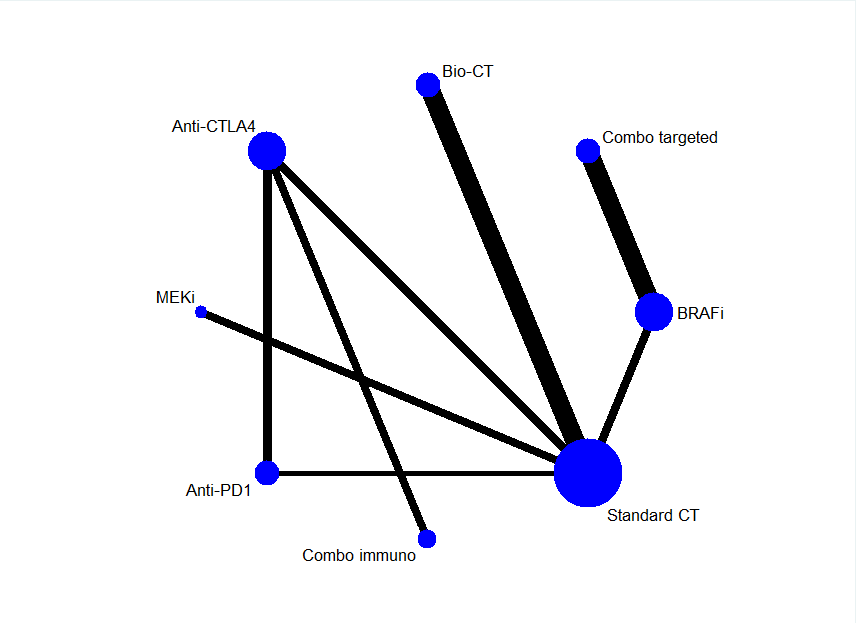
Systemic treatments for metastatic cutaneous melanoma
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Single centre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 56. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and the outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated metastatic melanoma. Participants randomised: 305. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: reported in a separate analysis (Beusterien 2003). The addition of subcutaneous histamine dihydrochloride to IL‐2. treatment improved median quality‐adjusted survival duration and did not adversely affect QoL. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports include all expected outcomes. However, no protocol is available and thus it is unclear if all planned outcomes are reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 395. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Based on permuted blocks within strata, with dynamic balancing within main institutions and their affiliate networks". Comment: This method ensured low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated metastatic melanoma. Participants randomised: 124. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Two patients were randomized, but did not receive therapy and were evaluated as progressive disease". Comment: There was insufficient information about completeness of outcome data to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 229. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: no significant difference was observed between treatment arms. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "An independent centralized radiologic committee (two radiologists not involved in the study) performed a blinded review of all radiologic files of patients who had CR, PR, or stable disease on the investigator’s evaluation. Imaging of patients declared progressive disease (PD) as a best response were not reviewed." Comment: It was unclear if this method was sufficient to ensure low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced in across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 132. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 37. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: not reported. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 266. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 36. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomization". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Unclear risk | There was insufficient information to permit judgment. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 151. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Resected advanced regional and distant metastasis from cutaneous melanoma. Number of participants: 136. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: 10 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 771. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available (24 months minimum follow‐up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were centrally randomly assigned in a 1:1 ratio in blocks of four". Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An independent panel blinded to treatment assignment reviewed all radiologic responses." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 393. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients meeting the enrollment criteria were randomly assigned in blocks within each country." Comment: This method ensured low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 50. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Unclear risk | There was insufficient information to permit judgment. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 450. | |
| Interventions | Four‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | High risk | One arm (D) was closed early and participants' data were not analysed. |
| Other bias | Unclear risk | There was insufficient information to permit judgment. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated metastatic melanoma. Number of participants:270. | |
| Interventions | Four‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Unclear risk | There was insufficient information to permit judgment. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated metastatic melanoma. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Particpants with brain metastasis: excluded. Median follow‐up: not available. Note: Both progression‐free survival and overall survival appeared longer in the subset of participants who developed an adverse event of hypertension while receiving ramucirumab. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One hundred and six patients were enrolled and randomised". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | High risk | There was a potential conflict of interest for some authors and the funding body which likely resulted in bias to the study methodology. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Number of randomised participants: 240. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: cross‐over to polychemotherapy was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 51. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Particpants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated metastatic melanoma. Participants randomised: 60. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 31 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol (provided by the trial principal investigator) and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated metastatic melanoma. Participants randomised: 149. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Incidence of CNS metastasis. Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 46 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed centrally... using a minimisation method". Comment: randomisation method was adequate |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol (provided by the trial principal investigator) and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Metastatic melanoma not previously treated with either Dacarbazine or C. parvum. Randomised participants: 49. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization data". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Metastatic melanoma not previously treated with either tamoxifen or dacarbazine. Randomised participants: 117. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation was made on the basis of randomly permuted blocks of two within strata". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 125. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation was made on the basis of randomly permuted blocks of two within strata". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Previously treated (no treatment in the previous 4 weeks) and untreated metastatic melanoma. Participants randomised: 140. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 415. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Particpants randomised: 286. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: was not allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Unclear risk | There was insufficient information to permit judgment. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated metastatic melanoma harbouring no mutations in KRAS, NRAS, BRAF, or c‐kit genes. Participants randomised: 114. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple stratified randomization with permuted blocks of size 2 was used to create a prospective randomization schedule". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment of patients was performed by designated personnel at each participating site in a double‐blind fashion such that the investigator and patient did not know the treatment assignment" Comment: Allocation was likely concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Number of participants: 181. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: no significant difference was noted between arms. Participants with brain metastasis: included. Median follow‐up: 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned, using permuted blocks". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 260. | |
| Interventions | Four arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized through a computerized procedure of permuted blocks centralized at the coordinating center" Comment: Randomisation method wad adequate. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated metastatic melanoma. Number of participants: 117. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase I‐II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 150. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned patients". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Particpants with metastasised melanoma after complete metastasectomy. Randomised participants: 139. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. | |
| Notes | Cross‐over: not reported. Quality of life: evaluation of the quality of life was insufficient because of the low feedback rate of the questionnaires. Participants with brain metastasis: not reported. Median follow‐up: 46 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...permuted block (size 12) randomization list" Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II/III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Previously treated metastatic melanoma. Participants randomised: 306. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not investigated. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized using an interactive voice response system". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: This method makes low the risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: This method makes low the risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Number of participants: 183. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 52 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 64. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 73. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 258. | |
| Interventions | Four‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Number of randomised participants: 81. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "All measurements for response were confirmed by one of the coauthors (C.A.), who also was responsible for collection of data from individual centers." Comment: It was unclear if this method was sufficient to ensure low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published report include all expected outcomes. However, no protocol is available and thus it is unclear if all planned outcomes are reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase I‐II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma harboring activating mutations of BRAF. Participants randomised: 162. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: Dabrafenib 150 mg twice daily + trametinib 2 mg was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: enrolled if at least a 3‐month history of stable disease. Median follow‐up: 14 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published report include all expected outcomes. However, no protocol is available and thus it is unclear if all planned outcomes are reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Previoulsy treated and untreated metastatic melanoma with a V600E or V600K BRAF mutation. Participants randomised: 322. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: Cross‐over to trametinib was allowed at disease progression. Quality of life: QoL analysis was reported in a separated study (Schadendorf 2014). Trametinib was associated with less functional impairment, smaller declines in health status, and less exacerbation of symptoms than dacarbazine. Participants with brain metastasis: included when brain disease was stable. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A blinded, independent central review of tumor assessments was performed." Comment: This method makes low the risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Metastatic melanoma not previously treated with either chemotherapy or MAP kinase pathway‐targeted drugs. Participants randomised: 823. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: This method makes low the risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: This method makes low the risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II/III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Untreated or previously treated (dacarbazine, temozolomide, IL‐2, and/or IFN‐α) metastatic melanoma. Randomised participants: 294. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: This method makes low the risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: This method makes low the risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 94. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 30. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Tumour response | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgement. |
| Other bias | Unclear risk | There was insufficient information to permit judgement. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Previuos treatment not reported. Randomised participants: 36. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated metastatic wild‐type BRAF melanoma. Randomised participants: 83 | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using a variable block size". Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...masking". Comment: There was insufficient information about allocation concealment to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blind". Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double blind". Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Previosuly treated metastatic melanoma. Randomised participants: 336. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: 5 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned 1:1 to treatment with tasisulam or paclitaxel''. Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | High risk | There is a potential conflict of interest for some authors and the funding body which likely caused bias in the study methodology . |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 290. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study. | |
| Participants | Previously treated metastatic melanoma progressing under either temozolomide or dacarbazine. Participants randomised: 270. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple, stratified randomization with permuted blocks of size 4 was used to create a prospective randomization schedule that was implemented in a telephone based interactive voice recognition system". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment of eligible patients was performed by designated personnel at each participating site using the IVRS in a double‐blind fashion such that the investigator, sponsor, and patient did not know the treatment assignment". Comment: Likely that allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blind". Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double blind". Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma with BRAF V600E mutation. Participants randomised: 250. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: Cross‐over to dabrafenib 150 mg twice daily was allowed at disease progression. Quality of life: Dabrafenib had functional and symptomatic benefit compared to dacarbazine (Grob 2014). Participants with brain metastasis: excluded unless they were without evidence of active central nervous system metastases for more than 3 months after surgery or stereotactic radiosurgery. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centrally located, computerised, interactive, voice activated response system controlled assignment of patient treatment". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Although investigators were aware of treatment group when assessing progression‐free survival, a masked independent review committee (IRC) reviewed all scans and, per protocol, had to confirm progression before patients crossed over from dacarbazine to dabrafenib". Comment: Likely that allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 529. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized... via a centralized system". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...independent radiologic review". Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study | |
| Participants | HLA‐A*0201–positive metastatic melanoma which had progressed during systemic treatment . Participants randomised: 676. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: Ipilimumab did not have a detrimental effect on QoL during the treatment induction phase (Revicki 2012). Participants with brain metastasis: participants with active, untreated metastases in the central nervous were excluded. Median follow‐up: 21 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Double‐blind”. Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Double‐blind”. Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated (1 chemotherapy was allowed) metastatic melanoma. Patients randomised: 245. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 13 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Stratified randomization based on permuted blocks within strata with dynamic institution balancing was used." Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment assignments were obtained from the central randomization desk at the ECOG coordinating center." Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Tumor responses were determined by the investigators using RECIST (Response Evaluation Criteria in Solid Tumors) criteria and were audited as a part of ECOG‐ACRIN (American College of Radiology Imaging Network) standard procedures." Comment: It was unclear if this method was sufficient to ensure low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Previously treated metastatic melanoma. Participants randomised: 117. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival Tumour response Toxicity | |
| Notes | Cross‐over: was not allowed. Quality of life: No significant difference in the quality of life could be found. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "After the first five patients, it was decided... that the centres have the option to enrol patients on a treatment preference basis (patients’ choice) ". Comment: This domain was assessed at high risk of selection bias because initially enrolled participants were randomly assigned to either chemotherapy or best supportive care, but enrolment was slow and allocation appeared to be based on physician's choice. |
| Allocation concealment (selection bias) | High risk | Quote: "...patients' choice". Comment: Unlikely that allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "There was no centralized review of the radiology files provided." Comment: Overall, there was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Single centre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 219. | |
| Interventions | Four‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Metastatic melanoma untreated or previously treated with no more than one previous systemic chemotherapy. Randomised participants: 65. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 294. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned without stratification". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "There was no centralized review of the radiologic files provided." Comment: It was unclear if this method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 80. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Double‐blind”. Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Double‐blind”. Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated and previously treated metastatic melanoma. Randomised participants: 138. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: > 2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients randomized". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 363. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 3.4 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blinded study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 214. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 13 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random assignment was performed using an interactive voice response system". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | No sufficient information to judge |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 74. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 132. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Tumour response | |
| Notes | Cross‐over: cross‐over was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: not reported. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | No other sources of bias found. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 34. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma with BRAF V600 mutations. Participants randomised: 495. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: participants with previously treated brain metastases were eligible if they had at least a 3‐week history of stable disease. Median follow‐up: 7 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "We performed a blinded, independent central review of tumor assessments." Comment: It is unclear if this method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 945. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: participants with inactive brain metastasis were excluded. Median follow‐up: > 9 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Double‐blind”. Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Double‐blind”. Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Quote: "Data on overall survival are insufficiently mature to present". Comment: Low risk of selective reporting. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Participants underwent surgery for locally advanced or metastatic melanoma. Randomised participants: 815. | |
| Interventions | HLA‐A2–positive (serologically defined)
HLA‐A2–negative group
| |
| Outcomes | Progression‐free survival. Overall survival. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: participants who underwent surgery for brain metastasis were included. Median follow‐up: 82 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was conducted centrally by using permuted blocks within strata". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Placebo‐controlled”. Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Placebo‐controlled”. Comment: The method ensured low risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants:102. | |
| Interventions | Two‐arm study:
Treatment schedules:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: participants with symptomatic brain metastasis were excluded. Median follow‐up: 45 months. Note: Both biochemotherapy schedules were compared with a non‐randomised group of participants who received chemotherapy alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma with BRAF Val600Glu or Val600Lys mutations. Participants randomised: 423. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: Dabrafenib and trametinib resulted in better preservation of health‐related quality of life and pain improvement compared to dabrafenib monotherapy (Schadendorf 2015). Participants with brain metastasis: participants with previously treated brain metastases were eligible if they had at least a 12‐week history of stable disease. Median follow‐up: 9 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centrally located, computerised, interactive, voice activated response system controlled the random assignment". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators, site staff, and patients were unaware of assignment throughout the study, and masking was maintained by using tablets and bottles of active drug and placebo that were identical in appearance. At the time of the primary analysis, only the sponsor and those assessing the data were made aware of treatment group assignments." Comment: Allocation likely concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Double blind”. Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Double blind”. Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 42. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Tumour response. Toxicity. | |
| Notes | Cross‐over: not available. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...before randomisation". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | No other sources of bias found. |
| Study characteristics | ||
| Methods | Phase III parallel RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 57. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: cross‐over to polychemotherapy was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random table of numbers". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 488. | |
| Interventions | Five‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". "The randomization list was produced by the Internal Quality Control Unit of Biostatistics and Data Management". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was blinded and centralized". Comment: This method ensured low risk of selection bias. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Independent, blinded evaluation of tumor images was performed by Fondazione Biomedica Europea." Comment: It was unclear if this method ensured low the risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated or previously treated (only treatments other than a nitrosurea was allowed) metastatic melanoma. Randomised participants: 62. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma with BRAF V600E e V600K mutations. Participants randomised: 675. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: Cross‐over to vemurafenib was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded when metastases to the central nervous system had progressed or required treatment in the previous 3 months. Median follow‐up: 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned using an interactive voice recognition system supported by an independent vendor". Comment: Randomisation method adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients and investigators were aware of treatment allocation" Comment: An independent review committee (IRC) had to confirm progression before participants crossed over from dacarbazine to dabrafenib. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Secondary endpoints not reported will be subject of future publications. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 101. | |
| Interventions | Two.arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple stratified randomization with permuted blocks of size 4 was used by the sponsor to create a prospective randomization schedule that was provided to the vendor for the telephone‐based interactive voice recognition system". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment of eligible patients was performed by designated personnel at each participating site using the interactive voice recognition system in a double‐blind fashion" Comment: Likely that allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 305. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: Temozolomide therapy significantly improved health‐related QoL (Kiebert 2003). Participants with brain metastasis: excluded. Median follow‐up: not available. Cost analysis: Temozolomide was associate with incremented cost‐effectiveness (Hillner 2000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was no sufficient information to judge. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 241. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Site‐specific randomization codes were produced electronically for each stratified group". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "...site personnel called a central randomization desk". Comment: This method ensured low risk of selection bias. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was no sufficient information to judge. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated and previously treated metastatic melanoma. Randomised participants: 346. | |
| Interventions | Three‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Mediano follow‐up: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized sequentially 1:1:1 using a computer‐based model". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated metastatic melanoma. Randomised participants: 53. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Participants with liver metastasis were also excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment assignments were provided to the investigators by a Research Nurse using sealed envelopes." Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 120. | |
| Interventions | Three‐arm study:
| |
| Outcomes | Tumour response. | |
| Notes | Cross‐over: allowed. Quality of life: not reported. Participants with brain metastasis: included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated". Comments: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 56. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Tumour response. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated and previously treated (1 chemotherapy was allowed) metastatic melanoma. Participants randomised: 81. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: cross‐over to open‐label elesclomol plus paclitaxel was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 3 months (for censored participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...using an interactive voice‐response system". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators and patients were blinded with respect to treatment assignment; unblinded site pharmacists were responsible for reconstituting study drugs at the pharmacy at each site". This method ensured low risk of selection bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blind". This method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double blind". This method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 129. | |
| Interventions | Four‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: quote "Patients in the blinded dacarbazine‐containing arms who could not tolerate dacarbazine were allowed to cross‐over to open‐label 10 mg/kg intetumumab monotherapy, and those on dacarbazine monotherapy who experienced progressive disease (PD) were allowed to cross over to open‐label dacarbazine plus10 mg/kg intetumumab". Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 24 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was stratified". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...blinded". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...blinded". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blind study. Multicentre trial. | |
| Participants | Untreated and previously treated (1 chemotherapy was allowed) metastatic melanoma. Participants randomised: 651. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not reported. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned patients". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blind". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 859. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 19 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation, performed centrally at the EORTC Headquarters, was stratified by performance status (0 versus 1) and institution, using a minimisation technique". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase I dose‐escalation parallel‐group RCT. Double‐blinded study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 142. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: cross‐over was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: > 11 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind trial". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind trial". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 120. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 195. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 93. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was performed centrally". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated metastatic melanoma. Number of randomised participants: 28. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 104. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: quote "Patients experiencing disease progression in the TMZ alone arm were permitted to continue study treatment by changing to the LM/TMZ combination". Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were to be randomly assigned". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | High risk | Quote: "In the course of the trial, it became apparent that MGMT persisted in tumor biopsy samples taken 24 to 72 hours after the end of cycle 1 LM/TMZ. Therefore, the trial was extended by 20 patients, with the LM dose in those assigned combination treatment being increased to 60 mg/d, then to 80 mg/d". |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 76. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: cross‐over to combination therapy was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: included (quote: "controlled brain metastasis"). Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 655. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: cross‐over to tremelimumab was not allowed for participants who progressed during standard chemotherapy. Cross‐over to ipilimumab was allowed at disease progression. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Tumor data assessed by investigators were reviewed by the sponsor to ensure compliance with RECIST criteria." Comment: It was unclear if this method ensured low risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Metastatic melanoma progressing after treatment with ipilimumab or BRAF and/or MEK inhibitors. Participants randomised: 540. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Quality of life: pembrolizumab had smaller decrements in the individual function and symptoms scales. Cross‐over: cross‐over to pembrolizumab after progression under investigation‐choice systemic chemotherapy was allowed. Participants who crossed‐over were randomly assigned to receive either 2 mg/kg or 10 mg/kg pembrolizumab. Participants with brain metastasis: excluded. Median follow‐up: 10 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation with a block size of six in each stratum was used". Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual treatment assignment between pembrolizumab and chemotherapy was open label; investigators and patients were masked to assignment to pembrolizumab dose". Comment: Allocation likely concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Investigators and patients were masked to assignment to pembrolizumab dose". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "investigators were masked to assignment to pembrolizumab dose". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated metastatic melanoma. Randomised participants: 47. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | High risk | Quote: "The study was stopped after the inclusion of approximately 50%". Comment: High risk of bias due to the trial stopping after approximately 50% of the planned participants were enrolled. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 165. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: allowed at disease progression. Quality of life: investigated in a separate analysis (Chiarion‐Sileni 2003). Biochemotherapy worsened significantly quality of life compared to chemotherapy. Participants with brain metastasis: excluded. Median follow‐up: 17 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...system of random permuted blocks within the strata (oncologic center variable) was used with a block size of four." Comment: Adequate randomisation method used. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available and thus it is unclear if all planned outcomes are reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 119. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients... were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blinded study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 502. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: A paper reported on quality‐adjusted time without symptoms of disease or toxicity of treatment (Q‐TWiST) (Sherrill 2013). Particpants treated with ipilimumab had little benefit in quality‐adjusted survival during the first year. The benefits of ipilimumab has increased with extended survival after 2, 3, and 4 years. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Double‐blind”. Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Double‐blind”. Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Untreated metastatic melanoma with BRAF mutations. Randomised participants: 91. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: Participants with either brain or spinal cord metastasis were eligible when asymptomatic, treated, and stable off treatment for > 3 months. Median follow‐up: 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by central interactive voice response system (1:1 ratio, block size four)." Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients, investigators, and the study team were masked to the treatment assigned." Comment: Allocation was likely concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma with BRAF V600E e V600K mutations. Participants randomised: 704. | |
| Interventions | Two arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: This was reported in a separated analysis (Grob 2015). Combination of dabrafenib and trametinib adds a clear benefit over monotherapy with vemurafenib. Participants with brain metastasis: participants with previously treated brain metastases were eligible if they had at least a 12‐week history of stable disease. Median follow‐up: 10 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blinded study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma without BRAF mutations. Participants randomised: 418. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not investigated. Participants with brain metastasis: participants with active brain metastasis were excluded. Median follow‐up: 9 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Double‐blind”. Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double‐blind”. Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Metastatic melanoma that had no more than one previous systemic therapy for advanced disease (CTLA‐4, PD‐1, or PD‐L1 inhibitors were not allowed). Participants randomised: 834. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported Participants with brain metastasis: participants with brain metastasis were excluded when they had active metastasis. Median follow‐up: > 9 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 88. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 102. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 42 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization between the two study arms was performed by the central data management office". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Double‐blinded study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 204. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: participants were allowed to cross‐over to tamoxifen‐based regimen at disease progression. Participants with brain metastasis: participants with brain metastasis were eligible if they had completed planned surgery/radiotherapy, did not require glucocorticosteroids at study entry, and had stable disease in the brain at a repeat computed tomography (CT) scan 2 weeks before randomisation. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Before randomization, patients were stratified". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 108. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: 22 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The study was externally monitored". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 185. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: 41 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Stratified randomization was performed with the use of random block sizes to ensure balance with respect to a potentially important prognostic feature." Comment: Randomisation method was adequate. |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...blinded central radiologic review". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 92. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients... were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment. |
| Other bias | Unclear risk | There was insufficient information to permit judgment. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated (only one chemotherapy line was allowed) metastatic melanoma. Participants randomised: 85. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All responses were independently reviewed by the study's principal investigators and by a single radiologist". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. Multicentre trial. | |
| Participants | Untreated metastatic melanoma. Participants randomised: 322. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: 9 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Comment: Risk was likely low because this was a multicentre trial with centralised randomisation |
| Allocation concealment (selection bias) | Low risk | Risk was likely low because this was a multicentre trial with centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 79. | |
| Interventions | Two‐arm trial:
All participants who had disease progression were treated with dacarbazine 250 mg/m² IV daily on days 1 to 5 and actinomycin D 1.5 mg/m² IV on day 1 every 3 weeks. | |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: > 36 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: However, there was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 170. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: analysis of quality of life was reported in a different article (Coates 1993). There was no statistically significant difference in quality of life between treatment arms. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised centrally using a dynamic randomisation technique." Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 377. | |
| Interventions | Three‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The composition of the series... was prepared by the coordinating center". Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "The envelopes... were opened at the moment of choice of treatment." Comment: This method ensured low risk of selection bias. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for exclusions not reported. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Randomised participants: 103. Untreated and previously treated metastatic melanoma. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 60. | |
| Interventions | Three‐arm trials:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: 13 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...closed envelope random number technique". Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated and previously treated (only drugs other than dacarbazine were allowed) metastatic melanoma. Randomised participants: 106. | |
| Interventions | Four‐arm trial:
| |
| Outcomes | Overall survival. Progression‐free survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: > 17 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization was performed at the Finnish Cancer Registry and stratified for treatment arm by institution." Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 184. | |
| Interventions | Four‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised." Comment: There was insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was no information sufficient to judge. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Previously treated metastatic melanoma. Both BRAF mutant and non‐mutant tumours were included. Randomised participants: 405. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded when brain metastases were active. Median follow‐up: 8 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used permuted blocks (block size of six) within each stratum for randomisation." Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "using an interactive voice response system." Comment: This method ensured low risk of selection bias. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "those doing tumour assessments were masked to treatment assignment". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 95. | |
| Interventions | Three‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Double‐blind study. | |
| Participants | Previously treated metastatic melanoma. Participants randomised: 217. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Overall survival. Tumour response. Toxicity. | |
| Notes | Cross‐over: not allowed. Quality of life: not reported. Participants with brain metastasis: included. Median follow‐up: 9 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done with a permuted block procedure" Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients, treating doctors, and doctors’ staff were unaware of the dose to which patients were assigned, whereas pharmacists were unmasked". Comment: This statement makes low the risk of selection bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blinded". Comment: The method ensured low risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double blinded". Comment: The method ensured low risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | No differences between protocol and published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase III parallel‐group RCT. Open label study. | |
| Participants | Untreated metastatic melanoma. Randomised participants: 61. | |
| Interventions | Two‐arm study:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: There were no differences in quality of life between treatment groups. Cross‐over: not allowed. Participants with brain metastasis: excluded. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random permuted blocks method" Comment: This method ensured low risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Phase II parallel‐group RCT. Open label study. | |
| Participants | Previoulsy treated metastatic melanoma. Randomised participants: 40. | |
| Interventions | Two‐arm trial:
| |
| Outcomes | Progression‐free survival. Overall survival. Tumour response. Toxicity. | |
| Notes | Quality of life: not reported. Cross‐over: not allowed. Participants with brain metastasis: included. Median follow‐up: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: There was insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | As an open label study, no blinding of participants or personnel was possible. However, we believe that in this setting (metastatic melanoma), with the treatments tested and outcomes assessed, the knowledge of which intervention was received or administered (rather than the intervention itself), could not affect the outcomes under investigation. Therefore, we judged the risk of performance bias as low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Published reports included all expected outcomes. However, no protocol was available so it was unclear if all planned outcomes were reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Abbreviations: BCG ‐ Bacillus Calmette‐Guérin; BCNU ‐ 1,3‐bis(2‐chloroethyl)‐1‐nitrosourea; CCNU ‐ lomustine; ECOG ‐ Eastern Cooperative Oncology Group; CR ‐ complete response; G‐CSF ‐ granulocyte‐colony stimulating factor; IFN ‐ interferon‐alpha; IFN‐α ‐ interferon‐alpha; IL‐2 ‐ interleukin‐2; IM ‐ intramuscular; IV ‐ intravenous; MAP ‐ mitogen‐activated protein; MGMT ‐ methylguanine‐DNA methyltransferase; NA ‐ not applicable; PEG‐IFN ‐ pegylated interferon; PR ‐ partial response; QoL ‐ quality of life; RCT ‐ randomised controlled trial; SC ‐ subcutaneous.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| This study investigated mechanisms of interaction between interleukin‐2 and histamine in a subgroup of 19 participants enrolled in a trial. This study was excluded because study endpoints did not match inclusion criteria; the study was about drug interaction and not patient survival or tumour response or toxicity. | |
| This study is not an RCT. | |
| This study is not an RCT. | |
| This study is not an RCT. | |
| This study investigated adjuvant therapy following radical resection of lymph node metastasis (participants were not affected with early stage and not advanced/metastatic melanoma). | |
| This study is not an RCT. | |
| This study investigated the effect of dopamine for reducing renal toxicity caused by interleukin‐2. | |
| This study is not an RCT. | |
| This study is not an RCT. | |
| This study reported a retrospective analysis of participants who had experienced a complete tumour response in RCTs from the Central Oncology Group. | |
| This RCT did not investigate systemic treatments for metastatic disease. Participants were randomised to receive a local treatment, liver infusion, for hepatic metastasis. | |
| This article is a commentary on preliminary findings of a RCT already included in this review (Schwartzentruber 2011a). | |
| This study did not investigate systemic treatment. It tested direct injection of an oncolytic herpes simplex virus type 1 encoding granulocyte macrophage colony‐stimulating factor into accessible melanoma lesions. | |
| This study is not an RCT. | |
| This study is not an RCT. | |
| This study analysed selected participants experiencing long‐term survival in Hodi 2010a. | |
| This study investigated whole brain radiotherapy associated with fotemustine compared to fotemustine alone, and thus did not test effectiveness of systemic treatment. | |
| This study investigated both participants with early stage and metastatic melanoma. This study was excluded because tumour stage of enrolled participants did not match our inclusion criteria (no separate findings for different stages were reported and thus we could not include even part of the results). | |
| This study is not an RCT. | |
| This study is not an RCT. | |
| This study was not an RCT. | |
| This study randomised participants with metastatic melanoma treated with bevacizumab to receive local interleukin‐2 injections. | |
| This study gathered data from three different RCTs and focused on adverse events. This study was excluded because it is a secondary analysis pooling data from one RCT, Hodi 2010a, already included in this systematic review. | |
| This study enrolled both participants with metastatic melanoma and metastatic renal cell carcinoma. Information specifically regarding melanoma was not reported for any study endpoint. |
RCT ‐ randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Study name | A phase 3 study to compare efficacy and safety of masitinib to dacarbazine in the treatment of patients with non‐resectable or metastatic stage 3 or stage 4 melanoma carrying a mutation in the juxta membrane domain of C‐Kit. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 200. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | January 2011. |
