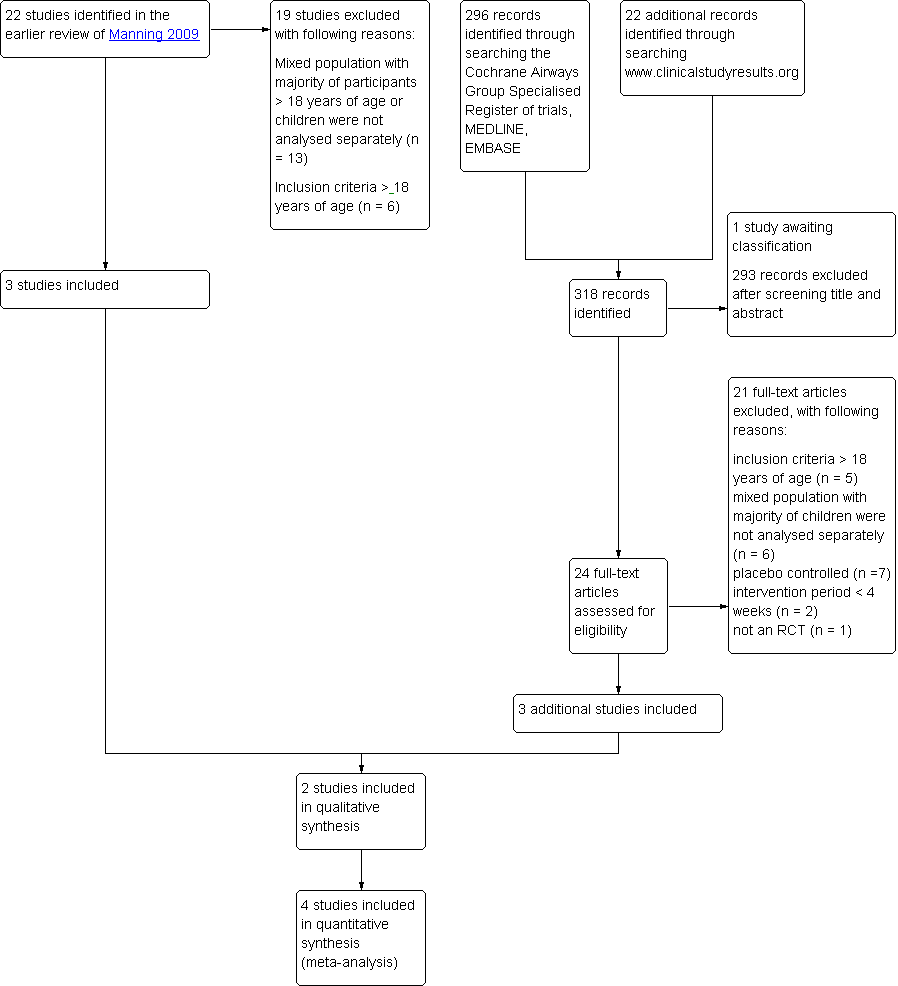
Ciclesonida versus otros corticosteroides inhalados para el asma crónica en niños
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Design: randomised controlled trial following a baseline period of 2 to 4 weeks (rescue medication only) and an intervention period of 12 weeks Location and number of centres: not reported | |
| Participants | Number screened: not reported Number randomised: 512 Number completed: not reported Age: children and adolescents (4 to 15 years) with predominantly moderate‐to‐severe asthma Gender: not reported Asthma severity: forced expiratory volume in 1 second (FEV1) 50‐90% of predicted Inclusion/exclusion criteria: not reported | |
| Interventions | Ciclesonide 160 μg (ex‐actuator; N = 254) once daily in the evening Fluticasone 88 μg twice daily (176 μg/day, ex‐actuator; N = 258) Delivery: both medications were administered via a metered‐dose inhaler with spacer (AeroChamber Plus®) Inhalation technique: not reported Treatment period: 12 weeks (following 2 to 4 weeks' baseline period rescue medication only) Allowed asthma medication: not reported | |
| Outcomes | FEV1 from baseline to the end of the treatment period, morning peak expiratory flow, median percentage of asthma symptom‐ and rescue medication‐free days and incidence of adverse events | |
| Notes | Incomplete data since this study was only published as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not enough information |
| Other bias | Unclear risk | Not enough information |
| Methods | Design: randomised, double‐blind, 2 parallel‐group study Location and number of centres: not reported | |
| Participants | Number screened: not reported Number randomised: 420 Number completed: not reported Age: 7 to 12 years Gender: not reported Asthma severity: FEV1 50‐90% of predicted Inclusion/exclusion criteria: not described | |
| Interventions | 1. Ciclesonide once daily (160 µg/day) 2. Fluticasone twice daily (176 µg/day) Delivery: not reported Inhalation technique: not reported Treatment period: 12 weeks (following 2 to 4 weeks baseline period rescue medication only) Allowed asthma medication: not reported | |
| Outcomes | Forced expiratory volume in 1 second (FEV1) (mL), peak expiratory flow (PEF) (L/minute), asthma symptom scores, rescue medication use, asthma exacerbation | |
| Notes | Incomplete data since this study was only published as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not enough information |
| Other bias | Unclear risk | Not enough information |
| Methods | Design: 12‐week, randomised, multicentre, double‐blind, double‐dummy, 2‐arm, parallel group study, with a 2‐ to 4‐week baseline period Location and number of centres: 51 centres in Europe, South Africa and Canada | |
| Participants | Number screened: 728 enrolled Age: median 10 years Gender: 331 boys; 180 girls | |
| Interventions | 1. Ciclesonide 100 μg twice daily Delivery: hydro‐fluoroalkane metered dose inhaler Inhalation technique: adequate inhalation technique no details described | |
| Outcomes | FEV1, clinic peak expiratory flow (PEF), a.m. PEF, p.m. PEF, symptoms, rescue medication usage, adverse events | |
