (0.87 to 1.20) | 0.94 (0.77 to 1.15) |
| 12%E | 0.99 (0.91 to 1.07) | 0.96 (0.79 to 1.18) | 0.91 (0.79 to 1.04) | 0.94 (0.85 to 1.04) | 0.95 (0.72 to 1.25) | 0.99 (0.88 to 1.12) | 1.02 (0.87 to 1.20) | 0.95 (0.82 to 1.10) |
| 13%E | 1.02 (0.83 to 1.25) | 0.93 (0.63 to 1.38) | 0.78 (0.61 to 1.00) | 0.87 (0.73 to 1.04) | 0.72 (0.50 to 1.03) | 0.54 (0.29 to 1.00) | 1.06 (0.76 to 1.48) | 0.84 (0.63 to 1.12) |
| CHD coronary heart disease | ||||||||
| Quality assessment | No of participants (study event rate%) | Effect | Quality | Importance | ||||||||
| No of studies | Design 1 | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||
| All‐cause mortality (follow‐up mean 56 months1) | ||||||||||||
| 12 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1377/22819 (6%) | 1899/33039 (5.7%) | RR 0.97 (0.9 to 1.05) | 17 fewer (from 57 fewer to 29 more) | ♁♁♁O | CRITICAL |
| Cardiovascular mortality (follow‐up mean 53 months1) | ||||||||||||
| 12 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 483/21844 | 613/31577 | RR 0.95 | 10 fewer | ♁♁♁O MODERATE | CRITICAL |
| Cardiovascular events (follow‐up mean 52 months1) | ||||||||||||
| 13 | RCTs | no serious risk of bias2 | serious inconsistency7 | no serious indirectness4 | no serious imprecision8 | none6 | 1774/21791 | 2603/31509 | RR 0.83 | 138 fewer (from 33 fewer to 228 fewer) | ♁♁♁O MODERATE | CRITICAL |
| Fatal and non‐fatal myocardial infarction (follow‐up mean 55 months1) | ||||||||||||
| 11 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 717/21725 | 997/31442 | RR 0.90 | 32 fewer (from 63 fewer to 3 more) | ♁♁♁O MODERATE | CRITICAL |
| Non‐fatal myocardial infarction (follow‐up mean 55 months1) | ||||||||||||
| 9 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none9 | 547/21559 | 801/31275 | RR 0.95 | 13 fewer (from 51 fewer to 33 more) | ♁♁♁O | CRITICAL |
| Stroke (follow‐up 59 mean months1) | ||||||||||||
| 8 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none9 | 453/20602 | 672/30350 | RR 1.00 | 0 fewer (from 25 fewer to 25 more) | ♁♁♁O | CRITICAL |
| CHD mortality (follow‐up mean 65 months1) | ||||||||||||
| 10 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none 6 | 401/21714 | 485/31445 | RR 0.98 | 30 fewer (from 25 fewer to 23 more) | ♁♁♁O | CRITICAL |
| CHD events (follow‐up mean 59 months1) | ||||||||||||
| 12 | RCTs | no serious risk of bias2 | serious inconsistency10 | no serious indirectness4 | serious imprecision5 | none 6 | 1346/21743 | 1961/31456 | RR 0.87 | 80 fewer (from 160 fewer to 19 more) | ♁♁OO | CRITICAL |
| 1Minimum study duration was 24 months. 2These large RCTs of relatively long duration were well randomised and almost half had good allocation concealment (the rest were unclear). Blinding was only well‐conducted in 1 RCT, however blinding is very difficult in trials of dietary fat intake. Incomplete outcome data were variable, and most included studies had systematic differences in care (i.e. intervention group had more time or attention than the control group). These risks to validity were combined with risks from imprecision, and outcomes were downgraded once for a combination of both issues. We noted no other biases. We noted that the level of compliance with interventions involving long‐term behaviour change, such as those used in these studies, can vary widely. This is likely to attenuate the pooled effect and bias it towards the null. 3No important heterogeneity; I² ≤ 30% 4These RCTs directly assessed the effect of lower vs usual saturated fat intake on health outcomes of interest. Participants included men and women with and without CVD at baseline (also some participants with CVD risk factors like diabetes, or at risk of cancers). 5 The 95% CI crosses 1.0 and does not exclude important benefit or harm 6The funnel plot did not suggest any small study (publication) bias 7Potentially important heterogeneity was identified; I² = 65%. However, the heterogeneity was partly explained by the degree of saturated fat reduction, and the degree of cholesterol lowering achieved (in subgrouping and in meta‐regression). 8The 95% CI does not cross 1.0 or a threshold of important harm. 9Too few studies to reliably assess publication bias (< 10 RCTs). 10Important heterogeneity; I² = 66% | ||||||||||||
