Ciclesonida versus otros corticosteroides inhalados para el asma crónica en niños
Resumen
Antecedentes
Los corticosteroides inhalados (CSI) son la base del tratamiento de mantenimiento para el asma en los niños. En especial entre los padres existen inquietudes con respecto a la seguridad de los CSI debido a que los estudios en niños han mostrado una reducción del crecimiento. Los CSI de partículas pequeñas dirigidas a las vías respiratorias más pequeñas han mejorado el depósito en los pulmones y se puede lograr un control efectivo del asma con dosis diarias inferiores.
La ciclesonida es un CSI relativamente nuevo. Este CSI de partículas pequeñas es un profármaco que se convierte en las vías respiratorias a un metabolito activo, por lo que potencialmente presenta menos efectos secundarios locales (infección en la garganta) y sistémicos (reducción del crecimiento). Se puede inhalar una vez al día y, por lo tanto, posiblemente mejora el cumplimiento.
Objetivos
Evaluar la eficacia y los efectos adversos de la ciclesonida comparada con otros CSI en el tratamiento del asma crónica en los niños.
Métodos de búsqueda
Se hicieron búsquedas en el Registro de ensayos del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group) con términos predefinidos. Se hicieron búsquedas adicionales en MEDLINE (vía PubMed), EMBASE y Clinicalstudyresults.org. Las búsquedas se actualizaron hasta el 7 de noviembre de 2012.
Criterios de selección
Fueron elegibles para la revisión los estudios controlados aleatorios de grupos paralelos o cruzados (crossover). Se incluyeron los estudios que compararon ciclesonida con otros corticosteroides a dosis nominalmente equivalentes o dosis inferiores de ciclesonida.
Obtención y análisis de los datos
Dos revisores evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos. Se estableció contacto con los autores de los estudios para obtener información adicional. Se obtuvo información de los ensayos acerca de los efectos adversos.
Resultados principales
En esta revisión se incluyeron seis estudios (3256 niños de cuatro a 17 años de edad). Dos estudios se publicaron solo como resúmenes de congresos. La ciclesonida se comparó con budesonida y fluticasona.
Ciclesonida comparada con budesonida (cociente de dosis 1:2): los síntomas del asma y los efectos adversos fueron similares en ambos grupos. Los resultados agrupados no mostraron diferencias significativas en los niños que presentaron una exacerbación (cociente de riesgos [CR] 2,20; intervalo de confianza [IC] del 95%: 0,75 a 6,43). Ambos estudios informaron que los niveles de cortisol en orina en 24 horas mostraron una disminución estadísticamente significativa en el grupo de budesonida en comparación con el grupo de ciclesonida.
Ciclesonida comparada con fluticasona (cociente de dosis 1:1): no se encontraron diferencias significativas para el resultado síntomas del asma. Los resultados agrupados no mostraron diferencias significativas en el número de pacientes con exacerbaciones (CR 1,37; IC del 95%: 0,58 a 3,21) y los datos de un estudio que no se pudieron agrupar en el metanálisis informaron números similares de pacientes con exacerbaciones en ambos grupos. Ninguno de los estudios encontró diferencias en los efectos adversos. No se encontraron diferencias significativas en los niveles de cortisol en orina en 24 horas entre los grupos (diferencia de medias 0,54 nmol/mmol; IC del 95%: ‐5,92 a 7,00).
Un estudio evaluó ciclesonida versus fluticasona (cociente de dosis 1:2) y mostró resultados similares entre los dos corticosteroides para los síntomas del asma. El número de niños con exacerbaciones fue significativamente mayor en el grupo de ciclesonida (CR 3,57; IC del 95%: 1,35 a 9,47). No se encontraron diferencias significativas en los efectos adversos (CR 0,98; IC del 95%: 0,81 a 1,14) ni en los niveles de cortisol en orina en 24 horas (diferencia de medias 1,15 nmol/mmol; IC del 95%: 0,07 a 2,23).
La calidad de las pruebas se consideró "baja" para los resultados síntomas del asma y eventos adversos y "muy baja" para el resultado exacerbaciones en la comparación ciclesonida versus budesonida (cociente de dosis 1:1). La calidad de las pruebas se calificó "moderada" para el resultado síntomas del asma, "muy baja" para el resultado exacerbaciones y "baja" para el resultado eventos adversos en la comparación ciclesonida versus fluticasona (cociente de dosis 1:1). En la comparación ciclesonida versus fluticasona (cociente de dosis 1:2) la calidad se calificó "baja" para el resultado síntomas del asma y "muy baja" para las exacerbaciones y los eventos adversos (cociente de dosis 1:2).
Conclusiones de los autores
No fue posible demostrar ni refutar una mejoría en los síntomas del asma, las exacerbaciones ni los efectos secundarios de la ciclesonida versus budesonida y fluticasona y no está claro el equilibrio entre los efectos beneficiosos y perjudiciales del uso de ciclesonida en lugar de budesonida o fluticasona. Por lo tanto, al tomar decisiones definitivas también se debe considerar el uso de los recursos o los costos de diferentes CSI.
Se necesitan ensayos de superioridad a más largo plazo para identificar la utilidad y la seguridad de la ciclesonida comparada con otros CSI. Además estos estudios deben tener el poder estadístico adecuado para evaluar resultados relevantes para los pacientes (exacerbaciones, síntomas del asma, calidad de vida y efectos secundarios). Se necesitan estudios que comparen la ciclesonida una vez al día con otro CSI dos veces al día para evaluar las ventajas de la ciclesonida como un profármaco que se puede administrar una vez al día con un cumplimiento posiblemente mayor, lo que da lugar a un mejor control del asma y a menos efectos secundarios.
PICOs
Resumen en términos sencillos
Ciclesonida en comparación con budesonida y fluticasona para el tratamiento del asma en los niños
El asma es una enfermedad frecuente en la infancia. La mayoría de los niños con asma crónica reciben tratamiento con corticosteroides inhalados (CSI) para controlar la inflamación de las vías respiratorias y reducir los síntomas del asma. Aunque estos fármacos se consideran muy seguros y efectivos no todos los niños logran un control total del asma y algunos padres están preocupados por la posibilidad de una reducción del crecimiento o de efectos secundarios locales como ronquera. El desafío para los CSI más nuevos es lograr un mejor control del asma con menos efectos secundarios. Lo anterior se podría lograr mediante el uso de CSI de partículas pequeñas, lo que da lugar a un mejor depósito en los pulmones debido a que penetran más profundo en las vías respiratorias pequeñas. Por lo tanto, el control del asma se podría lograr con dosis diarias inferiores y con menos efectos secundarios. En los niños podría ser aún más importante el tamaño de las partículas de los CSI debido a que sus vías respiratorias son más pequeñas.
La ciclesonida es un CSI nuevo de partículas pequeñas. El tamaño más pequeño de las partículas puede dar lugar a que el corticosteroide penetre más profundamente en los pulmones. Las ventajas potenciales son una dosis necesaria inferior para lograr el control del asma, una dosis una vez al día en lugar de dos veces al día y la reducción de los efectos secundarios locales (candidiasis bucal) y sistémicos (supresión del crecimiento).
Se encontraron seis estudios que compararon ciclesonida con budesonida o fluticasona en 3256 niños (de cuatro a 17 años de edad) con asma crónica. Después de tres meses de tratamiento con ciclesonida en comparación con budesonida o fluticasona no fue posible encontrar diferencias relevantes en los síntomas del asma, las exacerbaciones ni los efectos secundarios. En un estudio se evaluó ciclesonida comparada con una dosis doble de fluticasona y no se encontraron diferencias en los síntomas del asma, el uso de medicación de rescate ni los efectos adversos. Sin embargo, los niños que recibieron ciclesonida presentaron más exacerbaciones del asma que los niños del grupo de fluticasona.
Los resultados de esta revisión con respecto a la eficacia y la seguridad de la ciclesonida comparada con otros CSI no son concluyentes. Se encontraron relativamente pocos estudios, se compararon diferentes inhaladores y el período de tratamiento y de seguimiento (12 semanas) fue demasiado corto para la evaluación de resultados relevantes como las exacerbaciones y el retardo del crecimiento. Los estudios futuros deben prestar atención a esos aspectos.
Authors' conclusions
Summary of findings
| Ciclesonide versus budesonide (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Budesonide (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptoms | See comment | See comment | Not estimable | 1024 | ⊕⊕⊝⊝ | Both studies used a 5‐point scale, but insufficient data were reported to allow meta‐analysis |
| Patients with exacerbations | 12 per 1000 | 26 per 1000 | RR 2.2 | 1024 | ⊕⊝⊝⊝ | |
| Adverse events | See comment | See comment | Not estimable | 1024 | ⊕⊕⊝⊝ | The data could not be meta‐analysed because the definitions of adverse events were too diverse |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 In one study the dose of budesonide was much higher than what is commonly prescribed in clinical practice. | ||||||
| Ciclesonide versus fluticasone (dose ratio 1:1) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:1) | Ciclesonide | |||||
| Asthma symptoms | See comment | See comment | Not estimable | 1468 | ⊕⊕⊕⊝ | 2 studies used a 5‐point scale and 1 study did not provide details how asthma symptoms were measured. Data could not be pooled due to diversity in scales |
| Patients with exacerbations | 18 per 1000 | 24 per 1000 | RR 1.37 | 1003 | ⊕⊝⊝⊝ | |
| Adverse events | See comment | See comment | Not estimable | 1560 | ⊕⊕⊝⊝ | Adverse events were defined differently across studies therefore results could not be pooled |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two fully published studies were sponsored by the manufacturer and at least one of the authors of each study was an employee of the manufacturer that sponsored the study. | ||||||
| Ciclesonide versus fluticasone (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptom | The mean asthma symptom in the control groups was | The mean asthma symptom in the intervention groups was | 482 | ⊕⊕⊝⊝ | Estimates are medians indicating data was skewed | |
| Patients with exacerbations | 20 per 1000 | 70 per 1000 | RR 3.48 | 502 | ⊕⊝⊝⊝ | |
| Adverse events | 476 per 1000 | 471 per 1000 | RR 0.99 (0.89 to 1.08) | 502 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Based on one study that was underpowered for a non‐inferiority trial. | ||||||
Background
Description of the condition
Asthma is the most common chronic disease of childhood with a prevalence of 8% to 15% (Masoli 2004). It is a chronic inflammatory disease affecting the whole airway system, including the small airways (Hamid 1997). Daily inhaled corticosteroids (ICS) are the cornerstone of treatment of chronic asthma and in recent guidelines ICS are recommended for all patients except those with mild, intermittent symptoms (British Thoracic Society 2011; GINA 2011).
