Inmunoterapia para el carcinoma metastásico de células renales
Appendices
Appendix 1. Cochrane Library (Wiley.com) search strategy
1. MeSH descriptor: [Carcinoma, Renal Cell] explode all trees and with qualifier(s): [Drug therapy ‐ DT, Immunology ‐ IM, Pathology ‐ PA, Prevention & control ‐ PC, Therapy ‐ TH]
2. MeSH descriptor: [von Hippel‐Lindau Disease] explode all trees and with qualifier(s): [Drug therapy ‐ DT, Immunology ‐ IM, Pathology ‐ PA, Prevention & control ‐ PC, Therapy ‐ TH]
3. advance* NEAR (renal OR kidney OR nephron*) NEAR (cancer* OR neoplasm* OR carcinoma* OR tumour* OR tumour*)
4. advance* NEAR (renal or kidney or nephron*) NEXT cell NEAR cancer*
5. metasta* NEAR (renal or kidney or nephron*) NEAR (cancer* or neoplasms* or carcinoma* or tumour* or tumour*)
6. metasta* NEAR (renal or kidney or nephron*) NEXT cell NEAR cancer*
7. local* NEAR advance* NEAR (renal or kidney or nephro*) NEAR (cancer* or neoplasms* or carcinoma* or tumour* or tumour*)
8. local* NEAR advance* NEAR (renal or kidney or nephro*) NEXT cell NEAR cancer*
9. "clear cell type" NEXT/3 carcinom*
10. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11. MeSH descriptor: [Immunotherapy, Active] explode all trees
12. MeSH descriptor: [Immunization, Passive] explode all trees
13. MeSH descriptor: [Adoptive Transfer] explode all trees
14. MeSH descriptor: [Cancer Vaccines] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
15. MeSH descriptor: [Antigens, Neoplasm] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
16. MeSH descriptor: [Antibodies, Monoclonal] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
17. MeSH descriptor: [Hematopoietic Stem Cell Transplantation] explode all trees
18. MeSH descriptor: [Immunomodulation] explode all trees and with qualifier(s): [Drug effects ‐ DE]
19. MeSH descriptor: [Immunologic Factors] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
20. MeSH descriptor: [Immunosuppressive Agents] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
21. MeSH descriptor: [Superantigens] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
22. MeSH descriptor: [Antigens, Neoplasm] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
23. vaccin*:ti,ab
24. (immune?ation OR immunotherap* OR immunosuppress* OR immunotox*):ti,ab
25. tum*r NEXT antigen*:ti,ab
26. adjuvants:ti,ab OR adoptive T cell therap*:ti,ab
27. (car or "containment and autonomic regulation") NEXT therapy
28. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29. MeSH descriptor: [Interferon‐alpha] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
30. MeSH descriptor: [Interleukin‐2] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
31. MeSH descriptor: [Interleukin‐7] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
32. MeSH descriptor: [Interleukin‐12] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
33. MeSH descriptor: [Interleukin‐15] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
34. MeSH descriptor: [Dendritic Cells] explode all trees
35. MeSH descriptor: [BCG Vaccine] explode all trees
36. MeSH descriptor: [Cytokine‐Induced Killer Cells] explode all trees
37. MeSH descriptor: [Recombinant Proteins] explode all trees
38. vaccin* NEXT/2 (BCG or "Bacillus Calmette Guerin" or Calmette*):ti,ab
39. (DNA or mRNA or cellular or peptid* or protein or genetic) NEXT/2 vaccin*:ti,ab
40. Dendritic cell NEXT/2 bas* NEXT/3 vaccin*:ti,ab
41. immun* NEXT/2 checkpoint:ti,ab
42. Cytotoxic T lymphocyt* or CTL:ti,ab
43. inhibit* NEXT/2 (receptor* or antibod*):ti,ab
44. (humanized or agonistic) NEXT antibod*.ab
45. Anti NEXT/2 (CTLA or "PD‐L1" or "PD‐1" or "sLAG‐3"):ti,ab
46. ("cytotoxic T lymphocytes antigen" or "soluble human LAG‐3 protein"):ti,ab
47. (ipilimumab or tremelimumab or nivolumab or naptumomab or estafenox):ti,ab
48. (IMP321 or IMA901 or AGS‐003 or CMDSC or ABR‐217620 or ANYARA):ti,ab
49. activating receptor*:ti,ab
50. ("denileukin difitox" or reniale or "recombinant human interleukin" or lymphocine):ti,ab
51. "autologous tumour lysate" NEXT/2 "loaded dendritic cells":ti,ab
52. HSPPC NEXT/3 vaccin*:ti,ab
53. mRNA NEXT transfected NEXT dendritic NEXT cell*:ti,ab
54. (co NEXT stimulation) or (co NEXT inhibitation):ti,ab
55. activat* NEXT killer NEXT cell*:ti,ab
56. ("CD4+" or regulat*) NEXT/3 "T" NEXT cell*:ti,ab
57. myeloid NEXT deriv* NEXT/3 suppressor* NEXT cell*:ti,ab
58. (tum*r NEXT associat* NEXT macrophag*) or TAM:ti,ab
59. "CpG" NEXT oligonucleotid*:ti,ab
60. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59
61. 28 or 60
62. 10 and 61
Appendix 2. MEDLINE (Ovid) search strategy
1. exp Carcinoma, Renal Cell/dt, pa, th, im, pc [Drug Therapy, Pathology, Therapy, Immunology, Prevention & Control]
2. von Hippel‐Lindau Disease/dt, pa, th, im, pc [Drug Therapy, Pathology, Therapy, Immunology, Prevention & Control]
3. (advance* adj6 (renal OR kidney OR nephron$) adj6 (cancer$ OR neoplasms$ OR carcinoma$ OR tumour$ OR tumour$)).mp
4. (advance* adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp.