| Quality assessment | No of participants (study event rate %) | Effect | Quality | Importance | ||||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||||
| All‐cause mortality (follow‐up mean 56 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 406/2123 | 418/2115 | RR 0.96 (0.82 to 1.13) | 79 fewer (from 360 fewer to 256 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular mortality (follow‐up mean 55 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | serious inconsistency7 | no serious indirectness4 | serious imprecision5 | none6 | 266/2123 | 287/2128 | RR 0.95 (0.73 to 1.25) | 67 fewer (from 364 fewer to 337 more) | ♁♁OO | CRITICAL | ||
| Cardiovascular events (follow‐up mean 53 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | serious inconsistency8 | no serious indirectness4 | no serious imprecision9 | none6 | 390/1953 | 494/1942 | RR 0.73 (0.58 to 0.92) | 687 fewer (from 204 fewer to 1068 fewer) | ♁♁♁O | CRITICAL | ||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 53 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 269/1953 | 322/1942 | RR 0.83 (0.67 to 1.02) | 282 fewer (from 547 fewer to 33 more) | ♁♁♁O | CRITICAL | ||
| Non‐fatal myocardial infarction (follow‐up mean 53 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 104/1875 | 129/1863 | RR 0.8 (0.63 to 1.03) | 138 fewer (from 256 fewer to 21 more) | ♁♁♁O | CRITICAL | ||
| Stroke (follow‐up mean 63 months1) | ||||||||||||||
| 4 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | very serious10 | none6 | 17/856 | 24/850 | RR 0.68 (0.37 to 1.27) | 90 fewer (from 178 fewer to 76 more) | ♁OOO | CRITICAL | ||
| CHD mortality (follow‐up mean 36 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 240/2147 | 251/2151 | RR 0.98 (0.74 to 1.28) | 23 fewer (from 303 fewer to 327 more) | ♁♁♁O | CRITICAL | ||
| CHD events (follow‐up mean 53 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | serious inconsistency11 | no serious indirectness4 | serious imprecision5 | none6 | 329/1956 | 408/1944 | RR 0.76 (0.57 to 1.0) | 504 fewer (from 902 fewer to 0 more) | ♁♁OO | CRITICAL | ||
| * Polyunsaturated fatty acids replacing saturated fatty acids in individual studies were predominantly of plant origin. 1 Minimum study duration was 24 months. 2 These large RCTs of relatively long duration were well randomised but fewer than half had good allocation concealment (the rest were unclear). Blinding was only well‐conducted in 1 RCT, however blinding is very difficult in trials of dietary fat intake. In about half the included studies, it was unclear if outcome data were incomplete and most studies had systematic differences in care (i.e. intervention group had more time or attention than the control group). We noted no other biases. Not downgraded for bias, however we note that the level of compliance with interventions involving long‐term behaviour change, such as those used in many of these studies, can vary widely. This is likely to attenuate the pooled effect and bias it towards the null. 3 No important heterogeneity; I² < 50% 6 Too few studies to reliably assess publication bias (< 10 RCTs). 7 Important heterogeneity; I² = 55% 8 Important heterogeneity; I² = 69%.Subgrouping suggested greater effects on cardiovascular events with greater reduction in SFA intake, higher baseline SFA intake and greater serum total cholesterol reduction (meta‐regression not carried out). 9 95% CI does not cross threshold of important benefit or harm. | ||||||||||||||
| Quality assessment | No of participants (study event rate%) | Effect | Quality | Importance | |||||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | |||||
| All‐cause mortality (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 3/26 | 1/26 | RR 3.0 (0.33 to 26.99) | 769 more (from 258 fewer to 9996 more) | ♁OOO | CRITICAL | |||
| Cardiovascular mortality (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 3/26 | 1/26 | RR 3.0 (0.33 to 26.99) | 769 more (from 258 fewer to 9996 more) | ♁OOO | CRITICAL | |||
| Cardiovascular events (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 11/26 | 11/26 | RR 1.0 (0.53 to 1.89) | 0 fewer (from 1988 fewer to 3765 more) | ♁OOO | CRITICAL | |||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 7/26 | 5/26 | RR 1.4 (0.51 to 3.85) | 769 more (from 942 fewer to 5481 more) | ♁OOO | IMPORTANT | |||
