Inmunoterapia para el carcinoma metastásico de células renales
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: 2‐arm, parallel‐group, double‐blind, placebo‐controlled RCT. Study dates: recruitment from October 2006 to March 2008, follow‐up to March 2009, range of follow‐up 12 to 29 months. Setting: multicentre, international, phase III. Countries: France, Germany, Israel, Poland, Romania, Russia, Spain, UK, Ukraine, US. | |
| Participants | Inclusion criteria: people with histologically confirmed advanced or metastatic clear‐cell RCC with prior nephrectomy who required first‐line treatment, aged ≥ 18 years, measurable disease, KPS ≥ 80%, MSKCC 0‐2, life expectancy > 12 weeks. Exclusion criteria: cerebral metastases, prior exposure to MVA‐5T4, known allergy to vaccinia vaccinations or egg proteins, pregnancy. Sample size:732. Age (years, median with range): group 1: 58 (18 to 86); group 0: 58 (24 to 85). Sex (M/F, %): group 1: 69.6/30.4; group 0: 65.1/34.9. Prognostic factors:
| |
| Interventions | Group 1 (n = 365): MVA‐5T4 (modified vaccinia Ankara encoding the tumour antigen 5T4). Group 0 (n = 367): placebo. Both as IM injection into the deltoid muscle at weeks 1, 3, 6, 9, 13, 17, 21, 25, 33, 41, 49, 57, 65. Cointerventions (standard‐of‐care according to local practice):
| |
| Outcomes | OS (primary outcome) How measured: active follow‐up. Time points measured: censored to March 2009. Time points reported: Kaplan‐Meier survival curves over up to 30 months, HR (with 95% CI), median. Subgroups: risk prognosis and standard of care (no comparison group 1 vs group 0 reported). AEs, grade ≥ 3 (primary safety outcome) How measured: NCI‐CTC (treatment‐emergent SAE all, grade 3, 4, 5). Time points measured and reported: not reported. Subgroups: not reported. QoL not evaluated. PFS (secondary outcome) How measured: not reported. Time points measured: week 26. Time points reported: not reported. Subgroups: not reported. Tumour remission (secondary outcome) How measured: complete response, partial response, stable disease. Time points measured and reported: week 26. Subgroups: not reported. | |
| Funding sources | Sponsored by Oxford BioMedica. | |
| Declarations of interest | RH, WHS, SN: named inventors on several Oxford BioMedica patents. REH: minor consultancy role for Oxford BioMedica. | |
| Notes | Trial registration: NCT00397345, at the recommendation of the data safety monitoring board, the sponsor terminated the administration of MVA‐5T4/placebo to participants in July 2008 due to little or no prospect of demonstrating a significant survival benefit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratification for standard of care, severity of disease and geographic location. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias assumed due to blinding of participants and study personnel. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias assumed due to blinding of participants and study personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed due to blinding of participants, study personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed due to blinding of participants, study personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on OS and PFS assumed, analysis with active follow‐up on survival data after termination of drug administration with no further description, no differences in censoring detected. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on safety outcomes assumed, assessment on the basis of all participants as randomized. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on tumour remission assumed, assessment on the basis of all participants as randomized. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | High risk | PFS was mentioned as secondary outcome, but no information provided. |
| Other bias | High risk | Safety monitoring board recommended stopping in July 2008 because there was little prospect of demonstrating a significant benefit in OS, second‐line therapies in 32% of placebo and 29% of MVA‐5T4 patients. |
| Methods | Study design: 2‐arm, parallel‐group, double‐blind, placebo‐controlled RCT. Study dates: randomization from June 2004 to October 2005, clinical cutoff: September 2006, median follow‐up of 13.3 months (0 to 25.6) (clinical cutoff), median follow‐up for OS: group 1: 21, group 0: 23 months to September 2008. Setting: multicentre, international, phase III. Countries: Europe (Belgium, Czech Republic, Finland, France, Germany, Hungary, Israel, Italy, Netherlands, Norway, Poland, Russia, Spain, Switzerland, UK), Asia (Russia, Singapore, Taiwan), Australia. | |
| Participants | Inclusion criteria: people with measurable or non‐measurable, predominantly clear‐cell mRCC with no prior systemic therapy, prior total or partial nephrectomy, KPS ≥ 70%; aged ≥ 18 years; normal hepatic, haematopoietic and renal function, and only minimal proteinuria. Exclusion criteria: prior systemic treatment for mRCC, recent major surgical procedures, evidence of brain metastases, ongoing full‐dose oral or parenteral anticoagulant or antiplatelet aggregation treatment, uncontrolled hypertension on medication, clinically significant cardiovascular disease, chronic corticosteroid treatment. Sample size:649. Age (years, median with range): group 1: 61 (30 to 82); group 0: 60 (18 to 81). Sex (M/F, %): group 1: 68/32; group 0: 73/27. Prognostic factors:
| |
| Interventions | Group 1 (n = 322): IFN‐α + placebo IFN α‐2a (Hoffmann‐La Roche Ltd, Basel, Switzerland) 9 MIU 3 times/week SC for maximum 52 weeks or until disease progression, unacceptable toxicity or withdrawal of consent, an initial dose < 9 MIU permitted if the recommended dose was reached within the first 2 weeks of treatment, dose reduction; to 6 MIU or 3 MIU to manage AE attributable to IFN‐α. Placebo: every 2 weeks until disease progression, unacceptable toxicity or withdrawal of consent. Group 0 (n = 327): IFN‐α + bevacizumab IFN α‐2a (Hoffmann‐La Roche Ltd, Basel, Switzerland) 9 MIU 3 times/week SC for maximum 52 weeks or until disease progression, unacceptable toxicity or withdrawal of consent, an initial dose < 9 MIU permitted if the recommended dose was reached within the first 2 weeks of treatment, dose reduction; to 6 MIU or 3 MIU to manage AE attributable to IFN‐α. Bevacizumab (Hoffmann‐La Roche Ltd, Basel, Switzerland) 10 mg/kg IV every 2 weeks until disease progression, unacceptable toxicity or withdrawal of consent, no dose reduction permitted. Cointerventions (standard‐of‐care according to local practice): not reported. | |
| Outcomes | OS (primary outcome) How measured: investigator‐assessed, time between the date of randomization and death due to any cause, censoring on the day of last follow‐up or the last day of study administration if no follow‐up was done. Time points measured: during treatment and follow‐up before unblinding with cross‐over and second‐line therapies. Time points reported: Kaplan‐Meier curves over up to 24 months, median OS, number of deaths at data cutoff before cross‐over and second‐line therapies. Subgroups: MSKCC score. AEs, grade ≥ 3 (secondary outcome) How measured: investigator‐assessed, ongoing documentation of AEs (CTCAE v.3.0), physical examination, electrocardiography, urinalysis, measurement of blood pressure. Time points measured: at each visit, weekly monitoring in participants who developed ≥ grade 3 hypertension, 24‐hour urine collection if protein was observed with a dipstick analysis. Time points reported: frequency of participants with AEs, SAEs (safety population), AEs with grade ≥ 3, most commonly reported AEs with grade ≥ 3 up to 28 days after the last dose, deaths due to AEs. Subgroups: not reported. QoL not evaluated PFS (secondary outcome) How measured: time between randomization and first documented disease progression or death due to any cause, investigator‐assessment and independent review committee. Time points measured: every 8 weeks up to week 32 and every 12 weeks thereafter until disease progression. Time points reported: Kaplan‐Meier survival curves for up to 24 months, median PFS. Subgroups: age, sex, MSKCC score, baseline VEGF, number of metastatic sites. Tumour remission (secondary outcome) How measured: assessment by the investigator with RECIST, non‐measurable lesions were used to define complete response and disease progression only. Time points measured: every 8 weeks up to week 32, every 12 weeks thereafter until disease progression, responses had to be confirmed by a second assessment ≥ 4 weeks after the first response was recorded. Time points reported: best tumour response for participants with measurable disease. Subgroups: not reported. | |
| Funding sources | Hoffmann‐La Roche Ltd. | |
| Declarations of interest | BE: consulted for and received honoraria from Roche, Bayer, Wyeth, Pfizer, Inate and Antigenics. SB: consulted for Roche, Pfizer, Wyeth and Bayer. AR: acted as an adviser for Bayer, Pfizer, GSK, Novartis and Wyeth. NM: employee of and owns stock of Roche. BM: received honoraria and research funding from Roche. | |
| Notes | BO17705E, NCT00738530 (registered October 2008), preplanned interim analysis with significant benefit in OS, unblinding, data and safety monitoring board recommended cross‐over of participants from the placebo to the bevacizumab group, differences in availability of new second‐line therapies in countries might confound OS results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No selection bias assumed, the randomization list bases on block design procedure, stratified by country and MSKCC risk group. |
| Allocation concealment (selection bias) | Low risk | No selection bias assumed, central allocation by an interactive voice recognition system, list kept in a secure location, not available to any person directly involved in the study. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on subjective outcomes assumed, blinding of participants and study personnel with same route and timing as bevacizumab until preplanned interim analysis and data cutoff. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on objective outcomes assumed, blinding of participants and study personnel with same route and timing as bevacizumab until preplanned interim analysis and data cutoff. |
| Blinding of outcome assessment (detection bias) | Low risk | No performance bias on subjective outcomes assumed, blinding of participants and study personnel with same route and timing as bevacizumab until preplanned interim analysis and data cutoff. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed due to blinding of participants, study personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on OS and PFS assumed due similar censoring between treatment groups, 251 deaths and 505 progression events from 649 participants had occurred at the time of data cutoff. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on safety outcomes assumed, all participants who included at least 1 dose of bevacizumab were compared to participants who received no bevacizumab. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on tumour remission assumed, all participants with measurable disease at baseline were included. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | Low risk | No reporting bias on tumour remission assumed, no reporting bias assumed, all preplanned outcomes reported. |
| Other bias | High risk | Cross‐over of 13 (4%) participants from group 0 to group 1, 49 (15%) participants in group 1 and 64 (20%) participants in group 0 received second‐line therapy with tyrosine kinase inhibitors. |
| Methods | Study design: 3‐arm, parallel‐group, open‐label RCT. Study dates: recruitment from July 2003 to April 2005, follow‐up until second interim analysis after 446 deaths (June 2006), range of follow‐up: 14 to 36 months. Setting: multicentre, international, phase III. Countries: Global Advanced Renal Cell Carcinoma (ARCC) Trial including countries of all continents, specifically: Argentina, Australia, Canada, Czech Republic, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Netherlands, Poland, Russia, Serbia, Montenegro, Slovakia, South Africa, Spain, Sweden, Taiwan, Turkey, Ukraine, UK, US. | |
| Participants | Inclusion criteria: histologically confirmed advanced RCC (stage IV or recurrent disease), KPS ≥ 60, no previous systemic therapy, measurable disease, adequate bone marrow, renal and hepatic functions (neutrophil count > 1500 cells/mm3, platelet count > 100,000 cells/mm3, haemoglobin count > 8 g/dL. People with a history of brain metastases if their condition was neurologically stable and they did not require corticosteroids after surgical resection or radiotherapy. Exclusion criteria: serum creatinine level ≤ 1.5 times ULN; aspartate aminotransferase level ≤ 3 times ULN (≤ 5 times if liver metastases present); total bilirubin level ≤ 1.5 times ULN; fasting level of total cholesterol ≤ 350 mg/dL, triglyceride level ≤ 400 mg/dL. Sample size:626. Age (years, median with range): group 1: 59 (32 to 82); group 1a: 60 (23 to 86); group 0: 58 (32 to 81). Sex (M/F, %): group 1: 69/31; group 1a: 71/28; group 0: 66/33. Prognostic factors:
| |
| Interventions | Group 1 (n = 210): IFN‐α + temsirolimus Temsirolimus (Wyeth Research, 15 mg IV weekly, 30‐minute infusion) + IFN‐α (Roferon‐A, Roche, starting dose 3 MU 3 times/week for week 1 and 6 MU SC 3 times/week thereafter). Group 1a (n = 207): IFN‐α IFN‐α starting dose of 3 MU SC 3 times/week for the first week, dose was raised to 9 MU 3 times/week for the second week and to 18 MU 3 times/week for week 3, if tolerated. Participants who were unable to tolerate 9 MU or 18 MU received the highest tolerable dose (3 MU, 4.5 MU or 6 MU). Group 0 (n = 209): temsirolimus Temsirolimus 25 mg IV weekly 30‐minute infusion. Cointerventions for participants treated with temsirolimus (standard‐of‐care according to local practice): premedication with diphenhydramine 25 mg to 50 mg IV or a similar histamine H1 blocker given approximately 30 minutes before each weekly temsirolimus infusion as prophylaxis against an allergic reaction. | |