Description of the intervention
ICS reduce inflammation in the lungs by modulating the inflammatory response of the lung by binding to the glucocorticoid receptor and suppressing the expression of pro‐inflammatory genes. With asthmatic inflammation occurring in all airways including the small airways, the challenge of ICS treatment has now focused mainly on targeting the small airways (Gelfand 2009; Lahzami 2008). Small‐particle drugs (median diameter 1.5 µm) penetrate better in the small airways and improve total lung deposition in adults, more so when inhaled with slower inspiratory flows (Usmani 2005).
Potential adverse drug effects of ICS can be divided into local (such as oral candidiasis and hoarseness) and systemic (adrenal and growth suppression) effects. Particularly in children growth is still a major concern for parents and clinicians. Several longitudinal studies evaluating the effect of ICS on growth have shown a small decrease in growth velocity (approximately 1 to 2 cm) during the first year of treatment (Peters 2006). However, long‐term follow‐up studies show no change in final adult height (Brand 2001). A chronic disease such as asthma may lead to suppressed growth as children with asthma enter puberty at a later age (Brand 2001).
How the intervention might work
The most widely available ICS are beclomethasone dipropionate (BDP), budesonide and fluticasone propionate. Chlorofluorocarbon‐BDP and budesonide are considered equipotent; fluticasone is considered twice as potent compared to chlorofluorocarbon‐BDP and budesonide. Additionally fluticasone and hydrofluoroalkane‐BDP are regarded as equipotent to ciclesonide and the recommended dosage of the Global Initiative for Asthma (GINA), Global Strategy for Asthma Management and Prevention 2011 guideline, is based on this equipotency (GINA 2011). Almost all ICS are registered for twice‐daily use, except for budesonide, which is also registered for once‐daily use. No consistent significant or clinically relevant differences in effectiveness among available ICS have been identified (Adams 2007). One systematic review comparing ICS with small particles (HFA‐BDP) with fluticasone showed no significant difference on forced expiratory volume in one second (FEV1) and peak expiratory flow (PEF) at a dose ratio of 1:1 (Lasserson 2006). There are concerns about adrenal suppression with fluticasone given to children at doses greater than 400 μg/day (Adams 2007; Masoli 2004). Studies reporting on cases of acute adrenal insufficiency in children are almost invariably in children receiving fluticasone and not beclomethasone or budesonide (Eijkemans 2011; Todd 2002). In addition, children receiving fluticasone at half the daily dose of budesonide or beclomethasone appear to have a higher risk of pharyngitis (Adams 2007).
Ciclesonide is a relatively new drug with several potential advantages over the currently used ICS. It is inhaled as a pro‐drug, which is converted in the airways to an active metabolite (des‐ciclesonide) and therefore with potentially less local and systemic side effects. As both ciclesonide and its active metabolite des‐ciclesonide are highly protein bound (˜ 99%), this results in a low proportion of free, unbound drug in the circulation. The 100‐fold greater glucocorticoid receptor binding affinity of des‐ciclesonide compared to ciclesonide may be the explanation for the prolonged local anti‐inflammatory action in the lung and its clinical efficacy with once‐daily dosing. Because of extensive first‐pass metabolism, the systemic availability of des‐ciclesonide is less than 1%. For a detailed overview we refer to paper published by Dahl (Dahl 2006). Furthermore, from a pressurised metered dose inhaler (pMDI), ciclesonide consists of small particles with a volume median diameter of 1.9 µm (compared to a volume median diameter of 3.5 µm for fluticasone, 2.8 µm for budesonide and 1.9 µm for HFA‐BDP) (De Vries 2009). Because of smaller particle size and lower plume velocity, ciclesonide has a better delivery to the small airways and consequently, effective asthma control could be achieved at lower daily doses.
Ciclesonide is registered for once‐daily use. Mean adherence rates may decline with increased frequency of dosing and therefore a once‐daily use could lead to better compliance compared to twice‐daily use (Guest 2005; Osterberg 2005; Price 2010). Particularly in adolescents, adherence to treatment is a major problem. Ciclesonide has been approved in Europe for children 12 years of age and older. The drug is delivered by a metered dose inhaler (MDI) and registered for use with the AeroChamber Plus® spacer.
Why it is important to do this review
The Cochrane Airways Group decided to split the existing review entitled "Ciclesonide versus other inhaled steroids for chronic asthma" (Manning 2009) into a review restricted to children and one restricted to adults. The effect of ciclesonide compared to placebo is subject of another Cochrane review (Manning 2008).
Objectives
To assess the efficacy and adverse effects of ciclesonide compared to other ICS in the management of chronic asthma in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT) comparing ciclesonide with another ICS. We included trials of parallel group design and cross‐over trials with a wash‐out period of two weeks or more. Available unpublished data were considered.
Types of participants
Children (younger than 18 years) with physician‐diagnosed chronic asthma in all settings (general practice, outpatient departments, emergency departments and hospitalised) were eligible for inclusion. Trials that included children as well as adults (aged 18 years and older) were included provided that the data on children were reported separately.
Studies with participants with pulmonary diagnosis other than asthma were excluded.
Types of interventions
This review includes studies that have compared ciclesonide with other ICS at equivalent and lower doses of ciclesonide. The intervention period had to be at least four weeks. Concomitant therapies for asthma, such as short‐acting beta2‐agonists (rescue therapy), theophyllines, long‐acting beta2‐agonists (salmeterol or formoterol), and inhaled anticholinergics were permitted provided that the dose and type of drug remained stable and were the same in both groups and was not introduced at the start of the trial as part of the study protocol. Studies involving anti‐leukotrienes (e.g. singular, accolate), combination inhalers (fluticasone‐salmeterol and budesonide‐formoterol) or other airway anti‐inflammatory asthma therapy (e.g. cromones) were excluded.
Types of outcome measures
Primary outcomes
-
Asthma symptoms: asthma symptom score and number of days without symptoms and use of rescue medication.
-
(Severe) asthma exacerbations defined as:
-
hospital admission;
-
visit to emergency department;
-
need for additional course of corticosteroids;
-
a combination of the above.
-
-
Adverse effects: oropharyngeal candidiasis, sore throat, symptoms of hoarseness, growth, lower‐leg growth, adrenal insufficiency, plasma cortisol, urinary cortisol excretion.
Secondary outcomes
-
Quality of life.
-
Compliance.
-
Change in lung function (FEV1, Mid expiratory flow 25‐75%)
-
Airway inflammation assessed by biopsy, lavage or exhaled nitric oxide (fraction of nitric oxide in exhaled air (FeNO))
Search methods for identification of studies
Electronic searches
We identified from the Cochrane Airways Group (CAG) Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched records in the Specialised Register coded as 'asthma' using the following terms:
ciclesonide* or Alveso* or pregnenedione* or CIC
We searched the CAG trials register from June 2007 up to November 2012. Additional searches in MEDLINE and EMBASE were undertaken using the strategies in Appendix 2 for articles published more recently (2007 to 2012).
Searching other resources
Included and excluded studies of the earlier review that included adults as well as children (Manning 2009) were checked if data concerning children were reported separately. Reference lists of all primary studies and review articles were reviewed for additional references. The manufacturer of ciclesonide (ALTANA Pharma and Nycomed) and authors of identified trials were contacted and asked to identify other published and unpublished studies.
We searched www.clinicalstudyresults.org for trial reports of CIC (December 2011).
Data collection and analysis
Selection of studies
Two review authors (NB and BLR) screened the title and abstract of each citation identified for eligibility. Articles that appeared to meet the inclusion criteria were retrieved in full text. Published abstracts of trials and trials published in a language other than English were also included. Then, based on the full text of the articles, NB and BLR independently established whether each study met the inclusion criteria of the review. Disagreement was solved by discussion.
Data extraction and management
Two review authors extracted the data from the included studies independently of each other. We attempted to contact study authors to identify additional papers, confirm data for accuracy and completeness.
We extracted data concerning the following characteristics of the included studies: study design; patient characteristics such as age, gender, asthma severity, inclusion and exclusion criteria, setting; diagnosis and diagnostic criteria used; characteristics of the interventions such as ICS type, dose, duration of study, method of delivery (MDI with or without spacer, breath actuated inhaler (BAI) or dry powder inhaler (DPI)); inhalation technique (breath hold after inhalation from DPI or BAI, inhalation from spacer with single breath followed by breath hold or tidal breathing) and reported outcome measures.
Assessment of risk of bias in included studies
Risk of bias of the included studies was independently assessed by two review authors according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was solved by discussion. The following items were assessed:
-
adequate sequence generation;
-
allocation concealment;
-
blinding (patient reported/subjective outcomes: asthma symptoms, adverse effects, quality of life, compliance);
-
blinding (other outcomes);
-
incomplete outcome data addressed (patient reported/subjective outcomes: asthma symptoms, adverse effects, quality of life, compliance);
-
incomplete outcome data addressed (other outcomes);
-
free of selective reporting;
-
free of other bias? (e.g. baseline differences).
Measures of treatment effect
A mean difference (MD) and 95% confidence interval (CI) was calculated for continuous variables measured on identical metrics. A standardised mean difference (SMD) was used for the continuous variables that addressed the same type of outcome, but were measured on different scales.
For dichotomous outcomes, we calculated a risk ratio (RR).
Unit of analysis issues
The unit of analysis was the patient.
Dealing with missing data
We contacted the authors of trials in which relevant data or information was missing that was needed for data synthesis and analyses.
Assessment of heterogeneity
Heterogeneity was assessed by comparing clinical characteristics of the included studies such as type of patients (age, gender, asthma severity, etc.), intervention (dose, inhalation technique, duration, etc.), comparison and outcome measures. Clinical homogeneity was discussed by the authors of this review and included experts in the field. Based on this discussion we decided whether pooling of results was sensible. Statistical heterogeneity was first assessed by visual inspection of the forest plots. We also applied the Chi2 test for homogeneity and we calculated the I2 statistic. To increase the power of the test for homogeneity we used a P < 0.1 for rejecting the null‐hypothesis of homogeneity. Interpretation of the statistical heterogeneity was according to the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and was as follows:
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
When interpreting the results of the test for homogeneity and the I2 statistic, we took into account the size of the studies that were included in the meta‐analysis.
Assessment of reporting biases
We planned to visually inspect funnel plots to assess reporting bias if we had been able to combine 10 or more trials in a forest plot.
Data synthesis
We only considered data of clinically homogeneous studies eligible to be combined. We hypothesised that the individual studies that evaluated the effect of ciclesonide estimated a common effect and therefore we chose to combine the results using a fixed‐effect model. If statistical heterogeneity was observed (Chi2: P < 0.1 and I2 > 30%) a sensitivity analysis using a random‐effects model was applied, to determine whether variation between the studies affected the pooled estimate. Furthermore, evidence of statistical heterogeneity prompted exploration of factors that can explain heterogeneity such as clinical or methodological characteristics of studies.
Summary of findings table
We created 'Summary of findings' (SoF) tables for each comparison and primary outcomes. We used GRADE‐profiler software to generate SoF tables that included the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the overall quality of evidence on relevant (primary) outcomes (asthma symptoms, exacerbations and adverse effects) (Schunemann 2011a; Schunemann 2011b). Two review authors (SK and NB) independently graded the body of evidence. According to GRADE, RCTs start as high‐quality evidence. There are five reasons for downgrading the quality of a body of evidence for a specific outcome: limitations in design, indirectness of evidence, inconsistency, imprecision of results and high probability of publication bias. All these items were scored and reasons for downgrading were explicitly stated. Overall quality of evidence was graded 'high', 'moderate' or 'low' based on the likelihood of further research changing our confidence in the estimate of effect. We resolved discrepancies by consensus among two review authors (SK and NB).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses, provided we had sufficient data, according to age (< six years and ≥ six years), asthma severity, dose of ciclesonide and delivery device (identical or different devices used for ciclesonide and BDP/budesonide/fluticasone) as well as inhalation manoeuvre.
Sensitivity analysis
Sensitivity analyses were planned to test the robustness of the results based on the results of the 'Risk of bias' assessment, provided we had sufficient data. We planned to repeat analyses with studies that scored a low risk of bias for allocation concealment, blinding (outcome: asthma symptoms, adverse effects, quality of life, compliance) or incomplete follow‐up (outcome: asthma symptoms, adverse effects, quality of life, compliance).
Results
Description of studies
Results of the search
Of the included and excluded studies of the existing Cochrane review of Manning 2009 three studies met our inclusion criteria (Pedersen 2006; Vermeulen 2007; von Berg 2007). The updated search yielded 296 citations and an additional search of the website www.clinicalstudyresults.org yielded 22 references. After screening of title and abstracts, the full text of 24 studies was assessed. Two reports identified by the search of www.clinicalstudy.org were reports of studies identified in the search of the databases (Agertoft 2010; Pedersen 2009). Of all identified studies, one study published in full text (Pedersen 2009) and two studies published as abstracts (Hiremath 2006; Paunovic 2010) met our inclusion criteria. Therefore, a total of six studies were included into this review. An overview of the selection process is shown in Figure 1.
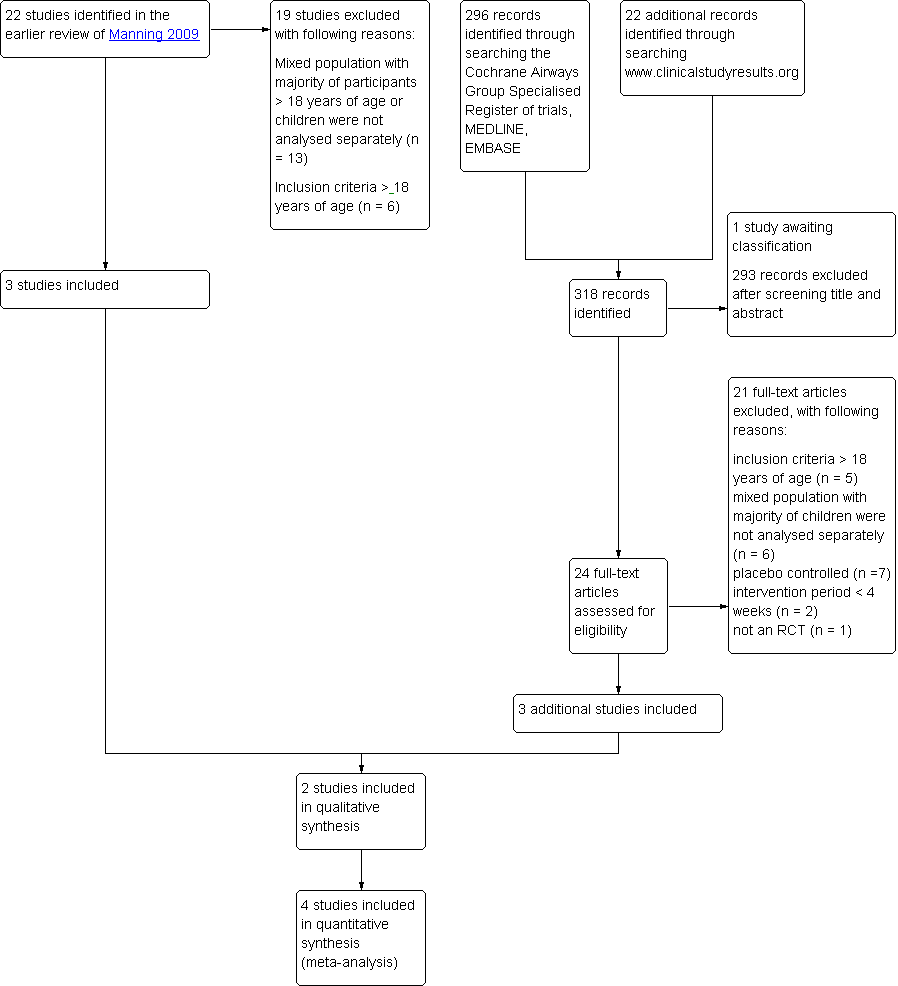
Study flow diagram.
In the previous review, four of the 13 ongoing studies were identified that potentially met our inclusion criteria. To date, three studies have been completed. One study was published but did not separately describe the data of children younger than 18 years of age (Postma 2011) and one study only including adults was excluded (van den Berge 2009). One study is awaiting classification since no full reports were available of the study data (see Characteristics of studies awaiting classification table). One study originally found in the National Research Register record could not be found in the registers archives, and contact details of the author were no longer up to date (GIWA 2003). No references were found to published data of this study and therefore this study is regarded as obsolete.
To retrieve additional data we contacted all contact authors of the included studies. Two of them replied and re‐directed us to the pharmaceutical companies involved. We did not get a reply from the companies on our request for additional data.
Included studies
The characteristics of the six included studies are presented in the Characteristics of included studies table.
Two studies were described as randomised double‐blind parallel group designs (Hiremath 2006; Paunovic 2010) and four studies as randomised double‐blind double‐dummy parallel group designs (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007). All were designed as non‐inferiority studies on lung function.
The six studies randomised 3256 children with asthma and included children between the age of 4 and 17 years. One study did not specify how asthma was diagnosed (von Berg 2007), whereas the other studies diagnosed asthma according to either the guidelines of the American Thoracic Society (ATS) (American Thoracic Society 1987) or the GINA 2003 classification (Pedersen 2006; Pedersen 2009; Vermeulen 2007). There was insufficient information on how asthma was diagnosed in two studies (Hiremath 2006; Paunovic 2010). The children in the fully published studies had suffered from asthma for at least six months.
In the six included studies, two different comparisons were assessed. Ciclesonide was compared to budesonide (Vermeulen 2007; von Berg 2007) or fluticasone (Hiremath 2006; Paunovic 2010; Pedersen 2006; Pedersen 2009) (see Table 1). All treatment periods were 12 weeks and outcomes were measured before and after the intervention period. The dose and delivery of the interventions varied between studies (see Table 1). Ciclesonide was delivered via MDIs in all studies.
| Study ID | Ciclesonide dose | Comparator ICS | Application | Inhalation technique | Treatment period |
| Ciclesonide versus budesonide | |||||
| 160 μg OD (ex‐actuator; equivalent to 200 μg ex‐valve) 2 x 80 μg puffs in the evening | Budesonide 400 μg OD 2 x 200 μg puffs | Ciclesonide: HFA‐MDI with an AeroChamber®; Budesonide: Turbohaler® | Not described | 12 weeks | |
| 320 μg OD (ex‐actuator; equivalent to 2 puffs of 200 μg ex‐valve) 2 x 160 μg puffs administered in the evening | Budesonide 800 μg OD (4 inhalations of | Ciclesonide: HFA‐MDI without spacer Budesonide: Turbohaler® | Not described | 12 weeks | |
| Ciclesonide versus fluticasone | |||||
| 160 μg OD | Fluticasone 88 μg BID | MDI with spacer, AeroChamber Plus® | Not described | 12 weeks | |
| 160 μg OD | Fluticasone 88 μg BID | No information provided | Not described | 12 weeks | |
| 80 μg BID (ex‐actuator; equivalent to 100 μg BID ex‐valve) | Fluticasone 88 μg BID (ex‐actuator dose, equivalent to 100 μg BID ex‐valve) | HFA‐MDI without spacer | Adequate inhalation technique no details described | 12 weeks | |
| 80 or 160 μg OD (ex‐actuator; equivalent to 100 and 200 μg ex‐valve) administered in the evening | Fluticasone 88 μg BID (176 ex‐actuator; equivalent to 100 μg BID ex‐valve) in the morning and evening | HFA 134‐MDI without spacer | Good inhalation technique, no details described | 12 weeks | |
BID: twice daily; ex‐actuator: drugs that leaves the inhaler; ex‐valve: drugs that leaves the metering chamber valve; HFA‐MDI: hydrofluoroalkane‐propelled metered dose inhaler; ICS: inhaled corticosteroid; MDI: metered dose inhaler; OD: once daily.
All studies assessed our primary outcome asthma symptoms. Five studies assessed exacerbations and one study did not address this outcome at all (Hiremath 2006). None of the studies specifically defined asthma exacerbations that conformed to our definition as hospital admissions or visits to an emergency department or additional course of corticosteroids and the description of exacerbation varied between studies. Four studies defined asthma exacerbations as increasing asthma symptoms requiring change or addition of patient's medication other than increasing rescue medication (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007) and in one of these studies the patients with exacerbations were withdrawn (Vermeulen 2007) and in two studies no definitions of exacerbations were described. One study did not report adverse events (Paunovic 2010). None of the studies reported on compliance.
The four fully published studies were all supported or sponsored by the manufacturer of ciclesonide. In all studies at least one of the authors was an employee of the manufacturer that sponsored the study.
Excluded studies
We excluded 21 records (see Characteristics of excluded studies). Two studies were excluded because the intervention period in these studies was two weeks (Agertoft 2010; Matsunaga 2009) and, therefore, did not met our criteria of at least four weeks.
Risk of bias in included studies
The risk of bias of the included studies is summarised in Figure 2. The risk of bias was unclear for the two studies that were published as conference abstracts as no information was available to make a definite judgement on the different items (Hiremath 2006; Paunovic 2010). Our judgements for the remaining four studies that were published in full text are discussed below per item.
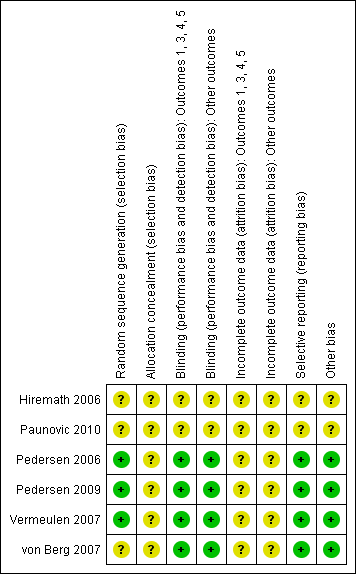
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The randomisation method was clearly described and adequate in three studies (Pedersen 2006; Pedersen 2009; Vermeulen 2007). One study did not provide sufficient information (von Berg 2007). No study described allocation concealment and therefore the risk of bias for this item was deemed unclear.
Blinding
The four fully published studies were described as double‐blind and double‐dummy. Therefore, risk of bias was assessed as low for both subjective outcomes and other outcomes (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007).
Incomplete outcome data
Loss to follow‐up was reported in all four studies. In three studies, 4% of the randomised patients did not complete the study (Pedersen 2009; Vermeulen 2007; von Berg 2007), only one study gave a clear description of the number of patients per group and the reasons for loss to follow‐up (von Berg 2007). In one study, 8% of the patients randomised terminated the study prematurely. The number of patients per group was described; however, no reasons for loss to follow‐up were reported (Pedersen 2006). All studies described that an intention‐to‐treat (ITT) analysis was performed but no details of the analyses were provided and it was not specified which values were imputed in the analyses. Therefore, risk of bias for the items regarding incomplete outcome data were deemed unclear. Two of the four studies reported the number of patients that violated the study protocol (Pedersen 2009; von Berg 2007). The percentage of patients that violated the study protocol was similar in the three different groups in the study of Pedersen 2009 (ciclesonide 80 μg = 6%; 160 μg = 7%; fluticasone 88 μg = 6%). In the study of von Berg 2007 the percentage of patients that violated the study protocol was also similar, 14% of the ITT population in both groups. Two studies did not provide detailed information on study protocol violations (Pedersen 2006; Vermeulen 2007).
Selective reporting
The four fully published studies all reported the outcomes that were specified in their method section (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007).
Other potential sources of bias
None of the four studies showed any obvious baseline differences, therefore for all studies risk of other biases were rated low.
Effects of interventions
See: Summary of findings for the main comparison Ciclesonide versus budesonide (dose ratio 1:2) for chronic asthma in children; Summary of findings 2 Ciclesonide versus fluticasone (dose ratio 1:1) for chronic asthma in children; Summary of findings 3 Ciclesonide versus fluticasone (dose ratio 1:2) for chronic asthma in children
Ciclesonide versus budesonide
Two studies assessed the effect of ciclesonide compared to budesonide both administered once daily at dose ratios of 1:2. The dose of both ciclesonide and budesonide in one study (Vermeulen 2007) was twice the dose of the other study (von Berg 2007). Ciclesonide was delivered using a hydrofluoroalkane‐propelled metered dose inhaler (HFA‐MDI) with the AeroChamber® spacer in one study (von Berg 2007) and without a spacer in the other study (Vermeulen 2007). In both studies, the comparator drug, budesonide, was deliver using a Turbohaler®.
Both studies were designed to assess non‐inferiority of ciclesonide versus budesonide. One study used the per protocol (PP) population to test for non‐inferiority (Vermeulen 2007) and one study based the primary analysis on the PP population and used the ITT population to confirm the results (von Berg 2007). One study set non‐inferiority limits for lung function outcomes (FEV1: ‐150 mL; forced vital capacity (FVC): ‐150 mL and PEF: ‐20 L/minute), percentage of days without asthma symptoms and rescue medication (‐8%) and quality of life measured with Standardized Pediatric Asthma Quality of Life Questionnaire (PAQLQ(S)) scores (‐0.5%) (Vermeulen 2007). One study set the non‐inferiority acceptance limit for the outcome FEV1 at ‐100 mL, using the lower limit of 95% CI for differences between treatment groups (von Berg 2007). The studies were considered to be clinically similar and therefore data were pooled when possible. The results are shown in Table 2 (see summary of findings Table for the main comparison).
| Dose | CIC 160 μg OD versus BUD 400 μg OD | CIC 320 μg OD versus BUD 800 μg OD |
| Dose ratio | 1:2 | 1:2 |
| Study | ||
| Primary outcomes | ||
| Asthma symptoms: asthma symptom score (sum score) | ITT: MD 0.01, 95% CI ‐0.14 to 0.16 PP: MD 0.03, 95% CI ‐0.20 to 0.25 Non‐inferiority acceptance limit = 0.3 | Median change from baseline (no CIs reported) ITT: CIC: ‐0.07; BUD: ‐0.14 PP: CIC: ‐0.07; BUD: ‐0.14 |
| Asthma symptoms: use of rescue medication (puff/day) | ITT: MD 0.06 puffs/day, 95% CI ‐0.26 to 0.38 | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days | ITT: CIC: mean 73%; BUD: mean 70% No difference between groups | ITT and PP: CIC: median 84%; BUD: median 85% Lower limit of the between difference was ‐1.4% and above non‐inferiority limit of ‐8% |
| Exacerbations: patients with exacerbations* | ITT: RR 2.71, 95% CI 0.61 to 12.11; Analysis 1.1 | ITT: RR 1.69, 95% CI 0.36 to 8.00; Analysis 1.1 |
| Adverse events: patients with adverse events | Adverse events were reported in 38% of patients in both groups | ITT: RR** 1.44, 95% CI 0.96 to 2.18 |
| Adverse events: change in body height | Mean change from baseline (least square mean) CIC: 1.18 cm; BUD: 0.70 cm | Not assessed |
| Adverse events: 24‐hour urine cortisol adjusted for creatinine | ITT: 2.99 nmol/mmol creatinine; P < 0.0001, one‐sided (decrease greater in the BUD group) | ITT: significant difference between groups (lower level in BUD group) |
| Secondary outcomes | ||
| Quality of life: PAQLQ(S) | ITT: MD ‐0.11, 95% CI ‐0.12 to 0.10, one‐sided superiority; Analysis 1.2 Non‐inferiority acceptance limits = not provided PP not reported | ITT: MD (least square mean) 0.01, 95% CI ‐0.14 to 0.16; Analysis 1.2 Non‐inferiority acceptance limit = ‐0.5% PP results were similar |
| Quality of life: PACQLQ | ITT: MD ‐0.08, 95% CI ‐0.27 to 0.11, one‐sided superiority Non‐inferiority acceptance limit not provided PP not reported | Not assessed |
| Compliance | Not assessed | Not assessed |
| Lung function: FEV1 (L) | ITT: MD (least square means) ‐0.019 L, 95% CI ‐0.059 to 0.022; Analysis 1.3 PP: MD (least square means) ‐0.034 L, 95% CI ‐75 to 10 Non‐inferiority acceptance limit = ‐100 mL | ITT: MD (least square means) ‐0.03 L, 95% ‐0.14 to 0.8; Analysis 1.3 PP: MD (least square means) ‐0.02 L, 95% CI ‐0.13 to 0.1 Non‐inferiority acceptance limit = ‐150 mL |
| Airway inflammation | Not assessed | Not assessed |
BUD: budesonide; CI: confidence interval; CIC: ciclesonide; ITT: intention to treat analysis; MD: mean difference; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol; RR: risk ratio.