| Non‐fatal myocardial infarction (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 6/26 | 5/26 | RR 1.2 (0.42 to 3.45) | 385 more (from 1115 fewer to 4711 more) | ♁OOO | IMPORTANT | |||
| Stroke | |||||||||||||||
| 0 | No studies identified reporting this outcome | ||||||||||||||
| CHD mortality (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 3/26 | 1/26 | RR 3 (0.33 to 26.99) | 769 more (from 258 fewer to 9996 more) | ♁OOO | IMPORTANT | |||
| CHD events (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 9/26 | 6/26 | RR 1.5 (0.62 to 3.61) | 1154 more (from 877 fewer to 6023 more) | ♁OOO | IMPORTANT | |||
| 1This single, very small RCT of relatively long duration was well randomised, but had an unclear risk of bias in terms of allocation concealment and incomplete outcome data, and lacked participant blinding; however blinding is very difficult in trials of dietary fat intake. We downgraded it for serious risk of bias. 3This RCT directly assessed the effect of reducing saturated fat, and replacing it with other dietary sources of energy, compared to usual diet, on health outcomes of interest. Participants included men with CVD at baseline. 4The 52 participants in the relevant arms of this trial experienced relatively few events. As a result, there were wide to very wide confidence intervals. In addition, the 95% CI crosses 1.0 and does not exclude important benefit or harm. 5Too few studies to reliably assess publication bias (< 10 RCTs). | |||||||||||||||
| Quality assessment | No of participants (study event rate %) | Effect | Quality | Importance | ||||||||||
| No of studies | Design1 | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||||
| All‐cause mortality (follow‐up mean 48 months2) | ||||||||||||||
| 6 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 1080/21715 | 1597/31954 | RR 0.98 (0.91 to 1.05) | 10 fewer (from 45 fewer to 25 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular mortality (follow‐up mean 46 months2) | ||||||||||||||
| 6 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 316/20740 | 429/30492 | RR 0.99 (0.86 to 1.14) | 1 fewer (from 20 fewer to 20 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular events (follow‐up mean 46 months2) | ||||||||||||||
| 6 | RCTs | no serious risk of bias3 | serious inconsistency9 | no serious indirectness5 | serious imprecision6 | none7 | 1512/20740 | 2273/30492 | RR 0.93 (0.79 to 1.08) | 52 fewer (from 157 fewer to 60 more) | ♁♁OO | CRITICAL | ||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 51 months2) | ||||||||||||||
| 4 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 572/20674 | 820/30425 | RR 0.96 (0.86 to 1.06) | 11 fewer (from 38 fewer to 16 more) | ♁♁♁O | IMPORTANT | ||
| Non‐fatal myocardial infarction (follow‐up mean 60 months2) | ||||||||||||||
| 3 | RCTs | no serious risk of bias3 | serious inconsistency9 | no serious indirectness5 | serious imprecision6 | none7 | 470/20559 | 718/30309 | RR 0.99 (0.73 to 1.35) | 2 fewer (from 64 fewer to 83 more) | ♁♁OO | IMPORTANT | ||
| Stroke (follow‐up mean 60 months2) | ||||||||||||||
| 4 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 435/19656 | 648/29410 | RR 1.01 (0.9 to 1.13) | 2 more (from 22 fewer to 29 more) | ♁♁♁O | IMPORTANT | ||
| CHD mortality (follow‐up mean 60 months2) | ||||||||||||||
| 3 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 255/20559 | 331/30309 | RR 1.01 (0.86 to 1.18) | 1 more (from 15 fewer to 20 more) | ♁♁♁O | IMPORTANT | ||
| CHD events (follow‐up mean 51 months2) | ||||||||||||||
| 5 | RCTs | no serious risk of bias3 | serious inconsistency8 | no serious indirectness5 | serious imprecision6 | none7 | 1140/20677 | 1706/30427 | RR 0.98 (0.83 to 1.14) | 11 fewer (from 95 fewer to 79 more) | ♁♁OO | IMPORTANT | ||
| 1There was insufficient information across all studies to make any determination about the type of carbohydrate used as replacement. 2Minimum study duration was 24 months. 4No important heterogeneity; I² = 0%. 6The 95% CI crosses 1.0 and does not exclude important benefit or harm. 7Too few studies to reliably assess publication bias (< 10 RCTs). 8Important heterogeneity; I² = 55%. | ||||||||||||||
