Formación de los profesionales de la asistencia sanitaria en la prevención de las úlceras por presión
Appendices
Appendix 1. Appendix 1: Search Strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Education, Professional EXPLODE ALL AND INREGISTER
2 MESH DESCRIPTOR Education, Continuing EXPLODE ALL AND INREGISTER
3 (professional* near5 (educat* or training)) AND INREGISTER
4 ((educat* or training) near5 professional*) AND INREGISTER
5 ((nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*) near5 (educat* or training)) AND INREGISTER
6 ((educat* or training) near5 (nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*)) AND INREGISTER
7 (("Allied Health Occupation*" or "Health Occupation*") near5 (educat* or training)) AND INREGISTER
8 (educat* or training) near5 ("Allied Health Occupation*" or "Health Occupation*") AND INREGISTER
9 ((education* or training) next program*) AND INREGISTER
10 (program* next (education* or training)) AND INREGISTER
11 (seminar* or workshop* or course* or open learning) AND INREGISTER
12 ((written or printed or oral) next information) AND INREGISTER
13 information next (written or printed or oral) AND INREGISTER
14 (leaflet* or booklet* or pamphlet* or poster*) AND INREGISTER
15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
16 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER
17 (pressure next (ulcer* or sore* or injur*)) AND INREGISTER
18 ((ulcer* or sore* or injur*) next pressure) AND INREGISTER
19 (decubitus next (ulcer* or sore*)) AND INREGISTER
20 ((ulcer* or sore*) next decubitus) AND INREGISTER
21 ((bed next sore*) or bedsore*) AND INREGISTER
22 #17 OR #16 OR #18 OR #19 OR #20 OR #21
23 #15 AND #22
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Education, Professional] explode all trees
#2 MeSH descriptor: [Education, Continuing] explode all trees
#3 (professional* near/5 (educat* or training)):ti,ab,kw
#4 ((nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*) near/5 (educat* or training)):ti,ab,kw
#5 (("Allied Health Occupation*" or "Health Occupation*") near/5 (educat* or training)):ti,ab,kw
#6 ((education* or training) next program*):ti,ab,kw
#7 (seminar* or workshop* or course* or open learning):ti,ab,kw
#8 ((written or printed or oral) next information):ti,ab,kw
#9 (leaflet* or booklet* or pamphlet* or poster*):ti,ab,kw
#10 {or #1‐#9}
#11 MeSH descriptor: [Pressure Ulcer] explode all trees
#12 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw
#13 (decubitus next (ulcer* or sore*)):ti,ab,kw
#14 ((bed next sore*) or bedsore*):ti,ab,kw
#15 {or #11‐#14}
#16 {and #10, #15} in Trials
Ovid MEDLINE
1. exp Education, Professional/
2. exp Education, Continuing/
3. (professional* adj5 (educat* or training)).tw.
4. ((nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*) adj5 (educat* or training)).tw.
5. (("Allied Health Occupation*" or "Health Occupation*") adj5 (educat* or training)).tw.
6. ((education* or training) adj program*).tw.
7. (seminar* or workshop* or course* or open learning).tw.
8. ((written or printed or oral) adj information).tw.
9. (leaflet* or booklet* or pamphlet* or poster*).tw.
10. or/1‐9
11. exp Pressure Ulcer/
12. (pressure adj (ulcer* or sore* or injur*)).tw.
13. (decubitus adj (ulcer* or sore*)).tw.
14. (bedsore* or bed sore*).tw.
15. or/11‐14
16. and/10,15
17. randomised controlled trial.pt.
18. controlled clinical trial.pt.
19. randomi?ed.ab.
20. placebo.ab.
21. clinical trials as topic.sh.
22. randomly.ab.
23. trial.ti.
24. or/17‐23
25. exp animals/ not humans.sh.
26. 24 not 25
27. 16 and 26
Ovid Embase
1 exp Allied health education/
2 exp Vocational education/
3 exp Clinical education/
4 exp Education, Continuing/
5 (professional* adj5 (educat* or training)).tw.
6 ((nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*) adj5 (educat* or training)).tw.
7 (("Allied Health Occupation*" or "Health Occupation*") adj5 (educat* or training)).tw.
8 ((education* or training) adj program*).tw.
9 (seminar* or workshop* or course* or open learning).tw.
10 ((written or printed or oral) adj information).tw.