* Exacerbations were defined as an increasing asthma symptoms requiring change or addition of patient's medication other than increasing rescue medication.
** Adverse events that needed treatment, reported in over 2% of patients in CIC or BUD group of safety population (N = 403).
Primary outcomes
Two studies on 1024 children found no significant differences between the groups regarding the outcome asthma symptoms (symptom scores, asthma symptom and rescue medication‐free days) (see Table 2). Asthma symptom scores were assessed using 5‐point scales where a score of 0 represented no asthma symptoms and a score of 4 very bad symptoms, unable to carry out daily activities. One study reported asthma symptom scores as a median change from baseline, which indicates skewed data and therefore we did not perform a meta‐analysis for this outcome.
Pooled data for exacerbations (as defined in the original studies) showed no significant difference between ciclesonide versus budesonide (RR 2.20, 95% CI 0.75 to 6.43; two studies; 1024 children) (Analysis 1.1).
The occurrence of adverse effects was similar in both treatment groups in one study on 621 children. Pharyngitis was one of the most reported adverse effect (ciclesonide: 6.0%; budesonide: 6.8%) in this study (von Berg 2007). Adverse effects likely to be related to treatment were low in the study comparing ciclesonide 320 μg versus budesonide 800 μg; 0.7% and 0.8% for ciclesonide and budesonide, respectively. This study also reported treatment emergent adverse effects (including pharyngitis, asthma aggravated, nasopharyngitis, upper respiratory tract infections) that were reported in more than 2% of the patients per group in a safety population (N = 403) and found no difference between ciclesonide and budesonide (RR 1.44, 95% CI 0.96 to 2.18) (Vermeulen 2007). Pooling of data was not possible because definition of adverse effects were very different between the studies.
One study reported the outcome changes in body height after 12 weeks of intervention. The measurements were taken only in some centres and selection criteria and procedures of the subgroup of patients was not described. Height was measured by stadiometry in 58 patients of the ciclesonide 160 μg group and 26 in the budesonide 400 μg group. The study reported that the increase in height was significantly bigger in the ciclesonide compared to the budesonide group (1.18 cm versus 0.70 cm, respectively) (von Berg 2007).
In the study that compared ciclesonide 160 μg once daily versus budesonide 400 μg once daily, one patient in each treatment group terminated participation due to serious adverse effects, but the author did not specify the nature of these effects (von Berg 2007).
Both studies (1024 children) reported that 24‐hour urine cortisol adjusted for creatinine levels showed a significant decrease in the budesonide group compared to the ciclesonide group, but no numerical data were reported.
Secondary outcomes
Both studies measured quality of life on the PAQLQ(S). One study used the interview version (von Berg 2007) and in the other study the PAQLQ(S) was self‐administered (Vermeulen 2007). Patients answered questions using a 7‐point scale where a score of 1 indicated maximum impairment and 7 indicated no impairment. Pooled results showed no significant differences between the groups (RR ‐0.00, 95% CI ‐0.09 to 0.09; two studies; 1010 children) (Analysis 1.2). One study on 621 children also assessed quality of life using the self‐administered Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ). Carers answered questions using a 7‐point scale, where a score of 1 indicated maximum impairment and 7 indicated no impairment, and reported one‐sided superiority of ciclesonide but did not provide acceptance limits (von Berg 2007).
Pooled result of FEV1 (higher scores indicates better lung function) showed no significant MD between groups (RR ‐0.02, 95% CI ‐0.10 to 0.05; two studies; 1021 children) (Analysis 1.3).
Compliance and airway inflammation were not formally assessed in either of the studies comparing ciclesonide versus budesonide.
Ciclesonide versus fluticasone propionate
Four studies assessed the effect of ciclesonide versus fluticasone (Hiremath 2006; Paunovic 2010; Pedersen 2006; Pedersen 2009) at a dose ratio of 1:1 and one study also assessed a dose ratio of 1:2 (ciclesonide 80 μg once daily compared to the fluticasone 88 μg twice daily; Pedersen 2009). Ciclesonide was administered once daily in all but one study that administered ciclesonide 80 μg twice a day (Pedersen 2006). One study did not report how either of the study drugs were delivered (Paunovic 2010). In one study both ciclesonide and fluticasone were delivered using an MDI with the AeroChamber Plus® spacer (Hiremath 2006) and in the other two studies both drugs were delivered using an HFA‐MDI without a spacer (Pedersen 2006; Pedersen 2009). Two studies were designed to assess non‐inferiority of ciclesonide. Both studies performed a PP analysis and used an ITT analysis to test for robustness of the results. In both studies, the non‐inferiority limits were set for the primary endpoint FEV1 at ‐0.100 L (Pedersen 2006; Pedersen 2009). In the study by Pedersen 2009, non‐inferiority limits were also set at 0.5 for PAQLQ(S) and PACQLQ scores; and +0.30 scores for asthma symptom score sum.
Of the four studies that assessed a dose ratio of 1:1, the study that administered ciclesonide 80 μg twice daily (Pedersen 2006) was considered to be clinically similar to the studies that administered ciclesonide 160 μg once daily (Hiremath 2006; Paunovic 2010; Pedersen 2009). Therefore, we pooled the data of these studies where possible. The results are shown in Table 3.
| Dose | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg OD vs. FP 88 μg BID |
| Dose ratio | 1:1 | 1:1 | 1:1 | 1:1 | 1:2 |
| Study | |||||
| Primary outcomes | |||||
| Asthma symptoms: asthma symptom score | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐0.29 to 0.14 | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP *: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score | Not assessed | Asthma symptom score decreased and was similar in both groups | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP **: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score |
| Asthma symptoms: use of rescue medication | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐1.23 to 2.12 | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.13; FP: ‐1.29 PP: CIC: ‐1.14; FP: ‐1.29 All P < 0.0001 | Not assessed | Use of rescue medication decreased and was similar in both groups | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.20; FP: ‐1.29 PP: CIC: ‐1.21; FP: ‐1.29 All P < 0.0001 |
| Asthma symptoms: a sthma symptom‐free days | Median difference (Hodges Lehmann point estimate) ITT: ‐1.01, 95% CI ‐4.60 to 2.46 PP: ‐1.01, 95% CI ‐4.82 to 2.51 | Not assessed | Not assessed | Not assessed | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days combined | Not assessed | Mean percentage was high and did not differ significantly between the treatment groups (PP) | Median CIC: 91.5%; FP: 94% P = 0.1320 (2‐sided between treatments) | Not assessed | PP: mean percentage was high and did not differ between the treatment groups |
| Exacerbations: number of patients with exacerbations | RR 1.26, 95% CI 0.34 to 4.66; Analysis 2.1 | RR 1.45, 95% CI 0.47 to 4.49; Analysis 2.1 | Not assessed | CIC: 2.3%; FP: 2.2% | RR 3.57, 95% CI 1.35 to 9.47; Analysis 2.1 |
| Adverse events: % of patients with adverse events | A similar percentage of patients reported adverse events | RR 0.88, 95% CI 0.72 to 1.07; Analysis 2.2 | The incidence of adverse events was similar in both groups | Not assessed | RR 0.98, 95% CI 0.81 to 1.17; Analysis 2.2 |
| Adverse events: cortisol 24‐hour urine sample (nmol/mmol) | ITT: difference between 2 groups was not statistically significant ITT and restricted ITT (which included only those urine cortisol measurements with a corresponding urine creatinine value within the normal range) A statistically significant difference in favour of CIC was seen in the restricted ITT analysis (P = 0.006). The findings were similar for patients who were ICS‐naive and patients who had received ICS prior to study entry although the differences were numerically greater in previously ICS‐naive patients | Safety analysis**: MD 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00; Analysis 2.3 | Not assessed | Not assessed | Safety analysis**: MD 1.15 nmol/mmol, 95% CI 0.07 to 2.23; Analysis 2.3 |
| Secondary outcomes | |||||
| Quality of life: PAQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 | Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 |
| Quality of life: PACQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 | Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 |
| Compliance | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Change in lung function: FEV1 (L) | ITT: MD (least square means) 0.0 L, 95% CI ‐0.042 to 0.042; Analysis 2.4 PP: MD (least square means) 0.001, 95% ‐0.044 to 0.046 | ITT: MD (least square means) ‐0.02 L, 95% CI ‐0.07 to 0.04; Analysis 2.4 PP: MD (least square means) ‐0.026, 95% CI ‐0.086 to 0.34 | Improvement similar between groups no point estimates | Improvement similar between groups no point estimates | ITT: MD (least square means) ‐0.05 L, 95% CI ‐0.11 to 0.01; Analysis 2.4 PP: MD (least square means) ‐0.056, 95% CI ‐0.12 to ‐0.004 |
| Airway inflammation | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
BID: twice daily; CI: confidence interval; CIC: ciclesonide; FP: fluticasone; ICS: inhaled corticosteroid; ITT: intention to treat analysis; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol analysis.