| Outcomes | OS (primary outcome) How measured: investigator assessed, time between date of randomization and date of death. Time points measured: not reported. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median with 95% CI, HR with 95% CI. Subgroups: prior nephrectomy, KPS (≤ 70, > 70). AEs, grade ≥3 How measured: AEs (NCI‐CTC 3.0) occurring in ≥ 20% of participants in any group (all grades and grade 3 or 4), number of AEs, grade 3 or 4 per treatment group, any visit at which the participant reported a symptomatic NCI‐CTC (v.3) grade 3 or 4. Time points measured: weekly or biweekly. Time points reported: treatment period. Subgroups: not evaluated. QOL How measured: EQ‐5D and EQ‐VAS questionnaire (self‐report). Time points measured: at screening, week 12, week 32. Time points reported: week 12, withdrawal or last recorded visit (only IFN‐α vs temsirolimus) (Yang 2010). Subgroups: prior nephrectomy. PFS (secondary outcome) How measured: determined by the site investigators' assessment and a blinded assessment of imaging studies (performed by Bio‐Imaging Technologies, not shown), time between date of randomization and date of disease progression or death, whichever occurred first. Time points measured: not reported. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median with 95% CI, HR with P value. Subgroups: not reported. Tumour remission (secondary outcome) How measured: CT scans of the chest, abdomen and pelvis; radionuclide bone scan MRI or CT scan of the brain; classification into participants with stable disease or objective response (RECIST); % participants who had a confirmed objective response (complete or partial) as their best response to treatment. Time points measured: before treatment, repeated at 8‐week intervals. Time points reported: 24 weeks. Subgroups: not reported. | |
| Funding sources | Wyeth Research, Cambridge, MA, US. | |
| Declarations of interest | GH: financial support from Pfizer and Wyeth; MC, RF, IGHS‐W, RJM: financial support from Wyeth; JD: financial support from Novartis, Chiron, Bayer, Onyx, Pfizer and Wyeth; AK: financial support from Bayer and Wyeth; DMcD: financial support from Bayer, Onyx, Genentech and Novartis. TO'T, SL and LM: full‐time employee of Wyeth Research. | |
| Notes | Trial registration: NCT00065468, study was stopped as a result of the second predefined interim analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified block randomization. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | High risk | Different delivery of the interventions, no placebo‐controlled trial, participants and physicians not blinded. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) | High risk | Kaplan‐Meier estimates of blinded assessment for PFS not shown, outcome assessors not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on PFS or OS assumed due to small censoring rates. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias on safety outcomes assumed, Inclusion of all participants as treated. |
| Incomplete outcome data (attrition bias) | High risk | Differences in postbaseline tumour assessment (group 1: 74% vs group 1a: 80% vs group 0: 92%). |
| Incomplete outcome data (attrition bias) | High risk | High risk of attrition on QoL due to high differences in completion rates between treatment groups. |
| Selective reporting (reporting bias) | Low risk | No reporting bias assumed, nearly all outcomes reported (besides quality‐adjusted time without symptoms or toxicity). |
| Other bias | Low risk | Early stop for benefit, no other risk of bias assumed. |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Study dates: enrolment between August 2004 and October 2005, median duration of treatment: 6 months, range 1 month to 15 months. Setting: inpatients, multicentre (101 centres), international, phase III. Countries: Australia, Brazil, Canada, Europe, US. | |
| Participants | Inclusion criteria: people with histologically confirmed clear‐cell mRCC; aged ≥ 18 years; no previous treatment with systemic therapy for RCC; measurable disease; ECOG Performance Status 0 to 1; adequate haematological, coagulation, hepatic, renal and cardiac function. Exclusion criteria: brain metastases, uncontrolled hypertension or clinically significant cardiovascular events or disease during the preceding 12 months. Sample size:750. Age (years, median with range): group 1: 59 (34 to 85); group 0: 62 (27 to 87). Sex (M/F, %): group 1: 72/28; group 0: 71/29. Prognostic factors:
| |
| Interventions | Group 1 (n = 375): IFN‐α IFN‐α‐2a (Roche) SC injection 3 times/week on non‐consecutive days at 3 MU per dose during first week, 6 MU per dose during second week and 9 MU per dose thereafter) until the occurrence of disease progression, unacceptable AEs or withdrawal of consent. Group 0 (n = 375): sunitinib Sunitinib orally 50 mg once daily in 6‐week cycles consisting of 4 weeks of treatment followed by 2 weeks without treatment. Cointerventions (standard‐of‐care according to local practice): not specified. | |
| Outcomes | OS (secondary endpoint) How measured: not specified. Time points measured: during follow‐up; off‐study every 2 months. Time points reported: Kaplan‐Meier survival curves over up to 36 months (data from Motzer 2009), median with 95% CI, unstratified HR and stratified HR with 95% CI. Subgroups: MSKCC criteria; previous nephrectomy; ECOG Performance Status; lactate dehydrogenase level; time since diagnosis; haemoglobin level; corrected serum calcium level; number of metastatic sites; bone, lung and liver metastases. AEs, grade ≥ 3 (not specified) How measured: CTCAE v.3.0. Time points measured and reported: not reported, no summarized frequencies reported. Subgroups: not reported. QoL (secondary outcome) How measured: FKSI‐15, FACT‐G, EQ‐5D, EQ‐VAS ‐ completion, if > 80% of items in FACT‐G and > 50% of items in FKSI completed. Time points measured and reported: before randomization, on days 1 and 28 of each cycle, overall mean score after 17 weeks (Cella 2008). Subgroups: not reported. PFS (primary endpoint) How measured: time from randomization to the first documentation of objective disease progression or to death from any cause, whichever occurred first with a blinded central review of radiological images. Time points measured: during follow‐up (central review to September 2007). Time points reported: Kaplan‐Meier survival curves over up to 14 months (Motzer 2007), median PFS with 95% CI; HR with 95% CI (central review and investigator‐assessed). Subgroups: MSKCC criteria, previous nephrectomy, age, sex, ECOG Performance Status, lactate dehydrogenase level, time since diagnosis, haemoglobin level, corrected serum calcium level. Tumour remission (secondary outcome) How measured: RECIST with blinded central review of radiological images, complete and partial response, stable disease. Time points measured: day 28 of cycles 1 through 4, and every two cycles thereafter until the end of treatment. Time points reported: independent central review to September 2007 (interim analysis), investigator‐assessed update results (not included). Subgroups: not reported. | |
| Funding sources | IFN‐α‐2a (Roferon‐A, Roche) and sunitinib were provided by Pfizer. | |
| Declarations of interest | RJM: research grants from Pfizer and Genentech, consulting fees from Wyeth and lecture fees from Bayer Pharmaceuticals; TEH: consulting and lecture fees from Pfizer, Bayer Pharmaceuticals and Onyx Pharmaceuticals; DM: consulting fees from Pfizer and Wyeth Pharmaceuticals and lecture fees from Pfizer; RMB: research grants from Pfizer, Bayer Pharmaceuticals, Genentech, Genzyme and Bristol‐Myers Squibb and consulting and lecture fees from Pfizer, Bayer Pharmaceuticals, Onyx Pharmaceuticals and Genentech; OR: consulting and lecture fees from Pfizer; SO: consulting and lecture fees from Pfizer; SN: consulting fees from Pfizer and Bayer Pharmaceuticals; RAF: research grants from Pfizer, consulting fees from Pfizer and Onyx Pharmaceuticals, and lecture fees from Pfizer and Bayer Pharmaceuticals, STK, IC, PWB, CMB: full‐time employees of Pfizer and equity owners in the company. | |
| Notes | Registration: NCT00098657 and NCT00083889, protocol amendment (February 2006), cross‐over of 7% (n = 25) participants from IFN‐α to sunitinib. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk of selection bias due to stratified randomization according to baseline levels of lactate dehydrogenase (> 1.5 vs ≤ 1.5 ULN), ECOG Performance Status (0 vs 1), and previous nephrectomy (yes vs no) with random permuted blocks of 4. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not possible due to different routes of interventions, participants and physicians were not blinded. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded central review of radiological images used to assess primary endpoint and ORR, no blinded assessment of AEs and QoL reported. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included, similar censoring. |
| Incomplete outcome data (attrition bias) | Low risk | All treated participants included. |
| Incomplete outcome data (attrition bias) | High risk | Images of 88 (12%) participants had not been assessed by a central review at the time of interim analysis and were assessed by investigators (results not included). |
| Incomplete outcome data (attrition bias) | Low risk | Completion rates for FKSI, FACT‐G and EQ‐5D questionnaires: 95% with at least 1 postbaseline assessment and slightly lower rates in the group with IFN‐α. |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes from the protocol reported. |
| Other bias | High risk | After the interim analysis, participants in the IFN‐α group with progressive disease were allowed to cross over to the sunitinib group and 25 participants crossed over which may influence OS. |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Study dates: randomization October 2012 to March 2014, data cutoff: June 2015, minimal follow‐up of 14 months. Setting: multicentre (146 centres), international, phase III. Countries: North America (US, Canada), Western Europe (Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Norway, Poland, Romania, Spain, Sweden, UK), South America (Argentina, Brazil) and Asia (Israel, Japan). | |
| Participants | Inclusion criteria: people with histologically confirmed advanced or mRCC with a clear‐cell component, aged ≥ 18 years, measurable disease, prior treatment with 1 or 2 antiangiogenic therapies, ≤ 3 previous systemic therapies with cytokines and cytotoxic drugs and disease progression during or > 1 treatment regimen within 6 months before study enrolment, KPS ≥ 70. Exclusion criteria: central nervous system metastasis, previous treatment with an mTOR inhibitor, condition requiring treatment with glucocorticoids (equivalent to > 10 mg of prednisone > 10 mg daily). Sample size:821. Age (years, median with range): group 1: 62 (23‐88); group 0: 62 (18‐86). Sex (M/F, %): group 1: 77/23; group 0: 74/26. Prognostic factors:
| |
| Interventions | Group 1 (n = 410): nivolumab Nivolumab 3 mg/kg IV every 2 weeks, no dose modifications permitted). Group 0 (n = 411): everolimus Everolimus 10 mg orally, every day, dose modifications permitted. Cointerventions (standard‐of‐care according to local practice): not specified. | |
| Outcomes | OS (primary endpoint) How measured: time from randomization to the date of death. Time points measured: every 8 weeks for the first year, and then every 12 weeks until disease progression or discontinuation of treatment, after discontinuation of treatment, participants followed every 3 months for assessment of survival and subsequent anticancer therapy. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median with 95% CI, unstratified HR with 98.5% CI. Subgroups: MSKCC prognostic score, previous antiangiogenic regimens, region, age, gender. AEs, grade ≥ 3 (secondary endpoint) How measured: NCI‐CTC AE V4.0. Time points measured: at each clinic visit. Time points reported: overall frequency of all and most common treatment‐related AEs (fatigue, pruritus, stomatitis, anaemia) and AEs grade 3/4 including treatment‐related deaths. Subgroups: not reported. QoL (secondary endpoint) How measured: health‐related QoL assessments with FACT FKSI‐DRS and a resulting summary score and EQ‐5D. Time points measured: baseline, after randomization but before cycle 1 of therapy, on day 1 of each cycle, at the first 2 follow‐up visits (each assessment before physician contact, treatment doses and any procedures), about 30 and 100 days after last dose, EQ‐5D: additional at each of the 10 survival follow‐ups visits (every 3 months). Time points reported: FKSI‐DRS and EQ‐5D (utility index and VAS) with completion rates at baseline, mean change from baseline to weeks 4 to 104, clinically important improvements, time to improvement (Cella 2016). Subgroups: not reported. PFS (secondary endpoint) How measured: time from randomization to first documented RECIST‐defined tumour progression or death from any cause. Time points measured: CT and MRI at baseline, every 8 weeks for the first year and then every 12 weeks until disease progression or discontinuation of treatment. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median. Subgroups: not reported. Tumour remission (secondary endpoint) How measured: evaluated by the investigator (RECIST 1.1), number of randomized participants with a complete or partial response. Time points measured: CT and MRI at baseline, every 8 weeks for the first year and then every 12 weeks until disease progression or discontinuation of treatment. Time points reported: overall response. Subgroups: not reported. | |
| Funding sources | Bristol Myers Squibb. | |
| Declarations of interest | RJM: honoraria from Bayer, Pfizer, Novartis and GlaxoSmithKline. SG: fees for consulting and serving on advisory boards from Bristol‐Myers Squibb, Novartis, Bayer, Sanofi‐Aventis, Astellas, Xcenda and Onclive; grant support from Bristol‐Myers Squibb, Novartis, Bayer, Pfizer, Acceleron, Merck and Agensys. HJH: grant support from Pfizer, Newlink Genetics, GlaxoSmithKline and SFJ Pharmaceuticals. SST: fees for serving on advisory boards from Prometheus; consulting fees from Amgen; grant support through his institution from Prometheus, Argos Therapeutics, Immatics Biotechnologies, Novartis and Exelixis. GP: fees for serving on advisory boards from Janssen and Novartis; lecture fees from Astellas and Pfizer; grant support from Bayer. ERP: fees for serving on advisory boards from Merck, Dendreon, GlaxoSmithKline, Pfizer, Astellas, Novartis and Genentech; grant support from AstraZeneca, Eli Lilly, Merck, Dendreon, GlaxoSmithKline, Acceleron and Pfizer. TKC: fees for consulting and for serving on advisory boards from GlaxoSmithKline, Novartis, Pfizer, Merck, AstraZeneca, Bayer and Prometheus; grant support through his institution from Bristol‐Myers Squibb, GlaxoSmithKline, Novartis, Exelixis, Pfizer, Merck, Roche, AstraZeneca, TRACON Pharmaceuticals and Peloton. HG: fees for serving on advisory boards from Novartis, Bayer, Sanofi‐Aventis, Astellas and Pfizer. FD: grant support from Novartis, Pfizer and GlaxoSmithKline. PB: honoraria from GlaxoSmithKline, Pfizer and Orion. JW: fees for serving on advisory boards, paid to his institution, from Bristol‐Myers Squibb, Novartis, GlaxoSmithKline, Roche and Amgen. YT: fees for serving on advisory boards from ONO Pharmaceuticals and Pfizer; honoraria and grant support from ONO Pharmaceuticals, Novartis and Pfizer. TCG: fees for consulting and serving on advisory boards from Boehringer Ingelheim, Merck Serono, Novartis, Pfizer, GlaxoSmithKline, Merck Sharp & Dohme, Bayer HealthCare, Roche, Bristol‐Myers Squibb, Eli Lilly and Janssen‐Cilag; honoraria from Boehringer Ingelheim, Merck Serono, Novartis, Pfizer, GlaxoSmithKline, Bayer, Roche, Eli Lilly, Janssen‐Cilag, Sanofi‐Aventis; travel support from Boehringer Ingelheim, Merck Serono, Pfizer, Roche and Eli Lilly; owning stock in Bayer. FAS: fees for serving on advisory boards from Pfizer, GlaxoSmithKline and Novartis; lecture fees from GlaxoSmithKline. CK: fees for serving on advisory boards from Pfizer, Novartis, Sanofi‐Aventis, Bayer and Seattle Genetics; lecture fees from Pfizer and Novartis. AR: lecture fees from Merck Sharp & Dohme. JSS, LAX, IMW: employees of and hold stock in Bristol‐Myers Squibb. PS: reports receiving consulting fees from Jounce Therapeutics, Amgen, Bristol‐Myers Squibb, GlaxoSmithKline and AstraZeneca/MedImmune; also founder of and holds stock in Jounce Therapeutics. | |