5. (metasta* adj6 (renal or kidney or nephron$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp
6. (metasta* adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp
7. (local* adj6 advance* adj6 (renal or kidney or nephro*) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp
8. (local* adj6 advance* adj6 (renal or kidney or nephro*) adj cell adj6 cancer$).mp
9. ("clear cell type" adj3 carcinom$).mp
10. or/1‐9
11. exp Immunotherapy, Active /ae, tu [therapeutic use]
12. Immunization, Passive/ae, tu [Adverse Effects, Therapeutic Use]
13. Adoptive Transfer/ or adoptive T cell therap$.ti,ab
14. Cancer Vaccines /ae, tu
15. exp Antigens, Neoplasm /ae, tu
16. exp Antibodies, Monoclonal /ae, tu
17. Hematopoietic Stem Cell Transplantation
18. exp Immunomodulation/de [Drug Effects]
19. Immunologic Factors/ ae, tu
20. Immunosuppressive Agents/ae, tu
21. exp Superantigens/ae, tu
22. exp Antigens, Neoplasm/ae, tu
23. vaccin$.ti,ab
24. (immuni#ation OR immunotherap$ OR immunosuppress$ OR immunotox$).ti,ab
25. ((tumour or tumour) adj antigen$).ti,ab
26. Adjuvants.ti,ab
27. ((car or "containment and autonomic regulation") adj therapy).mp
28. or/11‐27
29. Interferon‐alpha/tu OR Interleukin‐2/tu OR Interleukin‐7/tu OR Exp Interleukin‐12/tu OR Interleukin‐15/tu
30. Exp Dendritic Cells/ OR BCG vaccine/
31. exp Cytokine‐Induced Killer Cells/de
32. exp Recombinant Proteins/tu
33. (vaccin$ adj2 (BCG or "Bacillus Calmette Guerin" or Calmette$)).ti,ab
34. ((DNA or mRNA or cellular or peptid$ or protein or genetic) adj2 vaccin$).ti,ab
35. (Dendritic cell adj2 bas$ adj3 vaccin$).ti,ab
36. (immun$ adj2 checkpoint).ti,ab
37. (Cytotoxic T lymphocyt$ or CTL).ti,ab
38. (inhibit$ adj2 (receptor$ or antibod$)).ti,ab
39. (humanized or agonistic) adj antibod$.ab
40. (Anti adj2 (CTLA or "PD‐L1" or "PD‐1" or "sLAG‐3")).ti,ab
41. ("cytotoxic T lymphocytes antigen" or "soluble human LAG‐3 protein").ti,ab
42. (ipilimumab or tremelimumab or nivolumab or naptumomab or estafenox).ti,ab
43. (IMP321 or IMA901 or AGS‐003 or CMDSC or ABR‐217620 or ANYARA).ti,ab
44. (activating receptor$).ti,ab
45. ("denileukin difitox" or reniale or "recombinant human interleukin" or lymphocine).ti,ab
46. ("autologous tumour lysate" adj2 "loaded dendritic cells").ti,ab
47. (HSPPC adj3 vaccin$).ti,ab
48. (mRNA transfected dendritic cell$).ti,ab
49. ((co adj stimulation) or (co adj inhibitation)).ti,ab
50. (activat$ adj killer adj cell$).ti,ab
51. ((CD4+ or regulat$) adj3 T adj cell$).ti,ab
52. (myeloid adj deriv$ adj3 suppressor$ adj cell$).ti,ab
53. ((tumour adj associat$ macrophag$) or (tumour adj associat$ macrophag$) or TAM).ti,ab
54. (CpG adj oligonucleotid$).ti,ab
55. or/29‐54
56. 28 or 55
57. randomised controlled trial.pt.
58. controlled clinical trial.pt.
59. randomised.ab.
60. placebo.ab.
61. drug therapy.fs.
62. randomly.ab.
63. trial.ab.
64. groups.ab.
65. or/57‐64
66. exp animals/ not humans/
67. 65 not 66
68. 10 and 56 and 67
Appendix 3. Embase (Ovid) search strategy
1 kidney carcinoma/
2 von Hippel Lindau disease/
3 (advance$ adj6 (renal or kidney or nephron$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp.
4 (advance$ adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp.
5 (metasta$ adj6 (renal or kidney or nephron$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp.
6 (metasta$ adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp.
7 (local$ adj6 advance$ adj6 (renal or kidney or nephro$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp.
8 (local$ adj6 advance$ adj6 (renal or kidney or nephro$) adj cell adj6 cancer$).mp.
9 ("clear cell type" adj3 carcinom$).mp.
10 or/1‐9
11 exp immunotherapy/
12 exp passive immunization/
13 adoptive transfer.ti,ab.
14 adoptive t cell therap*.ti,ab.
15 cancer vaccine/
16 exp tumour antigen/
17 exp monoclonal antibody/
18 exp hematopoietic stem cell transplantation/
19 immunomodulation/
20 immunologic factor/
21 exp immunosuppressive agent/
22 superantigen/
23 alpha interferon/
24 interleukin 2/
25 interleukin 7/
26 interleukin 12/
27 interleukin 15/
28 exp dendritic cell/
29 BCG vaccine/
30 vaccin$.ti,ab.
31 (immuni#ation or immunotherap$ or immunosuppress$ or immunotox$).ti,ab.
32 ((tumour or tumour) adj antigen$).ti,ab.