| Notes | Analysis of co‐variance included age and randomisation values as co‐variates and sex, treatment, and region/country as fixed factors Funding: Grant sponsor: ALTANA Pharma AG, Konstanz, Germany. This study was supported by ALTANA Pharma, Konstanz, Germany. The authors would like to thank Pro Ed Communications, Inc., Beachwood, also all Medicus International, London, UK for their editorial assistance. Editorial support was funded by ALTANA Pharma. Dr. Søren Pedersen has received remuneration for lectures from AstraZeneca and GlaxoSmithKline and served as a paid consultant for ALTANA Pharma and AstraZeneca. Ilse Theron is an employee of ALTANA Madaus Ltd, Woodmead, South Africa. Dr. Renate Engelstatter is an employee of ALTANA Pharma AG, Konstanz, Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer‐generated list (Program RANDOM) provided to the study centres by ALTANA Pharma AG (Konstanz, Germany)" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the investigator nor anyone at the study centre knew whether ciclesonide or fluticasone was administered" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the investigator nor anyone at the study centre knew whether ciclesonide or fluticasone was administered" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | Small differences in baseline characteristics |
| Methods | Design: 12‐week, randomised, double‐blind, double‐dummy, 3‐arm, parallel‐group study, following a 2– to 4‐week run‐in period Location and number of centres: 50 centres in Brazil, Germany, Hungary, Poland, Portugal and South Africa | |
| Participants | Number screened: 904 enrolled Number randomised: 744 randomised and entered treatment period Number completed: 33 patients terminated study, 711 completed (of the 744, 50 violated protocol leaving 694 in per protocol population) Age: 6 to 11 years; median age in each group 9 years (range: 6 to 11). Gender: 170 boys; 161 girls Inclusion criteria: outpatients aged 6 to 11 years with a history of persistent bronchial asthma, for ≥ 6 months were eligible for participation. To be entered into the treatment period, patients were required to have a forced expiratory volume in 1 second (FEV1) 50–90% of predicted and a FEV1 reversibility of ≥ 12% after inhalation of salbutamol 200 to 400 mg at the end of the run‐in period. In addition, patients had to present asthma symptoms on at least 6 of the last 10 consecutive days of the baseline period, or to use at least 8 puffs of rescue medication within the last 10 consecutive days of the baseline period. Furthermore, patients had to demonstrate a good inhalation technique when using a metered dose inhaler (MDI) without a spacer Exclusion criteria: a history of near‐fatal asthma that required intubation; a respiratory tract infection or asthma exacerbation within the last 30 days prior to study entry; more than 2 inpatient hospitalisations for asthma in the previous year; use of systemic corticosteroids during the study, within the last 30 days prior to study entry or for more than 60 days in the previous 2 years | |
| Interventions | 1. Ciclesonide MDI (80 μg once daily) (N = 252) 2. Ciclesonide 160 MDI (160 μg once daily) (N = 242) Both: in the evening (ex‐actuator; equivalent to 100 and 200 μg ex‐valve) 3. Fluticasone MDI (88 μg twice daily) (N = 250) ‐ fluticasone 176 (ex‐actuator; equivalent to 100 μg twice daily ex‐valve) in the morning and evening without a spacer Delivery: administered via HFA134‐a MDIs Inhalation technique: good inhalation technique, no details described Treatment period: a run‐in period (of at least 2 weeks and up to 4 weeks), in which eligible patients discontinued previous inhaled corticosteroids (ICS) and other controller medications followed by a 12‐week treatment period Allowed asthma medication: rescue medication salbutamol, patients were allowed to continue regular nasal corticosteroids at a constant dose | |