| Notes | Registration: NCT01668784 (CheckMate025). Study was stopped early due to the results of a planned interim analysis by the independent data monitoring committee showing significant benefit for OS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk of selection bias assumed due to block randomization, stratified by region, MSKCC prognostic risk group and the number of previous antiangiogenic therapy regimens (1 or 2) for advanced RCC. |
| Allocation concealment (selection bias) | Low risk | Central randomization. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) | Low risk | Low risk of attrition bias assumed, high completeness of follow‐up with similar censoring in between treatment groups. |
| Incomplete outcome data (attrition bias) | Low risk | Low risk of attrition bias assumed, safety analysis bases on all treated participants. |
| Incomplete outcome data (attrition bias) | High risk | High risk of bias on attrition bias for tumour remission assumed due to different numbers of non‐evaluated participants (6% with nivolumab vs 12% with everolimus). |
| Incomplete outcome data (attrition bias) | Low risk | High completion rates (≥ 80% in the first year) with no differences between treatment groups. |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes from the protocol reported. |
| Other bias | High risk | Stopped early for benefit in OS, subsequent systematic therapies (group 1: 55%; group 0: 63%) with cross‐over (25% from group 1 to group 0; 1.7% from group 0 to group 1). |
| Methods | Study design: 3‐arm, parallel‐group, open‐label RCT. Study dates: randomization from May 2008 to May 2009, median follow‐up 23.2 months, study completion date February 2012. Setting: multicentre (24 centres), national, phase II. Country: France. | |
| Participants | Inclusion criteria: histologically confirmed mRCC of all histological subtypes except papillary carcinomas; aged ≥ 18 years; ECOG Performance Status 0 to 2; measurable metastases; liver, renal and haematological functions in the range of 1.5 to 2 times above or below normal values; normal lipid and glycaemic concentrations; normal cardiac function within 6 weeks before randomization. Exclusion criteria: brain metastases, hypertension, systemic treatment for the disease, history of arterial or venous thrombosis in the past 6 months. Sample size: 171. Age (years, median with range): group 1: 62 (40 to 79); group 0a: 62 (33 to 83); group 0b: 61 (33 to 83). Sex (M/F, %): group 1: 66/34; group 0a: 74/26; group 0b: 76/24. Prognostic factors (all randomized participants):
| |
| Interventions | Group 1 (n = 41): IFN‐α + bevacizumab IFN‐α 9 mIU SC 3 times/week + bevacizumab 10 mg/kg IV every 2 weeks. Group 0 (n = 42): sunitinib Sunitinib 50 mg/day for 4 weeks, followed by 2 weeks off. Group 0a (n = 88): temsirolimus + bevacizumab (excluded, not standard treatment). Temsirolimus 25 mg IV weekly + bevacizumab 10 mg/kg IV every 2 weeks. Treatments continued until disease progression, unacceptable toxicity or protocol violation. Cointerventions (standard‐of‐care according to local practice): not specified. | |
| Outcomes | OS (secondary endpoint) How measured: time from randomization to death from any cause. Time points measured: during follow‐up. Time points reported: 12‐months OS (study is ongoing for long‐term OS). Subgroups: not reported. AEs, grade ≥ 3 (secondary endpoint) How measured:participants on study medication were assessed (NCI‐CTCAE v.3.0), data safety monitoring committee. Time points measured: at day 15 and then at least every 6 weeks over 48 weeks. Time points reported: main types of AEs (all grades and grade ≥ 3), frequency of AEs and SAEs ≥ 3. Subgroups: not reported. QoL not evaluated PFS (primary endpoint) How measured: time from randomization to disease progression or death from any cause (central reviewed data), 4 follow‐up CT scans according to RECIST 1.0. Time points measured: baseline and then every 12 weeks over 48 weeks with 4 follow‐up CT scans. Time points reported: Kaplan‐Meier survival curves over up to 30 weeks, median PFS with 95% CI. Subgroups: not reported. Tumour remission (secondary endpoint) How measured: thoracic, abdominal and pelvic CT scan, brain MRI or CT and bone scan. Time points measured: baseline and then every 12 weeks. Time points reported: best response. Subgroups: not reported. | |
| Funding sources | French Ministry of Health and Wyeth Pharmaceuticals. | |
| Declarations of interest | SN: honoraria from Novartis, Wyeth, Pfizer, GlaxoSmithKline and Roche; research funding from Wyeth, Roche and Novartis. DP: honoraria from Bayer, Eli Lilly and Roche. JOB: honoraria from Amgen; consultant with Novartis. LG, BL: honoraria from Novartis. BE: honoraria from Bayer, Roche, Pfizer, Genentech, Novartis, GlaxoSmithKline and Aveo; consultant with Bayer, Pfizer and Roche. All other authors declared no conflicts of interest. | |
| Notes | Registration: NCT00619268 (TORAVA). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk of selection bias assumed, computer‐generated list, permutated blocks, stratification by participating centre and performance status. |
| Allocation concealment (selection bias) | Low risk | Low risk of selection bias assumed due to central allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and investigators were unmasked. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) | Low risk | Masked central review of CT scans done in 89% of all randomized participants. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assumed due to high completeness of, and similar censoring in, different treatment groups during follow‐up. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assumed, all participants who received at least 1 dose of the study drug were included. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assumed, response was reported for all participants. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | High risk | Information on long‐term OS and QoL not published despite planning in protocol. |
| Other bias | High risk | Blocked randomization in centres in an unblinded trial, differences in second‐line treatment after study treatment failure because of toxicity or progression with lower rates of second‐line therapies with sunitinib (48%) compared to 68% to 69% in other groups. |
| Methods | Study design: 2‐arm, parallel‐group RCT. Study dates: randomization from October 2003 to July 2005, data cutoff March 2009, median follow‐up among surviving participants 46.2 months. Setting: multicentre, international. Countries: Canada, US. | |
| Participants | Inclusion criteria: people with mRCC; clear‐cell histological component confirmed by local pathology review; no prior systemic therapy for RCC; KPS ≥ 70%; aged ≥ 18 years; adequate bone marrow, hepatic and renal function; serum creatinine ≤ 1.5 times ULN. Exclusion criteria: central nervous system metastases; NYHA class II to IV heart failure; bleeding within 6 months; blood pressure that could not be controlled < 160/90 mmHg with medication; history of venous thrombosis within 1 year or arterial thrombosis within 6 months or who required ongoing therapeutic anticoagulation; uncontrolled thyroid function; pregnancy; requirement for systemic corticosteroids greater than physiological replacement doses or delayed healing wounds, ulcers or bone fractures. Sample size:732. Age (years, median with IQR): group 1: 61 (56 to 70); group 0: 62 (55 to 70). Sex (M/F, %): group 1: 73/27; group 0: 66/34. Prognostic factors:
| |
| Interventions | Group 1 (n = 363): IFN‐α IFN‐α‐2a (Intron; Schering‐Plough, Kenilworth, NJ), provided by the NCI Cancer Therapy Evaluation Program, 9 MU SC 3 times/week (non‐consecutive days). Dose reduction to 6 MU and 3 MU if IFN‐related toxicity present. Group 0 (n = 369): IFN‐α + bevacizumab IFN‐α‐2a (Intron; Schering‐Plough, Kenilworth, NJ), provided by the NCI Cancer Therapy Evaluation Program, 9 MU SC 3 times/week (non‐consecutive days). Dose reduction to 6 MU and 3 MU if IFN‐related toxicity present. Bevacizumab (provided by the NCI Cancer Therapy Evaluation Program) 10 mg/kg IV every 2 weeks. Cointerventions (standard‐of‐care according to local practice): not specified. | |
| Outcomes | OS (primary outcome) How measured: time from registration to death from any cause. Time points measured: during treatment and follow‐up. Time points reported: Kaplan‐Meier curves over up to 60 months, median OS. Subgroups: nephrectomy, MSKCC, liver metastases, age, gender. AEs, grade ≥ 3 (secondary outcome) How measured: ongoing documentation of AEs (CTCAE v.3.0). Time points measured: baseline, every 12 weeks. Time points reported: frequency of participants with AEs grade ≥ 3, deaths due to AEs, treatment‐related AEs to March 2009. Subgroups: not reported. QoL not evaluated PFS (secondary outcome) How measured: time between randomization and date of progression or death, investigator assessment of x‐rays. Time points measured: baseline, every 12 weeks. Time points reported: Kaplan‐Meier survival curves for up to 60 months, median PFS. Subgroups: number of adverse risk factors. Tumour remission (secondary outcome) How measured: investigator assessment of x‐rays, RECIST criteria. Time points measured: baseline, every 12 weeks. Time points reported: overall response rate. Subgroups: not reported. | |
| Funding sources | Supported in part by National Cancer Institute to the Cancer and Leukemia Group B (CALGB) (Grant No. CA31946, CA33601) and by National Cancer Institute (Grants No. CA60138, CA41287, CA47642, CA45808, CA77440, CA14985, CA77202). | |
| Declarations of interest | BIR: consultant or advisory role: Genentech. WMS: consultant or advisory role: Genentech; research funding: Genentech. JP: consultant or advisory role: Genentech; honoraria: Genentech. JD: consultant or advisory role: Genentech and Novartis; honoraria: Pfizer, Novartis; research funding: Novartis, Genentech, Pfizer. DAV: research funding: Genentech. | |
| Notes | Registration: NCT00072046 (CALGB 90206). Results on PFS and overall response rate published in Rini 2008, no cross‐over was permitted for participants randomly assigned to IFN‐α monotherapy, a substantial percentage of participants in both arms received systemic anticancer therapy subsequent to progression (62% of participants on IFN‐ monotherapy and 54% of participants on bevacizumab + IFN‐α) (mostly with sunitinib or sorafenib). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random block design, stratified by nephrectomy status and number of adverse prognostic factors. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded trial and no independent review of x‐rays could potentially have contributed to the improved PFS and overall response rate. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assumed due to similar censoring, 657/732 (90%) participants experienced progression or death. |
| Incomplete outcome data (attrition bias) | Low risk | Based on all participants who were eligible for evaluation for toxicity (362/363 from the intervention and 347/369 from the control group, reasons not reported). |
| Incomplete outcome data (attrition bias) | Unclear risk | No data reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | Low risk | No differences in outcomes to the protocol. |
| Other bias | High risk | Treatment with second‐line systemic anticancer therapy subsequent to progression (62% of participants on IFN‐α monotherapy and 54% of participants on bevacizumab + IFN‐α) might bias OS. |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Study dates: December 2010 to July 2015 (study start to study completion date according to the trial registration). Setting: multicentre, international, phase III. Countries: Europe (France, Germany, Hungary, Italy, Poland, Russia, UK, Netherlands, Romania, Norway), US. | |
| Participants | Inclusion criteria: metastatic or locally advanced (or both) RCC with clear‐cell histology (histological confirmation by local pathologist required), aged > 18 years, HLA‐A*02‐positive type, candidates for a first‐line therapy with sunitinib, favourable or intermediate‐risk (favourable risk: none, intermediate risk: 1 or 2 of the following criteria applied: haemoglobin < LLN, serum corrected calcium > ULN, KPS < 80%, time from initial diagnosis to initiation of therapy < 1 year, absolute neutrophil count > ULN, platelets > ULN), women who were postmenopausal or surgically sterile or practiced medically acceptable method of contraception, men willing to use contraception or had undergone vasectomy. Exclusion criteria: prior systemic therapy for metastatic disease; history of or current brain metastases; abnormal ≥ CTC grade 3 laboratory values for haematology, liver and renal function; metastatic second malignancy; localized second malignancy expected to influence the person's lifespan; history or evidence of systemic autoimmune disease; known HIV infection; active infections requiring oral or IV antibiotics; any other known infection with a biological agent that can cause a severe disease and posed a severe danger to laboratory personnel working on participants' blood or tissue; received study drug within any clinical study within 4 weeks before sunitinib start; serious intercurrent illness, which according to the investigator, posed an undue risk for the person when participating in the trial; < 12 months since myocardial infarction, severe or unstable angina, coronary or peripheral artery bypass graft or cerebrovascular event. Sample size:339. Age (years, mean): group 1: 62.2; group 0: 59.8. Sex (M/F, %): group 1: 70/30; group 0: 65/35. Prognostic factors:
| |
| Interventions | Group 1 (n = 204): IMA901 + sunitinib Single infusion of cyclophos 300 mg/m2 3 days prior to first vaccination, 10 intradermal vaccination IMA901 + GM‐CSF 75 µg. 1 cycle sunitinib prior to randomization, sunitinib 50 mg orally (4 weeks/2 weeks off). Group 0 (n = 135): sunitinib alone 1 cycle sunitinib prior to randomization, sunitinib 50 mg orally (4 weeks/2 weeks off). Cointerventions (standard‐of‐care according to local practice): not specified. | |
| Outcomes | OS (primary endpoint) How measured: investigator‐assessed. Time points measured: not reported. Time points reported: Kaplan‐Meier‐curves over 42 months, median OS, log rank P value. Subgroups: favourable and intermediate risk. AEs, grade ≥ 3 (secondary endpoint) How measured: investigator‐assessed, AEs, physical examinations, vital signs, haematology, clinical chemistry, urinalysis and electrocardiographic changes. Time points measured: not reported. Time points reported: most frequent (≥ 10% of participants) AEs. Subgroups: not reported. QoL: not measured. PFS (secondary endpoint) How measured: RECIST 1.1, central review and investigator analysis. Time points measured: not reported. Time points reported: Kaplan‐Meier‐curves over 24 months, median PFS, log rank P value. Subgroups: not reported. Tumour remission (secondary endpoint) How measured: RECIST 1.1, images collected centrally and interpreted by independent radiologists and oncologists who assessed the tumour images without being informed about participant's treatment and the local assessment of site investigators. Time points measured: not reported. Time points reported: best objective response. Subgroups: not reported. | |
| Funding sources | Immatics Biotechnologies GmbH, Pfizer. | |
| Declarations of interest | CR, HS, TW: shareholders of Immatics biotechnologies GmbH. JL, DM, RM, AM, JF, AK: employees of Immatics biotechnologies GmbH. | |
| Notes | Registration: NCT01265901. Published as abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Missing information on generation of randomization, 3:2 block randomization, stratified by factors included risk group, nephrectomy and region (Western EU, US, Central Eastern EU). |
| Allocation concealment (selection bias) | Low risk | Low risk of selection bias assumed due to central allocation via fax or email (or both). |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. |
| Blinding of participants and personnel (performance bias) | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) | Low risk | No performance bias, assessment of response by blinded assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assessed due to similar censoring in the treatment groups. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assessed due to reporting in the safety population of all treated participants. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition bias assessed due to reporting in all randomized participants. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes reported. |
| Other bias | Low risk | No other bias identified. |
AE: adverse event; CI: confidence interval; CT: computer tomography; CTC: Common Terminology Criteria; ECOG: Eastern Cooperative Oncology Group; EQ‐5D: EuroQol 5‐Dimension; EQ‐VAS: EuroQol Visual Analogue Scale; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; FKSI‐15: 15‐item Kidney Symptom Index; FKSI‐DRS: Kidney Symptom Index ‐ Disease‐Related Symptoms; HR: hazard ratio; IL: interleukin; IM: intramuscular; IFN‐α: interferon‐α; IV: intravenous; KPS: Karnovsky Performance Status; LLN: lower limit of normal; mIU: milli‐international unit; mRCC: metastatic renal cell carcinoma; MRI: magnetic resonance imaging; MSKCC: Memorial Sloan‐Kettering Cancer Center; MU: million units; n: number of participants; NCI‐CTC: National Cancer Institute Common Terminology Criteria; NYHA: New York Heart Association; OS: overall survival; PFS: progression‐free survival; QoL: quality of life; RCC: renal cell carcinoma; RCT: randomized controlled trial; RECIST: Response Evaluation Criteria In Solid tumours; SAE: serious adverse event; SC: subcutaneous; ULN: upper limit of normal; VAS: visual analogue score; VEGF: vascular endothelial growth factor.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± 13 cRA). | |
| No comparison to current standard therapy as defined in review protocol (autologous tumour cells + BCG vs norprogesterone). | |
| Not an RCT. | |
| Not an RCT. | |
| No comparison to current standard therapy as defined per review protocol (IFN‐α + IL‐2 vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α + 5‐FU vs tamoxifen). | |
| No comparison to current standard therapy as defined per review protocol (SC IL‐2/SC IFN‐α + IL‐2/IV 5‐FU vs SC IL‐2/SC IFN‐α + IL‐2/IV 5‐FU/OP 13 cRA vs SC IFN‐α/IV vinblastine). | |
| Not mostly mRCC (stage IV) patients. | |
| No comparison to current standard therapy as defined per review protocol (IL‐2 + IFN‐α + PO 13cRA/inhaled + inhaled IL‐2 vs IL‐2 + IFN‐α + PO 13cRA/inhaled). | |
| Secondary publication to Hudes 2007. | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 vs IFN‐α vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐͎β IV different doses). | |
| Secondary publication to Escudier 2007. | |
| No comparison to current standard therapy as defined in review protocol (sorafenib + high‐dose IFN‐α vs sorafenib + low‐dose IFN‐α). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α + 5‐FU vs misletoe lectin). | |
| Not an RCT. | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs vinblastine). | |
| Secondary publication to Motzer 2007. | |
| Secondary publication to Motzer 2007. | |
| Secondary publication to Motzer 2007. | |
| Secondary publication to Motzer 2015a. | |
| No comparison to current standard therapy as defined in review protocol (pharmacodynamic study with nivolumab). | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs aspirin). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± IFN‐γ). | |
| No comparison to current standard therapy as defined in review protocol (combined chemotherapy ± IFN‐α). | |
| Study of mixed solid tumours with no separate analysis of mRCC patients. | |
| No comparison to current standard therapy as defined in review protocol (reduced dose IL‐2 ± histamine). | |
| Study of mixed solid tumours with no separate analysis of mRCC patients. | |
| No comparison to current standard therapy as defined in review protocol (LMI vaccination + cyclophosphamide + IL‐2 vs LMI + cyclophosphamide vs LMI). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐γ + IFN‐α vs IFN‐γ). | |
| Secondary publication to Hudes 2007. | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs pulmonal irradiation + vincristine + bleomycin). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± naptumomab estafenox). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs first‐line sorafenib). | |
| Secondary publication to Escudier 2007. | |
| Preliminary publication to Negrier 2011. | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (TIL vaccination + IL‐2 vs IL‐2). | |
| Secondary publication to Hudes 2007. | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± initial nephrectomy). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + IFN‐γ vs IFN‐γ). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± vinblastine). | |
| No comparison to current standard therapy as defined in study protocol (IFN‐α ± 13 cRA). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α different doses). | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐γ vs placebo). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs IFN‐α + IL‐2 + 5‐FU). | |
| Not an RCT. | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + tamoxifen vs tamoxifen). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 vs IFN‐α). | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (first‐line sorafenib + IFN‐α vs sorafenib). | |
| No immunotherapeutic intervention (CRLX101 + bevacizumab). | |
| No comparison to current standard therapy as defined in review protocol (high IFN‐α vs low‐dose IFN‐α). | |
| Secondary publication to Rini 2015. | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± cimetidine). | |
| No comparison to current standard therapy as defined in review protocol (high‐dose vs low‐dose IFN‐α). | |
| No comparison to current standard therapy as defined in review protocol (LAK vaccination + IL‐2 vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine vs medroxyprogesterone acetate). | |
| Secondary publication to Hudes 2007. | |
| No comparison to current standard therapy as defined in review protocol (LAK vaccination + IL‐2 vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (reduced‐dose IL‐2 ± melatonin). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + GM‐CSF vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (CIK vaccination vs IL‐2 + IFN‐α). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IFN‐γ). | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| Study of mixed solid tumours with no separate analysis of mRCC patients. | |
| No comparison to current standard therapy as defined in review protocol (reduced‐dose IL‐2 vs LAK). | |
| No comparison to current standard therapy as defined in review protocol (high‐dose IL‐2 vs reduced‐dose IL‐2 + IFN‐α). | |
| Secondary publication to Escudier 2007. | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± initial nephrectomy). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± 13 cRA). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2). | |
| Secondary publication to Motzer 2007. | |
| Preliminary study to Motzer 2015a. | |
| No comparison to current standard therapy as defined in review protocol (dose‐response study of nivolumab). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs medroxyprogesterone acetate). | |
| No comparison to current standard therapy as defined in review protocol (IV IFN‐α vs SC IFN‐α). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 vs IL‐2 + epidoxorubicin). | |
| No comparison to current standard therapy as defined in review protocol (sorafenib + IFN‐α vs sorafenib + gemcitabine ‐ early termination of study because of slow accrual with no data analysis). | |
| Stopped early due to slow accrual, no data analysis performed (sorafenib + IFN‐α vs sorafenib). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 ± 5‐FU). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2 vs IFN‐α vs medroxyprogesterone acetate). | |
| No comparison to current standard therapy as defined in review protocol (IV IL‐2 + IFN‐α vs SC IL‐2 + IFN‐α ). | |
| No immunotherapeutic intervention (sorafenib vs placebo). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± vinblastine). | |
| No comparison to current standard therapy as defined in review protocol (cimetidine + ALT vaccination vs cimetidine). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± vinblastine). | |
| Secondary publication to Motzer 2007. | |
| No comparison to current standard therapy as defined in review protocol (maintenance therapy after disease progression). | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (study A. no RCT; study B: IL‐2 + SRL172 vaccination vs IL‐2). | |
| Secondary publication to Motzer 2007. | |
| No comparison to current standard therapy as defined in review protocol (nephrectomy ± plasma). | |
| Secondary publication to Motzer 2007. | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± medroxyprogesterone acetate). | |
| No immunotherapeutic intervention. | |
| No comparison to current standard therapy as defined in review protocol (first‐line sorafenib + IL‐2 vs sorafenib). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine vs vinblastine). | |
| No comparison to current standard therapy as defined in review protocol (high‐dose vs low‐dose IFN‐α). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine ± medroxyprogesterone acetate). | |
| No comparison to current standard therapy (IFN + bevacizumab vs everolimus + bevacizumab). | |
| Preliminary publication to Motzer 2007. | |
| Protocol to Rini 2010. | |
| Preliminary publication without OS to Rini 2010. | |
| No immunotherapeutic intervention (sorafenib ± AMG 386). | |
| No comparison to current standard therapy (IFN + bevacizumab vs temsirolimus + bevacizumab). | |
| No comparison to current standard therapy as defined in review protocol (LAK vaccination + IL‐2 vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (dose‐response study of siltuximab). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α ± coumarin + cimetidine). | |
| No comparison to current standard therapy as defined in review protocol (postoperative + preoperative IL‐2 vs postoperative IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (AV vaccination + IFN‐α + IFN‐γ (together with AV) vs AV + IFN‐α + IFN‐γ (after initiation of AV)). | |
| Preliminary study to Motzer 2015a. | |
| No comparison to current standard therapy as defined in review protocol (autologous vaccine ± GM‐CSF). | |
| Study of mixed solid tumours with no separate analysis of mRCC patients. | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α vs medroxyprogesterone acetate). | |
| No immunotherapeutic intervention (pazopanib vs placebo). | |
| Secondary publication to Escudier 2007. | |
| No comparison to current standard therapy as defined in review protocol (intermediate‐dose vs low‐dose IFN‐α). | |
| No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine vs IFN‐α). | |
| No immunotherapeutic intervention (CRLX101 + bevacizumab vs SOC). | |
| No comparison to current standard therapy as defined in review protocol (cyclophosphamide + IMA 901 vaccination vs IMA 901 vaccination). | |
| Not an RCT. | |
| No comparison to current standard therapy as defined in review protocol (continuous IL‐2 vs bolus IL‐2 + LAK vaccination). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐β vs IL‐2). | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 + PEG‐IL‐2 vs IL‐2). | |
| No comparison to current standard therapy as defined in review protocol (IL‐2 different doses). | |
| Not an RCT. | |
| Secondary publication to Hudes 2007. | |
| Not mostly mRCC (stage IV) patients (adjuvant study). | |
| No comparison to current standard therapy as defined in review protocol (DC‐CIK vaccination vs IL‐2 + IFN‐α). |
5‐FU: 5‐fluorouracil; ALT: alanine transaminase; CIK: cytokine‐induced killer; DC‐CIK: dendritic cell cytokine‐induced killer; GM‐CSF: granulocyte‐macrophage colony‐stimulating factor; IL: interleukin; IV: intravenous; LAK: lymphokine‐activated killer; mRCC: metastatic renal cell carcinoma; PEG‐IL‐2: pegylated interferon‐2; PO: per os (orally); RCT: randomized controlled trial; SC: subcutaneous; SOC: standard of care.