11 (leaflet* or booklet* or pamphlet* or poster*).tw.
12 or/1‐11
13 exp Pressure Ulcer/
14 (pressure adj (ulcer* or sore* or injur*)).tw.
15 (decubitus adj (ulcer* or sore*)).tw.
16 (bedsore* or bed sore*).tw.
17 or/13‐16
18 and/12,17
19 Randomized controlled trials/
20 Single‐Blind Method/
21 Double‐Blind Method/
22 Crossover Procedure/
23 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
24 (doubl* adj blind*).ti,ab.
25 (singl* adj blind*).ti,ab.
26 or/19‐25
27 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
28 human/ or human cell/
29 and/27‐28
30 27 not 29
31 26 not 30
32 18 and 31
EBSCO CINAHL Plus
S31 S17 AND S30
S30 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
S29 TI allocat* random* or AB allocat* random*
S28 MH "Quantitative Studies"
S27 TI placebo* or AB placebo*
S26 MH "Placebos"
S25 TI random* allocat* or AB random* allocat*
S24 MH "Random Assignment"
S23 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S22 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S21 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S20 TI clinic* N1 trial* or AB clinic* N1 trial*
S19 PT Clinical trial
S18 MH "Clinical Trials+"
S17 S11 AND S16
S16 S12 OR S13 OR S14 OR S15
S15 TI decubitus or AB decubitus
S14 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* )
S13 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* )
S12 (MH "Pressure Ulcer+")
S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10
S10 TI ( leaflet* or booklet* or pamphlet* or poster* ) OR AB ( leaflet* or booklet* or pamphlet* or poster* )
S9 TI ( "written information" or "printed information" or "oral information" ) OR AB ( "written information" or "printed information" or "oral information" )
S8 TI ( seminar* or workshop* or course* or "open learning" ) OR AB ( seminar* or workshop* or course* or "open learning" )
S7 TI ( education* program* or training program* ) OR AB ( education* program* or training program* )
S6 TI ("Allied Health Occupation*" or "Health Occupation*" n5 (educat* or training)) or AB ("Allied Health Occupation*" or "Health Occupation*" n5 (educat* or training))
S5 TI ((nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*) n5 (educat* or training)) or AB ( (nurs* or doctor* or physiotherap* or therapist* or surgeon* or domiciliar* or practitioner*) n5 (educat* or training))
S4 TI ( professional* N5 (educat* or training) ) OR AB ( professional* N5 (educat* or training) )
S3 (MH "Education, Allied Health+")
S2 (MH "Education, Health Sciences+")
S1 (MH "Education, Continuing+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
"education" AND "pressure ulcer"
"education" AND "pressure ulcer prevention"
"healthcare professional" AND "pressure ulcer"
"healthcare professional" AND "pressure ulcer prevention"
World Health Organization International Clinical Trials Registry Platform
"pressure ulcers" AND "education" AND "healthcare professional"
"pressure ulcer prevention" AND "education" and "healthcare professional"
Appendix 2. Appendix 2: Assessment of risk of bias (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
-
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
-
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
-
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
-
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following.
-
Insufficient information to permit judgement of low or high risk of bias.
-
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
-
No missing outcome data.
-
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
-
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
-
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
-
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
-
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
-
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
-
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
-
The study did not address this outcome
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
-
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
-
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
-
Not all of the study’s prespecified primary outcomes have been reported.
-
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
-
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
-
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
-
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
-
had a potential source of bias related to the specific study design used; or
-
has been claimed to have been fraudulent; or
-
had some other problem.
Unclear
There may be a risk of bias, but there is either:
-
insufficient information to assess whether an important risk of bias exists; or
-
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Appendix 3 Risk of bias assessment (cluster randomised controlled trials)
In cluster‐randomised trials, particular biases to consider include: (i) recruitment bias; (ii) baseline imbalance; (iii) loss of clusters; (iv) incorrect analysis; and (v) comparability with individually randomised trials.
(i) Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited.