| Contact information | Jean Jaques Grob, [email protected] |
| Notes | ‐ |
| Study name | Phase 3 trial in subjects with metastatic melanoma comparing 3 mg/kg ipilimumab versus 10 mg/kg ipilimumab. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 700. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | January 2012. |
| Contact information | 88 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | Study comparing the efficacy of MEK162 versus dacarbazine in unresectable or metastatic NRAS mutation‐positive melanoma. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 397. |
| Interventions | Two‐arm study:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | July 2013. |
| Contact information | 167 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | Study comparing combination of LGX818 plus MEK162 versus vemurafenib and LGX818 monotherapy in BRAF mutant melanoma (COLUMBUS). |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 900. |
| Interventions | Four‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | September 2013. |
| Contact information | 230 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | Ipilimumab with or without dabrafenib, trametinib, and/or nivolumab in treating patients with melanoma that is metastatic or cannot be removed by surgery |
| Methods | Phase I RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 40. |
| Interventions | Five‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | August 2013. |
| Contact information |
|
| Notes | ‐ |
| Study name | Safety and efficacy study of vemurafenib and high‐dose interferon alfa‐2b in melanoma (12‐107) |
| Methods | Phase I/II RCT |
| Participants | Metastatic melanoma. Estimated enrolment: 63. |
| Interventions | Three‐arm study:
|
| Outcomes | Primary outcomes:
Secondary outcome:
|
| Starting date | September 2013. |
| Contact information | John Kirkwood, MD, [email protected] |
| Notes | ‐ |
| Study name | A phase I/II study to assess the safety and efficacy of MK‐3475 in combination with trametinib and dabrafenib in subjects with advanced melanoma. |
| Methods | Phase I/II RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 177. |
| Interventions | Four‐arm trial:
|
| Outcomes | Primary outcomes:
Secondary outcome:
|
| Starting date | May 2014. |
| Contact information | Toll Free Number 1‐888‐577‐8839 |
| Notes | ‐ |
| Study name | A randomized phase III trial of dabrafenib + trametinib followed by ipilimumab + nivolumab at progression vs. ipilimumab + nivolumab followed by dabrafenib + trametinib at progression in patients with advanced BRAFV600 mutant melanoma. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 300. |
| Interventions | Four‐arm study:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | July 2015 |
| Contact information | Michael Atkins, ECOG‐ACRIN Cancer Research Group |
| Notes | ‐ |
| Study name | Randomized phase III study comparing a non‐myeloablative lymphocyte depleting regimen of chemotherapy followed by infusion of tumor infiltrating lymphocytes and interleukin‐2 to standard ipilimumab treatment in metastatic melanoma. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 162. |
| Interventions | Two arm trial:
|
| Outcomes | Primary outcome:
Secondary outcome:
Other outcome measure:
|
| Starting date | September 2014. |
| Contact information | John BAG Haanen, [email protected] |
| Notes | ‐ |
| Study name | Randomized phase II/III study of nivolumab plus ipilimumab plus sargramostim versus nivolumab plus ipilimumab in patients with unresectable stage III or stage IV melanoma. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma with brain metastasis. Estimated enrolment: 400. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | September 2015. |
| Contact information | Frank Hodi, ECOG‐ACRIN Cancer (Eastern Co‐operative Oncology Group‐American College of Radiology Imaging Network) Research Group |
| Notes | ‐ |
| Study name | A phase III, randomized, double‐blind study of adjuvant immunotherapy with nivolumab versus ipilimumab after complete resection of stage IIIb/c or stage IV melanoma in subjects who are at high risk for recurrence (CheckMate 238: CHECKpoint Pathway and nivoluMAb Clinical Trial Evaluation 238). |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma with brain metastasis. Estimated enrolment: 800. |
| Interventions | Two‐arm study:
|
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | March 2015. |
| Contact information | 136 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | An open label non randomized access study of trametinib for patients with advanced unresectable (stage IIIc) or distant metastatic (stage IV) BRAF V600E/K mutation positive cutaneous melanoma. |
| Methods | Phase III non‐RCT. |
| Participants | Metastatic melanoma with brain metastasis. Estimated enrolment: 250. |
| Interventions | Single arm study: participants will receive trametinib 2 mg orally once daily and, where appropriate, in combination with dabrafenib 150 mg orally twice daily. |
| Outcomes | Primary outcomes:
|
| Starting date | March 2015. |
| Contact information | USA GSK Clinical Trials Call Center, [email protected] |
| Notes | ‐ |
| Study name | A randomized, phase III study of fotemustine versus the combination of fotemustine and ipilimumab or the combination of ipilimumab and nivolumab in patients with metastatic melanoma with brain metastasis (NIBIT‐m²). |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma with brain metastasis. Estimated enrolment: 168. |
| Interventions | Three‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | December 2012 |
| Contact information | Anna Maria Di Giacomo, PhD, MD, [email protected] |
| Notes | ‐ |
| Study name | A phase III randomized trial comparing physician/patient choice of either high dose interferon or ipilimumab to MK‐3475 (pembrolizumab) in patients with high risk resected melanoma. |
| Methods | Phase III RCT. |
| Participants | Participants who underwent surgery for distant metastasis. Estimated enrolment: 1378. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | October 2015. |
| Contact information | 314 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | Clinical trial of nivolumab (BMS‐936558) combined with ipilimumab followed by nivolumab monotherapy as first‐line therapy of subjects with histologically confirmed stage III (unresectable) or stage IV melanoma. CheckMate 401: CHECKpoint Pathway and nivoluMAb Clinical Trial Evaluation 401 |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma with brain metastasis. Estimated enrolment: 615. |
| Interventions | Two‐arm study:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | December 2015. |
| Contact information | 41 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | Phase 2 study comparing pembrolizumab with intermittent/short‐term dual MAPK pathway inhibition plus pembrolizumab in patients harboring the BRAFV600 mutation. |
| Methods | Phase II RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 32. |
| Interventions | Four‐arm trial:
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | January 2016. |
| Contact information |
|
| Notes | ‐ |
| Study name | Phase IIIb/IV, randomized, double blinded, study of nivolumab 3 mg/kg in combination with ipilimumab 1 mg/kg vs nivolumab 1 mg/kg in combination with ipilimumab 3 mg/kg in subjects with previously untreated, unresectable or metastatic melanoma. |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 304. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | March 2016. |
| Contact information | 52 recruiting sites (available at clinicaltrials.gov). |
| Notes | ‐ |
| Study name | A phase III randomized, double‐blind, placebo‐controlled study of pembrolizumab (MK‐3475) in combination with epacadostat or placebo in subjects with unresectable or metastatic melanoma (Keynote‐252 / ECHO‐301). |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 600. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | June 2016. |
| Contact information | Merck Sharp & Dohme Corp 1‐888‐577‐8839 |
| Notes | ‐ |
| Study name | A randomized phase III trial of the duration of anti‐PD‐1 therapy in metastatic melanoma (STOP‐GAP). |
| Methods | Phase III RCT. |
| Participants | Metastatic melanoma. Estimated enrolment: 550. |
| Interventions | Two‐arm trial:
|
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | June 2016. |
| Contact information | Janet Dancey, [email protected] |
| Notes | ‐ |
B‐Raf – a protein; C‐Kit – a protein; CR ‐ complete response; CTCAE ‐ Common Terminology Criteria for Adverse Events; EORTC QLQC30 ‐ European Organization for Research and Treatment quality of life questionnaire (version 3.0); EQ‐5D ‐ EuroQol‐5D; irAEs – immune‐related adverse events; IV ‐ intravenously; MAPK ‐ mitogen‐activated protein kinase; mWHO ‐ modified WHO criteria; NRAS ‐ neuroblastoma RAS viral oncogene; PR ‐ partial response; PD‐1 ‐ an inhibitory receptor located on the surface of the T‐cells; PD‐L1 – programmed death‐ligand 1; RCT – randomised controlled trial; SC ‐ subcutaneously; SD ‐ stable disease; Vs – versus.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Overall survival Show forest plot | 6 | 594 | Hazard Ratio (IV, Random, 95% CI) | 0.99 [0.85, 1.16] |
| Analysis 1.1  Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 1: Overall survival | ||||
| 1.2 Progression‐free survival Show forest plot | 5 | 398 | Hazard Ratio (IV, Random, 95% CI) | 1.07 [0.91, 1.25] |
| Analysis 1.2  Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 2: Progression‐free survival | ||||
| 1.3 Tumour response Show forest plot | 14 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.02, 1.58] |
| Analysis 1.3  Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 3: Tumour response | ||||
| 1.4 Toxicity (≥ G3) Show forest plot | 3 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.44, 2.71] |
| Analysis 1.4  Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Overall survival Show forest plot | 4 | 643 | Hazard Ratio (IV, Random, 95% CI) | 1.03 [0.80, 1.33] |
| Analysis 2.1  Comparison 2: Chemotherapy ± tamoxifen, Outcome 1: Overall survival | ||||
| 2.2 Progression‐free survival Show forest plot | 2 | 475 | Hazard Ratio (IV, Random, 95% CI) | 1.06 [0.93, 1.22] |
| Analysis 2.2  Comparison 2: Chemotherapy ± tamoxifen, Outcome 2: Progression‐free survival | ||||
| 2.3 Tumour response Show forest plot | 4 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.94, 1.89] |
| Analysis 2.3  Comparison 2: Chemotherapy ± tamoxifen, Outcome 3: Tumour response | ||||
| 2.4 Toxicity (≥ G3) Show forest plot | 1 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.28] |
| Analysis 2.4  Comparison 2: Chemotherapy ± tamoxifen, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Overall survival Show forest plot | 3 | 1313 | Hazard Ratio (IV, Random, 95% CI) | 0.98 [0.85, 1.12] |
| Analysis 3.1  Comparison 3: Temozolomide versus dacarbazine, Outcome 1: Overall survival | ||||
| 3.2 Progression‐free survival Show forest plot | 3 | 1313 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.74, 1.03] |
| Analysis 3.2  Comparison 3: Temozolomide versus dacarbazine, Outcome 2: Progression‐free survival | ||||
| 3.3 Tumour response Show forest plot | 3 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.73] |
| Analysis 3.3  Comparison 3: Temozolomide versus dacarbazine, Outcome 3: Tumour response | ||||
| 3.4 Toxicity (≥ G3) Show forest plot | 2 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.35] |
| Analysis 3.4  Comparison 3: Temozolomide versus dacarbazine, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Overall survival Show forest plot | 11 | 1785 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.73, 1.04] |
| Analysis 4.1  Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 1: Overall survival | ||||
| 4.2 Progression‐free survival Show forest plot | 6 | 1272 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.74, 1.01] |
| Analysis 4.2  Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 2: Progression‐free survival | ||||
| 4.3 Tumour response Show forest plot | 15 | 2419 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.12, 1.66] |
| Analysis 4.3  Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 3: Tumour response | ||||
| 4.4 Toxicity (≥ G3) Show forest plot | 3 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.37, 7.95] |
| Analysis 4.4  Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Overall survival Show forest plot | 2 | 644 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.82, 1.11] |
| Analysis 5.1  Comparison 5: Chemotherapy ± interleukin‐2, Outcome 1: Overall survival | ||||
| 5.2 Progression‐free survival Show forest plot | 1 | 363 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.70, 1.08] |
| Analysis 5.2  Comparison 5: Chemotherapy ± interleukin‐2, Outcome 2: Progression‐free survival | ||||
| 5.3 Tumour response Show forest plot | 3 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| Analysis 5.3  Comparison 5: Chemotherapy ± interleukin‐2, Outcome 3: Tumour response | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Overall survival Show forest plot | 7 | 1307 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.06] |
| Analysis 6.1  Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 1: Overall survival | ||||
| 6.2 Progression‐free survival Show forest plot | 6 | 964 | Hazard Ratio (IV, Random, 95% CI) | 0.90 [0.83, 0.99] |
| Analysis 6.2  Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 2: Progression‐free survival | ||||
| 6.3 Tumour response Show forest plot | 7 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.11, 1.67] |
| Analysis 6.3  Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 3: Tumour response | ||||
| 6.4 Toxicity (≥ G3) Show forest plot | 2 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.14, 1.61] |
| Analysis 6.4  Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Overall survival Show forest plot | 5 | 1118 | Hazard Ratio (IV, Random, 95% CI) | 0.96 [0.83, 1.10] |
| Analysis 7.1  Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 1: Overall survival | ||||
| 7.2 Progression‐free survival Show forest plot | 4 | 775 | Hazard Ratio (IV, Random, 95% CI) | 0.86 [0.76, 0.99] |
| Analysis 7.2  Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 2: Progression‐free survival | ||||
| 7.3 Tumour response Show forest plot | 5 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.15, 1.83] |
| Analysis 7.3  Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 3: Tumour response | ||||
| 7.4 Toxicity (≥ G3) Show forest plot | 1 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.87] |
| Analysis 7.4  Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Overall survival Show forest plot | 2 | 154 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.61, 1.25] |
| Analysis 8.1  Comparison 8: Chemotherapy ± Bacille Calmette‐Guérin (BCG), Outcome 1: Overall survival | ||||
| 8.2 Tumour response Show forest plot | 6 | 770 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.12] |
| Analysis 8.2  Comparison 8: Chemotherapy ± Bacille Calmette‐Guérin (BCG), Outcome 2: Tumour response | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Overall survival Show forest plot | 4 | 242 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.74, 1.22] |
| Analysis 9.1  Comparison 9: Chemotherapy ± Corynebacterium parvum, Outcome 1: Overall survival | ||||
| 9.2 Tumour response Show forest plot | 7 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.38] |
| Analysis 9.2  Comparison 9: Chemotherapy ± Corynebacterium parvum, Outcome 2: Tumour response | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Overall survival Show forest plot | 2 | 1157 | Hazard Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.01] |
| Analysis 10.1  Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 1: Overall survival | ||||
| 10.2 Progression‐free survival Show forest plot | 1 | 502 | Hazard Ratio (IV, Random, 95% CI) | 0.76 [0.63, 0.92] |
| Analysis 10.2  Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 2: Progression‐free survival | ||||
| 10.3 Tumour response Show forest plot | 2 | 1157 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.92, 1.77] |
| Analysis 10.3  Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 3: Tumour response | ||||
| 10.4 Toxicity (≥ G3) Show forest plot | 2 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.19, 2.42] |
| Analysis 10.4  Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Overall survival Show forest plot | 2 | 784 | Hazard Ratio (IV, Random, 95% CI) | 0.83 [0.52, 1.33] |
| Analysis 11.1  Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 1: Overall survival | ||||
| 11.2 Progression‐free survival Show forest plot | 2 | 785 | Hazard Ratio (IV, Random, 95% CI) | 1.06 [0.75, 1.51] |
| Analysis 11.2  Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 2: Progression‐free survival | ||||
| 11.3 Tumour response Show forest plot | 2 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.47] |
| Analysis 11.3  Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 3: Tumour response | ||||
| 11.4 Toxicity (≥ G3) Show forest plot | 2 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.11] |
| Analysis 11.4  Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Overall survival Show forest plot | 1 | 418 | Hazard Ratio (IV, Random, 95% CI) | 0.42 [0.37, 0.48] |
| Analysis 12.1  Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 1: Overall survival | ||||
| 12.2 Progression‐free survival Show forest plot | 2 | 957 | Hazard Ratio (IV, Random, 95% CI) | 0.49 [0.39, 0.61] |
| Analysis 12.2  Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 2: Progression‐free survival | ||||
| 12.3 Tumour response Show forest plot | 3 | 1367 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [2.38, 4.92] |
| Analysis 12.3  Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 3: Tumour response | ||||
| 12.4 Toxicity (≥ G3) Show forest plot | 3 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.31, 0.97] |
| Analysis 12.4  Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Overall survival Show forest plot | 1 | 834 | Hazard Ratio (IV, Random, 95% CI) | 0.63 [0.60, 0.66] |
| Analysis 13.1  Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 1: Overall survival | ||||
| 13.2 Progression‐free survival Show forest plot | 2 | 1465 | Hazard Ratio (IV, Random, 95% CI) | 0.54 [0.50, 0.60] |
| Analysis 13.2  Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 2: Progression‐free survival | ||||
| 13.3 Tumour response Show forest plot | 2 | 1465 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [2.01, 3.04] |
| Analysis 13.3  Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 3: Tumour response | ||||
| 13.4 Toxicity (≥ G3) Show forest plot | 2 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.91] |
| Analysis 13.4  Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Progression‐free survival Show forest plot | 2 | 738 | Hazard Ratio (IV, Random, 95% CI) | 0.40 [0.35, 0.46] |
| Analysis 14.1  Comparison 14: Anti‐PD1 monoclonal antibodies and anti‐CTLA4 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies alone, Outcome 1: Progression‐free survival | ||||
| 14.2 Tumour response Show forest plot | 2 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [2.07, 5.92] |
| Analysis 14.2  Comparison 14: Anti‐PD1 monoclonal antibodies and anti‐CTLA4 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies alone, Outcome 2: Tumour response | ||||
| 14.3 Toxicity (≥ G3) Show forest plot | 2 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.92] |
| Analysis 14.3  Comparison 14: Anti‐PD1 monoclonal antibodies and anti‐CTLA4 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies alone, Outcome 3: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Overall survival Show forest plot | 3 | 1194 | Hazard Ratio (IV, Random, 95% CI) | 1.00 [0.88, 1.14] |
| Analysis 15.1  Comparison 15: Chemotherapy ± sorafenib, Outcome 1: Overall survival | ||||
| 15.2 Progression‐free survival Show forest plot | 3 | 1194 | Hazard Ratio (IV, Random, 95% CI) | 0.89 [0.73, 1.09] |
| Analysis 15.2  Comparison 15: Chemotherapy ± sorafenib, Outcome 2: Progression‐free survival | ||||
| 15.3 Tumour response Show forest plot | 3 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.91, 1.50] |
| Analysis 15.3  Comparison 15: Chemotherapy ± sorafenib, Outcome 3: Tumour response | ||||
| 15.4 Toxicity (≥ G3) Show forest plot | 3 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.26] |
| Analysis 15.4  Comparison 15: Chemotherapy ± sorafenib, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Overall survival Show forest plot | 1 | 651 | Hazard Ratio (IV, Random, 95% CI) | 1.10 [0.92, 1.32] |
| Analysis 16.1  Comparison 16: Chemotherapy ± elesclomol, Outcome 1: Overall survival | ||||
| 16.2 Progression‐free survival Show forest plot | 2 | 732 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.50, 1.13] |
| Analysis 16.2  Comparison 16: Chemotherapy ± elesclomol, Outcome 2: Progression‐free survival | ||||
| 16.3 Tumour response Show forest plot | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.98, 3.50] |
| Analysis 16.3  Comparison 16: Chemotherapy ± elesclomol, Outcome 3: Tumour response | ||||
| 16.4 Toxicity (≥ G3) Show forest plot | 1 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.00, 1.50] |
| Analysis 16.4  Comparison 16: Chemotherapy ± elesclomol, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 17.1 Overall survival Show forest plot | 2 | 324 | Hazard Ratio (IV, Random, 95% CI) | 0.60 [0.45, 0.81] |
| Analysis 17.1  Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 1: Overall survival | ||||
| 17.2 Progression‐free survival Show forest plot | 2 | 324 | Hazard Ratio (IV, Random, 95% CI) | 0.69 [0.52, 0.92] |
| Analysis 17.2  Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 2: Progression‐free survival | ||||
| 17.3 Tumour response Show forest plot | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.96, 3.03] |
| Analysis 17.3  Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 3: Tumour response | ||||
| 17.4 Toxicity (≥ G3) Show forest plot | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.09, 5.32] |
| Analysis 17.4  Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 18.1 Overall survival Show forest plot | 2 | 925 | Hazard Ratio (IV, Random, 95% CI) | 0.40 [0.28, 0.57] |
| Analysis 18.1  Comparison 18: Single agent BRAF inhibitor, Outcome 1: Overall survival | ||||
| 18.2 Progression‐free survival Show forest plot | 2 | 925 | Hazard Ratio (IV, Random, 95% CI) | 0.27 [0.21, 0.34] |
| Analysis 18.2  Comparison 18: Single agent BRAF inhibitor, Outcome 2: Progression‐free survival | ||||
| 18.3 Tumour response Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 6.78 [4.84, 9.49] |
| Analysis 18.3  Comparison 18: Single agent BRAF inhibitor, Outcome 3: Tumour response | ||||
| 18.4 Toxicity (≥ G3) Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.48, 3.33] |
| Analysis 18.4  Comparison 18: Single agent BRAF inhibitor, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 19.1 Overall survival Show forest plot | 3 | 496 | Hazard Ratio (IV, Random, 95% CI) | 0.85 [0.58, 1.25] |
| Analysis 19.1  Comparison 19: Single agent MEK inhibitor, Outcome 1: Overall survival | ||||
| 19.2 Progression‐free survival Show forest plot | 3 | 496 | Hazard Ratio (IV, Random, 95% CI) | 0.58 [0.42, 0.80] |
| Analysis 19.2  Comparison 19: Single agent MEK inhibitor, Outcome 2: Progression‐free survival | ||||
| 19.3 Tumour response Show forest plot | 3 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.35, 2.99] |
| Analysis 19.3  Comparison 19: Single agent MEK inhibitor, Outcome 3: Tumour response | ||||
| 19.4 Toxicity (≥ G3) Show forest plot | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.08, 2.41] |
| Analysis 19.4  Comparison 19: Single agent MEK inhibitor, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 20.1 Overall survival Show forest plot | 4 | 1784 | Hazard Ratio (IV, Random, 95% CI) | 0.70 [0.59, 0.82] |
| Analysis 20.1  Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 1: Overall survival | ||||
| 20.2 Progression‐free survival Show forest plot | 4 | 1784 | Hazard Ratio (IV, Random, 95% CI) | 0.56 [0.44, 0.71] |
| Analysis 20.2  Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 2: Progression‐free survival | ||||
| 20.3 Tumour response Show forest plot | 4 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.20, 1.46] |
| Analysis 20.3  Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 3: Tumour response | ||||
| 20.4 Toxicity (≥ G3) Show forest plot | 4 | 1774 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| Analysis 20.4  Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 4: Toxicity (≥ G3) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 21.1 Overall survival Show forest plot | 4 | 1458 | Hazard Ratio (IV, Random, 95% CI) | 0.82 [0.67, 0.99] |
| Analysis 21.1  Comparison 21: Immunostimulating agents, Outcome 1: Overall survival | ||||
| 21.2 Progression‐free survival Show forest plot | 4 | 1458 | Hazard Ratio (IV, Random, 95% CI) | 0.92 [0.74, 1.14] |
| Analysis 21.2  Comparison 21: Immunostimulating agents, Outcome 2: Progression‐free survival | ||||
| 21.3 Tumour response Show forest plot | 4 | 1451 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.60, 2.50] |
| Analysis 21.3  Comparison 21: Immunostimulating agents, Outcome 3: Tumour response | ||||
| 21.4 Toxicity (≥ G3) Show forest plot | 4 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.08] |
| Analysis 21.4  Comparison 21: Immunostimulating agents, Outcome 4: Toxicity (≥ G3) | ||||