* = In this study analyses were based on PP population and analysis of ITT population was used to confirm results, description of the results are unclear but we assumed it to be based on analysis of PP population.
** = safety analysis excluded patients with concurrent nasal, ophthalmological or dermatological corticosteroid treatment.
Primary outcomes
Dose ratio 1:1
In two studies on 1048 children asthma symptom scores were assessed using a 5‐point scale where a score of 0 represented no asthma symptoms and a score of 4 represented very bad symptoms, unable to carry out daily activities (Pedersen 2006; Pedersen 2009). The results could not be pooled since data were reported as medians and this indicates skewed data. The other two studies on 932 children did not provide information on how asthma symptoms were measured (Hiremath 2006; Paunovic 2010) (see: summary of findings Table 2).
No significant differences were found in asthma symptoms and rescue medication‐free days (four studies; 1934 children) (Hiremath 2006; Paunovic 2010; Pedersen 2006; Pedersen 2009) and non‐inferiority of ciclesonide was confirmed (limit was set at 0.3) for asthma symptom scores in one study on 492 children (Pedersen 2009) (see Table 3).
Pooled data of two studies comparing ciclesonide 160 μg versus fluticasone 88 μg twice daily showed no significant difference in number of patients with exacerbations (RR 1.37, 95% CI 0.58 to 3.21; two studies; 1003 children) (Analysis 2.1) (Pedersen 2006; Pedersen 2009). One study on 420 children reported that the number of patients with exacerbations was similar in both the ciclesonide and fluticasone groups (2.3% and 2.2%, respectively) (Paunovic 2010).
One study on 492 children reported that five (2.1%) children treated with ciclesonide 160 μg and two (0.8%) children treated with fluticasone 88 μg twice daily discontinued the study prematurely due to asthma exacerbation (Pedersen 2009).
No significant difference in number of patients with adverse events were found between ciclesonide 160 μg and fluticasone 88 μg twice daily (RR 0.88, 95% CI 0.72 to 1.07; one study; 492 children) (Analysis 2.2) (Pedersen 2009). The other two studies on 1023 children reported that adverse effects were similar in both groups (Hiremath 2006; Pedersen 2006) and one study did not assess adverse effects (Paunovic 2010).
The outcome 24‐hour urine cortisol adjusted for creatinine levels was reported in one study. No significant differences were found for ciclesonide compared to fluticasone (MD 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00; one study; 492 children) (Analysis 2.3).
Dose ratio 1:2
In one study on 502 children, no significant differences were found in asthma symptoms and rescue medication‐free days. For asthma symptom sum scores non‐inferiority (limit was set at 0.3) was confirmed (Pedersen 2009)
The number of exacerbations was significantly higher in the ciclesonide 80 μg once‐daily group compared to the fluticasone 88 μg twice‐daily group (RR 3.57, 95% CI 1.35 to 9.47; one study; 502 children) (Analysis 2.1) (Pedersen 2009).
Thirteen (5.2%) participants treated with ciclesonide 80 μg and two (0.8%) treated with fluticasone 88 μg discontinued the study prematurely due to asthma exacerbation (Pedersen 2009).
No significant differences in number of patients with adverse effects were found between ciclesonide 80 μg once daily and fluticasone 88 μg twice daily (RR 0.98, 95% CI 0.81 to 1.1; one study; 502 children) (Analysis 2.2) (Pedersen 2009).
No significant difference was found for 24‐hour urine cortisol adjusted for creatinine levels in ciclesonide 80 μg once daily versus fluticasone 88 μg twice daily (MD 1.15 nmol/mmol, 95% CI 0.07 to 2.23; one study; 502 children) (Analysis 2.3).
Secondary outcomes
Dose ratio 1:1
Quality of life measured by the PAQLQ and the PACQLQ was reported in one study on 492 children (Pedersen 2009). Patients and carers answered questions using a 7‐point scale where a score of 1 indicated maximum impairment and 7 indicated no impairment.
Non‐inferiority was confirmed for both measurements for ciclesonide compared to fluticasone (P < 0.0001, one‐sided) (Pedersen 2009). Non‐inferiority limits were set at ‐0.5 for the PAQLQ scores and 15 for the PACQLQ scores. The other studies did not formally assess quality of life.
Pooled data of two studies showed no significant difference in FEV1 between ciclesonide 160 μg and fluticasone 88 μg (‐0.01 L, 95% CI ‐0.04 to 0.02; two studies; 1000 children) (Analysis 2.4).
None of the studies formally assessed outcomes on compliance or airway inflammation.
Dose ratio 1:2
Quality of life was measured by the PAQLQ(S) and the PACQLQ. Patients and carers answered questions using a 7‐point scale where a score of 1 indicated maximum impairment and 7 indicated no impairment. Non‐inferiority of ciclesonide versus fluticasone was confirmed for both measurements (P < 0.0001, one‐sided) (Pedersen 2009).
Results were similar in both groups and non‐significant for FEV1 (higher FEV1 indicates better lung function) and non‐inferiority was confirmed (MD ‐0.05 L, 95% CI ‐0.11 to 0.01; one study; 499 children) (Analysis 2.4) (limits set at ‐100 L) (Pedersen 2009).
The outcomes compliance or airway inflammation were not formally assessed.
It was not possible to conduct subgroup or sensitivity analyses due to lack of sufficient data.
Discussion
Summary of main results
In this review we assessed the efficacy and safety of ciclesonide compared to other ICS (budesonide and fluticasone) at a dose ratio 1:1 and 1:2 in the treatment of children younger than 18 years of age with chronic asthma. We found six studies including 3256 children that met our inclusion criteria.
We found no significant differences in efficacy between ciclesonide and fluticasone or budesonide for asthma symptoms and exacerbations after 12 weeks of treatment, except for one study comparing ciclesonide versus fluticasone (1:2) that found significantly more exacerbations in the ciclesonide group. Adherence was not assessed in the studies.
With regards to safety, local side effects such as pharyngitis were seen in both treatment groups with no significant differences, even in the study using a very high dose of budesonide (800 μg) administered once daily. Looking at systemic side effects, one study showed a significant improvement in height in the ciclesonide group compared to the budesonide group after 12 weeks of intervention, but measurements were only performed in a subset of patients. Studies assessing 24‐hour urinary cortisol levels showed either less suppression (ciclesonide versus budesonide) or no significant difference (ciclesonide versus fluticasone).
Overall completeness and applicability of evidence
Only six studies fulfilled our inclusion criteria, of which two studies were only published in abstract form, with limited details available concerning the participants enrolled, definition of outcome measures and trial methodology. The studies were mainly performed in Eastern European countries and South‐Africa, where fewer children might have received ICS treatment before enrolment in the studies than in western European countries. Patients included in the published studies were aged four to 15 years and diagnosed with chronic moderate‐to‐severe asthma according to ATS/GINA criteria, with a relatively poor FEV1 as a requirement at study entry in most of the included studies. No studies comparing ciclesonide versus HFA‐BDP were found. Different doses of both ciclesonide and comparator ICS were used; ciclesonide 80 to 320 μg, budesonide 400 to 800 μg, and fluticasone 88 to 176 μg in a pMDI‐AeroChamber Plus® combination (fluticasone, ciclesonide), as a pMDI without a spacer (fluticasone, ciclesonide) or DPI (budesonide). Current evidence is insufficient to recommend the optimal doses of ICS. Studies comparing different ICS doses could not reveal a clear dose‐response relationship in terms of efficacy and safety in children with mild‐to‐moderate asthma (Zhang 2011). However, all ICS doses in the studies were within accepted ranges for children. Fluticasone and ciclesonide are not registered with the AeroChamber Plus®; further, the use of a pMDI without a spacer is discouraged with children. Because all these different combinations were used, it is not known which part of the effect can be attributed to the ICS used and which part to the inhaler used and the conclusions are only valid for the chosen comparisons.
In all studies for the outcome adverse effects, 24‐hour urinary cortisol levels was measured. The clinical relevance of lower 24‐hour urinary cortisol levels for patients and practitioners is unclear and more important is the ability of the adrenal cortex to be able to respond to stressful circumstances, such as an infection, fever, etc. The most appropriate test would then be the more invasive low‐dose adrenocorticotropic hormone (ACTH) (Synacthen) stimulation test, which is more sensitive in detecting adrenal impairment (Crowley 1991; Lipworth 1999). However, for relevant systemic adverse effects, such as growth and adrenal insufficiency, a follow‐up period of 12 weeks is too short.
One study was published assessing the long‐term safety of ciclesonide (Skoner 2008). This RCT was not included in this review, because it compared ciclesonide to placebo. Mean linear growth velocity and 24‐hour urinary cortisol levels were similar in the three groups after one year. However, this study could not provide enough reassurance about safety, as considerable concern was expressed about compliance of the children as their asthma was very mild and the study failed to show any benefit of ciclesonide in terms of lung function or asthma control (Chapman 2008; Malozowski 2008).
All studies included in this review were designed as non‐inferiority trials. The allowance of setting pre‐defined non‐inferiority acceptance limits the concern is that drugs that are less effective will be classified as non‐inferior or as effective as the control drug. A trial showing non‐inferiority of the experimental drug suggests that the experimental drug is as good as the standard treatment. However, the width of the pre‐defined margins of inferiority has to be taken into account when interpreting the results of these trials individually. Wide margins can result in concluding that the experimental treatment is equally beneficial when it is really less beneficial. Additionally, non‐inferiority should be assessed for relevant outcomes, with a sufficiently long treatment and follow‐up period.