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | NIVOSWITCH: a Randomized Phase II Study with NIVOlumab or Continuation of Therapy as an Early SWITCH Approach in Patients with Advanced or Metastatic Renal Cell Carcinoma (RCC) and Disease Control after 3 Months of Treatment with a Tyrosine Kinase Inhibitor. |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Setting: multicentre, international, phase II. Countries: Europe. |
| Participants | Main inclusion criteria: aged ≥ 18 years, either gender, histological confirmation of RCC with a clear‐cell component, ECOG Performance Status 0 to 2, metastatic or locally advanced RCC with clear‐cell component, not amenable to surgery with curative intention, first‐line treatment with a TKI for 10 to 12 weeks (limited to sunitinib or pazopanib), people with measurable disease (RECIST 1.1), adequate blood count, liver‐enzymes, and renal function. Main exclusion criteria: prior systemic therapy other than 10 to 12 weeks SOC TKI treatment, complete remission or progression during SOC TKI first‐line treatment, termination of first‐line treatment with TKI due to intolerance, prior therapy with antitumour vaccines, anti‐PD‐L1, anti‐PD1, anti‐CTLA‐4, or other immunomodulatory antitumour agents, known chronic infection and intercurrent illness. Sample size planned: 244. |
| Interventions | Group 1: nivolumab after TKI (sunitinib or pazopanib) and disease control. Group 0: pazopanib after TKI (sunitinib or pazopanib) and disease control. |
| Outcomes | Primary outcome: 24 months OS. Secondary outcomes: best overall response, PFS, QoL, safety, other. |
| Starting date | September 2016. |
| Contact information | AIO‐Studien‐gGmbH, Dr Aysun Karatas, info@aio‐studien‐ggmbh.de. |
| Notes | Sponsor protocol no: AIO‐NZK‐0116. |
| Trial name or title | ADAPT: an International Phase 3 Randomised Trial of Autologous Dendritic Cell Immunotherapy (AGS‐003) Plus Standard Treatment of Advanced Renal Cell Carcinoma (NCT01582672). |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Setting: multicentre, international, phase III. Countries: Canada, Czech Republic, Hungary, Israel, Italy, Spain, UK, US. |
| Participants | Main inclusion criteria: aged ≥ 18 years; either gender; histological confirmation of advanced RCC with predominantly clear‐cell histology; advanced disease; metastatic disease (measurable or non‐measurable per RECIST 1.1); people who were candidates for standard first‐line therapy initiating with sunitinib; time from diagnosis to treatment < 1 year; KPS ≥ 70%; life expectancy ≥ 6 months; resolution of all acute toxic effects of prior radiotherapy or surgical procedures to grade ≤ 1 (NCI‐CTC 4.0); adequate haematological, renal, hepatic and coagulation function; negative serum pregnancy test for women with reproductive potential and agreement of both men and women of reproductive potential to use a reliable form of contraception during the study and for 12 weeks after the last dose of study drug. Main exclusion criteria: prior systemic therapy of any type for RCC, including immunotherapy, chemotherapy, hormonal or investigational therapy; prior history of malignancy within the preceding 3 years, except for adequately treated in situ carcinomas or non‐melanoma skin cancer; adequately treated early‐stage breast cancer, superficial bladder cancer and non‐metastatic prostate cancer with a normal PSA; history of, or known, brain metastases; spinal cord compression, carcinomatous meningitis or evidence of brain or leptomeningeal disease; people with ≥ 4 of the following risk factors: haemoglobin < LLN, corrected calcium > 10 mg/dL, KPS < 80%, neutrophils > ULN, platelets > ULN, planned or elective surgical treatment postnephrectomy for the direct management of RCC, within 28 days before visit 1, NCI CTCAE grade 3 haemorrhage < 28 days before day 0, clinically significant comorbidities. Sample size planned:450. |
| Interventions | Group 1: AGS‐003 + standard treatment (sunitinib). Group 0: standard treatment (sunitinib). |
| Outcomes | Primary outcome: OS, duration from randomization to death. Secondary outcomes: PFS, tumour response, AEs. |
| Starting date | November 2012. |
| Contact information | Robert Figlin, MD, principal Investigator. |
| Notes | Final data collection date for primary outcome measure: April 2017. |
| Trial name or title | (CheckMate 214): A Phase 3, Randomised, Open‐Label Study of Nivolumab Combined with Ipilimumab versus Sunitinib Monotherapy in Subjects with Previously Untreated, Advanced or Metastatic Renal Cell Carcinoma (NCT02231749). |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Setting: multicentre, international, phase III. Countries: Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, Colombia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Israel, Italy, Japan, Korea, Republic of, Mexico, Netherlands, Poland, Spain, Sweden, Taiwan, Turkey, UK, US. |
| Participants | Main inclusion criteria: aged ≥ 18 years, either gender, histological confirmation of RCC with a clear‐cell component, advanced or metastatic (AJCC Stage IV) RCC, no prior systemic therapy for RCC with predefined exceptions (regular adjuvant or neoadjuvant therapy), KPS ≥ 70%, measurable disease (RECIST 1.1), archival or recent tumour tissue. Exclusion criteria: cerebral metastases; prior systemic treatment with VEGF or VEGF receptor targeted therapy; prior treatment with an anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibody or any other antibody or drug specifically targeting T‐cell costimulation or checkpoint pathways; any active or recent history of a known or suspected autoimmune disease or recent history of a syndrome that required systemic corticosteroids or immunosuppressive medications except for syndromes which would not be expected to recur in the absence of an external trigger; vitiligo or type 1 diabetes mellitus or residual hypothyroidism due to autoimmune thyroiditis only requiring hormone replacement are permitted to enrol, any condition requiring systemic treatment with corticosteroids or other immunosuppressive medications within 14 days prior to first dose of study drug; inhaled steroids and adrenal replacement steroid doses > 10 mg daily; prednisone equivalents are permitted in the absence of active autoimmune disease. Sample size planned:1070. |
| Interventions | Group 1: nivolumab 3 mg/kg + ipilimumab 1 mg/kg. Group 0: sunitinib 50 mg. |
| Outcomes | Primary outcome: OS, PFS (coprimary). Secondary outcomes: ORR, safety. |
| Starting date | October 2014. |
| Contact information | Sponsor: Bristol‐Myers Squibb. |
| Notes | Estimated primary completion date: May 2019. |
| Trial name or title | CARMENA: Randomised Phase III Trial Evaluating the Importance of Nephrectomy in Patients Presenting with Metastatic Renal Cell Carcinoma Treated with Sunitinib. |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Setting: phase III, multicentre, national. Countries: France. |
| Participants | Main inclusion criteria: aged ≥ 18 years, either gender, ECOG Performance Status 0 or 1, biopsy (primary tumour or metastases) confirming the diagnosis of clear‐cell carcinoma, documented metastatic disease, absence of prior systemic treatment for kidney cancer including antiangiogenic, tumour amenable to nephrectomy in the opinion of the patient's urologist, patients for whom the indication of sunitinib is considered according to the recommendation rules given by national health authorities of participating countries, prescription of sunitinib in the circumstances of the study is considered as a standard treatment, people with predefined adequate organ function. Main exclusion criteria: prior systemic treatment for kidney cancer, bilateral kidney cancer, pregnant or breastfeeding women, specified comorbidities, symptomatic brain metastases. Sample size planned: 576. |
| Interventions | Group 1: nephrectomy + sunitinib. Group 0: sunitinib. |
| Outcomes | Primary outcome: OS. Secondary outcome: ORR, PFS. |
| Starting date | September 2009. |
| Contact information | Principal Investigator: Arnaud Mejean, MD PhD, [email protected]. |
| Notes | Estimated primary completion date: September 2019. |
| Trial name or title | IMmotion 150: a Phase II, Randomised Study of Atezolizumab Administered as Monotherapy or In Combination with Bevacizumab versus Sunitinib In Patients with Untreated Advanced Renal Cell Carcinoma. |
| Methods | Study design: 3‐arm, parallel‐group, open‐label RCT. Setting: phase II, multicentre, international. Countries: Czech Republic, France, Germany, Italy, Poland, Romania, Spain, UK, US. |
| Participants | Main inclusion criteria: aged ≥18 years, either gender, unresectable advanced or metastatic renal cell carcinoma with component of clear‐cell histology or component of sarcomatoid histology that has not been previously treated with any systemic agents (or both), including treatment in the adjuvant setting, measurable disease, as defined by RECIST v1.1, KPS ≥ 70, adequate haematological and end‐organ function as defined by protocol. Main exclusion criteria: cerebral metastases; radiotherapy for RCC within 28 days prior to cycle 1; uncontrolled pleural effusion, pericardial effusion or ascites; pregnancy and lactating women; life expectancy < 12 weeks. Sample size planned: 305. |
| Interventions | Group 1: atezolizumab + bevacizumab. Group 1.1: atezolizumab (following PD ‐ atezolizumab + bevacizumab). Group 0: sunitinib (following PD ‐ atezolizumab + bevacizumab). |
| Outcomes | Primary outcome: PFS central reading. Secondary outcomes: PFS investigator assessed, ORR, OS. |
| Starting date | January 2014. |
| Contact information | Sponsor: Hoffmann La Roche. |
| Notes | Estimated primary completion date: September 2018. |
| Trial name or title | A Phase I/II Study to Assess the Safety and Efficacy of Pazopanib and MK 3475 in Subjects with Advanced Renal Cell Carcinoma. |
| Methods | Study design: part 1. non‐randomized dose escalation, part 2: 3‐arm, parallel‐group, open‐label RCT. Setting: multicentre, international. Countries: UK, US. |
| Participants | Inclusion criteria: aged ≥ 18 years, either gender, people with histologically confirmed advanced or mRCC, measurable disease, no prior systemic therapy, ECOG Performance Status 0 or 1, adequate organ function. Exclusion criteria: cerebral metastases, active autoimmune disease, pregnancy, history of a malignancy (other than the disease under treatment in the study) within 5 years. Sample size planned:228. |
| Interventions | Group 1: MK 3475 (pembrolizumab) + pazopanib. Group 1.1: MK 3475 (pembrolizumab). Group 0: pazopanib. |
| Outcomes | Primary outcome: part 2: PFS. Secondary outcomes: part 2: ORR, OS, safety. |
| Starting date | December 2013. |
| Contact information | Sponsor: Novartis Pharmaceuticals. |
| Notes | Estimated primary completion date: May 2021. |
| Trial name or title | KEYNOTE 029 ‐ a Phase I/II Clinical Trial to Study the Safety and Tolerability of MK‐3475 + Pegylated Interferon Alfa‐2b (PEG‐IFN) and MK‐3475 + Ipilimumab (IPI) in Subjects with Advanced Melanoma (MEL) and Renal Cell Carcinoma (RCC). |
| Methods | Study design: 3‐arm, parallel‐group, open‐label RCT. Setting: multicentre, international. Countries: Australia, New Zealand, US. |
| Participants | Inclusion criteria: aged ≥ 18 years, either gender, people with histologically confirmed advanced or metastatic melanoma or RCC, RCC participants must have received ≥ 1 prior line of therapy for metastatic disease, ECOG Performance Status of 0 or 1, adequate organ function. Exclusion criteria: cerebral metastases, diagnosis of immunodeficiency or receiving systemic steroid therapy, additional malignancy, active infection requiring systemic therapy, pregnancy, breastfeeding women. Sample size planned:343. |
| Interventions | Group 1: pembrolizumab + PegIFN‐2b. Group 1.1: pembrolizumab + ipilimumab. Group 0: pembrolizumab. |