(ii) Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
(iii) Occasionally, complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
(iv) Many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a ‘unit of analysis error’ and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
(v) In a meta‐analysis including both cluster and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by a Cochrane Review of hip protectors. The cluster trials showed large positive effect, whereas individually randomised trials did not show any clear benefit. One possibility is that there was a ‘herd effect’ in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such ‘contamination’ would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and ‘herd effects’ may be different for different types of cluster.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
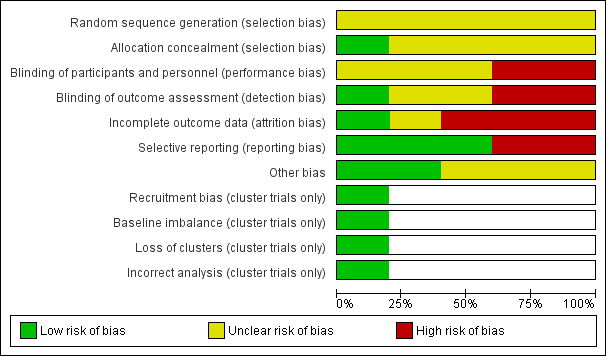
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
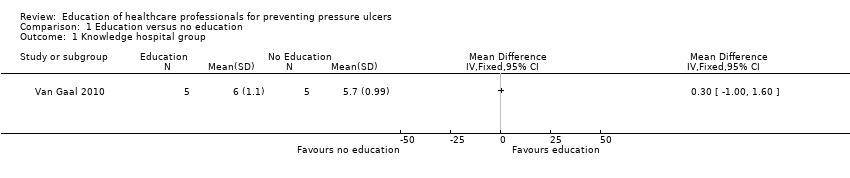
Comparison 1 Education versus no education, Outcome 1 Knowledge hospital group.

Comparison 1 Education versus no education, Outcome 2 Knowledge nursing‐home group.

Comparison 2 Training, monitoring and observation vs monitoring and observation, Outcome 1 Pressure ulcer developed.

Comparison 3 Training monitoring and observation vs observation alone, Outcome 1 Pressure ulcer developed.

Comparison 4 Monitoring and observation vs observation alone, Outcome 1 Pressure ulcer developed.

Comparison 5 Education via didactic lecture versus video, Outcome 1 Knowledge.
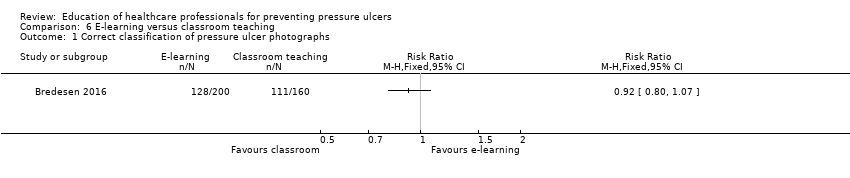
Comparison 6 E‐learning versus classroom teaching, Outcome 1 Correct classification of pressure ulcer photographs.
| Education compared to no education for preventing pressure ulcers | ||||||
| Patient or population: staff caring for patients at risk of pressure ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No education | Education | |||||
| Knowledge in hospital group | Mean knowledge score with no education was 5.7 | Mean knowledge score was 0.30 units higher (1.0 lower to 1.6 higher) | 10 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in health professionals' knowledge depending on whether they receive education or no education on pressure ulcer prevention | |
| Knowledge in nursing‐home group | Mean knowledge score with no education was 5.1 | Mean knowledge score was 0.30 units higher (0.77 lower to 1.37 higher) | 10 | ⊕⊝⊝⊝ | ||
| Change in health professionals' clinical behaviour | Not reported | |||||
| Incidence of new pressure ulcers | Not reported | |||||
| Severity of pressure ulcers | Not reported | |||||
| Patient‐reported outcomes | Not reported | |||||
| Carer‐reported outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1Downgraded four times: serious limitations due to performance bias, detection bias and attrition bias; indirectness due to use of a non validated instrument to assess knowledge; serious imprecision due to a wide confidence interval and small sample size. | ||||||
| Training, monitoring and observation compared to monitoring and observation for preventing pressure ulcers | ||||||
| Patient or population: staff caring for patients at risk of pressure ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Monitoring and observation | Training, monitoring and observation | |||||
| Change in health professionals' knowledge | Not reported | |||||
| Change in health professionals' clinical behaviour | Not reported | |||||
| Incidence of new pressure ulcers | Study population | RR 0.63 | 345 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in pressure ulcer incidence when using different components of educational intervention such as training, monitoring and observation compared with monitoring and observation | |
| 183 per 1000 | 115 per 1000 | |||||
| Severity of new pressure ulcers | No data were presented by the study author | |||||
| Patient‐reported outcomes | Insufficient data within the study report to further interrogate this outcome | |||||
| Carer‐reported outcomes | Insufficient data within the study report to further interrogate this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1Downgraded three times: very serious limitations due to performance, detection and reporting bias; serious imprecision due to wide confidence interval. | ||||||
| Training, monitoring and observation compared to observation alone for preventing pressure ulcers | ||||||
| Patient or population: staff caring for patients at risk of pressure ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation alone | Training monitoring and observation | |||||
| Change in health professionals' knowledge | Not reported | |||||
| Change in health professionals' clinical behaviour | Not reported | |||||