33 Adjuvants.ti,ab.
34 ((car or "containment and autonomic regulation") adj therapy).mp.
35 (vaccin$ adj2 (BCG or "Bacillus Calmette Guerin" or Calmette$)).ti,ab.
36 ((DNA or mRNA or cellular or peptid$ or protein or genetic) adj2 vaccin$).ti,ab.
37 (Dendritic cell adj2 bas$ adj3 vaccin$).ti,ab.
38 (immun$ adj2 checkpoint).ti,ab.
39 (Cytotoxic T lymphocyt$ or CTL).ti,ab.
40 (inhibit$ adj2 (receptor$ or antibod$)).ti,ab.
41 ((humanized or agonistic) adj antibod$).ab.
42 (Anti adj2 (CTLA or "PD‐L1" or "PD‐1" or "sLAG‐3")).ti,ab.
43 ("cytotoxic T lymphocytes antigen" or "soluble human LAG‐3 protein").ti,ab.
44 (ipilimumab or tremelimumab or nivolumab or naptumomab or estafenox).ti,ab.
45 (IMP321 or IMA901 or AGS‐003 or CMDSC or ABR‐217620 or ANYARA).ti,ab.
46 activating receptor$.ti,ab.
47 ("denileukin difitox" or reniale or "recombinant human interleukin" or lymphocine).ti,ab.
48 ("autologous tumour lysate" adj2 "loaded dendritic cells").ti,ab.
49 (HSPPC adj3 vaccin$).ti,ab.
50 mRNA transfected dendritic cell$.ti,ab.
51 ((co adj stimulation) or (co adj inhibitation)).ti,ab.
52 (activat$ adj killer adj cell$).ti,ab.
53 ((CD4+ or regulat$) adj3 T adj cell$).ti,ab.
54 (myeloid adj deriv$ adj3 suppressor$ adj cell$).ti,ab.
55 ((tumour adj associat$ macrophag$) or (tumour adj associat$ macrophag$) or TAM).ti,ab.
56 (CpG adj oligonucleotid$).ti,ab.
57 or/11‐56
58 10 and 57
59 Crossover Procedure/
60 double‐blind procedure/
61 randomised controlled trial/
62 single‐blind procedure/
63 (random$ or factorial$ or crossover$ or cross over$ or placebo$ or assign$ or allocat$ or volunteer$).mp.
64 ((doubl$ or singl$) adj blind$).mp.
65 or/59‐64
66 exp animal/ not human.sh.
67 65 not 66
68 58 and 67
Appendix 4. ClinicalTrials.gov search strategy
Search strategies in Advanced Search
Search 1:
Interventions: Immunotherapy OR Immunization OR Cancer Vaccines OR tumour antigen OR monoclonal antibody OR hematopoietic stem cell transplantation OR immunosuppressive agent OR superantigen OR adoptive transfer
Conditions: metastatic renal cell carcinoma
Search 2:
Interventions: tumour‐associated peptides OR interferon alpha OR interleukin 2 OR interleukin 7 OR interleukin 12 OR interleukin 15 OR dendritic cell OR BCG vaccine OR DNA vaccine OR mRNA vaccine OR cellular vaccine
Conditions: metastatic renal cell carcinoma
Search 3:
Interventions: peptide vaccine OR peptid vaccine OR protein vaccine OR genetic vaccine OR IMP321 OR IMA901 OR AGS‐003 OR CMDSC OR ABR‐217620 OR ANYARA
Conditions: metastatic renal cell carcinoma
Search 4:
Interventions: immune checkpoint inhibitor OR CTLA OR PD‐L1 OR PD‐1 OR sLAG‐3 OR cytotoxic T lymphocytes antigen OR soluble human LAG‐3 protein OR ipilimumab OR tremelimumab OR nivolumab OR atezolizumab OR pembrolizumab OR pidilizumab OR durvalumab
Conditions: metastatic renal cell carcinoma
Appendix 5. WHO ICTRP search strategy
In the Condition:
metastatic renal cell carcinoma
In the Intervention:
Immunotherapy OR Immunization OR Cancer Vaccines OR tumour antigen OR monoclonal antibody OR hematopoietic stem cell transplantation OR immunosuppressive agent OR superantigen OR tumour‐associated peptides OR interferon alpha OR interleukin 2 OR interleukin 7 OR interleukin 12 OR interleukin 15 OR dendritic cell OR BCG vaccine OR DNA vaccine OR mRNA vaccine OR cellular vaccine OR peptide vaccine OR peptid vaccine OR protein vaccine OR genetic vaccine OR IMP321 OR IMA901 OR AGS‐003 OR CMDSC OR ABR‐217620 OR ANYARA OR adoptive transfer OR immune checkpoint inhibitor OR CTLA OR PD‐L1 OR PD‐1 OR sLAG‐3 OR cytotoxic T lymphocytes antigen OR soluble human LAG‐3 protein OR ipilimumab OR tremelimumab OR nivolumab OR atezolizumab OR pembrolizumab OR pidilizumab OR durvalumab
Appendix 6. EORTC search strategy
We browsed the website www.eortc.be/protoc/listprot.asp?kind=sites&site=24 and screened all trials by tumour site (kidney cancer).