| Outcomes | Primary outcome: dose‐limiting toxicities, AE, PFS. Secondary outcomes: ORR, OS. |
| Starting date | March 2014. |
| Contact information | Sponsor: Merck Sharp & Dohme Corp. |
| Notes | Estimated primary completion date: April 2017. |
| Trial name or title | A Pilot Randomised Tissue‐Based Study Evaluating Anti‐PD1 Antibody or Anti‐PD1 + Bevacizumab or Anti‐PD1 + Anti‐CTLA‐4 in Patients with Metastatic Renal Cell Carcinoma who are Eligible for Cytoreductive Nephrectomy, Metastasectomy or Post‐Treatment Biopsy. |
| Methods | Study design: 3‐arm, parallel‐group, double‐blind RCT. Setting: multicentre. Countries: US. |
| Participants | Main inclusion criteria: informed consent; histologically or cytologically confirmed clear‐cell mRCC who are eligible for cytoreductive nephrectomy; metastasectomy or post‐treatment biopsy; diagnosis must be confirmed by pathologist review of screening biopsy; measurable disease defined as a lesion that can be accurately measured in at least 1 dimension and measures ≥ 15 mm with conventional techniques or ≥ 10 mm with more sensitive techniques such as MRI or spiral CT scan; prior treatment for RCC including prior surgery, radiotherapy, immunotherapy with IL‐2 or interferon (but not anti‐PD1 or anti‐CTLA‐4); target therapy with RTK inhibitors/mTOR inhibitors, such as sunitinib, sorafenib, pazopanib, axitinib, everolimus and temsirolimus (but not bevacizumab) or chemotherapy allowed; ECOG Performance Status 0 or 1. Main exclusion criteria: any other malignancy from which the person has been disease‐free for < 2 years, except for non‐melanoma skin cancer, in situ carcinoma of any site, organ allografts, major surgical procedure, open biopsy or significant traumatic injury with poorly healed wound within 6 weeks prior to first dose of study drug; or anticipation of need for major surgical procedure during the course of the study (other than in protocol); autoimmune disease; known history of testing positive for HIV or known AIDS; positive test for hepatitis B virus or positive test for hepatitis C virus. Sample size planned: 60. |
| Interventions | Group 1: nivolumab. Group 1.1: nivolumab + bevacizumab. Group 1.2: nivolumab + ipilimumab. |
| Outcomes | Primary outcome: safety. Secondary outcomes: immunological changes in tumour tissue, ORR. |
| Starting date | November 2014. |
| Contact information | Padmanee Sharma, MD, PhD. |
| Notes | Estimated primary data: November 2018. |
| Trial name or title | A Phase III, Open‐Label, Randomised Study of Atezolizumab (Anti‐PD‐L1 Antibody) in Combination with Bevacizumab versus Sunitinib in Patients with Untreated Advanced Renal Cell Carcinoma. |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT. Setting: multicentre, international, phase III. Countries: Australia, Bosnia and Herzegovina, Brazil, Canada, Czech Republic, Denmark, France, Germany, Italy, Japan, Korea, Republic of, Mexico, Poland, Russian Federation, Serbia, Singapore, Spain, Taiwan, Thailand, Turkey, Ukraine, UK, US. |
| Participants | Inclusion criteria: aged ≥ 18 years, definitive diagnosis of unresectable locally advanced or mRCC with clear‐cell histology or a component of sarcomatoid carcinoma (or both), with no prior treatment in the metastatic setting, evaluable MSKCC risk score, measurable disease (RECIST 1.1), KPS ≥ 70%, adequate haematological and end‐organ function. Exclusion criteria: radiotherapy for RCC within 14 days prior to treatment; central nervous system disease; uncontrolled pleural effusion, pericardial effusion or ascites; uncontrolled hypercalcaemia; any other malignancies within 5 years except for low‐risk prostate cancer or those with negligible risk of metastasis or death; life expectancy < 12 weeks. Sample size planned:830. |
| Interventions | Group 1: atezolizumab (MPDL3280A ‐ PD‐L1 AB) + bevacizumab. Group 0: sunitinib. |
| Outcomes | Primary outcome: PFS, OS. Secondary outcomes: ORR, safety, pharmacokinetics. |
| Starting date | May 2015. |
| Contact information | |
| Notes | Primary results expected: June 2020. |
| Trial name or title | An Open‐Label, Randomised, Controlled, Multicenter, Phase II Study Evaluating Safety and Efficacy of Intratumourally Administered Intuvax Pre‐nephrectomy Followed by Sunitinib Post‐Nephrectomy, Compared to Sunitinib Post‐Nephrectomy in Metastatic Renal Cell Carcinoma Patients (MERECA). |
| Methods | Study design: 2‐arm, parallel‐group, open‐label RCT, 2:1 randomization. Setting: multicentre, phase II. Countries: Sweden. |
| Participants | Main inclusion criteria: aged ≥ 18 years, either gender, informed consent, recent (< 6 months) diagnosed RCC with at least 1 CT‐verified metastasis, planned resection of primary tumour, primary tumour diameter ≥ 4 cm, candidate for standard first‐line therapy with sunitinib, adequate haematological parameters and liver function. Main exclusion criteria: life expectancy < 4 months; active autoimmune disease requiring treatment with systemic immunosuppressive agents, e.g. inflammatory bowel disease, multiple sclerosis, sarcoidosis, psoriasis, autoimmune haemolytic anaemia, rheumatoid arthritis, systemic lupus erythematosus, vasculitis, Sjögren's syndrome, scleroderma and autoimmune hepatitis; treatment with systemic corticosteroids within 7 days before screening, known cardiomyopathy or clinical significant finding in electrocardiography at screening (or both); KPS < 70%. Sample size planned:90. |
| Interventions | Group 1: intuvax + nephrectomy + sunitinib. Group 0: nephrectomy + sunitinib. |
| Outcomes | Primary outcome: median OS from randomization for high‐risk patients, 18‐month OS in the intermediate‐risk mRCC patients. Secondary outcomes: safety, PFS. |
| Starting date | April 2015. |
| Contact information | |
| Notes | Estimated primary completion date: February 2018. |
| Trial name or title | JAVELIN RENAL 101 ‐ A Phase 3, Multinational, Randomised, Open‐Label, Parallel‐Arm Study of Avelumab (MSB0010718C) in Combination with Axitinib (Inlyta(Registered)) versus Sunitinib (Sutent(Registered)) Monotherapy in the First‐Line Treatment of Patients with Advanced Renal Cell Carcinoma. |
| Methods | Study design: parallel‐arm, open‐label RCT. Setting: multicentre, international phase III. Countries: Japan, US. |
| Participants | Main inclusion criteria: aged ≥ 18 years; either gender; histologically or cytologically confirmed advanced or mRCC with clear‐cell component; availability of a recent formalin‐fixed, paraffin‐embedded tumour tissue block; ≥ 1 measurable lesion (RECIST 1.1) not previously irradiated; ECOG Performance Status 0 or 1; adequate bone marrow function, renal and liver functions. Main exclusion criteria: prior systemic therapy directed at advanced or mRCC; prior adjuvant or neoadjuvant therapy for RCC if disease progression or relapse has occurred during or within 12 months after the last dose of treatment; prior immunotherapy with IL‐2, IFN‐α or anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibody (including ipilimumab) or any other antibody or drug specifically targeting T cell costimulation or immune checkpoint pathways; prior therapy with axitinib or sunitinib (or both) and any prior therapies with other VEGF pathway inhibitors; known severe hypersensitivity reactions to monoclonal antibodies (grade ≥ 3), any history of anaphylaxis or uncontrolled asthma; any of the following in the previous 6 months: myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure, cerebrovascular accident, transient ischaemic attack, deep vein thrombosis or symptomatic pulmonary embolism; vaccination within 4 weeks of the first dose of avelumab and while on trial is prohibited except for administration of inactivated vaccines. Sample size planned: 583. |
| Interventions | Group 1: avelumab in combination with axitinib. Group 0: sunitinib. |
| Outcomes | Primary outcome: PFS. Secondary outcomes: OS, ORR, QoL, safety. |
| Starting date | March 2016. |
| Contact information | Pfizer CT.gov call centre. |
| Notes | Estimated primary completion date: June 2018. |
| Trial name or title | Nivolumab and Stereotactic Ablative Radiation Therapy versus Nivolumab Alone for Metastatic Renal Cancer. |
| Methods | Study design: parallel‐arm, open‐label RCT. Setting: national, phase II. Countries: US. |
| Participants | Main inclusion criteria: aged 18 to 100 years, either gender, pathological diagnosis of metastatic RCC with clear‐cell component, measurable disease in at least 2 non‐radiated sites, progression or intolerance to ≥ 1 prior systemic antiangiogenic therapy, ECOG Performance Status 0, 1, 2 or 3, adequate organ and marrow function. Main exclusion criteria: major surgery within 2 weeks prior to first dose; prior treatment with any anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibody or any other antibody or drug specifically targeting T‐cell costimulation or checkpoint pathways; active known or suspected autoimmune disease; history of hypersensitivity to monoclonal antibodies. Sample size planned: 87. |
| Interventions | Group 1: nivolumab. Group 0: nivolumab with radiation. |
| Outcomes | Primary outcome: ORR. Secondary outcomes: OS, PFS, safety, other. |
| Starting date | June 2016. |
| Contact information | Raquibu Hannan, MD, PhD, tel: +1 214‐645‐8525. |
| Notes | Contact: Jean Wu, RN, MSN, OCN. |
| Trial name or title | KEYNOTE‐426 ‐ Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK‐3475) in Combination with Axitinib versus Sunitinib Monotherapy in Participants with Renal Cell Carcinoma. |
| Methods | Study design: parallel‐arm, open‐label RCT. Setting: multicentre, international phase III. Countries: Japan, Hungary, Spain, Korea, Russia, US. |
| Participants | Main inclusion criteria: aged ≥ 18 years, either gender, histologically or cytologically confirmed advanced or mRCC (stage IV) with clear‐cell component with or without sarcomatoid features, availability of an archival tumour tissue sample or fresh biopsy, measurable disease (RECIST 1.1), no previously systemic therapy, KPS ≥ 70%. Main exclusion criteria: prior treatment with VEGF receptor or mTOR targeting agents; prior treatment with anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2 or other immunotherapy; known severe hypersensitivity reactions; active autoimmune disease; active infection; major surgery within 4 weeks prior to randomization; heart failure NYHA III or IV. Sample size planned: 840. |
| Interventions | Group 1: pembrolizumab and axitinib. Group 0: sunitinib. |
| Outcomes | Primary outcome: PFS, OS. Secondary outcomes: ORR, safety. |
| Starting date | September 2016. |
| Contact information | Merck Sharp & Dohme Corp. |
| Notes | Estimated primary completion date: December 2019; EudraCT: 2016‐000588‐17. |
AE: adverse event; AJCC: American Joint Committee on Cancer; CT: computer tomography; CTLA‐4: cytotoxic T‐lymphocyte antigen 4; ECOG: Eastern Cooperative Oncology Group; IL: interleukin; IFN‐α: interferon‐α; KPS: Karnovsky Performance Status; LLN: lower limit of normal; mRCC: metastatic renal cell carcinoma; MRI: magnetic resonance imaging; MSKCC: Memorial Sloan‐Kettering Cancer Center; mTOR: mammalian target of rapamycin; NCI‐CTC: National Cancer Institute Common Terminology Criteria; NCI CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NYHA: New York Heart Association; ORR: objective response rate; PD: programmed death; PD‐1: programmed death‐1; PFS: progression‐free survival; PSA: prostate‐specific antigen; QoL: quality of life; RCC: renal cell carcinoma; RCT: randomized controlled trial; RECIST: Response Evaluation Criteria In Solid tumours; SOC: standard of care; ULN: upper limit of normal; VEGF: vascular endothelial growth factor.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.13, 1.51] |
| Analysis 1.1  Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality. | ||||