| Incidence of new pressure ulcers | Study population | RR 1.21 | 325 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in pressure ulcer incidence when using different components of educational intervention such as training, monitoring and observation compared with observation alone | |
| 94 per 1000 | 114 per 1000 | |||||
| Severity of new pressure ulcers | Not reported | |||||
| Patient‐reported outcomes | Insufficient data within the study report to further interrogate this outcome | |||||
| Carer‐reported outcomes | Insufficient data within the study report to further interrogate this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1Downgraded three times: very serious limitations due to performance, detection and reporting bias; serious imprecision due to wide confidence interval. | ||||||
| Monitoring and observation compared to observation alone for preventing pressure ulcers | ||||||
| Patient or population: staff caring for patients at risk of pressure ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation alone | Monitoring and observation | |||||
| Change in health professionals' knowledge | Not reported | |||||
| Change in health professionals' clinical behaviour | Not reported | |||||
| Incidence of new pressure ulcers | Study population | RR 1.93 | 232 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in pressure ulcer incidence when using different components of educational intervention such as monitoring and observation compared with observation alone | |
| 94 per 1000 | 182 per 1000 | |||||
| Severity of new pressure ulcers | No data are presented by the study author | |||||
| Patient reported outcomes | Insufficient data within the study report to further interrogate this outcome | |||||
| Carer reported outcomes | Insufficient data within the study report to further interrogate this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1Downgraded three times: very serious limitation due to performance, detection and reporting bias; serious imprecision due to wide confidence interval. | ||||||
| Education versus video for preventing pressure ulcers | ||||||
| Patient or population: staff caring for patients at risk of pressure ulcers Comparison: didactic lecture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Didactic education | Video education | |||||
| Change in health professionals' knowledge | Mean knowledge score with didactic education was 84.62 | Mean knowledge score was 4.60 units higher (3.8 units to 6.12 units higher) | 102 | ⊕⊝⊝⊝ | It is uncertain whether education delivered in different formats such as didactic or video‐based format makes a difference to health professionals' knowledge of pressure ulcer prevention | |
| Change in health professionals' clinical behaviour | Not reported | |||||
| Incidence of new pressure ulcers | Not reported | |||||
| Severity of pressure ulcers | Not reported | |||||
| Patient‐reported outcomes | Not reported | |||||
| Carer‐reported outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1Downgraded three times: serious limitation due to unclear risk of selection, performance and detection bias; very serious imprecision due to small sample size and wide confidence intervals. | ||||||
| E‐learning compared with classroom education for preventing pressure ulcers | ||||||
| Patient or population: staff caring for patients at risk of pressure ulcers Settings: hospitals and nursing homes Intervention: e‐learning Comparison: classroom education | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Classroom education | E‐learning | |||||
| Knowledge of pressure ulcer classification | Study population | RR 0.92 (0.80 to 1.07) | 18 participants | very low1 | It is uncertain whether education delivered in different formats such as e‐learning or classroom‐based format makes a difference to health professionals' knowledge of pressure ulcer prevention | |
| 694 per 1000 | 638 per 1000 | |||||
| Change in health professionals' clinical behaviour | Not reported | |||||
| Incidence of new pressure ulcers | Not reported | |||||
| Severity of pressure ulcers | Not reported | |||||
| Patient‐reported outcomes | Not reported | |||||
| Carer‐reported outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1Downgraded three times: serious limitations due to high risk of attrition and selective reporting bias; unclear risk of performance, selection, detection and other bias; serious imprecision due to small sample size and wide confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knowledge hospital group Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Knowledge nursing‐home group Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer developed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer developed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer developed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knowledge Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Correct classification of pressure ulcer photographs Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