Appendix 7. Web of Science Core Collection ‐ Meeting Abstracts search strategy
Search in "TOPIC" field:
"metastatic renal cell carcinoma" OR (metastatic NEAR/2 (renal or kidney or nephron*) NEAR/2 (cancer* or neoplasms* or carcinoma* or tumour* or tumour*))
AND
Immunotherapy OR Immunization OR Cancer Vaccines OR adoptive transfer OR tumour antigen OR monoclonal antibody OR hematopoietic stem cell transplantation OR immunosuppressive agent OR superantigen OR tumour‐associated peptides OR interferon alpha OR interleukin 2 OR interleukin 7 OR interleukin 12 OR interleukin 15 OR dendritic cell OR BCG vaccine OR DNA vaccine OR mRNA vaccine OR cellular vaccine OR peptide vaccine OR peptid vaccine OR protein vaccine OR genetic vaccine OR IMP321 OR IMA901 OR AGS‐003 OR CMDSC OR ABR‐217620 OR ANYARA
Refine to Document Type: Meeting Abstract

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Interferon‐α (IFN‐α) alone versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, outcome: 1.1 1‐year mortality.

Forest plot of comparison: 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, outcome: 3.1 1‐year mortality.
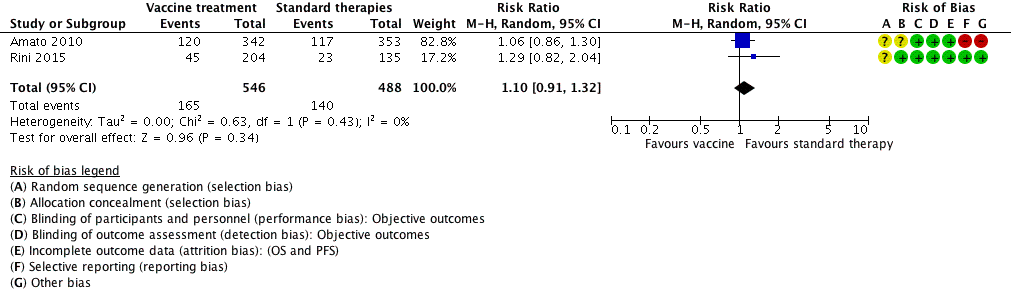
Forest plot of comparison: 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, outcome: 5.1 1‐year mortality.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
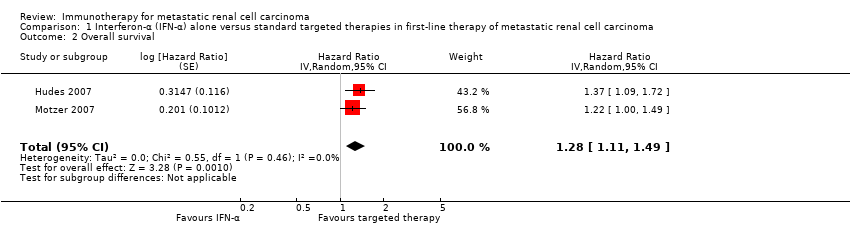
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Quality of life.
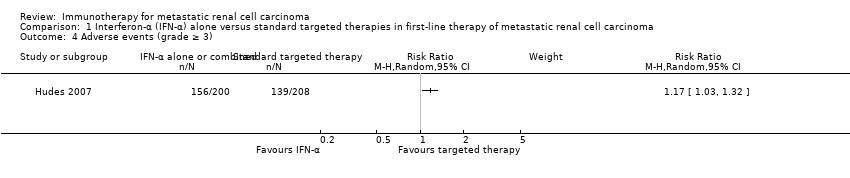
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3).

Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Progression‐free survival.
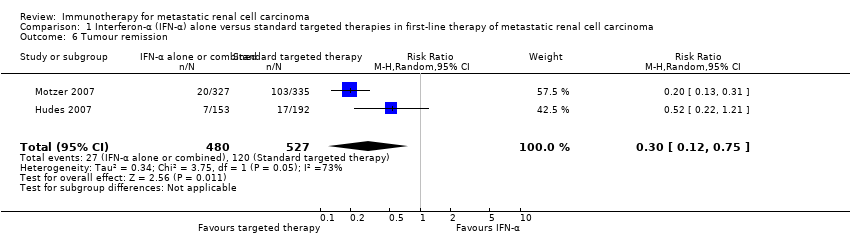
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 6 Tumour remission.
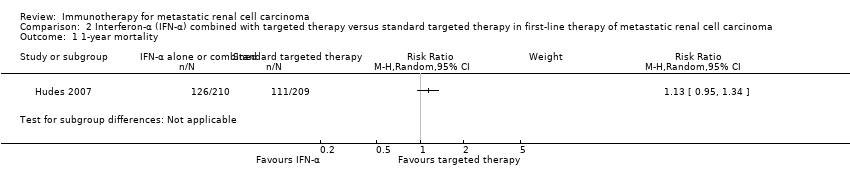
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
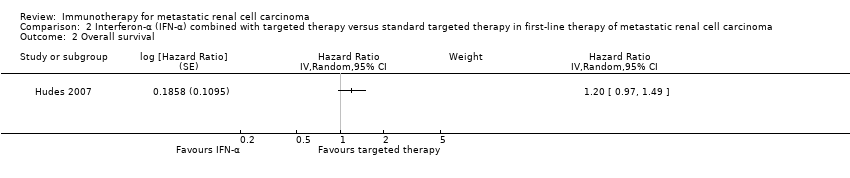
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.
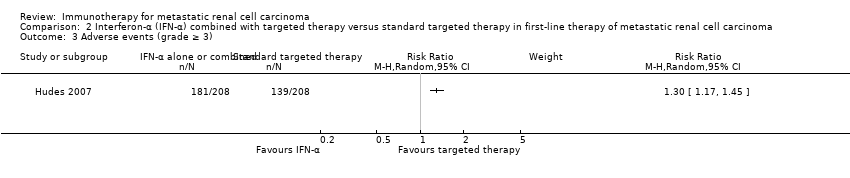
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).