RAS‐RAF‐MEK‐ERK pathway. Copyright © 2018 Claire Gorry: reproduced with permission.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
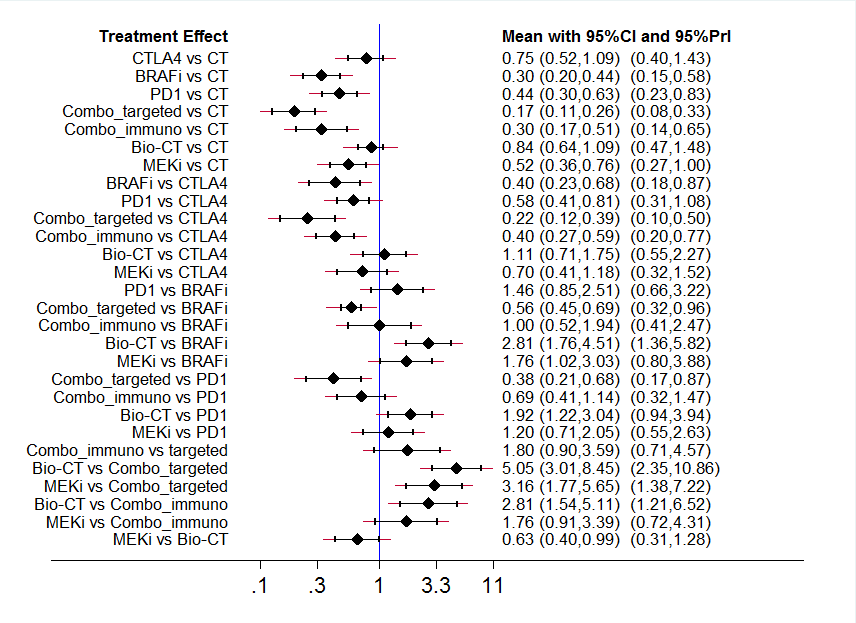
Interval plot: network meta‐analysis results for progression‐free survival. The network included eight treatment modalities. The effect measure is reported as hazard ratio (HR). CI: confidence interval; PrI: predictive interval.
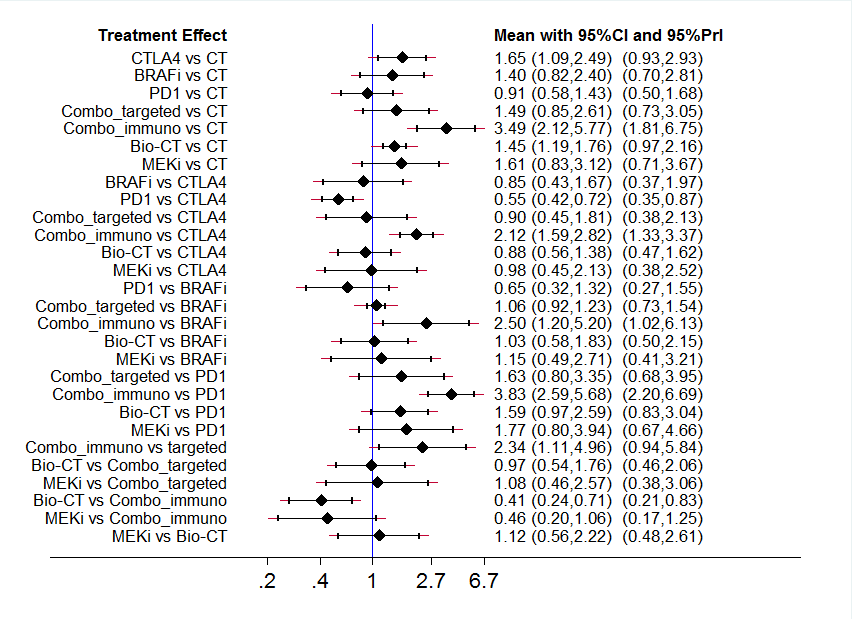
Interval plot: network meta‐analysis results for high grade toxicity. The network included eight treatment modalities. The effect measure is reported as relative risk (RR). CI: confidence interval; PrI: predictive interval.

