Reduction in saturated fat intake for cardiovascular disease
Appendices
Appendix 1. Search strategies March 2014
CENTRAL
#1 lipid near (low* or reduc* or modifi*)
#2 cholesterol* near (low* or modifi* or reduc*)
#3 (#1 or #2)
#4 MeSH descriptor: [Nutrition Therapy] explode all trees
#5 diet* or food* or nutrition*
#6 (#4 or #5)
#7 (#3 and #6)
#8 fat* near (low* or reduc* or modifi* or animal* or saturat* or unsaturat*)
#9 MeSH descriptor: [Diet, Atherogenic] explode all trees
#10 MeSH descriptor: [Diet Therapy] explode all trees
#11 (#7 or #8 or #9 or #10)
#12 MeSH descriptor: [Cardiovascular Diseases] this term only
#13 MeSH descriptor: [Heart Diseases] explode all trees
#14 MeSH descriptor: [Vascular Diseases] explode all trees
#15 MeSH descriptor: [Cerebrovascular Disorders] this term only
#16 MeSH descriptor: [Brain Ischemia] explode all trees
#17 MeSH descriptor: [Carotid Artery Diseases] explode all trees
#18 MeSH descriptor: [Dementia, Vascular] explode all trees
#19 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees
#20 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
#21 MeSH descriptor: [Intracranial Hemorrhages] explode all trees
#22 MeSH descriptor: [Stroke] explode all trees
#23 coronar* near (bypas* or graft* or disease* or event*)
#24 cerebrovasc* or cardiovasc* or mortal* or angina* or stroke or strokes or tia or ischaem* or ischem*
#25 myocardi* near (infarct* or revascular* or ischaem* or ischem*)
#26 morbid* near (heart* or coronar* or ischaem* or ischem* or myocard*)
#27 vascular* near (peripheral* or disease* or complication*)
#28 heart* near (disease* or attack* or bypas*)
#29 (#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28)
#30 (#11 and #29)
MEDLINE OVID
1. (lipid$ adj5 (low$ or reduc$ or modifi$)).mp.
2. (cholesterol$ adj5 (low$ or modific$ or reduc$)).mp.
3. 1 or 2
4. exp Nutrition Therapy/
5. (diet$ or food$ or nutrition$).mp.
6. 4 or 5
7. 3 and 6
8. (fat adj5 (low$ or reduc$ or modifi$ or animal$ or saturat$ or unsatur$)).mp.
9. exp Diet, Atherogenic/
10. exp Diet Therapy/
11. 7 or 8 or 9 or 10
12. cardiovascular diseases/ or exp heart diseases/ or exp vascular diseases/
13. cerebrovascular disorders/ or exp brain ischemia/ or exp carotid artery diseases/ or exp dementia, vascular/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or exp stroke/
14. (coronar$ adj5 (bypas$ or graft$ or disease$ or event$)).mp.
15. (cerebrovasc$ or cardiovasc$ or mortal$ or angina$ or stroke or strokes).mp.
16. (myocardi$ adj5 (infarct$ or revascular$ or ischaemi$ or ischemi$)).mp.
17. (morbid$ adj5 (heart$ or coronar$ or ischaem$ or ischem$ or myocard$)).mp.
18. (vascular$ adj5 (peripheral$ or disease$ or complication$)).mp.
19. (heart$ adj5 (disease$ or attack$ or bypass$)).mp.
20. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21. 11 and 20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. drug therapy.fs.
27. randomly.ab.
28. trial.ab.
29. groups.ab.
30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. exp animals/ not humans.sh.
32. 30 not 31
33. 21 and 32
34. 33
35. limit 34 to yr="2010 ‐Current"
36. limit 35 to "core clinical journals (aim)"
EMBASE OVID
1. cardiovascular diseases/ or exp heart diseases/ or exp vascular diseases/
2. cerebrovascular disorders/ or exp brain ischemia/ or exp carotid artery diseases/ or exp dementia, vascular/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or exp stroke/
3. (coronar$ adj5 (bypas$ or graft$ or disease$ or event$)).mp.
4. (cerebrovasc$ or cardiovasc$ or mortal$ or angina$ or stroke or strokes).mp.
5. (myocardi$ adj5 (infarct$ or revascular$ or ischaemi$ or ischemi$)).mp.
6. (morbid$ adj5 (heart$ or coronar$ or ischaem$ or ischem$ or myocard$)).mp.
7. (vascular$ adj5 (peripheral$ or disease$ or complication$)).mp.
8. (heart$ adj5 (disease$ or attack$ or bypass$)).mp.
9. or/1‐8
10. (lipid$ adj5 (low$ or reduc$ or modifi$)).mp.
11. (cholesterol$ adj5 (low$ or modific$ or reduc$)).mp.
12. 10 or 11
13. (diet$ or food$ or eat$ or nutrition$).mp.
14. exp nutrition/
15. 13 or 14
16. 12 and 15
17. (fat adj5 (low$ or reduc$ or modifi$ or animal$ or saturat$ or unsatur$)).mp.
18. exp lipid diet/ or exp fat intake/ or exp low fat diet/
19. 16 or 17 or 18
20. 9 and 19
21. random$.tw.
22. factorial$.tw.
23. crossover$.tw.
24. cross over$.tw.
25. cross‐over$.tw.
26. placebo$.tw.
27. (doubl$ adj blind$).tw.
28. (singl$ adj blind$).tw.
29. assign$.tw.
30. allocat$.tw.
31. volunteer$.tw.
32. crossover procedure/
33. double blind procedure/
34. randomized controlled trial/
35. single blind procedure/
36. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35
37. (animal/ or nonhuman/) not human/
38. 36 not 37
39. 20 and 38
40. (2010* or 2011* or 2012* or 2013* or 2014*).em.
41. 39 and 40
42. limit 41 to priority journals

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Please note that while Rose 1965 (Rose corn oil 1965; Rose olive 1965) and WHI 2006 (WHI with CVD 2006; WHI without CVD 2006) each appear twice in this summary, they are each a single trial. Rose 1965 was a 3‐arm trial and we have used the two intervention arms separately in the review, while WHI 2006 provided some data separately for people with or without CVD at baseline.

Forest plot of comparison: 1 SFA reduction vs usual diet ‐ health events, outcome: 1.1 All‐cause mortality.
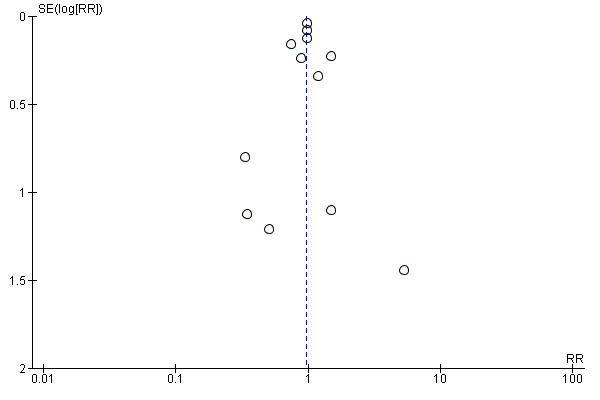
Funnel plot of comparison: fat modification or reduction vs usual diet ‐ total mortality.
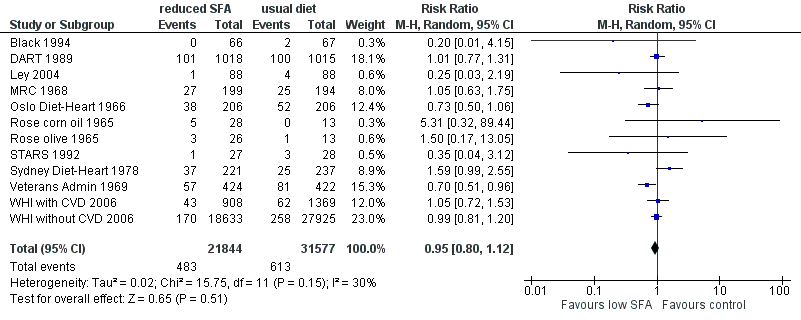
Forest plot of comparison: 1 SFA reduction vs usual diet ‐ Primary outcomes, outcome: 1.2 Cardiovascular mortality.

Forest plot of comparison: 1 SFA reduction vs usual diet ‐ Primary outcomes, outcome: 1.3 Combined cardiovascular events.

Funnel plot of comparison: fat modification or reduction vs usual diet ‐ combined cardiovascular events.

Exploring saturated fat cut‐offs.
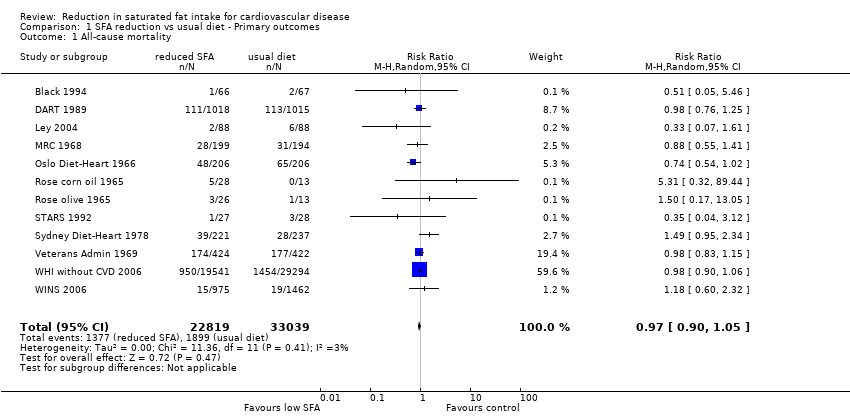
Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 1 All‐cause mortality.

Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 2 Cardiovascular mortality.