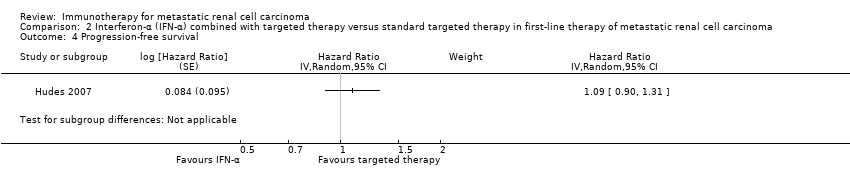
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.

Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).

Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.
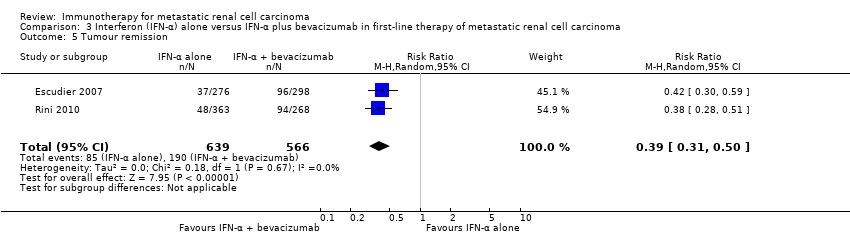
Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Adverse events (grade ≥ 3).

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Progression‐free survival.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Tumour remission.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.

Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 1 1‐year mortality.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 2 Overall survival.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 3 Quality of life.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3).
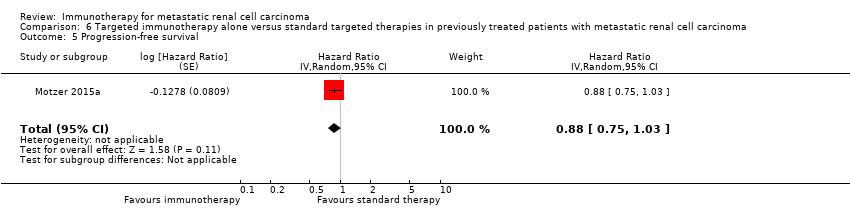
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 5 Progression‐free survival.

Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 6 Tumour remission.
| IFN‐α alone versus standard targeted therapy for mRCC | ||||||
| Patient population: previously untreated patients with mRCC Settings: phase III, international, multicentre, open‐label Intervention: IFN‐α alone Comparison: standard targeted therapy (sunitinib or temsirolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapy | Risk difference with IFN‐α alone (95% CI) | |||||
| 1‐year mortality | Lowa | RR 1.3 | 1166 | ⊕⊕⊕⊝ | ‐ | |
| 150 per 1000 | 45 more per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 84 more per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 165 more per 1000 | |||||
| QoL | The mean QoL in the control group was | MD 5.58 lower | ‐ | 730 | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control group was | MD 3.27 lower (4.18 to 2.36 lower) | ‐ | 730 | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control group was | MD 1.98 lower (2.51 to 1.46 lower) | ‐ | 730 | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control group was | MD 0.06 lower (0.12 lower to 0 higher) | ‐ | 1002 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| QoL | The mean QoL in the control groups was | MD 4.68 lower (6.53 to 2.83 lower) | ‐ | 1002 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| Adverse events (grade ≥ 3) | 668 per 1000 | 114 more per 1000 | RR 1.17 | 408 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for selection bias and performance bias due to cross‐over. | ||||||
| IFN‐α alone or combined with targeted therapy compared to standard targeted therapy in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patients with mRCC Setting: phase III, international, multicentre, open‐label Intervention: IFN‐α combined with targeted therapy Comparison: standard targeted therapy (temsirolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapy | Risk difference with IFN‐α combined with targeted therapy (95% CI) | |||||
| 1‐year mortality | Lowa | RR 1.13 | 419 | ⊕⊕⊕⊝ | ‐ | |
| 150 per 1000 | 20 more per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 36 more per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 71 more per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) | 668 per 1000 | 200 more per 1000 | RR 1.30 | 416 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. | ||||||
| IFN‐α alone versus IFN‐α + bevacizumab in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patient with mRCC Setting: phase III, international, multicentre, Escudier 2007: double‐blind, placebo‐controlled; Rini 2010: open‐label Intervention: IFN‐α alone Comparison: IFN‐α alone + bevacizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard therapy (IFN‐α + bevacizumab) | Risk difference with IFN‐α alone (95% CI) | |||||
| 1‐year mortality | Low | RR 1.17 | 1381 | ⊕⊕⊝⊝ | ‐ | |
| 150 per 1000 | 25 more per 1000 | |||||
| Moderate | ||||||
| 280 per 1000 | 48 more per 1000 | |||||
| High | ||||||
| 550 per 1000 | 93 more per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: up to 28 days after last dose to 65 months | 705 per 1000 | 162 fewer per 1000 | RR 0.77 | 1350 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for selection bias and performance bias due to substantial cross‐over. | ||||||
| IFN‐α + bevacizumab versus targeted therapies in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patients with mRCC Setting: phase II, national (France), multicentre, open‐label Intervention: IFN‐α + bevacizumab Comparison: standard targeted therapies (sunitinib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapies | Risk difference with IFN‐α + bevacizumab (95% CI) | |||||
| 1‐year mortality Follow‐up: 23.2 months | Lowa | RR 0.37 | 83 | ⊕⊕⊝⊝ | ‐ | |