| Quality assessment | No of participants (study event rate%) | Effect | Quality | Importance | ||||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||||
| All‐cause mortality (follow‐up mean 50 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1079/21688 | 1594/31926 | RR 0.98 (0.91 to 1.06) | 10 fewer (from 45 fewer to 30 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular mortality (follow‐up mean 48 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 315/20713 | 426/30464 | RR 0.99 (0.86 to 1.14) | 1 fewer (from 20 fewer to 20 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular events (follow‐up mean 48 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1504/20713 | 2253/30464 | RR 0.98 (0.9 to 1.06) | 15 fewer (from 74 fewer to 44 more) | ♁♁♁O | CRITICAL | ||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 56 months1) | ||||||||||||||
| 3 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 571/20647 | 818/30397 | RR 0.96 (0.86 to 1.07) | 11 fewer (from 38 fewer to 19 more) | ♁♁♁O | IMPORTANT | ||
| Non‐fatal myocardial infarction (follow‐up mean 60 months1) | ||||||||||||||
| 3 | RCTs | no serious risk of bias2 | serious inconsistency7 | no serious indirectness4 | serious imprecision5 | none6 | 470/20559 | 718/30309 | RR 0.99 (0.73 to 1.35) | 2 fewer (from 64 fewer to 83 more) | ♁♁OO | IMPORTANT | ||
| Stroke (follow‐up mean 72 months1) | ||||||||||||||
| 3 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 435/19629 | 647/29382 | RR 1.01 (0.89 to 1.15) | 2 more (from 24 fewer to 33 more) | ♁♁♁O | IMPORTANT | ||
| CHD mortality (follow‐up mean 60 months1) | ||||||||||||||
| 3 | randomised trials | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 255/20559 | 331/30309 | RR 1.01 (0.86 to 1.18) | 1 more (from 15 fewer to 20 more) | ♁♁♁O | IMPORTANT | ||
| CHD events (follow‐up mean 56 months1) | ||||||||||||||
| 4 | randomised trials | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1137/20647 | 1696/30397 | RR 0.99 (0.88 to 1.12) | 6 fewer (from 67 fewer to 67 more) | ♁♁♁O | IMPORTANT | ||
| 1Minimum study duration was 24 months. 5The 95% CI crosses 1.0 and does not exclude important benefit or harm. 6Too few studies to reliably assess publication bias (< 10 RCTs). 7Important heterogeneity; I² = 75%. | ||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 12 | 55858 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 2 Cardiovascular mortality Show forest plot | 12 | 53421 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.12] |
| 3 Combined cardiovascular events Show forest plot | 13 | 53300 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarctions Show forest plot | 11 | 53167 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 2 Non‐fatal MI Show forest plot | 9 | 52834 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.13] |
| 3 Stroke Show forest plot | 8 | 50952 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 4 CHD mortality Show forest plot | 10 | 53159 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.15] |
| 5 CHD events Show forest plot | 12 | 53199 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.74, 1.03] |
| 6 Diabetes diagnoses Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol, mmol/L Show forest plot | 14 | 7115 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.36, ‐0.13] |
| 2 LDL cholesterol, mmol/L Show forest plot | 5 | 3291 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| 3 HDL cholesterol, mmol/L Show forest plot | 6 | 5147 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 4 Triglycerides, mmol/L Show forest plot | 7 | 3845 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.21, 0.04] |
| 5 total cholesterol /HDL ratio Show forest plot | 3 | 2985 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 6 LDL /HDL ratio Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Lp(a), mmol/L Show forest plot | 2 | 2882 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 8 Insulin sensitivity Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 HbA1c (glycosylated haemoglobin), % | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 GTT (glucose tolerance test), glucose at 2 hours, mmol/L | 3 | 249 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.55, ‐0.82] |
| 8.3 HOMA | 1 | 2832 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |