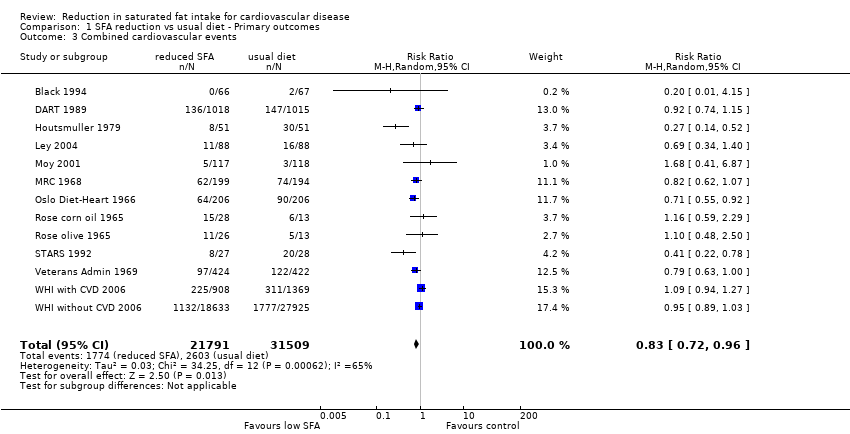
Comparison 1 SFA reduction vs usual diet ‐ Primary outcomes, Outcome 3 Combined cardiovascular events.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 1 Myocardial infarctions.
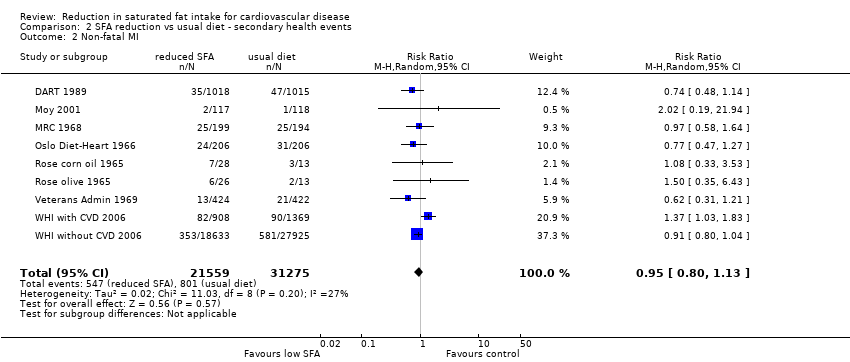
Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 2 Non‐fatal MI.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 3 Stroke.
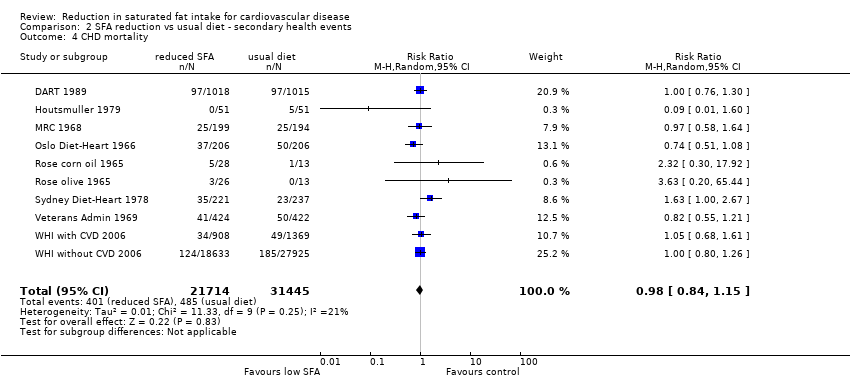
Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 4 CHD mortality.

Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 5 CHD events.
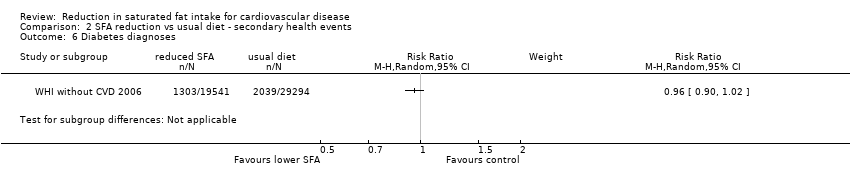
Comparison 2 SFA reduction vs usual diet ‐ secondary health events, Outcome 6 Diabetes diagnoses.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 1 Total cholesterol, mmol/L.
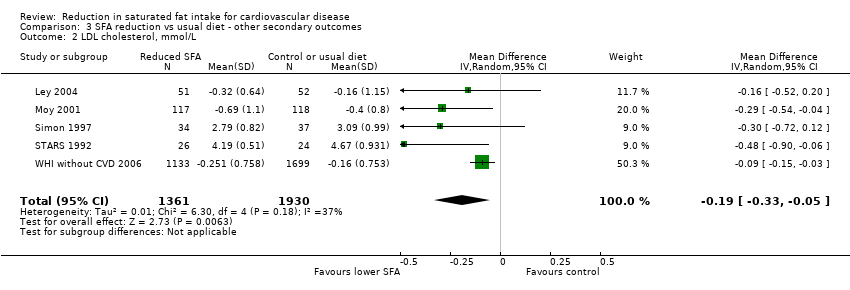
Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 2 LDL cholesterol, mmol/L.
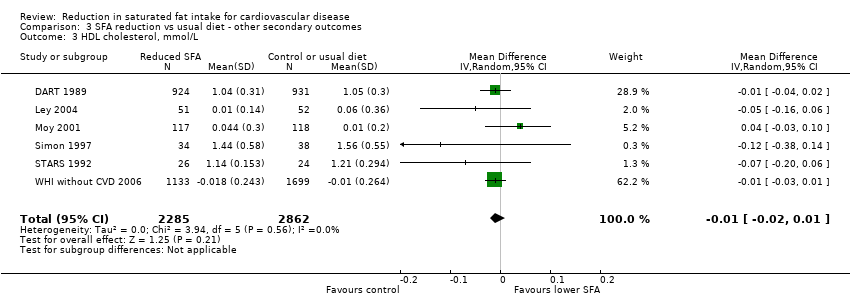
Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 3 HDL cholesterol, mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 4 Triglycerides, mmol/L.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 5 total cholesterol /HDL ratio.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 6 LDL /HDL ratio.

Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 7 Lp(a), mmol/L.
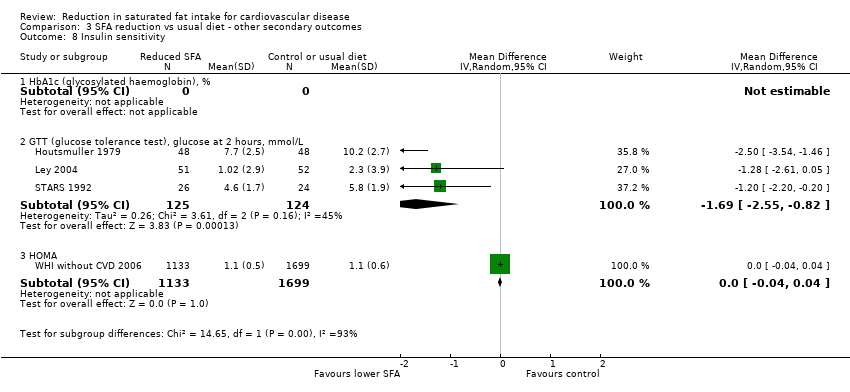
Comparison 3 SFA reduction vs usual diet ‐ other secondary outcomes, Outcome 8 Insulin sensitivity.
| Low saturated fat compared with usual saturated fat for CVD risk | |||||
| Patient or population: people at any baseline risk of CVD Intervention: reduction of saturated fat intake Comparison: usual saturated fat intake | |||||
| Outcomes | Relative effect | Absolute effects (per 10,000) | No of Participants | Quality of the evidence | Comments |
| All‐cause mortality follow‐up mean duration 56 months1 | RR 0.97 (0.90 to 1.05) | 17 fewer (from 57 fewer to 29 more) | 55,858 | ⊕⊕⊕⊝ | Critical importance |
| Cardiovascular mortality follow‐up mean duration 53 months1 | RR 0.95 (0.80 to 1.12) | 10 fewer | 53,421 | ⊕⊕⊕⊝ | Critical importance |
| Combined cardiovascular events follow‐up mean duration 52 months1 | RR 0.83 (0.72 to 0.96) | 138 fewer (from 33 fewer to 228 fewer) | 53,300 | ⊕⊕⊕⊝ | Critical importance |
| Myocardial infarctions follow‐up mean duration 55 months | RR 0.90 (0.80 to 1.01) | 32 fewer (from 63 fewer to 3 more) | 53,167 | ⊕⊕⊕⊝ | Critical importance |
| Non‐fatal MI follow‐up mean duration 55 months1 | RR 0.95 (0.80 to 1.13) | 13 fewer (from 51 fewer to 33 more) | 52,834 | ⊕⊕⊕⊝ | Critical importance |
| Stroke follow‐up mean duration 59 months1 | RR 1.00 (0.89 to 1.12) | 0 fewer (from 25 fewer to 25 more) | 50,952 | ⊕⊕⊕⊝ | Critical importance |
| CHD mortality follow‐up mean duration 65 months1 | RR 0.98 (0.84 to 1.15) | 3 fewer (from 25 fewer to 23 more) | 53,159 | ⊕⊕⊕⊝ | Critical importance |
| CHD events follow‐up mean duration 59 months1 | RR 0.87 (0.74 to 1.03) | 80 fewer (from 160 fewer to 19 more) | 53,199 | ⊕⊕⊝⊝ | Critical importance |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Minimum study duration was 24 months 2These large RCTs of relatively long duration were well randomised but fewer than half had good allocation concealment (the rest were unclear). Blinding was only well‐conducted in 1 RCT, however blinding is very difficult in trials of dietary fat intake. Incomplete outcome data were variable, and most included studies had systematic differences in care (i.e. intervention group had more time or attention than the control group). We noted no other biases. We downgraded each study once for a combination of these issues around validity and issues around precision. The level of compliance with interventions involving long‐term behaviour change, such as those used in these studies, can vary widely. This is likely to attenuate the pooled effect and bias it towards the null. 3No important heterogeneity; I² ≤ 30% 4These RCTs directly assessed the effect of lower vs usual saturated fat intake on health outcomes of interest. Participants included men and women with and without CVD at baseline (also some participants with CVD risk factors like diabetes, or at risk of cancers). 5The 95% CI crosses 1.0 and does not exclude important benefit or harm. 6The funnel plot did not suggest any small‐study (publication) bias. 7Potentially important heterogeneity was identified; I² = 65%. However, the heterogeneity was partly explained by the degree of saturated fat reduction, and the degree of cholesterol lowering achieved (in subgrouping and in meta‐regression). For this reason we did not downgrade the study for inconsistency. 8The 95% CI does not cross 1.0 or a threshold of important harm. 9Too few studies to reliably assess small‐study bias (< 10 RCTs) 10Important heterogeneity; I² = 66%. | |||||
| Reference | Population | CVD risk category | Is intervention delivered to Individual or group? | intervention given by? | Face‐to‐face or other? | Number of visits | Is intervention advice only or other intervention? |
| People with non‐melanoma skin cancer | Low | Unclear | Dietitian | Face‐to‐face | 8 x weekly classes then monthly follow‐up sessions | Advice (behaviour techniques learning) | |
| Men recovering from a MI | High | Individual | Dietitian | Face‐to‐face | 9 | Advice (diet advice, recipes and encouragement) | |
| Adults with newly‐diagnosed diabetes | Moderate | Unclear | Dietitian | Unclear | Unclear | Advice? | |
| People with impaired glucose intolerance or high normal blood glucose | Moderate | Small group | Unclear | Face‐to‐face | Monthly meetings | Advice (education, personal goal setting, self‐monitoring) | |
| Middle‐aged siblings of people with early CHD, with at least 1 CVD risk factor | Moderate | Individual | Trained nurse | Face‐to‐face | 6 ‐ 8 weekly for 2 years | Advice (individualised counselling sessions) | |
| Free‐living men who have survived a 1st MI | High | Individual | Dietitian | Face‐to‐face | Unclear | Advice and supplement (soy oil) | |
| Men with previous MI | High | Individual | Dietitian | Face‐to‐face and other | Unclear | Advice and supplement (food) | |
| Newly‐diagnosed non‐insulin‐dependent diabetics | Moderate | Individual | Diabetes dietitian | Face‐to‐face | After 1 month then at 3‐month intervals | Advice | |
| Men (?) with angina or following MI | High | Unclear | Unclear | Unclear | Follow‐up clinic monthly, then every 2 months | Advice and supplement (oil) | |
| Men (?) with angina or following MI | High | Unclear | Unclear | Unclear | Follow‐up clinic monthly, then every 2 months | Advice and supplement (oil) | |
| Women with a high risk of breast cancer | Low | Individual followed by individual or group | Dietitian | Face‐to‐face | Bi‐weekly over 3 months followed by monthly | Advice (individualised eating plan and counselling sessions) | |
| Men with angina referred for angiography | High | individual | Dietitian | Face‐to‐face | Clinic visits at 3‐months intervals | Advice | |
| Men with angina referred for angiography | High | Individual | Unclear | Face‐to‐face | 3 times in 1st year and twice annually thereafter | Advice | |
| Men living at the Veterans Administration Center | Low | Individual | Unclear (whole diet provided) | N/A | N/A | Diet provided | |
| Post‐menopausal women aged 50 ‐ 79 with CVD at baseline | High | Group | Nutritionists | Face‐to‐face | 18 sessions/ 1st yr and quarterly maintenance sessions after | Advice | |
| Post‐menopausal women aged 50 ‐ 79 without CVD at baseline | Low | Group | Nutritionists | Face‐to‐face | 18 sessions/ 1st yr and quarterly maintenance sessions after | Advice | |
| Women with localised resected breast cancer | Low | Individual followed by group | Dietitian | Face‐to‐face | 8 bi‐weekly sessions, then 3‐monthly contact and optional monthly sessions | Advice | |
| N/A: not applicable | |||||||
| Participants | All‐cause mortality | CV mortality | CVD events | MI | Non‐fatal MI | Stroke | CHD mortality | CHD events | Diabetes Diagnoses | |
| 133 | 133 | 133 | 133 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2033 | 2033 | 2033 | 2033 | 2033 | 2033 | 0 | 2033 | 2033 | 0 | |
| 102 | 0 | 0 | 102 | 102 | 0 | 0 | 102 | 102 | 0 | |
| 176 | 176 | 176 | 176 | 176 | 0 | 176 | 0 | 176 | 0 | |
| 267 | 0 | 0 | 235 | 235 | 235 | 235 | 0 | 267 | 0 | |
| 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 0 | |
| 412 | 412 | 412 | 412 | 412 | 412 | 412 | 412 | 412 | 0 | |
| 249 (data not provided by arm) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 41 | 41 | 41 | 41 | 41 | 41 | 0 | 41 | 41 | 0 | |
| 39 | 39 | 39 | 39 | 39 | 39 | 0 | 39 | 39 | 0 | |
| 194 (data not provided by arm) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 60 | 55 | 55 | 55 | 55 | 0 | 55 | 0 | 55 | 0 | |
| 458 | 458 | 458 | 0 | 0 | 0 | 0 | 458 | 0 | 0 | |
| 846 | 846 | 846 | 846 | 846 | 846 | 846 | 846 | 846 | 0 | |
| 2277 | 0 | 2277 | 2277 | 0 | 2277 | 2277 | 2277 | 2277 | 0 | |
| 48835 | 48835 | 46558 | 46558 | 48835 | 46558 | 46558 | 46558 | 46558 | 48835 | |
| 2437 | 2437 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total Participants | 58509 | 55858 | 53421 | 53300 | 53167 | 52834 | 50952 | 53159 | 53204 | 48835 |
| Percent of participants for this outcome | 100% | 95% | 91% | 91% | 91% | 90% | 87% | 91% | 91% | 83% |
| These numbers are the numbers of participants in each study who were available for assessment of outcomes within meta‐analysis (not necessarily the number of participants randomised within the trial). | ||||||||||
| Participants | Total cholesterol | LDL cholesterol | HDL cholesterol | Triglycerides | TG/HDL ratio | Total cholesterol/HDL ratio | LDL/HDL ratio | LP (a) | Insulin sensitivity | |
| 133 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2033 | 1855 | 0 | 1855 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 102 | 96 | 0 | 0 | 96 | 0 | 0 | 0 | 0 | 96 | |
| 176 | 103 | 103 | 103 | 103 | 0 | 103 | 0 | 0 | 103 | |
| 267 | 0 | 235 | 235 | 235 | 0 | 0 | 0 | 0 | 0 | |
| 393 | 177 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 412 | 329 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 249 | 58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 41 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 39 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 194 | 72 | 71 | 72 | 71 | 0 | 0 | 0 | 0 | 0 | |
| 55 | 50 | 50 | 50 | 50 | 0 | 50 | 50 | 50 | 50 | |
| 458 | 458 | 0 | 0 | 458 | 0 | 0 | 0 | 0 | 0 | |
| 846 | 843 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2277 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 48835 | 2832 | 2832 | 2832 | 2832 | 0 | 2832 | 0 | 2832 | 2832 | |
| 2437 | 196 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total Participants | 58952 | 7115 | 3291 | 5147 | 3845 | 0 | 2985 | 50 | 2882 | 3081 |
| Percent of participants for this outcome | 100% | 12% | 6% | 9% | 7% | 0% | 5% | 0.1% | 5% | 5% |
| These numbers are the numbers of participants in each study who were available for assessment of outcomes within meta‐analysis (not necessarily the number of participants randomised within the trial). | ||||||||||
| Analysis | RR (95% CI) of all‐cause mortality | I² | No. of events | No. of participants | |
| Main analysis | 0.97 (0.90 to 1.05) | 3% | 3276 | > 55,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.97 (0.89 to 1.06) | 11% | 3231 | > 53,000 |
| SFA significantly reduced | 0.99 (0.92 to 1.06) | 0% | 3095 | > 54,000 | |
| TC significantly reduced | 0.96 (0.83 to 1.11) | 33% | 2871 | > 52,000 | |
| Minus WHI | 0.96 (0.84 to 1.11) | 12% | 872 | > 7000 | |
| Mantel‐Haenszel fixed‐effect | 0.98 (0.91 to 1.04) | 3% | 3276 | > 55,000 | |
| Peto fixed‐effect | 0.97 (0.90 to 1.05) | 16% | 3276 | > 55,000 | |
| SFA: saturated fatty acids | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of all‐cause mortality | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.79 | PUFA replacement | 0.96 (0.82 to 1.13) | 26% | 824 | > 4000 |
| MUFA replacement | 3.00 (0.33 to 26.99) | N/A | 4 | 52 | |
| CHO replacement | 0.98 (0.91 to 1.05) | 0% | 2677 | > 53,000 | |
| Protein replacement | 0.98 (0.91 to 1.06) | 0% | 2673 | > 53,000 | |
| Subgroup by duration, P = 0.60 | Up to 24 months | 0.99 (0.78 to 1.26) | 0% | 236 | > 2000 |
| > 24 to 48 months | 0.96 (0.83 to 1.12) | 0% | 414 | > 1000 | |
| > 48 months | 1.00 (0.79 to 1.27) | 55% | 2618 | > 52,000 | |
| Unclear duration | 0.33 (0.07 to 1.61) | N/A | 8 | > 100 | |
| Subgroup by baseline SFA, P = 0.48 | Up to 12%E SFA | 1.18 (0.60 to 2.32) | N/A | 34 | > 2400 |
| > 12 to 15%E SFA | 1.01 (0.86 to 1.19) | 26% | 2706 | > 51,000 | |
| > 15 to 18%E SFA | 0.35 (0.04 to 3.12) | N/A | 4 | 55 | |
| > 18%E SFA | 0.98 (0.83 to 1.15) | N/A | 351 | 846 | |
| Subgroup by SFA change, P = 0.31 | Up to 4%E SFA difference | 1.02 (0.88 to 1.19) | 26% | 2737 | > 53,000 |
| > 4 to 8%E SFA difference | 0.41 (0.08 to 2.07) | 0% | 7 | > 100 | |
| > 8%E SFA difference | 0.98 (0.83 to 1.15) | N/A | 351 | > 800 | |
| Subgroup by sex, P = 0.40 | Men | 0.96 (0.83 to 1.11) | 13% | 830 | > 4000 |
| Women | 0.98 (0.91 to 1.06) | 0% | 2438 | > 51,000 | |
| Mixed, men and women | 0.33 (0.07 to 1.61) | N/A | 8 | 176 | |
| Subgroup by CVD risk, P = 0.40 | Low CVD risk | 0.98 (0.91 to 1.05) | 0% | 2792 | > 52,000 |
| Moderate CVD risk | 0.33 (0.07 to 1.61) | N/A | 8 | 176 | |
| Existing CVD | 0.97 (0.90 to 1.05) | 33% | 476 | > 3000 | |
| Subgroup by serum TC reduction, P = 0.85 | TC reduced by ≥ 0.2 mmol/L | 0.96 (0.81 to 1.14) | 32% | 823 | > 4000 |
| TC reduced by < 0.2 mmol/L | 0.98 (0.91 to 1.06) | 0% | 2450 | > 51,000 | |
| Unclear TC change | 0.51 (0.05 to 5.46) | N/A | 3 | > 100 | |
| Subgroup by decade of publication, P = 0.28 | 1960s | 0.92 (0.80 to 1.07) | 2% | 532 | > 1700 |
| 1970s | 1.49 (0.95 to 2.34) | N/A | 67 | 458 | |
| 1980s | 0.98 (0.76 to 1.25) | N/A | 224 | 2033 | |
| 1990s | 0.41 (0.08 to 2.07) | 0% | 7 | 188 | |
| 2000s | 0.98 (0.83 to 1.15) | 5% | 2446 | > 51,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CVD mortality | I² | No. of events | No. of participants | |
| Main analysis | 0.95 (0.80 to 1.12) | 30% | 1096 | >53000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.96 (0.81 to 1.13) | 32% | 1089 | > 53,000 |
| SFA significantly reduced | 0.96 (0.79 to 1.18) | 42% | 945 | > 52,000 | |
| TC significantly reduced | 1.00 (0.86 to 1.16) | 19% | 942 | > 52,000 | |
| Minus WHI | 0.92 (0.72 to 1.18) | 40% | 563 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.95 (0.85 to 1.07) | 30% | 1096 | > 53,000 | |
| Peto fixed‐effect | 0.95 (0.84 to 1.08) | 41% | 1096 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CVD mortality | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.79 | PUFA replacement | 0.95 (0.73 to 1.25) | 55% | 553 | > 4000 |
| MUFA replacement | 3.00 (0.33 to 26.99) | N/A | 4 | 52 | |
| CHO replacement | 0.99 (0.86 to 1.14) | 0% | 745 | > 51,000 | |
| Protein replacement | 0.99 (0.86 to 1.14) | 0% | 741 | > 51,000 | |
| Subgroup by duration, P = 0.33 | Up to 24 months | 1.26 (0.54 to 2.94) | 26% | 213 | > 2000 |
| > 24 to 48 months | 0.79 (0.57 to 1.08) | 14% | 194 | > 1000 | |
| > 48 months | 1.01 (0.79 to 1.30) | 54% | 685 | > 49,000 | |
| Unclear duration | 0.25 (0.03 to 2.19) | N/A | 5 | > 100 | |
| Subgroup by baseline SFA, P = 0.13 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.04 (0.88 to 1.24) | 19% | 803 | > 51,000 | |
| > 15 to 18%E SFA | 0.35 (0.04 to 3.12) | N/A | 4 | 55 | |
| > 18%E SFA | 0.70 (0.51 to 0.96) | N/A | 138 | 846 | |
| Subgroup by SFA change, P = 0.08 | Up to 4%E SFA difference | 1.05 (0.89 to 1.24) | 21% | 801 | >51000 |
| > 4 to 8%E SFA difference | 0.29 (0.05 to 1.70) | 0% | 6 | > 100 | |
| > 8%E SFA difference | 0.70 (0.51 to 0.96) | N/A | 152 | > 900 | |
| Subgroup by sex, P = 0.45 | Men | 0.96 (0.73 to 1.25) | 48% | 559 | > 4000 |
| Women | 1.00 (0.84 to 1.19) | 0% | 533 | > 48,000 | |
| Mixed, men and women | 0.25 (0.03 to 2.19) | NA | 5 | 176 | |
| Subgroup by CVD risk, p=0.26 | Low CVD risk | 0.84 (0.60 to 1.16) | 54% | 568 | > 47,000 |
| Moderate CVD risk | 0.25 (0.03 to 2.19) | NA | 5 | 176 | |
| Existing CVD | 1.04 (0.83 to 1.31) | 33% | 524 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.57 | TC reduced by ≥ 0.2 mmol/L | 0.95 (0.73 to 1.25) | 55% | 553 | > 4000 |
| TC reduced by < 0.2 mmol/L | 1.00 (0.84 to 1.18) | 0% | 542 | > 49,000 | |
| Unclear TC change | 0.20 (0.01 to 4.15) | N/A | 2 | > 100 | |
| Subgroup by decade of publication, P = 0.04 | 1960s | 0.78 (0.63 to 0.97) | 2% | 289 | > 1700 |
| 1970s | 1.59 (0.99 to 2.55) | N/A | 62 | 458 | |
| 1980s | 1.01 (0.77 to 1.31) | N/A | 201 | > 2000 | |
| 1990s | 0.29 (0.05 to 1.70) | 0% | 6 | 188 | |
| 2000s | 0.99 (0.83 to 1.18) | 0% | 538 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CVD events | I² | No. of events | No. of participants | |
| Main analysis | 0.83 (0.72 to 0.96) | 65% | 4377 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.84 (0.72 to 0.97) | 69% | 4354 | > 52,000 |
| SFA significantly reduced | 0.91 (0.79 to 1.04) | 53% | 4012 | > 52,000 | |
| TC significantly reduced | 0.81 (0.68 to 0.98) | 77% | 4092 | > 52,000 | |
| Minus WHI | 0.75 (0.61 to 0.91) | 51% | 932 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.93 (0.88 to 0.98) | 65% | 4377 | > 53,000 | |
| Peto fixed‐effect | 0.92 (0.86 to 0.98) | 72% | 4377 | > 53,000 | |
| SFA: saturated fatty ac5d | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CVD events | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.14 | PUFA replacement | 0.73 (0.58 to 0.92) | 69% | 884 | > 3000 |
| MUFA replacement | 1.00 (0.53 to 1.89) | NA | 22 | 52 | |
| CHO replacement | 0.93 (0.79 to 1.08) | 57% | 3785 | > 51,000 | |
| Protein replacement | 0.98 (0.90 to 1.06) | 15% | 3757 | > 51,000 | |
| Subgroup by duration, P = 0.15 | Up to 24 months | 0.96 (0.78 to 1.16) | 0% | 330 | > 2000 |
| > 24 to 48 months | 0.73 (0.56 to 0.95) | 50% | 383 | > 1000 | |
| > 48 months | 0.93 (0.79 to 1.11) | 75% | 3599 | > 49,000 | |
| Unclear duration | 0.43 (0.17 to 1.08) | NA | 65 | > 200 | |
| Subgroup by baseline SFA, P = 0.13 | Up to 12%E SFA | NA | |||
| > 12 to 15%E SFA | 0.98 (0.91 to 1.05) | 6% | 3765 | > 51,000 | |
| > 15 to 18%E SFA | 0.41 (0.22 to 0.78) | NA | 28 | 55 | |
| > 18%E SFA | 0.79 (0.63 to 1.00) | NA | 219 | 846 | |
| Subgroup by SFA change, P = 0.005 | Up to 4%E SFA difference | 0.98 (0.91 to 1.05) | 6% | 3763 | > 51,000 |
| > 4 to 8%E SFA difference | 0.40 (0.22 to 0.74) | 0% | 30 | > 100 | |
| > 8%E SFA difference | 0.79 (0.63 to 1.00) | NA | 219 | > 800 | |
| Subgroup by sex, P = 0.05 | Men | 0.80 (0.69 to 0.93) | 24% | 859 | > 3000 |
| Women | 1.00 (0.88 to 1.14) | 60% | 3445 | > 48,000 | |
| Mixed, men and women | 0.59 (0.23 to 1.49) | 71% | 73 | > 500 | |
| Subgroup by CVD risk, P = 0.67 | Low CVD risk | 0.89 (0.75 to 1.06) | 40% | 3130 | > 47,000 |
| Moderate CVD risk | 0.59 (0.23 to 1.49) | 71% | 73 | > 500 | |
| Existing CVD | 0.86 (0.71 to 1.05) | 63% | 1174 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.03 | TC reduced by ≥ 0.2 mmol/L | 0.74 (0.59 to 0.92) | 63% | 887 | > 4000 |
| TC reduced by < 0.2 mmol/L | 0.99 (0.90 to 1.08) | 15% | 3488 | > 49,00 | |
| Unclear TC change | 0.20 (0.01 to 4.15) | NA | 2 | > 100 | |
| Subgroup by decade of publication, P , 0.0001 | 1960s | 0.79 (0.69 to 0.91) | 0% | 546 | > 1700 |
| 1970s | 0.27 (0.14 to 0.52) | NA | 38 | 102 | |
| 1980s | 0.92 (0.74 to 1.15) | NA | 283 | > 2000 | |
| 1990s | 0.40 (0.22 to 0.74) | 0% | 30 | 188 | |
| 2000s | 0.99 (0.89 to 1.11) | 25% | 3480 | > 49,000 | |
| CHO: carbohydrate | |||||
| Regression factor | No. of studies | Constant | Coefficient (95% CI) | P value | Proportion of between study variation explained |
| Change in SFA as %E | 8 | 0.01 | 0.05 (‐0.03 to 0.13) | 0.16 | 89% |
| Change in SFA as % of control | 8 | 0.26 | 0.01 (‐0.01 to 0.03) | 0.14 | 89% |
| Baseline SFA as %E | 8 | 0.68 | ‐0.06 (‐0.15 to 0.04) | 0.19 | 81% |
| Change in TC, mmol/L | 12 | 0.03 | 0.69 (0.05 to 1.33) | 0.04 | 99% |
| Change in PUFA as %E | 5 | ‐0.01 | ‐0.02 (‐0.08 to 0.03) | 0.25 | 100% |
| Change in MUFA as %E | 5 | ‐0.26 | ‐0.03 (‐0.14 to 0.09) | 0.50 | ‐87% |
| Change in CHO as %E | 7 | ‐0.11 | ‐0.00 (‐0.05 to 0.05) | 0.92 | ‐273% |
| Change in total fat intake as %E | 9 | ‐0.17 | ‐0.01 (‐0.03 to 0.01) | 0.28 | 100% |
| Gender* | 13 | ‐0.17 | ‐0.14 (‐0.63 to 0.35) | 0.55 | ‐13% |
| Study duration | 13 | ‐0.47 | 0.00 (‐0.01 to 0.02) | 0.76 | ‐24.8 |
| CVD risk at baseline** | 13 | ‐0.44 | 0.03 (‐0.48 to 0.55) | 0.89 | ‐39% |
| *0 = women, 1 = mixed, 2 = men | |||||
| Analysis | RR (95% CI) of any MI | I² | No. of events | No. of participants | |
| Main analysis | 0.90 (0.80 to 1.01) | 10% | 1714 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.89 (0.78 to 1.02) | 17% | 1707 | > 53,000 |
| SFA significantly reduced | 0.94 (0.85 to 1.04) | 0% | 1520 | > 52,000 | |
| TC significantly reduced | 0.89 (0.76 to 1.05) | 26% | 1561 | > 52,000 | |
| Minus WHI | 0.85 (0.73 to 0.98) | 1% | 608 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.92 (0.84 to 1.01) | 10% | 1714 | > 53,000 | |
| Peto fixed‐effect | 0.92 (0.83 to 1.01) | 31% | 1714 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of any MI | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.48 | PUFA replacement | 0.83 (0.67 to 1.02) | 29% | 591 | > 3000 |
| MUFA replacement | 1.40 (0.51 to 3.85) | N/A | 12 | 52 | |
| CHO replacement | 0.96 (0.86 to 1.06) | 0% | 1392 | > 51,000 | |
| Protein replacement | 0.96 (0.86 to 1.07) | 0% | 1389 | > 51,000 | |
| Subgroup by duration, P = 0.78 | Up to 24 months | 0.95 (0.77 to 1.17) | 0% | 300 | > 2000 |
| > 24 to 48 months | 0.83 (0.64 to 1.06) | 0% | 207 | > 1000 | |
| > 48 months | 0.81 (0.54 to 1.24) | 78% | 1194 | > 49,000 | |
| Unclear duration | 0.41 (0.02 to 7.73) | 71% | 13 | > 200 | |
| Subgroup by baseline SFA, P = 0.50 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 0.96 (0.87 to 1.07) | 0% | 1392 | > 51,000 | |
| > 15 to 18%E SFA | 0.52 (0.05 to 5.39) | N/A | 3 | 55 | |
| > 18%E SFA | 0.76 (0.55 to 1.05) | N/A | 125 | 846 | |
| Subgroup by SFA change, P = 0.50 | Up to 4%E SFA difference | 0.96 (0.87 to 1.07) | 0% | 1392 | > 51,000 |
| > 4 to 8%E SFA difference | 0.52 (0.05 to 5.39) | N/A | 3 | 55 | |
| > 8%E SFA difference | 0.76 (0.55 to 1.05) | N/A | 125 | > 800 | |
| Subgroup by sex, P = 0.35 | Men | 0.85 (0.73 to 0.98) | 0% | 592 | > 3000 |
| Women | 0.97 (0.86 to 1.09) | N/A | 1106 | > 48,000 | |
| Mixed, men and women | 0.75 (0.13 to 4.47) | 51% | 16 | > 500 | |
| Subgroup by CVD risk, P = 0.96 | Low CVD risk | 0.90 (0.72 to 1.13) | 49% | 1231 | > 49,000 |
| Moderate CVD risk | 0.75 (0.13 to 4.47) | 51% | 16 | > 500 | |
| Existing CVD | 0.87 (0.74 to 1.03) | 0% | 467 | > 2000 | |
| Subgroup by serum TC reduction, P = 0.12 | TC reduced by ≥ 0.2 mmol/L | 0.83 (0.70 to 0.98) | 9% | 592 | > 4000 |
| TC reduced by < 0.2 mmol/L | 0.98 (0.87 to 1.10) | 0% | 1122 | > 49,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P = 0.23 | 1960s | 0.80 (0.64, 1.00) | 10% | 313 | 1731 |
| 1970s | 0.08(0.0, 1.33) | N/A | 6 | 102 | |
| 1980s | 0.91 (0.73, 1.14) | N/A | 276 | 2033 | |
| 1990s | 0.52 (0.05, 5.39) | N/A | 3 | 55 | |
| 2000s | 0.98 (0.87, 1.10) | 0% | 1116 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of non‐fatal MI | I² | No. of events | No. of participants | |
| Main analysis | 0.95 (0.80 to 1.13) | 27% | 1348 | > 52,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.95 (0.80 to 1.13) | 27% | 1348 | > 52,000 |
| SFA significantly reduced | 0.91 (0.72 to 1.25) | 60% | 1225 | > 51,000 | |
| TC significantly reduced | 0.97 (0.79 to 1.19) | 45% | 1296 | > 51,000 | |
| Minus WHI | 0.81 (0.64 to 1.04) | 0% | 242 | > 3000 | |
| Mantel‐Haenszel fixed‐effect | 0.94 (0.85 to 1.05) | 27% | 1348 | > 52,000 | |
| Peto fixed‐effect | 0.94 (0.84 to 1.05) | 27% | 1348 | > 52,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of non‐fatal MI | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.62 | PUFA replacement | 0.80 (0.63 to 1.03) | 0% | 233 | > 3000 |
| MUFA replacement | 1.20 (0.42 to 3.45) | N/A | 11 | 52 | |
| CHO replacement | 0.99 (0.73 to 1.35) | 75% | 1188 | > 50,000 | |
| Protein replacement | 0.99 (0.73 to 1.35) | 75% | 1188 | > 50,000 | |
| Subgroup by duration, P = 0.64 | Up to 24 months | 0.83 (0.57 to 1.22) | 0% | 103 | > 2000 |
| > 24 to 48 months | 0.82 (0.53 to 1.27) | 10% | 84 | > 1000 | |
| > 48 months | 1.01 (0.74 to 1.38) | 73% | 1161 | > 49,000 | |
| Unclear duration | |||||
| Subgroup by baseline SFA, P = 0.43 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.00 (0.75 to 1.35) | 64% | 1191 | > 51,000 | |
| > 15 to 18%E SFA | |||||
| > 18%E SFA | 0.62 (0.31 to 1.21) | N/A | 34 | 846 | |
| Subgroup by SFA change, P = 0.43 | Up to 4%E SFA difference | 1.00 (0.75 to 1.35) | 64% | 1191 | > 51,000 |
| > 4 to 8%E SFA difference | |||||
| > 8%E SFA difference | 0.62 (0.31 to 1.21) | N/A | 34 | > 800 | |
| Subgroup by sex, P = 0.35 | Men | 0.81 (0.63 to 1.03) | 0% | 239 | > 3000 |
| Women | 1.10 (0.73 to 1.64) | 85% | 1106 | > 48,000 | |
| Mixed, men and women | 2.02 (0.19 to 21.94) | N/A | 3 | > 200 | |
| Subgroup by CVD risk, P = 0.61 | Low CVD risk | 0.87 (0.68 to 1.12) | 19% | 968 | > 47,000 |
| Moderate CVD risk | 2.02 (0.19 to 21.94) | N/A | 3 | > 200 | |
| Existing CVD | 1.00 (0.76 to 1.31) | N/A | 377 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.14 | TC reduced by ≥ 0.2 mmol/L | 0.80 (0.62 to 1.03) | 0% | 234 | > 3000 |
| TC reduced by < 0.2 mmol/L | 1.11 (0.77 to 1.60) | 71% | 1114 | > 48,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P = 0.34 | 1960s | 0.84 (0.62, 1.13) | 0% | 157 | 1743 |
| 1970s | |||||
| 1980s | 0.74 (0.48, 1.14) | NA | 82 | 2033 | |
| 1990s | |||||
| 2000s | 1.11 (0.76, 1.61) | 71% | 1109 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of stroke | I² | No. of events | No. of participants | |
| Main analysis | 1.00 (0.89 to 1.12) | 0% | 1125 | > 50,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 1.00 (0.90 to 1.12) | 0% | 1119 | > 50,000 |
| SFA significantly reduced | 0.99 (0.88 to 1.12) | 2% | 1120 | > 50,000 | |
| TC significantly reduced | 1.02 (0.91 to 1.14) | 0% | 1084 | > 49,000 | |
| Minus WHI | 0.63 (0.35 to 1.14) | 0% | 49 | > 2000 | |
| Mantel‐Haenszel fixed‐effect | 0.99 (0.89 to 1.11) | 0% | 1125 | > 50,000 | |
| Peto fixed‐effect | 0.99 (0.88 to 1.13) | 18% | 1125 | > 50,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of stroke | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.69 | PUFA replacement | 0.68 (0.37 to 1.27) | 0% | 41 | > 1700 |
| MUFA replacement | |||||
| CHO replacement | 1.01 (0.90 to 1.13) | 0% | 1083 | > 49,000 | |
| Protein replacement | 1.01 (0.89 to 1.15) | 11% | 1082 | > 49000 | |
| Subgroup by duration, P = 0.17 | Up to 24 months | 1.01 (0.06 to 15.93) | N/A | 2 | > 200 |
| > 24 to 48 months | 0.57 (0.30 to 1.11) | 0% | 36 | > 900 | |
| > 48 months | 1.02 (0.91 to 1.14) | 0% | 1079 | > 49,000 | |
| Unclear duration | 0.20 (0.02 to 1.68) | N/A | 6 | 196 | |
| Subgroup by baseline SFA, P = 0.36 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.01 (0.90 to 1.13) | 0% | 1084 | > 49,000 | |
| > 15 to 18%E SFA | 0.35 (0.01 to 8.12) | N/A | 1 | 55 | |
| > 18%E SFA | 0.59 (0.30 to 1.15) | N/A | 35 | 846 | |
| Subgroup by SFA change, P = 0.36 | Up to 4%E SFA difference | 1.01 (0.90 to 1.13) | 0% | 1084 | > 49,000 |
| > 4 to 8%E SFA difference | 0.35 (0.01 to 8.12) | N/A | 1 | 55 | |
| > 8%E SFA difference | 0.59 (0.30 to 1.15) | N/A | 35 | > 800 | |
| Subgroup by sex, P = 0.35 | Men | 0.63 (0.33 to 1.18) | 0% | 39 | > 1300 |
| Women | 1.02 (0.91 to 1.14) | 0% | 1076 | > 48,000 | |
| Mixed, men and women | 0.37 (0.07 to 1.97) | 0% | 8 | > 400 | |
| Subgroup by CVD risk, P = 0.42 | Low CVD risk | 0.86 (0.52 to 1.42) | 59% | 597 | > 47,000 |
| Moderate CVD risk | 0.37 (0.07 to 1.97) | 0% | 8 | > 400 | |
| Existing CVD | 1.01 (0.86 to 1.18) | 0% | 518 | > 2000 | |
| Subgroup by serum TC reduction, P = 0.24 | TC reduced by ≥ 0.2 mmol/L | 0.70 (0.38 to 1.28) | 0% | 43 | > 1900 |
| TC reduced by < 0.2 mmol/L | 1.01 (0.89 to 1.15) | 11% | 1082 | > 49,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P=0.79 | 1960s | 0.92 (0.31, 2.69) | 23% | 40 | 1651 |
| 1970s | |||||
| 1980s | |||||
| 1990s | 0.35 (0.01, 8.12) | N/A | 1 | 55 | |
| 2000s | 1.01 (0.90, 1.13) | 0% | 1084 | > 49,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CHD mortality | I² | No. of events | No. of participants | |
| Main analysis | 0.98 (0.84 to 1.15) | 21% | 886 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.98 (0.84 to 1.15) | 21% | 886 | > 53,000 |
| SFA significantly reduced | 1.02 (0.87 to 1.20) | 17% | 735 | > 52,000 | |
| TC significantly reduced | 1.00 (0.83 to 1.20) | 33% | 786 | > 52,000 | |
| Minus WHI | 0.97 (0.76 to 1.24) | 37% | 494 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.98 (0.86 to 1.12) | 21% | 886 | > 53,000 | |
| Peto fixed‐effect | 0.98 (0.85 to 1.13) | 39% | 886 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CHD mortality | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.80 | PUFA replacement | 0.98 (0.74 to 1.28) | 49% | 491 | > 4000 |
| MUFA replacement | 3.00 (0.33 to 26.99) | N/A | 4 | 52 | |
| CHO replacement | 1.01 (0.86 to 1.18) | 0% | 586 | > 50,000 | |
| Protein replacement | 1.01 (0.86 to 1.18) | 0% | 586 | > 50,000 | |
| Subgroup by duration, P = 0.33 | Up to 24 months | 1.02 (0.78 to 1.33) | 0% | 203 | > 2000 |
| > 24 to 48 months | 0.87 (0.64 to 1.19) | N/A | 141 | > 1000 | |
| > 48 months | 1.03 (0.79 to 1.34) | 52% | 537 | > 49,000 | |
| Unclear duration | 0.09 (0.01 to 1.60) | N/A | 5 | > 100 | |
| Subgroup by baseline SFA, P = 0.35 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 1.06 (0.90 to 1.25) | 11% | 644 | > 51,000 | |
| > 15 to 18%E SFA | |||||
| > 18%E SFA | 0.82 (0.55 to 1.21) | N/A | 91 | > 800 | |
| Subgroup by SFA change, P = 0.35 | Up to 4%E SFA difference | 1.06 (0.90 to 1.25) | 11% | 644 | > 51,000 |
| > 4 to 8%E SFA difference | |||||
| > 8%E SFA difference | 0.82 (0.55 to 1.21) | N/A | 91 | > 800 | |
| Subgroup by sex, P = 0.26 | Men | 0.98 (0.79 to 1.23) | 30% | 489 | > 4000 |
| Women | 1.01 (0.83 to 1.24) | 0% | 392 | > 48,000 | |
| Mixed, men and women | 0.09 (0.01 to 1.60) | N/A | 5 | > 100 | |
| Subgroup by CVD risk, P = 0.23 | Low CVD risk | 0.95 (0.78 to 1.16) | 0% | 400 | > 47,000 |
| Moderate CVD risk | 0.09 (0.01 to 1.60) | N/A | 5 | > 100 | |
| Existing CVD | 1.03 (0.83 to 1.27) | 22% | 481 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.73 | TC reduced by ≥ 0.2 mmol/L | 0.96 (0.75 to 1.24) | 42% | 491 | > 4000 |
| TC reduced by < 0.2 mmol/L | 1.02 (0.83 to 1.25) | 0% | 395 | > 48,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P = 0.62 | 1960s | 0.84 (0.66, 1.06) | 23% | 40 | 1651 |
| 1970s | 0.54 (0.03, 9.26) | 75% | 63 | 560 | |
| 1980s | 1.00 (0.76, 1.30) | N/A | 194 | 2033 | |