Ranking plot. Ranking plot representing simultaneously the efficacy (progression‐free survival) on the X axis and the acceptability (the inverse of toxicity) on the Y axis. The network included eight treatments for patients with metastatic melanoma. Optimal treatment should be characterised by both high efficacy and acceptability and should be in the right upper corner of this graph.

Comparison adjusted funnel plot for network meta‐analysis of progression‐free survival

Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 1: Overall survival

Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 2: Progression‐free survival

Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 3: Tumour response

Comparison 1: Polychemotherapy versus single agent chemotherapy, Outcome 4: Toxicity (≥ G3)

Comparison 2: Chemotherapy ± tamoxifen, Outcome 1: Overall survival

Comparison 2: Chemotherapy ± tamoxifen, Outcome 2: Progression‐free survival

Comparison 2: Chemotherapy ± tamoxifen, Outcome 3: Tumour response

Comparison 2: Chemotherapy ± tamoxifen, Outcome 4: Toxicity (≥ G3)

Comparison 3: Temozolomide versus dacarbazine, Outcome 1: Overall survival

Comparison 3: Temozolomide versus dacarbazine, Outcome 2: Progression‐free survival

Comparison 3: Temozolomide versus dacarbazine, Outcome 3: Tumour response

Comparison 3: Temozolomide versus dacarbazine, Outcome 4: Toxicity (≥ G3)

Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 1: Overall survival

Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 2: Progression‐free survival

Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 3: Tumour response

Comparison 4: Chemotherapy ± interferon‐alpha, Outcome 4: Toxicity (≥ G3)

Comparison 5: Chemotherapy ± interleukin‐2, Outcome 1: Overall survival

Comparison 5: Chemotherapy ± interleukin‐2, Outcome 2: Progression‐free survival

Comparison 5: Chemotherapy ± interleukin‐2, Outcome 3: Tumour response

Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 1: Overall survival

Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 2: Progression‐free survival

Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 3: Tumour response

Comparison 6: Chemotherapy ± interferon‐alpha and interleukin‐2, Outcome 4: Toxicity (≥ G3)

Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 1: Overall survival

Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 2: Progression‐free survival

Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 3: Tumour response

Comparison 7: Chemotherapy ± interferon‐alpha and interleukin‐2 (first line), Outcome 4: Toxicity (≥ G3)

Comparison 8: Chemotherapy ± Bacille Calmette‐Guérin (BCG), Outcome 1: Overall survival

Comparison 8: Chemotherapy ± Bacille Calmette‐Guérin (BCG), Outcome 2: Tumour response

Comparison 9: Chemotherapy ± Corynebacterium parvum, Outcome 1: Overall survival

Comparison 9: Chemotherapy ± Corynebacterium parvum, Outcome 2: Tumour response

Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 1: Overall survival

Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 2: Progression‐free survival

Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 3: Tumour response

Comparison 10: Anti‐CTLA4 monoclonal antibodies (first line), Outcome 4: Toxicity (≥ G3)

Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 1: Overall survival

Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 2: Progression‐free survival

Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 3: Tumour response

Comparison 11: Anti‐CTLA4 monoclonal antibodies ± other immunostimulating agents (second line), Outcome 4: Toxicity (≥ G3)

Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 1: Overall survival

Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 2: Progression‐free survival

Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 3: Tumour response

Comparison 12: Anti‐PD1 monoclonal antibodies versus chemotherapy, Outcome 4: Toxicity (≥ G3)

Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 1: Overall survival

Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 2: Progression‐free survival

Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 3: Tumour response

Comparison 13: Anti‐PD1 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies, Outcome 4: Toxicity (≥ G3)