| 150 per 1000 | 95 fewer per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 176 fewer per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 347 fewer per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: 48 weeks | 595 per 1000 | 107 more per 1000 | RR 1.18 | 82 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for reporting and performance bias due to differences in second‐line treatment. | ||||||
| Vaccine treatment versus standard therapies in first‐line therapy of mRCC | ||||||
| Patient population: previously untreated patients with mRCC Setting: phase III, international, multicentre, double‐blind, placebo‐controlled (Amato 2010), open‐label (Rini 2015) Intervention: vaccine treatment (MVA‐5T4 or IMA0901) Comparison: placebo and standard therapies (IL‐2, IFN‐α and sunitinib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard therapies | Risk difference with vaccine treatment (95% CI) | |||||
| 1‐year mortality | Lowa | RR 1.10 | 1034 | ⊕⊕⊝⊝ | ‐ | |
| 150 per 1000 | 15 more per 1000 | |||||
| Moderatea | ||||||
| 280 per 1000 | 28 more per 1000 | |||||
| Higha | ||||||
| 550 per 1000 | 55 more per 1000 | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: not reported | 241 per 1000 | 39 more per 1000 | RR 1.16 | 1065 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not downgraded for performance bias, borderline decision due to second‐line therapies in one study. | ||||||
| Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with mRCC | ||||||
| Patient population: previously treated patients with mRCC Setting: phase III, international, multicentre, open‐label Intervention: targeted immunotherapy (nivolumab) alone Comparison: standard targeted therapies (everolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapies | Risk difference with targeted immunotherapy alone (95% CI) | |||||
| 1‐year mortality | 341 per 1000 | 102 fewer per 1000 | RR 0.70 | 821 | ⊕⊕⊕⊝ | ‐ |
| Quality of life: Clinically relevant improvement in FKSI‐DRS | 367 per 1000 | 187 more per 1000 (from 103 more to 287 more) | RR 1.51 (1.28 to 1.78) | 704 | ⊕⊕⊕⊝ | ‐ |
| Quality of life: clinically relevant improvement in EQ‐5D VAS | 391 per 1000 | 145 more per 1000 (from 63 more to 238 more) | RR 1.37 (1.16‐1.61) | 703 (1 study) | ⊕⊕⊕⊝ | ‐ |
| Adverse events (grade ≥ 3) | 365 per 1000 | 179 fewer per 1000 | RR 0.51 | 803 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for performance bias due to cross‐over. | ||||||
| Study ID | Comparison (group 1 vs group 0) | Median OS (95% CI) (months) | 1‐year mortality | Comments | ||
| Group 1 | Group 0 | Group 1 | Group 0 | |||
| 1 (IFN‐α alone vs standard targeted therapies) | 7.3 (6.1 to 8.8) | 10.9 (8.6 to 12.7) | 70% | 53% | From curves. | |
| 21.8 (17.9 to 26.9) | 26.4 (23.0 to 32.9) | 12.3% | 10.1% | Numbers reported. | ||
| 2 (IFN‐α + targeted therapies vs standard targeted therapies) | 8.4 (6.6 to 10.3) | 10.9 (8.6 to 12.7) | 60% | 53% | From curves. | |
| 3 (IFN‐α alone vs IFN‐α + bevacizumab) | 21.3 | 23.3 | 32% | 26% | From curves. | |
| 17.4 (14.4 to 20.0) | 18.3 (16.5 to 22.5) | 39% | 34% | From curves with numbers and censoring marks. | ||
| 4 (IFN‐α + bevacizumab vs standard targeted therapies) | Not reported | Not reported | 10% | 26% | Reported. | |
| 5 (Vaccine treatment vs standard therapies) | 19.2 | 20.1 | 35% | 33% | From curves with censoring. | |
| 33.1 | Not reached | 17% | 22% | Numbers reported. | ||
| 6 (Targeted immunotherapy alone vs targeted standard therapy) | 25.0 (21.8 to NE) | 19.6 (17.6 to 23.1) | 24% | 34% | From curves with numbers with censoring marks. | |
| CI: confidence interval; IFN‐α: interferon‐α; NE: not estimable; OS: overall survival. | ||||||
| Comparison (group 1 vs group 0) | Study | Subgroup | Sample size | Treatment effects (95% CI) or P values |
| 1 (IFN‐α alone vs standard targeted therapies) | Prior nephrectomy | 278 | HR 1.2 (0.9 to 1.6) | |
| Prior nephrectomy | 674 | HR 1.2 (0.95 to 1.5) | ||
| Pooled | Prior nephrectomy | 952 | HR 1.2 (1.0 to 1.43), I2 = 0% | |
| No prior nephrectomy | 138 | HR 1.7 (1.1 to 2.5) | ||
| No prior nephrectomy | 76 | HR 1.23 (0.8 to 2.1) | ||
| Pooled | No prior nephrectomy | 214 | HR 1.48 (1.1 to 2.0), I2 = 1% | |
| KPS ≤ 70 | 340 | HR 1.39 (1.11 to 1.75) | ||
| KPS > 70 | 75 | HR 0.93 (0.53 to 1.67) | ||
| With poor risk | 48 | HR 1.15 (0.83 to 2.78) | ||
| Intermediate risk | 421 | HR 1.27 (1.00 to 1.62) | ||
| ECOG Performance Status 0 | 460 | HR 1.1 (0.87 to 1.5) | ||
| ECOG Performance Status 1 | 290 | HR 1.4 (1.05 to 1.7) | ||
| 3 (IFN‐α alone vs IFN‐α + bevacizumab) | Favourable risk | 180 | HR 1.09 (0.73 to 1.61), P = 0.6798 | |