| Outcomes | Change in FEV1 (L), peak expiratory flow (PEF) (L/minute), PD20FEV1 to methacholine (bronchial provocation test with methacholine to assess the provocative dose producing a 20% fall of FEV1) was performed at a subgroup of sites, Pediatric Asthma Quality of Life Questionnaire (PAQLQ) and Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), asthma symptom scores and use of rescue medication (salbutamol), safety was assessed by adverse effect reporting, physical examination, vital signs and laboratory investigations, including haematology, urinalysis and biochemistry | |
| Notes | Analysis of co‐variance included treatment, gender and centre pool as fixed factors and baseline value and age as co‐variates Funding: Professor S. Pedersen has received consultancy fees and lecture honoraria from Nycomed and GlaxoSmithKline, and has worked on research projects supported by Nycomed, GlaxoSmithKline and AstraZeneca. Dr R. Engelstatter and Dr S. Hirsch are employees of Nycomed. Dr H.‐J. Weber was an employee of Nycomed at the time of writing of the manuscript. Professor A. Emeryk has received consultancy fees from Nycomed and lecture honoraria from Nycomed, GlaxoSmithKline and AstraZeneca, and has | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "…a 1:1:1 randomisation scheme by means of a computer generated randomisation list.…." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind and double‐dummy design |
| Blinding (performance bias and detection bias) | Low risk | Ciclesonide provided in the evening 1 or 2 puffs and fluticasone was administered in the morning and evening |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | No obvious baseline differences |
| Methods | Design: 12‐week, randomised, double‐blind, double‐dummy, parallel‐group study, following a 2‐week run‐in period Location and number of centres: 31 centres in Europe and South Africa | |
| Participants | Number screened: 431 Gender: 272 boys; 131 girls Exclusion criteria: oral corticosteroids within 4 weeks of study entry; concomitant severe diseases; relevant lung diseases or clinically relevant abnormal laboratory values; > 10 cigarette pack‐year smoking history; females of child‐bearing potential without contraception | |
| Interventions | 1. Ciclesonide 400 μg once daily Delivery: HFA‐MDI (ciclesonide); Turbohaler® dry powder inhaler (DPI) (budesonide) Inhalation technique: not described | |
| Outcomes | FEV1; peak expiratory flow (PEF); 24‐hour urinary free cortisol concentrations | |
| Notes | Analysis of co‐variance included baseline value, treatment, age, sex and country pool as co‐variates or factors (not specified) Funding: this study (EudraCT No: 2004‐ 001233‐41) was sponsored by ALTANA Pharma. ALTANA Pharma had a role in the study design, the collection, analysis and interpretation of the data and was involved in the writing of the report and the decision to submit the manuscript. The co‐authors H. Rauerc and R. Engelstatter were both employees of ALTANA Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation list was generated by the sponsor using a multiplicative congruential pseudo‐random number generator with modulus 231‐1 (Program RANDOM based on Fishman and Moore" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind and double‐dummy design |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy but ciclesonide was administered in 2 puffs with metered dose inhaler (MDI) and budesonide with Turbohaler® device 4 inhalations |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | No obvious baseline differences |