| 1990s | |||||
| 2000s | 1.01 (0.83, 1.24) | 0% | 392 | > 48,000 | |
| CHO: carbohydrate | |||||
| Analysis | RR (95% CI) of CHD events | I² | No. of events | No. of participants | |
| Main analysis | 0.87 (0.74 to 1.03) | 66% | 3307 | > 53,000 | |
| Sensitivity analyses | Stated aim to reduce SFA | 0.87 (0.74 to 1.03) | 66% | 3307 | > 53,000 |
| SFA significantly reduced | 1.95 (0.82 to 1.10) | 49% | 2988 | > 52,000 | |
| TC significantly reduced | 0.85 (0.70 to 1.03) | 75% | 3034 | > 52,000 | |
| Minus WHI | 0.80 (0.61 to 1.03) | 59% | 758 | > 4000 | |
| Mantel‐Haenszel fixed‐effect | 0.93 (0.87 to 0.99) | 66% | 3307 | > 53,000 | |
| Peto fixed‐effect | 0.92 (0.86 to 0.99) | 72% | 3307 | > 53,000 | |
| SFA: saturated fatty acid | |||||
| Analysis, P value for subgroup differences | RR (95% CI) of CHD events | I² | No. of events | No. of participants | |
| Subgroup by replacement P = 0.28 | PUFA replacement | 0.76 (0.57 to 1.00) | 71% | 737 | > 3000 |
| MUFA replacement | 1.50 (0.62 to 3.61) | N/A | 15 | 52 | |
| CHO replacement | 0.98 (0.83 to 1.14) | 55% | 2846 | > 51,000 | |
| Protein replacement | 0.99 (0.88 to 1.12) | 41% | 2833 | > 51,000 | |
| Subgroup by duration, P = 0.72 | Up to 24 months | 1.01 (0.76 to 1.35) | 5% | 307 | > 2000 |
| > 24 to 48 months | 0.79 (0.55 to 1.14) | 50% | 251 | > 1000 | |
| > 48 months | 0.93 (0.76 to 1.14) | 79% | 2703 | > 49,000 | |
| Unclear duration | 0.60 (0.10 to 3.58) | 81% | 46 | > 200 | |
| Subgroup by baseline SFA, P = 0.09 | Up to 12%E SFA | N/A | |||
| > 12 to 15%E SFA | 0.99 (0.88 to 1.12) | 34% | 2837 | > 51,000 | |
| > 15 to 18%E SFA | 0.30 (0.09 to 0.98) | N/A | 13 | 60 | |
| > 18%E SFA | 0.77 (0.56 to 1.04) | N/A | 138 | > 800 | |
| Subgroup by SFA change, P = 0.09 | Up to 4%E SFA difference | 0.99 (0.88 to 1.12) | 34% | 2837 | > 51,000 |
| >4 to 8%E SFA difference | 0.30 (0.09 to 0.98) | N/A | 13 | 60 | |
| >8%E SFA difference | 0.77 (0.56 to 1.04) | N/A | 138 | > 800 | |
| Subgroup by sex, P = 0.39 | Men | 0.84 (0.70 to 1.02) | 35% | 708 | > 3000 |
| Women | 1.02 (0.84 to 1.23) | 77% | 2549 | > 48,000 | |
| Mixed, men and women | 0.88 (0.18 to 4.36) | 76% | 50 | > 500 | |
| Subgroup by CVD risk, P = 0.95 | Low CVD risk | 0.90 (0.76 to 1.05) | 33% | 2236 | > 47,000 |
| Moderate CVD risk | 0.88 (0.18 to 4.36) | 76% | 50 | > 500 | |
| Existing CVD | 0.94 (0.75 to 1.17) | 61% | 1021 | > 5000 | |
| Subgroup by serum TC reduction, P = 0.06 | TC reduced by ≥ 0.2 mmol/L | 0.75 (0.58 to 0.99) | 65% | 738 | > 4000 |
| TC reduced by < 0.2 mmol/L | 1.03 (0.87 to 1.21) | 44% | 2569 | > 49,000 | |
| Unclear TC change | |||||
| Subgroup by decade of publication, P < 0.001 | 1960s | 0.84 (0.68, 1.05) | 30% | 419 | 1731 |
| 1970s | 0.27 (0.14, 0.52) | N/A | 38 | 102 | |
| 1980s | 0.91 (0.73, 1.14) | N/A | 276 | 2033 | |
| 1990s | 0.33 (0.10, 1.09) | N/A | 13 | 57 | |
| 2000s | 1.03 (0.86, 1.23) | 48% | 2561 | > 49,000 | |
| CHO: carbohydrate | |||||
| Outcome | Effect (95% CI) | I² | No. of studies (participants) | Differential effect by replacement? | Summary |
| TC, mmol/L | ‐0.24 (‐0.36 to ‐0.13) | 60% | 13 (7115) | No, P = 0.20 | TC reduced |
| LDL, mmol/L | ‐0.19 (‐0.33 to ‐0.05) | 37% | 5 (3291) | No, P = 0.16 | LDL reduced |
| HDL, mmol/L | ‐0.01 (‐0.02 to 0.01) | 0% | 7 (5183) | No, P = 0.99 | No effect |
| TG, mmol/L | ‐0.08 (‐0.21 to 0.04) | 51% | 7 (3845) | No, P = 0.12 | No effect |
| TG/HDL ratio | N/A | N/A | N/A | N/A | No data |
| TC/HDL ratio | ‐0.10 (‐0.33 to 0.13) | 24% | 3 (2985) | No, P = 0.45 | No effect |
| LDL/HDL ratio | ‐0.36 (‐0.92 to 0.20) | N/A | 1 (50) | N/A | Unclear |
| Lipoprotein (a), mmol/L | 0.00 (‐0.00 to 0.00) | 0% | 2 (2882) | No, P = 1.00 | No effect |
| Diabetes diagnosis | RR 0.96 (0.90 to 1.02) | N/A | 1 (> 48,000; 3342 diagnoses) | No, P = 1.00 | No effect |
| HbA1c, % | N/A | N/A | N/A | N/A | No data |
| Glucose 2 hrs post GTT, mmol/L | ‐1.69 (‐2.55 to ‐0.82) | 45% | 3 (249) | N/A | Reduced |
| HOMA | 0.00 (‐0.04 to 0.04) | NA | 1 (2832) | N/A | No effect |
| GTT: glucose tolerance test | |||||
| Outcome | Effect (95% CI) | I² | No. of studies (participants) | Differential effect by replacement? | Summary |
| Cancer diagnoses | RR 0.94 (0.83 to 1.07) | 33% | 4 (> 52,000; 5476 diagnoses) | No, P = 0.33 | No effect |
| Cancer deaths | RR 1.00 (0.61 to 1.64) | 49% | 5 (> 52,000; 2472 deaths) | No, P = 0.94 | No effect |
| Body weight, kg | MD ‐1.97 (‐3.67 to ‐0.27) | 72% | 6 (4541) | No, P = 1.00 | Weight loss |
| BMI, kg/m² | MD ‐0.50 (‐0.82 to ‐0.19) | 55% | 6 (5553) | No, P = 0.41 | BMI reduced |
| Systolic BP, mmHg | MD ‐0.19 (‐1.36 to 0.97) | 0% | 5 (3812) | No, P = 0.97 | No effect |
| Diastolic BP, mmHg | MD ‐0.36 (‐1.03 to 0.32) | 0% | 5 (3812) | No, P = 1.00 | No effect |
| BMI: body mass index | |||||
| Cut‐ off | RR of all‐cause mortality | RR of CVD mortality | RR of CVD events | RR of MI | RR of non‐fatal MI | RR of stroke | RR of CHD mortality | RR of CHD events |
| 7%E | 1.11 (0.58 to 2.12) | 0.20 (0.01 to 4.15) | 0.20 (0.01 to 4.15) | N/A | N/A | N/A | N/A | N/A |
| 8%E | 1.11 (0.58 to 2.12) | 0.20 (0.01 to 4.15) | 0.20 (0.01 to 4.15) | N/A | N/A | N/A | N/A | N/A |
| 9%E | 0.99 (0.84 to 1.15) | 0.69 (0.51 to 0.94) | 0.79 (0.62 to 0.99) | 0.76 (0.55 to 1.05) | 0.62 (0.31 to 1.21) | 0.59 (0.30 to 1.15) | 0.82 (0.55 to 1.21) | 0.77 (0.56 to 1.04) |
| 10%E | 0.99 (0.90 to 1.09) | 0.97 (0.74 to 1.26) | 0.89 (0.74 to 1.07) | 0.93 (0.80 to 1.08) | 0.99 (0.69 to 1.41) | 1.00 (0.89 to 1.12) | 1.05 (0.83 to 1.32) | 0.93 (0.75 to 1.14) |
| 11%E | 1.00 (0.88 to 1.12) | 0.95 (0.73 to 1.24) | 0.88 (0.74 to 1.05) | 0.94 (0.84 to 1.06) | 0.99 (0.69 to 1.41) | 0.98 (0.83 to 1.14) | 1.02 (0.87 to 1.20) | 0.94 (0.77 to 1.15) |
| 12%E | 0.99 (0.91 to 1.07) | 0.96 (0.79 to 1.18) | 0.91 (0.79 to 1.04) | 0.94 (0.85 to 1.04) | 0.95 (0.72 to 1.25) | 0.99 (0.88 to 1.12) | 1.02 (0.87 to 1.20) | 0.95 (0.82 to 1.10) |
| 13%E | 1.02 (0.83 to 1.25) | 0.93 (0.63 to 1.38) | 0.78 (0.61 to 1.00) | 0.87 (0.73 to 1.04) | 0.72 (0.50 to 1.03) | 0.54 (0.29 to 1.00) | 1.06 (0.76 to 1.48) | 0.84 (0.63 to 1.12) |
| CHD coronary heart disease | ||||||||
| Quality assessment | No of participants (study event rate%) | Effect | Quality | Importance | ||||||||
| No of studies | Design 1 | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||
| All‐cause mortality (follow‐up mean 56 months1) | ||||||||||||
| 12 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1377/22819 (6%) | 1899/33039 (5.7%) | RR 0.97 (0.9 to 1.05) | 17 fewer (from 57 fewer to 29 more) | ♁♁♁O | CRITICAL |
| Cardiovascular mortality (follow‐up mean 53 months1) | ||||||||||||
| 12 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 483/21844 | 613/31577 | RR 0.95 | 10 fewer | ♁♁♁O MODERATE | CRITICAL |
| Cardiovascular events (follow‐up mean 52 months1) | ||||||||||||
| 13 | RCTs | no serious risk of bias2 | serious inconsistency7 | no serious indirectness4 | no serious imprecision8 | none6 | 1774/21791 | 2603/31509 | RR 0.83 | 138 fewer (from 33 fewer to 228 fewer) | ♁♁♁O MODERATE | CRITICAL |
| Fatal and non‐fatal myocardial infarction (follow‐up mean 55 months1) | ||||||||||||
| 11 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 717/21725 | 997/31442 | RR 0.90 | 32 fewer (from 63 fewer to 3 more) | ♁♁♁O MODERATE | CRITICAL |
| Non‐fatal myocardial infarction (follow‐up mean 55 months1) | ||||||||||||
| 9 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none9 | 547/21559 | 801/31275 | RR 0.95 | 13 fewer (from 51 fewer to 33 more) | ♁♁♁O | CRITICAL |
| Stroke (follow‐up 59 mean months1) | ||||||||||||
| 8 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none9 | 453/20602 | 672/30350 | RR 1.00 | 0 fewer (from 25 fewer to 25 more) | ♁♁♁O | CRITICAL |