| 2 Overall survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.28 [1.11, 1.49] | |
| Analysis 1.2 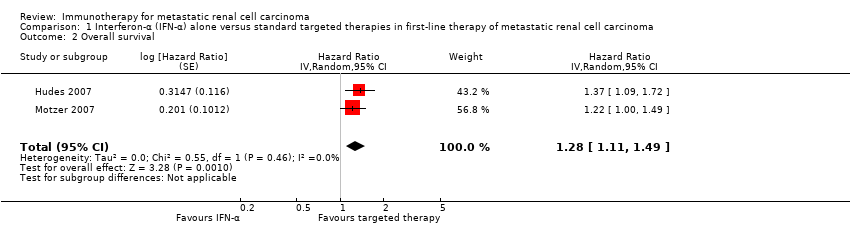 Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival. | ||||
| 3 Quality of life Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Quality of life. | ||||
| 3.1 FACT‐G | 1 | 730 | Mean Difference (Random, 95% CI) | ‐5.58 [‐7.25, ‐3.91] |
| 3.2 FKSI‐15 | 1 | 730 | Mean Difference (Random, 95% CI) | ‐3.27 [‐4.18, ‐2.36] |
| 3.3 FKSI‐DRS | 1 | 730 | Mean Difference (Random, 95% CI) | ‐1.98 [‐2.51, ‐1.45] |
| 3.4 EQ‐5D | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.12, ‐0.00] |
| 3.5 EQ‐VAS | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐4.68 [‐6.53, ‐2.83] |
| 4 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 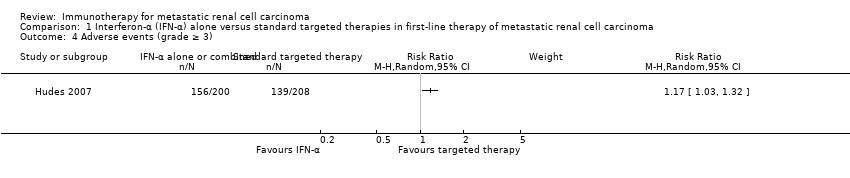 Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3). | ||||
| 5 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 2.23 [1.79, 2.77] | |
| Analysis 1.5  Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Progression‐free survival. | ||||
| 6 Tumour remission Show forest plot | 2 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.75] |
| Analysis 1.6 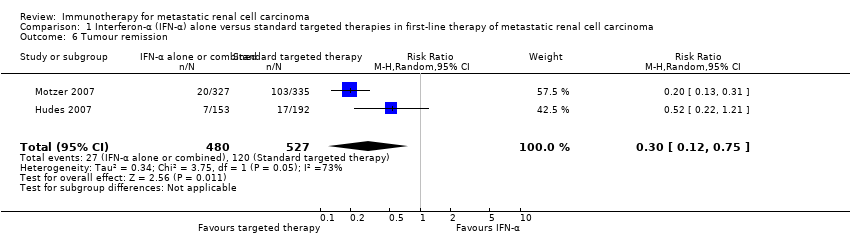 Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 6 Tumour remission. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1 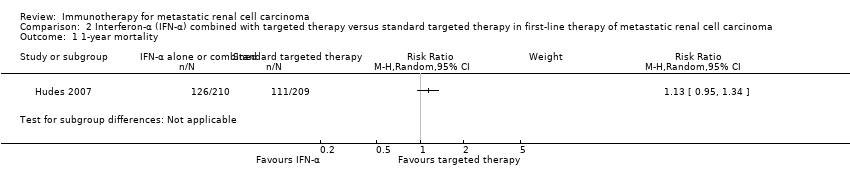 Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality. | ||||
| 2 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 2.2 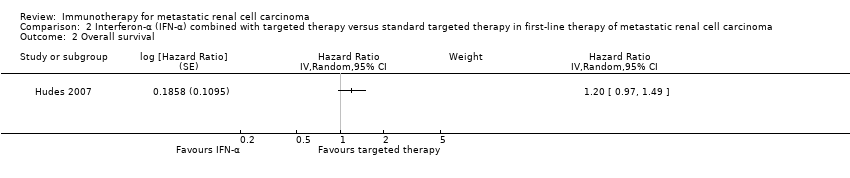 Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival. | ||||
| 3 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3 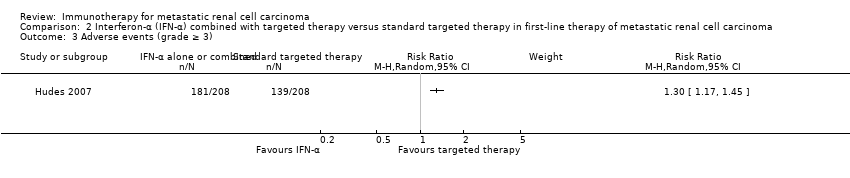 Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3). | ||||
| 4 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 2.4 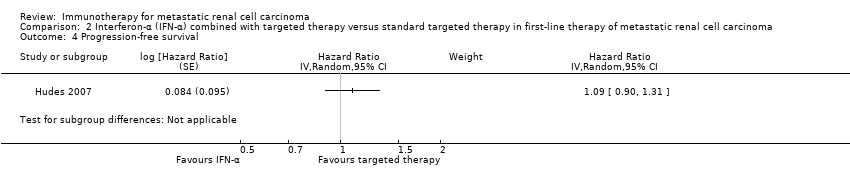 Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival. | ||||
| 5 Tumour remission Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.36] |
| Analysis 3.1  Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality. | ||||
| 2 Overall survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.13 [1.00, 1.28] | |
| Analysis 3.2  Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival. | ||||
| 3 Adverse events (grade ≥ 3) Show forest plot | 2 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.71, 0.84] |
| Analysis 3.3  Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3). | ||||
| 4 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.53 [1.36, 1.73] | |
| Analysis 3.4  Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival. | ||||
| 5 Tumour remission Show forest plot | 2 | 1205 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.31, 0.50] |
| Analysis 3.5 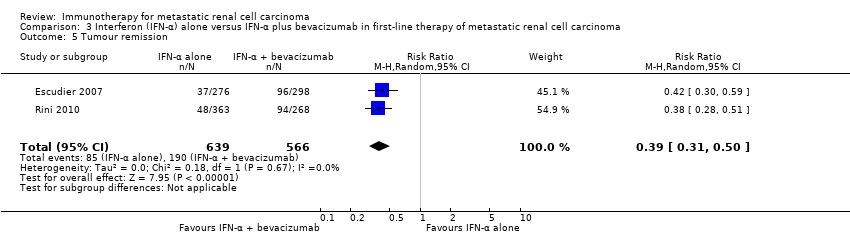 Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality. | ||||
| 2 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Adverse events (grade ≥ 3). | ||||
| 3 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Progression‐free survival. | ||||
| 4 Tumour remission Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Tumour remission. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
| Analysis 5.1  Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality. | ||||
| 2 Overall survival Show forest plot | 2 | 1071 | Hazard Ratio (Fixed, 95% CI) | 1.14 [0.96, 1.37] |
| Analysis 5.2  Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival. | ||||
| 3 Adverse events (grade ≥ 3) Show forest plot | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.97, 1.39] |
| Analysis 5.3  Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3). | ||||
| 4 Progression‐free survival Show forest plot | 1 | 339 | Hazard Ratio (Random, 95% CI) | 1.05 [0.87, 1.27] |
| Analysis 5.4  Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival. | ||||
| 5 Tumour remission Show forest plot | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| Analysis 5.5  Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.87] |
| Analysis 6.1  Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 1 1‐year mortality. | ||||
| 2 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.60, 0.89] | |
| Analysis 6.2  Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 2 Overall survival. | ||||
| 3 Quality of life Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 3 Quality of life. | ||||
| 3.1 Clinical important MID in FKSI‐DRS | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.28, 1.78] |
| 3.2 Clinical important MID in EQ‐5D‐VAS | 1 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.16, 1.61] |
| 4 Adverse events (grade ≥ 3) Show forest plot | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.40, 0.65] |
| Analysis 6.4  Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3). | ||||
| 5 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.88 [0.75, 1.03] | |
| Analysis 6.5 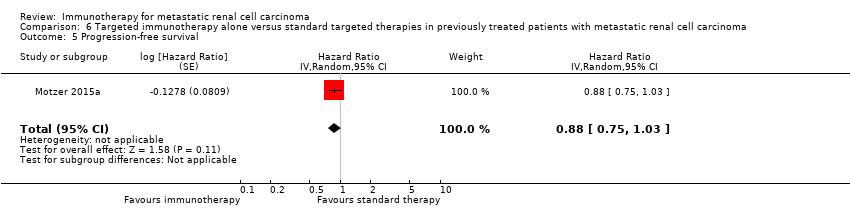 Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 5 Progression‐free survival. | ||||
| 6 Tumour remission Show forest plot | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 4.39 [2.84, 6.80] |
| Analysis 6.6  Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 6 Tumour remission. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Interferon‐α (IFN‐α) alone versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, outcome: 1.1 1‐year mortality.

Forest plot of comparison: 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, outcome: 3.1 1‐year mortality.
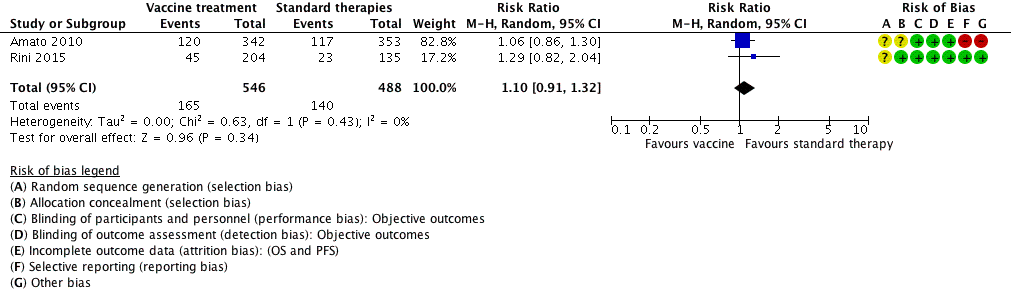
Forest plot of comparison: 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, outcome: 5.1 1‐year mortality.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Quality of life.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3).

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Progression‐free survival.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 6 Tumour remission.

Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).

Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.

Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Adverse events (grade ≥ 3).

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Progression‐free survival.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Tumour remission.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 3 Quality of life.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3).

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 5 Progression‐free survival.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 6 Tumour remission.
| IFN‐α alone versus standard targeted therapy for mRCC | ||||||
| Patient population: previously untreated patients with mRCC Settings: phase III, international, multicentre, open‐label Intervention: IFN‐α alone Comparison: standard targeted therapy (sunitinib or temsirolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapy | Risk difference with IFN‐α alone (95% CI) | |||||
| 1‐year mortality | Lowa | RR 1.3 | 1166 | ⊕⊕⊕⊝ | ‐ | |
| 150 per 1000 | 45 more per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 84 more per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 165 more per 1000 | |||||
| QoL | The mean QoL in the control group was | MD 5.58 lower | ‐ | 730 | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control group was | MD 3.27 lower (4.18 to 2.36 lower) | ‐ | 730 | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control group was | MD 1.98 lower (2.51 to 1.46 lower) | ‐ | 730 | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control group was | MD 0.06 lower (0.12 lower to 0 higher) | ‐ | 1002 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control groups was | MD 4.68 lower (6.53 to 2.83 lower) | ‐ | 1002 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| Adverse events (grade ≥ 3) | 668 per 1000 | 114 more per 1000 | RR 1.17 | 408 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for selection bias and performance bias due to cross‐over. | ||||||
| IFN‐α alone or combined with targeted therapy compared to standard targeted therapy in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patients with mRCC Setting: phase III, international, multicentre, open‐label Intervention: IFN‐α combined with targeted therapy Comparison: standard targeted therapy (temsirolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapy | Risk difference with IFN‐α combined with targeted therapy (95% CI) | |||||
| 1‐year mortality | Lowa | RR 1.13 | 419 | ⊕⊕⊕⊝ | ‐ | |
| 150 per 1000 | 20 more per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 36 more per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 71 more per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) | 668 per 1000 | 200 more per 1000 | RR 1.30 | 416 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. | ||||||
| IFN‐α alone versus IFN‐α + bevacizumab in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patient with mRCC Setting: phase III, international, multicentre, Escudier 2007: double‐blind, placebo‐controlled; Rini 2010: open‐label Intervention: IFN‐α alone Comparison: IFN‐α alone + bevacizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard therapy (IFN‐α + bevacizumab) | Risk difference with IFN‐α alone (95% CI) | |||||
| 1‐year mortality | Low | RR 1.17 | 1381 | ⊕⊕⊝⊝ | ‐ | |
| 150 per 1000 | 25 more per 1000 | |||||
| Moderate | ||||||
| 280 per 1000 | 48 more per 1000 | |||||
| High | ||||||
| 550 per 1000 | 93 more per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: up to 28 days after last dose to 65 months | 705 per 1000 | 162 fewer per 1000 | RR 0.77 | 1350 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for selection bias and performance bias due to substantial cross‐over. | ||||||
| IFN‐α + bevacizumab versus targeted therapies in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patients with mRCC Setting: phase II, national (France), multicentre, open‐label Intervention: IFN‐α + bevacizumab Comparison: standard targeted therapies (sunitinib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapies | Risk difference with IFN‐α + bevacizumab (95% CI) | |||||
| 1‐year mortality Follow‐up: 23.2 months | Lowa | RR 0.37 | 83 | ⊕⊕⊝⊝ | ‐ | |
| 150 per 1000 | 95 fewer per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 176 fewer per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 347 fewer per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: 48 weeks | 595 per 1000 | 107 more per 1000 | RR 1.18 | 82 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for reporting and performance bias due to differences in second‐line treatment. | ||||||
| Vaccine treatment versus standard therapies in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patients with mRCC Setting: phase III, international, multicentre, double‐blind, placebo‐controlled (Amato 2010), open‐label (Rini 2015) Intervention: vaccine treatment (MVA‐5T4 or IMA0901) Comparison: placebo and standard therapies (IL‐2, IFN‐α and sunitinib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard therapies | Risk difference with vaccine treatment (95% CI) | |||||
| 1‐year mortality | Lowa | RR 1.10 | 1034 | ⊕⊕⊝⊝ | ‐ | |
| 150 per 1000 | 15 more per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 28 more per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 55 more per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: not reported | 241 per 1000 | 39 more per 1000 | RR 1.16 | 1065 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not downgraded for performance bias, borderline decision due to second‐line therapies in one study. | ||||||
| Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with mRCC | ||||||
| Patient population: previously treated patients with mRCC Setting: phase III, international, multicentre, open‐label Intervention: targeted immunotherapy (nivolumab) alone Comparison: standard targeted therapies (everolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapies | Risk difference with targeted immunotherapy alone (95% CI) | |||||