| Methods | Design: 12‐week, randomised, double‐blind, double‐dummy, 2‐arm, parallel‐group study, following a 2‐ to 4‐week run‐in period Location and number of centres: 59 centres in Europe and South Africa | |
| Participants | Number screened: 774 Gender: 395 boys; 226 girls Exclusion criteria: history of life‐threatening asthma, concomitant severe diseases; 2 or more hospitalisations for asthma within previous 12 months; asthma exacerbation during 4 weeks before baseline; systemic corticosteroids during 30 days before baseline; use of systemic corticosteroids for more than 60 days within the previous 2 years; participation in another study within 30 days before baseline. No other asthma medication permitted during study | |
| Interventions | 1. Ciclesonide 200 μg once daily Delivery: ciclesonide: hydro‐fluoroalkane metered dose inhaler (HFA‐MDI) (+ AeroChamber®); budesonide: Pulmicort Turbohaler® Inhalation technique: not described | |
| Outcomes | FEV1, peak expiratory flow, asthma symptoms, rescue medication, bone growth, 24‐hour urinary cortisol, adverse events | |
| Notes | Analysis of co‐variance included baseline value at randomisations visit and age as co‐variates Funding: this study was funded and sponsored by ALTANA Pharma. The authors would like to thank ProEd Communications, Inc., Beachwood Ohio and Medicus International, London, UK, for their editorial assistance. Editorial support was funded by ALTANA Pharma. The co‐authors Renate Engelstatter Stefan Leichtl, Stefan Hellbardt and Thomas D. Bethke were employees of ALTANA Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomised at a ratio of 2:1…" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind and double‐dummy design |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy, not specified who was blinded Ciclesonide and budesonide were administered in the evening via an HFA‐MDI with an AeroChamber Plus® spacer and Pulmicort Turbohaler®, respectively |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | No obvious baseline differences |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Children not analysed separately | |
| Treatment < 4 weeks | |
| Children not analysed separately | |
| Placebo controlled | |
| Children not analysed separately | |
| Placebo controlled | |
| Children not analysed separately | |
| Included patients > 18 years of age | |
| Included patients > 18 years of age | |
| Included patients > 18 years of age | |
| Placebo controlled | |
| Included patients > 18 years of age | |
| Children not analysed separately | |
| Included patients > 18 years of age | |
| Not a randomised controlled trial | |
| Treatment < 4 weeks | |
| Placebo controlled | |
| Children not analysed separately | |
| Placebo controlled | |
| Children not analysed separately | |
| Placebo controlled | |
| Children not analysed separately | |
| Included patients > 18 years of age |
Characteristics of studies awaiting assessment [author‐defined order]
Jump to:
| Methods | Randomised controlled trial, double‐blind, study duration consists of a baseline period (2 to 4 weeks) and a treatment period (12 weeks) |