| CHD mortality (follow‐up mean 65 months1) | ||||||||||||
| 10 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none 6 | 401/21714 | 485/31445 | RR 0.98 | 30 fewer (from 25 fewer to 23 more) | ♁♁♁O | CRITICAL |
| CHD events (follow‐up mean 59 months1) | ||||||||||||
| 12 | RCTs | no serious risk of bias2 | serious inconsistency10 | no serious indirectness4 | serious imprecision5 | none 6 | 1346/21743 | 1961/31456 | RR 0.87 | 80 fewer (from 160 fewer to 19 more) | ♁♁OO | CRITICAL |
| 1Minimum study duration was 24 months. 2These large RCTs of relatively long duration were well randomised and almost half had good allocation concealment (the rest were unclear). Blinding was only well‐conducted in 1 RCT, however blinding is very difficult in trials of dietary fat intake. Incomplete outcome data were variable, and most included studies had systematic differences in care (i.e. intervention group had more time or attention than the control group). These risks to validity were combined with risks from imprecision, and outcomes were downgraded once for a combination of both issues. We noted no other biases. We noted that the level of compliance with interventions involving long‐term behaviour change, such as those used in these studies, can vary widely. This is likely to attenuate the pooled effect and bias it towards the null. 3No important heterogeneity; I² ≤ 30% 4These RCTs directly assessed the effect of lower vs usual saturated fat intake on health outcomes of interest. Participants included men and women with and without CVD at baseline (also some participants with CVD risk factors like diabetes, or at risk of cancers). 5 The 95% CI crosses 1.0 and does not exclude important benefit or harm 6The funnel plot did not suggest any small study (publication) bias 7Potentially important heterogeneity was identified; I² = 65%. However, the heterogeneity was partly explained by the degree of saturated fat reduction, and the degree of cholesterol lowering achieved (in subgrouping and in meta‐regression). 8The 95% CI does not cross 1.0 or a threshold of important harm. 9Too few studies to reliably assess publication bias (< 10 RCTs). 10Important heterogeneity; I² = 66% | ||||||||||||
| Quality assessment | No of participants (study event rate %) | Effect | Quality | Importance | ||||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||||
| All‐cause mortality (follow‐up mean 56 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 406/2123 | 418/2115 | RR 0.96 (0.82 to 1.13) | 79 fewer (from 360 fewer to 256 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular mortality (follow‐up mean 55 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | serious inconsistency7 | no serious indirectness4 | serious imprecision5 | none6 | 266/2123 | 287/2128 | RR 0.95 (0.73 to 1.25) | 67 fewer (from 364 fewer to 337 more) | ♁♁OO | CRITICAL | ||
| Cardiovascular events (follow‐up mean 53 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | serious inconsistency8 | no serious indirectness4 | no serious imprecision9 | none6 | 390/1953 | 494/1942 | RR 0.73 (0.58 to 0.92) | 687 fewer (from 204 fewer to 1068 fewer) | ♁♁♁O | CRITICAL | ||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 53 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 269/1953 | 322/1942 | RR 0.83 (0.67 to 1.02) | 282 fewer (from 547 fewer to 33 more) | ♁♁♁O | CRITICAL | ||
| Non‐fatal myocardial infarction (follow‐up mean 53 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 104/1875 | 129/1863 | RR 0.8 (0.63 to 1.03) | 138 fewer (from 256 fewer to 21 more) | ♁♁♁O | CRITICAL | ||
| Stroke (follow‐up mean 63 months1) | ||||||||||||||
| 4 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | very serious10 | none6 | 17/856 | 24/850 | RR 0.68 (0.37 to 1.27) | 90 fewer (from 178 fewer to 76 more) | ♁OOO | CRITICAL | ||
| CHD mortality (follow‐up mean 36 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 240/2147 | 251/2151 | RR 0.98 (0.74 to 1.28) | 23 fewer (from 303 fewer to 327 more) | ♁♁♁O | CRITICAL | ||
| CHD events (follow‐up mean 53 months1) | ||||||||||||||
| 7 | RCTs | no serious risk of bias2 | serious inconsistency11 | no serious indirectness4 | serious imprecision5 | none6 | 329/1956 | 408/1944 | RR 0.76 (0.57 to 1.0) | 504 fewer (from 902 fewer to 0 more) | ♁♁OO | CRITICAL | ||
| * Polyunsaturated fatty acids replacing saturated fatty acids in individual studies were predominantly of plant origin. 1 Minimum study duration was 24 months. 2 These large RCTs of relatively long duration were well randomised but fewer than half had good allocation concealment (the rest were unclear). Blinding was only well‐conducted in 1 RCT, however blinding is very difficult in trials of dietary fat intake. In about half the included studies, it was unclear if outcome data were incomplete and most studies had systematic differences in care (i.e. intervention group had more time or attention than the control group). We noted no other biases. Not downgraded for bias, however we note that the level of compliance with interventions involving long‐term behaviour change, such as those used in many of these studies, can vary widely. This is likely to attenuate the pooled effect and bias it towards the null. 3 No important heterogeneity; I² < 50% 6 Too few studies to reliably assess publication bias (< 10 RCTs). 7 Important heterogeneity; I² = 55% 8 Important heterogeneity; I² = 69%.Subgrouping suggested greater effects on cardiovascular events with greater reduction in SFA intake, higher baseline SFA intake and greater serum total cholesterol reduction (meta‐regression not carried out). 9 95% CI does not cross threshold of important benefit or harm. | ||||||||||||||
| Quality assessment | No of participants (study event rate%) | Effect | Quality | Importance | |||||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | |||||
| All‐cause mortality (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 3/26 | 1/26 | RR 3.0 (0.33 to 26.99) | 769 more (from 258 fewer to 9996 more) | ♁OOO | CRITICAL | |||
| Cardiovascular mortality (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 3/26 | 1/26 | RR 3.0 (0.33 to 26.99) | 769 more (from 258 fewer to 9996 more) | ♁OOO | CRITICAL | |||
| Cardiovascular events (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 11/26 | 11/26 | RR 1.0 (0.53 to 1.89) | 0 fewer (from 1988 fewer to 3765 more) | ♁OOO | CRITICAL | |||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 7/26 | 5/26 | RR 1.4 (0.51 to 3.85) | 769 more (from 942 fewer to 5481 more) | ♁OOO | IMPORTANT | |||
| Non‐fatal myocardial infarction (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 6/26 | 5/26 | RR 1.2 (0.42 to 3.45) | 385 more (from 1115 fewer to 4711 more) | ♁OOO | IMPORTANT | |||
| Stroke | |||||||||||||||
| 0 | No studies identified reporting this outcome | ||||||||||||||
| CHD mortality (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 3/26 | 1/26 | RR 3 (0.33 to 26.99) | 769 more (from 258 fewer to 9996 more) | ♁OOO | IMPORTANT | |||
| CHD events (follow‐up mean 24 months) | |||||||||||||||
| 1 | RCTs | serious risk of bias1 | no serious inconsistency2 | no serious indirectness3 | very serious imprecision4 | none5 | 9/26 | 6/26 | RR 1.5 (0.62 to 3.61) | 1154 more (from 877 fewer to 6023 more) | ♁OOO | IMPORTANT | |||