| 1‐year mortality | 341 per 1000 | 102 fewer per 1000 | RR 0.70 | 821 | ⊕⊕⊕⊝ | ‐ |
| Quality of life: Clinically relevant improvement in FKSI‐DRS | 367 per 1000 | 187 more per 1000 (from 103 more to 287 more) | RR 1.51 (1.28 to 1.78) | 704 | ⊕⊕⊕⊝ | ‐ |
| Quality of life: clinically relevant improvement in EQ‐5D VAS | 391 per 1000 | 145 more per 1000 (from 63 more to 238 more) | RR 1.37 (1.16‐1.61) | 703 (1 study) | ⊕⊕⊕⊝ | ‐ |
| Adverse events (grade ≥ 3) | 365 per 1000 | 179 fewer per 1000 | RR 0.51 | 803 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for performance bias due to cross‐over. | ||||||
| Study ID | Comparison (group 1 vs group 0) | Median OS (95% CI) (months) | 1‐year mortality | Comments | ||
| Group 1 | Group 0 | Group 1 | Group 0 | |||
| 1 (IFN‐α alone vs standard targeted therapies) | 7.3 (6.1 to 8.8) | 10.9 (8.6 to 12.7) | 70% | 53% | From curves. | |
| 21.8 (17.9 to 26.9) | 26.4 (23.0 to 32.9) | 12.3% | 10.1% | Numbers reported. | ||
| 2 (IFN‐α + targeted therapies vs standard targeted therapies) | 8.4 (6.6 to 10.3) | 10.9 (8.6 to 12.7) | 60% | 53% | From curves. | |
| 3 (IFN‐α alone vs IFN‐α + bevacizumab) | 21.3 | 23.3 | 32% | 26% | From curves. | |
| 17.4 (14.4 to 20.0) | 18.3 (16.5 to 22.5) | 39% | 34% | From curves with numbers and censoring marks. | ||
| 4 (IFN‐α + bevacizumab vs standard targeted therapies) | Not reported | Not reported | 10% | 26% | Reported. | |
| 5 (Vaccine treatment vs standard therapies) | 19.2 | 20.1 | 35% | 33% | From curves with censoring. | |
| 33.1 | Not reached | 17% | 22% | Numbers reported. | ||
| 6 (Targeted immunotherapy alone vs targeted standard therapy) | 25.0 (21.8 to NE) | 19.6 (17.6 to 23.1) | 24% | 34% | From curves with numbers with censoring marks. | |
| CI: confidence interval; IFN‐α: interferon‐α; NE: not estimable; OS: overall survival. | ||||||
| Comparison (group 1 vs group 0) | Study | Subgroup | Sample size | Treatment effects (95% CI) or P values |
| 1 (IFN‐α alone vs standard targeted therapies) | Prior nephrectomy | 278 | HR 1.2 (0.9 to 1.6) | |
| Prior nephrectomy | 674 | HR 1.2 (0.95 to 1.5) | ||
| Pooled | Prior nephrectomy | 952 | HR 1.2 (1.0 to 1.43), I2 = 0% | |
| No prior nephrectomy | 138 | HR 1.7 (1.1 to 2.5) | ||
| No prior nephrectomy | 76 | HR 1.23 (0.8 to 2.1) | ||
| Pooled | No prior nephrectomy | 214 | HR 1.48 (1.1 to 2.0), I2 = 1% | |
| KPS ≤ 70 | 340 | HR 1.39 (1.11 to 1.75) | ||
| KPS > 70 | 75 | HR 0.93 (0.53 to 1.67) | ||
| With poor risk | 48 | HR 1.15 (0.83 to 2.78) | ||
| Intermediate risk | 421 | HR 1.27 (1.00 to 1.62) | ||
| ECOG Performance Status 0 | 460 | HR 1.1 (0.87 to 1.5) | ||
| ECOG Performance Status 1 | 290 | HR 1.4 (1.05 to 1.7) | ||
| 3 (IFN‐α alone vs IFN‐α + bevacizumab) | Favourable risk | 180 | HR 1.09 (0.73 to 1.61), P = 0.6798 | |
| Favourable risk | 192 | HR 1.11 (0.8 to 1.56), P = 0.5189 | ||
| Pooled | Favourable risk | 372 | HR 1.10 (0.85 to 1.43), I2 = 0% | |
| Intermediate risk | 392 | HR 1.20 (0.95 to 1.54), P = 0.1230 | ||
| Intermediate risk | 465 | HR 1.15 (0.94 to 1.41), P = 0.1688 | ||
| Pooled | Intermediate risk | 857 | HR 1.18 (1.01 to 1.37), I2 = 0% | |
| Poor risk | 59 | HR 1.18 (0.68 to 2.04), P = 0.5594 | ||
| Poor risk | 75 | HR 1.33 (0.82 to 2.17), P = 0.2439 | ||
| Pooled | Poor risk | 124 | HR 1.27 (0.88 to 1.82), I2 = 0% | |
| Prior nephrectomy | 620 | HR 1.10 (0.93 to 1.32), P = 0.2871 | ||
| No prior nephrectomy | 112 | HR 1.54 (1.02 to 2.27), P = 0.0381 | ||
| 5 (Vaccine treatment vs standard therapies) | Favourable risk, treated with IL‐2 (SOC) | 100 | HR 0.54 (0.30 to 0.98), P = 0.046 | |
| Favourable risk, treated with IFN‐α (SOC) | 206 | P > 0.05 | ||
| Good prognosis, treated with sunitinib (SOC) | 119 | P > 0.05 | ||
| Intermediate prognosis, treated with IL‐2 (SOC) | 70 | P > 0.05 | ||
| Intermediate prognosis, treated with IFN‐α (SOC) | 169 | P > 0.05 | ||
| Intermediate prognosis, treated with sunitinib (SOC) | 65 | P > 0.05 | ||
| Favourable risk (n = 92) | 92 | HR 0.82, P = 0.59 | ||
| Intermediate risk (n = 240) | 240 | HR 1.52, P < 0.05 | ||
| 6 (Targeted immunotherapy alone vs standard targeted therapies) | Favourable risk group (MSKCC risk group) | 293 | HR 0.89 (0.59 to 1.32) | |
| Intermediate risk group (MSKCC risk group) | 404 | HR 0.76 (0.58 to 0.99) | ||
| Poor risk group (MSKCC risk group) | 124 | HR 0.47 (0.30 to 0.73) | ||
| 1 previous antiangiogenic regimen | 591 | HR 0.71 (0.56 to 0.90) | ||
| 2 previous antiangiogenic regimens | 230 | HR 0.89 (0.61 to 1.29) | ||
| CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; IFN‐α: interferon‐α; IL: interleukin; KPS: Karnovsky Performance Score; MSKCC: Memorial Sloan‐Kettering Cancer Center; n: number of participants; SOC: standard of care. | ||||
| Comparison (group 1 vs group 0) | Study (reported in) | Measurement instrument | Group 1 | Group 0 | Favours | Difference (95% CI) or P values |
| 1 (IFN‐α alone vs standard targeted therapies) | Motzer 2007 (reported in Cella 2008) | FACT‐G total score, mean postbaseline score over 17 weeks | 76.8 (n = 357) | 82.3 (n = 373) | Group 0 | ‐5.58 (‐7.25 to ‐3.91) |
| Motzer 2007 (reported in Cella 2008) | FKSI‐15, mean postbaseline score over 17 weeks | 42.1 (n = 357) | 45.3 (n = 373) | Group 0 | ‐3.27 (‐4.18 to ‐2.36) | |
| Motzer 2007 (reported in Cella 2008) | FKSI‐DRS, mean postbaseline score over 17 weeks | 27.4 (n = 357) | 29.4 (n = 373) | Group 0 | ‐1.98 (‐2.51 to ‐1.46) | |
| Hudes 2007, (reported in Yang 2010) | EQ‐5D Index, mean score on treatment | 0.492 (n = 115) | 0.590 (n = 157) | Group 0 | ‐0.099 (95% CI ‐0.162 to ‐0.036) | |
| Motzer 2007 (reported in Cella 2008) | EQ‐5D Index, mean postbaseline score over 17 weeks | 0.725 (n = 357) | 0.762 (n = 373) | Group 0 | ‐0.0364 (‐0.0620 to ‐0.0109) | |
| Pooled EQ‐5D | 472 | 530 | Group 0 | ‐0.06 (‐0.12 to 0), I2 = 69% | ||
| Hudes 2007, (reported in Yang 2010) | EQ‐VAS, mean score on treatment | 58.83 (n = 115) | 63.33 (n = 157) | Group 0 | ‐4.50 (‐8.184 to ‐0.819) | |
| Motzer 2007, (reported in Cella 2008) | EQ‐VAS, mean postbaseline score over 17 weeks | 68.7 (n = 357) | 73.4 (n = 373) | Group 0 | ‐4.74 (‐6.87 to ‐2.60) | |
| Pooled EQ‐VAS | 472 | 530 | Group 0 | ‐4.68 (‐6.53 to ‐2.83), I2 = 0% | ||
| 6 (Targeted immunotherapy alone vs standard targeted therapies) | Motzer 2015a (reported in Cella 2016) | FKSI‐DRS, mean score at baseline | 30.2 ± 4.4 (n = 362) | 30.1 ± 4.8 (n = 344) | ‐ | Difference in mean change 1.6 (1.4 to 1.9), P < 0.0001 |
| FKSI‐DRS, mean change from baseline to week 28 | 0.4 ± 5 (n = 164) | ‐1.2 ± 4 (n = 122) | Group 1 | |||
| FKSI‐DRS, mean change from baseline to week 52 | 1.6 ± 4 (n = 97) | ‐1.0 ± 6 (n = 63) | Group 1 | |||
| FKSI‐DRS, mean change from baseline to week 104 | 3.5 ± 4.1 (n = 20) | 0.2 ± 6 (n = 9) | Group 1 | |||
| Clinically important improvement from baseline by ≥ 2 FKSI‐DRS points | 200 (55%)/361 | 126 (37%)/343 | Group 1 | RR 1.51 (1.28 to 1.78); P < 0.0001 | ||
| Time to clinically important improvement ≥ 2 FKSI‐DRS points | Median: 4.7 months (3.7 to 7.5) | Median not reached | Group 1 | HR 1.66 (1.33 to 2.08); P < 0.0001 | ||
| Clinically important improvement from baseline by ≥ 3 FKSI‐DRS points | 148 (41%)/361 | 95 (28%)/343 | Group 1 | RR 1.48 (1.20 to 1.83); P = 0.0002 | ||
| Time to clinically important improvement ≥ 3 FKSI‐DRS points | Median not estimable | Median not estimable | Group 1 | HR 1.61 (1.24 to 2.09); P < 0.0003 | ||
| EQ‐5D utility index, mean score at baseline | 0.78 ± 0.24 (n = 362) | 0.78 ± 0.21 (n = 344) | ‐ | No significant differences in proportion of participants with clinical important improvements (P = 0.070) or time to improvement (P = 0.86). | ||
| EQ‐5D utility index, mean change from baseline to week 28 | 0.052 ± 0.22 (n = 164) | ‐0.03 ± 0.2 (n = 122) | Group 1 | |||
| EQ‐5D utility index, mean change from baseline to week 52 | 0.06 ± 0.1 (n = 98) | ‐0.01 ± 0.2 (n = 63) | Group 1 | |||
| EQ‐5D utility index, mean change from baseline to week 104 | 0.13 ± 0.7 (n = 20) | ‐0.02 ± 0.15 (n = 9) | Group 1 | |||
| EQ‐5D VAS, mean score at baseline | 73.3 ± 18.5 (n = 362) | 72.5 ± 18.7 (n = 344) | ‐ | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 28 | 5 ± 13 (n = 164) | ‐3 ± 11 (n = 122) | Group 1 | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 52 | 7 ± 15 (n = 98) | ‐2 ± 16 (n = 63) | Group 1 | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 104 | 9 ± 9 (n = 20) | 1 ± 18 (n = 9) | Group 1 | ‐ | ||
| Clinically important improvement from baseline by ≥ 7 EQ‐5D VAS points | 192 (53%)/360 | 134(39%)/343 | ‐ | RR 1.37 (1.16 to 1.61); P = 0.0001 | ||
| Time to clinically important improvement | Median: 6.5 months (3.9 to 12.2) | Median: 23.1 months (15.4 to not estimated) | ‐ | HR 1.37 (1.10 to 1.71) | ||
| CI: confidence interval; EQ‐5D Index: EuroQol 5‐Dimension (MID 0.06 to 0.08, Pickard 2007); EQ‐VAS: EuroQol Visual Analog Scale (MID 7, Pickard 2007); FACT‐G: Functional Assessment of Cancer Therapy ‐ General (MID 4 points for better rating and 8 points for worse rating, Ringash 2007); FKSI‐15: FACT‐Kidney Symptom Index (MID 3 points, Cella 1997); FKSI‐DRS: FACT‐Kidney Symptom Index Disease Related Symptoms (MID 2 points, Cella 1997); HR: hazard ratio; MID: minimal important difference; n: number of participants. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.13, 1.51] |
| 2 Overall survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.28 [1.11, 1.49] | |
| 3 Quality of life Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 FACT‐G | 1 | 730 | Mean Difference (Random, 95% CI) | ‐5.58 [‐7.25, ‐3.91] |
| 3.2 FKSI‐15 | 1 | 730 | Mean Difference (Random, 95% CI) | ‐3.27 [‐4.18, ‐2.36] |
| 3.3 FKSI‐DRS | 1 | 730 | Mean Difference (Random, 95% CI) | ‐1.98 [‐2.51, ‐1.45] |
| 3.4 EQ‐5D | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.12, ‐0.00] |
| 3.5 EQ‐VAS | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐4.68 [‐6.53, ‐2.83] |
| 4 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 2.23 [1.79, 2.77] | |
| 6 Tumour remission Show forest plot | 2 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 5 Tumour remission Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.36] |
| 2 Overall survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.13 [1.00, 1.28] | |
| 3 Adverse events (grade ≥ 3) Show forest plot | 2 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.71, 0.84] |
| 4 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.53 [1.36, 1.73] | |
| 5 Tumour remission Show forest plot | 2 | 1205 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.31, 0.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Tumour remission Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
| 2 Overall survival Show forest plot | 2 | 1071 | Hazard Ratio (Fixed, 95% CI) | 1.14 [0.96, 1.37] |
| 3 Adverse events (grade ≥ 3) Show forest plot | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.97, 1.39] |
| 4 Progression‐free survival Show forest plot | 1 | 339 | Hazard Ratio (Random, 95% CI) | 1.05 [0.87, 1.27] |
| 5 Tumour remission Show forest plot | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.87] |
| 2 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.60, 0.89] | |
| 3 Quality of life Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Clinical important MID in FKSI‐DRS | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.28, 1.78] |
| 3.2 Clinical important MID in EQ‐5D‐VAS | 1 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.16, 1.61] |
| 4 Adverse events (grade ≥ 3) Show forest plot | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.40, 0.65] |
| 5 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.88 [0.75, 1.03] | |
| 6 Tumour remission Show forest plot | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 4.39 [2.84, 6.80] |