| Participants | Children aged 4 to 15 years Main inclusion criteria: history of persistent bronchial asthma for at least 6 months, forced expiratory volume in one second (FEV1) 50‐90% of predicted Main exclusion criteria: concomitant severe diseases or diseases which are contraindications for the use of inhaled corticosteroids; chronic obstructive pulmonary disease (chronic bronchitis or emphysema), other relevant lung diseases causing alternating impairment in lung function, or a combination; respiratory tract infection or asthma exacerbation within the last 30 days prior to entry into the study; history of life‐threatening asthma; premature birth; current smoking; smoking history with either ≥ 10 pack‐years; pregnancy; intention to become pregnant during the course of the study; breast feeding; lack of safe contraception |
| Interventions | Ciclesonide 200 μg/day Fluticasone propionate 200 μg/day |
| Outcomes | Primary outcome measures: FEV1 absolute values Secondary outcome measures: FEV1 as % of predicted, peak expiratory flow (PEF) from spirometry, diary‐based morning and evening PEF, diary‐based symptom score, diary‐based salbutamol metered dose inhaler (MDI) use, diurnal PEF fluctuation, drop‐out rate due to asthma exacerbations, time until asthma exacerbation, number of symptom‐free and rescue medication‐free days, number of days with asthma control, physical examination, vital signs, laboratory work‐up, adverse events |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with exacerbations Show forest plot | 2 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.75, 6.43] |
| Analysis 1.1  Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 1 Patients with exacerbations. | ||||
| 2 Quality of life PAQLQ (S) Show forest plot | 2 | 1010 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| Analysis 1.2 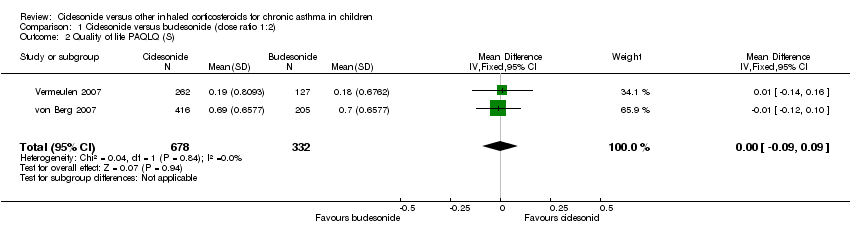 Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 2 Quality of life PAQLQ (S). | ||||
| 3 FEV1 least square means (L) Show forest plot | 2 | 1021 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
| Analysis 1.3  Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 3 FEV1 least square means (L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with exacerbations Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 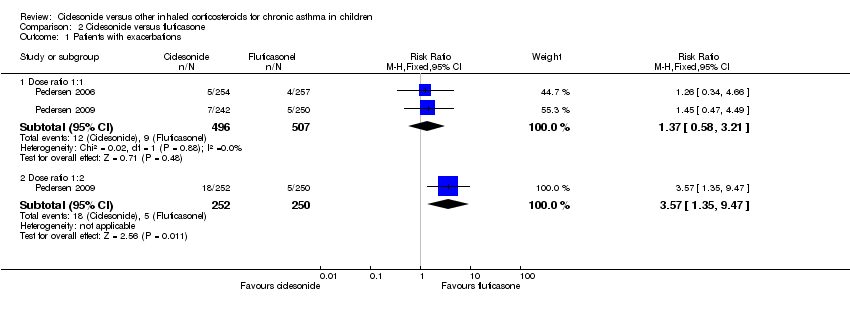 Comparison 2 Ciclesonide versus fluticasone, Outcome 1 Patients with exacerbations. | ||||
| 1.1 Dose ratio 1:1 | 2 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.58, 3.21] |
| 1.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.35, 9.47] |
| 2 Adverse events: number of patients with adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 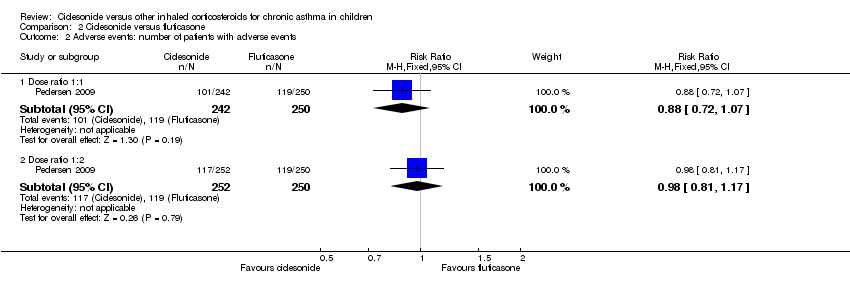 Comparison 2 Ciclesonide versus fluticasone, Outcome 2 Adverse events: number of patients with adverse events. | ||||
| 2.1 Dose ratio 1:1 | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 2.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.17] |
| 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 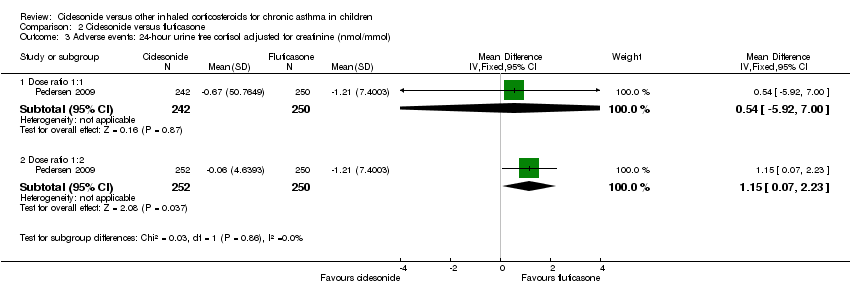 Comparison 2 Ciclesonide versus fluticasone, Outcome 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol). | ||||
| 3.1 Dose ratio 1:1 | 1 | 492 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐5.92, 7.00] |
| 3.2 Dose ratio 1:2 | 1 | 502 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.07, 2.23] |
| 4 Generic FEV1 least square mean (L) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Ciclesonide versus fluticasone, Outcome 4 Generic FEV1 least square mean (L). | ||||
| 4.1 Dose ratio 1:1 | 2 | 1000 | Mean Difference (Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 4.2 Dose ratio 1:2 | 1 | 499 | Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.11, 0.01] |
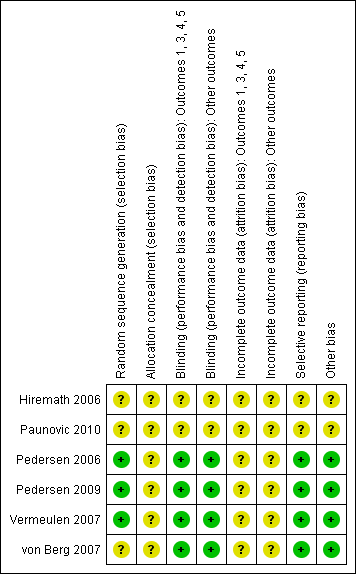
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 1 Patients with exacerbations.

Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 2 Quality of life PAQLQ (S).

Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 3 FEV1 least square means (L).

Comparison 2 Ciclesonide versus fluticasone, Outcome 1 Patients with exacerbations.

Comparison 2 Ciclesonide versus fluticasone, Outcome 2 Adverse events: number of patients with adverse events.

Comparison 2 Ciclesonide versus fluticasone, Outcome 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol).

Comparison 2 Ciclesonide versus fluticasone, Outcome 4 Generic FEV1 least square mean (L).
| Ciclesonide versus budesonide (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Budesonide (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptoms | See comment | See comment | Not estimable | 1024 | ⊕⊕⊝⊝ | Both studies used a 5‐point scale, but insufficient data were reported to allow meta‐analysis |
| Patients with exacerbations | 12 per 1000 | 26 per 1000 | RR 2.2 | 1024 | ⊕⊝⊝⊝ | |
| Adverse events | See comment | See comment | Not estimable | 1024 | ⊕⊕⊝⊝ | The data could not be meta‐analysed because the definitions of adverse events were too diverse |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 In one study the dose of budesonide was much higher than what is commonly prescribed in clinical practice. | ||||||
| Ciclesonide versus fluticasone (dose ratio 1:1) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:1) | Ciclesonide | |||||
| Asthma symptoms | See comment | See comment | Not estimable | 1468 | ⊕⊕⊕⊝ | 2 studies used a 5‐point scale and 1 study did not provide details how asthma symptoms were measured. Data could not be pooled due to diversity in scales |
| Patients with exacerbations | 18 per 1000 | 24 per 1000 | RR 1.37 | 1003 | ⊕⊝⊝⊝ | |
| Adverse events | See comment | See comment | Not estimable | 1560 | ⊕⊕⊝⊝ | Adverse events were defined differently across studies therefore results could not be pooled |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two fully published studies were sponsored by the manufacturer and at least one of the authors of each study was an employee of the manufacturer that sponsored the study. | ||||||
| Ciclesonide versus fluticasone (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptom | The mean asthma symptom in the control groups was | The mean asthma symptom in the intervention groups was | 482 | ⊕⊕⊝⊝ | Estimates are medians indicating data was skewed | |
| Patients with exacerbations | 20 per 1000 | 70 per 1000 | RR 3.48 | 502 | ⊕⊝⊝⊝ | |
| Adverse events | 476 per 1000 | 471 per 1000 | RR 0.99 (0.89 to 1.08) | 502 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Based on one study that was underpowered for a non‐inferiority trial. | ||||||
| Study ID | Ciclesonide dose | Comparator ICS | Application | Inhalation technique | Treatment period |
| Ciclesonide versus budesonide | |||||
| 160 μg OD (ex‐actuator; equivalent to 200 μg ex‐valve) 2 x 80 μg puffs in the evening | Budesonide 400 μg OD 2 x 200 μg puffs | Ciclesonide: HFA‐MDI with an AeroChamber®; Budesonide: Turbohaler® | Not described | 12 weeks | |
| 320 μg OD (ex‐actuator; equivalent to 2 puffs of 200 μg ex‐valve) 2 x 160 μg puffs administered in the evening | Budesonide 800 μg OD (4 inhalations of | Ciclesonide: HFA‐MDI without spacer Budesonide: Turbohaler® | Not described | 12 weeks | |
| Ciclesonide versus fluticasone | |||||
| 160 μg OD | Fluticasone 88 μg BID | MDI with spacer, AeroChamber Plus® | Not described | 12 weeks | |
| 160 μg OD | Fluticasone 88 μg BID | No information provided | Not described | 12 weeks | |
| 80 μg BID (ex‐actuator; equivalent to 100 μg BID ex‐valve) | Fluticasone 88 μg BID (ex‐actuator dose, equivalent to 100 μg BID ex‐valve) | HFA‐MDI without spacer | Adequate inhalation technique no details described | 12 weeks | |
| 80 or 160 μg OD (ex‐actuator; equivalent to 100 and 200 μg ex‐valve) administered in the evening | Fluticasone 88 μg BID (176 ex‐actuator; equivalent to 100 μg BID ex‐valve) in the morning and evening | HFA 134‐MDI without spacer | Good inhalation technique, no details described | 12 weeks | |
| BID: twice daily; ex‐actuator: drugs that leaves the inhaler; ex‐valve: drugs that leaves the metering chamber valve; HFA‐MDI: hydrofluoroalkane‐propelled metered dose inhaler; ICS: inhaled corticosteroid; MDI: metered dose inhaler; OD: once daily. | |||||