Not all of the included studies in our review provided non‐inferiority acceptance limits for our primary outcomes. Additionally most of the non‐inferiority limits were hard to interpret for reasons such as unclear description of the outcome measure (asthma symptom scores) and no information available on clinical important difference of the questionnaire (PACQLQ). To help readers of this review interpret data of individual studies we provided pre‐defined non‐inferiority limits where possible.
The results of the primary studies are focused on non‐inferiority of ciclesonide versus another ICS. However, when data could be pooled non‐inferiority was not a concern anymore since the point estimate and CI are not influenced by the acceptance limits set in the individual studies. In addition, a meta‐analysis of non‐inferiority studies showed that drugs that were found non‐inferior in published RCTs were not shown to be systematically less effective than standard treatments (Soonawala 2010).
Quality of the evidence
Using recommendations in the Cochrane Handbook and from the GRADE working group, we judged that the quality of the evidence was 'low' for the outcomes asthma symptoms and adverse events and 'very low' for the outcome exacerbations for ciclesonide versus budesonide (dose ratio 1:1; Summary of findings table 1). The quality of evidence was graded 'moderate for the outcome asthma symptoms, 'very low' for the outcome exacerbations and 'low' for the outcome adverse events for ciclesonide versus fluticasone (dose ratio 1:1; Summary of findings table 2). For ciclesonide versus fluticasone (dose ratio 1:2) the quality was rated 'low' for the outcome asthma symptoms and 'very low' for exacerbations and adverse events (dose ratio 1:2; Summary of findings table 3).
The evidence was regarded TO BE indirect due to the fact that in all studies the outcomes were measured after a 12‐week intervention period, which was regarded as an insufficient period to expect an effect on the outcomes adverse events and exacerbations.
Potential biases in the review process
We used the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) to prevent or restrict the risk of bias in our review process. A comprehensive search of the literature searching several databases was conducted. We are confident that all relevant published studies for this review were found. We did attempt to find study protocols by searching www.clinicalstudyresults.org. We included six studies and therefore we could not generate funnel plots to identify publication bias. We contacted study authors in an attempt to find additional data, but did not receive any. Two review authors independently performed study selection, data collection, risk of bias and GRADE assessment to minimise bias. We did not write a protocol for this review but used the protocol of the review of Manning 2009. Any changes to this protocol are listed in the following section of this review (Differences between protocol and review).
Agreements and disagreements with other studies or reviews
Our findings are largely in keeping with other reviews on small particle size and reviews in adult patients. The systematic review by Manning 2009 comparing ciclesonide to other ICS in adults reached the same conclusions on efficacy outcomes; ciclesonide is equal to budesonide/fluticasone in terms of lung function end points, but for our primary outcomes this could not be established due to wide CIs (Manning 2009). The results of this review are also similar to the reported results on the outcomes FEV1 and quality of life in a narrative review that discusses ciclesonide as a treatment for asthma in adults and children (Dahl 2006). In this narrative review, the authors reported that in children the efficacy of ciclesonide was equivalent to fluticasone for the outcomes FEV1 and quality of life (Dahl 2006). A contrast with Manning 2009 and Dahl 2006 was the lower oral candidiasis with ciclesonide compared to fluticasone in adults, which we did not find in children. Other reviews comparing small‐particle‐size ICS with normal‐particle‐size ICS so far could not identify improved efficacy or safety on relevant end points compared to normal‐particle‐size ICS (Adams 2007).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 1 Patients with exacerbations.
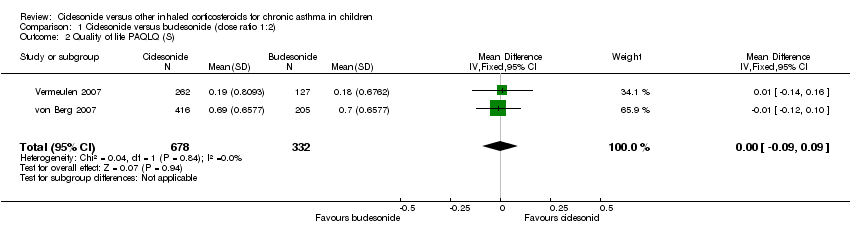
Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 2 Quality of life PAQLQ (S).

Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 3 FEV1 least square means (L).
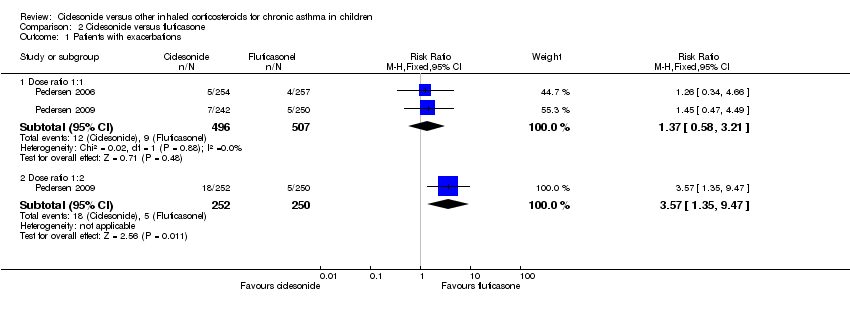
Comparison 2 Ciclesonide versus fluticasone, Outcome 1 Patients with exacerbations.
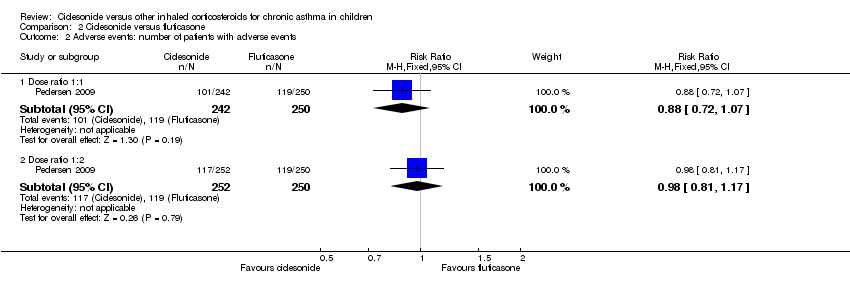
Comparison 2 Ciclesonide versus fluticasone, Outcome 2 Adverse events: number of patients with adverse events.
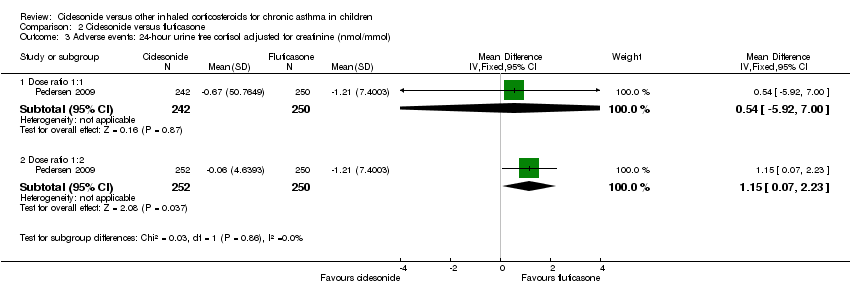
Comparison 2 Ciclesonide versus fluticasone, Outcome 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol).

Comparison 2 Ciclesonide versus fluticasone, Outcome 4 Generic FEV1 least square mean (L).