| Favourable risk | 192 | HR 1.11 (0.8 to 1.56), P = 0.5189 | ||
| Pooled | Favourable risk | 372 | HR 1.10 (0.85 to 1.43), I2 = 0% | |
| Intermediate risk | 392 | HR 1.20 (0.95 to 1.54), P = 0.1230 | ||
| Intermediate risk | 465 | HR 1.15 (0.94 to 1.41), P = 0.1688 | ||
| Pooled | Intermediate risk | 857 | HR 1.18 (1.01 to 1.37), I2 = 0% | |
| Poor risk | 59 | HR 1.18 (0.68 to 2.04), P = 0.5594 | ||
| Poor risk | 75 | HR 1.33 (0.82 to 2.17), P = 0.2439 | ||
| Pooled | Poor risk | 124 | HR 1.27 (0.88 to 1.82), I2 = 0% | |
| Prior nephrectomy | 620 | HR 1.10 (0.93 to 1.32), P = 0.2871 | ||
| No prior nephrectomy | 112 | HR 1.54 (1.02 to 2.27), P = 0.0381 | ||
| 5 (Vaccine treatment vs standard therapies) | Favourable risk, treated with IL‐2 (SOC) | 100 | HR 0.54 (0.30 to 0.98), P = 0.046 | |
| Favourable risk, treated with IFN‐α (SOC) | 206 | P > 0.05 | ||
| Good prognosis, treated with sunitinib (SOC) | 119 | P > 0.05 | ||
| Intermediate prognosis, treated with IL‐2 (SOC) | 70 | P > 0.05 | ||
| Intermediate prognosis, treated with IFN‐α (SOC) | 169 | P > 0.05 | ||
| Intermediate prognosis, treated with sunitinib (SOC) | 65 | P > 0.05 | ||
| Favourable risk (n = 92) | 92 | HR 0.82, P = 0.59 | ||
| Intermediate risk (n = 240) | 240 | HR 1.52, P < 0.05 | ||
| 6 (Targeted immunotherapy alone vs standard targeted therapies) | Favourable risk group (MSKCC risk group) | 293 | HR 0.89 (0.59 to 1.32) | |
| Intermediate risk group (MSKCC risk group) | 404 | HR 0.76 (0.58 to 0.99) | ||
| Poor risk group (MSKCC risk group) | 124 | HR 0.47 (0.30 to 0.73) | ||
| 1 previous antiangiogenic regimen | 591 | HR 0.71 (0.56 to 0.90) | ||
| 2 previous antiangiogenic regimens | 230 | HR 0.89 (0.61 to 1.29) | ||
| CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; IFN‐α: interferon‐α; IL: interleukin; KPS: Karnovsky Performance Score; MSKCC: Memorial Sloan‐Kettering Cancer Center; n: number of participants; SOC: standard of care. | ||||
| Comparison (group 1 vs group 0) | Study (reported in) | Measurement instrument | Group 1 | Group 0 | Favours | Difference (95% CI) or P values |
| 1 (IFN‐α alone vs standard targeted therapies) | Motzer 2007 (reported in Cella 2008) | FACT‐G total score, mean postbaseline score over 17 weeks | 76.8 (n = 357) | 82.3 (n = 373) | Group 0 | ‐5.58 (‐7.25 to ‐3.91) |
| Motzer 2007 (reported in Cella 2008) | FKSI‐15, mean postbaseline score over 17 weeks | 42.1 (n = 357) | 45.3 (n = 373) | Group 0 | ‐3.27 (‐4.18 to ‐2.36) | |
| Motzer 2007 (reported in Cella 2008) | FKSI‐DRS, mean postbaseline score over 17 weeks | 27.4 (n = 357) | 29.4 (n = 373) | Group 0 | ‐1.98 (‐2.51 to ‐1.46) | |
| Hudes 2007, (reported in Yang 2010) | EQ‐5D Index, mean score on treatment | 0.492 (n = 115) | 0.590 (n = 157) | Group 0 | ‐0.099 (95% CI ‐0.162 to ‐0.036) | |
| Motzer 2007 (reported in Cella 2008) | EQ‐5D Index, mean postbaseline score over 17 weeks | 0.725 (n = 357) | 0.762 (n = 373) | Group 0 | ‐0.0364 (‐0.0620 to ‐0.0109) | |
| Pooled EQ‐5D | 472 | 530 | Group 0 | ‐0.06 (‐0.12 to 0), I2 = 69% | ||
| Hudes 2007, (reported in Yang 2010) | EQ‐VAS, mean score on treatment | 58.83 (n = 115) | 63.33 (n = 157) | Group 0 | ‐4.50 (‐8.184 to ‐0.819) | |
| Motzer 2007, (reported in Cella 2008) | EQ‐VAS, mean postbaseline score over 17 weeks | 68.7 (n = 357) | 73.4 (n = 373) | Group 0 | ‐4.74 (‐6.87 to ‐2.60) | |
| Pooled EQ‐VAS | 472 | 530 | Group 0 | ‐4.68 (‐6.53 to ‐2.83), I2 = 0% | ||
| 6 (Targeted immunotherapy alone vs standard targeted therapies) | Motzer 2015a (reported in Cella 2016) | FKSI‐DRS, mean score at baseline | 30.2 ± 4.4 (n = 362) | 30.1 ± 4.8 (n = 344) | ‐ | Difference in mean change 1.6 (1.4 to 1.9), P < 0.0001 |
| FKSI‐DRS, mean change from baseline to week 28 | 0.4 ± 5 (n = 164) | ‐1.2 ± 4 (n = 122) | Group 1 | |||
| FKSI‐DRS, mean change from baseline to week 52 | 1.6 ± 4 (n = 97) | ‐1.0 ± 6 (n = 63) | Group 1 | |||
| FKSI‐DRS, mean change from baseline to week 104 | 3.5 ± 4.1 (n = 20) | 0.2 ± 6 (n = 9) | Group 1 | |||
| Clinically important improvement from baseline by ≥ 2 FKSI‐DRS points | 200 (55%)/361 | 126 (37%)/343 | Group 1 | RR 1.51 (1.28 to 1.78); P < 0.0001 | ||
| Time to clinically important improvement ≥ 2 FKSI‐DRS points | Median: 4.7 months (3.7 to 7.5) | Median not reached | Group 1 | HR 1.66 (1.33 to 2.08); P < 0.0001 | ||
| Clinically important improvement from baseline by ≥ 3 FKSI‐DRS points | 148 (41%)/361 | 95 (28%)/343 | Group 1 | RR 1.48 (1.20 to 1.83); P = 0.0002 | ||
| Time to clinically important improvement ≥ 3 FKSI‐DRS points | Median not estimable | Median not estimable | Group 1 | HR 1.61 (1.24 to 2.09); P < 0.0003 | ||