| Dose | CIC 160 μg OD versus BUD 400 μg OD | CIC 320 μg OD versus BUD 800 μg OD |
| Dose ratio | 1:2 | 1:2 |
| Study | ||
| Primary outcomes | ||
| Asthma symptoms: asthma symptom score (sum score) | ITT: MD 0.01, 95% CI ‐0.14 to 0.16 PP: MD 0.03, 95% CI ‐0.20 to 0.25 Non‐inferiority acceptance limit = 0.3 | Median change from baseline (no CIs reported) ITT: CIC: ‐0.07; BUD: ‐0.14 PP: CIC: ‐0.07; BUD: ‐0.14 |
| Asthma symptoms: use of rescue medication (puff/day) | ITT: MD 0.06 puffs/day, 95% CI ‐0.26 to 0.38 | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days | ITT: CIC: mean 73%; BUD: mean 70% No difference between groups | ITT and PP: CIC: median 84%; BUD: median 85% Lower limit of the between difference was ‐1.4% and above non‐inferiority limit of ‐8% |
| Exacerbations: patients with exacerbations* | ITT: RR 2.71, 95% CI 0.61 to 12.11; Analysis 1.1 | ITT: RR 1.69, 95% CI 0.36 to 8.00; Analysis 1.1 |
| Adverse events: patients with adverse events | Adverse events were reported in 38% of patients in both groups | ITT: RR** 1.44, 95% CI 0.96 to 2.18 |
| Adverse events: change in body height | Mean change from baseline (least square mean) CIC: 1.18 cm; BUD: 0.70 cm | Not assessed |
| Adverse events: 24‐hour urine cortisol adjusted for creatinine | ITT: 2.99 nmol/mmol creatinine; P < 0.0001, one‐sided (decrease greater in the BUD group) | ITT: significant difference between groups (lower level in BUD group) |
| Secondary outcomes | ||
| Quality of life: PAQLQ(S) | ITT: MD ‐0.11, 95% CI ‐0.12 to 0.10, one‐sided superiority; Analysis 1.2 Non‐inferiority acceptance limits = not provided PP not reported | ITT: MD (least square mean) 0.01, 95% CI ‐0.14 to 0.16; Analysis 1.2 Non‐inferiority acceptance limit = ‐0.5% PP results were similar |
| Quality of life: PACQLQ | ITT: MD ‐0.08, 95% CI ‐0.27 to 0.11, one‐sided superiority Non‐inferiority acceptance limit not provided PP not reported | Not assessed |
| Compliance | Not assessed | Not assessed |
| Lung function: FEV1 (L) | ITT: MD (least square means) ‐0.019 L, 95% CI ‐0.059 to 0.022; Analysis 1.3 PP: MD (least square means) ‐0.034 L, 95% CI ‐75 to 10 Non‐inferiority acceptance limit = ‐100 mL | ITT: MD (least square means) ‐0.03 L, 95% ‐0.14 to 0.8; Analysis 1.3 PP: MD (least square means) ‐0.02 L, 95% CI ‐0.13 to 0.1 Non‐inferiority acceptance limit = ‐150 mL |
| Airway inflammation | Not assessed | Not assessed |
| BUD: budesonide; CI: confidence interval; CIC: ciclesonide; ITT: intention to treat analysis; MD: mean difference; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol; RR: risk ratio. * Exacerbations were defined as an increasing asthma symptoms requiring change or addition of patient's medication other than increasing rescue medication. ** Adverse events that needed treatment, reported in over 2% of patients in CIC or BUD group of safety population (N = 403). | ||
| Dose | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg OD vs. FP 88 μg BID |
| Dose ratio | 1:1 | 1:1 | 1:1 | 1:1 | 1:2 |
| Study | |||||
| Primary outcomes | |||||
| Asthma symptoms: asthma symptom score | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐0.29 to 0.14 | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP *: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score | Not assessed | Asthma symptom score decreased and was similar in both groups | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP **: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score |
| Asthma symptoms: use of rescue medication | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐1.23 to 2.12 | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.13; FP: ‐1.29 PP: CIC: ‐1.14; FP: ‐1.29 All P < 0.0001 | Not assessed | Use of rescue medication decreased and was similar in both groups | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.20; FP: ‐1.29 PP: CIC: ‐1.21; FP: ‐1.29 All P < 0.0001 |
| Asthma symptoms: a sthma symptom‐free days | Median difference (Hodges Lehmann point estimate) ITT: ‐1.01, 95% CI ‐4.60 to 2.46 PP: ‐1.01, 95% CI ‐4.82 to 2.51 | Not assessed | Not assessed | Not assessed | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days combined | Not assessed | Mean percentage was high and did not differ significantly between the treatment groups (PP) | Median CIC: 91.5%; FP: 94% P = 0.1320 (2‐sided between treatments) | Not assessed | PP: mean percentage was high and did not differ between the treatment groups |