| Ciclesonide versus budesonide (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Budesonide (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptoms | See comment | See comment | Not estimable | 1024 | ⊕⊕⊝⊝ | Both studies used a 5‐point scale, but insufficient data were reported to allow meta‐analysis |
| Patients with exacerbations | 12 per 1000 | 26 per 1000 | RR 2.2 | 1024 | ⊕⊝⊝⊝ | |
| Adverse events | See comment | See comment | Not estimable | 1024 | ⊕⊕⊝⊝ | The data could not be meta‐analysed because the definitions of adverse events were too diverse |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 In one study the dose of budesonide was much higher than what is commonly prescribed in clinical practice. | ||||||
| Ciclesonide versus fluticasone (dose ratio 1:1) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:1) | Ciclesonide | |||||
| Asthma symptoms | See comment | See comment | Not estimable | 1468 | ⊕⊕⊕⊝ | 2 studies used a 5‐point scale and 1 study did not provide details how asthma symptoms were measured. Data could not be pooled due to diversity in scales |
| Patients with exacerbations | 18 per 1000 | 24 per 1000 | RR 1.37 | 1003 | ⊕⊝⊝⊝ | |
| Adverse events | See comment | See comment | Not estimable | 1560 | ⊕⊕⊝⊝ | Adverse events were defined differently across studies therefore results could not be pooled |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two fully published studies were sponsored by the manufacturer and at least one of the authors of each study was an employee of the manufacturer that sponsored the study. | ||||||
| Ciclesonide versus fluticasone (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptom | The mean asthma symptom in the control groups was | The mean asthma symptom in the intervention groups was | 482 | ⊕⊕⊝⊝ | Estimates are medians indicating data was skewed | |
| Patients with exacerbations | 20 per 1000 | 70 per 1000 | RR 3.48 | 502 | ⊕⊝⊝⊝ | |
| Adverse events | 476 per 1000 | 471 per 1000 | RR 0.99 (0.89 to 1.08) | 502 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Based on one study that was underpowered for a non‐inferiority trial. | ||||||
| Study ID | Ciclesonide dose | Comparator ICS | Application | Inhalation technique | Treatment period |
| Ciclesonide versus budesonide | |||||
| 160 μg OD (ex‐actuator; equivalent to 200 μg ex‐valve) 2 x 80 μg puffs in the evening | Budesonide 400 μg OD 2 x 200 μg puffs | Ciclesonide: HFA‐MDI with an AeroChamber®; Budesonide: Turbohaler® | Not described | 12 weeks | |
| 320 μg OD (ex‐actuator; equivalent to 2 puffs of 200 μg ex‐valve) 2 x 160 μg puffs administered in the evening | Budesonide 800 μg OD (4 inhalations of | Ciclesonide: HFA‐MDI without spacer Budesonide: Turbohaler® | Not described | 12 weeks | |
| Ciclesonide versus fluticasone | |||||
| 160 μg OD | Fluticasone 88 μg BID | MDI with spacer, AeroChamber Plus® | Not described | 12 weeks | |
| 160 μg OD | Fluticasone 88 μg BID | No information provided | Not described | 12 weeks | |
| 80 μg BID (ex‐actuator; equivalent to 100 μg BID ex‐valve) | Fluticasone 88 μg BID (ex‐actuator dose, equivalent to 100 μg BID ex‐valve) | HFA‐MDI without spacer | Adequate inhalation technique no details described | 12 weeks | |
| 80 or 160 μg OD (ex‐actuator; equivalent to 100 and 200 μg ex‐valve) administered in the evening | Fluticasone 88 μg BID (176 ex‐actuator; equivalent to 100 μg BID ex‐valve) in the morning and evening | HFA 134‐MDI without spacer | Good inhalation technique, no details described | 12 weeks | |
| BID: twice daily; ex‐actuator: drugs that leaves the inhaler; ex‐valve: drugs that leaves the metering chamber valve; HFA‐MDI: hydrofluoroalkane‐propelled metered dose inhaler; ICS: inhaled corticosteroid; MDI: metered dose inhaler; OD: once daily. | |||||
| Dose | CIC 160 μg OD versus BUD 400 μg OD | CIC 320 μg OD versus BUD 800 μg OD |
| Dose ratio | 1:2 | 1:2 |
| Study | ||
| Primary outcomes | ||
| Asthma symptoms: asthma symptom score (sum score) | ITT: MD 0.01, 95% CI ‐0.14 to 0.16 PP: MD 0.03, 95% CI ‐0.20 to 0.25 Non‐inferiority acceptance limit = 0.3 | Median change from baseline (no CIs reported) ITT: CIC: ‐0.07; BUD: ‐0.14 PP: CIC: ‐0.07; BUD: ‐0.14 |
| Asthma symptoms: use of rescue medication (puff/day) | ITT: MD 0.06 puffs/day, 95% CI ‐0.26 to 0.38 | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days | ITT: CIC: mean 73%; BUD: mean 70% No difference between groups | ITT and PP: CIC: median 84%; BUD: median 85% Lower limit of the between difference was ‐1.4% and above non‐inferiority limit of ‐8% |
| Exacerbations: patients with exacerbations* | ITT: RR 2.71, 95% CI 0.61 to 12.11; Analysis 1.1 | ITT: RR 1.69, 95% CI 0.36 to 8.00; Analysis 1.1 |
| Adverse events: patients with adverse events | Adverse events were reported in 38% of patients in both groups | ITT: RR** 1.44, 95% CI 0.96 to 2.18 |
| Adverse events: change in body height | Mean change from baseline (least square mean) CIC: 1.18 cm; BUD: 0.70 cm | Not assessed |
| Adverse events: 24‐hour urine cortisol adjusted for creatinine | ITT: 2.99 nmol/mmol creatinine; P < 0.0001, one‐sided (decrease greater in the BUD group) | ITT: significant difference between groups (lower level in BUD group) |
| Secondary outcomes | ||
| Quality of life: PAQLQ(S) | ITT: MD ‐0.11, 95% CI ‐0.12 to 0.10, one‐sided superiority; Analysis 1.2 Non‐inferiority acceptance limits = not provided PP not reported | ITT: MD (least square mean) 0.01, 95% CI ‐0.14 to 0.16; Analysis 1.2 Non‐inferiority acceptance limit = ‐0.5% PP results were similar |
| Quality of life: PACQLQ | ITT: MD ‐0.08, 95% CI ‐0.27 to 0.11, one‐sided superiority Non‐inferiority acceptance limit not provided PP not reported | Not assessed |
| Compliance | Not assessed | Not assessed |
| Lung function: FEV1 (L) | ITT: MD (least square means) ‐0.019 L, 95% CI ‐0.059 to 0.022; Analysis 1.3 PP: MD (least square means) ‐0.034 L, 95% CI ‐75 to 10 Non‐inferiority acceptance limit = ‐100 mL | ITT: MD (least square means) ‐0.03 L, 95% ‐0.14 to 0.8; Analysis 1.3 PP: MD (least square means) ‐0.02 L, 95% CI ‐0.13 to 0.1 Non‐inferiority acceptance limit = ‐150 mL |
| Airway inflammation | Not assessed | Not assessed |
| BUD: budesonide; CI: confidence interval; CIC: ciclesonide; ITT: intention to treat analysis; MD: mean difference; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol; RR: risk ratio. * Exacerbations were defined as an increasing asthma symptoms requiring change or addition of patient's medication other than increasing rescue medication. ** Adverse events that needed treatment, reported in over 2% of patients in CIC or BUD group of safety population (N = 403). | ||
| Dose | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg OD vs. FP 88 μg BID |
| Dose ratio | 1:1 | 1:1 | 1:1 | 1:1 | 1:2 |
| Study | |||||
| Primary outcomes | |||||
| Asthma symptoms: asthma symptom score | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐0.29 to 0.14 | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP *: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score | Not assessed | Asthma symptom score decreased and was similar in both groups | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP **: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score |
| Asthma symptoms: use of rescue medication | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐1.23 to 2.12 | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.13; FP: ‐1.29 PP: CIC: ‐1.14; FP: ‐1.29 All P < 0.0001 | Not assessed | Use of rescue medication decreased and was similar in both groups | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.20; FP: ‐1.29 PP: CIC: ‐1.21; FP: ‐1.29 All P < 0.0001 |
| Asthma symptoms: a sthma symptom‐free days | Median difference (Hodges Lehmann point estimate) ITT: ‐1.01, 95% CI ‐4.60 to 2.46 PP: ‐1.01, 95% CI ‐4.82 to 2.51 | Not assessed | Not assessed | Not assessed | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days combined | Not assessed | Mean percentage was high and did not differ significantly between the treatment groups (PP) | Median CIC: 91.5%; FP: 94% P = 0.1320 (2‐sided between treatments) | Not assessed | PP: mean percentage was high and did not differ between the treatment groups |
| Exacerbations: number of patients with exacerbations | RR 1.26, 95% CI 0.34 to 4.66; Analysis 2.1 | RR 1.45, 95% CI 0.47 to 4.49; Analysis 2.1 | Not assessed | CIC: 2.3%; FP: 2.2% | RR 3.57, 95% CI 1.35 to 9.47; Analysis 2.1 |
| Adverse events: % of patients with adverse events | A similar percentage of patients reported adverse events | RR 0.88, 95% CI 0.72 to 1.07; Analysis 2.2 | The incidence of adverse events was similar in both groups | Not assessed | RR 0.98, 95% CI 0.81 to 1.17; Analysis 2.2 |
| Adverse events: cortisol 24‐hour urine sample (nmol/mmol) | ITT: difference between 2 groups was not statistically significant ITT and restricted ITT (which included only those urine cortisol measurements with a corresponding urine creatinine value within the normal range) A statistically significant difference in favour of CIC was seen in the restricted ITT analysis (P = 0.006). The findings were similar for patients who were ICS‐naive and patients who had received ICS prior to study entry although the differences were numerically greater in previously ICS‐naive patients | Safety analysis**: MD 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00; Analysis 2.3 | Not assessed | Not assessed | Safety analysis**: MD 1.15 nmol/mmol, 95% CI 0.07 to 2.23; Analysis 2.3 |
| Secondary outcomes | |||||
| Quality of life: PAQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 | Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 |
| Quality of life: PACQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 | Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 |
| Compliance | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Change in lung function: FEV1 (L) | ITT: MD (least square means) 0.0 L, 95% CI ‐0.042 to 0.042; Analysis 2.4 PP: MD (least square means) 0.001, 95% ‐0.044 to 0.046 | ITT: MD (least square means) ‐0.02 L, 95% CI ‐0.07 to 0.04; Analysis 2.4 PP: MD (least square means) ‐0.026, 95% CI ‐0.086 to 0.34 | Improvement similar between groups no point estimates | Improvement similar between groups no point estimates | ITT: MD (least square means) ‐0.05 L, 95% CI ‐0.11 to 0.01; Analysis 2.4 PP: MD (least square means) ‐0.056, 95% CI ‐0.12 to ‐0.004 |
| Airway inflammation | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| BID: twice daily; CI: confidence interval; CIC: ciclesonide; FP: fluticasone; ICS: inhaled corticosteroid; ITT: intention to treat analysis; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol analysis. * = In this study analyses were based on PP population and analysis of ITT population was used to confirm results, description of the results are unclear but we assumed it to be based on analysis of PP population. ** = safety analysis excluded patients with concurrent nasal, ophthalmological or dermatological corticosteroid treatment. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with exacerbations Show forest plot | 2 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.75, 6.43] |
| 2 Quality of life PAQLQ (S) Show forest plot | 2 | 1010 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 3 FEV1 least square means (L) Show forest plot | 2 | 1021 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with exacerbations Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dose ratio 1:1 | 2 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.58, 3.21] |
| 1.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.35, 9.47] |
| 2 Adverse events: number of patients with adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Dose ratio 1:1 | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 2.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.17] |
| 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Dose ratio 1:1 | 1 | 492 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐5.92, 7.00] |
| 3.2 Dose ratio 1:2 | 1 | 502 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.07, 2.23] |
| 4 Generic FEV1 least square mean (L) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 Dose ratio 1:1 | 2 | 1000 | Mean Difference (Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 4.2 Dose ratio 1:2 | 1 | 499 | Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.11, 0.01] |