Comparison 14: Anti‐PD1 monoclonal antibodies and anti‐CTLA4 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies alone, Outcome 1: Progression‐free survival

Comparison 14: Anti‐PD1 monoclonal antibodies and anti‐CTLA4 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies alone, Outcome 2: Tumour response

Comparison 14: Anti‐PD1 monoclonal antibodies and anti‐CTLA4 monoclonal antibodies versus anti‐CTLA4 monoclonal antibodies alone, Outcome 3: Toxicity (≥ G3)

Comparison 15: Chemotherapy ± sorafenib, Outcome 1: Overall survival

Comparison 15: Chemotherapy ± sorafenib, Outcome 2: Progression‐free survival

Comparison 15: Chemotherapy ± sorafenib, Outcome 3: Tumour response

Comparison 15: Chemotherapy ± sorafenib, Outcome 4: Toxicity (≥ G3)

Comparison 16: Chemotherapy ± elesclomol, Outcome 1: Overall survival

Comparison 16: Chemotherapy ± elesclomol, Outcome 2: Progression‐free survival

Comparison 16: Chemotherapy ± elesclomol, Outcome 3: Tumour response

Comparison 16: Chemotherapy ± elesclomol, Outcome 4: Toxicity (≥ G3)

Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 1: Overall survival

Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 2: Progression‐free survival

Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 3: Tumour response

Comparison 17: Chemotherapy ± anti‐angiogenic drugs, Outcome 4: Toxicity (≥ G3)

Comparison 18: Single agent BRAF inhibitor, Outcome 1: Overall survival

Comparison 18: Single agent BRAF inhibitor, Outcome 2: Progression‐free survival

Comparison 18: Single agent BRAF inhibitor, Outcome 3: Tumour response

Comparison 18: Single agent BRAF inhibitor, Outcome 4: Toxicity (≥ G3)

Comparison 19: Single agent MEK inhibitor, Outcome 1: Overall survival

Comparison 19: Single agent MEK inhibitor, Outcome 2: Progression‐free survival

Comparison 19: Single agent MEK inhibitor, Outcome 3: Tumour response

Comparison 19: Single agent MEK inhibitor, Outcome 4: Toxicity (≥ G3)

Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 1: Overall survival

Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 2: Progression‐free survival

Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 3: Tumour response

Comparison 20: Combination of BRAF and MEK inhibitors versus single agent BRAF inhibitor, Outcome 4: Toxicity (≥ G3)