| Exacerbations: number of patients with exacerbations | RR 1.26, 95% CI 0.34 to 4.66; Analysis 2.1 | RR 1.45, 95% CI 0.47 to 4.49; Analysis 2.1 | Not assessed | CIC: 2.3%; FP: 2.2% | RR 3.57, 95% CI 1.35 to 9.47; Analysis 2.1 |
| Adverse events: % of patients with adverse events | A similar percentage of patients reported adverse events | RR 0.88, 95% CI 0.72 to 1.07; Analysis 2.2 | The incidence of adverse events was similar in both groups | Not assessed | RR 0.98, 95% CI 0.81 to 1.17; Analysis 2.2 |
| Adverse events: cortisol 24‐hour urine sample (nmol/mmol) | ITT: difference between 2 groups was not statistically significant ITT and restricted ITT (which included only those urine cortisol measurements with a corresponding urine creatinine value within the normal range) A statistically significant difference in favour of CIC was seen in the restricted ITT analysis (P = 0.006). The findings were similar for patients who were ICS‐naive and patients who had received ICS prior to study entry although the differences were numerically greater in previously ICS‐naive patients | Safety analysis**: MD 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00; Analysis 2.3 | Not assessed | Not assessed | Safety analysis**: MD 1.15 nmol/mmol, 95% CI 0.07 to 2.23; Analysis 2.3 |
| Secondary outcomes | |||||
| Quality of life: PAQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 | Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 |
| Quality of life: PACQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 | Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 |
| Compliance | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Change in lung function: FEV1 (L) | ITT: MD (least square means) 0.0 L, 95% CI ‐0.042 to 0.042; Analysis 2.4 PP: MD (least square means) 0.001, 95% ‐0.044 to 0.046 | ITT: MD (least square means) ‐0.02 L, 95% CI ‐0.07 to 0.04; Analysis 2.4 PP: MD (least square means) ‐0.026, 95% CI ‐0.086 to 0.34 | Improvement similar between groups no point estimates | Improvement similar between groups no point estimates | ITT: MD (least square means) ‐0.05 L, 95% CI ‐0.11 to 0.01; Analysis 2.4 PP: MD (least square means) ‐0.056, 95% CI ‐0.12 to ‐0.004 |
| Airway inflammation | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| BID: twice daily; CI: confidence interval; CIC: ciclesonide; FP: fluticasone; ICS: inhaled corticosteroid; ITT: intention to treat analysis; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol analysis. * = In this study analyses were based on PP population and analysis of ITT population was used to confirm results, description of the results are unclear but we assumed it to be based on analysis of PP population. ** = safety analysis excluded patients with concurrent nasal, ophthalmological or dermatological corticosteroid treatment. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with exacerbations Show forest plot | 2 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.75, 6.43] |
| 2 Quality of life PAQLQ (S) Show forest plot | 2 | 1010 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 3 FEV1 least square means (L) Show forest plot | 2 | 1021 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with exacerbations Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dose ratio 1:1 | 2 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.58, 3.21] |
| 1.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.35, 9.47] |
| 2 Adverse events: number of patients with adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Dose ratio 1:1 | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 2.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.17] |
| 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Dose ratio 1:1 | 1 | 492 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐5.92, 7.00] |
| 3.2 Dose ratio 1:2 | 1 | 502 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.07, 2.23] |
| 4 Generic FEV1 least square mean (L) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 Dose ratio 1:1 | 2 | 1000 | Mean Difference (Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 4.2 Dose ratio 1:2 | 1 | 499 | Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.11, 0.01] |