| EQ‐5D utility index, mean score at baseline | 0.78 ± 0.24 (n = 362) | 0.78 ± 0.21 (n = 344) | ‐ | No significant differences in proportion of participants with clinical important improvements (P = 0.070) or time to improvement (P = 0.86). | ||
| EQ‐5D utility index, mean change from baseline to week 28 | 0.052 ± 0.22 (n = 164) | ‐0.03 ± 0.2 (n = 122) | Group 1 | |||
| EQ‐5D utility index, mean change from baseline to week 52 | 0.06 ± 0.1 (n = 98) | ‐0.01 ± 0.2 (n = 63) | Group 1 | |||
| EQ‐5D utility index, mean change from baseline to week 104 | 0.13 ± 0.7 (n = 20) | ‐0.02 ± 0.15 (n = 9) | Group 1 | |||
| EQ‐5D VAS, mean score at baseline | 73.3 ± 18.5 (n = 362) | 72.5 ± 18.7 (n = 344) | ‐ | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 28 | 5 ± 13 (n = 164) | ‐3 ± 11 (n = 122) | Group 1 | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 52 | 7 ± 15 (n = 98) | ‐2 ± 16 (n = 63) | Group 1 | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 104 | 9 ± 9 (n = 20) | 1 ± 18 (n = 9) | Group 1 | ‐ | ||
| Clinically important improvement from baseline by ≥ 7 EQ‐5D VAS points | 192 (53%)/360 | 134(39%)/343 | ‐ | RR 1.37 (1.16 to 1.61); P = 0.0001 | ||
| Time to clinically important improvement | Median: 6.5 months (3.9 to 12.2) | Median: 23.1 months (15.4 to not estimated) | ‐ | HR 1.37 (1.10 to 1.71) | ||
| CI: confidence interval; EQ‐5D Index: EuroQol 5‐Dimension (MID 0.06 to 0.08, Pickard 2007); EQ‐VAS: EuroQol Visual Analog Scale (MID 7, Pickard 2007); FACT‐G: Functional Assessment of Cancer Therapy ‐ General (MID 4 points for better rating and 8 points for worse rating, Ringash 2007); FKSI‐15: FACT‐Kidney Symptom Index (MID 3 points, Cella 1997); FKSI‐DRS: FACT‐Kidney Symptom Index Disease Related Symptoms (MID 2 points, Cella 1997); HR: hazard ratio; MID: minimal important difference; n: number of participants. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.13, 1.51] |
| 2 Overall survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.28 [1.11, 1.49] | |
| 3 Quality of life Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 FACT‐G | 1 | 730 | Mean Difference (Random, 95% CI) | ‐5.58 [‐7.25, ‐3.91] |
| 3.2 FKSI‐15 | 1 | 730 | Mean Difference (Random, 95% CI) | ‐3.27 [‐4.18, ‐2.36] |
| 3.3 FKSI‐DRS | 1 | 730 | Mean Difference (Random, 95% CI) | ‐1.98 [‐2.51, ‐1.45] |
| 3.4 EQ‐5D | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.12, ‐0.00] |
| 3.5 EQ‐VAS | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐4.68 [‐6.53, ‐2.83] |
| 4 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 2.23 [1.79, 2.77] | |
| 6 Tumour remission Show forest plot | 2 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 5 Tumour remission Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.36] |
| 2 Overall survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.13 [1.00, 1.28] | |
| 3 Adverse events (grade ≥ 3) Show forest plot | 2 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.71, 0.84] |
| 4 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.53 [1.36, 1.73] | |
| 5 Tumour remission Show forest plot | 2 | 1205 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.31, 0.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Adverse events (grade ≥ 3) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Tumour remission Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 2 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
| 2 Overall survival Show forest plot | 2 | 1071 | Hazard Ratio (Fixed, 95% CI) | 1.14 [0.96, 1.37] |
| 3 Adverse events (grade ≥ 3) Show forest plot | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.97, 1.39] |
| 4 Progression‐free survival Show forest plot | 1 | 339 | Hazard Ratio (Random, 95% CI) | 1.05 [0.87, 1.27] |
| 5 Tumour remission Show forest plot | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1‐year mortality Show forest plot | 1 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.87] |
| 2 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.60, 0.89] | |
| 3 Quality of life Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Clinical important MID in FKSI‐DRS | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.28, 1.78] |
| 3.2 Clinical important MID in EQ‐5D‐VAS | 1 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.16, 1.61] |
| 4 Adverse events (grade ≥ 3) Show forest plot | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.40, 0.65] |
| 5 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.88 [0.75, 1.03] | |
| 6 Tumour remission Show forest plot | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 4.39 [2.84, 6.80] |