Comparison 21: Immunostimulating agents, Outcome 1: Overall survival

Comparison 21: Immunostimulating agents, Outcome 2: Progression‐free survival

Comparison 21: Immunostimulating agents, Outcome 3: Tumour response

Comparison 21: Immunostimulating agents, Outcome 4: Toxicity (≥ G3)
| Anti‐PD1 monoclonal antibodies compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: anti‐PD1 monoclonal antibodies Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Anti‐PD1 | |||||
| Overall survival† | 600 per 1000† | 320 per 1000† | HR 0.42 (0.37 to 0.48) | N = 418 | ⊕⊕⊕⊕ | ‐ |
| Progression‐free survival† | 850 per 1000† | 610 per 1000† | HR 0.49 (0.39 to 0.61) | N = 957 | ⊕⊕⊕⊝ | ‐ |
| Tumour response | 81 per 1000 | 277 per 1000 | RR 3.42 (2.38 to 4.92) | N = 1367 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 300 per 1000 | 165 per 1000 | RR0.55 (0.31 to 0.97) | N = 1360 | ⊕⊕⊝⊝ | ‐ |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). CI: confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. b Downgraded by one level: inconsistency (between‐study heterogeneity). c Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (CI includes both a meaningful benefit (relative risk reduction > 25%) and a small/null benefit (relative risk reduction < 10%)). | ||||||
| Anti‐PD1 monoclonal antibodies compared with anti‐CTLA4 monoclonal antibodies for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: anti‐PD1 monoclonal antibodies Comparison: anti‐CTLA4 monoclonal antibodies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Anti‐CTLA4 | Anti‐PD1 | |||||
| Overall survival† | 600 per 1000† | 438 per 1000† | HR 0.63 (0.60 to 0.66) | N = 764 | ⊕⊕⊕⊕ | ‐ |
| Progression‐free survival† | 850 per 1000† | 641 per 1000† | HR 0.54 (0.50 to 0.60) | n = 1465 | ⊕⊕⊕⊕ | ‐ |
| Tumour response | 157 per 1000 | 388 per 1000 | RR 2.47 (2.01 to 3.04) | N = 1465 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 398 per 1000 | 278 per 1000 | RR 0.70 (0.54 to 0.91) | N = 1465 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). CI: confidence interval; RR: risk ratio; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. b Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (CI includes both a meaningful benefit (relative risk reduction > 25%) and a small/null benefit (relative risk reduction < 10%). | ||||||
| Anti‐CTLA4 monoclonal antibodies plus chemotherapy compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: anti‐CTLA4 monoclonal antibodies plus chemotherapy (combo) Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative Effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Combo | |||||
| Overall survival† | 600 per 1000† | 524 per 1000† | HR 0.81 (0.65 to 1.01) | N = 1157 | ⊕⊕⊝⊝ | ‐ |
| Progression‐free survival† | 850 per 1000† | 763 per 1000† | HR 0.76 (0.63 to 0.92) | N = 502 | ⊕⊕⊕⊝ | ‐ |
| Tumour response | 100 per 1000 | 128 per 1000 | RR 1.28 (0.92 to 1.77) | N = 1157 | ⊕⊕⊕⊝ | ‐ |
| Toxicity (≥ G3) | 352 per 1000 | 595 per 1000 | RR 1.69 (1.19 to 2.42) | N = 1142 | ⊕⊕⊕⊝ | ‐ |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). CI: confidence interval; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (CI includes both a meaningful benefit (relative risk reduction > 25%) and a harmful effect). b Downgraded by one level: imprecision (CI includes both a meaningful benefit (relative risk reduction > 25%) and a small/null benefit (relative risk reduction < 10%)). c Downgraded by one level: imprecision (CI includes both a meaningful benefit (relative risk increase > 25%) and a harmful effect). d Downgraded by one level: inconsistency (between‐study heterogeneity). | ||||||
| Anti‐CTLA4 plus anti‐PD1 monoclonal antibodies compared with anti‐CTLA4 monoclonal antibodies for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: Anti‐CTLA4 plus Anti‐PD1 monoclonal antibodies (combo) Comparison: Anti‐CTLA4 monoclonal antibodies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Anti‐CTLA4 | Combo | |||||
| Overall survival | See comment | See comment | See comment | See comment | See comment | Outcome not measured |
| Progression‐free survival† | 750 per 1000† | 425 per 1000† | HR 0.40 (0.35 to 0.46) | N = 738 | ⊕⊕⊕⊕ | ‐ |
| Tumour response | 182 per 1000 | 636 per 1000 | RR 3.50 (2.07 to 5.92) | N = 738 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 521 per 1000 | 818 per 1000 | RR 1.57 (0.85 to 2.92) | N = 764 | ⊕⊕⊝⊝ | ‐ |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. progression rates). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year progression‐free survival rate = 25%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. b Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (CI includes both a meaningful harm (relative risk increase > 25%) and a beneficial effect) | ||||||
| BRAF inhibitors compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: BRAF inhibitors Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | BRAF inhibitors | |||||
| Overall survival† | 600 per 1000† | 307 per 1000† | HR 0.40 (0.28 to 0.57) | N = 925 | ⊕⊕⊕⊕ | ‐ |
| Progression‐free survival† | 850 per 1000† | 401 per 1000† | HR 0.27 (0.21 to 0.34) | N = 925 | ⊕⊕⊕⊕ | ‐ |
| Tumour response | 82 per 1000 | 556 per 1000 | RR 6.78 (4.84 to 9.49) | N = 925 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 341 per 1000 | 433 per 1000 | RR 1.27 (0.48 to 3.33) | N = 408 | ⊕⊕⊝⊝ | ‐ |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. b Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (CI includes both a meaningful harm (relative risk increase > 25%) and a meaningful benefit (relative risk reduction > 25%)). | ||||||
| MEK inhibitors compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: MEK inhibitors Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | MEK inhibitors | |||||
| Overall survival† | 600 per 1000† | 541 per 1000† | HR 0.85 (0.58 to 1.25) | N = 496 | ⊕⊕⊝⊝ | ‐ |
| Progression‐free survival† | 850 per 1000† | 667 per 1000† | HR 0.58 (0.42 to 0.80) | N = 496 | ⊕⊕⊕⊝ | ‐ |
| Tumour response | 138 per 1000 | 277 per 1000 | RR 2.01 (1.35 to 2.99) | N = 496 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 413 per 1000 | 665 per 1000 | RR 1.61 (1.08 to 2.41) | N = 91 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (CI includes both a meaningful benefit (relative risk reduction > 25%) and a harmful effect). b Downgraded by one level: inconsistency (between‐study heterogeneity). c Not downgraded: high‐quality evidence. d Downgraded by one level: imprecision (sample size lower than optimal information size, calculated to be equal to 400 participants). | ||||||
| BRAF plus MEK inhibitors compared with BRAF inhibitors for the treatment of metastatic melanoma | ||||||
| Patient or population: people cutaneous melanoma Settings: hospital (metastatic disease) Intervention: BRAF inhibitor plus MEK inhibitor (combo) Comparison: BRAF inhibitor | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| BRAF inhibitor | Combo | |||||
| Overall survival† | 350 per 1000† | 260 per 1000† | HR 0.70 (0.59 to 0.82) | N = 1784 | ⊕⊕⊕⊕ | ‐ |
| Progression‐free survival† | 700 per 1000† | 490 per 1000† | HR 0.56 (0.44 to 0.71) | N = 1784 | ⊕⊕⊕⊝ | ‐ |
| Tumour response | 494 per 1000 | 652 per 1000 | RR 1.32 (1.20 to 1.46) | N = 1784 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 495 per 1000 | 500 per 1000 | RR 1.01 (0.85 to 1.20) | N = 1774 | ⊕⊕⊕⊝ | ‐ |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). CI confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 65%. Assumed risk in the control population: 1‐year progression‐free survival rate = 30%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. b Downgraded by one level: inconsistency (between‐study heterogeneity). | ||||||
| Anti‐angiogenic drugs plus chemotherapy compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: Anti‐angiogenic drug plus chemotherapy (combo) Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Combo | |||||
| Overall survival† | 600 per 1000† | 423 per 1000† | HR 0.60 (0.45 to 0.81) | N = 324 | ⊕⊕⊕⊝ | ‐ |
| Progression‐free survival† | 850 per 1000† | 730 per 1000† | HR 0.69 (0.52 to 0.92) | N = 324 | ⊕⊕⊕⊝ | ‐ |
| Tumour response | 104 per 1000 | 178 per 1000 | RR 1.71 (0.96 to 3.03) | N = 324 | ⊕⊕⊕⊝ | ‐ |
| Toxicity (≥ G3) | 272 per 1000 | 185 per 1000 | RR 0.68 (0.09 to 5.32) | N = 324 | ⊕⊕⊝⊝ | ‐ |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Downgraded by one level: imprecision (sample size lower than optimal information size, calculated to be equal to 400 participants). b Downgraded by two levels: inconsistency (between‐study heterogeneity) and imprecision (sample size lower than optimal information size, calculated to be equal to 400 participants). | ||||||
| Biochemotherapy compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: biochemotherapy (chemotherapy combined with both interferon‐alpha and interleukin‐2) Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Biochemotherapy | |||||
| Overall survival† | 600 per 1000† | 577 per 1000† | HR 0.94 (0.84 to 1.06) | N = 1317 | ⊕⊕⊕⊕ | ‐ |
| Progression‐free survival† | 850 per 1000 ° | 818 per 1000† | HR 0.90 (0.83 to 0.99) | N = 964 | ⊕⊕⊕⊕ | ‐ |
| Tumour response | 192 per 1000 | 262 per 1000 | RR 1.36 (1.12 to 1.66) | N = 770 | ⊕⊕⊕⊕ | ‐ |
| Toxicity (≥ G3) | 631 per 1000 | 852 per 1000 | RR 1.35 (1.14 to 1.61) | N = 631 | ⊕⊕⊕⊕ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. | ||||||
| Polychemotherapy compared with chemotherapy for the treatment of metastatic melanoma | ||||||
| Patient or population: people with cutaneous melanoma Settings: hospital (metastatic disease) Intervention: polychemotherapy Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Polychemotherapy | |||||
| Overall survival† | 600 per 1000† | 596 per 1000† | HR 0.99 (0.85 to 1.16) | N = 594 | ⊕⊕⊕⊕ | ‐ |
| Progression‐freesurvival† | 850 per 1000† | 869 per 1000† (822 to 907) | HR 1.07 (0.91 to 1.25) | N = 398 (n = 5) | ⊕⊕⊕⊕ | ‐ |
| Tumour response | 143 per 1000 | 182 per 1000 | RR 1.27 (1.02 to 1.58) | N = 1885 | ⊕⊕⊕⊝ | ‐ |
| Toxicity (≥ G3) | 189 per 1000 | 372 per 1000 | RR 1.97 (1.44 to 2.71) | N = 390 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). † Numbers presented refer to event rates (i.e. death rates and progression rates). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk in the control population: 1‐year overall survival rate = 40%. Assumed risk in the control population: 1‐year progression‐free survival rate = 15%. Assumed risk in the control population: tumour response rate across control arms of included trials. Assumed risk in the control population: toxicity rate across control arms of included trials. a Not downgraded: high‐quality evidence. b Downgraded by one level: imprecision (CI includes both a meaningful benefit (relative risk increase > 25%) and a small/null benefit (relative risk increase < 10%)). c Downgraded by one level: imprecision (sample size lower than optimal information size, calculated to be equal to 400 participants). | ||||||
| Term | Explanation |
|---|---|
| Actinomycin‐D | A polypeptide used as an antibiotic and antineoplastic agent as a result of its ability to inhibit transcription |
| AJCC TNM staging | This is the most widely used tumour staging classification system, which has been developed and constantly updated by the American Joint Committee on Cancer (AJCC) for describing the extent of disease progression in people with cancer. It uses in part the TNM scoring system: tumour size, lymph nodes affected, metastases. Individuals affected by specific tumour type are assigned to categories describing risk of death |
| AJCC TNM stage III | People at this disease stage have melanoma metastasis in their regional lymph node (i.e. the first lymph nodes draining the skin area affected by the melanoma) |
| AJCC TNM stage IIIC | Stage IIIC is a higher risk subgroup among people with lymph node metastasis. The category includes people with all primary tumour stages (T stages) and those with clinically positive lymph nodes, or 4 or more positive lymph nodes |
| AJCC TNM stage IV | People with this disease stage have melanoma metastasis to distant sites (e.g. lung, liver, brain, bone) |
| Anti‐angiogenic agents | Drugs aimed to disrupt tumour vascularisation and reduce blood supply to malignant cells; examples include bevacizumab and endostar |
| Antigen | A substance that invokes the body's immune response |
| Aranoza | An alkylating agent that is used as a chemotherapy drug for various cancers including melanoma as part of combination chemotherapy regimens |
| Bacille Calmette‐Guérin (BCG) | BCG is a vaccine used in the prevention of tuberculosis. However, it is also a form of cancer immunotherapy with established effects in superficial (non‐muscle invading) bladder cancer |
| Bevacizumab | Bevacizumab (Avastin) is an angiogenesis inhibitor approved for use for people with various metastatic cancers. Bevacizumab acts through blockade of vascular endothelial growth factor A (VEGF‐A) that prevents development of new vessels necessary for tumours to grow |
| Bleomycin | An antineoplastic agent used in chemotherapy regimens for various tumours. Belomycin acts through cleavage of DNA within cells |
| Biochemotherapy | A combination of chemotherapy plus immunostimulating cytokines, such as interleukin‐2 and interferon‐alpha |
| Bosentan | An endothelin receptor inhibitor that causes reduced DNA synthesis and promotes apoptosis through competitive antagonism with the anti‐apoptotic factor endothelin‐1, often secreted by cancer cells in an autocrine or paracrine manner |
| BRAF | A gene that makes a protein called B‐Raf. BRAF is involved in sending signals within cells that direct their growth. In some cancers, this gene has mutated (Melanoma Institute Australia 2017) |
| Carmustine | An alkylating agent that prevents DNA replication and cell proliferation used in chemotherapy for various cancers |
| Cobimetinib | An inhibitor of MAPK kinase (MEK) approved for use in metastatic melanoma with BRAF V600E/K mutation usually in combination with a BRAF inhibitor |
| Corynebacterium parvum | C parvum is an aerobic, gram positive bacterium that has been reported to have antineoplastic potential |
| Cyclophosphamide | An alkylating agent used in auto‐immune diseases and various tumours as a chemotherapy drug |
| Cytokine | Small proteins produced by a broad range of cells that are important in cell signalling; they are immunostimulating agents |
| Cytotoxic | Cell killing |
| CTLA4 (cytotoxic T‐cell lymphocyte‐associated antigen‐4) | CTLA4 is a receptor located on the surface of T‐cells that down regulates the immune system (an immune checkpoint). The inhibition of this receptor with monoclonal antibodies, such as ipilimumab and tremelimumab, 'unleashes' the immune response to fight against malignant cells |
| Dabrafenib | An inhibitor of the BRAF kinase that has been approved for people with advanced melanoma carrying the BRAF V600E mutation |
| Dacarbazine | A chemotherapy drug that belongs to the family of alkylating agents that is used in the treatment of various cancers, including melanoma |
| Dendritic cell | These are antigen‐presenting cells that link the innate to the adaptive immune systems via processing antigens and presenting them to T‐lymphocytes. Their role is crucial for proper functioning of vaccines, including cancer vaccines |
| Elesclomol | A drug that causes the accumulation of reactive oxygen species to trigger apoptosis in cancer cells via oxidative stress. It is approved for use for people with metastatic melanoma |
| Endostar | A modified recombinant human endostatin that acts as an anti‐angiogenic agent to prevent the formation of new blood vessels that are necessary for tumour growth and survival |
| Fotemustine | A chemotherapy drug that belongs to the family of alkylating agents and has been approved for the treatment of metastatic melanoma |
| G3 and G4 | G3 (grade 3) and G4 (grade 4) toxicity refers to the highest degree of adverse events due to a systemic treatment. This system grades the toxicity related to a given system or organ (e.g. hepatic, cardiac, haematologic) |
| gp100 | A known melanoma antigen that can be applied to develop a cancer vaccine through processing and presentation by dendritic cells to lymphocytes |
| Granulocyte macrophage ‐ colony‐stimulating factor (GM‐CSF) | A cytokine that stimulates stem cells to give rise to granulocytes and monocytes and boosts the immune system |
| Hydroxyurea | A chemotherapy agent that acts through reducing the generation of deoxyribonucleotides, the building blocks of DNA, to inhibit adequate synthesis of DNA. It is used as a chemotherapy drug for people with myeloproliferative disorders |
| Immune checkpoints | Signalling proteins that protect against auto‐immunity and regulate the immune response; these checkpoints can be hijacked by cancer cells to evade T‐cell‐mediated death, i.e. stopping an immune response to the tumour. CTLA4 and PD1 are both immune checkpoints |
| Immune checkpoint inhibitors | Drugs that override the signalling/activation of immune checkpoints to encourage cytotoxic T‐cell recognition of cancer (i.e. an immune response). These are monoclonal antibodies blocking either CTLA4 or PD1 (two immune checkpoints), known as anti‐CTLA4 and anti‐PD1 monoclonal antibodies |
| Immunomodulating | Stimulates or suppresses the immune system |
| Immunostimulating | Stimulates an immune response |
| Interferon‐alpha | Interferon‐alpha is used for the postoperative treatment of people with AJCC TNM stages II (primary tumour at high risk of disease progression with negative lymph nodes) and III (positive lymph nodes) and to enhance the efficacy of chemotherapy in those who have metastatic melanoma |
| Interleukin‐2 | Interleukin‐2 is a protein that regulates the activities of leucocytes (particularly lymphocytes) that are responsible for immunity. The receptor for interleukin‐2 is expressed by lymphocytes. A recombinant form of human interleukin‐2 has been approved by the FDA for the treatment of melanoma and renal cell cancer |
| Lomustine | An oral alkylating chemotherapeutic agent used mainly to treat brain tumours because it crosses the blood‐brain barrier |
| MEK | Mitogen‐activated protein kinase (MEK) is part of the MAPK signalling pathway (see 'RAS‐RAF‐MEK‐ERK pathway' below), which is activated in melanoma |
| Monoclonal antibodies | Monoclonal antibodies are a type of targeted drug therapy; they work by recognising and finding specific proteins on cancer cells (they work in different ways depending on the protein they are targeting) (Cancer Research UK 2017) |
| Oblimersen | A bcl‐2 antisense oligodeoxynucleotide that reduces cancer cell survival and proliferation by blocking the generation of the anti‐apoptotic protein bcl‐2 thus promoting programmed cell death in cancer cells |
| Oncogene | A gene thats activation or over expression favours cancer growth |
| Paclitaxel | A chemotherapy agent targeting the protein tubulin. The drug interferes with the dynamics of microtubule formation and breakdown leading to problems during cell division and triggering of apoptosis. DHA‐ and nab‐paclitaxel are modified forms of the drug |
| PD1 (programmed cell death protein‐1) | PD1 is a receptor located on the surface of the T‐cells that down regulates the immune system (an immune checkpoint). The inhibition of this receptor with monoclonal antibodies, such as nivolumab and pembrolizumab, 'unleashes' immune response to fight against malignant cells |
| PF‐3512676 | An synthetic oligonucleotide that acts as a Toll‐like receptor‐9 (TLR‐9) agonist. It is used as an immunomodulatory agent alone, or in combination with chemotherapy, to boost anti‐tumour effects by enhancing B‐cell proliferation and antigen‐specific antibody production and cytokine secretion |
| Polychemotherapy | A combination of multiple chemotherapeutic agents |
| Procarbazine | An alkylating agent used as an antineoplastic chemotherapy drug in various tumours such as glioblastoma multiforme and Hodgkin's lymphoma |
| Programmed death‐1 (PD‐1) | PD‐1 is an inhibitory receptor located on the surface of the T‐cells that down regulates the immune system when bound by its ligands (PD‐L1 and PD‐L2, often found on cancer cells). The inhibition of this receptor with monoclonal antibodies, such as pembrolizumab and nivolumab, releases the brake on immune cells thus allowing them to freely fight malignant cells |
| Ramucirumab | A human monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2) to block VEGF binding and thus inhibit angiogenesis. It is approved for use in advanced gastric adenocarcinoma and metastatic non‐small cell lung carcinoma |
| RAS‐RAF‐MEK‐ERK pathway | This is also known as 'MAPK/ERK pathway', which is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the nucleus of the cell (where DNA is located). When one of the proteins in the pathway is mutated, it can be stuck in the 'on' or 'off' position, which is a necessary step in the development of many cancers, including melanoma. Drugs, such as BRAF and MEK inhibitors, can reverse this switch |
| Small‐molecule inhibitors | Low molecular weight drugs targeting molecules mutated or overexpressed in tumours; examples include BRAF inhibitors (which block the BRAF protein) or MEK inhibitors (which block the MEK protein) |
| Sorafenib | An inhibitor of various tyrosine protein kinases including RAF |
| Selumetinib | An inhibitor of the MAPK kinase (MEK) downstream of BRAF |
| T‐cell | A white blood cell type, which plays a key role in immunity |
| Tasisulam | A small‐molecule agent that induces apoptosis through the intrinsic mitochondrial pathway |
| Tamoxifen | A cytostatic hormonal therapeutic agent used mainly as a treatment for oestrogen receptor positive breast cancer. Tamoxifen acts through competing with oestrogen for its receptor thus reducing oestrogen‐related effects in breast tissue such as DNA synthesis and cell proliferation |
| Temozolomide | An oral alkylating agent that can be used in chemotherapy regimens for various cancers such as glioblastoma multiforme |
| Trametinib | An inhibitor of MAPK kinase (MEK) 1 and 2 approved for use in people with V600E‐mutated metastatic melanoma |
| Vemurafenib | A small‐molecule inhibitor of mutated BRAF, an oncogene involved in cell survival or proliferation |
| Vincristine | An anti‐mitotic agent that binds tubulin thus preventing cell proliferation and triggering apoptosis |
| Vindesine | An anti‐mitotic agent that acts by targeting microtubules and preventing cell division thus useful as a chemotherapy drug in various cancers |
| Vitespen | A tumour‐derived heat shock protein that is used as an adjuvant in cancer immunotherapy |
| Study ID | Reason for exclusion from meta‐analysis |
|---|---|
| Single study investigating tasisulam | |
| Single study investigating bosentan | |
| Single study comparing dacarbazine and best supportive care | |
| Single study investigating dendritic cells therapy | |
| Single study investigating histamine with interleukin‐2 | |
| Different polychemotherapy regimens not compared in other studies | |
| Different polychemotherapy regimens not compared in other studies | |
| Different polychemotherapy regimens not compared in other studies | |
| Different PEG‐interferon schedules tested | |
| Inpatient and outpatient interleukin‐2‐based regimens not compared in other studies | |
| Different lenalidomide schedules not compared in other studies | |
| Different polychemotherapy regimens not compared in other studies | |
| Study comparing biochemotherapy versus biotherapy | |
| Study comparing alternating and sequential biochemotherapy and chemotherapy | |
| Single study investigating Indomethacine with interferon | |
| Different single‐agent chemotherapy regimens not compared in other studies | |
| Different polychemotherapy regimens not compared in other studies | |
| Different temozolomide and interferon schedules tested | |
| Different polychemotherapy regimens not compared in other studies | |
| Different interferon‐based regimens not compared in other studies | |
| Different biochemotherapy regimens not compared in other studies | |
| Single study investigating chemotherapy and COX‐2 inhibitor | |
| Single study comparing interleukin‐2 with versus without interferon‐alpha | |
| Different ipilimumab schedules tested | |
| Single study comparing fotemustine and dacarbazine | |
| Single study testing Intetumumab | |
| Single study testing lomeguatrib | |
| Single study testing nab‐paclitaxel | |
| Single study testing oblimersen | |
| Single study testing DHA‐paclitaxel | |
| Single study testing PF‐3512676 | |
| Single study testing ramucirumab | |
| Single study testing dacarbazine and C parvum after surgery | |
| Single study testing vindesine after surgery | |
| Single study testing GM‐CSF and a polypeptide vaccination after surgery | |
| Single study testing lenalidomide | |
| Single study testing veliparib | |
| Single study testing vetaspen |
| Comparison | Experimental (class of) drug | Study ID |
|---|---|---|
| Polychemotherapy versus single agent chemotherapy | Polychemotherapy | |
| Biochemotherapy versus chemotherapy | Interferon‐alpha | |
| Interleukin‐2 | ||
| Interleukin‐2 plus interferon‐alpha | ||
| Immune checkpoint inhibitors versus chemotherapy (or other immune checkpoint inhibitors) | Anti‐CTLA4 monoclonal antibodies | |
| Anti‐PD1 monoclonal antibodies | ||
| Anti‐CTLA4 plus anti‐PD1 monoclonal antibodies | ||
| Small‐molecule targeted drugs versus chemotherapy (or other small‐molecule targeted drugs) | BRAF inhibitors | |
| MEK inhibitors | ||
| BRAF plus MEK inhibitors | ||
| Chemotherapy with versus without other agents | Bacille Calmette‐Guérin (BCG) | |
| Corynebacterium parvum | ||
| Tamoxifen | ||
| Anti‐angiogenic drugs | ||
| Sorafenib | ||
| Elesclomol | ||
| Single agent chemotherapy versus other single agent chemotherapy | Temozolomide | |
| Hodi 2010a; Hodi 2014; Maio 2010; Schwartzentruber 2011a were included in a meta‐analysis of immunostimulating agents. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Overall survival Show forest plot | 6 | 594 | Hazard Ratio (IV, Random, 95% CI) | 0.99 [0.85, 1.16] |
| 1.2 Progression‐free survival Show forest plot | 5 | 398 | Hazard Ratio (IV, Random, 95% CI) | 1.07 [0.91, 1.25] |
| 1.3 Tumour response Show forest plot | 14 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.02, 1.58] |
| 1.4 Toxicity (≥ G3) Show forest plot | 3 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.44, 2.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Overall survival Show forest plot | 4 | 643 | Hazard Ratio (IV, Random, 95% CI) | 1.03 [0.80, 1.33] |
| 2.2 Progression‐free survival Show forest plot | 2 | 475 | Hazard Ratio (IV, Random, 95% CI) | 1.06 [0.93, 1.22] |
| 2.3 Tumour response Show forest plot | 4 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.94, 1.89] |
| 2.4 Toxicity (≥ G3) Show forest plot | 1 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Overall survival Show forest plot | 3 | 1313 | Hazard Ratio (IV, Random, 95% CI) | 0.98 [0.85, 1.12] |
| 3.2 Progression‐free survival Show forest plot | 3 | 1313 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.74, 1.03] |
| 3.3 Tumour response Show forest plot | 3 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.73] |
| 3.4 Toxicity (≥ G3) Show forest plot | 2 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Overall survival Show forest plot | 11 | 1785 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.73, 1.04] |
| 4.2 Progression‐free survival Show forest plot | 6 | 1272 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.74, 1.01] |
| 4.3 Tumour response Show forest plot | 15 | 2419 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.12, 1.66] |
| 4.4 Toxicity (≥ G3) Show forest plot | 3 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.37, 7.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Overall survival Show forest plot | 2 | 644 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.82, 1.11] |
| 5.2 Progression‐free survival Show forest plot | 1 | 363 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 5.3 Tumour response Show forest plot | 3 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Overall survival Show forest plot | 7 | 1307 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 6.2 Progression‐free survival Show forest plot | 6 | 964 | Hazard Ratio (IV, Random, 95% CI) | 0.90 [0.83, 0.99] |
| 6.3 Tumour response Show forest plot | 7 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.11, 1.67] |
| 6.4 Toxicity (≥ G3) Show forest plot | 2 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.14, 1.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Overall survival Show forest plot | 5 | 1118 | Hazard Ratio (IV, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 7.2 Progression‐free survival Show forest plot | 4 | 775 | Hazard Ratio (IV, Random, 95% CI) | 0.86 [0.76, 0.99] |
| 7.3 Tumour response Show forest plot | 5 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.15, 1.83] |
| 7.4 Toxicity (≥ G3) Show forest plot | 1 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Overall survival Show forest plot | 2 | 154 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.61, 1.25] |
| 8.2 Tumour response Show forest plot | 6 | 770 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Overall survival Show forest plot | 4 | 242 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.74, 1.22] |
| 9.2 Tumour response Show forest plot | 7 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Overall survival Show forest plot | 2 | 1157 | Hazard Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.01] |
| 10.2 Progression‐free survival Show forest plot | 1 | 502 | Hazard Ratio (IV, Random, 95% CI) | 0.76 [0.63, 0.92] |
| 10.3 Tumour response Show forest plot | 2 | 1157 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.92, 1.77] |
| 10.4 Toxicity (≥ G3) Show forest plot | 2 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.19, 2.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Overall survival Show forest plot | 2 | 784 | Hazard Ratio (IV, Random, 95% CI) | 0.83 [0.52, 1.33] |
| 11.2 Progression‐free survival Show forest plot | 2 | 785 | Hazard Ratio (IV, Random, 95% CI) | 1.06 [0.75, 1.51] |
| 11.3 Tumour response Show forest plot | 2 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.47] |
| 11.4 Toxicity (≥ G3) Show forest plot | 2 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Overall survival Show forest plot | 1 | 418 | Hazard Ratio (IV, Random, 95% CI) | 0.42 [0.37, 0.48] |
| 12.2 Progression‐free survival Show forest plot | 2 | 957 | Hazard Ratio (IV, Random, 95% CI) | 0.49 [0.39, 0.61] |
| 12.3 Tumour response Show forest plot | 3 | 1367 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [2.38, 4.92] |
| 12.4 Toxicity (≥ G3) Show forest plot | 3 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.31, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Overall survival Show forest plot | 1 | 834 | Hazard Ratio (IV, Random, 95% CI) | 0.63 [0.60, 0.66] |
| 13.2 Progression‐free survival Show forest plot | 2 | 1465 | Hazard Ratio (IV, Random, 95% CI) | 0.54 [0.50, 0.60] |
| 13.3 Tumour response Show forest plot | 2 | 1465 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [2.01, 3.04] |
| 13.4 Toxicity (≥ G3) Show forest plot | 2 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Progression‐free survival Show forest plot | 2 | 738 | Hazard Ratio (IV, Random, 95% CI) | 0.40 [0.35, 0.46] |
| 14.2 Tumour response Show forest plot | 2 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [2.07, 5.92] |
| 14.3 Toxicity (≥ G3) Show forest plot | 2 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Overall survival Show forest plot | 3 | 1194 | Hazard Ratio (IV, Random, 95% CI) | 1.00 [0.88, 1.14] |
| 15.2 Progression‐free survival Show forest plot | 3 | 1194 | Hazard Ratio (IV, Random, 95% CI) | 0.89 [0.73, 1.09] |
| 15.3 Tumour response Show forest plot | 3 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.91, 1.50] |
| 15.4 Toxicity (≥ G3) Show forest plot | 3 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Overall survival Show forest plot | 1 | 651 | Hazard Ratio (IV, Random, 95% CI) | 1.10 [0.92, 1.32] |
| 16.2 Progression‐free survival Show forest plot | 2 | 732 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.50, 1.13] |
| 16.3 Tumour response Show forest plot | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.98, 3.50] |
| 16.4 Toxicity (≥ G3) Show forest plot | 1 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.00, 1.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 17.1 Overall survival Show forest plot | 2 | 324 | Hazard Ratio (IV, Random, 95% CI) | 0.60 [0.45, 0.81] |
| 17.2 Progression‐free survival Show forest plot | 2 | 324 | Hazard Ratio (IV, Random, 95% CI) | 0.69 [0.52, 0.92] |
| 17.3 Tumour response Show forest plot | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.96, 3.03] |
| 17.4 Toxicity (≥ G3) Show forest plot | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.09, 5.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 18.1 Overall survival Show forest plot | 2 | 925 | Hazard Ratio (IV, Random, 95% CI) | 0.40 [0.28, 0.57] |
| 18.2 Progression‐free survival Show forest plot | 2 | 925 | Hazard Ratio (IV, Random, 95% CI) | 0.27 [0.21, 0.34] |
| 18.3 Tumour response Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 6.78 [4.84, 9.49] |
| 18.4 Toxicity (≥ G3) Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.48, 3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 19.1 Overall survival Show forest plot | 3 | 496 | Hazard Ratio (IV, Random, 95% CI) | 0.85 [0.58, 1.25] |
| 19.2 Progression‐free survival Show forest plot | 3 | 496 | Hazard Ratio (IV, Random, 95% CI) | 0.58 [0.42, 0.80] |
| 19.3 Tumour response Show forest plot | 3 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.35, 2.99] |
| 19.4 Toxicity (≥ G3) Show forest plot | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.08, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 20.1 Overall survival Show forest plot | 4 | 1784 | Hazard Ratio (IV, Random, 95% CI) | 0.70 [0.59, 0.82] |
| 20.2 Progression‐free survival Show forest plot | 4 | 1784 | Hazard Ratio (IV, Random, 95% CI) | 0.56 [0.44, 0.71] |
| 20.3 Tumour response Show forest plot | 4 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.20, 1.46] |
| 20.4 Toxicity (≥ G3) Show forest plot | 4 | 1774 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 21.1 Overall survival Show forest plot | 4 | 1458 | Hazard Ratio (IV, Random, 95% CI) | 0.82 [0.67, 0.99] |
| 21.2 Progression‐free survival Show forest plot | 4 | 1458 | Hazard Ratio (IV, Random, 95% CI) | 0.92 [0.74, 1.14] |
| 21.3 Tumour response Show forest plot | 4 | 1451 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.60, 2.50] |
| 21.4 Toxicity (≥ G3) Show forest plot | 4 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.08] |