| 1This single, very small RCT of relatively long duration was well randomised, but had an unclear risk of bias in terms of allocation concealment and incomplete outcome data, and lacked participant blinding; however blinding is very difficult in trials of dietary fat intake. We downgraded it for serious risk of bias. 3This RCT directly assessed the effect of reducing saturated fat, and replacing it with other dietary sources of energy, compared to usual diet, on health outcomes of interest. Participants included men with CVD at baseline. 4The 52 participants in the relevant arms of this trial experienced relatively few events. As a result, there were wide to very wide confidence intervals. In addition, the 95% CI crosses 1.0 and does not exclude important benefit or harm. 5Too few studies to reliably assess publication bias (< 10 RCTs). | |||||||||||||||
| Quality assessment | No of participants (study event rate %) | Effect | Quality | Importance | ||||||||||
| No of studies | Design1 | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||||
| All‐cause mortality (follow‐up mean 48 months2) | ||||||||||||||
| 6 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 1080/21715 | 1597/31954 | RR 0.98 (0.91 to 1.05) | 10 fewer (from 45 fewer to 25 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular mortality (follow‐up mean 46 months2) | ||||||||||||||
| 6 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 316/20740 | 429/30492 | RR 0.99 (0.86 to 1.14) | 1 fewer (from 20 fewer to 20 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular events (follow‐up mean 46 months2) | ||||||||||||||
| 6 | RCTs | no serious risk of bias3 | serious inconsistency9 | no serious indirectness5 | serious imprecision6 | none7 | 1512/20740 | 2273/30492 | RR 0.93 (0.79 to 1.08) | 52 fewer (from 157 fewer to 60 more) | ♁♁OO | CRITICAL | ||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 51 months2) | ||||||||||||||
| 4 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 572/20674 | 820/30425 | RR 0.96 (0.86 to 1.06) | 11 fewer (from 38 fewer to 16 more) | ♁♁♁O | IMPORTANT | ||
| Non‐fatal myocardial infarction (follow‐up mean 60 months2) | ||||||||||||||
| 3 | RCTs | no serious risk of bias3 | serious inconsistency9 | no serious indirectness5 | serious imprecision6 | none7 | 470/20559 | 718/30309 | RR 0.99 (0.73 to 1.35) | 2 fewer (from 64 fewer to 83 more) | ♁♁OO | IMPORTANT | ||
| Stroke (follow‐up mean 60 months2) | ||||||||||||||
| 4 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 435/19656 | 648/29410 | RR 1.01 (0.9 to 1.13) | 2 more (from 22 fewer to 29 more) | ♁♁♁O | IMPORTANT | ||
| CHD mortality (follow‐up mean 60 months2) | ||||||||||||||
| 3 | RCTs | no serious risk of bias3 | no serious inconsistency4 | no serious indirectness5 | serious imprecision6 | none7 | 255/20559 | 331/30309 | RR 1.01 (0.86 to 1.18) | 1 more (from 15 fewer to 20 more) | ♁♁♁O | IMPORTANT | ||
| CHD events (follow‐up mean 51 months2) | ||||||||||||||
| 5 | RCTs | no serious risk of bias3 | serious inconsistency8 | no serious indirectness5 | serious imprecision6 | none7 | 1140/20677 | 1706/30427 | RR 0.98 (0.83 to 1.14) | 11 fewer (from 95 fewer to 79 more) | ♁♁OO | IMPORTANT | ||
| 1There was insufficient information across all studies to make any determination about the type of carbohydrate used as replacement. 2Minimum study duration was 24 months. 4No important heterogeneity; I² = 0%. 6The 95% CI crosses 1.0 and does not exclude important benefit or harm. 7Too few studies to reliably assess publication bias (< 10 RCTs). 8Important heterogeneity; I² = 55%. | ||||||||||||||
| Quality assessment | No of participants (study event rate%) | Effect | Quality | Importance | ||||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Reduced saturated fat intake | Usual saturated fat intake | Relative effect | Absolute effects (per 10,000) | ||||
| All‐cause mortality (follow‐up mean 50 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1079/21688 | 1594/31926 | RR 0.98 (0.91 to 1.06) | 10 fewer (from 45 fewer to 30 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular mortality (follow‐up mean 48 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 315/20713 | 426/30464 | RR 0.99 (0.86 to 1.14) | 1 fewer (from 20 fewer to 20 more) | ♁♁♁O | CRITICAL | ||
| Cardiovascular events (follow‐up mean 48 months1) | ||||||||||||||
| 5 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1504/20713 | 2253/30464 | RR 0.98 (0.9 to 1.06) | 15 fewer (from 74 fewer to 44 more) | ♁♁♁O | CRITICAL | ||
| Fatal and non‐fatal myocardial infarction (follow‐up mean 56 months1) | ||||||||||||||
| 3 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 571/20647 | 818/30397 | RR 0.96 (0.86 to 1.07) | 11 fewer (from 38 fewer to 19 more) | ♁♁♁O | IMPORTANT | ||
| Non‐fatal myocardial infarction (follow‐up mean 60 months1) | ||||||||||||||
| 3 | RCTs | no serious risk of bias2 | serious inconsistency7 | no serious indirectness4 | serious imprecision5 | none6 | 470/20559 | 718/30309 | RR 0.99 (0.73 to 1.35) | 2 fewer (from 64 fewer to 83 more) | ♁♁OO | IMPORTANT | ||
| Stroke (follow‐up mean 72 months1) | ||||||||||||||
| 3 | RCTs | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 435/19629 | 647/29382 | RR 1.01 (0.89 to 1.15) | 2 more (from 24 fewer to 33 more) | ♁♁♁O | IMPORTANT | ||
| CHD mortality (follow‐up mean 60 months1) | ||||||||||||||
| 3 | randomised trials | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 255/20559 | 331/30309 | RR 1.01 (0.86 to 1.18) | 1 more (from 15 fewer to 20 more) | ♁♁♁O | IMPORTANT | ||
| CHD events (follow‐up mean 56 months1) | ||||||||||||||
| 4 | randomised trials | no serious risk of bias2 | no serious inconsistency3 | no serious indirectness4 | serious imprecision5 | none6 | 1137/20647 | 1696/30397 | RR 0.99 (0.88 to 1.12) | 6 fewer (from 67 fewer to 67 more) | ♁♁♁O | IMPORTANT | ||
| 1Minimum study duration was 24 months. 5The 95% CI crosses 1.0 and does not exclude important benefit or harm. 6Too few studies to reliably assess publication bias (< 10 RCTs). 7Important heterogeneity; I² = 75%. | ||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 12 | 55858 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 2 Cardiovascular mortality Show forest plot | 12 | 53421 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.12] |
| 3 Combined cardiovascular events Show forest plot | 13 | 53300 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarctions Show forest plot | 11 | 53167 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 2 Non‐fatal MI Show forest plot | 9 | 52834 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.13] |
| 3 Stroke Show forest plot | 8 | 50952 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 4 CHD mortality Show forest plot | 10 | 53159 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.15] |
| 5 CHD events Show forest plot | 12 | 53199 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.74, 1.03] |
| 6 Diabetes diagnoses Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol, mmol/L Show forest plot | 14 | 7115 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.36, ‐0.13] |
| 2 LDL cholesterol, mmol/L Show forest plot | 5 | 3291 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| 3 HDL cholesterol, mmol/L Show forest plot | 6 | 5147 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 4 Triglycerides, mmol/L Show forest plot | 7 | 3845 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.21, 0.04] |
| 5 total cholesterol /HDL ratio Show forest plot | 3 | 2985 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 6 LDL /HDL ratio Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Lp(a), mmol/L Show forest plot | 2 | 2882 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 8 Insulin sensitivity Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 HbA1c (glycosylated haemoglobin), % | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 GTT (glucose tolerance test), glucose at 2 hours, mmol/L | 3 | 249 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.55, ‐0.82] |
| 8.3 HOMA | 1 | 2832 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |


